Abstract
Background & Aims
Pancreatic adenocarcinoma has been associated with several familial cancer syndromes that also predispose to other malignancies. Younger ages of onset of pancreatic cancer (PC) have been reported in families with these syndromes.
Methods
Six hundred twenty-four consecutive patients (probands) from the Mayo Clinic Pancreatic Cancer Patient Registry who completed questionnaires were analyzed for family history of cancer and cigarette smoking. The ages at diagnosis of those probands who reported a family history (first- or second-degree relative) of PC, breast, ovarian, colorectal cancer, or melanoma were compared with those probands who did not. Multivariable regression analyses were performed with age at diagnosis as the primary outcome variable.
Results
As expected, smokers had a younger median age of onset of PC than nonsmokers in dose-dependent fashion (P = .0003). After controlling for tobacco exposure and gender, those probands with a family history of breast (−3.23 years, P = .001), ovarian (− 5.63 years, P = .005), colorectal (− 3.19 years, P = .002) cancers, and melanoma (− 5.75 years, P = .017) had a younger age of onset of PC than those who did not. Those with a family history of PC (−.61 years, P = .65) exhibited no difference. Probands reporting other cancers in relatives showed no difference (+.78 years, P = .49) in age of onset of PC.
Conclusion
A family history of cancers (breast, ovarian, colorectal, melanoma) associated with specific cancer syndromes that are known to contribute also to PC risk is associated with a younger onset of PC. A family history of PC does not appear to affect age of onset of PC.
Pancreatic cancer (PC) is a highly lethal, common malignancy, accounting for an annual incidence between 1–10/100,0001 and a mortality rate of 96%.2 Of the approximately 30,000 incident cases diagnosed each year in the United States, 5%–10% have a family history of PC,3 and there are ongoing efforts to study genetic factors.4,5 Although PC is predominantly a cancer of the elderly, it is of great interest and relevance that approximately 20% of patients diagnosed with PC are younger than the age of 60 years.6 This represents a significant proportion of potential years of life lost from PC. Younger patients are likely the best candidates for early surgical intervention if the cancer is caught early enough. It would be of great value to understand the identifiable risk profile for young-onset PC, so that it could be incorporated into strategies for prevention or screening.
There is some evidence to date that genetic predisposition to PC is associated with a younger age of onset of disease. James et al7 reported an analysis of 30 patients with familial pancreatic cancer (FPC) in a cohort of 826 patients seen at Roswell Park Cancer Institute. The age of onset of those with FPC was younger (57.6 years) versus sporadic PC patients (61.0), although the difference was not statistically significant. Notably, the FPC group had a higher proportion of ever-smokers (87% vs 66%).
Hahn et al8 described 64 PC patients from 26 families with FPC, defined as kindreds containing at least 2 affected first-degree relatives. The median age at diagnosis was 60 years (range, 33–81 years), with 14 patients (22%) younger than 50 years at diagnosis and 50% younger than 60 years at diagnosis. This is substantially younger than for non-FPC patients (20% younger than 60 years in Surveillance, Epidemiology, and End Results 9 database6), although a referral bias cannot be excluded. In a report from the Pancreatic Cancer Genetic Epidemiology Consortium (PACGENE), of which Mayo Clinic is a participant, affected probands from PC families were significantly younger than patients in the Surveillance, Epidemiology, and End Results database at diagnosis of PC (64.8 vs 70.0 years, P < .001). The age of onset was progressively younger as more relatives carried the diagnosis of PC.9
Families known to carry BRCA2 mutations had a median age of onset of PC similar to those families without mutations (59 years; range, 42–78 years).8 Lorenzo Bermejo and Hemminki10 reported data from the population-based Swedish Family-Cancer Database regarding history of breast cancer and risk for PC. Families with 2 or more cases of breast cancer younger than age 50 years had an increased risk for PC younger than the age of 50 years (standardized incidence ratio [SIR], 5.50; 95% confidence interval [CI], 1.43–14.2), but not PC overall. Smoking status was not reported.
In Familial Atypical Multiple Mole Melanoma syndrome (FAMMM), Lynch et al11 reported 5 families with identified mutations in the causal gene CDKN2A/p16. There were 14 cases of PC reported in these families with a median age of onset of 62 years (range, 46–78 years), which again is suggestive of a younger onset of disease in this familial syndrome.
Park et al12 reported 33 Korean and 42 Dutch families meeting the Amsterdam Criteria for Hereditary Non-Polyposis Colorectal Cancer. In the Korean families, 4 patients were diagnosed with PC at a mean age of 44.7 years. No PC cases were reported in the Dutch families. Again, this age of onset was substantially decreased over PC in population-based data, in which the mean age of onset is 65 years.13
A population-based study of 27,005 PC patients reported in abstract form that tobacco smoking lowered the age of onset of PC.14 The ages of onset of disease among never-smokers (73.0 years) and ever-smokers (67.0 years) were highly statistically significant (P < .001). In addition, Rulyak et al15 have reported an earlier age of onset by nearly a decade (59.6 vs 69.1 years, P = .01) among smoking members in FPC families. Therefore, in our proposed analysis of age of diagnosis by family history, smoking status was included in the analysis.
Methods
Six hundred twenty-four consecutive primary pancreatic adenocarcinoma patients (probands) from the Mayo Clinic Pancreatic Cancer Patient Registry were included in this study, diagnosed from October 2000–April 2005. Patients were enrolled by using ultra-rapid case ascertainment, meaning they were entered into the study at the time of their Mayo Clinic visit by a study coordinator in the hospital, in Gastroenterology clinic, or in Gastrointestinal Oncology clinic. Any patients missed through this approach were recruited by mail. This is in contrast to population-based PC studies that use rapid case ascertainment (retrospectively recruiting patients identified from tumor registries) for PC, in which up to 55% of potential patients might be deceased by the time they are contacted.16 More than 70% of all patients with pancreatic adenocarcinoma seen at Mayo Clinic during this time period have been enrolled in the study. On enrollment, a risk-factor and family history questionnaire is self-completed, and the diagnosis of PC is clinically confirmed. Age at time of diagnosis is computed from date of birth to date of medically confirmed diagnosis of pancreatic adenocarcinoma.
Of 895 total study participants, 271 patients who did not complete the questionnaire were excluded from the analysis. Six hundred twenty-four patients who returned questionnaires were included in the analysis. Mean age at diagnosis was 66.2 years, 55% were male, and 96% were white. Sixty-one percent of probands reported some smoking history, whereas 62% of never-smokers reported cohabitation with a regular smoker (environmental tobacco smoke [ETS]).
Self-reported family history (limited to first- or second-degree relatives) of any cancer (excluding non-melanoma skin cancers) was included in the analysis. Gender and smoking intensity were included in the multivariate analysis, comparing those who had family history of the cancers of interest versus those who did not. Because of the potential confounding of an age-at-diagnosis analysis with a pack-year tobacco exposure variable (older patients would likely have higher pack-year totals), smoking intensity was chosen as the tobacco variable for the analysis. Smoking intensity incorporated both ETS and personal smoking history. Increasing smoking intensity was categorized in the following manner: never-smoker with no ETS, never-smoker with ETS, smoker with 0–1/2 pack-per-day at maximum usage in lifetime, smoker with >1/2–1 pack per day, and smoker with >1 packs per day. ETS exposure was defined as patient report on the questionnaire that their mother and/or father smoked cigarettes, or if the patient’s spouse smoked cigarettes.
Statistical Methods
Kaplan-Meier survival analyses (with diagnosis of PC as the event and age at diagnosis of PC as the time to event) were implemented to examine the univariate associations between potential risk factors (smoking intensity and family history of various cancers) and the age of PC onset. Kaplan-Meier curves were used to visualize the trend in age of onset, and log-rank tests were used to test any apparent univariate associations for statistical significance. Linear regression models were used to estimate the mean effect on age of onset for potential risk factors after adjusting for important covariates. All tests were two-sided, and P values ≤.05 were considered statistically significant. All analyses were performed by using SAS Version 8.2 (SAS Institute, Cary, NC).
Results
Among the 624 probands, median onset of PC was 66.2 years; 342 (55%) were male, 382 (61%) reported any history of smoking, 74 (12%) reported a family history of PC, 15 (2%) reported a family history of breast cancer, 29 (5%) ovarian cancer, 131 (21%) colorectal cancer, and 21 (3%) melanoma. As expected, ever-smoker patients had a younger median age of onset of PC than never-smokers (−3.2 years, P = .042), and a dose-dependent effect of tobacco exposure on age of onset was demonstrated (P = .0003).
The ages of diagnosis of PC by gender were not different (67.4 years/female, 67.5 years/male; P = .31). The ages of onset of those probands who reported family history (first- or second-degree relatives) of pancreatic, breast, ovarian, melanoma, colorectal, lung, bladder, and head/neck cancer were compared with those probands who did not. Multivariable regression analyses were performed with age of onset as the primary outcome variable, adjusted for gender and smoking intensity for each malignancy. The patients excluded from the analysis who did not return questionnaires were similar to those included with regard to race distribution, gender, age, and stage distribution.
After controlling for tobacco exposure and gender, those probands with a family history of breast, ovarian, colorectal cancers, and melanoma had a younger age of onset than those probands who did not (Table 1, Figure 1). Those with a family history of PC (−.61 years, P = .65) had a similar age of onset to those who did not. Those probands with at least 2 other PCs in their family (N = 8) tended to be younger at diagnosis (−7.75 years), but this did not reach statistical significance (P = .056).
Table 1.
Effect of Family History of Specific Malignancies on Age of Diagnosis of Pancreatic Adenocarcinoma, Controlled for Smoking Intensity and Gender
| Cancer | N | Median age at diagnosis of PC (y) | Corrected difference in age (y) | 95% CI | P value |
|---|---|---|---|---|---|
| Breast | 155 | 65.6 | −3.23 | −5.19 to −1.27 | .001 |
| Ovarian | 29 | 57.9 | −5.63 | −9.59 to −1.68 | .005 |
| Colorectal | 131 | 62.7 | −3.19 | −5.27 to −1.13 | .002 |
| Melanoma | 21 | 65.1 | −5.75 | −10.5 to −1.01 | .017 |
| Breast, ovarian, colorectal, or melanoma | 263 | 64.3 | −3.73 | −5.44 to −.2.02 | <.001 |
| All others besides breast, ovarian, colorectal, melanoma | 227 | 69.4 | +.78 | −1.46 to +3.03 | .49 |
| Pancreatic | 74 | 65.5 | −.61 | −3.26 to +2.04 | .65 |
| Bladder | 33 | 64.9 | −1.83 | −5.60 to +1.95 | .34 |
| Lung | 141 | 64.0 | −1.91 | −3.97 to +.15 | .07 |
| Head/neck | 39 | 66.1 | +43 | −3.08 to +3.93 | .81 |
| No history of cancer | 134 | 69.6 | +.26 | −.81 to +3.35 | .23 |
Figure 1.
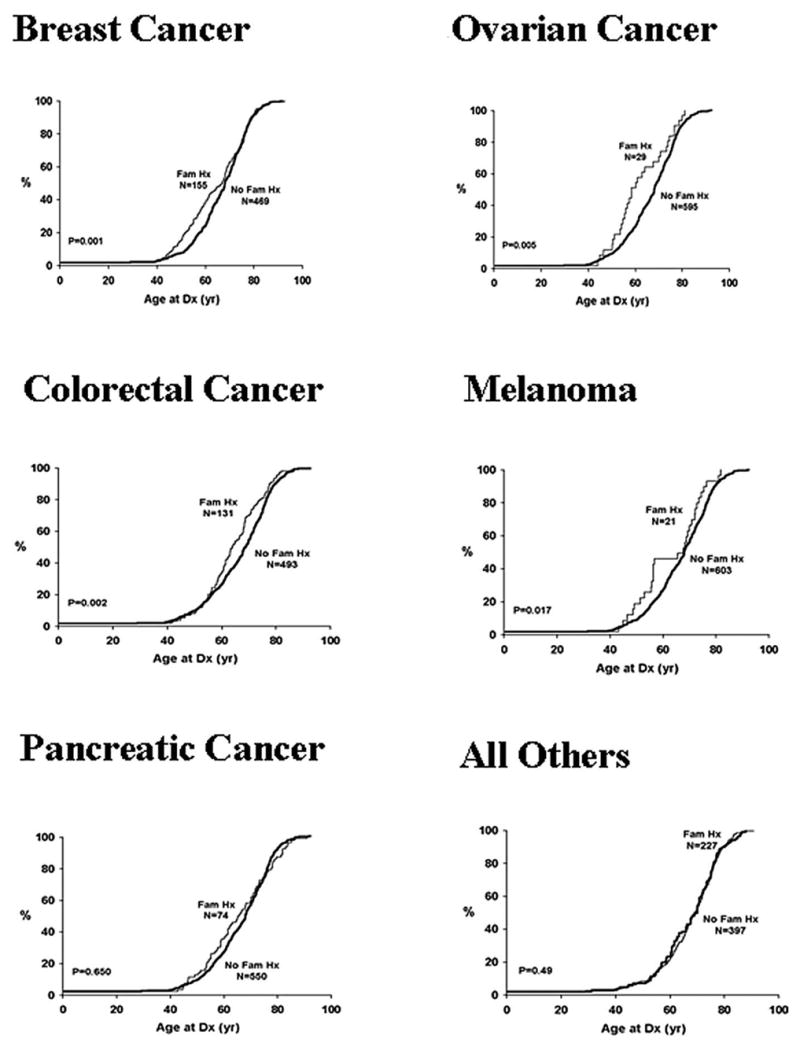
Age of onset of PC family history of selected cancers. All Others includes all cancers reported by probands except breast cancer, ovarian cancer, colorectal cancer, and melanoma.
No difference was seen in age of onset of PC for patients with a family history of other smoking-related cancers: bladder (−1.83 years, P = .34), head/neck (+.43, P = .81), or lung cancer (−1.91, P = .07). In addition, ages of diagnosis of PC among all other patients reporting any family history of cancer besides pancreatic, colorectal, breast, ovarian cancer, or melanoma (n = 227) were compared with those who reported no such history (n = 134) (Figure 1). No difference was noted (+.78 years, P = .49) in age at diagnosis of PC.
Discussion
In an analysis intended to identify underlying factors conferring a younger onset of PC, we found that several cancers known to be associated with familial cancer syndromes that include PC might be important. Patients reporting a family history (first- or second-degree relative) of breast cancer, ovarian cancer, colorectal cancer, or melanoma have a younger age of onset of PC, after taking into account reported smoking intensity and gender. Surprisingly, having a family history of PC was not associated with a younger age at diagnosis, although probands with more than 1 affected family member trended toward a younger age of onset. Family history of all other cancers combined and other smoking-related cancers (bladder, head/neck, and lung cancer) also showed no difference in the age of onset of PC in our probands.
Possibilities for this association include shared genetic risk factors such as the familial cancer syndromes described earlier, shared environmental factors, reporting bias, and chance. The cancer family syndromes that have been identified are thought to be relatively rare. However, when present, they could shift the age of onset enough to give the results found. Interestingly, studies involving breast cancer patients note an earlier age of onset not only in patients with known BRCA1/2 mutations but also in those with relatives affected with breast cancer in the absence of detected mutations.17
A shared genetic association, however, would likely increase overall risk for the respective cancers in families with PC, and no increase in risk for breast (SIR, .73), ovarian (SIR, .90), colorectal (SIR, .83) cancers, and melanoma (SIR, .65) was seen in our prior studies in relatives of PC patients.18 However, a trend was noted in families of PC patients diagnosed younger than the age of 60 years toward an increased risk for ovarian cancer (SIR, 2.20; 95% CI, .72–5.12), melanoma (SIR, 1.73; 95% CI, .70–3.57), and colorectal cancer (SIR, 1.37; 95% CI, .80–2.19). Although not statistically significant, these data could be expected if a subset of patients who carry shared genetic risk for pancreatic and these other cancers went on to develop pancreatic cancer at a young age. Hemminki and Li1 in a population registry in Sweden showed increased risk for lung cancer (SIR, 3.14; 95% CI, 1.86–4.97) in offspring of PC patients diagnosed younger than the age of 60 years. Bladder cancer (1.57), melanoma (1.92), endometrial (1.97), colon (1.46), rectal (1.45), and PC all showed trends toward increased risk, but they did not reach significance. Smoking status was not included in that analysis. Further studies assessing the impact of genetic mutation carriers in young-onset PC will be necessary to determine the full contribution of these genetic syndromes to young-onset PC. In the absence of definitive genetic testing, these family history associations provide some insight into potential underlying genetic predispositions. We included first- and second-degree relatives to maximize the number of relatives who have achieved ages of risk for the relevant cancers, whereas limiting the analysis to first-degree relatives would have enabled us only to have parents included in the analysis, because children of most patients would not have reached the peak ages of risk for most cancers, and siblings might or might not be a potential source of bias, on the basis of the age of the proband.
Shared environmental factors are another possible explanation, and our adjustment for smoking intensity helped to address this common risk factor. The lack of association of tobacco-related cancers and age of onset along with the dose-dependent effect of tobacco on age of onset in our study support our approach to correct the analysis for smoking. Although unknown risk factors could theoretically affect our results, no other environmental factor is known at this time to affect age of onset, and hence no further corrections were applied in our study. Reporting bias of family history of cancers could also affect the results, but prior validation of our family history reporting showed high sensitivity (86.4%) and specificity (100%) for reporting of cancer in first-degree relatives of PC patients.18 Finally, there is a possibility that our results are due to chance. However, our sample is probably the most comprehensive and unbiased sample of self-reported family history of cancer by PC patients to date. Our ultra-rapid ascertainment ensures that we obtained data from the majority of all PC patients approached at our institution. The patterns of associations observed are also inconsistent with chance associations.
The earlier age of onset in patients with a family history of these cancers has implications for genetic counseling. Persons with family histories of breast cancer, ovarian cancer, melanoma, or colorectal cancer appear to be at risk for PC at a younger age. As screening and prevention studies and strategies emerge for PC, identifying populations at risk, especially those at a younger age, will be vital for targeting these interventions.
Interestingly, we did not observe that a family history of PC alone affects age of onset, a finding that was not expected, given prior reports of a younger age of onset among FPC kindreds.7,15,19 No prior studies had controlled for smoking status, which could potentially be the sole cause for these reports of younger ages of onset in FPC.
Conclusion
A family history of cancers seen in familial cancer syndromes that are known to contribute to inherited risk (breast, ovarian, melanoma, colorectal), including PC, is associated with a younger onset of PC, when corrected for smoking and gender. A family history of PC alone does not appear to affect age of onset. Further research is needed in families known to have BRCA2, p16, Peutz-Jeghers syndrome, and mismatch repair mutations before any counseling recommendations can be made regarding their risk for earlier-onset PC.
Abbreviations in this paper
- CI
confidence interval
- ETS
environmental tobacco smoke
- FPC
familial pancreatic cancer
- PC
pancreatic cancer
- SIR
standardized incidence ratio
Footnotes
The Mayo Clinic Pancreatic Cancer Patient Registry is funded by a SPORE grant from the National Cancer Institute (NCI), Bethesda, MD (P50 CA102701). R.R.M. is supported by an R25T fellowship grant from NCI (R25T 92049).
The authors gratefully acknowledge the contributions of all of the patients who participated in the study, without whom they would not be able to carry out this research; and Tammy Dahl, RN and Kathy Liffrig for enrolling patients into the registry.
References
- 1.Hemminki K, Li X. Familial and second primary pancreatic cancers: a nationwide epidemiologic study from Sweden. Int J Cancer. 2003;103:525–530. doi: 10.1002/ijc.10863. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. Available from: http://caonline.amcancersoc.org/cgi/content/abstract/54/1/8. [DOI] [PubMed] [Google Scholar]
- 3.Brentnall TA. Cancer surveillance of patients from familial pancreatic cancer kindreds. Med Clin North Am. 2000;84:707–718. doi: 10.1016/s0025-7125(05)70253-4. [DOI] [PubMed] [Google Scholar]
- 4.Petersen GM, Hruban RH. Familial pancreatic cancer: where are we in 2003? J Natl Cancer Inst. 2003;95:180–181. doi: 10.1093/jnci/95.3.180. [DOI] [PubMed] [Google Scholar]
- 5.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. Available from: http://www.sciencedirect.com/science/article/B6X28-4846CSN-1/2/d47b3ea271949c5b9187010acb2e2659. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) program. www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Public-Use.
- 7.James TA, Sheldon DG, Rajput A, et al. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer. 2004;101:2722–2726. doi: 10.1002/cncr.20700. [DOI] [PubMed] [Google Scholar]
- 8.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 9.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo Bermejo J, Hemminki K. Risk of cancer at sites other than the breast in Swedish families eligible for BRCA1 or BRCA2 mutation testing. Ann Oncol. 2004;15:1834–1841. doi: 10.1093/annonc/mdh474. Available from: http://annonc.oxfordjournals.org/cgi/content/abstract/15/12/1834. [DOI] [PubMed] [Google Scholar]
- 11.Lynch HT, Brand RE, Hogg D, et al. Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: the familial atypical mole melanoma-pancreatic carcinoma syndrome. Cancer. 2002;94:84–96. doi: 10.1002/cncr.10159. [DOI] [PubMed] [Google Scholar]
- 12.Park JG, Park YJ, Wijnen JT, et al. Gene-environment interaction in hereditary nonpolyposis colorectal cancer with implications for diagnosis and genetic testing. Int J Cancer. 1999;82:516–519. doi: 10.1002/(sici)1097-0215(19990812)82:4<516::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16:1–16. doi: 10.1016/s0889-8588(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 14.Brand RE, Gorchow A, Brand RM. Gastrointestinal Cancers Symposium. Hollywood, FL: January 27–29, 2005. The effect of smoking on the age of pancreatic adenocarcinoma diagnosis. [Google Scholar]
- 15.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–1299. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 16.Hoppin JA, Tolbert PE, Holly EA, et al. Pancreatic cancer and serum organochlorine levels. Cancer Epidemiol Biomarkers Prev. 2000;9:199–205. Available from: http://cebp.aacrjournals.org/cgi/content/abstract/9/2/199. [PubMed] [Google Scholar]
- 17.Dite GS, Jenkins MA, Southey MC, et al. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst. 2003;95:448–457. doi: 10.1093/jnci/95.6.448. [DOI] [PubMed] [Google Scholar]
- 18.McWilliams RR, Rabe KG, Olswold C, et al. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer. 2005;104:388–394. doi: 10.1002/cncr.21166. [DOI] [PubMed] [Google Scholar]
- 19.Luttges J, Stigge C, Pacena M, et al. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer. 2004;100:173–182. doi: 10.1002/cncr.11860. [DOI] [PubMed] [Google Scholar]


