Abstract
Aging is the most prominent risk factor for Parkinson’s disease. Yet, consensus of how advancing age may predispose the dopamine (DA) system to parkinsonism is lacking. Three age-ranges of female rhesus monkeys, 8–9, 15–17 and 21–31 years, received unilateral DA depletion with intracarotid 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Morphological and biochemical analyses of DA-depleted and intact hemispheres revealed three primary findings: 1) The intact striatum exhibited age-related declines in dopamine (DA) and homovanillic acid (HVA) that were present by middle-age; 2) In the MPTP-treated striatum, the compensatory increase in DA activity was absent in old monkeys; and, 3) Age-associated morphological changes included declines in the density of tyrosine hydroxylase (TH) positive fibers in striatum, decreased nigral soma size and optical density of TH, but no significant loss of neurons. These findings suggest that aging produces changes in the nigrostriatal DA system that approach the threshold for expression of parkinsonian features, and that progressive impairment of plasticity may be central to the role of aging in development of parkinsonism.
Keywords: aging, dopamine, substantia nigra, parkinsonism, MPTP, monkey
The mesostriatal dopamine (DA) system exhibits dramatic degenerative changes in Parkinson’s disease (PD). The morphological and biochemical patterns of these changes are well characterized and consistent (Fahn, 2003). Aging is a well-established risk factor for PD (Granieri et al., 1991; Mayeux et al., 1995; Morens et al., 1996; Tanner and Goldman, 1996; Totaro et al., 2005). In contrast to PD, the pattern of aging-related changes in the DA system vary considerably among species (e.g. Morgan and Finch, 1988). Even within a species variability exists in the exact pattern of changes reported and whether age-related changes could be a precursor to the degeneration seen in PD. For nonhuman primates, there tends to be agreement that declines in striatal DA content accompany advancing age, but associated changes in midbrain DA neuron numbers and function are a source of disagreement (Goldman-Rakic and Brown, 1981; Irwin et al., 1994; Pakkenberg et al., 1995; Emborg et al., 1998; Gerhardt et al., 2002; McCormack et al., 2004). In addition, while inclusion of young adult, middle-aged, and elderly individuals is considered proper experimental design for studies of the biology of aging, in reality this can be difficult to accomplish and many reports in the literature compare only two of these age groups. As a consequence significant gaps exist in our understanding of the development of changes in the DA system over the lifespan in primates. In the present study we were fortunate to have available for study three age ranges of female rhesus monkeys: 8–9 years (N=3), 15–17 years (N=4), and 21–31 years (N=6). It is documented that rhesus monkeys exhibit aging-related changes consistent with a rate of aging 3:1 as compared to humans (Andersen et al., 1999). Accordingly, our groups of animals correspond to humans aged 24–27 years, 45–51 years, and 63–93 years. We contend these correspond to generally held views of young adulthood, middle-age, and advanced age. In the present study we treated monkeys with unilateral intracarotid infusion of the DA neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to create unilateral degeneration of the mesostriatal DA system. Our goal was not to revisit the previously established aging-related increased vulnerability to MPTP toxicity (Ovadia et al., 1995; McCormack et al., 2004), but rather to study aging-related differences and similarities in the response to equivalent insult to the DA system. Therefore, the MPTP dose was adjusted for monkeys of differing age to yield a constant behavioral endpoint: complete disuse of the contralateral arm and hand. Cell counts confirm that this MPTP dosing regimen produced the expected severe depletion of tyrosine hydroxylase (TH) positive neurons in substantia nigra ipsilateral to treatment while maintaining cell counts in the contralateral hemisphere that did not differ from those in untreated monkeys. Thus, after establishing our behavioral endpoint, we compared the morphology and biochemistry of the DA system ipsilateral to MPTP treatment to document aging-related changes in the response to lesion and the hemisphere contralateral to MPTP treatment to document changes due to aging itself.
Materials and Methods
Experimental Subjects and MPTP Treatment
Subjects were female rhesus monkeys (macaca mulatta) weighing 5.3–10.1kg. Three age groups were studied: young adult (8–9yr) N=3, middle-aged (15–17yr) N=4, and aged (21–31yr) N=6. All animals were born in captivity with chronological age documented by birth records. Animals were housed in individual primate cages and cared for in the AALAC approved Biological Resources Laboratory at the University of Illinois-Chicago. All monkeys were treated with unilateral intracarotid administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine as previously described (Emborg et al., 2001) (MPTP-HCl, Sigma Chemical, St. Louis, MO, first dose = 3.5 mg for monkeys ≥ 7kg body weight, 3.0 mg for monkeys <7kg body weight, 2.3 mg for monkeys >17 years of age, re-treatment, when necessary to achieve behavioral endpoint, = 2.0 mg) (Table 1). Treatment resulted in equivalent behavioral signs in all subjects, principally characterized by complete disuse of the forelimb contralateral to infusion. No obvious impairment of contralateral leg function was noted, and none of the treated monkeys exhibited spontaneous rotational behavior. Care and use of these animals was in compliance with all applicable laws and regulations as well as principles expressed in the National Institutes of Health, United States Public Health Service Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Care and Use Committees of the University of Illinois-Chicago and Rush University Medical Center where the live animal aspects of the study were conducted.
Table 1.
Experimental Subjects and MPTP Dose.
| Animal I.D. | Age (yr) | Weight (kg) | MPTP (mg) |
|---|---|---|---|
| Young | |||
| 6965 | 8 | 6.0 | 3.0; 2.0 |
| 6966 | 8 | 5.3 | 3.0 |
| 7009 | 9 | 6.4 | 3.0; 2.0 |
| Middle-aged | |||
| 6337 | 17 | 10.0 | 3.5 |
| 6327 | 17 | 6.4 | 3.0 |
| 6339 | 14 | 9.5 | 3.5; 2.0 |
| 7014 | 17 | 7.3 | 3.5 |
| Old | |||
| 6724 | 31 | 7.2 | 2.3 |
| 6725 | 30 | 7.3 | 2.3; 2.0 |
| 6254 | 26.5 | 6.9 | 2,3; 2.0 |
| 6334 | 20.5 | 10.1 | 2.3 |
| 6826 | 24.5 | 8.9 | 2.3 |
| 6823 | 25 | 7.4 | 2.3 |
Measurement of Spontaneous Activity
Prior to MPTP treatment and at 3 months after treatment, monkeys were transferred from their home cage to a cage equipped with a Plexiglas front panel for video tape recording of spontaneous activity. Activity was recorded for 30 minutes with no observers in the room. Videotapes were analyzed off-line by an examiner blinded to the experimental condition using motion tracking and analysis software (Ethovision 2.3, Noldus, Wageningen, The Netherlands). The analysis provides an objective measurement of kinematic parameters. In the present study we analyzed total distance moved (cm) as an index of general motor activity.
Tissue Collection
Three months following induction of behavioral symptoms, animals were killed by pentobarbital overdose (50 mg/kg with effect confirmed by absence of corneal reflex) and perfused with ice-cold physiological saline. Perfusion both serves to reduce the temperature of the tissue, aiding in stabilization of tissue catecholamines, and removes potential contamination of samples by catecholamines in blood. Brains were removed, and each forebrain was divided into coronal slabs of 4 mm thickness on ice. Tissue punches, 1.3 mm in diameter, were taken from standard locations (Sladek et al., 1995) in the dorsal-lateral caudate nucleus and putamen at precommissural and commissural levels of the striatum (Figure 1). Punches were frozen on dry ice and stored at −80 degrees C until processing with high performance liquid chromatography (HPLC) for detection of dopamine (DA) and homovanillic acid (HVA). Measures of 3,4-dihydroxyphenylacetic acid (DOPAC) also were obtained during HPLC analysis. When present, DOPAC amounts were <5% of HVA levels and in many samples were below the level of detection. Since DOPAC levels were not consistently obtained and HVA is the metabolite of greater relevance in nonhuman primate studies, we chose not to present DOPAC levels. After collection of fresh tissue punches for HPLC, remaining tissue blocks through the striatum and the brain caudal to the striatum was immersion fixed in 4% paraformaldehyde solution and sunk in 30% sucrose solution in preparation for sectioning and immunocytochemical staining for TH.
Figure 1.
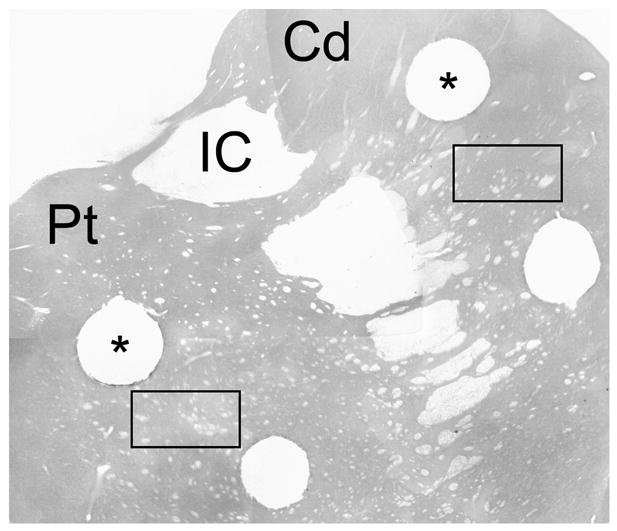
Locations of tissue punches for HPLC and regions analyzed for THir fiber staining. Tissue punches, 1.3mm in diameter (*), were taken from the dorsal lateral caudate nucleus and putamen at precommissural and commissural levels of the striatum for analysis of DA and HVA. A single 50X magnification microscopic field (rectangle) in caudate and putamen was analyzed for intensity of THir fiber staining in four coronal tissue sections from each subject. Tissue punches in ventral medial caudate and putamen were analyzed for striatal trophic factor activity reported in Collier et. al., 2005. Abbreviations: Cd = Caudate nucleus, IC = internal capsule, Pt = putamen.
Dopamine Biochemistry
Levels of DA and HVA were analyzed using HPLC. Punches of caudate nucleus and putamen were dissected out, placed in vials with 200 μl of an anti-oxidant solution (perchlorate and sodium metabisulfite) and immediately frozen on powdered dry ice and stored at –80°C. At the time of analysis samples were thawed and homogenized using an ultrasonic tissue homogenizer. Supernatants were separated and detected via HPLC and pellets were reserved for protein determination (Pierce BCA Protein Reagent Kit). Samples were simultaneously examined for DA and HVA using a 12 channel coulometric array detector (CoulArray 5200, ESA) attached to a Waters 2695 Solvent Delivery System. All procedures within the laboratory were carried out at GLP quality control levels utilizing appropriate 6-point standard curves and interspersed quality control samples to ensure HPLC calibration.
Immunocytochemical Analysis
Fixed tissue was sectioned on a freezing microtome in the coronal plane at 50μm section thickness. Staining for TH (mouse antiTH monoclonal, 1:4,000, Chemicon International, Temecula, CA) as a marker for DA neurons and their processes was visualized by using the Vectastain ABC protocol (Vector Laboratories, Burlingame, CA). Stereological estimates of TH stained substantia nigra (SN) neurons were performed using a BX52 Olympus microscope (Olympus America Inc.) equipped with Microbrightfield stereological software and a Microfire CCD camera (Optronics, Goleta, CA). The camera settings were maintained throughout the entirety of the experiment. Using the optical fractionator procedure, neurons were sampled in a uniform, systematic, but random design. The region of interest was outlined under low magnification (1.25X), demarcating SN by the caudal edge of the mammillary bodies rostrally, the cerebral peduncle ventrally and laterally, and the lateral edge of the third cranial nerve rootlets medially. Approximately, 5% of the outlined region was sampled with a series of counting frames that were randomly and systematically distributed through the region of interest. Using a 60X magnification lens with a 1.4 N/A the section thickness was empirically determined by first bringing the top of the section into focus and then using the Microbrightfield program to step through the z-axis in 1μm increments until the very bottom of the section was in focus. Once the top of the section was in focus, the z-plane was lowered at 1–2 μm intervals and cell counts were made according to stereological principles while focusing down through the z-axis. Care was taken to ensure that the bottom and top guard zones were never included in the analysis. Eight to ten equally spaced sections (1 in 6 series) were sampled from each subject. After obtaining the cell counts the stereological software generates accurate estimates of the total number of cells in the whole cell population based on stereological principles and equations. Additional image analysis was performed on TH stained neurons sampled from three cytoarchitectural subdivisions of the SN: dorsal tier, ventral tier, and pars lateralis. As few as 10 cells from the lesioned hemisphere and up to 80 cells from the intact hemisphere were sampled from a single 50X magnification field positioned in the center of these regions from 3 tissue sections at the level of the third cranial nerve rootlets. Sampling at this level provides the clearest demarcation of these subregions. Sampling of a single microscopic field placed in the center of these regions insures that the cells measured are within the region, but precludes more complete sampling. Both intensity of TH staining and soma area were quantified using computer-assisted imaging (Sigma Scan/Sigma Scan Pro software, Jandel Scientific, San Rafael, CA). The TH intensity measure is semi-quantitative. Sections from all monkeys were processed simultaneously for TH immunoreactivity, ensuring constant reaction conditions. As such, variations in expression of TH immunoreactivity by individual cells while not appropriate as a linear correlate of protein expression can be used in assessment of relative differences among cell regions and age groups. The staining intensity value for each cell represented the average of TH immunoreactivity over the cross-sectional area of each cell expressed relative to the staining intensity of white matter in the cerebral peduncle in the same tissue section. Measures of staining intensity and soma area for each SN subregion were averaged for the 3 tissue sections to provide a single mean value for each cell region for each monkey. These mean values were used for statistical analysis across age groups. Finally, the average intensity of TH fiber staining in the striatum was determined using the same software package. A single 50X magnification microscopic field in the center of the caudate nucleus and putamen of each subject (Figure 1) was assessed in four coronal tissue sections (at 300μm intervals) immediately rostral to the decussation of the anterior commisssure and expressed relative to staining intensity of white matter in the internal capsule in the same tissue section. Measures of staining intensity of fibers were averaged across tissue sections to provide a single mean value for each structure for each monkey. These mean values were used for statistical analysis across age groups.
Statistics
Comparisons of motor activity scores, regional levels of DA and HVA in tissue punches, stereological cell number estimates, intensity of TH staining in nigral neurons and striatal neuropil, and TH neuron soma area were analyzed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test (Statview software). Motor activity was analyzed with a repeated measures design (Super ANOVA software).
Results
Spontaneous locomotion in a cage used for videotaping was assessed over a 30 minute duration recording session prior to MPTP treatment and at 3 months after treatment. Total distance traveled was computed using the Ethovision software program. At baseline, young adult, middle-aged, and aged monkeys were progressively less active (Figure 2). All monkeys were unilaterally depleted of striatal DA by MPTP treatment to achieve the behavioral endpoint of complete disuse of the contralateral arm and hand. All monkeys held the affected arm in a flexed posture and would not retrieve food with this hand. Overt impairment of the contralateral lower limb was not detected, but not explicitly tested, nor did subjects exhibit spontaneous rotational behavior. Statistical analysis revealed significant differences attributable to age (F(2,5)=6.91, p<0.04), treatment (pre- and post-MPTP, F(1,5)=41.09, p<0.002) and the age × treatment interaction (F(2,5)=9.89, p<0.02). Post-hoc analysis indicated that significant differences were attributable to the large decrease in baseline spontaneous activity in aged monkeys compared to young monkeys (-79%, p=0.005). After MPTP treatment, further reductions in activity were observed for all age groups, but none of these changes reached statistical significance.
Figure 2.
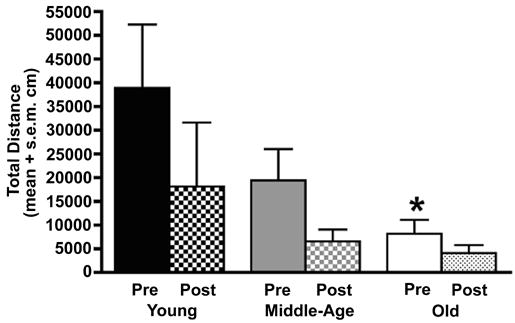
Spontaneous locomotor activity progressively decreases with advancing age. Data represents total distance traveled (cm) in a video recording cage over a thirty minute interval prior to MPTP treatment (Pre) and 3 months after induction of unilateral parkinsonism (Post). Prior to MPTP treatment, spontaneous activity was noted to decrease progressively with advancing age, reaching statistical significance for the comparison of young adult animals to aged animals (* p=0.005). Further decreases in motor activity were noted after MPTP treatment, but these were not statistically different between age groups.
Measurement of DA and the metabolite HVA from both intact and DA-depleted caudate nucleus and putamen revealed aging-related changes. For the intact hemisphere, both caudate and putamen showed statistically significant declines in DA that began in middle-age (caudate -55%, putamen –54%) and were sustained in old age (caudate -43%, putamen –30%) (Figure 3) (Caudate: F(5,42)=41.98, p<0.0001; p<0.0001 for comparison of young intact to middle-aged and aged intact; Putamen: F(5,42)=32.90, p<0.0001; p<0.0001 for comparison of young intact to middle-aged and aged intact). HVA also was significantly reduced from young adult levels in the caudate nucleus and putamen of middle-aged (caudate -46%, putamen –50%) and aged monkeys (caudate –20%, putamen -30%)(Figure 3)(Caudate: F(5,42)=29.83, p<0.0001; p<0.05 for comparison of young intact to middle-aged and aged intact; Putamen: F(5,42)=24.19, p<0.0001; p≤0.0001 for comparison of young intact to middle-aged and aged intact). No differences among age groups were detected for the HVA/DA ratio in either striatal structure.
Figure 3.
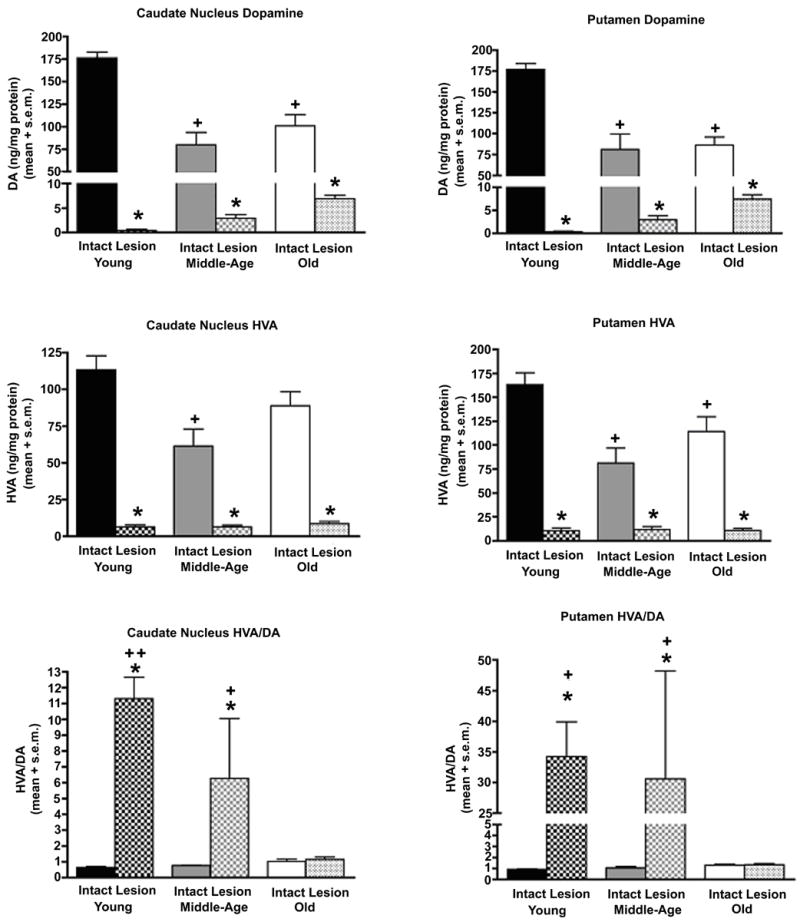
With advancing age, striatal DA content declines and the capacity to increase DA activity in response to insult is lost in the oldest monkeys. Significant aging-related decreases in DA content of the caudate nucleus and putamen of the intact hemisphere were detected in middle-age and sustained in old age. As expected, dramatic loss of striatal DA accompanied MPTP exposure in the opposite hemisphere (caudate and putamen: + p<0.0001 for comparison of young intact to middle-aged and aged intact; * p<0.0001 for comparison of lesioned hemisphere to intact hemisphere). Decreases in striatal HVA also accompanied aging and exposure to MPTP (caudate and putamen: + p<0.05 for comparison of young intact to middle-aged and aged intact; * p<0.0001 for comparison of lesioned hemisphere to intact hemisphere). Large increases in striatal DA activity followed exposure to MPTP in young and middle-aged monkeys, but this compensatory response was completely absent in aged monkeys (Caudate: * p<0.005 for comparison of intact to lesioned hemispheres, + p<0.009 for comparison of middle-age lesion to old lesion, ++ p<0.04 for comparison of young lesion to both middle-age and old lesion. Putamen: * p<0.005 for comparison of intact to lesioned hemispheres, + p<0.003 for comparison of young and middle-age lesion to old lesion).
In the DA-depleted striatum, there were the expected dramatic decreases in DA (-92-99%) and HVA (-85-94%) in all age groups as compared to their intact hemisphere (Figure 3)(Caudate and Putamen DA and HVA p<0.0001 for comparison of lesioned hemisphere to intact hemisphere). No statistically significant differences in the extent of depletion were found between the age groups. This confirms that equivalent damage to the DA system was achieved in animals of all age groups. The most dramatic aging-related change in DA biochemistry was found for the HVA/DA ratio. In both caudate nucleus and putamen, the anticipated significant increase in DA activity in response to MPTP-induced degeneration was present in young and middle-aged monkeys, but completely absent in the oldest age group (Figure 3)(Caudate: F(5,41)=7.42, p<0.0001; p<0.005 for comparison of intact to lesioned hemispheres, p<0.009 for comparison of middle-age lesion to old lesion, p<0.04 for comparison of young lesion to both middle-age and old lesion; Putamen: F(5,41)=5.85, p<0.0005; p<0.005 for comparison of intact to lesioned hemispheres, p<0.003 for comparison of young and middle-age lesion to old lesion).
Light microscopy for TH immunoreactive (THir) neurons of the substantia nigra pars compacta (SNC) in the intact hemisphere viewed at low magnification suggested the presence of a dramatic aging-related decline in neuron number and dendritic neuropil (Figure 4). However, when SNC THir neuron numbers were quantified using unbiased stereology, no differences in neuron counts were found across age groups (Figure 6). Marked decreases in cell numbers accompanied MPTP treatment (Figures 4 & 5) and these were not different with increasing chronological age (Figure 6) (F(5,16)=40.86, p<0.0001; p<0.0001 for comparison of intact to lesioned hemisphere for all ages).
Figure 4.
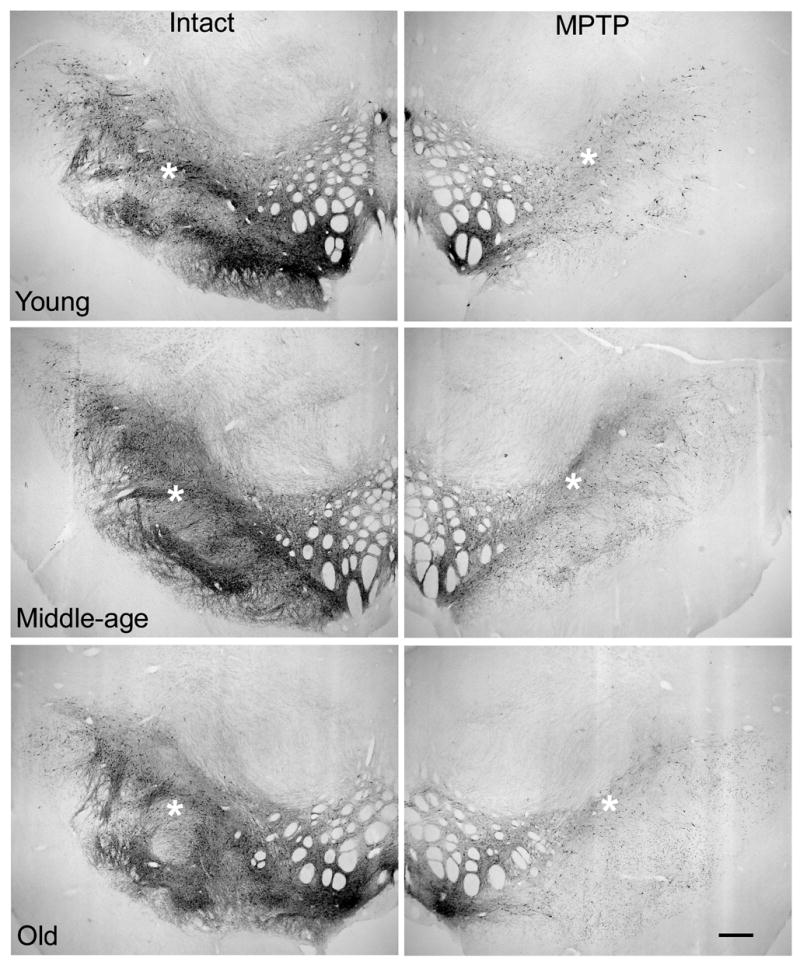
Low magnification views of TH immunoreactivity in SNC of the intact hemisphere suggest a decrease in cell number and neuropil density accompany advancing age (calibration bar=600μm). However, quantitation of THir cell numbers with stereology indicates no aging-related loss of neurons. The difference in appearance at low magnification was accounted for by a progressive increase in the frequency of THir neurons that stained lightly and decreased in cross-sectional area with advancing age (Figure 5). The expected dramatic loss of THir SNC neurons accompanied MPTP exposure in the ipsilateral hemisphere. * denote regions associated with high magnification views presented in Figure 5.
Figure 6.
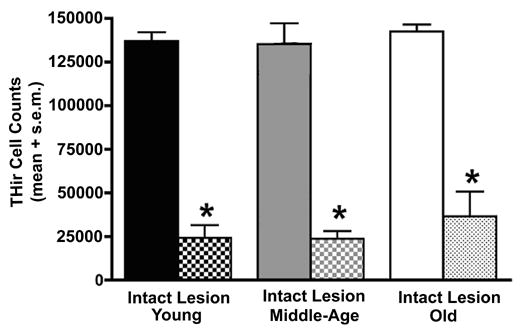
Counts of THir neurons using stereology reveal no aging-related change in the intact hemisphere of monkeys. The expected significant loss of THir neurons accompanied exposure to MPTP. The magnitude of this loss was not different among age groups (* p<0.0001 for comparison of intact to lesioned hemisphere).
Figure 5.
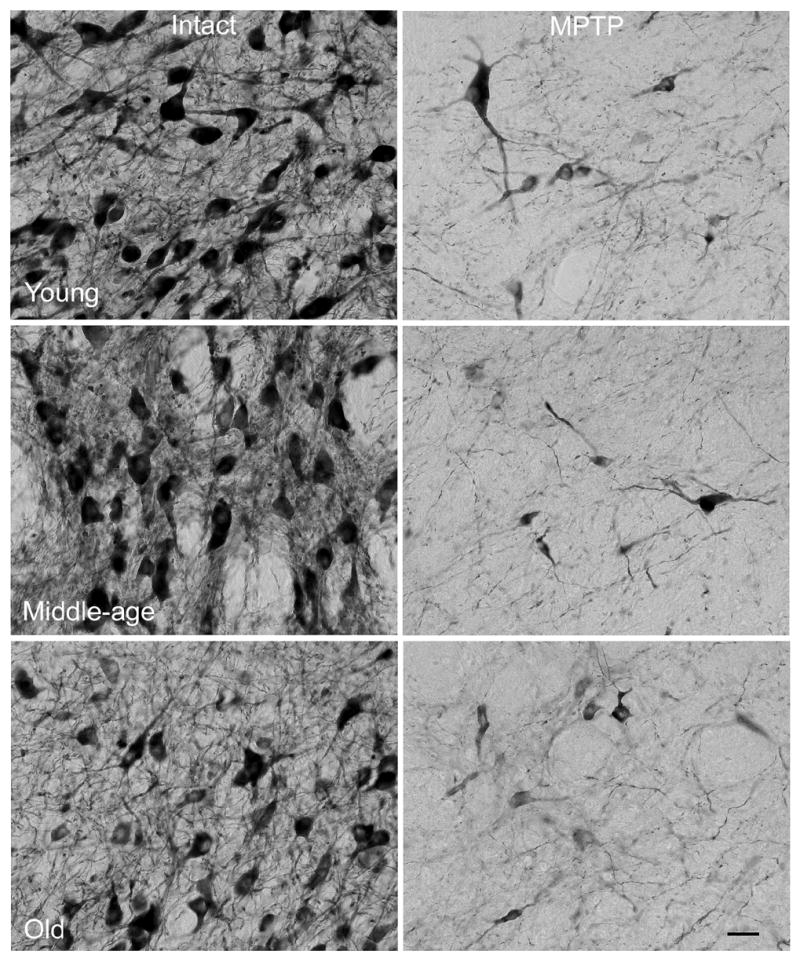
With advancing age, the number of THir SNC neurons that stain lightly and exhibit lower cross-sectional area increase in the intact hemisphere. While THir intensity and soma area are preserved through middle-age, old subjects exhibited increased variability in these measures. SNC neurons surviving the MPTP insult in the ipsilateral hemisphere exhibited variable further declines in THir intensity and soma area. * in Figure 4 depict locations of higher magnification views presented here. Calibration bar = 50μm.
Despite the absence of overt cell loss in SNC with advancing age, analysis of THir neuron staining intensity and soma area revealed some aging-related changes (Figures 5 & 7). These changes accounted for the dramatic difference in appearance of SNC in the intact hemisphere at low magnification. For this analysis, cells were separately sampled in the main cytoarchitectural subdivisions of SNC: the dorsal tier, ventral tier, and pars lateralis (Figure 7). For dorsal tier and ventral tier neurons, an aging-related decrease in THir intensity was detected in the intact hemisphere of the oldest monkeys as compared to young adult monkeys (dorsal tier: F(5,16)=4.26, p<0.02; ventral tier: F(5,16)=3.84, p<0.02; p<0.02 for comparison of young to old). Neurons in pars lateralis exhibited no statistically significant decline in THir intensity (p=0.053), but showed significant declines in soma area in both middle-aged and aged monkeys (F(5,16)=7.40, p<0.001; p<0.03 for comparison of young to middle-age and old). In general, both THir intensity and soma area decreased in surviving neurons following MPTP treatment, but only some of these changes achieved statistical significance. In pars lateralis, MPTP treatment produced significant declines in soma area in young adult and aged monkeys, with this decrease exaggerated in the oldest group (F(5,16)=7.40, p<0.001; p<0.03 for comparison of intact to MPTP-treated, p<0.04 for comparison of young MPTP treated to old MPTP-treated). No significant changes in THir intensity accompanied the declines in soma area in pars lateralis. In addition, aged monkeys exhibited a significant decrease in THir soma area in dorsal tier neurons after MPTP treatment (F(5,16)=3.43, p<0.03; p<0.01 for comparison of aged intact to aged MPTP-treated).
Figure 7.
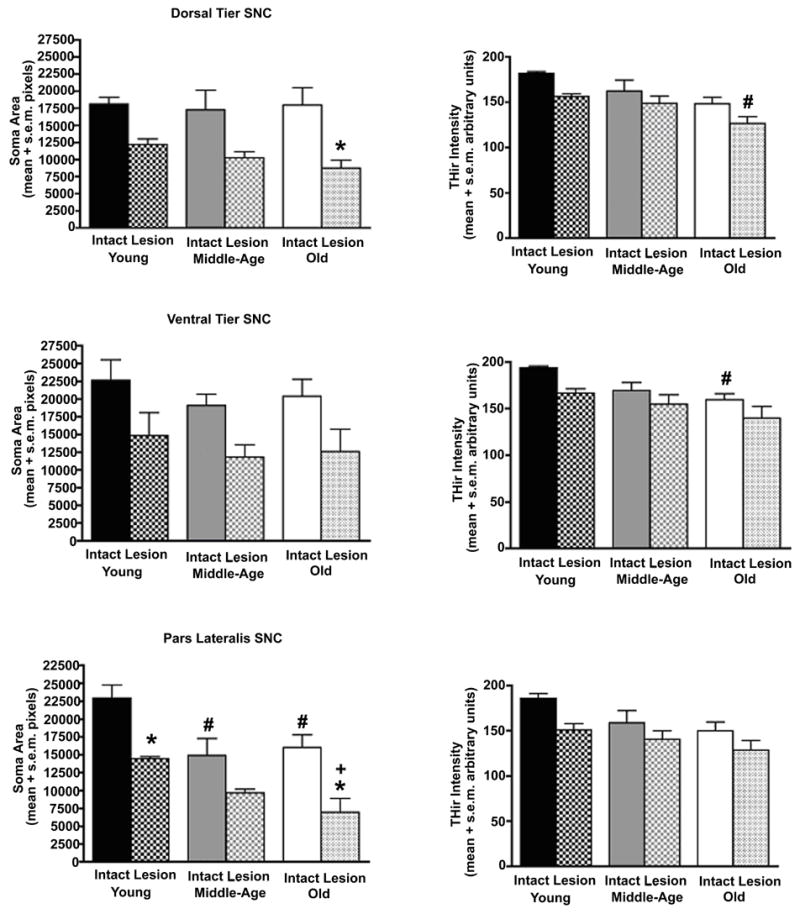
Aging and exposure to MPTP produce specific reductions in soma area and TH staining intensity in the three major subdivisions of SNC. For dorsal tier and ventral tier neurons of the intact hemisphere, there was an aging-related reduction in intensity of THir that reached statistical significance for the comparison of young adult monkeys to aged monkeys (dorsal tier and ventral tier: # p<0.02 for comparison of young to old). For pars lateralis neurons an aging-related decline in THir was evident, but did not attain statistical significance (p=0.053). For these cells the change expressed was decreased soma cross-sectional area. This decline was detectable in middle-age and sustained in old age (# p<0.03). In general MPTP treatment was associated with further decreases in both THir intensity and soma area in all regions in all age groups. Statistically significant decreases in soma area were detected for dorsal tier neurons of aged monkeys (* p<0.01) and pars lateralis neurons of young and old monkeys (* p<0.03). This cell shrinkage after MPTP exposure was exaggerated in the oldest animals when compared to young adult animals (+ p<0.04).
Analysis of THir fiber staining in striatum also revealed aging-related changes. With advancing age the intensity of fiber staining decreased in both caudate nucleus and putamen, with these declines being more pronounced and expressed earlier in the putamen (Figure 8)(F(5,20)=6.37, p<0.002; Caudate: p<0.01 for comparison of young to old; Putamen: p<0.04 for comparison of young to middle-aged and old). THir fibers did not measure above levels of background in central caudate and putamen in the MPTP-treated hemisphere.
Figure 8.
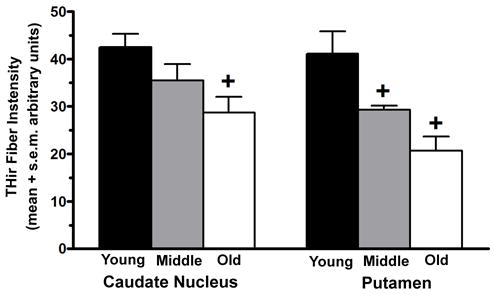
The intensity of THir fiber staining decreased in caudate nucleus and putamen with advancing age in the intact hemisphere. Decreased fiber staining was more pronounced and expressed earlier in the putamen (+ Caudate: p<0.01 for comparison of young to old; Putamen: p<0.04 for comparison of young to middle-aged and old).
Discussion
The incidence of Parkinson’s disease increases dramatically with advancing chronological age (Mayeux et al., 1995; Tanner and Goldman, 1996; Totaro et al., 2005). Yet, surprisingly little is known about whether the aging primate DA system responds differently to insults and if such changes might contribute to the expression of parkinsonian syndromes. Indeed, some believe that the pattern of changes in the DA system in PD and aging are distinct and may bear no relationship one to the other (Fearnley and Lees, 1991; Kish et al., 1992; Kubis et al., 2000). It was the goal of the present study to revisit this comparison.
Choices were made in the design of our study, and the findings presented need to be interpreted within that context. First, intracarotid administration of MPTP was used to provide the opportunity to analyze biochemical and morphological correlates of age-related responses to toxic insult (brain hemisphere ipsilateral to MPTP infusion) and DA system changes attributable to aging itself (brain hemisphere contralateral to MPTP infusion) in the same cohort of monkeys. Disagreement exists in the literature as to whether this model produces a purely unilateral DA depletion, or produces variable damage to the contralateral DA system (e.g. Pate et al., 1993). Consensus suggests that contralateral effects are associated with increasing doses of MPTP and need not be an inevitable consequence of intracarotid administration (Bankiewicz et al., 1986; Guttman et al., 1990; Palomobo et al., 1990; Oiwa et al., 2003). Our MPTP doses were on the low end of the spectrum for rhesus monkeys and comparisons of SNC THir cell counts and THir intensity from the intact hemisphere of our treated animals do not differ from the same measures in untreated young, middle-aged, and aged monkeys analyzed in our other ongoing studies (Kanaan et al., 2006). Second, MPTP treatment was used to achieve the same behavioral endpoint in monkeys of all ages: complete disuse of the contralateral arm and hand. This approach precludes analysis of aging-related changes in sensitivity to MPTP toxicity, assessed by comparing the response of subjects of different age to the same MPTP dose, and shifts the focus to aging-related changes associated with a comparable insult produced by age-adjusted MPTP doses as defined by a behavioral endpoint. Third, brain samples were collected at a single time point, three months after establishing behavioral dysfunction. As such, the short-term dynamics of the response to MPTP were lost in favor of evaluation of a relatively stable response to damage that potentially models the biology of the DA system in individuals of different ages expressing parkinsonism and embarking upon a course of therapy.
Our analysis of striatal DA and HVA confirmed previous studies documenting aging-related declines (Goldman-Rakic and Brown, 1981; Irwin et al., 1994; Gerhardt et al., 2002; McCormack et al., 2004.) and these were associated with a progressive decrease in spontaneous motor activity. In addition, we found that significant decreases in DA content in caudate nucleus and putamen already were expressed in middle-age and sustained in advanced age (McCormack et al., 2004). Following MPTP treatment, the expected significant depletions of DA in caudate and putamen were observed in the ipsilateral striatum, and the extent of depletion did not vary among age groups. This supports our contention that MPTP dosing adjusted to attain a common behavioral endpoint resulted in an equivalent toxic insult for comparison across age groups. In response to equivalent insult, the most dramatic aging-related change was in DA activity in response to MPTP-induced degeneration. While the well-documented compensatory increase in DA activity reflected in the HVA/DA ratio (Zigmond et al., 1990) was present in young adult and middle-aged monkeys after MPTP treatment, this biochemical compensation was completely absent in monkeys of advanced age. Increased DA activity was a highly variable response in middle-aged animals yet probably accounts for the relative preservation of motor performance in this age group despite the presence of significant decreases in striatal DA content. In contrast, the absence of compensatory changes in DA activity in the oldest monkeys was a consistent finding and in combination with sustained decreases in DA content likely contributes to the significant deficits in motor activity.
Disagreement exists among previous studies regarding morphological correlates in SNC DA neurons that accompany aging-related declines in striatal DA (Pakkenberg et al., 1995; Emborg et al., 1998; Gerhardt et al., 2002; McCormack et al., 2004). In our hands, estimates of SNC THir cell numbers in the intact hemisphere using unbiased stereology found no loss of neurons across the three age groups. The difference between the present study and some previous ones may reflect differences in detectability of TH. The loss of THir perikarya seen in aged monkeys in some studies likely reflects a phenotypic down-regulation of TH to undetectable levels. Environmental and genetic differences among colonies of aged monkeys may result in some animals displaying TH levels below the level of detection in some studies but not others. The decrease in TH optical density seen in our study supports that view. As expected, dramatic loss of these neurons accompanied MPTP treatment, but the magnitude of cell loss did not differ with aging.
Despite the absence of evidence for overt DA neuron loss in the intact hemisphere with advancing age, some significant decreases in soma area and THir intensity were detected. For this analysis, neuron cell bodies were sampled in the main cytoarchitectural subdivisions of SNC: dorsal tier, ventral tier, and pars lateralis. These regions are documented to exhibit different degrees of cell loss in PD, with neurons of ventral tier and pars lateralis being more prone to degeneration and dorsal tier neurons being more resistant (German et al., 1989; Fearnley and Lees, 1991; Damier et al., 1999). This pattern holds true for susceptibility to MPTP-induced degeneration. While such regional distinctions have not been extensively studied with regard to aging, one often cited report indicates that SNC cell loss in human aging follows an opposite pattern, with most degeneration occurring in the dorsal tier (Fearnley and Lees, 1991). We detected aging-related changes in all three SNC subdivisions. Statistically significant changes in dorsal and ventral tier neurons consisted of decreased TH immunoreactivity, while changes in pars lateralis consisted of cell shrinkage. In all subdivisions, further declines in soma area and THir were associated with MPTP treatment. Thus, despite the absence of detectable loss of SNC neurons with advancing age, progressive declines in TH expression or soma size likely represent a neuronal response to accumulating damage since the same changes are exaggerated by exposure to MPTP. No major differences in intensity of THir and soma area were found for vulnerable ventral tier and resistant dorsal tier neurons. Both with aging and in response to MPTP exposure, the previously characterized vulnerable pars lateralis neurons exhibited the most pronounced changes.
Aging-related changes in TH expression in SNC cell bodies was accompanied by decreased THir fiber staining in caudate and putamen. Decreased fiber staining was more pronounced in the putamen and was detectable in middle-age. Differences in THir fiber staining in caudate nucleus only was significant for the comparison of young to old animals. This pattern of changes is consistent with declines in THir fibers in putamen preceding decreased TH expression at the level of SNC cell bodies. Decreased THir in fibers was reduced significantly in middle-age, but decreased THir in neuronal soma only reached statistical significance in old subjects.
The etiology of idiopathic PD remains obscure and a focus of considerable debate. Indeed, it is likely that PD is a family of syndromes rather than a unitary entity. Individuals may arrive at a symptomatic state via any number of combinations of genetic risks and accumulated insults to the DA system. Conceptual models of PD have evolved from expression of unsuccessful aging, to the Calne-Langston hypothesis of an insult superimposed upon aging-related changes (Calne and Langston, 1983), to the multiple-hit hypothesis that combines more than one environmental or genetic event with aging-related changes (Thiruchelvam et al., 2003, 2004; Carvey et al., 2006; Ling et al., 2006). In these models, the aging-related change identified is progressive depletion of striatal DA, approaching the threshold for PD symptoms over decades of the lifespan. This remains a likely event and in our study of nonhuman primates striatal DA depletion is significant even in middle-age. However in addition, the current study identifies a dramatic loss of nigrostriatal biochemical compensatory mechanisms in aged individuals. We previously have documented that these same aged animals exhibit saturation of striatal trophic compensation, rendering old animals incapable of increasing striatal trophic activity in response to MPTP-induced DA depletion (Collier et al., 2005). In our view, this loss of compensatory mechanisms may be more central to the role of aging in the development of PD. With advancing age this loss of plasticity renders the organism less capable of recovering from the lifelong series of insults to the DA system that lead with increasing probability to expression of parkinsonian signs.
Acknowledgments
The authors gratefully acknowledge the talents and contributions of Barbara Blanchard, Nicholas Campbell, Michelle Gartland, Nicholas Kanaan, Jeff Moriano, James Stansell, Drs. Marina Emborg, Liza Leventhal, and Ben Roitberg. This work was supported by NIH award AG17092 (TJC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen AH, Zhang Z, Zhang M, Gash DM, Avison MJ. Age-associated changes in rhesus CNS composition identified by MRI. Brain Res. 1999;829:90–98. doi: 10.1016/s0006-8993(99)01343-8. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW. Aetiology of Parkinson’s disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Punati A, Newman MB. Cell Transplant. Vol. 15. 2006. Progressive dopamine neuron loss in Parkinson’s disease: The multiple hit hypothesis; pp. 239–250. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Ling ZD, Carvey PM, Fletcher-Turner A, Yurek DM, Sladek JR, Jr, Kordower JH. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exper Neurol. 2005;191:S60–S67. doi: 10.1016/j.expneurol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122 (Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Shin P, Roitberg B, Sramek JG, Chuy Y, Stebbins GT, Hamilton JS, Suzdak PD, Steiner JP, Kordower JH. Systemic administration of the immunophilin ligand GPI 1046 in MPTP-treated monkeys. Exp Neurol. 2001;168:171–182. doi: 10.1006/exnr.2000.7592. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. In: Federoff HJ, Burke RE, Fahn S, Fiskum G, editors. Parkinson’s disease. The life cycle of the dopamine neuron. Vol. 991. Annals of the New York Academy of Sciences; New York: 2003. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Aging and Parkinson’s disease substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Guttman M, Fibiger HC, Jakubovic A, Calne DB. Intracarotid 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration: biochemical and behavioral observations in a primate model of hemiparkinsonism. J Neurochem. 1990;54:1329–1334. doi: 10.1111/j.1471-4159.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy GM, Jr, Di Monte DA, Sandy MS, Langston JW. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegen. 1994;3:251–265. [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Accumulation of intracellular inclusions in dopaminergic subregions of the midbrain during normal aging in the rhesus monkey: relevance in differential susceptibility to degeneration. Soc Neurosci. 2006 Abstract 75.1. [Google Scholar]
- Kish SJ, Shannak K, Rajput A, Deck JHN, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–648. doi: 10.1111/j.1471-4159.1992.tb09766.x. [DOI] [PubMed] [Google Scholar]
- Kubis N, Faucheux BA, Ransmayr G, et al. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain. 2000;123:366–373. doi: 10.1093/brain/123.2.366. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong CW, Snyder JA, Lipton JW, Carvey PM. Exp Neurol. 2006. Feb 24, Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, Tang MX, Lantigua R, Wilder D, Gurland B, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA, Delfani K, Irwin I, DeLanney LE, Langston WJ, Janson AM. Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol. 2004;471:387–395. doi: 10.1002/cne.20036. [DOI] [PubMed] [Google Scholar]
- Morgan DG, Finch CE. Dopaminergic changes in the basal ganglia. A generalized phenomenon of aging in mammals. In: Joseph JA, editor. Central determinants of age-related declines in motor function. Vol. 515. Annals of the New York Academy of Sciences; New York: 1988. pp. 145–160. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS. Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes. Front Biosci. 2003;8:a155–166. doi: 10.2741/1104. [DOI] [PubMed] [Google Scholar]
- Ovadia A, Zhang Z, Gash DM. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiol Aging. 1995;16:931–937. doi: 10.1016/0197-4580(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Pakkenberg H, Andersen BB, Burns RS, Pakkenberg B. A stereological study of substantia nigra in young and old rhesus monkeys. Brain Res. 1995;693:201–206. doi: 10.1016/0006-8993(95)00678-j. [DOI] [PubMed] [Google Scholar]
- Palomobo E, Porrino LF, Bankiewicz KS, Crane AM, Sokoloff L, Kopin IJ. Local cerebral glucose utilization in monkeys with hemiparkinsonism induced by intracarotid infusion of the neurotoxin MPTP. J Neurosci. 1990;10:860–869. doi: 10.1523/JNEUROSCI.10-03-00860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate BD, Kawamata T, Yamada T, McGeer EG, Hewitt KA, Snow BJ, Ruth TJ, Calne DB. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann Neurol. 1993;34:331–338. doi: 10.1002/ana.410340306. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr, Elsworth J, Taylor JR, Roth RH, Redmond DE., Jr . Methods in Cell Transplantation. RB Landes; Austin TX: 1995. Techniques for neural transplantation in non-human primates; pp. 391–408. [Google Scholar]
- Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro R, Marini C, Pistoia F, Sacco S, Russo T, Carolei A. Prevalence of Parkinson’s disease in the L’Aquila district, central Italy. Acta Neurol Scand. 2005;112:24–28. doi: 10.1111/j.1600-0404.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of Parkinson’s disease. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]


