Abstract
Inverted DNA repeats are known to cause genomic instabilities. Here we demonstrate that double-strand DNA breaks (DSBs) introduced a large distance from inverted repeats in the yeast (Saccharomyces cerevisiae) chromosome lead to a burst of genomic instability. Inverted repeats located as far as 21 kb from each other caused chromosome rearrangements in response to a single DSB. We demonstrate that the DSB initiates a pairing interaction between inverted repeats, resulting in the formation of large dicentric inverted dimers. Furthermore, we observed that propagation of cells containing inverted dimers led to gross chromosomal rearrangements, including translocations, truncations, and amplifications. Finally, our data suggest that break-induced replication is responsible for the formation of translocations resulting from anaphase breakage of inverted dimers. We propose a model explaining the formation of inverted dicentric dimers by intermolecular single-strand annealing (SSA) between inverted DNA repeats. According to this model, anaphase breakage of inverted dicentric dimers leads to gross chromosomal rearrangements (GCR). This “SSA-GCR” pathway is likely to be important in the repair of isochromatid breaks resulting from collapsed replication forks, certain types of radiation, or telomere aberrations that mimic isochromatid breaks.
Genetic instability is associated with most tumor cells, and fusions between chromosomes or chromatids is a common source of chromosome aberrations found in such cells. Fusions between chromatids can be initiated by simultaneous breakage of the two chromatids or by the loss of telomere capping (13, 35). The outcome of fusions depends on the location of the fusion site. Thus, fusions between acentric fragments lead to gene amplifications due to missegregation (21, 36). Fusions between fragments containing centromeres lead to the formation of dicentric chromosomes that break during anaphase, when the two centromeres are pulled in opposite directions (breakage-fusion bridge [BFB] events). BFB events often lead to BFB cycles characterized by continued breakages and fusions. The BFB cycle, which was originally described by McClintock for maize (32), is repeated until newly acquired telomeres stabilize the broken chromosomes (13, 35). The process of broken chromosomes acquiring telomeres (the exit from BFB) creates different chromosomal rearrangements, including translocations, deletions, and amplifications (25, 35, 39, 46).
The mechanisms responsible for initiating chromosome or chromatid fusions are not clear. Although several studies indicate an important role for nonhomologous end joining in this process (33, 41), fusions can efficiently occur in a number of nonhomologous end joining-defective mutants (11), implicating the involvement of alternative mechanisms. Junctions of chromatid fusions sometimes have short regions of homology (26), suggesting the possible involvement of a homology-driven repair mechanism. Our present knowledge of fusions is based mainly on cells with defects in telomere length regulation (42). It is possible that fusions initiated internally on the chromosome would have different properties. In the majority of studies, fusions are initiated over several cell generations, making it difficult to distinguish the initial fusion events from those that perpetuate the BFB cycles.
The final outcome of BFB events and cycles depends on the choice and the efficiency of the repair pathway that restores telomeres to broken chromosomes. Telomeres in human tumor cells are frequently restored by translocations, which can be formed in at least two different ways (2, 26, 39). First, the telomere could be restored by “duplicative translocations,” which often lead to genetic imbalance. Such duplicative translocations are likely to be a consequence of break-induced replication (BIR), a mechanism in which a region of homology at the broken end invades a region of homology on a different chromosome and then copies the invaded chromosome to the end. This type of repair has been demonstrated to take place in yeast (Saccharomyces cerevisiae) (4, 29, 34) but not in mammalian cells. Another pathway, called NRT (for nonreciprocal translocation), results in the nonreciprocal transfer of information from donor to recipient, which leaves the donor chromosome broken (14, 18, 25, 39). This type of repair might proceed via incomplete BIR (where copying is interrupted before reaching the end of the chromosome) followed by crossing over (10). As a result of this repair pathway, DNA breaks are transferred from one chromosome to another, leading to cascades of DNA instability (39). Finally, broken chromosomes could be repaired by de novo telomere additions, resulting in a chromosome with a terminal deletion (9, 12, 39, 44, 51).
In this study, we followed chromatid fusions initiated by HO-generated double-strand DNA breaks (DSBs) induced at the same positions on sister chromatids. This type of break can be also caused by replication fork collapse (52) or by exposure to high-linear energy transfer radiation (3, 20). Alternatively, such breaks can be mimicked by the “uncapping” of telomeres (13). We observed that HO-generated DSBs led to sister chromatid fusions mediated by a homology-driven recombination between nonallelic Ty elements. These fusions, which form inverted dicentric dimeric chromosomes in dividing cells, give rise to various types of other chromosome aberrations, including translocations. In our previous studies, we demonstrated HO-induced chromosome rearrangements that we interpreted as Rad51p-independent BIR events (28, 30). In the study described below, we demonstrate that the HO-induced break on chromosome III results in the formation of an inverted dimer (ID) as a consequence of single-strand annealing (SSA) rather than BIR. Processing of this dicentric chromosome generates subsequent translocations that are likely to reflect Rad51p-independent BIR, although other types of homologous recombination cannot be excluded.
MATERIALS AND METHODS
Yeast strains and plasmids.
The haploid strains used in this study were isogenic or congenic to AM919 (Fig. 1A), which has the genotype MATa ade1 ura3-52 leu2-3,112 THR4 lys5 chrIII::URA3 hmlΔ::ADE1 hmrΔ::ADE1 ade3::GAL::HO. This strain is a URA3 derivative of EI515 published in the work of Malkova et al. (28). The haploid strains YLS23 and AM133 (40) and diploid strains YLS101 (40) and AM792 (29) were described previously. Other diploid strains used in this study were isogenic to YLS101. Complete genotypes for all strains and details of their construction are in the supplemental material.
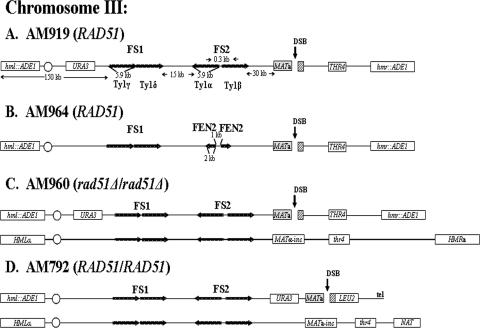
FIG. 1.
Arrangement of chromosome III markers in strains used to study effect of inverted repeats on DSB repair. (A) RAD51 haploid strain AM919. A DSB (black vertical arrow) is induced at MATa by a galactose-inducible HO gene. HML and HMR are replaced by ADE1. URA3 is inserted 133 kb from the left telomere, about 67 kb proximal to MATa. FS2 consists of two Ty1 elements (labeled Ty1α and Ty1β) in inverted orientation (24) and located 30 kb proximal to MAT. FS1 consists of two Ty1 elements (labeled Ty1γ and Ty1δ) in direct orientation located 57 kb centromere proximal to MATa. (B) RAD51 haploid strain AM964. This strain is similar to AM919 but with an inverted repeat of FEN2 replacing FS2. The distance between the two inverted copies of FEN2 is 1 kb. (C) rad51Δ/rad51Δ diploid strain AM960. In this strain, one copy of chromosome III (shown at the top) is the same as the chromosome in AM919. The other homologue contains MATα-inc, which cannot be cut by HO, and also has a mutant copy of the thr4 gene. This chromosome is about 20 kb longer than the homologue from AM919. (D) RAD51 diploid strain AM792. In this strain, the MATa-containing chromosome is truncated by insertion of the LEU2 gene fused to telomere (tel) sequences (29). The other homologue is similar to that of AM960, except that the HMR gene is replaced by the NAT (nourseothricin [Clonat] resistance) gene.
Media and growth conditions.
Rich medium (yeast extract-peptone-dextrose [YEPD]), synthetic complete medium with bases and amino acids omitted as specified, and sporulation media were as described previously (15). YEP-lactate and YEP-galactose (YEP-Gal) contained 1% yeast extract, 2% Bacto peptone medium supplemented with 3.7% lactic acid (pH 5.5), or 2% (wt/vol) galactose, respectively. Cultures were grown at 30°C.
Analysis of DNA repair.
In order to monitor the repair of HO-induced DSBs, we harvested logarithmically growing cells grown in YEP-lactate and plated them on YEP-Gal. The resulting colonies were then replica plated onto omission media to examine the heterozygous ADE1, THR4, LEU2, and URA3 markers of these strains. The kinetics of DSB repair were examined as described previously (29). To arrest cells at the G2 stage, we performed the experiments in the presence of nocodazole (USB) at a concentration of 0.015 mg/ml. We used 50-ml cultures for Southern analysis, extracting DNA by the glass bead-phenol-sodium dodecyl sulfate protocol (17). For pulsed-field gel electrophoresis (PFGE), chromosomal plugs were prepared using a contour-clamped homogenous electric field genomic DNA plug kit (Bio-Rad). Some experiments were performed in the presence of α-factor (Zymo Research) at a concentration of 10 μg/ml or in the presence of 4-amino-1-(tert-butyl)-3-(1′-naphthylmethyl)pyrazolo[3,4-d] pyrimidine (1-NMPP1; Toronto Research Chemicals, Inc.) at a concentration of 5 μM. The compound 1-NMPP1 is an ATP analogue which inhibits the function of the mutant protein encoded by the cdc7-as3 allele (19).
For Southern analysis, DNA was digested with the appropriate restriction enzymes, and the resulting fragments were separated on a 0.8% agarose gel. Southern blotting was carried out by standard procedures (7). PFGE was done using genomic DNA embedded in plugs of 1% agarose. The DNA was subsequently examined by Southern analysis. The blots were probed with appropriate DNA fragments labeled with 32P (see the supplemental material for a complete list of probes used for hybridization). Blots were analyzed by using a Molecular Dynamics PhosphorImager.
Comparative genomic hybridization analysis.
DNA preparation and subsequent microarray analysis were done according to procedures described in the work of Lemoine et al. (24). Arrays were analyzed using GenePix Pro 4.1 (Axon Instruments) and CGH Miner (http://www-stat.stanford.edu/∼wp57/CGH-Miner/). Genomic ratios and copy numbers of the amplified regions were estimated using cluster along chromosomes analysis (48).
DNA combing and fluorescent in situ hybridization.
Genomic DNA for molecular combing was prepared as described previously (36). The probes, comprised of a set of 7-kb-long fragments, were obtained by PCR amplification from genomic DNA of AM919 as a template. Probe 3 (see Fig. 3A) consisted of two 7-kb PCR fragments corresponding to two positions on chromosome III (153772 to 160772 and 160748 to 167789, respectively). Probe 6 (see Fig. 3A) consisted of two 7-kb fragments corresponding to positions 134574 to 141274 and 141250 to 148547. As shown below (see Fig. 3C), six full-length molecules were detected and measured.
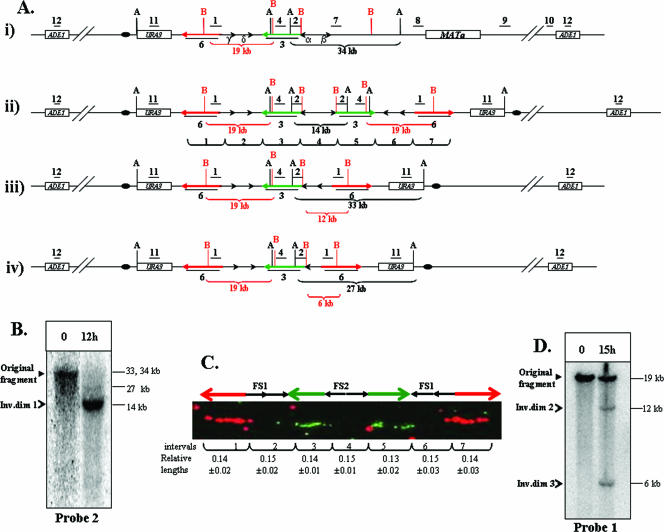
FIG. 3.
Analysis of inverted dicentric dimers formed by interaction between inverted repeats of Ty1. (A) Expected structures of chromosome III of AM919 and repair intermediates derived from this chromosome. (i) The structure of chromosome III of AM919 before the formation of inverted dicentric dimers. Underlined numbers (1, 2, 4, 7, 8, 9, 10, 11, and 12) indicate the positions of probes used to analyze the region (see the supplemental material for a detailed description of all probes). The positions of AvrII (black A) and BspEI (red B) restriction sites are shown; only those sites relevant to the Southern analysis described below are depicted. (ii) The structure of ID1 (370-kb chromosome), resulting from recombination between Ty1α and Ty1β. Probe 3 (shown as a green arrow) is a 14-kb-long probe specific to the region between FS1 and FS2. Probe 6 (shown as a red arrow) is a 14.3-kb-long probe specific to the region centromere proximal to FS1. (iii) The structure of ID2, resulting from recombination between Ty1α and Ty1δ. (iv) The structure of ID3, resulting from recombination between Ty1α and Ty1γ. (B) Analysis of large inverted dimers (Inv.dim) by gel electrophoresis. DNA was extracted from preinduction (0-h) and postinduction (12-h) samples of AM919. The samples were digested with AvrII, and the resulting fragments were separated by gel electrophoresis and examined by Southern analysis with probe 2. As expected from the maps shown in panel A, the DNA fragment in the uninduced sample was about 34 kb. The expected fragment size from the 370-kb ID1 is 14 kb. The expected fragments for ID2 and ID3 are about 33 and 27 kb, respectively. Since the appearance of the 340-kb intermediates is delayed compared to that of the 370-kb intermediate, only small amounts of the 33- and 27-kb fragments are present 12 h after DSB induction. (C) Analysis of large inverted dimers by DNA combing. DNA was extracted from AM919 cells 12 h after the addition of galactose. This DNA was stretched and hybridized to the fluorescent probe 3 (green arrow) and probe 6 (red arrow). The relative lengths of hybridizing regions and the gaps between them were calculated as a fraction of the length between the termini of the red arrows (96 kb). (D) Southern analysis of ID2 and ID3. DNA was extracted from preinduction (0-h) and postinduction (15-h) samples of AM919. The samples were digested with BspEI, and the resulting fragments were examined by Southern analysis using probe 1. Fragments of the expected sizes were found for the wild-type, ID2, and ID3 chromosomes (19, 12, and 6 kb, respectively).
RESULTS
Inverted repeats (IRs) mediate aberrant repair of DSBs introduced at a distant chromosomal site.
We first examined the repair of a single DSB introduced by a galactose-inducible HO endonuclease at the MATa locus on chromosome III in the haploid yeast strain AM919 (Fig. 1A). These DSBs could not be repaired by homologous recombination with the potential donor cassettes (HML and HMR), because these sequences were replaced in this strain by the ADE1 gene. There are two pairs of Ty elements on chromosome III proximal to MATa, identical to the Ty1 repeats described in reference 50 and also in reference 24, where they were named FS2 and FS1, respectively. FS2 is located 30 kb proximal to MATa and consists of two Ty1 elements (Ty1α and Ty1β) (Fig. 1A) in inverted orientation. FS1 is located 57 kb proximal to the MAT locus and includes two Ty1 elements (Ty1γ and Ty1δ) (Fig. 1A) in direct orientation. The positions and orientations of FS1 and FS2 in our strains were confirmed by PCR analysis (data not shown).
DSBs were induced by the addition of galactose to the media. Nocodazole was used to prevent mitotic divisions. We examined the structure of chromosome III by PFGE before the induction of DSBs as well as 3 and 22 h after the addition of galactose. In the experiment shown in Fig. 2A, URA3 was used as a hybridization probe which detects both chromosome V (the native location of URA3) and chromosome III. Two repair products (of about 340 and 370 kb in size) were present 22 h after DSB induction (Fig. 2A, left panel). Similar results were obtained when the ADE1 gene, which hybridizes to both chromosomes I and III, was used as a hybridization probe (data not shown). The intensities of the larger and the smaller repair products were about two-thirds and one-third, respectively, of the intensity of the unbroken chromosome. Since the sum of intensities of both repair products was approximately 96% of the intensity of the unbroken chromosome III, most broken molecules were repaired. In addition, we found that with a higher resolution of separation of the DNA fragments, the 340-kb repair product was comprised of two different species of very similar sizes (data not shown).
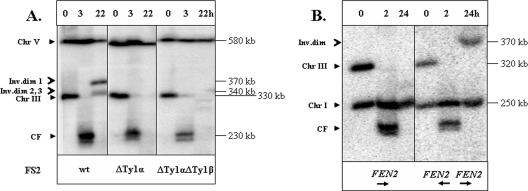
FIG. 2.
Interaction between inverted Ty1 elements leads to chromosome rearrangements. In this figure, the repair of DSBs in AM919 and related RAD51 strains was examined by PFGE. (A) Formation of chromosome (Chr) rearrangements depends on the presence of an intact FS2. DNA was prepared for PFGE at intervals (0, 3, and 22 h) after the induction of a DSB at MATa in the RAD51 haploid strains AM919, AM903 (ΔTy1α), and AM936 (ΔTy1α ΔTy1β). Southern blots were probed with URA3, which hybridizes with its normal locus on chromosome V and to a URA3 insertion on chromosome III (Fig. 1A). In AM919, two repair products were detected, one of about 370 kb (inverted dimer [Inv.dim] 1) and another of 340 kb. The smaller product was shown subsequently to represent two products of similar size (inverted dimers 2 and 3). The fragment labeled CF is the chromosome fragment resulting from deletion of the sequences centromere distal to the HO cleavage site. An additional CF band, observed 3 h after the addition of galactose, corresponds to the processed (partially single-stranded) form of cut fragment (K. VanHulle and A. Malkova, unpublished observation). (B) Formation of chromosome rearrangements is stimulated by an inverted repeat of the FEN2 gene. Experiments similar to those described for panel A were performed with the haploid strains AM915 (containing a deletion of FS2) and AM964 (containing an inverted repeat of FEN2 replacing FS2). Southern blots were probed with ADE1. A repair product of approximately 370 kb was observed in AM964 but not in AM915. wt, wild type.
The 340- and 370-kb repair products were analyzed by hybridization with probes specific to different regions of chromosome III (positions shown in Fig. 3Ai). Both the 340- and 370-kb products showed hybridization to the probes specific to the regions located centromere proximal to FS2 (probes 12, 11, 1, 4, and 2) and did not hybridize with probes located centromere distal to FS2 (probes 7 to 10) (Fig. 3Ai). Therefore, we hypothesized that the repair products resulted from recombination events involving FS2. Supporting this conclusion, formation of both repair products was completely abolished in the isogenic strain lacking FS2 (Fig. 2A, right panel). Formation of both repair products was also abolished in a strain that lacked one (Ty1α) of the two FS2 Ty elements (Fig. 2A, center panel).
Finally, we asked if the ability to form the repair products is specific to the Ty elements or is a property common to other IRs. We replaced the FS2 region by the FEN2 gene inserted in an orientation inverted to FEN2 in its normal location, thus creating a 2-kb inverted repeat with a spacer of 1 kb (AM964; Fig. 1B). After HO induction of DSBs, we analyzed DNA at different time points by PFGE; the ADE1 gene, which hybridizes to both chromosomes I and III, was used as a hybridization probe. We observed a 370-kb repair product (Fig. 2B) similar to that observed for strains with an intact FS2. Thus, this type of repair product is a common property of inverted repeats.
Ty-mediated DSB repair leads to the formation of inverted dimers.
We hypothesized that the 370- and 340-kb repair products were inverted dicentric dimers. Based on their sizes and their hybridization patterns (see above), we predicted that the larger repair product (inverted dimer 1 [ID1]) would contain two copies of the left part of chromosome III (from the left telomere to the FS2-proximal region) in inverted orientation (Fig. 3Aii). This structure could be formed by recombination between Ty1α and Ty1β (Fig. 4Ci), resulting in a palindromic chromosome with FS2 as the center of the palindrome (Fig. 4Di). The structure of ID1 was confirmed by Southern blot analysis. We found (Fig. 3B) that the formation of the repair products coincides with the appearance of a novel 14-kb AvrII restriction fragment hybridizing to the FS2-specific probe (probe 2), while the initial 34-kb fragment disappears. This result is consistent with the predicted formation of ID1 (Fig. 3Aii).
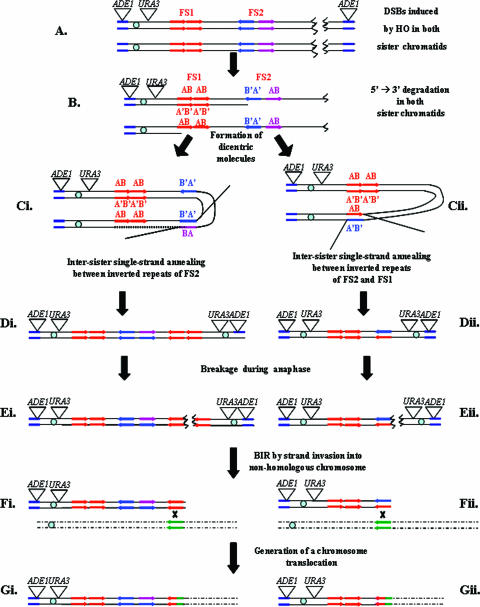
FIG. 4.
Model to explain the generation of inverted dicentric dimers and their subsequent processing into translocations. The designations AB and A'B' indicate the orientations of Ty elements; the A' strand is complementary to A and the B' strand is complementary to B. In this diagram, we depict the formation of only one type of chromosome aberration, a translocation. Other types of aberrations can be generated by similar mechanisms, as discussed in the text. Ty1α and Ty1β, blue and purple arrows.
The structure of ID1 was confirmed by a DNA-combing technique (Fig. 3C), wherein stretched DNA molecules isolated from the samples containing repair products were hybridized to two 14-kb fluorescent probes (probes 3 and 6) (Fig. 3Aii). In the original strain (AM919), each probe hybridizes to the unique region of chromosome III. Probe 3 hybridizes to the region between FS2 and FS1, and probe 6 hybridizes to the region centromere proximal to FS1. The expected pattern of hybridization for ID1 (Fig. 3Aii) is red-green-green-red, with the areas of hybridization interrupted by gaps corresponding to the pairs of Ty elements. The relative lengths of areas of red and green hybridization, as well as the gap sizes, were consistent with the map shown in Fig. 3Aii.
The sizes of the smaller repair products (about 340 kb) were consistent with a recombination event that involved either Ty1δ and Ty1α (ID2) or Ty1γ and Ty1α (ID3). In Fig. 3Aiii and Fig. 3Aiv, we show the sizes of the BspEI fragments (detected by probe 1) for recombination events involving Ty1δ and Ty1α (predicted size of 12 kb) and Ty1γ with Ty1α (predicted size of 6 kb), respectively. The right side of Fig. 4 shows the details of the recombination event involving the Ty1γ with Ty1α. In summary, we demonstrate that inverted DNA repeats channel the repair of distantly located DSBs into the formation of different species of large inverted dimers and that repair of the broken molecules is highly efficient.
Formation of inverted dimers occurs with kinetics suggestive of an SSA mechanism.
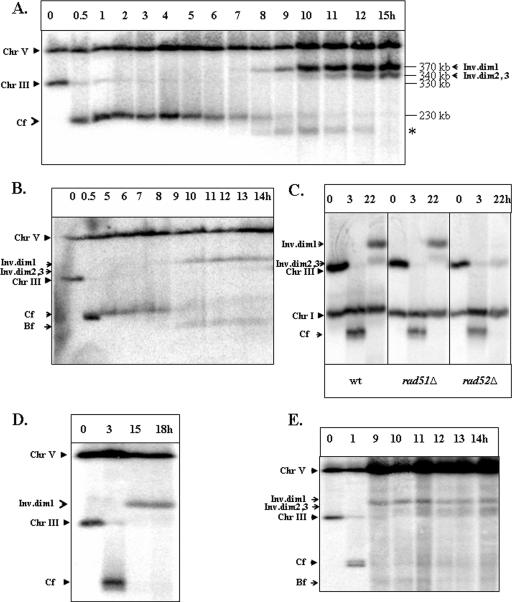
The kinetics of formation of the repair products were analyzed in an experiment in which samples were removed every hour after galactose addition and analyzed by PFGE (Fig. 5A). Accumulation of ID1 occurred between 8 and 12 hours after DSB induction, which corresponds to the time predicted for a 5′-to-3′ DNA strand resection to pass through FS2 based on a 4- to 5-kb/hour rate of degradation (47). In the experiment shown in Fig. 5A and in similar experiments, a 5′-to-3′ DNA resection reached the region of FS2 by 10 h after the addition of galactose in approximately 80 to 90% of the cells (confirmed by the loss of EcoRI and of HindIII restriction sites located in immediate proximity, centromere distal to FS2 [data not shown]). The ID2 and ID3 chromosomes accumulated between 10 and 15 hours, which is close to the time predicted for a 5′-to-3′ DNA strand resection to pass through FS1. The kinetics of the appearance of repair products did not depend on the time of nocodazole addition relative to the induction of the DSB (Fig. 5A; also see Fig. S1 in the supplemental material). The close correspondence between the kinetics of appearance of the repair products and the time required for 5′-to-3′ resection suggests that Ty1-mediated repair occurs via the SSA repair pathway (Fig. 4). In AM919 cells that were not treated with nocodazole, the same repair intermediates appeared (although in lower amounts) with approximately the same kinetics (Fig. 5B). In addition, the same repair intermediates were observed for cells for which reentry into the cell cycle after repair was prevented by means other than nocodazole (see Fig. S1G in the supplemental material). Thus, the formation of dicentric structures is not a consequence of nocodazole treatment.
FIG. 5.
Kinetics and genetic requirements of Ty-mediated formation of inverted dimers. (A) Kinetics of formation of inverted dimers in AM919. DNA was prepared at intervals after the induction of a DSB at MATa in the RAD51 haploid strain AM919. Nocodazole was added 2 h following the addition of galactose. Southern blots were probed with URA3, which hybridizes to chromosome (Chr) V (its native locus) and to URA3 inserted about 15 kb from CEN3. The repair products (inverted dimers [Inv.dim] 1, 2, and 3) are indicated. Repair products of the same size were also observed after probing the same blots with an ADE1-specific probe located at the left end of chromosome III (data not shown). The nature of the band marked with asterisk is yet to be identified. (B) An experiment similar to the one shown in panel A was performed using AM919 cells that had not been treated with nocodazole. (C) Genetic requirements for formation of inverted dicentric dimers in haploid strains. The formation of inverted dimers was compared in AM919 (wild-type [wt]), EI517 (rad52Δ), and YLS73 (rad51Δ) strains arrested with nocodazole. Southern blots were probed with ADE1, which hybridized to ADE1 inserted at HML and HMR, and to its native locus on chromosome I. Dimer formation is independent of Rad51p but dependent on Rad52p. (D) Formation of inverted dimers in the rad51Δ/rad51Δ diploid strain AM960 treated with nocodazole. DNA was prepared for PFGE at intervals after the induction of DSBs at MATa in AM960 cells (Fig. 1C) arrested with nocodazole. Southern blots were hybridized to the URA3-specific probe. (E) Formation of inverted dimers in the rad51Δ/rad51Δ diploid strain AM960 that was not treated with nocodazole. DNA was prepared at intervals after induction of DSBs in AM960 cells that were not treated with nocodazole. Bf indicates DNA fragments that are likely to reflect breakage of dicentrics; Cf indicates chromosome fragments resulting from deletion of the sequences centromere distal to the HO cleavage site.
Formation of inverted dimers requires RAD52 but not RAD51.
Genetic control of IR-mediated repair was investigated for rad52 and rad51 mutant strains isogenic to the wild-type strain AM919. In a rad52Δ mutant (EI517), the formation of inverted dimers was abolished (Fig. 5C, right panel). The faint band at a position corresponding to about 330 kb is observed in all gels and likely represents a small amount of uncut chromosome III. Thus, we conclude that RAD52 is essential for the formation of inverted dimers. In contrast, in the rad51Δ strain (YLS73), both the 370- and 340-kb repair products were present (Fig. 5C, center panel). The amounts of ID products in the rad51Δ strain were not significantly different from the amounts observed for the wild-type strain. We conclude that the formation of inverted dimers depends on RAD52 but is RAD51 independent. These genetic requirements are a hallmark of the SSA pathway (37), consistent with our conclusion that inverted dimers are formed via SSA.
We also observed inverted dicentric dimers in the nocodazole-arrested rad51Δ diploid strain AM960 (Fig. 5D). In AM960 (shown in Fig. 1C), DSBs introduced at MATa cannot be efficiently repaired by homologous recombination with the intact homologue due to the absence of RAD51 (29). The sizes and relative amounts of the inverted dicentric intermediates observed in these rad51Δ diploids were similar to those observed in the rad51Δ haploid. Finally, we examined the kinetics of repair intermediates in AM960 cells that were not arrested with nocodazole. The same intermediates were observed for these cells, and the kinetics of their appearance were similar to those observed for AM919 (Fig. 5, compare panel E to panel B).
Mitotic propagation of cells containing inverted dicentric dimers leads to chromosomal rearrangements.
When cells containing dicentric inverted dimers are allowed to enter mitosis, one would expect the dicentric to undergo breakage during anaphase, thereby generating one chromosome with a deletion and one chromosome with an inverted duplication of a large chromosomal region (Fig. 4Ei and Eii). We hypothesized that these BFB events would result in gross chromosomal rearrangements (GCRs). This prediction was tested in both RAD51 and rad51Δ cells by inducing DSBs at the MATa locus, monitoring the resultant phenotypes of the various repair outcomes, and analyzing chromosome rearrangements.
Analysis of repair outcomes in RAD51 strains by PFGE.
Any chromosome aberrations that deleted the sequences distal to the HO cleavage site would be inviable in RAD51 haploid cells. To circumvent this problem, we induced DSBs and chromosome rearrangements in the RAD+ (AM919) haploid and recovered the repair products by mating these cells to a MATα-inc ade1 thr4 strain (α-parent). Two closely related MATα-inc ade1 thr4 strains, YLS23 and AM133 (28, 40), were used as α-parents. DSB formation was induced in nocodazole-arrested AM919 cells (treated for 12 h), after which the cells were washed to remove nocodazole and plated on a lawn of α-parent cells. In our subsequent discussion, we will refer to the diploids formed by the mating of AM919 and YLS23 as AM1000a and to the diploids formed by the mating of AM919 and AM133 as AM1000b diploids; different numbers after the strain names indicate independently formed isolates.
Wild-type (AM919) cells in which the DSB was effectively induced and repaired by the mechanisms diagrammed in Fig. 4 would be expected to lose the MATa locus as well as the THR4 locus. Haploid strains that lack the MAT locus mate efficiently to MATα strains (23, 45, 49). The resulting diploids, which lack a functional MATa locus, mate as MATα strains. Consequently, we screened for Ade+ diploids that expressed MATα information. Forty-seven percent of such strains were Thr−, while 53% were Thr+.
We analyzed 25 AM1000 Thr+ strains by PFGE, using ADE1 or URA3 as hybridization probes. Chromosome III in α-parents is about 20 kb longer than chromosome III in AM919 (see Fig. S3 in the supplemental material); although the nature of the polymorphism is not understood, it is located centromere distal to THR4 (unpublished data). In 18 of the Thr+ strains, the chromosome III with the ADE1 and URA3 genes was the size of chromosome III from AM919. Such strains presumably reflect gene conversion events in which information from the MATa locus was deleted and replaced with MATα information derived from α-parents. In five of the Thr+ strains, the ADE1 probe hybridized to both copies of chromosome III, one of the size expected for the AM919 strain and one of the size expected for α-parents. This result is consistent with a gene conversion event at the MAT locus associated with a crossover centromere proximal to THR4 (see Fig. S3 in the supplemental material). In two of the Thr+ strains, there were multiple rearrangements involving chromosome III that were not characterized further.
We also examined 37 Thr− diploids. In 23 of the diploids, the chromosome III that contained the ADE1 and URA3 genes was about 350 kb, instead of the original size of 330 kb. The simplest explanation for these strains is that they reflect repair of the HO-induced DSB by a BIR event involving the α-parent-derived homologue. In 12 of the diploids, PFGE showed a chromosome III with a size different from that of the unrearranged chromosomes in AM1000 (Fig. 6A). Such strains could represent either intrachromosomal deletions or duplications of chromosome III or interchromosomal rearrangements (translocations). The microarray analysis described below demonstrated both intra- and interchromosomal changes. In the remaining two strains, the URA3-containing copy of chromosome III was about 330 kb instead of 350 kb. These structures are consistent with a coconversion of the MAT and THR4 loci as a consequence of gap repair of the HO-induced break. To confirm our conclusions based on PFGE, we examined DNA isolated from AM1000a and AM1000b strains by use of DNA microarrays (24).
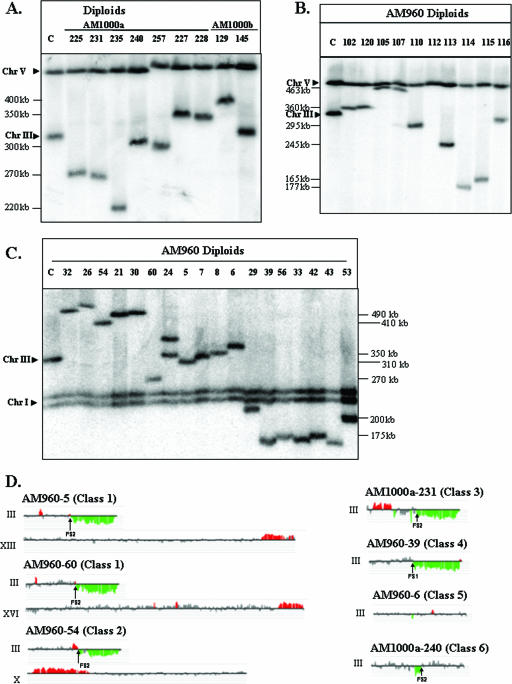
FIG. 6.
Chromosome rearrangements in strains with HO-induced DSBs. (A) Chromosome (Chr) alterations in derivatives of the RAD51 diploid strains AM1000a and AM1000b. DSBs were induced in the nocodazole-arrested RAD51 haploid AM919. The nocodazole was removed, and the haploids were mated to a RAD51 strain of the opposite mating type. We performed PFGE analysis of several independent diploids that were phenotypically Ade+ Thr− and expressed MATα information. The chromosomal DNA was analyzed with the URA3 probe (which hybridizes to both chromosomes III and V), although similar results were obtained with the ADE1-specific probe (data not shown). In many of the strains, the repair products were different in size from the normal-length chromosome IIIs, indicating a chromosome rearrangement. (B) Chromosome alterations in nocodazole-treated derivatives of the rad51 diploid strain AM960. DSBs were induced in the nocodazole-arrested rad51Δ diploid strain AM960. The nocodazole was removed, the cells were plated on YEP-plus-galactose medium, and the survivors were examined by PFGE. The chromosome III with the HO site was detected using URA3 as the hybridization probe, although similar results were obtained with the ADE1 probe (data not shown). The majority of the repair products were different in size from the two “normal” chromosome IIIs in AM960 (330 and 350 kb). (C) Chromosome alterations in derivatives of the rad51Δ diploid strain AM960 that were not treated with nocodazole. DSBs were induced in AM960 by plating on a galactose-containing medium, and we examined the consequences of these breaks in the surviving cells by PFGE. The chromosome III with the HO site was detected using ADE1 as the hybridization probe, although similar results were obtained with the URA3 probe (data not shown). The majority of the repair products were different in size from the two “normal” chromosome IIIs in AM960 (330 and 350 kb). The lanes labeled “C” in panels A, B, and C contained DNA from AM960 cells in which the HO site was not cleaved. (D) Genomic microarray analysis of chromosomal rearrangements. We depict gene dosages (CGH Miner format) for chromosomes in strains representing various classes of repair outcomes (see Table S1 in the supplemental material for details). Only chromosome III and rearranged chromosomes (containing chromosomal regions with changed gene dosage) are shown. Genomic DNA was isolated from strains with potential chromosome rearrangements and labeled with a Cy5-labeled fluorescent nucleotide; DNA from a reference strain (AM960) was labeled with Cy3 nucleotide. The two samples were mixed and hybridized to DNA microarrays that contained all yeast genes. Green indicates approximately twofold less DNA in the experimental strain relative to that in the control, and red indicates about 1.5-fold more DNA in the experimental strain. Small regions of red or green color (less than three open reading frames) were not considered significant. Also, regions of red or green color that were disrupted by gray-colored regions were not considered significant.
Analysis of repair outcomes in the rad51 diploid AM960 by PFGE.
Repair events in the rad51 AM960 diploids were examined for cells treated with nocodazole for 18 h before being plated on galactose-containing medium and for cells that were plated on galactose-containing medium without prior treatment with nocodazole. In both types of experiments, all colonies formed on the galactose-containing medium were MATα Thr−, as expected since the HO cleavage site is centromere proximal to the THR4 locus. To analyze the outcomes of IR-mediated repair, we selected Ade+ Ura+ or colonies that had Ade+ Ura+ sectors, as such colonies are likely to represent repair events that occurred centromere distal to URA3. These repair events were likely to be IR mediated, as they occurred in a close proximity to FS1 and FS2.
In the nocodazole-treated cells, 76% of the colonies were either Ade+ Ura+ or had Ade+ Ura+ sectors. We examined 28 independent Ade+ repair events using PFGE. In 19 of the 20 events, chromosome III was altered in size (examples in Fig. 6B). About 5% of the nocodazole-treated cells gave rise to viable colonies. In contrast, when we plated untreated rad51Δ (AM960) cells on medium containing galactose, about 98% formed viable colonies. In untreated AM960 cells, consistent with our previous results (30), 74% of colonies were Ade+ Ura+ or contained Ade+ Ura+ sectors. Thus, in these cells, the URA3 gene located proximal to FS1 was conserved, suggesting that repair took place near FS1 and FS2. About 90% of the Ade+ Ura+ isolates had a chromosome III of altered size (Fig. 6C). Note that the hybridization probes used to detect chromosome III in the experiments shown in Fig. 6B and C were different.
Microarray analysis of repair outcomes in the wild-type and rad51 strains.
Although the results from the PFGE analysis demonstrated that the repair events often generated GCRs, the nature of these chromosome rearrangements was not clear until we performed microarray analysis. DNA was isolated from each strain and hybridized (in competition with differentially labeled DNA from a wild-type strain) to a microarray containing all of the yeast genes. This type of experiment (comparative genome hybridization) allows one to determine the extent of deletions and duplications in strains with repaired DSBs. We analyzed 30 strains by microarrays, 10 derived from AM1000a and AM1000b and 20 from AM960. Most of the AM1000a and AM1000b strains that we chose to analyze had a chromosome III that was different in size from the unrearranged copies of chromosome III (330 and 350 kb), but we also examined a few strains in which chromosome III was unaltered in size (AM1000b-12, -13, and -15). We chose to examine by microarrays the derivatives of AM960 that were not treated with nocodazole. Since the cell viability in the untreated cells was very high, the detected events should be reflective of the entire population of cells.
Based on the PFGE and microarray analyses, most (22) of the strains could be grouped into six classes (Fig. 6A, C, and D; also see Table S1 and Fig. S4 and S5 in the supplemental material). The remaining eight strains had more complicated rearrangements that could not be readily explained as a consequence of a single repair event. These strains were not characterized further. The microarray analysis detects chromosomal regions that are duplicated or deleted. In all of our diploid isolates, the duplicated regions have representation about 50% greater than that in the progenitor diploid, and the deleted regions are represented about 50% less.
Class 1 strains had nonreciprocal translocations. All of these repair outcomes were monosomic for the right arm of chromosome III (distal to FS2 or FS1) and contained an amplification (duplication) of sequences located on a nonhomologous chromosome. By microarray analysis, the deletion breakpoints on III were adjacent to the Ty elements of FS1 or FS2, and the duplication breakpoints on the nonhomologous chromosome were adjacent to a Ty element or a solo delta element (the long terminal repeat at the ends of Ty elements). This pattern suggests that a nonreciprocal translocation was created by homologous recombination (BIR or related mechanisms) between a Ty1 element within FS1 or FS2 with a Ty element or a solo delta element on a nonhomologous chromosome. For example, in AM960-5, the predicted translocation involved a Ty element in FS2 on chromosome III recombining with a delta element on chromosome XIII (Fig. 6C and D; also see Table S1 in the supplemental material). The predicted size of this translocation was 305 kb, close to the observed size of the novel chromosome (310 kb). Although our conclusions for most of the class 1 strains are based on PFGE and microarray analysis, we also did detailed Southern analysis of one of these strains (AM960-60) to confirm the structure (see the supplemental material).
Five of the six class 1 strains could be explained by a single BIR event involving a Ty on III and a Ty or delta element on a nonhomologous chromosome. The microarray pattern observed for AM960-21 (Fig. 6C; also see Table S1 and Fig. S4 and S5 in the supplemental material), however, indicated a single deleted region on chromosome III (breakpoint at FS2) and two triplicated regions: an internal duplication on chromosome V and a terminal duplication on chromosome XII. This pattern could result from the repair of a DSB located near FS2 by invading a Ty element on chromosome V, followed by dissociation of the invading end after duplicating about 50 kb of DNA and reinvasion into a delta element on chromosome XII. The observed size of the rearranged chromosome was consistent with this hypothesis.
The chromosome rearrangements of class 2 were also nonreciprocal translocations. In this class, however, adjacent to the FS2-associated deletion of sequences from chromosome III, the adjacent region (between FS1 and FS2) was duplicated (Fig. 6D; also see Table S1 and Fig. S4 and S5 in the supplemental material). This pattern is likely to reflect the breakage of a dicentric chromosome of the type shown in Fig. 3Aii. If a break occurred in the region labeled “6” in this figure, the resulting end could invade a Ty element on a nonhomologous chromosome. The translocation product would have two copies of the region between FS1 and FS2. The structures of the translocations in the two class 2 strains (AM960-8 and AM960-54) were confirmed by detailed Southern analysis and/or PCR analysis (see the supplemental material). Their structures represent very strong arguments for the hypothesis that some of the chromosome rearrangements reflect BFB-mediated repair. Similar types of chromosome rearrangements have been observed to be associated with an inverted pair of 300-bp repeats inserted onto yeast chromosome V (36).
In class 3 strains (Fig. 6D; also see Table S1 and Fig. S4 and S5 in the supplemental material), there is a deletion of the sequences distal to FS2 and a duplication of sequences from the opposite chromosome arm. The most likely mechanism to generate such a strain is a break at a Ty element in FS2 that is repaired by a BIR event using the Ty element on the left arm of III as the point of invasion. The observed size of chromosome III in these strains (275 kb) is consistent with this hypothesis. Chromosomes in which the same information exists on both chromosomes' arms are termed “isochromosomes.”
Class 4 strains had deletions with breakpoints near FS1 or FS2. No duplications were associated with the deletions. These strains may reflect a DSB near FS1 or FS2 in which the broken end was “capped” by de novo telomere addition. These sizes of the chromosomes were consistent with this hypothesis (Fig. 6C and D; also see Table S1 and Fig. S4 and S5 in the supplemental material).
In class 5 strains, since the size of chromosome III was either 330 kb or 350 kb (the sizes of the unrearranged chromosomes), we did not expect any deletions or duplications (Fig. 6A and C). The microarrays confirmed that expectation (Fig. 6D; also see Table S1 in the supplemental material). The sizes of the chromosomes could be explained as the result of repairing DSBs by a BIR event using the 350-kb homologue (AM1000b-12 and AM960-6), a gene conversion event involving the homologue (AM1000b-15), or a conversion event associated with crossing over (AM1000b-13).
Class 6 strains have intrachromosomal rearrangements, either deletions (AM1000b-145 and AM1000a-240) or duplications (AM1000b-129 and -14) (Fig. 6D; also see Table S1 and Fig. S4 and S5 in the supplemental material). These rearrangements can be interpreted as BIR events that proceed via one of two scenarios: (i) strand invasion of one Ty1 from FS1 into Ty1β of FS2, leading to a deletion; or (ii) strand invasion of Ty1β of FS2 into one Ty1 of FS1, leading to a duplication.
Since the breakpoints of the rearrangements in classes 3, 4, and 6 are all associated with FS1 and FS2, it is likely that these events reflect breakage of dicentrics, although we cannot exclude the possibility that some are a consequence of the repair of the HO-induced DSB.
In summary, in both RAD51 and rad51 strains, an HO-induced DSB can result in various types of chromosome aberrations. Most of these aberrations have breakpoints associated with Ty or delta elements. At least some of the rearrangements (class 2) are likely to be associated with breakage of a dicentric intermediate. It should be pointed out that some of the class 3, 4, and 6 rearrangements may have been derived from a class 2 rearrangement that underwent a secondary change, since the large inverted repeats associated with a class 2 rearrangement would be expected to be unstable (36).
IR-mediated interchromatid SSA efficiently competes with other DSB repair pathways.
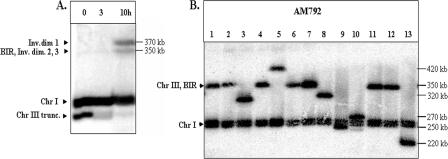
It could be argued that the high rate of chromosome rearrangements observed in the diploid AM960 occurs because the rad51 mutation reduces the likelihood of recombination with the homologue. We examined the consequences of an HO-induced DSB in a RAD51 diploid, AM792 (Fig. 1D). As shown in Fig. 1D, the chromosome III with the HO cleavage site is truncated, removing most of the chromosome III sequences centromere distal to the cleavage site. Most DSBs in strains in which one chromosome III has this truncation and one chromosome III has the wild-type arrangement are repaired by BIR (29). We induced DSBs in nocodazole-arrested AM792 (MATa/MATa-inc). Samples were taken every hour and analyzed by PFGE using the ADE1 probe hybridizing with the left arm of chromosome III. By 10 h, the amount of the truncated chromosome was substantially reduced and two repair products were observed (Fig. 7A). One repair product was about 370 kb and did not hybridize to either THR4- or URA3-specific probes (in AM792, URA3 is located distal to FS2 [Fig. 1D]). This product is similar, therefore, to ID1, which was observed in AM919 (Fig. 2A, left panel) and in a previous study (29). The smaller repair product (350 kb) could represent either a product similar to ID2 and ID3 (Fig. 2A, left panel) or a BIR product (29); alternatively, the 350-kb band could contain both types of products. To determine whether any of the observed repair products would result in chromosome rearrangements in viable cells, we induced DSBs in nocodazole-arrested AM792 cells, removed the nocodazole, and allowed the cells to recover on plates containing rich growth medium. We analyzed DNA from 28 strains derived from this treatment by PFGE (Fig. 7B). In nine of these strains, the size of the repaired chromosome III was different from the size expected from a BIR event. This result demonstrates that the repair intermediates that accumulate in nocodazole-arrested cells can give rise to chromosome rearrangements. A similar frequency of GCR events was observed in an experiment in which AM792 was not treated by nocodazole. In 9 out of 24 repair outcomes, the size of chromosome III was consistent with a GCR event, whereas 15 had a size consistent with BIR. Thus, the SSA-GCR pathway, which begins with dicentric formation and results in various GCRs, is efficient even when DSBs can be repaired by other means.
FIG. 7.
Repair intermediates and chromosome alterations in the RAD51/RAD51 diploid AM792. (A) Formation of inverted dimers (Inv.dim) in AM792. DNA was prepared for PFGE at intervals after induction of DSBs at MATa in nocodazole-arrested AM792. Southern blots were hybridized with ADE1-specific probe, which hybridizes to chromosome (Chr) I, to the truncated (trunc.) chromosome III, and to the repair products. The appearance of two repair products is shown. Inverted dimer 1 corresponds to the 370-kb dicentric dimer described for AM919. As expected, it does not hybridize to a THR4 probe (data not shown). The expected positions of the smaller repair intermediates (inverted dimers 2 and 3) are at the same positions expected for a BIR event, in which the truncated copy of chromosome III is repaired using the other homologue as a template. Thus, we cannot distinguish among these events. (B) DSB repair in AM792 leads to chromosome aberrations. DSBs were induced in nocodazole-arrested AM792 cells. The nocodazole was removed, and the cells were then plated to medium lacking adenine. DNA isolated from the resulting Ade+ colonies was analyzed by PFGE, followed by hybridization with an ADE1-specific probe. These strains had a chromosome III that was about 350 kb in length (suggestive of repair by BIR) or a chromosome III that was distinctly larger or smaller than 350 kb, indicating a chromosome rearrangement.
DISCUSSION
Several previous studies in yeast have characterized genomic rearrangements associated with closely linked inverted repeats (5, 6, 24, 27, 31, 36, 38). In these studies, the DSB that initiated genetic instability occurred at or in a close proximity to the inverted repeat. In the present study, we have shown that inverted repeats can lead to genetic instability by affecting the repair of distantly located DSBs that undergo extensive processing. The generation of chromosome rearrangements associated with a DSB located distantly from the inverted repeats involves several steps: (i) processing of the broken end to allow pairing of inverted repeats from different sister chromatids, resulting in formation of the dicentric repair intermediate; (ii) breakage of the intermediate to generate a recombinogenic substrate; and (iii) production of a stable rearranged chromosome either by homologous recombination or by telomere addition. Each of the steps will be discussed in detail below.
Inverted repeats promote the formation of inverted dicentric dimers.
The proposed mechanism by which the DSB at the MAT locus produces a dicentric repair intermediate is outlined in Fig. 4. Both sister chromatids undergo breakage at MATa (initiated in our experiments by HO) (Fig. 4A), followed by 5′-to-3′ degradation, resulting in the formation of long single-strand tails at the 3′ end that include inverted Ty repeats (Fig. 4B). The resulting molecules undergo intermolecular SSA in one of two configurations, either an FS2 Ty annealing with another FS2 Ty (Fig. 4Ci) or an FS2 Ty annealing with an FS1 Ty (Fig. 4Cii). Formation of inverted dicentric dimers could also be explained by “fold-back” mechanisms (27, 31) involving the Ty elements of FS2, followed by replication or by a BIR event in which the Ty1β on one chromatid invades the Ty1α on another chromatid. Since the “fold-back” mechanisms require a round of chromosomal replication following the intramolecular SSA, this mechanism cannot explain the formation of repair products in the presence of nocodazole or other chemicals that preclude cells from entering S phase. In the experiments without nocodazole, a large amount of repair product was formed before the recovery from G2/M arrest, thus excluding the “fold-back” mechanism. BIR also does not seem to be a likely explanation of our results because it is largely RAD51 dependent (8, 29). BIR also involves a 4- to 6-h delay at the step of initiation (29) and proceeds inefficiently through the centromere (34). Since the observed rearrangements are independent of RAD51, proceed with the kinetics expected from the 5′-to-3′ nuclease degradation, and include the centromere, we favor the mechanism shown in Fig. 4. For this mechanism, the formation of the dicentric intermediate requires considerable time. In our experiments, the time necessary for the formation of the intermediate is provided by making DSBs in cells arrested with nocodazole or in strains that cannot efficiently repair the DSB (haploid strains with the DSB in a single-copy sequence or strains with a rad51 mutation). Although it could be argued that these conditions are somewhat artificial, this pathway could also be important under natural conditions, such as the repair of an isochromatid break in a haploid cell (see below).
Dicentric inverted dimers initiate chromosome breakage.
The dicentric chromosomes with the inverted repeats would be expected to undergo breakage during anaphase. If the dicentric chromosome (Fig. 4Di) breaks asymmetrically, two types of monocentric products are expected, as shown in Fig. 4Ei and Eii. The larger fragment would retain a palindromic duplication, whereas the smaller fragment would not. As described above, the class 2 chromosome rearrangements are consistent with the existence of broken chromosome fragments that retain a palindromic duplication. Since both fragments lack telomeres, both would be unstable. The broken ends would be degraded unless stabilized by recombination acquisition of a telomere (as described below) or by de novo telomere addition.
Repair of DSBs by recombination.
In many of the RAD51 diploid AM1000a and AM1000b strains that survived the HO-induced cleavage, the chromosome III structure indicated that the DSB had been repaired by strand invasion into the uncleaved chromosome III of the α-parent, leading to gene conversion or BIR (AM1000b-12, -13, and -15). In some AM1000 strains, however, novel chromosome rearrangements were detected, including a nonreciprocal translocation (AM1000a-257), formation of chromosome III isochromosomes (AM1000a-225 and -231), and intrachromosomal duplications and deletions on chromosome III (AM1000a-240 and AM1000b-14, -129, and -145). All of these rearrangements had Ty or delta elements at the breakpoints. We therefore suggest that in these strains the chromosome rearrangement was a consequence of BIR event between a Ty or delta located on the right arm of chromosome III and a Ty or delta element located on a nonhomologous chromosome (AM1000a-257), a Ty element on the left arm of chromosome III (AM1000a-225 and -231), or a nonallelic Ty or delta element on the right arm of chromosome III (AM1000a-240 and AM1000b-14, -129, and -145).
In the rad51 strain AM960, as observed for AM1000, most of the chromosome rearrangements had Ty or delta elements at the breakpoints of the rearrangements. As discussed above, the translocations can be explained by BIR events involving a Ty or delta located on chromosome III and a Ty or delta located on a nonhomologous chromosome. Such events would be expected to produce a deletion of chromosome III and a duplication of sequences from the nonhomologous chromosome, as observed. In addition to BIR events, two other types of homologous recombination could result in a translocation: reciprocal recombination and half crossovers. A reciprocal exchange would be expected to produce two translocation products. If both segregated into the same cell, then no change in gene dosage would be observed by the microarray analysis. If one translocation product segregated into a cell with three untranslocated chromosomes, microarray analysis would detect a deletion and a duplication. A half crossover is a fusion between two chromosome fragments to generate one whole chromosome (16). If the translocation product segregates into a cell that has only one copy of the two homologues, then two deletions would be detectable by the microarrays. If the translocation product segregates into a cell that has two copies of one homologue and one copy of the other, however, one would observe a deletion and a duplication.
Although the small number of events examined in the RAD51 AM1000a and AM1000b strains prevents the exclusion of any of the three homologous recombination mechanisms described above, similar events from other studies appear to be a consequence of BIR (24, 36). Previously, we described DSB repair in rad51 diploids and proposed that the repair occurs via Rad51p-independent BIR (28); this repair event was dependent on a region termed “FBI” (30), which was later determined to be equivalent to FS2 (M. Bellinger and A. Malkova, unpublished observation). In the present study, we find that the HO-induced DSB results in the formation of a dicentric inverted dimer by a process that involves SSA rather than BIR. The chromosome rearrangements resulting from the processing of this dimer, however, often are homologous recombination events. In some rare cases (class 5), these events proceeded via recombination with a homologous chromosome. In other cases, the recombination events involved Ty elements on nonhomologous chromosomes and generated strains with both deletions and duplications. As discussed above, such events could represent Rad51p-independent BIR, Rad51p-independent reciprocal recombination followed by a particular type of chromosome segregation, or formation of half crossovers. Although we cannot exclude any of these mechanisms, since the rate of HO-induced reciprocal recombination is greatly reduced in rad51 strains (28) and since the double-deletion pattern characteristic of half crossovers was not observed, some level of Rad51p-independent BIR is likely. Finally, we note that six strains obtained in our study displayed complicated chromosome rearrangements that could not be explained as the consequence of a single repair event. Such strains could have chromosome alterations (such as giant palindromes) that result in ongoing chromosome instability (1, 36).
HO-induced chromatid fusions: implications for understanding chromatid fusions that produce instability in the human genome.
If a chromosome with a DSB is replicated, the cell will contain an isochromatid break (both sisters broken at the same position). Such lesions may occur naturally as a consequence of replication problems (43, 52) or be induced by certain types of radiation (3, 20). In addition, defects in telomere replication often result in “uncapped” sister chromatids that mimic isochromatid breaks (13, 35). In our study, we have generated isochromatid breaks using the HO site-specific endonuclease. As discussed in the introduction, isochromatid breaks in mammalian cells are often repaired by fusions of the broken ends. In general, the mechanism of these fusions has not been determined. In our study, we propose a mechanism of chromatid fusions involving single-strand annealing between inverted repeats located far away from the break. Since inverted repeats are very common in the human genome (22), we suggest that this mechanism might also apply to chromatid fusions in human cells.
Summary.
We have shown that a DSB located at a distance from inverted repeats can stimulate the formation of dicentric DNA repair intermediates that involve these repeats. These dicentric chromosomes are subsequently processed by mechanisms that lead to chromosome aberrations including nonreciprocal translocations, intrachromosomal deletions, and duplications. Given the very large number of repetitive DNA sequences in the mammalian genome, this “SSA-GCR” pathway is likely to contribute to genome instability in higher organisms.
Supplementary Material
Acknowledgments
We thank James E. Haber for his support and suggestions (the project was originally started in his laboratory, where it was supported by NIH grant GM20056 to J.E.H.). We thank Dmitry Gordenin and Gregory Ira for their suggestions throughout this work and for comments on the manuscript. We are thankful to Carol Newlon and James Theis for their analysis of the structure of chromosome III and for helpful discussion pertinent to this subject.
This work was supported by ACS institutional grant IRG-84-002-22, IUPUI RSFG grant, and NIH grant 1R15GM074657-01A1 to A.M.; NIH grants GM24110 and GM52319 to T.D.P.; and NSF grant MCB-0417088 to K.L.
Footnotes
Published ahead of print on 22 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Admire, A., L. Shanks, N. Danzl, M. Wang, U. Weier, W. Stevens, E. Hunt, and T. Weinert. 2006. Cycles of chromosome instability are associated with a fragile site and are increased by defects in DNA replication and checkpoint controls in yeast. Genes Dev. 20:159-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artandi, S. E., S. Chang, S.-L. Lee, S. Alson, and G. L. Gottlieb. 2000. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641-645. [DOI] [PubMed] [Google Scholar]
- 3.Behr, T., M. Behe, M. Stabin, E. Wehrmann, C. Apostolidis, R. Molinet, F. Strutz, A. Fayyazi, E. Wieland, S. Gratz, L. Koch, D. Goldenberg, and W. Becker. 1999. High-linear energy transfer (LET) α versus low-LET β emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213 Bi-versus 90 Y-labeled CO17-1A Fab fragments in a human colonic cancer model. Cancer Res. 59:2635-2643. [PubMed] [Google Scholar]
- 4.Bosco, G., and J. E. Haber. 1998. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 150:1037-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, D. K., D. Gillespie, and B. Steele. 2002. Formation of large palindromic DNA by homologous recombination of short inverted repeat sequences in Saccharomyces cerevisiae. Genetics 161:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler, D. K., L. E. Yasuda, and M. C. Yao. 1996. Induction of large DNA palindrome formation in yeast: implications for gene amplification and genome stability in eukaryotes. Cell 87:1115-1122. [DOI] [PubMed] [Google Scholar]
- 7.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, A. P., and L. S. Symington. 2004. RAD51-dependent break-induced replication in yeast. Mol. Cell. Biol. 24:2344-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diede, S. L., and D. E. Gottschling. 1999. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerase α and δ. Cell 99:723-733. [DOI] [PubMed] [Google Scholar]
- 10.Difilippantonio, M. J., S. Petersen, H. T. Chen, R. Johnson, M. Jasin, R. Kanaar, T. Ried, and A. Nussenzweig. 2002. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J. Exp. Med. 196:469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson, D. O., and F. Alt. 2001. DNA double strand double strand break repair and chromosomal translocation: lesson from animal models. Oncogene 20:5572-5579. [DOI] [PubMed] [Google Scholar]
- 12.Fouladi, B., D. Miller, L. Sabatier, and J. P. Murnane. 2000. The relationship between spontaneous telomere loss and chromosome instability in a human tumor cell line. Neoplasia 2:540-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilley, D., H. Tanaka, and B. S. Herbert. 2005. Telomere dysfunction in aging and cancer. Int. J. Biochem. Cell Biol. 37:1000-1013. [DOI] [PubMed] [Google Scholar]
- 14.Gollin, S. M. 2001. Chromosome alterations in squamous cell carcinomas of the head and neck: window to the biology of disease. Head Neck 23:238-253. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology. Academic Press, San Diego, CA.
- 16.Haber, J. E., and M. Hearn. 1985. Rad52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosome loss. Genetics 111:7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, A. M., and J. E. Haber. 1999. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell 96:415-424. [DOI] [PubMed] [Google Scholar]
- 18.Ingvarsson, S. 1999. Molecular genetics of breast cancer progression. Semin. Cancer Biol. 9:277-288. [DOI] [PubMed] [Google Scholar]
- 19.Ira, G., A. Pellicioli, A. Balijja, X. Wang, S. Fioranu, W. Carotenuto, G. Liberi, D. A. Bressan, L. Wan, N. M. Hollingsworth, J. E. Haber, and M. Foiani. 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawata, T., H. Ito, T. Uno, M. Saito, S. Yamamoto, Y. Furusawa, M. Durante, K. George, H. Wu, and F. A. Cucinotta. 2004. G2 chromatid damage and repair kinetics in normal human fibroblast cells exposed to low- or high-LET radiation. Cytogenet. Genome Res. 104:211-215. [DOI] [PubMed] [Google Scholar]
- 21.Kaye, J. A., J. A. Melo, S. K. Cheung, M. B. Vaze, J. E. Haber, and D. Toczyski. 2004. DNA breaks promote genomic instability by impending proper chromosome segregation. Curr. Biol. 14:2096-2106. [DOI] [PubMed] [Google Scholar]
- 22.Kolomietz, E., M. S. Meyn, A. Pandita, and J. A. Squire. 2002. The role of Alu repeat clusters as mediators of recurrent chromosomal aberrations in tumors. Genes Chromosomes Cancer 35:97-112. [DOI] [PubMed] [Google Scholar]
- 23.Kramer, K. M., and J. E. Haber. 1993. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 7:2345-2356. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine, F. J., N. P. Degtyareva, K. Lobachev, and T. D. Petes. 2005. Chromosomal translocations in yeast induced by low levels of DNA polymerase: a model for chromosome fragile sites. Cell 120:587-598. [DOI] [PubMed] [Google Scholar]
- 25.Lo, A. W., L. Sabatier, B. Fouladi, G. Pottier, M. Ricoul, and J. P. Murnane. 2002. DNA amplification by breakage/fusion/bridge cycles initiated by spontaneous telomere loss in a human cancer cell line. Neoplasia 4:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo, A. W., C. N. Sprung, B. Fouladi, M. Pedram, L. Sabatier, M. Ricoul, G. E. Reynolds, and J. P. Murnane. 2002. Chromosome instability as a result of double-strand breaks near telomeres in mouse embryonic stem cells. Mol. Cell. Biol. 22:4836-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobachev, K. S., D. A. Gordenin, and M. A. Resnick. 2002. The mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell 108:183-193. [DOI] [PubMed] [Google Scholar]
- 28.Malkova, A., E. L. Ivanov, and J. E. Haber. 1996. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc. Natl. Acad. Sci. USA 93:7131-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malkova, A., M. Naylor, M. Yamaguchi, G. Ira, and J. Haber. 2005. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol. Cell. Biol. 25:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malkova, A., L. Signon, C. B. Schaefer, M. L. Naylor, J. F. Theis, C. S. Newlon, and J. E. Haber. 2001. RAD51-independent break-induced replication to repair a broken chromosome depends on a distant enhancer site. Genes Dev. 15:1055-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maringele, L., and D. Lydall. 2004. Telomerase and recombination independent immortalization of budding yeast. Genes Dev. 18:2663-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClintock, B. 1941. The stability of broken ends of chromosomes in Zea Mays. Genetics 26:234-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mieczkowski, P. A., J. O. Mieczkowska, M. Dominska, and T. D. Petes. 2003. Genetic regulation of telomere fusions in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100:10854-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow, D. M., C. Connelly, and P. Hieter. 1997. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147:371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murnane, J. P., and L. Sabatier. 2004. Chromosome rearrangements resulting from telomere dysfunction and their role in cancer. Bioassays 26:1164-1174. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan, V., P. A. Mieczkowski, H. M. Kim, T. D. Petes, and K. V. Lobachev. 2006. The pattern of gene amplification is determined by the chromosomal location of hairpin-capped breaks. Cell 125:1283-1296. [DOI] [PubMed] [Google Scholar]
- 37.Paques, F., and J. E. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63:349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rattray, A. J., B. K. Shafer, B. Neelam, and J. Strathern. 2005. A mechanism of palindromic gene amplification in Saccharomyces cerevisiae. Genes Dev. 19:1390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabatier, L., M. Ricoul, G. Pottier, and J. P. Murnane. 2005. The loss of a single telomere can result in instability of multiple chromosomes in a tumor cell line. Mol. Cancer Res. 3:139-150. [DOI] [PubMed] [Google Scholar]
- 40.Signon, L., A. Malkova, M. L. Naylor, H. Klein, and J. E. Haber. 2001. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol. Cell. Biol. 21:2048-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smogorzewska, A., J. Karlseder, H. Holtgreve-Grez, A. Jauch, and T. De Lange. 2002. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr. Biol. 12:1635-1644. [DOI] [PubMed] [Google Scholar]
- 42.Soler, D., A. Genesca, G. Arnedo, J. Egozcue, and L. Tussell. 2005. Telomere dysfunction drives chromosomal instability in human mammary epithelial cells. Genes Chromosomes Cancer 44:339-350. [DOI] [PubMed] [Google Scholar]
- 43.Sonoda, E., M. S. Sasaki, J. M. Buerstedde, O. Bezzubova, A. Shinohara, H. Ogawa, M. Takata, Y. Yamaguchi-Iwai, and S. Takeda. 1998. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17:598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprung, C. N., G. E. Reynolds, M. Jasin, and J. P. Murnane. 1999. Chromosome healing in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 96:6781-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strathern, J., J. Hicks, and I. Herskowitz. 1981. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J. Mol. Biol. 147:357-372. [DOI] [PubMed] [Google Scholar]
- 46.Toledo, F., G. Buttin, and M. Debatisse. 1993. The origin of chromosome rearrangements at early stages of AMPD2 gene amplification in Chinese hamster cells. Curr. Biol. 1993:255-264. [DOI] [PubMed] [Google Scholar]
- 47.Vaze, M. B., A. Pellicioli, S. E. Lee, G. Ira, G. Liberi, A. Arbel-Eden, M. Foiani, and J. E. Haber. 2002. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol. Cell 10:373-385. [DOI] [PubMed] [Google Scholar]
- 48.Wang, P., Y. Kim, J. Pollack, B. Narasimhan, and R. Tibshirani. 2005. A method for calling gains and losses in array CGH data. Biostatistics 6:45-58. [DOI] [PubMed] [Google Scholar]
- 49.Weiffenbach, B., and J. E. Haber. 1981. Homothallic mating type switching generates lethal chromosome breaks in rad52 strains of Saccharomyces cerevisiae. Mol. Cell. Biol. 1:522-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wicksteed, B. L., I. Collins, A. Dershowitz, L. I. Stateva, R. P. Green, S. G. Oliver, A. J. Brown, and C. S. Newlon. 1994. A physical comparison of chromosome III in six strains of Saccharomyces cerevisiae. Yeast 10:39-57. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, J.-Q., E. K. Monson, S.-C. Teng, V. P. Schulz, and V. A. Zakian. 2000. Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science 289:771-774. [DOI] [PubMed] [Google Scholar]
- 52.Zou, H., and R. Rothstein. 1997. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell 90:87-96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.