Abstract
The basic helix-loop-helix TAL-1/SCL essential for hematopoietic development is also required during vascular development for embryonic angiogenesis. We reported that TAL-1 acts positively on postnatal angiogenesis by stimulating endothelial morphogenesis. Here, we investigated the functional consequences of TAL-1 silencing in human primary endothelial cells. We found that TAL-1 knockdown caused the inhibition of in vitro tubulomorphogenesis, which was associated with a dramatic reduction in vascular endothelial cadherin (VE-cadherin) at intercellular junctions. Consistently, silencing of TAL-1 as well as of its cofactors E47 and LMO2 down-regulated VE-cadherin at both the mRNA and the protein level. Endogenous VE-cadherin transcription could be activated in nonendothelial HEK-293 cells by the sole concomitant ectopic expression of TAL-1, E47, and LMO2. Transient transfections in human primary endothelial cells derived from umbilical vein (HUVECs) demonstrated that VE-cadherin promoter activity was dependent on the integrity of a specialized E-box associated with a GATA motif and was maximal with the coexpression of the different components of the TAL-1 complex. Finally, chromatin immunoprecipitation assays showed that TAL-1 and its cofactors occupied the VE-cadherin promoter in HUVECs. Together, these data identify VE-cadherin as a bona fide target gene of the TAL-1 complex in the endothelial lineage, providing a first clue to TAL-1 function in angiogenesis.
During development, hematopoietic precursors and endothelial cells (ECs) arise in close association from a common precursor, the hemangioblast. Although the hemangioblast per se has not yet been identified in vivo, coexpression of blood and endothelial genes, as well as the dependence of both lineages on some of these shared genes, supports its existence. One such gene is TAL-1/SCL, hereafter referred to as TAL-1 (reviewed in reference 25). TAL-1, initially identified at the sites of chromosomal rearrangements in human acute T-cell leukemia, encodes a transcription factor from the basic-helix-loop-helix family. Gene knockout studies with mice have revealed the essential role of tal-1 in the establishment of the hematopoietic system (33, 37, 38, 41) and its specific requirement for erythroid and megakaryocytic lineage formation (15, 28).
To exert its hematopoietic functions, TAL-1 protein acts through both DNA-binding-dependent and -independent mechanisms (32, 36). TAL-1 forms heterodimers with the E basic-helix-loop-helix proteins E47 and HEB and binds to a specific E-box (16). TAL-1 can either activate or repress transcription, depending on its association with other essential hematopoietic transcription factors, such as GATA-1 or GATA-2 and LMO2 (5, 21, 44-46). TAL-1 also interacts with coactivators (p300 and p/CAF) and corepressors (mSin3A and ETO-2), the function of which is linked to histone acetyltransferases or deacetylases (12, 17, 18, 40).
Loss- and gain-of-function studies with different vertebrate models showed that TAL-1 is also involved in the formation of the vascular system (10, 11, 31, 32, 43). Tal-1−/− embryos, rescued for Tal-1 expression in primitive hematopoietic cells, exhibit defective yolk sac angiogenesis, due to an intrinsic defect in Tal-1−/− endothelial cells (43). In adults, TAL-1 expression is undetectable in quiescent endothelium but is present in newly formed blood vessels (20, 35), including tumor vasculature (4). Together, these observations associate TAL-1 activity with both developmental and adult angiogenesis. We previously reported that TAL-1 acts as a positive factor for postnatal angiogenesis by modulating the migration properties of ECs and activating the morphogenetic events that lead to tubular structures. Importantly, the expression of a dominant negative mutant of TAL-1 in ECs completely abolished in vitro morphogenesis, as well as in vivo angiogenesis (23).
To understand how TAL-1 modulates angiogenesis, we investigated the functional effects of TAL-1 silencing, mediated by RNA interference, in human primary ECs. We show here that TAL-1 knockdown completely impairs in vitro tubulogenesis by down-regulating vascular endothelial cadherin (VE-cadherin) expression at both the protein and the mRNA level. Moreover, we provide evidence that TAL-1, in association with its partners E47, LMO2, GATA-2, and Ldb1, up-regulates VE-cadherin gene expression through direct binding to the VE-cadherin promoter.
MATERIALS AND METHODS
Cell cultures.
Human primary endothelial cells derived from umbilical vein (HUVECs) were obtained from Cambrex (France), and ECs from human cord blood (UCB-ECs) were prepared and cultured as described previously (23). HEK-293 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum.
Reagents and antibodies.
Human epidermal growth factor, human-basic fibroblast growth factor (bFGF), and human vascular endothelial growth factor (VEGF) were purchased from Peprotech (France), and Matrigel and rat type I collagen were purchased from BD Biosciences (France). The following antibodies were used in this study: 3BTL73 and 2TL136, two mouse monoclonal antibodies (MAb) directed against human TAL-1 (35); MAb anti-β-actin (clone AC-15; Sigma); MAb anti-β-catenin (clone 14; Transduction Laboratories); MAb anti-E47 (clone G127-32) and MAb anti-CD31/PECAM (clone WM-59 BD) from Pharmingen; MAb anti-VE-cadherin (clone BV9 [22] and clone 75; Transduction Laboratories); MAb anti-N-cadherin (clone 32; BD Biosciences); polyclonal rabbit antibody anti-general transcription factor IIB (TFIIB) (sc-225; Santa Cruz Biotechnology, Inc.); and polyclonal goat anti-human LMO2 (AF2726; R&D Systems).
siRNA transfections.
Small interfering RNA (siRNA) transfections in ECs were carried out using Magnetofection technology (polyMag; OZ Biosciences, France). Two successive transfections were performed 24 h apart, with a 30 nM siRNA concentration. For E47 and LMO2 silencing, a mixture of two RNA duplexes was used. The sequences of duplex RNAs are presented in the supplemental material.
Proliferation assays.
HUVECs or UCB-ECs (4 × 104) were seeded in collagen-coated 24-well plates and transfected with siRNAs as described above. After 3 days in culture, the number of viable cells per well was estimated by an MTT (3-[4,5-dimethylthiazol-2-yl]-diphenyltetrazolium bromide) assay (Sigma), following the manufacturer's instructions.
In vitro three-dimensional (3D) tubulogenesis in collagen I gels.
HUVECs (8 × 104) in basal medium (MCDB131 with 1% fetal calf serum, 1× insulin transferrin supplement, 2 mM glutamine, and 25 mM NaHCO3) were mixed with neutralized collagen I cold solution in wells of a 24-well plate to get a 0.9-mg/ml collagen concentration. Capillary tube formation was stimulated by the addition of basal medium supplemented with phorbol myristate acetate (80 nM), VEGF, and bFGF (20 ng/ml). Photographs were taken on a Zeiss Axiovert 25 inverted-phase microscope coupled to a digital Canon power shot camera. The digitalized images were mounted using Adobe Photoshop and Adobe Illustrator.
In vitro two-dimensional (2D) angiogenesis on Matrigel.
HUVECs (8 × 104) in complete EC medium were added to wells of a 24-well plate coated with Matrigel (BD Biosciences) and incubated at 37°C for 24 h. When required, cells were recovered from Matrigel as described previously (23), and total RNAs were prepared with an RNeasy kit (QIAGEN).
Immunofluorescence analyses.
Twenty-four hours after siRNA transfections, HUVECs were trypsinized and seeded onto gelatin-coated coverslips for 24 h. Primary MAbs were revealed with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulins G (IgGs) (Sigma). Cell nuclei were counterstained with Hoechst bisbenzimine (Sigma). Single-plane images were captured using a MicroMax 1300 charge-coupled-device camera (Princeton Instruments, Trenton, NJ) driven by Metamorph (Universal Imaging, Westchester, PA). The images were imported as TIFF files that were mounted using Adobe Photoshop and Adobe Illustrator.
RT-qPCR.
Reverse transcription was carried out as described previously (23). PCR amplification reactions were performed using a light cycler (Roche). The primer sequences used for real-time quantitative PCR (RT-qPCR) are indicated in the supplemental material.
Transient transfections.
The reporter constructs containing fragments of the human VE-cadherin promoter region upstream of the firefly luciferase gene have been described previously (34). Mutations of the E-boxes −101 and −784 and GATA −798 in the promoter were performed using a QuikChange II site-directed mutagenesis kit (Stratagene). Oligonucleotides used for these mutations are indicated in the supplemental material. Plasmid DNAs were transfected with Lipofectamine Plus (Life Technology) in HUVECs or precipitated with calcium phosphate in HEK-293 cells. The TK-RL plasmid encoding Renilla luciferase was cotransfected with firefly luciferase reporters to correct for transfection efficiency. Normalized firefly luciferase activities were determined using a dual luciferase kit (Promega). Human TAL-1 (wild type and Δ-bas [mutant that lacks a DNA-binding domain]) and E47 cDNAs were subcloned into pRc/CMV. The expression vector encoding hemagglutinin-tagged LMO-2 was provided by O. Bernard (Paris, France). The cDNAs encoding Ldb1 and GATA-2, provided by T. Hoang (Montreal, Quebec, Canada), were subcloned into pcDNA-3. The cDNAs encoding TAL-1 mutants SCL-FL and SCL-F-G (39), provided by C. Porcher (Oxford, United Kingdom), were subcloned in pcDNA-3.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed using exponentially growing HUVECs and are described in detail in the supplemental material. Cleared chromatin corresponding to 1 × 107 to 1.5 × 107 cells was incubated with antibodies against TAL-1, E47/E2A, GATA-2, LMO-2, and control IgGs. Aliquots of immunoprecipitated DNAs were analyzed in triplicate by real-time quantitative PCR with the indicated primers. The genomic region downstream of the VE-cadherin gene (+40932) was amplified as a negative control. Enrichment (n-fold) of target genomic regions immunoprecipitated by each specific antibody was normalized to the levels obtained with species-matched control IgGs and plotted as the increase over the level of enrichment at the negative-control region.
Statistical analysis.
All results are expressed as means ± standard deviations (SD). Significance of differences was determined with the Student t test, with significance at P of <0.05.
RESULTS
TAL-1 knockdown disrupts endothelial morphogenesis without affecting cell survival.
siRNA was used to reduce the levels of endogenous TAL-1 protein in HUVECs or UCB-ECs. An eGFP (gene encoding enhanced green fluorescent protein) siRNA, the sequence of which matches no known human gene, was used as a control. TAL-1 siRNA treatment suppressed the expression of TAL-1 protein to a level that was undetectable by immunoblotting (Fig. 1A, left). Both HUVECs and UCB-ECs carrying either TAL-1 siRNA or control siRNA grew at similar rates (Fig. 1A, right), indicating that TAL-1 is not essential for in vitro survival and growth of ECs. Moreover, TAL-1-silenced HUVECs seeded 48 h posttransfection onto coated glass coverslips formed a confluent cell monolayer similarly to control cells (Fig. 1B).
FIG. 1.
TAL-1 knockdown impairs in vitro morphogenesis. (A and B) TAL-1 silencing does not affect EC survival or growth. HUVECs or UCB-ECs transfected with TAL-1 or control siRNA (eGFP) were tested for their proliferative properties. (A) (Left) TAL-1 expression was assessed in whole-cell lysates (30 μg) by immunoblotting with the anti-TAL-1 MAb. The blot was reprobed with an anti-TFIIB antibody to control protein loading. (Right) Cells were transfected with the indicated siRNAs. MTT assays were carried out 48 h after the second transfection. Each bar is the mean ± SD of cell numbers relative to numbers of control siRNA-treated cells from three independent experiments performed in sextuplicate. NT, not transfected. (B) Forty-eight hours posttransfection, the same numbers of cells were seeded onto gelatin-coated coverslips. Shown are phase-contrast microscopic photographs after 24 h of culture, taken using a 10× objective. The experiments were repeated at least three times with similar results. (C) TAL-1 knockdown affects in vitro angiogenesis. HUVECs were transfected with TAL-1 or control eGFP siRNA and analyzed 24 h after the second transfection for their ability to produce in vitro capillary-like structures in 2D Matrigel culture or a tubular network in 3D collagen I gel. Shown are phase-contrast microscopic photographs, taken using a 10× objective, after 24 h of culture on Matrigel (left) or after 48 h in collagen I gel (right). The experiments were repeated at least three times with similar results.
We assessed the effects of TAL-1 knockdown on endothelial morphogenesis tested in 2D Matrigel cultures or in 3D collagen I gels. When seeded on 2D Matrigel, HUVECs treated with eGFP siRNA rapidly underwent morphogenesis, and by 24 h, virtually all cells had formed continuous cords with branching sprouts and anastomosis (Fig. 1C, left). TAL-1 siRNA-treated HUVECs also organized a network by 24 h. However, higher magnification revealed that the TAL-1-silenced ECs were still assembled into chains, albeit with poor cell-cell contacts, and did not undergo morphogenetic changes. When seeded within a 3D collagen gel with VEGF, bFGF, and phorbol myristate acetate, ECs align with each other, become polarized, and form tubular structures (6). Control ECs had formed an interconnected network of tubules 48 h after seeding (Fig. 1C, right). In contrast, TAL-1-silenced HUVECs did not form tubular structures, and similarly to the 2D culture, remained dispersed, nonpolarized, and without any cell-cell contact. These results indicate that the lack of TAL-1 does not affect EC survival or migration but impairs in vitro morphogenesis.
TAL-1 or E47 knockdown impairs junctional VE-cadherin distribution.
To assemble into tubular structures, ECs must establish contacts with their neighbors and with the extracellular matrix. Cell-cell adhesion is initiated and maintained by adherens junctions. The abnormal intercellular contacts observed in both 2D and 3D networks formed with ECs lacking TAL-1 (Fig. 1C) strongly suggested that TAL-1 knockdown might directly affect adherens junction formation. VE-cadherin is the adhesion component of endothelial adherens junctions and is directly involved in the formation and maintenance of cell-cell contacts (7). ECs also express platelet endothelial cell adhesion molecule (PECAM), which promotes homophilic adhesion between ECs. Previous studies have shown that antibodies blocking VE-cadherin or PECAM interactions impair HUVEC tubulogenesis in collagen I gels (48). These observations prompted us to assess whether TAL-1 knockdown alters VE-cadherin or PECAM expression at the cell surface. Strong VE-cadherin staining was observed at cell-cell contacts in control ECs (Fig. 2). In striking contrast, VE-cadherin staining was no longer present at sites of cell-cell contacts in TAL-1-silenced ECs. Conversely, both control and TAL-1-silenced ECs displayed similar results of PECAM and β-catenin staining at cell-cell contacts. β-Catenin recruitment at the intercellular region in the absence of VE-cadherin suggested that N-cadherin might have replaced VE-cadherin at cell-cell junctions in TAL-1-silenced ECs (29). Indeed, while control cells exhibited only a diffuse distribution of N-cadherin over the cell surface, TAL-1-silenced ECs displayed some N-cadherin clustering at cell-cell junctions in addition to nonjunctional distribution. These data indicated that TAL-1 silencing either disrupted VE-cadherin localization at intercellular junctions or decreased VE-cadherin content in ECs.
FIG. 2.
TAL-1 or E47 knockdown disrupts junctional VE-cadherin distribution. HUVECs were transfected with the indicated siRNA and, 24 h posttransfection, were trypsinized and seeded onto coverslips overnight. Cells were stained for VE-cadherin, PECAM, β-catenin, and N-cadherin, and the nuclei were counterstained. Micrographs were taken using a 40× objective. The pictures are representative of at least three independent experiments. Bar, 20 μM.
Dimerization of TAL-1 with E proteins is a prerequisite for all of its functions. Since E47 is the major dimerization partner of TAL-1 in ECs (23), we investigated whether E47 silencing might also influence VE-cadherin expression. E47-specific siRNAs dramatically decreased E47 protein expression (Fig. 3A, right) and produced the same effects as TAL-1 siRNA, i.e., a strong reduction of VE-cadherin staining at the cell-cell junctions (Fig. 2). Consistently, E47 knockdown also impaired in vitro tubulogenesis of HUVECs (see Fig. S1 in the supplemental material).
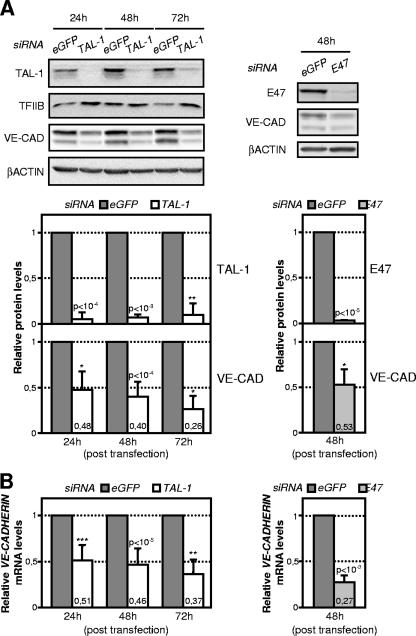
FIG. 3.
VE-cadherin expression is down-regulated in TAL-1- or E47-silenced ECs. HUVECs were transfected with the indicated siRNA. Whole-cell extracts and total RNAs were prepared at the indicated time points after transfection and monitored for VE-cadherin (VE-CAD) expression. (A) TAL-1, E47, and intracellular VE-cadherin protein expression levels were analyzed by immunoblotting. β-Actin or TFIIB expression was monitored for normalization of protein loading. Protein content from control siRNA-treated cells was arbitrarily set at 1. The images (top) are representative of three independent experiments, and the means ± SD of the indicated protein relative to that of β-actin are shown (bottom). (B) VE-cadherin mRNA levels were analyzed by RT-qPCR. The signals of VE-cadherin mRNA were normalized to that of GAPDH (gene encoding glyceraldehyde-3-phosphate dehydrogenase). Data shown are the means ± SD from three independent experiments. The VE-cadherin mRNA content from control siRNA-treated cells was arbitrarily set at 1.
We used immunoblotting to determine whether the absence of VE-cadherin at cell-cell junctions induced by TAL-1 or E47 knockdown was associated with variations in the amount of intracellular VE-cadherin. Indeed, TAL-1-silenced HUVECs showed significant decreases in VE-cadherin protein levels, namely, 52%, 60%, and 74% by 24, 48, and 72 h posttransfection, respectively, relative to results with control cells (Fig. 3A, left). Similarly, E47 silencing resulted in a 47% decrease in intracellular VE-cadherin protein levels by 48 h posttransfection (Fig. 3A, right). Thus, silencing of either TAL-1 or E47 reduces VE-cadherin expression.
In light of this, using RT-qPCR, we assessed whether this reflected decreased mRNA levels. VE-cadherin mRNA levels were significantly reduced in TAL-1-silenced ECs, namely, by 49%, 54%, and 63% at 24, 48, and 72 h posttransfection, respectively (Fig. 3B, left), compared to results with control cells. Similarly, E47 silencing induced a strong reduction over 70% of VE-cadherin mRNA levels (Fig. 3B, right).
Collectively, these data show that silencing of either TAL-1 or E47 disrupts endothelial intercellular junctions by down-regulating VE-cadherin expression at both the mRNA and the protein level.
LMO2 silencing down-regulates VE-cadherin expression.
In hematopoietic cells, the TAL-1/E heterodimers regulate gene expression through association with the LIM-only protein LMO2 (25). Given its key role in the endothelial lineage (47), we wondered if LMO2 also influences VE-cadherin expression. We reported that TAL-1 expression is modulated during the process of in vitro angiogenesis (23). We performed a time course analysis of VE-cadherin and LMO2 mRNA expression during in vitro angiogenesis on 2D Matrigel (Fig. 4A). Remarkably, both VE-cadherin and LMO2 mRNA levels paralleled those of TAL-1; they showed a strong decrease when cells were migrating to form the cell cord network, i.e., within the first 6 h, followed by an up-regulation during the morphogenetic events, i.e., the 12- to 24-h time points. These data strongly supported the possibility that LMO2 might also modulate VE-cadherin expression.
FIG. 4.
LMO-2 activity correlates with VE-cadherin up-regulation. (A) Time course analysis of TAL-1, VE-cadherin, and LMO-2 mRNA expression during in vitro angiogenesis. HUVECs were plated on Matrigel and cultured for 24 h. Cells were recovered at the indicated time points to prepare total RNA. Gene expression was analyzed by RT-qPCR. The signals of TAL-1, VE-cadherin, and LMO-2 mRNAs were normalized to that of GAPDH (gene encoding glyceraldehyde-3-phosphate dehydrogenase). Data shown are mRNA levels relative to the corresponding mRNA levels of exponentially growing cells, which were assigned a value of 1. Results from two independent experiments (Exp. 1 and Exp. 2) are shown. (B) LMO-2 silencing down-regulates VE-cadherin expression. HUVECs were transfected with control or LMO-2 siRNA. Forty-eight hours posttransfection, VE-cadherin protein expression was analyzed by immunoblotting; β-actin expression was used to normalize protein loading. The image (middle) is representative of at least three independent experiments. Data shown are VE-cadherin protein contents relative to that of control siRNA-treated cells, which was assigned a value of 1 (bottom). LMO-2 silencing (top) and VE-cadherin mRNA expression were monitored by RT-qPCR, and mRNA signals were normalized to that of GAPDH. Data shown are mRNA contents relative to that of control siRNA-treated cells, which was assigned a value of 1 (**, P < 0.01; ***, P < 0.001). (C) Ectopic coexpression of TAL-1, E47, and LMO-2 up-regulates VE-cadherin expression in nonendothelial cells. HEK-293 cells were cotransfected with the indicated expression vectors. The total amount of DNA (6.5 μg) was maintained constant with the addition of the corresponding empty vectors. eGFP vector (0.5 μg) was added to each point to control transfection efficiency. Total RNAs and whole-cell extracts were prepared 48 h posttransfection, and VE-cadherin expression was analyzed by RT-qPCR. The expression of ectopic proteins in whole-cell extracts (30 μg) was monitored by immunoblotting. A representative experiment is shown. VE-cadherin mRNA signals were normalized to those of GAPDH. Data shown are VE-cadherin mRNA contents relative to that of cells transfected with empty vectors, which was assigned a value of 1. Data are the means ± SD from at least three independent experiments (***, P < 0.001).
We assessed the effects of LMO2 silencing on VE-cadherin expression. LMO2 siRNA treatment efficiently suppressed LMO2 mRNA expression in HUVECs (Fig. 4B, top). LMO2 knockdown was associated with a 60% reduction in VE-cadherin expression at both the protein (Fig. 4B, middle) and the mRNA (Fig. 4B, bottom) level. Similarly to TAL-1- and E47-silenced HUVECs, LMO2-silenced cells failed to display VE-cadherin staining at the cell-cell junctions (not shown) and to generate tubules in 3D collagen gels (see Fig. S1 in the supplemental material).
Coexpression of TAL-1, E47, and LMO2 up-regulates the VE-cadherin gene in nonendothelial cells.
We investigated whether TAL-1, E47, and LMO2 could also influence endogenous VE-cadherin expression in a nonendothelial cell environment. We chose human embryonic kidney 293 cells (HEK-293) since they express neither TAL-1 nor LMO2 and they display low constitutive amounts of VE-cadherin mRNAs as detected by RT-qPCR. Basal transcription of VE-cadherin was insensitive to exogenous TAL-1 or E47, either alone or together, and to LMO2 alone (Fig. 4C). In contrast, coexpression of TAL-1 and E47 with LMO2 caused a sixfold increase in VE-cadherin mRNA levels. We tested the effects of several TAL-1 mutants in this activation: Δbas, which lacks a DNA-binding domain; TAL-1-FL, which is unable to form a heterodimer with E proteins (39); and TAL-1-FG, which fails to interact with LMO2 (39). The coexpression of each individual TAL-1 mutant with E47 and LMO2 failed to stimulate VE-cadherin expression. Thus, the binding of TAL-1 to DNA and its interaction with E47 as well as with LMO2 are required for VE-cadherin transcriptional activation in HEK-293 cells.
TAL-1, E47, and LMO2 regulate the VE cadherin promoter in HUVECs.
Our above-described results suggested that TAL-1, E47, and LMO2 regulate VE-cadherin expression at the transcriptional level. To test this possibility, we transfected various human VE-cadherin promoter reporter constructs in HUVECs. As described previously (34), all of these constructs were active, where the highest efficiency was observed with the longest promoter fragment, −3500/−5 (Fig. 5A). TAL-1 knockdown caused a significant decrease in the activity of all of the promoter fragments, compared with eGFP siRNA treatment (Fig. 5A, left), but did not affect the heterologous simian virus 40 (SV40) promoter (Fig. 5A, right).
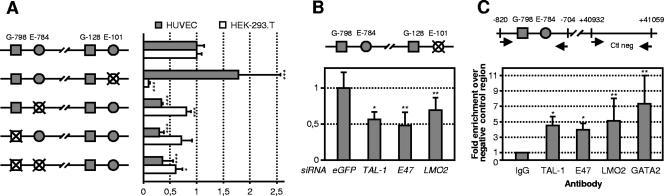
FIG. 5.
Silencing of TAL-1, E47, or LMO2 reduces VE-cadherin promoter activity. (A) (Top) HUVECs were transfected by control or TAL-1 siRNA and 24 h later with the indicated reporter containing fragments derived from the human VE-cadherin promoter, for which the negative number indicates the position of the first nucleotide of the gene segment relative to the transcription start site (+1), or with the pGL3 promoter reporter (SV40). Luciferase activity was assayed as described previously (24). Relative luciferase activities are presented as increases (n-fold) over the activity of the minimal promoter (the −166/−5 construct) treated with control eGFP siRNA, which was assigned a value of 1. Each bar is the mean ± SD from at least three independent experiments, each performed in triplicate. TAL-1 silencing was monitored by immunoblot analysis (not shown). (Bottom) The VE-cadherin promoter contains two conserved E-box-GATA motifs. Nucleotide sequences of the two highly conserved regions from the human VE-cadherin promoter are shown. The E-boxes E−784 and E−101 are indicated by dark-gray boxes and GATA-binding sites G−798 and G−128 by light-gray boxes. The ETS-binding sites (EBS) from the minimum promoter (34) are underlined. (B) HUVECs were transfected with the indicated siRNAs and either with the −1135/−5 reporter construct (left) or with the pGL3 promoter (right). E47 silencing was monitored by immunoblot analysis and LMO2 silencing by RT-qPCR (not shown). Data are the means ± SD from at least three independent experiments, each performed in triplicate, and are shown relative to the luciferase activity of cells transfected with control siRNA. (C) HUVECs were cotransfected with the −1135/−5 reporter construct and the indicated expression vectors. The total amount of DNA was maintained constant with the addition of the corresponding empty vectors. Data are the means ± SD from at least three independent experiments, each performed in triplicate, and are shown relative to the luciferase activity of cells transfected with empty vector, which was arbitrarily set at 1 (**, P < 0.01; ***, P < 0.001). Ectopic expression was monitored by Western blot analysis or RT-qPCR (see Fig. S2 in the supplemental material).
Comparative-genomics sequence analysis is a powerful technique for identifying important regulatory elements in genomic DNA. We aligned the upstream sequences of the VE-cadherin gene from several species (human, mouse, rabbit, rat, dog, and elephant) by using the UCSC genome bioinformatics site and found several highly conserved blocks. We searched for potential cis-acting binding sites in the human VE-cadherin promoter by using the Transcription Element Search System (TESS) and examined the alignment to verify whether these were conserved in all species tested. This study highlighted two highly conserved regions of interest within the VE-cadherin promoter (−150/−80 and −800/−770 [Fig. 5A]), which both contain an E-box consensus in close vicinity of a potential GATA-binding site. This particular E-box-GATA motif arrangement is present in the promoter of several hematopoietic genes found to be directly regulated by TAL-1-containing complexes (1, 5, 21, 27, 44-46).
For the next studies, we used the −1135/−5 reporter construct since it contains these two E-box-GATA motif elements. Significantly, E47 or LMO2 siRNA treatment also reduced the activity of the VE-cadherin promoter by 45% and 55%, respectively (Fig. 5B, left), but not the activity of the SV40 promoter (Fig. 5B, right). Thus, the VE-cadherin reporter transfection studies mirrored the effects of TAL-1, E47, or LMO2 silencing on endogenous VE-cadherin in HUVECs.
Given the presence of these two E-box-GATA motifs in the VE-cadherin promoter, we tested whether ectopic expression of the different components of the TAL-1 complex as described for hematopoietic cells may influence the VE-cadherin promoter. Transient transfections were carried out in HUVECs using the −1135/−5 construct (Fig. 5C). The expression of TAL-1, E47, GATA-2, or Ldb1 induced a significant increase in promoter activity, i.e., 1.7-, 2.6-, 2.2-, or 1.6-fold, respectively. However, maximal activation (fivefold) was observed with the coexpression of the five components, including LMO2. These results showed that TAL-1 in association with E47, LMO2, GATA-2, and Ldb1 activates the VE-cadherin promoter in endothelial cells.
VE-cadherin promoter activity depends on a specialized E-box-GATA element.
To assess the significance of the E-box motifs identified by sequence comparison analysis (E−101 and E−784 [Fig. 5A]), we disrupted these motifs in the context of the −1135/−5 reporter construct and tested the mutated promoter activity by using transient-transfection assays with HUVECs and HEK-293 cells (Fig. 6A). In HUVECs, the E−784 mutated promoter exhibited a strong reduction (66%) in activity compared to that of the wild-type promoter. In contrast, the mutation of the E−101 element produced a significant 80% increase in luciferase activity. Opposite observations were made with HEK-293 cells, in which the basal activity of the VE-cadherin reporter was abolished by the E−101 mutation but not affected by the E−784 mutation.
FIG. 6.
VE-cadherin promoter activity in endothelial cells is dependent on a specialized E-box-GATA element. (A) HUVECs or HEK-293 cells were transfected with either the wild-type −1135/−5 promoter construct or the −1135/−5 promoter construct with the indicated mutation(s). Three independent clones were tested for each mutation. Data are the means ± SD from three experiments performed in triplicate and are shown relative to the luciferase activity of cells transfected with the wild-type promoter construct, which was assigned a value of 1. (***, P < 0.001; *, P < 0.05.) (B) Silencing of TAL-1, E47, or LMO2 reduces the activity of the VE-cadherin promoter in the absence of functional E−101. HUVECs were transfected by the indicated siRNA and 24 h later with the VE-cadherin −1135/−5 promoter reporter in which E−101 was mutated. Relative luciferase activities are presented as increases (n-fold) over the activity of the E−101 mutated promoter treated with control GFP siRNA, which was assigned a value of 1. Each bar is the mean ± SD from three independent experiments, each performed in triplicate. TAL-1 and E47 silencing was monitored by immunoblot analysis and LMO2 silencing by RT-qPCR (not shown). **, P < 0.01; *, P < 0.05. (C) TAL-1, E47, LMO2, and GATA-2 are recruited to the VE-cadherin promoter in HUVECs. ChIP assays were performed on cross-linked chromatin from HUVECs, as described in Materials and Methods (see the supplemental material for a detailed description). Aliquots of immunoprecipitated DNAs were analyzed in triplicate by RT-qPCR with primers targeting the G−798/E−784 region. The genomic region downstream of the VE-cadherin gene (+40932) was amplified as a negative control. Enrichment (n-fold) of target genomic regions immunoprecipitated by each specific antibody was normalized to the levels obtained with species-matched control IgGs, assigned a value of 1, and plotted as the increase over the level of enrichment at the negative-control region. The error bars represent SD from at least two independent ChIP assays. **, P < 0.01; *, P < 0.05.
These experiments demonstrated that the E−784 element was required for endothelial-specific activity of the VE-cadherin promoter. In contrast, the E−101 element exerted negative effects on the promoter in endothelial cells, whereas it functioned as a positive element in nonendothelial cells.
Importantly, the mutation of GATA−798, located 8 bp upstream of E−784, also induced a strong 70% reduction in VE-cadherin promoter activity in HUVECs but not in HEK-293 cells (Fig. 6A). Disruption of both E−784 and G−798 provoked a similar 70% decrease in promoter activity in HUVECs, as did individual mutation of E−784 or G−798. Meaningfully, knockdown of TAL-1, E47, or LMO2 still induced a significant reduction in the activity of the mutated E−101 reporter construct, in which the G−798-E−784 motif was intact (Fig. 6B). Altogether, these assays identified a critical role for the G−798-E−784 motif for VE-cadherin promoter activity in endothelial cells.
TAL-1, E47, LMO2, and GATA-2 are recruited to the VE-cadherin promoter in HUVECs.
Our above-described observations were consistent with the possibility that TAL-1, E47, LMO2, and GATA-2 work together in a complex, as has been shown for erythroid cells (1, 5, 21, 27, 44-46), to activate the VE-cadherin promoter through binding to the G−798-E−784 motif. To test this possibility, chromatin immunoprecipitation assays were performed with HUVECs, using anti-TAL-1, anti-LMO-2, anti-E47, and anti-GATA-2 antibodies as well as control immunoglobulins. The amounts of specific genomic fragments in immunoprecipitated samples were determined individually by real-time quantitative PCR. Primers were chosen to amplify the VE-cadherin promoter region including the G−798-E−784 motif (Fig. 6C). The genomic region downstream of VE-cadherin (+40932 from the transcription start site) was used as a negative control in PCR analysis.
ChIP assays with anti-acetylated histone H3, which is a mark of active chromatin, were performed as a positive control for the quality of chromatin (not shown). As shown in Fig. 6C, TAL-1, E47, LMO2, and GATA-2 were all found to bind the G−798-E−784 region of the VE-cadherin promoter in HUVECs.
VE-cadherin silencing causes the same defects of in vitro tubulogenesis as TAL-1 silencing.
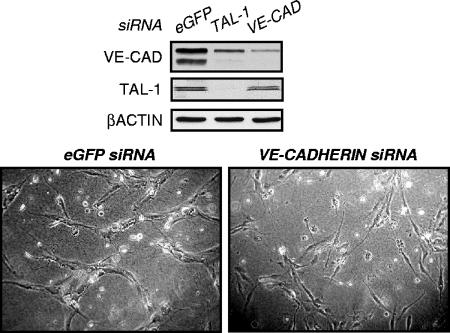
Our above-described results demonstrated that the morphogenetic defects resulting from silencing of TAL-1, E47, or LMO2 correlated with down-regulation of VE-cadherin expression. Meaningfully, a decrease in VE-cadherin expression mediated by VE-cadherin siRNA (Fig. 7) provoked a behavior of HUVECs during in vitro tubulogenesis similar to that of TAL-1-silenced ECs (Fig. 1C). Indeed, unlike control ECs, VE-cadherin-silenced ECs did not establish cell-cell contacts and did not form tubular structures. This indicates that VE-cadherin down-expression induced by depletion of TAL-1 or its cofactor is a major event accounting for an EC-deficient phenotype during in vitro tubulogenesis.
FIG. 7.
Knockdown of VE-cadherin (VE-CAD) impairs in vitro tubulogenesis. HUVECs were transfected twice with the indicated siRNA and analyzed 24 h after the second transfection for their ability to produce an in vitro tubular network in 3D collagen I gel. Shown are phase-contrast microscopic photographs, taken using a 10× objective, after 48 h of culture in collagen I gel. The residual protein expression was monitored by immunoblot analysis using 30 μg whole-cell extracts. A representative experiment of three is shown.
DISCUSSION
In addition to its essential role in hematopoiesis, TAL-1 is crucial for vascular development and more particularly for embryonic angiogenesis (43). We previously reported that TAL-1 acts as a positive factor for postnatal angiogenesis (23). Here, we investigated how TAL-1 modulates this process, utilizing siRNA technology to block TAL-1 expression in human primary ECs. Our data demonstrate that TAL-1 silencing impairs in vitro tubulogenesis and strongly reduces VE-cadherin expression. Accordingly, several approaches demonstrated a direct role of TAL-1 together with E47, LMO2, and GATA-2 in up-regulation of VE-cadherin in ECs. Our findings identify VE-cadherin as a bona fide target of the TAL-1 complex in the endothelial lineage.
TAL-1 mediates endothelial morphogenesis through regulation of VE-cadherin expression.
Capillary tube formation is a specialized endothelial cell function and is a prerequisite for the establishment of a continuous vessel lumen. ECs lacking TAL-1 are unable to initiate in vitro morphogenesis, defined here as the process whereby ECs assemble into cell cords in a 2D culture (Matrigel) or into tubes in a 3D matrix (collagen I). The defect was not due to perturbation in cell survival or growth in our experimental conditions, in agreement with our previous observations that overexpression of TAL-1 or a mutant that lacks the DNA-binding domain did not modify EC growth properties (23). However, this contradicts recent in vitro studies reporting a growth defect of Tal-1-deficient ECs (8, 9). This apparent discrepancy probably reflects the difference between transient silencing of TAL-1 and its total absence observed in ECs from the start.
TAL-1-silenced ECs do not develop normal cell-to-cell contacts, a prerequisite for initiating morphogenesis. Among the potential mediators, VE-cadherin stood out as an attractive candidate. VE-cadherin is an essential component in endothelial morphogenesis (3, 14), and the in vitro morphogenetic defects caused by TAL-1 silencing resemble that observed with antibodies blocking VE-cadherin adhesions (48). Here, we found that TAL-1 depletion leads to a vast decrease in VE-cadherin at the cell-cell junctions, which corresponds to diminished VE-cadherin transcription. VE-cadherin down-regulation appears as a major event accounting for the deficient phenotype of TAL-1-silenced ECs. Indeed, reduction in VE-cadherin mediated by VE-cadherin-targeting siRNA causes the same morphogenetic defects during the process of in vitro tubulogenesis.
TAL-1 acts together with LMO2, E47, and GATA-2 in a multiprotein complex to activate the VE-cadherin promoter.
In hematopoietic cells, TAL-1 transactivates transcription if all of its appropriate partners are recruited to the promoter. To activate hematopoietic-specific genes, TAL-1 requires the presence of E47, LMO2, Ldb1, and GATA-1 or GATA-2 (1, 5, 21, 27, 44-46). Given that all of these factors are present in ECs, we speculated that a similar multiprotein complex might positively regulate VE-cadherin.
Consistently, TAL-1, E47, and LMO-2 knockdowns in ECs cause similar strong reductions in endogenous VE-cadherin expression and reduce the activity of the VE-cadherin promoter in transient transfections in HUVECs. Meaningfully, ectopic coexpression of TAL-1 with E47 and LMO-2 in nonendothelial cells is able to up-regulate endogenous VE-cadherin transcription, whereas ectopic expression of each factor alone has no effect. From the data obtained with TAL-1 mutants, we conclude that, to exert this transcriptional function, TAL-1 is required to bind DNA and to interact with both E47 and LMO2.
Our experiments establish that TAL-1 acts jointly with E47, LMO2, and GATA-2 in a multiprotein complex to activate the VE-cadherin promoter. First, transient transfections demonstrate that VE-cadherin promoter activity in ECs is dependent on a specialized E-box (E−784) in close association with a proximal GATA element, as has been described for the promoter of target genes of the TAL-1 complex in erythroid cells. Importantly, the sequence of E-box−784 matches the preferred consensus E-box sequence (CAGATG) determined for the heterodimers TAL-1/E, where the half site “ATG” is bound by TAL-1 (16). Second, mutations that disrupt the E-box, the GATA element, or both provoke similar strong reductions in the activity of the promoter in ECs but not in nonendothelial cells. Third, exogenous expression of TAL-1, E47, GATA-2, or Ldb1 activates the VE-cadherin promoter in HUVECs; however, the maximal activation requires the concomitant expression of all components of the complex, including LMO2. Finally, ChIP experiments show that these different factors occupy this promoter region in HUVECs.
Complex regulation of VE-cadherin expression.
In this study, we identified a second highly conserved E-box (E−101) in the proximal promoter, which exerts a negative effect on the promoter in endothelial cells but is required for its basal activity in nonendothelial cells. Unlike the E−784 element, the palindromic sequence of E−101 (CAGCTG) should favor the binding of homodimers, such as E/E or other unknown factors, but not TAL-1 heterodimers. Accordingly, the activity of the promoter in which E−101 was disrupted was still affected by the depletion of TAL-1 or its partners in endothelial cells. The E−101 mutation rendered the promoter more active in ECs, suggesting the presence of one or several repressors that might interact directly with this element in the promoter. One candidate is SNAIL, a potent repressor that has been shown to inhibit E-cadherin expression through its binding to specific E-boxes in the promoter (2). Recently, SNAIL induction was correlated with VE-cadherin repression in specialized ECs during cardiac development (42), and importantly SNAIL was shown to directly repress the VE-cadherin proximal promoter in porcine aortic ECs (42).
Given its essential function in the vascular system, VE-cadherin must be tightly controlled through a complex interplay between positive and negative regulators, similarly to that of E-cadherin. Endothelial cells derived from Tal-1−/− embryonic bodies express VE-cadherin at their surface (28), indicating that the TAL-1 complex is not the sole transcriptional activator of the VE-cadherin promoter. Notably, ETS-1 protein activates the VE-cadherin proximal promoter through binding to two ETS-binding sites present in both mouse and human genes (13, 26, 34). Our studies suggest that dynamic fluctuations of TAL-1 and its partners on the VE-cadherin promoter likely contribute to the precise timing of VE-cadherin expression in ECs. Up-regulation of VE-cadherin coincided with the induction of both TAL-1 and LMO2 during in vitro angiogenesis (Fig. 4A). At the initiation of morphogenesis, increases in TAL-1 and LMO2 would trigger up-regulation of VE-cadherin, as well as other unidentified TAL-1 target genes. Although the elevation of VE-cadherin expression in angiogenesis has been documented previously (19, 30, 34), this is the first VE-cadherin expression study associating a morphogenetic event with a transcriptional mechanism.
The involvement of both TAL-1 and LMO2 in the up-regulation of VE-cadherin is strongly supported by the similar vascular defects in early development caused by their respective knockouts (3, 14, 43, 47). The deficiency of any of the three genes does not affect the assembly of ECs in the primitive vascular plexus but impairs their subsequent remodeling by angiogenesis, causing lethality at 9.5 days of gestation. Thus, it is possible that the phenotype alterations observed for Lmo2−/− and Tal-1−/− embryos might be the consequence of the absence of up-regulation of VE-cadherin during angiogenesis due to the lack of one of these two transcription factors.
In conclusion, our study demonstrates that VE-cadherin is an important component of the genetic program controlled by TAL-1 in the endothelial lineage. Establishing the compendium of TAL-1 transcriptional targets in ECs should help to determine whether TAL-1 is dedicated solely to the up-regulation of VE-cadherin expression or whether it also promotes the expression of other genes important for endothelial cell function.
Supplementary Material
Acknowledgments
We are indebted to Valérie Pinet and Philippe Couttet for helpful discussions and to Robert Hipskind for critical reading of the manuscript. We acknowledge Olivier Bernard, Trang Hoang, and Catherine Porcher for providing us with cDNA reagents and Gerlinde Layh-Schmitt for the anti-LMO2 antibody. We are grateful to Ignacio A. Romero and Babette Wekler for the hCMEC/D3 endothelial cell line. We thank Pierre Travo and Julien Cau (RIO Platform, Montpellier, France) for their constant help with imaging studies.
V.D. was a fellowship recipient of the Ligue Nationale Contre le Cancer (France) and is now funded by the Institut National du Cancer (INCa, France). E.C. was a recipient of the Association pour la Recherche sur le Cancer (ARC, France). R.E.-H. is supported by the Ligue Régionale contre le Cancer (Hérault, France) and D.M. by the Institut National de la Santé et de la Recherche Médicale (INSERM, France). This work was supported in part by grants from ARC and Cancéropôle Grand Sud-Ouest (France).
Footnotes
Published ahead of print on 22 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anguita, E., J. Hughes, C. Heyworth, G. A. Blobel, W. G. Wood, and D. R. Higgs. 2004. Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J. 23:2841-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2:84-89. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet, P., M. G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, A. Zanetti, A. Angellilo, V. Mattot, D. Nuyens, E. Lutgens, F. Clotman, M. C. de Ruiter, A. Gittenberger-de Groot, R. Poelmann, F. Lupu, J. M. Herbert, D. Collen, and E. Dejana. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147-157. [DOI] [PubMed] [Google Scholar]
- 4.Chetty, R., M. A. Dada, C. H. Boshoff, M. A. Comley, S. C. Biddolph, J. W. Schneider, D. Y. Mason, K. A. Pulford, and K. C. Gatter. 1997. TAL-1 protein expression in vascular lesions. J. Pathol. 181:311-315. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Kaminsky, S., L. Maouche-Chretien, L. Vitelli, M. A. Vinit, I. Blanchard, M. Yamamoto, C. Peschle, and P. H. Romeo. 1998. Chromatin immunoselection defines a TAL-1 target gene. EMBO J. 17:5151-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, G. E., K. J. Bayless, and A. Mavila. 2002. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268:252-275. [DOI] [PubMed] [Google Scholar]
- 7.Dejana, E. 2004. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5:261-270. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza, S. L., A. G. Elefanty, and G. Keller. 2005. SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development. Blood 105:3862-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ema, M., P. Faloon, W. J. Zhang, M. Hirashima, T. Reid, W. L. Stanford, S. Orkin, K. Choi, and J. Rossant. 2003. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 17:380-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gering, M., A. R. Rodaway, B. Gottgens, R. K. Patient, and A. R. Green. 1998. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17:4029-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gering, M., Y. Yamada, T. H. Rabbitts, and R. K. Patient. 2003. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development 130:6187-6199. [DOI] [PubMed] [Google Scholar]
- 12.Goardon, N., J. A. Lambert, P. Rodriguez, P. Nissaire, S. Herblot, P. Thibault, D. Dumenil, J. Strouboulis, P. H. Romeo, and T. Hoang. 2006. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 25:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gory, S., J. Dalmon, M. H. Prandini, T. Kortulewski, Y. de Launoit, and P. Huber. 1998. Requirement of a GT box (Sp1 site) and two Ets binding sites for vascular endothelial cadherin gene transcription. J. Biol. Chem. 273:6750-6755. [DOI] [PubMed] [Google Scholar]
- 14.Gory-Faure, S., M. H. Prandini, H. Pointu, V. Roullot, I. Pignot-Paintrand, M. Vernet, and P. Huber. 1999. Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126:2093-2102. [DOI] [PubMed] [Google Scholar]
- 15.Hall, M. A., D. J. Curtis, D. Metcalf, A. G. Elefanty, K. Sourris, L. Robb, J. R. Gothert, S. M. Jane, and C. G. Begley. 2003. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc. Natl. Acad. Sci. USA 100:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, H. L., L. Huang, J. T. Tsan, W. Funk, W. E. Wright, J. S. Hu, R. E. Kingston, and R. Baer. 1994. Preferred sequences for DNA recognition by the TAL1 helix-loop-helix proteins. Mol. Cell. Biol. 14:1256-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, S., and S. J. Brandt. 2000. mSin3A regulates murine erythroleukemia cell differentiation through association with the TAL1 (or SCL) transcription factor. Mol. Cell. Biol. 20:2248-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, S., Y. Qiu, R. W. Stein, and S. J. Brandt. 1999. p300 functions as a transcriptional coactivator for the TAL1/SCL oncoprotein. Oncogene 18:4958-4967. [DOI] [PubMed] [Google Scholar]
- 19.Hubank, M., and D. G. Schatz. 1994. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 22:5640-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallianpur, A. R., J. E. Jordan, and S. J. Brandt. 1994. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood 83:1200-1208. [PubMed] [Google Scholar]
- 21.Lahlil, R., E. Lecuyer, S. Herblot, and T. Hoang. 2004. SCL assembles a multifactorial complex that determines glycophorin A expression. Mol. Cell. Biol. 24:1439-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampugnani, M. G., M. Corada, L. Caveda, F. Breviario, O. Ayalon, B. Geiger, and E. Dejana. 1995. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J. Cell Biol. 129:203-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazrak, M., V. Deleuze, D. Noel, D. Haouzi, E. Chalhoub, C. Dohet, I. Robbins, and D. Mathieu. 2004. The bHLH TAL-1/SCL regulates endothelial cell migration and morphogenesis. J. Cell Sci. 117:1161-1171. [DOI] [PubMed] [Google Scholar]
- 24.Le Clech, M., E. Chalhoub, C. Dohet, V. Roure, S. Fichelson, F. Moreau-Gachelin, and D. Mathieu. 2006. PU.1/Spi-1 binds to the human TAL-1 silencer to mediate its activity. J. Mol. Biol. 355:9-19. [DOI] [PubMed] [Google Scholar]
- 25.Lecuyer, E., and T. Hoang. 2004. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp. Hematol. 32:11-24. [DOI] [PubMed] [Google Scholar]
- 26.Lelievre, E., V. Mattot, P. Huber, B. Vandenbunder, and F. Soncin. 2000. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene 19:2438-2446. [DOI] [PubMed] [Google Scholar]
- 27.McCormack, M. P., M. A. Hall, S. M. Schoenwaelder, Q. Zhao, S. Ellis, J. A. Prentice, A. J. Clarke, N. J. Slater, J. M. Salmon, S. P. Jackson, S. M. Jane, and D. J. Curtis. 2006. A critical role for the transcription factor Scl in platelet production during stress thrombopoiesis. Blood 108:2248-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikkola, H. K., J. Klintman, H. Yang, H. Hock, T. M. Schlaeger, Y. Fujiwara, and S. H. Orkin. 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421:547-551. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, P., L. Ruco, and E. Dejana. 1998. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J. Cell Biol. 140:1475-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, B. S., P. Argani, B. P. Cook, H. Liangfeng, S. D. Chartrand, M. Zhang, S. Saha, A. Bardelli, Y. Jiang, T. B. St. Martin, M. Nacht, B. A. Teicher, K. W. Klinger, S. Sukumar, and S. L. Madden. 2004. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 64:7857-7866. [DOI] [PubMed] [Google Scholar]
- 31.Patterson, L. J., M. Gering, and R. Patient. 2005. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood 105:3502-3511. [DOI] [PubMed] [Google Scholar]
- 32.Porcher, C., E. C. Liao, Y. Fujiwara, L. I. Zon, and S. H. Orkin. 1999. Specification of hematopoietic and vascular development by the bHLH transcription factor SCL without direct DNA binding. Development 126:4603-4615. [DOI] [PubMed] [Google Scholar]
- 33.Porcher, C., W. Swat, K. Rockwell, Y. Fujiwara, F. W. Alt, and S. H. Orkin. 1996. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86:47-57. [DOI] [PubMed] [Google Scholar]
- 34.Prandini, M. H., I. Dreher, S. Bouillot, S. Benkerri, T. Moll, and P. Huber. 2005. The human VE-cadherin promoter is subjected to organ-specific regulation and is activated in tumour angiogenesis. Oncogene 24:2992-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulford, K., N. Lecointe, K. Leroy-Viard, M. Jones, D. Mathieu-Mahul, and D. Y. Mason. 1995. Expression of TAL-1 proteins in human tissues. Blood 85:675-684. [PubMed] [Google Scholar]
- 36.Ravet, E., D. Reynaud, M. Titeux, B. Izac, S. Fichelson, P. H. Romeo, A. Dubart-Kupperschmitt, and F. Pflumio. 2004. Characterization of DNA-binding-dependent and -independent functions of SCL/TAL1 during human erythropoiesis. Blood 103:3326-3335. [DOI] [PubMed] [Google Scholar]
- 37.Robb, L., N. J. Elwood, A. G. Elefanty, F. Kontgen, R. Li, L. D. Barnett, and C. G. Begley. 1996. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15:4123-4129. [PMC free article] [PubMed] [Google Scholar]
- 38.Robb, L., I. Lyons, R. Li, L. Hartley, F. Kontgen, R. P. Harvey, D. Metcalf, and C. G. Begley. 1995. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl. Acad. Sci. USA 92:7075-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlaeger, T. M., A. Schuh, S. Flitter, A. Fisher, H. Mikkola, S. H. Orkin, P. Vyas, and C. Porcher. 2004. Decoding hematopoietic specificity in the helix-loop-helix domain of the transcription factor SCL/Tal-1. Mol. Cell. Biol. 24:7491-7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuh, A. H., A. J. Tipping, A. J. Clark, I. Hamlett, B. Guyot, F. J. Iborra, P. Rodriguez, J. Strouboulis, T. Enver, P. Vyas, and C. Porcher. 2005. ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol. Cell. Biol. 25:10235-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivdasani, R. A., E. L. Mayer, and S. H. Orkin. 1995. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373:432-434. [DOI] [PubMed] [Google Scholar]
- 42.Timmerman, L. A., J. Grego-Bessa, A. Raya, E. Bertran, J. M. Perez-Pomares, J. Diez, S. Aranda, S. Palomo, F. McCormick, J. C. Izpisua-Belmonte, and J. L. de la Pompa. 2004. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 18:99-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visvader, J. E., Y. Fujiwara, and S. H. Orkin. 1998. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 12:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitelli, L., G. Condorelli, V. Lulli, T. Hoang, L. Luchetti, C. M. Croce, and C. Peschle. 2000. A pentamer transcriptional complex including tal-1 and retinoblastoma protein downmodulates c-kit expression in normal erythroblasts. Mol. Cell. Biol. 20:5330-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadman, I. A., H. Osada, G. G. Grutz, A. D. Agulnick, H. Westphal, A. Forster, and T. H. Rabbitts. 1997. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 16:3145-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, Z., S. Huang, L. S. Chang, A. D. Agulnick, and S. J. Brandt. 2003. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol. Cell. Biol. 23:7585-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada, Y., R. Pannell, A. Forster, and T. H. Rabbitts. 2000. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 97:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, S., J. Graham, J. W. Kahn, E. A. Schwartz, and M. E. Gerritsen. 1999. Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am. J. Pathol. 155:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.