Abstract
Ras activation is crucial for lymphocyte development and effector function. Both T and B lymphocytes contain two types of Ras activators: ubiquitously expressed SOS and specifically expressed Ras guanyl nucleotide-releasing protein (RasGRP). The need for two activators is enigmatic since both are activated following antigen receptor stimulation. In addition, RasGRP1 appears to be dominant over SOS in an unknown manner. The crystal structure of SOS provides a clue: an unusual allosteric Ras-GTP binding pocket. Here, we demonstrate that RasGRP orchestrates Ras signaling in two ways: (i) by activating Ras directly and (ii) by facilitating priming of SOS with RasGTP that binds the allosteric pocket. Priming enhances SOS' in vivo activity and creates a positive RasGTP-SOS feedback loop that functions as a rheostat for Ras activity. Without RasGRP1, initiation of this loop is impaired because SOS' catalyst is its own product (RasGTP)—hence the dominance of RasGRP1. Introduction of an active Ras-like molecule (RasV12C40) in T- and B-cell lines can substitute for RasGRP function and enhance SOS' activity via its allosteric pocket. The unusual RasGRP-SOS interplay results in sensitive and robust Ras activation that cannot be achieved with either activator alone. We hypothesize that this mechanism enables lymphocytes to maximally respond to physiologically low levels of stimulation.
Ras proteins are membrane-anchored GTPases cycling between active GTP-bound and inactive GDP-bound states. Stimulation of the T-cell or B-cell antigen receptors (TCR or BCR) results in very robust Ras activation, much higher than that observed in other cell types (reviewed in reference 18). Optimal activation of Ras is essential for thymocyte and B-lymphocyte development and remains crucial in mature lymphocytes for effector functions: e.g., proliferation and cytokine production (18). Activated H-Ras, N-Ras, and K-Ras function as signaling branch points that couple to various effector molecules. The best-studied effectors are phosphoinositide 3′ kinase (PI3K), Ral guanine nucleotide dissociation stimulator (RalGDS), and RAF (8, 20, 35). In mature T cells, activation of Ras typically leads to recruitment and activation of RAF that is capable of inducing (via MEK and extracellular signal-regulated kinase [ERK] kinases) many cellular responses critical for lymphocyte function (12, 18). On the cell surface, the CD69 activation marker is induced following triggering of this pathway (18).
The cycle of Ras activation is under the control of guanine nucleotide exchange factors (GEFs) and Ras GTPase-activating proteins (RasGAPs). RasGEFs bind to guanine nucleotide-free Ras molecules during a process that physically dissociates GDP from Ras. Empty Ras subsequently binds to GTP, available at excess levels in the cell (8, 20). RasGTPases themselves demonstrate very low intrinsic GTP hydrolysis activity, but this activity is greatly enhanced by RasGAPs (28). Tumor formation as a consequence of loss of the RasGAP neurofibromin illustrates the importance of Ras inactivation (28). Although the mechanisms of signal-mediated RasGAP activation are an active area of research, some RasGAPs are clearly at an intersection of signaling pathways: e.g., the RasGAPs CAPRI and RASAL regulate Ras activity in a calcium-dependent fashion (23). As such, RasGAPs control the conversion of RasGTP to RasGDP.
Son of Sevenless 1 and 2 (SOS1 and -2, respectively) are ubiquitously expressed mammalian RasGEFs that are recruited to the membrane by the adapter Grb2 following growth factor or antigen receptor stimulation which can lead to activation of membrane-anchored Ras (6, 17, 24). The N-terminal SH3 domain of Grb2 plays a crucial role in this process by binding to the C-terminal proline-rich region of SOS (6, 17, 24), whereas the Grb2 SH2 domain properly localizes the complex to the membrane by binding to phosphorylated tyrosines on receptors or membrane-anchored adapters. Recently, Margarit et al. reported the crystal structure of the catalytic region of SOS complexed with Ras (26). Surprisingly, three proteins were identified in a ternary Ras-SOS-RasGTP complex. In addition to a classical GEF pocket that binds guanine nucleotide free-Ras, SOS was found to have an allosteric pocket, distal to the GEF pocket, specific for GTP-bound Ras (26). Loading this allosteric pocket with an active Ras molecule enhances the GEF activity of SOS in vitro (26), suggesting a possible positive feedback loop: SOS generates RasGTP, RasGTP enhances SOS GEF activity, etc. This model raises the question as to how the initial priming RasGTP in the allosteric pocket is generated.
A second family of RasGEFs, Ras guanyl nucleotide-releasing protein (RasGRP) has gained much attention. In addition to the brain, RasGRP1 and -3 are expressed predominantly in T and B lymphocytes: there are high levels of RasGRP1 and low levels of RasGRP3 in T lymphocytes, the reverse from most B lymphocytes (1, 15, 16, 32, 36-38). Thus, lymphocytes have potentially redundant means, via SOS or RasGRP, of activating Ras. In order to activate Ras, RasGRP1 and -3 need to be membrane recruited via diacylglycerol (DAG) and also to be phosphorylated by protein kinase C (PKC) family members that are likewise recruited to the membrane by DAG (1, 32, 38). We have recently reported that stimulation of T lymphocytes with phorbol esters, DAG mimics, or via TCR engagement leads to phosphorylation of RasGRP1 and activation of Ras-ERK (32). Phosphorylation of both RasGRP1 as well as ERK kinases was decreased by inhibitors, such as rottlerin, of DAG-responsive novel PKC family kinases (32). Although TCR engagement also leads to membrane recruitment of SOS, these data suggested that the DAG-PKC-RasGRP1-Ras pathway in T lymphocytes may play a more important in role in TCR-triggered Ras-ERK activation than SOS (32). Similar findings were reported for B lymphocytes (1, 38). RasGRP1-deficient thymocytes are developmentally arrested when Ras-ERK-dependent positive selection normally occurs (15), suggesting a unique role for RasGRP1 in T-lymphocyte Ras activation that cannot be compensated for by SOS proteins.
Intrigued by the apparent dominant RasGRP function, we set out to address why lymphocytes require two distinct types of GEFs that are activated via independent biochemical mechanisms, yet are regulated by the same antigen receptor. Here we demonstrate that both GEFs contribute to sensitive and robust Ras activation in stimulated T and B lymphocytes via an unusual RasGRP-RasGTP-SOS interplay that critically relies on intact RasGRP function.
MATERIALS AND METHODS
Cell lines, stimulations, and inhibitors.
Jurkat leukemic T cells and derived cell lines were generated and cultured as described before (32). Raji B cells were grown in RPMI supplemented with 10% fetal calf serum, glutamine, and penicillin-streptomycin (Pen/Strep); DT40 cells and derivative mutants were grown as described previously (30). Stimulations were carried out in phosphate-buffered saline at 37°C with phorbol myristate acetate (PMA) at 25 ng/ml, 1:1,000 C305 supernatant recognizing TCRβ, mouse anti-human CD25 antibody (ICN Biochemicals) at 1 μg/ml with goat anti-mouse antibody (Jackson Laboratories) at 10 μg/ml, and 1:1,000 M4 antibody recognizing the BCR on DT40 cells. Cells were preloaded for 30 min with the PKC inhibitors rottlerin at 20 μM (Calbiochem), Go6976 at 5 μM (Calbiochem), GF109203X at 5 μM (Sigma), and Ro318220 at 10 μM (Alexis) or dimethyl sulfoxide (DMSO) as a control before stimulations.
Plasmids.
RasGRP1 and green fluorescent protein (GFP) expression constructs and RasGRP1 RNA interference (RNAi) plasmids were described before (32). SOS1 and SOS1-F expression constructs were generated by shuttling SOS1 or SOS1-F from Rous sarcoma virus expression vectors with HindIII blunted/BamHI partial fragment introduced into pBS EcoRV/BamHI and pBS ClaI-blunted/NotI introduced into pEF6MycHisA EcoRV/NotI.
SOS1-cat constructs were generated by Pfx PCR introducing BamHI and NotI sites and a start codon into pEF6 MycHis A BamHI/NotI using the following primers: 5′-GAAGGATCCATGGAGCAGATGAGGCTGCCTAG-3′ and 5′-TGGCGGCCGCCTCATGGTACCTGGTCTTGGGTTTG-3′; for the F929A mutation, 5′-CTATTAATCCACCATGTGTGCCTGCATTTGGAATTTATCTC-3′ and 5′-GAGATAAATTCCAAATGCAGGCACACATGGTGGATTAATAG-3′; and for the W729E mutation, 5′-GAGGTAAAGCAATGAAAAAAGAGGTTGAATCCATCACT-3′ and 5′-AGTGATGGATTCAACCTCTTTTTTCATTTGGAATTTATCTC-3′. 5′-SOS1 was generated by Pfx PCR using SOS1-pEF6 as a template and the primers 5′-GAGGATCCTAGAATGCAGGCGCGCGCTGCCTAC-3′ and 5′-TGGCGGCCGCCTCATGGTACCTGGTCTTGGGTTCG-3′, introducing a BamHI site followed by the start codon and a NotI site destroying the stop codon. This PCR product was cloned into pEF6 MycHis A via BamHI/NotI. 5′-SOS1-F was generated by ligating a BamHI/EcoR1 partial 5′-SOS1 fragment from 5′-SOS1-Myc-pEF6A into BamHI/EcoRI-digested SOS1-cat-F-pEF6B. SOS1 RNAi constructs were generated using BglII/HindIII-digested pTER plasmid and the following oligonucleotides: for SOS-RNAi2607, 5′-GATCCCTGGTGTCCTTGAGGTTGTCTTCAAGAGAGACAACCTCAAGGACACCATTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAATGGTGTCCTTGAGGTTGTCTCTCTTGAAGACAACCTCAAGGACACCAGG-3′. RasV12 expression constructs were generated by shuttling RasV12 and derived mutants from pCDNA3.1 via BamHI/XbaI into pEF6MycHis A.
Western blot analysis.
Expression levels of various proteins were determined and quantitated by Western blot analysis of 1% NP-40 lysates using the appropriate antibodies as described before (32). In short, 1 × 106 cell equivalents was analyzed per sample with the following antibodies: RasGRP1 (A176), phospho-MEK1/2 (Ser217/221), phospho-p44/42 mitogen-activated protein (MAP) kinase (Thr204/Tyr204), MEK1/2 Myc-tag (9B11) (Cell Signaling), Erk-1 (C-16) and Erk-2 (C14) (Santa Cruz Biotechnology), α-tubulin (Sigma), and SOS1 and Ras (Upstate Biotechnologies). Proteins were visualized using Western Lightning chemiluminescence reagent plus (Perkin Elmer) and a Kodak Image Station 440CF and Kodak ID image analysis software 3.5 to quantify expression levels.
RNAi and siRNA.
Cells were transfected with the following pTER RNAi constructs (30 μg) or small interfering RNA (siRNA) duplexes (5 μg, high-performance purity, fully annealed from QIAGEN) and processed 4 days or 40 h later, respectively: siRNA duplexes, siRNA human RasGRP1 (GAUUGCUGCGAGUUUUCCAdTdT), siRNA human SOS (UGGUGUCCUUGAGGUUGUCdTdT), siRNA mouse Rasgrp1 (GAUCGCUGCAAGCUUUCCAdTdT; boldface indicates differences from the human sequence), and siRNA mouse Sos1 (UGGUGUCCUGGAAGUUGUCdTdT). Mouse target sequences were used as off-target controls in the human cells or cell lines.
FACS analysis.
Fluorescence-activated cell sorting (FACS) assays were carried out as described before (32) using allophycocyanin-conjugated CD69 and phycoerythrin (PE)-conjugated CD3 and CD4 antibodies (all from BD Biosciences).
Transfections/nucleofections.
Jurkat and derived cell lines were transfected as described before (32). In short, 20 × 106 cells in 0.3 ml of a mixture of RPMI, 10% fetal calf serum (FCS), and glutamine, without Pen/Strep, were transfected by electroporation using a Bio-Rad electroporator (Bio-Rad) set at 250 mV, 960 μF. Raji B cells were transfected in 0.3 ml of the mixture of RPMI, 15% FCS, and glutamine, without Pen/Strep, with a BTX Square Wave electroporator (BTX, Genetronics) set at 190 V for 22 ms with 2 pulses. DT40 B cells were transfected like Raji B cells, with the settings of 300 mV, 10 ms, and 1 pulse. All transfected cells were cultured for the first 16 h without antibiotics. For nucleofections, CD4 T cells were purified from peripheral blood from healthy donors using RosetteSep (StemCell Technologies, Inc.) following the manufacturer's instructions. CD4 T cells (8 × 106) were nucleofected with siRNA duplexes (5 μg; QIAGEN) using protocol U-14 and an Amaxa Nucleofector following Amaxa's instructions and resuspended in the mixture of 4 ml RPMI, 10% FCS, and glutamine. Cells were analyzed 40 h after nucleofection.
Ras activation assays.
Activation of Ras was analyzed by a RasGTP pull-down assay (15 μl of RAF-1 RBD agarose and 10 × 106 cells) according to the manufacturer's instructions (Upstate) as described before (32). In short, lysates were tumbled in the cold room with the agarose for 30 min and washed three times in ice-cold NP-40 lysis buffer, and the agarose was resuspended in sample buffer, boiled, and loaded.
RESULTS
Impairment of the PKC-RasGRP1 pathway is not compensated for by SOS function and results in decreased receptor-stimulated Ras-ERK activation.
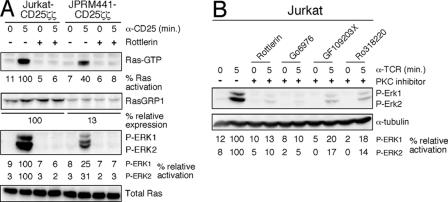
We used the RasGRP1-deficient Jurkat mutant (JPRM441) that we previously characterized and described in detail (32) together with siRNA or various PKC inhibitors to investigate the role of RasGRP1 and SOS1 in receptor-stimulated Ras-ERK activation. Although JPRM441 cells express no TCR, cross-linking of stably expressed CD25ζζ can be used as a surrogate TCR signal, as described previously (32). We observed that CD25ζζ stimulation of RasGRP1-deficient cells led to severely reduced levels of Ras and ERK activation in these cells compared to wild-type Jurkat T cells (Fig. 1A). Rottlerin, a PKC inhibitor, prevented CD25ζζ-triggered Ras-ERK activation in wild-type cells and in RasGRP1-deficient cells (Fig. 1A) as well as TCR-induced activation (data not shown). This inhibitory effect of rottlerin occurred, presumably, by inhibiting PKC-mediated activation of (residual) RasGRP1 and of the low level of RasGRP3 present in both Jurkat and JPRM441 cells (32). Similar inhibition was obtained with three additional well-characterized PKC inhibitors, Go6976, GF109203X, and Ro318220 (Fig. 1B) (14, 27). Such similar results with four PKC inhibitors (with different chemical moieties) strongly suggests that the blockade in ERK activation is caused by blocking PKC kinases and is not a result of off-target (non-PKC) inhibition, although we cannot formally rule out this possibility. We previously reported that the Grb2-SOS complex is properly recruited to phospho-LAT following cross-linking of CD25ζζ in RasGRP1-deficient cells (32). These results suggest that the RasGRP1 pathway plays a more dominant role in activating the Ras-ERK pathway than SOS and challenge the notion that SOS molecules play an important role in T-lymphocyte Ras-ERK activation.
FIG. 1.
PKC-RasGRP1 signaling dominates induced Ras-ERK activation. (A) Jurkat and JPRM441 T-cell lines were stimulated by cross-linking CD25ζζ with anti-CD25 (α-CD25) monoclonal antibody for the indicated time intervals in the presence of rottlerin or the DMSO control (−). The relative percentages of Ras and ERK activation and RasGRP1 expression were determined by normalizing for total Ras expression. Jurkat T cells express K- and N-Ras, both migrating as doublets and recognized by the anti-Ras antibody. All Ras-GTP pull-down assays were run on a separate minigel, typically using 10-fold more input material compared to the material presented in the Western blot analysis of the NP-40 whole-cell lysates. The result presented in panel A is a representative example of three independent experiments. Almost all panels presented in this study are representative examples of at least three independent experiments: exceptions are experiments shown in Fig. 6C and 7B that yielded very similar results in two independent experiments (one of which is shown). (B) Wild-type Jurkat T cells were stimulated through the TCR in the presence of rottlerin, Go6976, GF109203X, Ro318220, or DMSO as a control (−). Phosphorylation of ERK kinases was measured as in panel A, normalizing for α-tubulin expression. α-TCR, anti-TCR.
Reduction of RasGRP1 expression affects activation of the Ras-ERK pathway more strongly than does reduction of SOS1 expression.
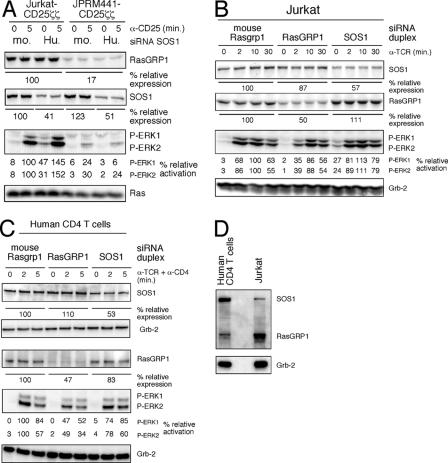
To test the importance of SOS, we identified an RNAi target sequence to effectively knock down SOS1 expression (data not shown). Partial reduction of SOS1 expression in Jurkat-CD25ζζ cells did not decrease the CD25ζζ-induced ERK activation (Fig. 2A). Rather, we consistently detected a modest increase in both the resting and stimulated samples. In RasGRP1-deficient JPRM441-CD25ζζ cells, SOS1 knockdown did not result in an increased response but instead resulted in a slight reduction of the already very reduced response (Fig. 2A). Similar results were obtained when wild-type Jurkat T cells were stimulated via the TCR: i.e., the knockdown of RasGRP1 (by 50%) resulted in a reduction of ERK phosphorylation, whereas knockdown of SOS1 (by 43%) did not (Fig. 2B). The lack of an effect of SOS1 knockdown on Ras signaling may in part be a reflection of the residual SOS1 expression in the population of cells tested. Nevertheless, these studies demonstrate that SOS function is at least not limiting for induced Ras activation and suggest that SOS1 in these cell lines has some suppressive function.
FIG. 2.
Knockdown of RasGRP1 affects induced Ras-ERK activation more profoundly than knockdown of SOS1. (A) siRNA duplexes for mouse (mo. [control]) or human (Hu.) SOS1 were introduced via transient transfection into wild-type Jurkat or mutant JPRM441 cells. The resultant populations were stimulated 40 h after transfection by cross-linking the stably expressed CD25ζζ and analyzed as in Fig. 1. The relative percentages of SOS1 and RasGRP1 expression as well as ERK phosphorylation were determined by normalizing for Ras expression. Both Jurkat and JPRM441 cells express similar levels of RasGRP3 and SOS2 (data not shown). α-CD25, anti-CD25. (B) Wild-type Jurkat cells were transfected with the indicated siRNA duplexes. Forty hours later, cells were stimulated by cross-linking the TCR and analyzed by Western blot analysis as in panel A, normalizing for Grb2 expression. α-TCR, anti-TCR. (C) Mouse Rasgrp1 (control), RasGRP1, and SOS1 siRNA duplexes were introduced into purified human CD4+ T cells using an Amaxa electroporator. Forty hours later, cells were stimulated by cross-linking the TCR and CD4. Expression of the indicated proteins, as well as phosphorylation of ERK kinases, was determined by Western blot analysis (the same samples were run on two separate gels, analyzing SOS1 and Grb2 on one gel and RasGRP1, P-ERK, and Grb2 on the other). The percentage of ERK activation and RasGRP1 and SOS1 expression levels were determined by normalizing for Grb2 expression. α-CD4, anti-CD4. (D) Expression levels of SOS1 and RasGRP1 were compared between human CD4 T cells and the Jurkat T-cell clone by combining the primary antibodies into one mixture. Grb2 normalizes for the amount of protein loaded.
We next confirmed these findings in CD4 T cells isolated from human peripheral blood nucleofected with siRNA duplexes targeting human RasGRP1 or SOS1 and, as a control, mouse Rasgrp1 (Fig. 2C). Similar to our findings in Jurkat T cells, the knockdown of RasGRP1 resulted in a more severe reduction of ERK phosphorylation induced by TCR and CD4 cross-linking, compared to SOS1 knockdown. Different from Jurkat T cells, reduction of SOS1 in CD4 T cells by itself consistently led to a mild reduction of induced ERK phosphorylation (Fig. 2C). The difference may lie in the expression levels of RasGRP1 and SOS1 in CD4 T cells versus Jurkat T cells; CD4 T cells express a higher level of SOS1 and a lower level of RasGRP1 and as a consequence may depend more on SOS1 (Fig. 2D).
Triggering of the Ras-ERK-CD69 pathway by membrane-targeted SOS1 relies on intact DAG-RasGRP1 function.
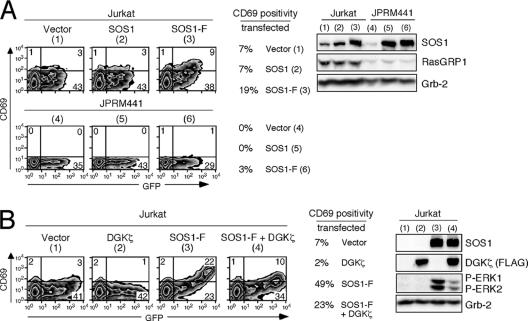
SOS1 is clearly important in many receptor tyrosine kinase pathways and has an essential and unique function during embryogenesis, since SOS1-deficient embryos die of early developmental defects (31). What then is SOS1's role in lymphocyte Ras activation? Our findings that SOS1 is membrane-recruited, yet is unable to compensate for a defective PKC-RasGRP-Ras-ERK pathway, would at first glance appear to be in apparent disagreement with published studies: targeting of SOS to the membrane is sufficient to activate the Ras pathway, independent of a stimulus, in cell lines (2) and in lymphocytes (19). We confirmed that membrane-targeted full-length SOS1 (SOS1-F) also induced moderate levels of CD69 expression on resting Jurkat cells, a reflection of cumulative Ras signal input (19% of CD69-positive cells normalized to GFP-expressing transfected cells; Fig. 3A). Non-membrane-targeted SOS1, included as a control, did not induce CD69 and demonstrated the same basal level of CD69 as vector-transfected Jurkat cells (7% of GFP-expressing cells; Fig. 3A). Similarly, SOS1-F, but not SOS1, overexpression induced CD69 expression in the Raji B cell line that expresses RasGRP3 and low levels of RasGRP1 (data not shown). Interestingly, the induction of CD69 by SOS1-F also relied on RasGRP1 since the same level of SOS1-F was not nearly as active in RasGRP1-deficient JPRM441 cells (19% versus 3%). Thus, SOS has the potential to activate the Ras pathway but appears to require the presence of RasGRP1 to do so effectively, even if SOS is targeted to the membrane.
FIG. 3.
Optimal activity of membrane-targeted SOS1 depends on RasGRP1 function and DAG levels. (A) Jurkat or JPRM441 cells were transiently transfected with 30 μg of the indicated expression plasmids together with 15 μg GFP expression construct. Sixteen hours later, cells were analyzed by FACS for GFP and CD69 expression and NP-40 lysates were prepared. Numbers inside the plots indicate percentages of cell populations in each quadrant. Note the diagonal pattern with SOS1-F in Jurkat cells. The percentage of CD69-positive cells in the transfected population was determined and is depicted (e.g., 9/(9 + 38) = 19%). Protein expression levels were determined as described before. (B) Jurkat cells were transiently transfected with 30 μg of SOS1-F plasmid or vector control, together with 30 μg FLAG-tagged DGKζ expression or vector control plasmids. All transfections included 15 μg GFP expression construct. Sixteen hours later, cells were analyzed as in panel A. SOS1-F expression was higher than in panel A (due to carrier effect of the other introduced plasmid) and induced a higher level of CD69.
RasGRP1 recruitment relies on the second messenger DAG. DAG levels in the membrane are regulated through production by phospholipase C-γ (PLC-γ) and turnover by three classes of enzymes (25). One mechanism of DAG metabolism is phosphorylation by diacyl glycerol kinases (DGKs), producing phosphatidic acid (25). Overexpression of DGKζ has been reported to decrease RasGRP-induced Ras activation (34). Conversely, DGKζ-deficient T cells demonstrate a reduction of TCR-induced phosphatidic acid production and enhanced TCR-induced ERK activation (39). To test whether the activity of membrane-targeted SOS1 relies on intact signals downstream of DAG, we coexpressed SOS1-F with DGKζ. DGKζ expression decreased the SOS1-F-induced CD69 expression and ERK phosphorylation in Jurkat T cells (from 49% to 23%; Fig. 3B). Thus, the activity of SOS1-F relies on intact RasGRP1 function and on sufficient levels of DAG, RasGRP1's upstream second messenger.
As we previously reported (32), the basal level of CD69 expression in JPRM441 cells was reduced compared to the basal level on wild-type Jurkat T cells (Fig. 3A; vector-transfected samples). Similar to the RasGRP1 deficiency, introduction of DGKζ expression in wild-type Jurkat cells reduced the basal level of CD69 compared to control vector introduction at higher levels of DGKζ expression, using GFP levels as a surrogate to assess expression levels (Fig. 3B, compare left two panels).
In B-lineage cells RasGRP and SOS function very similarly to activate the Ras-RAF-MEK-ERK pathway following antigen receptor stimulation.
A caveat to the experiments presented in Fig. 2A to C is the incomplete reduction of expression by RNAi-driven knockdown (in a population of transfected cells). To further explore the relative roles of RasGRP and SOS and to avoid partial reduction, we took advantage of the SOS- and RasGRP-deficient mutants of the chicken DT40 B-cell line, generated by homologous recombination (30). These mutants extended our observations to B-lineage cells and, importantly, have the advantage of possessing precisely targeted genetic deletions resulting in absolute nulls in terms of gene expression. Using SOS1 and SOS2 or RasGRP1 and RasGRP3 doubly deficient lines that express normal levels of surface BCR (30), we analyzed BCR-induced phosphorylation of ERK and the upstream MEK kinases.
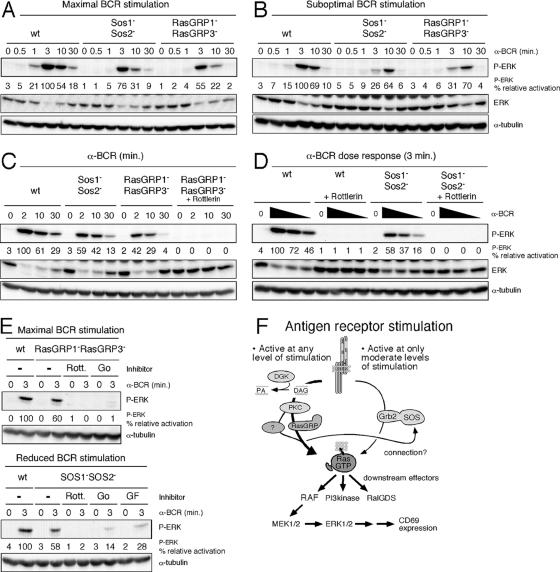
SOS1 and -2 dual deficiency in these B cells resulted in only moderate reduction of BCR-induced activation of ERK kinases (Fig. 4A). A very similar pattern was observed for the activation of upstream MEK kinases (data not shown). As reported previously (30), BCR stimulation of RasGRP1 and RasGRP3 doubly deficient B cells resulted in a greater reduction (but not absence) of ERK activation. These results are strikingly similar to our results with RasGRP1-deficient Jurkat T cells.
FIG. 4.
Suboptimal BCR stimulation reveals SOS function. (A and B) Wild-type (wt) or DT40 chicken B-cell lines with the indicated targeted gene deletions were stimulated for the indicated intervals with BCR-stimulating M4 monoclonal antibody at either a maximal dose (A [1:1,000]) or a suboptimal dose (B [1:20,000]). NP-40 lysates were analyzed by Western blotting. The same portion of the blot was blotted for P-ERK and stripped, blotted for P-MEK1/2 and stripped, blotted for MEK1/2 and stripped, and blotted for ERK. Blotting for ERK after P-ERK typically gives inverted patterns compared to P-ERK. Note that DT40 B cells express only one ERK protein (chicken ERK2). A separate portion of the same blot was blotted for α-tubulin. The relative percentage of ERK phosphorylation was determined by normalizing for α-tubulin. α-BCR, anti-BCR. (C) The indicated DT40 lines were pretreated with DMSO (control) or rottlerin, stimulated with a 1:1,000 dilution of M4 monoclonal antibody as indicated, and analyzed as in panels A and B. (D) Wild-type or Sos1− Sos2− DT40 chicken B cells were pretreated with DMSO (control) or rottlerin and stimulated with control phosphate-buffered saline (0) or decreasing dilutions of BCR-stimulating M4 monoclonal antibody (1:1,000, 1:5,000, and 1:20,000) and analyzed as before. Of note, rottlerin did not lead to general cell toxicity as BCR (or TCR- or CD25ζζ-)-induced phosphorylation of MAP kinases other than ERK was less affected (data not shown). (E) The indicated DT40 lines were pretreated with DMSO (control), rottlerin, Go6976, or GF109203X and maximally stimulated with a 1:100 dilution of M4 monoclonal antibody (different M4 batch from those used in panels A to D) or with a reduced amount of 1:500 as indicated, and analyzed as before. (F) Model of Ras-RAF-MEK-ERK activation following antigen receptor engagement on lymphocytes. The pathway downstream of DAG dominates lymphocyte Ras activation. Our collective data suggest an unknown dependence of SOS function on the DAG-PKC-RasGRP pathway.
However, under normal physiological circumstances, only limited numbers of receptors are engaged on lymphocytes. Therefore, we examined the effects of suboptimal antigen receptor engagement, using 20-fold-smaller amounts of the BCR-cross-linking antibody to mimic normal physiology. An overall reduction and a delay of the ERK phosphorylation were seen in either SOS- or RasGRP-deficient lines compared to wild-type DT40 B cells (Fig. 4B; note that the maximal response in wild-type cells occurs at 3 min, in contrast to 10 min in both mutant lines). This suggests that functional contributions of SOS, as well as RasGRP proteins, may be most relevant at low levels of receptor stimulation, consistent with more physiologic levels of stimulation.
Despite the absence of RasGRP1 and -3, the DAG-Ras-ERK (SOS independent) pathway is not completely impaired in RasGRP1 and RasGRP3 doubly deficient DT40 B cells, since PMA still triggers some ERK activation in these mutant cells (30). Similarly, PMA stimulation of Rasgrp1 and Rasgrp3 doubly deficient mouse B cells leads to some induction of ERK activation (11). Our observation that residual Ras-ERK signals exist in these BCR-stimulated mutant DT40 B cells may be a reflection of residual BCR-induced activity through the pathway downstream of DAG. To address this possibility, we used various PKC inhibitors as a tool to block PKC function downstream of the second messenger DAG. The observed residual BCR-induced ERK phosphorylation in RasGRP1 and RasGRP3 doubly deficient DT40 B cells was strongly inhibited by rottlerin, a PKC inhibitor (Fig. 4C). Thus there is a PKC-dependent pathway that is responsible for the residual Ras-ERK activation in RasGRP1 and RasGRP3 doubly deficient DT40 B cells.
The identity of this DAG- and PKC-dependent molecule that signals the Ras-ERK pathway in DAG-stimulated RasGRP1 and RasGRP3 doubly deficient DT40 B cells is unclear. We hypothesized that these cells may express chicken RasGRP4, which is known to be efficiently recruited by DAG, but we were unable to amplify this transcript from DT40 cells or identify the transcript in the chicken expression database (data not shown). Another possibility is that the residual activity is not coming from a RasGRP family member, but comes from other DAG-responsive signaling molecules (reviewed in reference 5). For instance, DAG in RasGRP1 and RasGRP3 doubly deficient DT40 cells may signal to PKC and protein kinase D. PKC-phosphorylated protein kinase D has been reported to phosphorylate the Ras interactor RIN1, leading to dissociation of RIN1 from Ras and allowing Ras to signal to RAF-MEK-ERK (5). Finally, it remains possible that lymphocytes express a third type of RasGEF. Future work will have to address these issues in both T and B lymphocytes since there may also be differences between the two cell types.
Since we did not know the identity of the molecule with possible similar function to RasGRP1 and -3, we investigated the synergy between the DAG-PKC-Ras-ERK and Grb2-SOS-Ras-ERK pathways using PKC inhibitors. Rottlerin not only blocked the reduced levels of ERK activation in SOS1 and SOS2 doubly deficient B cells, but it also blocked ERK activation in wild-type cells (Fig. 4D). Again, we verified these findings with two different PKC inhibitors, Go6976 and GF109203X, that also potently reduced BCR-induced ERK activation in RasGRP1 and RasGRP3 and SOS1 and SOS2 doubly deficient DT40 B cells, stimulated at either maximal or reduced levels, respectively (Fig. 4E). Thus, as in our T-lineage experiments, the DAG-PKC-Ras-ERK pathway plays a very dominant role in activated B-lineage cells.
The data presented in Fig. 1 through 4 indicate that the DAG-RasGRP pathway is crucial for Ras-ERK activation following antigen receptor stimulation of T and B lymphocytes. SOS molecules appear to play only a modest role for this pathway at high levels of receptor stimulation but seem more important at lower levels of receptor stimulation, where SOS may increase sensitivity to receptor stimulation (Fig. 4F). In addition, the results revealed a possible and unexpected connection between the DAG-PKC-RasGRP and Grb2-SOS pathways. This connection appears to be downstream of PKC (e.g., at the level of RasGRP1/3 and/or an unknown protein or downstream of it; Fig. 4F) and not at the level of PKC kinases; loss of RasGRP1 or RasGRP1 and -3 was not compensated for by a hypothetical direct PKC-SOS pathway that would completely bypass RasGRP function (Fig. 1A and 3A). This notion is strengthened by the observation that loss of RasGRP1 affected the potential of membrane-targeted SOS1 in JPRM441 cells (Fig. 3A).
The activity of SOS molecules is regulated by intramolecular inhibition.
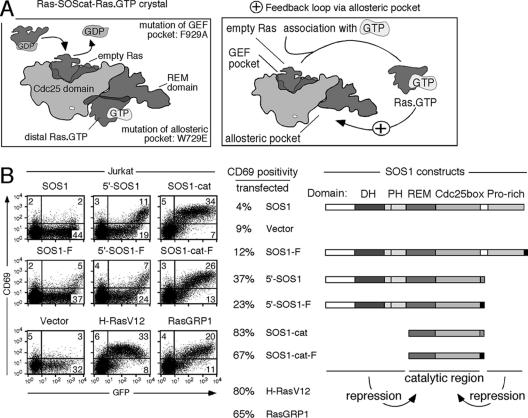
Our results thus far have demonstrated that full-length SOS (either membrane-targeted, exogenous, or endogenous molecules) has only a limited ability to trigger the Ras-ERK-CD69 pathway in the T- and B-cell lineages. SOS contains many protein domains. In addition to its centrally positioned RasGEF domain, SOS contains a flanking C-terminal proline-rich region that binds Grb2 and N-terminal Dbl homology and pleckstrin homology domains (Fig. 5B). Both flanking regions have been reported to dampen SOS activity (2, 10, 33). For instance, membrane targeting of SOS lacking the C-terminal proline-rich region resulted in more potent activation of the Ras pathway in NIH 3T3 cells (2). Also, N-terminally truncated versions of SOS exhibit stronger activity towards Ras in vitro (10, 33). Finally, the SOS catalytic domain itself may regulate its own RasGEF activity in a complex manner: the RasGEF catalytic domain of SOS contains an allosteric pocket specific for RasGTP, as previously identified in the crystal structure of SOS (26) (Fig. 5A). Mutation of this allosteric pocket (W729E) severely diminishes the GEF activity of SOS in vitro (33). Conversely, RasGTP loading of this pocket has been shown to improve SOS in vitro GEF activity, suggesting the possibility of a positive feedback loop (33) (Fig. 5A). Very recently, a similar positive feedback loop via SOS was demonstrated in the HeLa cervical carcinoma cell line (4).
FIG. 5.
Complex regulation of SOS activity. (A) Graphic representation of the published Ras-SOScat-Ras GTP crystal structure and proposed positive feedback loop based on this structure (26, 33). RasGDP binding to the GEF pocket results in displacement of the GDP group, leaving empty Ras bound to SOS. After association with GTP, RasGTP can bind to the other side of the SOS molecule, to the allosteric pocket. Loading of the allosteric pocket increases the in vitro GEF activity of a wild-type molecule but not that of a W729E mutant. (B) Twenty-five micrograms (each) of SOS1, SOS1-F, 5′-SOS1, and 5′-SOS1-F, as well as 10 μg of vector, SOS1-cat, SOS1-cat-F, H-RasV12, or RasGRP1 expression constructs (all in the same pEF6 vector backbone), was introduced together with 10 μg GFP plasmid. Sixteen hours later, populations were analyzed for GFP and CD69 expression (using anti-CD69APC antibody detectable in Fl4). The right panel gives a representation of the introduced SOS1 constructs as well as the resultant percentage of CD69-positive cells of all transfected, GFP-positive cells (determined as in Fig. 3A).
In Jurkat cells, similar to the results observed in NIH 3T3 cells (2), SOS molecules lacking the C-terminal proline-rich domain (5′-SOS1 or 5′-SOS1-F) were also more active than membrane-targeted, full-length SOS1 (Fig. 5B). In contrast to full-length SOS1, membrane targeting was not required for 5′-SOS1 to activate the Ras-ERK pathway. The minimal catalytically active domain of SOS1 (SOS1-cat; 83% activity) very potently activated the Ras-ERK-CD69 pathway (Fig. 5B), comparable to an active mutant of Ras that is frequently found in human cancer, H-RasV12 (80% activity). Again, addition of a farnesylation sequence (SOS1-cat-F) or, as a control, a mutated version (SOS1-cat-F*) that does not bind the membrane did not appear to increase the overall activity (Fig. 5B) (data not shown). These findings suggest that both the C-terminal proline-rich domain and the N-terminal Dbl-PH domains present in full-length SOS1 inhibit GEF activity in vivo (Fig. 5B). That full-length SOS, but not truncated SOS variants, requires the farnesylation sequence of H-Ras (localizing to the plasma and/or Golgi membranes) (29) indicates that full-length SOS is dependent on membrane localization, possibly to relieve an inhibitory constraint in the SOS molecule. Transfection of unmodified, full-length RasGRP1 also resulted in activation of the Ras-ERK pathway and CD69 upregulation (Fig. 5B). It thus appears that RasGRP1 (65% activity) possibly lacks intramolecular repression of its activity, in contrast to SOS1 (4% activity).
RasGTP binding to the allosteric pocket of SOS initiates a positive feedback loop that functions as an in vivo rheostat for Ras activation, regulated by RasGRP1.
The notion that (i) the catalytic domain of SOS contains GEF activity and that (ii) SOS1 can contribute to ERK activation at lower levels of stimulation yet cannot compensate for a defective PKC-RasGRP pathway was puzzling. The in vivo GEF activity of SOS is apparently regulated in a complex manner with some unknown role for DAG and RasGRP1.
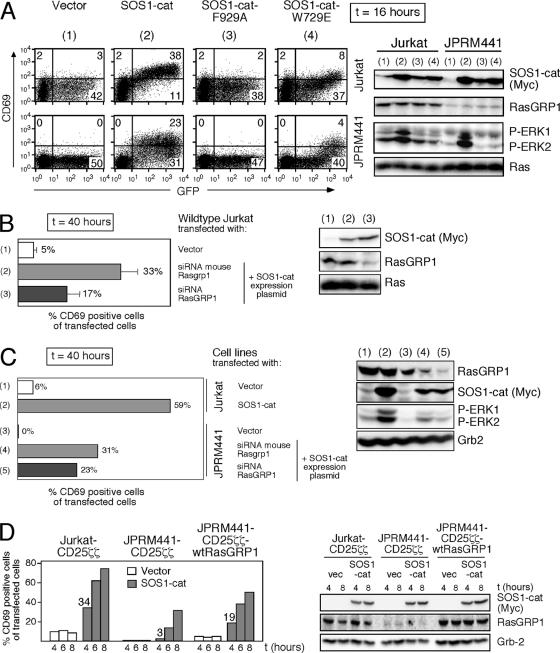
We decided to focus on the regulatory mechanisms that may lie within the catalytic domain of SOS itself and study the role of RasGRP1 (see Fig. 5A). The activity of SOS1-cat versions carrying either an inactivating point mutation in the GEF pocket (F929A) or a mutation in the allosteric RasGTP binding site (W729E) was tested in intact cells. Equivalent overexpression of SOS1-cat led to robust CD69 induction in wild-type Jurkat T cells, but to a more moderate response in RasGRP1-deficient JPRM441 cells (Fig. 6A). In contrast, the F929A point mutant was inactive in either cell line. Quite strikingly, SOS1-cat-W729E was also severely impaired in its ability to induce CD69 upregulation (Fig. 6A). In agreement with the CD69 upregulation, only SOS1-cat induced phosphorylation of ERK kinases (Fig. 6A). The same patterns of CD69 expression induced by the three SOS1-cat alleles were observed using the Raji B-cell line (data not shown). Thus, in vivo SOS function appears to rely on the intact allosteric pocket reported to bind RasGTP, suggesting that a positive feedback loop is functional in lymphocytes, similar to the recently published results with HeLa cells (4).
FIG. 6.
The isolated catalytic domain of SOS requires the allosteric pocket and RasGRP1 to efficiently trigger a Ras-ERK-CD69 pathway. (A) Ten micrograms of vector, SOS1-cat, SOS1-cat-F929A, or SOS1-cat-W729E expression constructs was transiently transfected into Jurkat or JPRM441 cells together with 10 μg of GFP plasmid. Sixteen hours later, cells were analyzed by FACS and Western blotting as described before. Numbers inside the plots indicate percentages of cell populations in each quadrant. (B) Wild-type Jurkat T cells were transiently cotransfected with 4 μg vector and 10 μg GFP plasmids or with 5 μg mouse Rasgrp1 or human RasGRP1 siRNA together with 4 μg SOS1-cat and 10 μg GFP plasmids. Transfected cells were analyzed for CD69 and GFP expression by FACS and for protein expression by Western blot analysis 40 h after transfection. The percentage of CD69-positive cells over all transfected cells was determined in three independent experiments and is plotted as mean values with error bars. Small amounts of SOS1-cat plasmid were introduced to minimize SOS1-cat expression before RasGRP1 knockdown occurs. (SOS1-cat starts to be expressed 4 h after transfection, but RasGRP1 knockdown is maximal only after 40 h.) (C) JPRM441 cells were transiently transfected with 4 μg vector and 10 μg GFP plasmids or with 5 μg mouse Rasgrp1 or human RasGRP1 siRNA together with 4 μg SOS1-cat and 10 μg GFP plasmids and analyzed as in panel B. As controls, wild-type Jurkat cells were transfected with 4 μg vector or SOS1-cat together with 5 μg vector and 10 μg GFP plasmids. Panel C is a representative example of two independent experiments. (D) Jurkat-CD25ζζ, JPRM441-CD25ζζ, or stably reconstituted JPRM441-CD25ζζ-wtRasGRP1 cells were transiently transfected with 30 μg of vector (vec) or SOS1-cat plasmids together with 15 μg of GFP expression construct and analyzed as in panel A at 4, 6, and 8 h posttransfection. The percentage of CD69-positive cells over all transfected cells was plotted for the various time points as a bar graph. Protein expression was determined as before using NP-40 lysates prepared at the indicated time points.
We confirmed the results in the JPRM441 line with the simultaneous introduction of SOS1-cat and RasGRP1 knockdown. SOS1-cat induced Ras-ERK-CD69 responses were blunted in wild-type Jurkat cells when RasGRP1 was simultaneously knocked down but not when control mouse Rasgrp1 siRNA (three mismatches with human sequence) was introduced (Fig. 6B). The potential of SOS1-cat to induce CD69 expression in RasGRP1-deficient JPRM441 cells was further dampened when their residual RasGRP1 expression was reduced via RasGRP1 siRNA (Fig. 6C, compare numbers 4 and 5). These results strongly suggested that the potency of the isolated catalytic region of SOS1 to activate the Ras-ERK-CD69 pathway is much greater in cells that express RasGRP1. Nevertheless, it is important to note that RasGRP1 expression is not an absolute requirement for SOS1-cat to induce some level of Ras activation. For instance, SOS1-cat introduced into the 293T fibroblast line, which does not express any RasGRP family members, led to moderate ERK activation (data not shown).
To examine the initiation of the positive feedback loop, early effects of SOS1-cat expression were examined. Although SOS1-cat eventually generated signaling events downstream of Ras when introduced in JPRM441-CD25ζζ cells, the initiation of the response was impaired compared to Jurkat-CD25ζζ cells, while the expression of SOS1-cat was not (Fig. 6D). In JPRM441-CD25ζζ cells stably reconstituted with RasGRP1 cDNA, initiation of the signal by SOS1-cat was partially rescued (Fig. 6D).
Basal signals downstream of DAG enhance the activity of the catalytic domain of SOS.
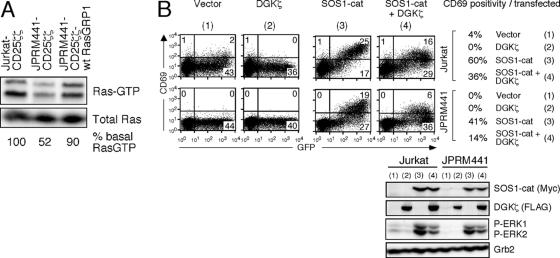
We hypothesized that RasGRP1 may facilitate initial priming of the allosteric site of SOS via the production of RasGTP. Based on published studies of RasGRP1 and -3 regulation, basal levels of DAG in the membrane are likely to allow for a low level of Ras activation in the resting state. Analysis of levels of RasGTP in resting cells demonstrated that RasGRP1-deficient cells indeed have a lower level of RasGTP in the basal state, compared to wild-type Jurkat cells, whereas the level of RasGTP in the RasGRP1-reconstituted mutant line is partially rescued (Fig. 7A). The reduction in basal RasGTP is a reflection of the RasGRP1 defect and not of an unknown mutation in the JPRM441 line since RasGRP1 siRNA in wild-type Jurkat cells also reduced the basal level of active Ras (data not shown). The smaller amount of RasGTP in the basal state in resting JPRM441 cells offers a possible mechanism for the lower sensitivity to the activating effects of SOS1-cat by these mutant cells: the allosteric pocket of the introduced SOS1-cat would be less efficiently loaded.
FIG. 7.
Basal DAG-RasGRP1 signaling is required for optimal SOS1-cat activity. (A) Analysis of basal Ras activity in the indicated unstimulated cell lines grown in 5% FCS. Relative percentages of RasGTP levels were determined by normalizing for total Ras levels by Western blot analysis. Note the K- and N-RasGTP doublets detectable on this high-percentage gel. Input for the RasGTP pulldown was 30-fold the material presented in the total Ras blot analyzing the NP-40 whole-cell lysates. (B) Jurkat and JPRM441 cells were transiently transfected with 5 μg of SOS1-cat plasmid or vector control plasmids, together with 30 μg FLAG-tagged DGKζ expression or vector control plasmids. All transfections included 15 μg GFP expression construct. Sixteen hours later, cells were analyzed as before. Panel B is a representative example of two independent experiments.
To confirm that the activity of SOS1-cat depends on the DAG-PKC-RasGRP1 pathway in the basal state, we again used introduction of DGKζ. As seen in Fig. 3B, expression of DGKζ alone decreased CD69 expression on wild-type Jurkat cells (from 4% to 0%) to a level similar to that detected on JPRM441 cells (Fig. 7B). CD69 upregulation and ERK phosphorylation induced by the catalytic domain of SOS1 (SOS1-cat) were similarly reduced by simultaneous introduction of DGKζ in both cell lines (Fig. 7B).
Taken together, these results demonstrate that SOS participates in a positive feedback loop in lymphocytes, confirming recent published results with HeLa cells (4). In addition, this feedback loop critically depends on RasGRP function and thereby creates a rheostat for in vivo Ras activation. These results also suggest that the connection between RasGRP and SOS is possibly via the product of the two GEFs, RasGTP.
An active Ras-like molecule can substitute for RasGRP1 function and enables SOS, via its allosteric pocket, to trigger the Ras pathway.
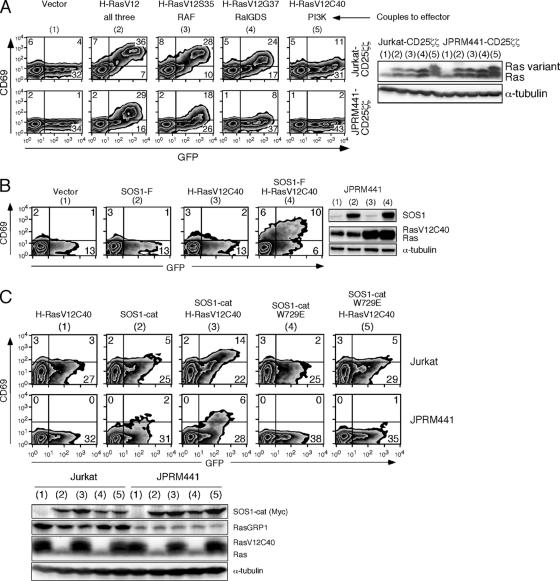
If RasGRP proteins in lymphocytes activate Ras directly but also indirectly by providing RasGTP for the allosteric pocket of SOS, then exogenous introduction of a RasGTP-like molecule that can dock in that pocket but lacks effector function should overcome SOS' dependency on RasGRP1. Altered versions of H-RasV12 have been used to dissect the Ras effector pathways. These variants are not inactive but couple to specific downstream pathways: H-RasV12S35 preferentially binds to RAF, H-RasV12G37 binds to RalGDS, and H-RasV12C40 binds to PI3K (20). The H-RasV12C40 variant resembles active Ras but inefficiently signals RAF (20). We utilized H-RasV12C40 to provide an active Ras-like molecule for the allosteric pocket of SOS in our cell lines. As reported for other cell systems (20), H-RasV12C40 was almost completely defective in activating the RAF-MEK-ERK-CD69 pathway in our T-cell lines, in sharp contrast to the activating effect of equal levels of H-RasV12, V12S35, or V12G37 (Fig. 8A). We next combined introduction of membrane-targeted SOS1 (SOS1-F) incapable of signaling in RasGRP1-deficient JPRM441 cells (Fig. 3A) with H-RasV12C40. As seen before, neither molecule alone was able to generate Ras-ERK signals, as measured by CD69 expression in the GFP-positive-transfected cells (Fig. 8B). However, introduction of this defective H-RasV12C40 molecule permitted SOS1-F to trigger the Ras-ERK-CD69 pathway in RasGRP1-deficient cells (Fig. 8B, right FACS plot). Thus, introduction of an active Ras mimetic that cannot bind to RAF (and therefore cannot activate the MEK-ERK pathway itself) enables the SOS-driven Ras-ERK-CD69 pathway.
FIG. 8.
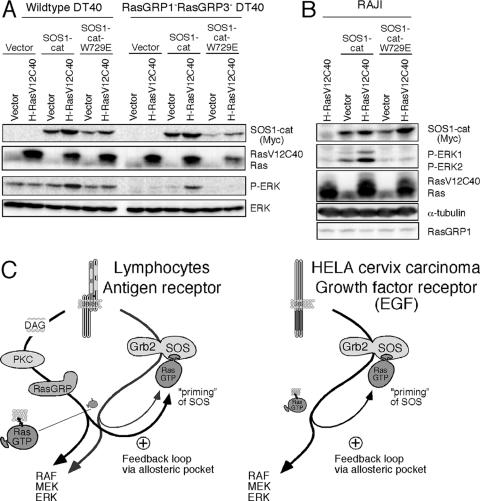
A RasGTP mimetic enhances in vivo GEF activity of SOS and can substitute for RasGRP1 deficiency. (A) The indicated H-RasV12 variants were tested in Jurkat and JPRM441 T cells. The effects of H-RasV12C40 on the Ras-ERK-CD69 pathway were tested by FACS analysis for CD69 expression 16 h after transient transfection (10 μg of RasV12 variants with 10 μg of GFP). (B) RasGRP1-deficient JPRM441 cells were transfected with the indicated combinations of H-RasV12C40 (10 μg) and/or SOS1-F (30 μg) together with GFP (10 μg) plasmids and analyzed for CD69 induction and protein expression 16 h later as described before. (C) Jurkat and JPRM441 T cells were transfected with 2 μg of SOS1-cat or SOS1-cat-W729E and/or 5 μg H-RasV12C40, together with 5 μg GFP plasmids and analyzed for induction of CD69 expression by FACS 16 h after transfection.
We also expressed the isolated catalytic domain of SOS (SOS1-cat, at lower levels than in Fig. 6A) without the repressive flanking domains together with H-RasV12C40 in either wild-type Jurkat or RasGRP1-deficient JPRM441 cells (Fig. 8C). Only when combined did introduction of these molecules effectively induce CD69 expression. Recently, an independent study demonstrated that loading of the allosteric pocket with an H-RasG59E38 variant (similar to our H-RasV12C40 variant) increased the activity of the catalytic SOS domain in a COS1 cell line (4). Importantly, these synergistic effects towards the Ras-ERK-CD69 pathway that we observed were largely lost when H-RasV12C40 was coexpressed with SOS-cat-W729E, the mutant with inactivation of the allosteric RasGTP pocket crucial for the positive feedback on SOS (Fig. 8C). Thus, the catalytic domain of SOS alone is sufficient for the observed synergy with H-RasV12C40 and the active Ras mimetic does not enhance SOS activity independent of the allosteric pocket.
A RasGTP-SOS-RasGTP feedback loop is functional in both T and B lymphocytes.
Synergy between SOS and H-RasV12C40 was also observed in vivo in B cells. Alone, either molecule induced very little ERK activation in wild-type or RasGRP1 and RasGRP3 doubly deficient DT40 B cells (Fig. 9A). However, combined, SOS1-cat and H-RasV12C40 clearly triggered ERK phosphorylation that was not observed with the allosteric pocket mutant SOS1-cat-W729E in conjunction with H-RasV12C40 (Fig. 9A). The SOS1-cat/H-RasV12C40 synergy was most prominent in RasGRP1 and RasGRP3 doubly deficient DT40 B cells that demonstrated an overall lower level of basal ERK phosphorylation compared to wild-type cells. The same synergy was observed in SOS1 and SOS2 doubly deficient cells (data not shown) and in human Raji B cells (Fig. 9B, lane 3). Thus, the sensitivity and potency of antigen receptors engaged on either T or B lymphocytes for signaling through the Ras pathway are greatly enhanced by a unique interplay of RasGRP and SOS. The interplay efficiently starts a positive feedback loop that operates by priming and activating SOS with RasGTP via its allosteric pocket, resulting in the combined output of two distinct GEFs (Fig. 9C, left).
FIG. 9.
Synergy between H-RasV12C40 and SOS function requires an intact allosteric pocket in SOS and is present in both T and B lymphocytes. (A) Wild-type and RasGRP1 and -3 dually deficient DT40 cells were transfected with 5 μg of SOS1-cat, SOS1-cat-W729E, and/or 5 μg H-RasV12C40 expression constructs and analyzed for protein expression as described before. The anti-Ras antibody only weakly reacts with the endogenously expressed chicken Ras. (B) Raji B cells were transfected and analyzed as in panel A. Two micrograms of SOS1-cat or SOS1-cat-W729E and/or 6 μg of H-RasV12C40 expression constructs was used. (C) The left panel shows a proposed model of Ras signaling in lymphocytes leading to activation of the Ras-RAF-MEK-ERK pathway following antigen receptor engagement. The right panel shows the same positive feedback loop via SOS' allosteric pocket, which was recently demonstrated to enhance Ras-ERK signaling in vivo in EGF-stimulated HeLa cells (4).
DISCUSSION
Here we have demonstrated that RasGRP and SOS act in concert to establish very sensitive and robust Ras activation when antigen receptors are triggered. We found a functional interaction between RasGRP and SOS in intact cells which relies on an intact binding pocket specific for RasGTP in SOS that was previously identified in SOS' crystal structure (26). In stimulated cells, this allosteric pocket has a crucial function to augment the GEF activity of activated SOS. In our assays, we have established that effective Ras-RAF-ERK-CD69 activation through SOS relies on (i) membrane localization of the full-length SOS molecule, (ii) both intact GEF and allosteric pockets in SOS, (iii) the presence of DAG and RasGRP1, and (iv) synergistic actions of RasGTP and SOS.
Our experiments in intact cells demonstrate the existence of a positive feedback loop in vivo downstream of antigen receptors that had been suggested based on previous in vitro work (26). Very recently, the in vivo presence of this positive feedback loop via SOS was independently demonstrated. Boykevisch et al. demonstrated that loading of the allosteric pocket increased the amplitude and duration of ERK kinase activation following EGF stimulation of a HeLa cervical carcinoma cell line (4) (see Fig. 9C, right). Thus, this amplification loop that enhances Ras signaling is conserved in different cell types. However, here we also show that this feedback loop, via SOS in lymphocytes, functions as a Ras rheostat, profoundly influenced by RasGRP1. Without RasGRP1, cells have difficulty overcoming the resistance of this rheostat since the catalyst of SOS is SOS' own product, RasGTP. Thus, although it is possible to achieve Ras activation solely via SOS (e.g., in 293T cells or HeLa cells that do not express any RasGRP family members) (Roose et al., unpublished results), activation occurs in a slower and less sensitive manner. Hence, the dominant function of RasGRP molecules is revealed. This unique interplay between SOS and RasGRP also offers an explanation for the lack of compensation by SOS observed in vivo in the developmentally blocked RasGRP1 null thymocytes (15). In addition, RasGRP1 and SOS1 appear to regulate the basal level of RasGTP in resting cells. We are currently investigating the mechanism of basal Ras activation, its control, and its biological relevance.
Cellular compartmentalization of Ras activation adds another level of complexity to understanding Ras regulation. Recent analyses of the cellular localization of Ras activation suggest that, surprisingly, most Ras activation occurs on internal membranes, in a RasGRP-dependent manner, although there are cell-type-specific nuances (3, 7, 9). Nonetheless, our biochemical analyses and those of others reveal a dominant function of RasGRP in Ras activation. In addition, specific trafficking of not only Ras itself, but also, e.g., RasGRP and RasGAPs like CAPRI and RASAL, adds complexity to regulated and compartmentalized Ras activation in different cell types (reviewed in reference 29). A very recent, provocative, study implicates compartmentalization of Ras-MAP kinase signaling in setting the threshold of T-cell selection in the thymus (13). Future work will undoubtedly refine our current model of Ras activation (Fig. 9C, left) in a spatiotemporal manner.
At first glance, SOS appears to play a less important and somewhat redundant role in T and B lymphocytes since RasGRP is present. Under maximally stimulating conditions, Ras-ERK activation is relatively efficient, without evidence of SOS function (Fig. 2 and 4). However, at decreasing doses of stimuli that likely mimic more physiological levels of stimulation, a clear contribution of SOS to optimal Ras-ERK activation is observed. What is the benefit of expressing two distinct classes of GEFs that can be activated by the very same receptors, notably the TCR and BCR? SOS' characteristics may explain the advantage of expressing two different GEFs. The allosteric RasGTP-binding pocket of SOS is critical for SOS' potential to enhance Ras signal transduction as a bona fide GEF together with RasGRP when the antigen receptor is engaged. We hypothesize that this two-step mechanism of activating Ras with a positive feedback loop has the advantage of better control, enabling the cell to discriminate appropriate signals from noise. As such, expression of two different types of GEFs is a much safer option for lymphocytes than, e.g., expressing double the amount of RasGRP1, which is known to have the potential to cause T-cell malignancies (21, 22). At the same time, such a mechanism preserves the capacity of robust activation of Ras via two GEFs, even when only a limited number of antigen receptors on lymphocytes are engaged.
The resultant discriminating receptor sensitivity and large differential in Ras activation are characteristics beneficial to T and B lymphocytes, which are required to respond with great sensitivity and selectivity to exposure to pathogens. These studies reveal a remarkable interplay between two distinct types of Ras GEFs that (triggered by the same receptor) places greater control in the Ras pathway and enhances its sensitivity and robustness in a manner that cannot be achieved via either GEF alone.
Acknowledgments
We express our gratitude to Athena Lin and Martin McMahon for RasV12 and variants, Michael Karin for SOS1 and SOS1-F constructs, and Xiao-Ping Zhong and Gary Koretzky for the DGKζ construct, as well as Jim Stone and Stacey Stang for anti-RasGRP1 antibody. We are grateful to Susan Levin, Tomas Brdicka, Henry Bourne, and Martin McMahon for critically reading the manuscript. We thank Holger Sondermann, John Kuriyan, and the members of the Weiss lab for their suggestions and comments and S.L., M.M., T.E., and B.F. for Sunday cell culture.
J.R. is grateful for a grant from the Dutch Cancer Society (KWF). This work was supported in part by a grant from the NCI (CA 72531).
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Aiba, Y., M. Oh-hora, S. Kiyonaka, Y. Kimura, A. Hijikata, Y. Mori, and T. Kurosaki. 2004. Activation of RasGRP3 by phosphorylation of Thr-133 is required for B cell receptor-mediated Ras activation. Proc. Natl. Acad. Sci. USA 101:16612-16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronheim, A., D. Engelberg, N. Li, N. al-Alawi, J. Schlessinger, and M. Karin. 1994. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell 78:949-961. [DOI] [PubMed] [Google Scholar]
- 3.Bivona, T. G., I. Perez De Castro, I. M. Ahearn, T. M. Grana, V. K. Chiu, P. J. Lockyer, P. J. Cullen, A. Pellicer, A. D. Cox, and M. R. Philips. 2003. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424:694-698. [DOI] [PubMed] [Google Scholar]
- 4.Boykevisch, S., C. Zhao, H. Sondermann, P. Philippidou, S. Halegoua, J. Kuriyan, and D. Bar-Sagi. 2006. Regulation of ras signaling dynamics by Sos-mediated positive feedback. Curr. Biol. 16:2173-2179. [DOI] [PubMed] [Google Scholar]
- 5.Brose, N., A. Betz, and H. Wegmeyer. 2004. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr. Opin. Neurobiol. 14:328-340. [DOI] [PubMed] [Google Scholar]
- 6.Buday, L., and J. Downward. 1993. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73:611-620. [DOI] [PubMed] [Google Scholar]
- 7.Caloca, M. J., J. L. Zugaza, and X. R. Bustelo. 2003. Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J. Biol. Chem. 278:33465-33473. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, V. K., T. Bivona, A. Hach, J. B. Sajous, J. Silletti, H. Wiener, R. L. Johnson II, A. D. Cox, and M. R. Philips. 2002. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4:343-350. [DOI] [PubMed] [Google Scholar]
- 10.Corbalan-Garcia, S., S. M. Margarit, D. Galron, S.-S. Yang, and D. Bar-Sagi. 1998. Regulation of Sos activity by intramolecular interactions. Mol. Cell. Biol. 18:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlin, J. J., S. L. Stang, N. A. Dower, and J. C. Stone. 2005. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J. Immunol. 175:7179-7184. [DOI] [PubMed] [Google Scholar]
- 12.D'Ambrosio, D., D. A. Cantrell, L. Frati, A. Santoni, and R. Testi. 1994. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 24:616-620. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, M. A., E. Teixeiro, J. Gill, B. Hausmann, D. Roubaty, K. Holmberg, G. Werlen, G. A. Hollander, N. R. Gascoigne, and E. Palmer. 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444:724-729. [DOI] [PubMed] [Google Scholar]
- 14.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dower, N. A., S. L. Stang, D. A. Bottorff, J. O. Ebinu, P. Dickie, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1:317-321. [DOI] [PubMed] [Google Scholar]
- 16.Ebinu, J. O., D. A. Bottorff, E. Y. Chan, S. L. Stang, R. J. Dunn, and J. C. Stone. 1998. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280:1082-1086. [DOI] [PubMed] [Google Scholar]
- 17.Gale, N. W., S. Kaplan, E. J. Lowenstein, J. Schlessinger, and D. Bar-Sagi. 1993. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature 363:88-92. [DOI] [PubMed] [Google Scholar]
- 18.Genot, E., and D. A. Cantrell. 2000. Ras regulation and function in lymphocytes. Curr. Opin. Immunol. 12:289-294. [DOI] [PubMed] [Google Scholar]
- 19.Holsinger, L. J., D. M. Spencer, D. J. Austin, S. L. Schreiber, and G. R. Crabtree. 1995. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc. Natl. Acad. Sci. USA 92:9810-9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz, M. E., and F. McCormick. 1997. Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 7:75-79. [DOI] [PubMed] [Google Scholar]
- 21.Klinger, M. B., B. Guilbault, R. E. Goulding, and R. J. Kay. 2005. Deregulated expression of RasGRP1 initiates thymic lymphomagenesis independently of T-cell receptors. Oncogene 24:2695-2704. [DOI] [PubMed] [Google Scholar]
- 22.Li, J., H. Shen, K. L. Himmel, A. J. Dupuy, D. A. Largaespada, T. Nakamura, J. D. Shaughnessy, Jr., N. A. Jenkins, and N. G. Copeland. 1999. Leukaemia disease genes: large-scale cloning and pathway predictions. Nat. Genet. 23:348-353. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Q., S. A. Walker, D. Gao, J. A. Taylor, Y. F. Dai, R. S. Arkell, M. D. Bootman, H. L. Roderick, P. J. Cullen, and P. J. Lockyer. 2005. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+. J. Cell Biol. 170:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolnik, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 25.Luo, B., D. S. Regier, S. M. Prescott, and M. K. Topham. 2004. Diacylglycerol kinases. Cell. Signal. 16:983-989. [DOI] [PubMed] [Google Scholar]
- 26.Margarit, S. M., H. Sondermann, B. E. Hall, B. Nagar, A. Hoelz, M. Pirruccello, D. Bar-Sagi, and J. Kuriyan. 2003. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell 112:685-695. [DOI] [PubMed] [Google Scholar]
- 27.Matthews, S. A., E. Rozengurt, and D. Cantrell. 2000. Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J. Exp. Med. 191:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitin, N., K. L. Rossman, and C. J. Der. 2005. Signaling interplay in Ras superfamily function. Curr. Biol. 15:R563-R574. [DOI] [PubMed] [Google Scholar]
- 29.Mor, A., and M. R. Philips. 2006. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 24:771-800. [DOI] [PubMed] [Google Scholar]
- 30.Oh-hora, M., S. Johmura, A. Hashimoto, M. Hikida, and T. Kurosaki. 2003. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J. Exp. Med. 198:1841-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian, X., L. Esteban, W. C. Vass, C. Upadhyaya, A. G. Papageorge, K. Yienger, J. M. Ward, D. R. Lowy, and E. Santos. 2000. The Sos1 and Sos2 Ras-specific exchange factors: differences in placental expression and signaling properties. EMBO J. 19:642-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roose, J. P., M. Mollenauer, V. A. Gupta, J. Stone, and A. Weiss. 2005. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sondermann, H., S. M. Soisson, S. Boykevisch, S. S. Yang, D. Bar-Sagi, and J. Kuriyan. 2004. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell 119:393-405. [DOI] [PubMed] [Google Scholar]
- 34.Topham, M. K., and S. M. Prescott. 2001. Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism. J. Cell Biol. 152:1135-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita, S., N. Mochizuki, Y. Ohba, M. Tobiume, Y. Okada, H. Sawa, K. Nagashima, and M. Matsuda. 2000. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J. Biol. Chem. 275:25488-25493. [DOI] [PubMed] [Google Scholar]
- 37.Yang, Y., L. Li, G. W. Wong, S. A. Krilis, M. S. Madhusudhan, A. Sali, and R. L. Stevens. 2002. RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J. Biol. Chem. 277:25756-25774. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, Y., H. Liu, J. Coughlin, J. Zheng, L. Li, and J. C. Stone. 2005. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and RAS signaling systems in B cells. Blood 105:3648-3654. (First published 18 January 2005; doi: 10.1182/blood-2004-10-3916.) [DOI] [PubMed] [Google Scholar]
- 39.Zhong, X. P., E. A. Hainey, B. A. Olenchock, M. S. Jordan, J. S. Maltzman, K. E. Nichols, H. Shen, and G. A. Koretzky. 2003. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 4:882-890. [DOI] [PubMed] [Google Scholar]