Abstract
Insulin resistance is a defining feature of type 2 diabetes and the metabolic syndrome. While the molecular mechanisms of insulin resistance are multiple, recent evidence suggests that attenuation of insulin signaling by c-Jun N-terminal kinase (JNK) may be a central part of the pathobiology of insulin resistance. Here we demonstrate that the p85α regulatory subunit of phosphoinositide 3-kinase (PI3K), a key mediator of insulin's metabolic actions, is also required for the activation of JNK in states of insulin resistance, including high-fat diet-induced obesity and JNK1 overexpression. The requirement of the p85α regulatory subunit for JNK occurs independently of its role as a component of the PI3K heterodimer and occurs only in response to specific stimuli, namely, insulin and tunicamycin, a chemical that induces endoplasmic reticulum stress. We further show that insulin and p85 activate JNK by via cdc42 and MKK4. The activation of this cdc42/JNK pathway requires both an intact N terminus and functional SH2 domains within the C terminus of the p85α regulatory subunit. Thus, p85α plays a dual role in regulating insulin sensitivity and may mediate cross talk between the PI3K and stress kinase pathways.
Insulin resistance is an underlying feature of type 2 diabetes and the metabolic syndrome (41). Physiologic and epidemiologic studies have demonstrated a strong link between obesity and the development of insulin resistance (22, 43). Not surprisingly, the rise of type 2 diabetes in the United States over the last decade has paralleled the rapid rise in obesity (35). While there are multiple mechanisms involved in obesity-linked insulin resistance, one important mediator of this process is the stress kinase c-Jun N-terminal kinase (JNK), which is activated by insulin (32), cytokines (4), and endoplasmic-reticulum (ER) stress (38). Obesity and other insulin-resistant states trigger JNK-mediated phosphorylation of IRS-1 on serine residues, which attenuates insulin signaling through the phosphoinositide 3-kinase (PI3K) pathway (38, 39). The importance of this stress kinase is further illustrated by that fact that genetic deletion of JNK1 can prevent insulin resistance in severely obese mice (19).
The molecular machinery that enables JNK activation in insulin-resistant states is not well understood. One interesting, and perhaps counterintuitive, molecule involved in the activation of JNK is the p85α regulatory subunit of PI3K. PI3K is an obligate heterodimer, with an SH2 domain-containing regulatory subunit (p85) and a catalytic subunit (p110). The regulatory subunit mediates the binding, activation, and localization of the p110 catalytic subunit (2, 13, 55). While the p85 subunit is central in the metabolic actions of insulin as a component of the PI3K holoenzyme (46), it also plays a role as an independent negative regulator of insulin signaling. Indeed, a reduction in the level of the p85 subunit can prevent high-fat diet (HFD)-induced induced insulin resistance (49) and ameliorate diabetes in mice with heterozygous deletions of the insulin receptor and IRS-1 (31). Recently, we have also found that a liver-specific deletion of Pik3r1, the gene that encodes p85α and the smaller p55α and p50α subunits, enhances hepatic and peripheral insulin sensitivity (47).
Several studies have linked p85α circumstantially to the activation of JNK. For instance, cells lacking p85α have diminished JNK activation in response to insulin/IGF-1, and this is reversed with reexpression of p85α (52). The p85 regulatory subunit has also been identified as part of a complex that is involved in JNK activation (57) and has been shown to bind to activated forms of the small GTPase cdc42 (50, 56), which is an upstream activator of JNK (33). Moreover, muscle biopsies from obese diabetic patients found a strong positive correlation of p85 expression and JNK (3). Although these data suggest an intriguing and novel connection between the PI3K and stress kinase pathways, this cross talk has been difficult to address in vivo because mice carrying complete germ line deletions of the Pik3r1 gene that encodes p85α and its shorter isoforms p55α and p50α die perinatally (14).
To circumvent the problems and to investigate the links between p85α and JNK activation in vivo, we created mice with a liver-specific deletion of the Pik3r1 gene (L-Pik3r1KO). We found that mice lacking p85α in the liver have diminished hepatic activation of JNK and improved whole-body insulin sensitivity, even when fed an HFD. We demonstrate that p85α is required for the full activation of JNK by insulin or by the ER stress-inducing agent tunicamycin. The p85 regulatory subunits enable JNK activation in response to insulin through a cdc42-MKK4 pathway and require an intact N terminus and functional SH2 domains in the C terminus of the p85α regulatory subunit. Thus, p85α may regulate insulin sensitivity via cross talk with the stress kinase pathway.
MATERIALS AND METHODS
Animals and HFD.
All animals were housed on a 12-h light-dark cycle and fed standard rodent chow (Purina). High-fat chow (45% of the calories from fat) was purchased from Research Diets. All protocols for animal use and euthanasia were approved by the Animal Care and Use Committee of the Joslin Diabetes Center and Harvard Medical School in accordance with National Institutes of Health guidelines. All mice in this study were on a 129Sv-C57BL/6-FVB mixed genetic background, and littermates of the same mixed genetic background were used as controls.
Antibodies.
Rabbit polyclonal anti-IRS-1 antibody (IRS-1), anti-IRS-2 antibody (IRS-2), anti-IR antibody (IR), and pan-p85α antibody were generated as described previously (53). Rabbit polyclonal anti-Akt, anti-phospho-Akt (S473), anti-phospho-JNK, anti-JNK, anti-phospho-MKK4, and anti-MKK4 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-phosphoserine 307 IRS-1 antibody, cdc42 antibody, and phosphotyrosine (pTyr) antibody 4G10 were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Goat polyclonal anti-Akt1/2 antibody (Akt) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Recombinant adenoviruses.
The p50α and p55α adenoviruses were constructed as previously described (51). The constitutively active MKK4 adenovirus was purchased from CellBioLabs (San Diego, CA), and the dominant-negative (N17) and constitutively active (V12) cdc42 adenoviruses were a generous gift from James Bamburg (28). Wild-type human p85α and mutant forms lacking the SH3 domain (ΔSH3), the bcr homology domain (ΔBH), the p110 binding domain (ΔiSH2), or both the SH3 domain and the p110 binding domain (ΔΔp85) were made as described in the supplemental material. The R358A/R649A (RARA) construct of mouse p85α was made as previously described (29). To create the recombinant adenoviruses, each of the above constructs was ligated into an empty pShuttle vector with a CAG promoter and bovine growth hormone poly(A) sequence (CAGpShuttle). A new multiple cloning site was inserted into the vector containing the following restriction sites in sequence: NotI-NheI-SwaI-EcoRV-Hind3-SbfI-SalI. The adenoviruses were then produced according to the standard AdEasy protocol (16).
Cell culture and in vitro adenovirus infection.
Immortalized Pik3r1+/+ or Pik3r1−/−fibroblasts were prepared as previously described (52). The fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM) with 10% (vol/vol) fetal bovine serum in a humidified 5% CO2 environment at 37°C. Following 12 to 16 h of serum starvation, cells were treated with LY294002 compound for 30 min, tunicamycin for 4 h, or anisomycin for 4 h prior to a 100 nM insulin incubation for 15 min. Primary hepatocytes were grown in six-well plates (see protocol below) and treated with adenovirus at a multiplicity of infection (MOI) of 100 for 1 h at 37°C. Approximately 36 h after infection, primary hepatocytes were placed in serum-free DMEM for 12 h and then stimulated with 100 nM insulin for 15 min.
Primary hepatocyte isolation and culture.
Hepatocytes were isolated by collagenase digestion as described previously (7). Briefly, the mice were anesthetized with a 1.2% solution of 2,2,2-tribromoethanol, the portal vein was cannulated with a 25-gauge catheter, and the liver was perfused for 15 min at a rate of 7 ml/min with calcium-free perfusion buffer. The blanched liver was perfused with collagenase solution (200 U/ml) for 10 min at 7 ml/min to release hepatocytes from the extracellular matrix. The digested liver was excised and placed in preservation buffer, where the digested cells were gently scraped from the liver sac, washed, and purified with Percoll to remove dead cells and enrich the hepatocyte fraction. Typical viabilities were 85 to 90%, with cell yields of 1.0 × 106 to 1.5 × 106 cells/g of mouse. The isolated hepatocytes are then grown on six-well collagen-coated plates at a density of 0.5 × 106 to 1.0 × 106 cells/well in Advanced DMEM (Gibco) supplemented with glutamine, antibiotic cocktail, and 10% fetal bovine serum.
Metabolic studies.
For glucose tolerance testing (GTT), blood samples were obtained at 0, 15, 30, 60, and 120 min after intraperitoneal injection of 2 g/kg dextrose. Insulin tolerance tests were performed by injecting 1 U/kg insulin (Novolin; Novo Nordisk, Bagsværd, Denmark) intraperitoneally, followed by blood collection at 0, 15, 30, and 60 min after injection. Blood glucose values were determined with a One Touch II glucose monitor (Lifescan Inc., Milpitas, CA). Plasma insulin levels were measured by enzyme-linked immunosorbent assay with mouse insulin as the standard (Crystal Chem Inc., Chicago, IL).
In vivo adenovirus-mediated gene transfer and insulin stimulation.
Prior to tail vein injection, adenoviruses were purified on sequential cesium chloride gradients and dialyzed into phosphate-buffered saline containing 10% glycerol. For primary hepatocytes, insulin stimulation experiments were performed 48 h after infection with adenovirus at an MOI of 100. For animal studies, we injected 10- to 12-week-old male mice with an adenovirus dose of 5 × 108 PFU/g of body weight via the tail vein as described previously (48). Insulin stimulation of mice was performed on day 5 after injection, following an overnight fast. Briefly, the mice were anesthetized with Avertin (1.2% 2,2,2-tribromoethanol in phosphate-buffered saline) and injected with 5 U of regular human insulin (Novolin; Novo Nordisk, Bagsværd, Denmark) via the inferior vena cava. Five minutes after administration of the insulin bolus, tissues were removed and frozen in liquid nitrogen. Immunoprecipitation and immunoblot analysis of insulin signaling molecules were performed with tissue homogenates prepared in a tissue homogenization buffer that contained 25 mM Tris-HCl (pH 7.4), 10 mM Na3VO4, 100 mM NaF, 50 mM Na4P2O7, 10 mM EGTA, 10 mM EDTA, 2 mM phenylmethylsulfonyl fluoride, 1% Nonidet P-40 supplemented with the Complete Protease Inhibitor Cocktail (Roche). All protein expression data were quantified by densitometry with NIH Image software.
In vivo and in vitro cdc42 activation assay.
Mice were anesthetized and injected with 5 U of insulin via the portal vein. Three minutes after administration of the injection, the right lobe of the liver was quickly dissected and snap-frozen directly in liquid nitrogen. Approximately 200 to 300 mg of the liver sample was used to measure cdc42 activity in the livers with a p21-associated kinase 1 (PAK1) pull-down assay kit (Upstate). The kit was used essentially as directed but with the addition of 10 mM orthovanadate to the reaction buffer. Measurement of cdc42 activity from primary hepatocytes was also performed as described in the instructions supplied with the kit (Upstate).
Statistics.
Data are presented as the mean ± the standard error of the mean (SEM). Student's t test was used for statistical analysis between two groups, while statistical significance of differences among multiple treatment groups was determined by analysis of variance and Tukey's t test.
RESULTS
Blunted JNK activation in L-Pik3r1KO mice ameliorates obesity-induced diabetes.
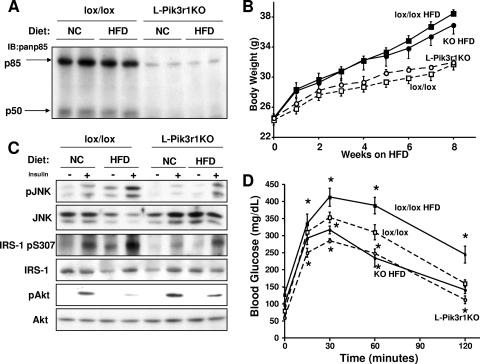
In previous studies, we had shown that cells lacking p85α exhibited a marked reduction in insulin-stimulated JNK activity (52). To better characterize this cross talk between p85α and JNK in vivo, we generated mice with a liver-specific deletion of Pik3r1 via the Cre-loxP system (L-Pik3r1KO) as described previously (40). Mice carrying a floxed exon 7, which encodes the N-terminal SH2 domain common to p85α and its alternatively spliced short forms p55α and p50α (30), were crossed with mice carrying the Cre transgene driven by the albumin promoter. Western blot assays of liver extracts of L-Pik3r1KO mice revealed an 80 to 90% decrease in p85α and a complete loss of p50α, the only one of the splice forms normally present in the liver, consistent with complete ablation of Pik3r1 in hepatocytes (Fig. 1A).
FIG. 1.
Loss of hepatic p85α protects mice from obesity-induced diabetes. (A) Western blot assays for expression of p85α and p50α with a pan-p85 antibody in the indicated animals and treatments. IB, immunoblot. (B) Six-week-old L-Pik3r1KO mice and lox/lox controls were fed normal chow (NC) or an HFD for a total of 8 weeks. The graph indicates body weight for each week on either diet. Symbols: open squares, lox/lox, NC; open circles, L-Pik3r1KO, NC; closed squares, lox/lox, HFD; closed circles, L-Pik3r1KO, HFD. (C) Western blot assays were performed against liver lysates of mice with the indicated genotypes and diets with the phosphoserine 473 Akt (pAkt) antibody, phospho-JNK (pJNK) antibody, and phosphoserine 307 (ps307) IRS-1 antibody. The phosphospecific antibody blots were stripped and reprobed with the appropriate antibodies to determine the total levels of the corresponding proteins. (D) GTTs of lox/lox or L-Pik3r1KO mice on NC or an HFD. *, P < 0.05 compared with lox/lox on NC. The error bars represent the SEM (n = 4 to 6).
In vivo obesity is a powerful physiologic activator of JNK, particularly in the liver (19). To investigate if p85α plays a role in JNK activation, we placed 6-week-old L-Pik3r1KO mice on an HFD (45% of the calories from fat) for a total of 8 weeks. At the end of the treatment, both lox/lox and L-Pik3r1KO mice were equally obese (Fig. 1B) and consumed the same amount of calories (see Fig. S1A in the supplemental material). In the lean lox/lox animals, insulin increased JNK activity by threefold. By contrast, L-Pik3r1KO animals showed a 60% reduction in insulin-simulated JNK activation (Fig. 1C). After high-fat feeding, basal and insulin-stimulated JNK activation increased by twofold in both lox/lox and L-Pik3r1KO animals but JNK activation in obese knockout (KO) animals reached only 50% the level observed in obese control animals (Fig. 1C). The attenuated JNK activity in both lean and obese L-Pik3r1KO mice directly correlated with decreased levels of serine phosphorylation of IRS-1 on residue 307, a site of JNK phosphorylation, and increased Akt activity compared to controls (Fig. 1C).
These reductions in stress kinase activation translated to marked improvements in glucose homeostasis. L-Pik3r1KO mice maintained lower fasting blood glucose and fasting serum insulin levels when fed either an HFD or normal chow (see Fig. S1B and C in the supplemental material). In addition, while obese lox/lox mice were severely glucose intolerant, obese L-Pik3r1KO mice exhibited normal-to-improved glucose tolerance even compared to control mice on normal chow (Fig. 1D). Thus, loss of p85α expression in the liver protected against obesity-induced insulin resistance and diabetes.
L-Pik3r1KO hepatocytes are resistant to JNK-induced insulin resistance.
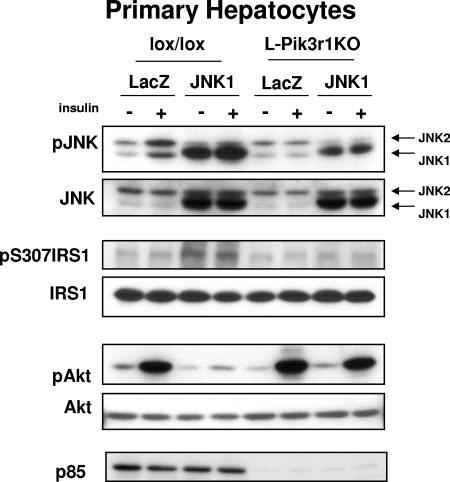
To determine whether JNK resistance in Pik3r1KO hepatocytes was a cell autonomous effect or due to a change in the hormonal milieu of the HFD-fed animals, primary hepatocytes were isolated from lox/lox or L-Pik3r1KO mice and JNK1 was overexpressed by adenovirus-mediated gene transfer. The JNK1 isoform was used because it is the only one of the three JNK isoforms that has been shown to have a significant role in mediating obesity-related insulin resistance in the liver (19). Following adenoviral infection, JNK1 was overexpressed by sixfold in both p85α KO and control primary hepatocytes (Fig. 2). This forced overexpression of JNK1 led to fivefold increases in JNK phosphorylation and serine phosphorylation of IRS-1 in lox/lox hepatocytes. By contrast, an equal level of JNK1 expression resulted in only a twofold increase in JNK activation in hepatocytes derived from L-Pik3r1KO mice (Fig. 2, P < 0.05 KO versus control cells). Likewise, overexpression of JNK1 reduced insulin-stimulated Akt phosphorylation in lox/lox hepatocytes by 85%, while the same level of overexpression in L-Pik3r1KO hepatocytes did not significantly reduce insulin-stimulated Akt signaling compared to that in LacZ-treated controls.
FIG. 2.
L-Pik3r1KO hepatocytes are JNK resistant. Primary hepatocytes were isolated from lox/lox mice or L-Pik3r1KO mice, infected with a JNK1-expressing adenovirus or a control LacZ adenovirus at an MOI of 100, and then treated with saline or 100 nM insulin after 12 h of serum starvation. Western blot assays were then performed against lysates from the treated hepatocytes with the indicated antibodies. The phosphospecific antibody blots were stripped and reprobed with the appropriate antibodies to determine the total levels of the corresponding proteins.
Pik3r1−/− cells exhibit impaired JNK activation during ER stress.
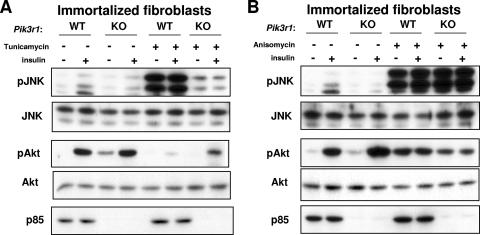
Insulin resistance in the liver is known to occur both through negative feedback from insulin itself (18) and by cross talk with other stimuli, such as cytokines and increased cellular stress (20). Obesity is known to induce JNK activity and insulin resistance through increased ER stress (38). To determine whether a reduced cellular response to ER stress could partially account for the decreased JNK activation and metabolic protection from HFD-induced glucose intolerance in L-Pik3r1KO mice, we treated WT and Pik3r1−/− cells with tunicamycin, a cytotoxin that induces ER stress (44), in the presence of insulin or saline controls (Fig. 3A). While Pik3r1−/− fibroblasts showed no differences in JNK activation in response to insulin, there was a marked difference in JNK activation in response to tunicamycin. Both WT and KO cells that were not treated with tunicamycin exhibited a modest twofold increase in JNK activation in response to insulin over their saline-treated counterparts. Akt was activated normally by insulin in both WT and KO cells not treated with tunicamycin.
FIG. 3.
Cells lacking p85 are resistant to ER stress. (A) WT or Pik3r1−/− immortalized fibroblasts were serum starved overnight before a 4-h incubation with tunicamycin as indicated. The cells were then treated with insulin for 15 min as indicated. Lysates from the treated cells were then analyzed by Western blot assay with phospho-JNK and phospho-Akt antibodies. (B) Cells were treated as in panel A, except with a 4-h incubation with anisomycin, as indicated.
Following induction of ER stress by tunicamycin in the hepatocytes, there was a marked difference in JNK activation. Thus, while WT cells displayed a 20-fold increase in JNK activation compared to untreated saline controls, KO cells only exhibited a fourfold enhancement of JNK activation, indicating that Pik3r1−/− cells are significantly protected from the ER stress response. Insulin had no appreciable incremental effect on JNK activation in tunicamycin-treated cells, presumably because insulin is a much weaker activator of JNK than tunicamycin. The activation of Akt was decreased in proportion to the increased JNK activation. For instance, while WT cells exhibited a 90% decrease in Akt activation compared to controls, Pik3r1−/− cells exhibited only a 50% decrease in Akt activation. Thus, the lack of p85 expression confers a degree of resistance to ER stress, and this may play a role in the improved metabolic homeostasis of HFD-fed L-Pik3r1KO mice.
JNK can be activated by many specific and nonspecific stimuli. To determine if the lack of p85 caused a systemic defect in JNK activation, we treated WT and KO cells with another potent JNK agonist, anisomycin, which induces JNK activity through a ribotoxic stress response (25). In contrast to the effect of tunicamycin, anisomycin strongly and equally induced JNK in both WT and KO cells (Fig. 3B). Interestingly, Akt was also activated by anisomycin, even in the absence of insulin, which is known to occur in response to some forms of more global cellular stress (42). Thus, p85 does not enable JNK activation in response to all capable stimuli but rather only to a subset of signals, which specifically includes the response to insulin and ER stress.
The p85α regulatory subunit enables insulin-mediated JNK activation via a cdc42/MKK4 pathway.
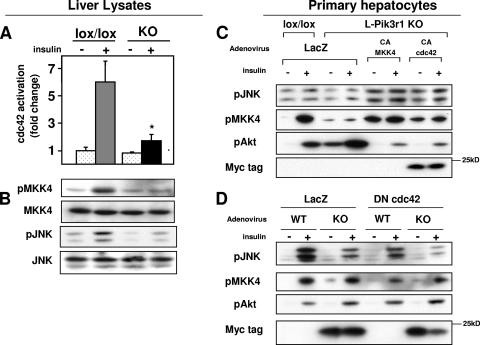
The defects in JNK activation by insulin in L-Pik3r1KO hepatocytes indicated that one of the products of the Pik3r1 gene might be regulating an upstream activator of JNK. One candidate effector is the small GTPase cdc42, which is known to activate both SEK1/MKK4 and JNK in response to insulin and other growth factors (15), and has been shown to interact with p85α (56), although it is unknown whether this physical interaction has any functional consequences in vivo. To assess activation of cdc42, we used the ability of this protein in its activated form to bind to PAK1 as assessed in a pull-down assay (54). This revealed that the level of activated cdc42 was reduced by ∼65% in KO animals (Fig. 4A). This decreased cdc42 activation correlated with a 75% decrease in MKK4 phosphorylation/activation (Fig. 4B). Since MKK4 is the direct upstream kinase of JNK, this decrease in MKK4 activation could account for the observed decrease in JNK activation.
FIG. 4.
Pik3r1 is required for insulin activation of the cdc42/MKK4/JNK pathway. (A) cdc42 activity as determined by PAK1 pull-down assay from liver lysates 3 min after administration of a 5-U portal bolus of insulin (see Materials and Methods). (B) Phosphospecific-antibody blots against phospho-MKK4 and phospho-JNK. The phosphospecific antibody blots were stripped and reprobed with the appropriate antibodies to determine the total levels of the corresponding proteins. (C) Primary hepatocytes isolated from lox/lox or L-Pik3r1KO mice were infected with either LacZ or constitutively active forms of MKK4 and cdc42 (myc tagged), serum starved overnight, and then stimulated with 100 nM insulin. Western blot assays were then performed with the indicated antibodies. (D) Primary hepatocytes of the indicated genotype were infected with either LacZ or a myc-tagged dominant negative cdc42 adenovirus, serum starved overnight, and then stimulated with 100 nM insulin.
To confirm that this cdc42/MKK4/JNK signaling pathway is intact in liver tissue, we performed a gain-of-function study in which we infected primary hepatocytes from L-Pik3r1KO and lox/lox control livers with adenoviruses expressing LacZ or constitutively active forms of MKK4 (CA-MKK4) and cdc42 (CA-cdc42). The hepatocytes were then stimulated with either insulin or a saline control (Fig. 4C). LacZ-treated L-Pik3r1KO hepatocytes showed a modest 20% decrease in JNK activation but maintained a 1.5-fold increase in Akt activation. The expression of activated forms of MKK4 or cdc42, however, enhanced JNK phosphorylation by 2- and 1.4-fold over L-Pik3r1KO cells treated with control LacZ adenovirus. This activation by cdc42 or MKK4 occurred in both saline- and insulin-treated cells, indicating that the unregulated signals from these mutant molecules were a stronger stimulus to JNK activation than insulin. As expected, uncontrolled activation of JNK by the constitutively activated cdc42/MKK4 pathway also led to significant reductions in Akt phosphorylation by insulin.
To further demonstrate the role of cdc42 in the activation of JNK, we performed a loss-of-function experiment where primary hepatocytes were infected with a dominant negative cdc42 adenovirus (DN-cdc42, Fig. 4D). Lox/lox hepatocytes treated with DN-cdc42 adenovirus caused a 30% decrease in JNK activation, which resulted in a small but reproducible 20% increase in Akt activation over control cells. On the other hand, when L-Pik3r1KO hepatocytes were infected with the DN-cdc42 adenovirus, JNK activity was decreased by 50% in response to insulin. Surprisingly, this did not result in further Akt activation over LacZ-treated KO cells.
Insulin-stimulated JNK activation occurs independently of PI3K activity.
The p85α regulatory subunit plays a critical role in regulating PI3K activity by stabilizing and properly localizing the p110 catalytic subunit of PI3K. We have previously shown that L-Pik3r1KO mice have a significant defect in PI3K activation because of decreased expression of p110 (47). Since small GTPases like cdc42 are known to be activated by PI3K-dependent mechanisms (8), we wanted to determine whether the decreased cdc42/JNK activation in L-Pik3r1KO livers merely reflects decreased PI3K activity or is due to some PI3K-independent aspect of p85α function. To this end, primary hepatocytes were isolated from lox/lox or L-Pik3r1KO mice and treated with the small-molecule PI3K inhibitor LY294002 in the presence of insulin or saline. Complete inhibition of PI3K signaling was demonstrated by the ablation of Akt activation in both lox/lox and KO cells after treatment with LY294002 (Fig. 5A). The inhibition of PI3K had no detectable effect on JNK activation, as insulin was able to activate JNK at levels normally found in WT or L-Pik3r1KO cells, where as L-Pik3r1KO hepatocytes showed a 75% decrease in insulin-mediated JNK activation compared to lox/lox controls.
FIG. 5.
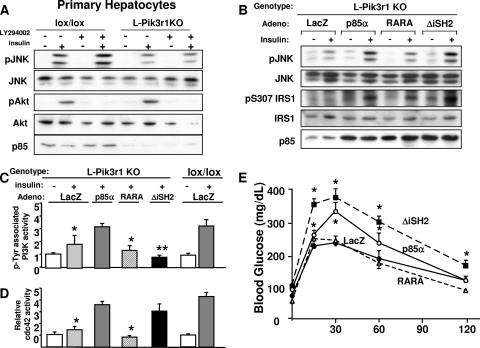
Activation of cdc42/JNK by p85α is not dependent upon PI3K activity. (A) Primary hepatocytes were isolated from lox/lox or L-Pik3r1KO mice (see Materials and Methods) and treated with insulin or the PI3K inhibitor LY294002 as indicated. JNK and Akt activities were then estimated with phosphospecific antibodies. The phosphospecific antibody blots were stripped and reprobed with the appropriate antibodies to determine the total levels of the corresponding proteins. (B) Purified recombinant adenoviruses (Adeno) were injected via tail vein into 10- to 12-week-old L-Pik3r1KO mice. Mice were injected with adenoviruses encoding control LacZ or WT p85α, RARA p85α, or ΔiSH2 p85α. Western blot assays were then performed against liver lysates of mice treated with adenovirus, with phospho-JNK, phosphoserine307, and pan-p85 antibodies to verify expression levels. Primary hepatocytes were isolated from lox/lox or L-Pik3r1KO mice and infected with the same adenoviruses, i.e., LacZ, WT p85α, RARA p85α, or ΔiSH2 p85α, and then PI3K (C) and cdc42 (D) activities were measured. *, P < 0.05 compared to insulin-stimulated lox/lox (E) GTT of mice whose livers were reconstituted with one of the indicated adenoviruses. The bars represent the SEM (n = 6 to 8).
To understand whether this PI3K-independent regulation of insulin-mediated cdc42/JNK activation could extend to mouse tissues in vivo, we reconstituted the livers of L-Pik3r1KO animals with either WT p85α or one of two p85α mutants that are incapable of activating PI3K. One mutant substitutes a FLAG tag for the p110 binding region of p85, which exists in the inter-SH2 domain (ΔiSH2; see Fig. S2 in the supplemental material) (11). When overexpressed in cells or in mouse livers, the ΔiSH2 construct has a dominant negative effect (34). The other p85 mutant contains arginine-to-alanine substitutions in critical residues in both SH2 domains in the C terminus (R358A/R649A, RARA; see Fig. S2 in the supplemental material); this mutant is able to bind p110 but cannot bind to phosphorylated IRS proteins, which is required for the proper activation and localization of the PI3K holoenzyme (17).
Interestingly, both JNK activity and serine phosphorylation of IRS-1 were restored by the expression of either WT p85α or the ΔiSH2 mutant but not by the RARA mutant (Fig. 5B). This activation of JNK could not have occurred by a PI3K-dependent mechanism, since the ΔiSH2 mutant drastically inhibited total PI3K in these livers, while the reexpression of WT p85α restored PI3K back to levels of lox/lox animals (Fig. 5B). These experiments suggest that p85α expression may be specifically required for JNK activation, at least in the liver. Interestingly, the RARA mutant, which also cannot activate PI3K, was also unable to activate JNK, indicating that functional SH2 domains are necessary for p85-JNK cross talk.
To further determine the extent to which PI3K activity is necessary to activate cdc42, the same p85α mutants were expressed in L-Pik3r1KO primary hepatocytes and insulin-stimulated cdc42 activity was measured (Fig. 5C). As with the JNK activity in whole livers, both the WT and ΔiSH2 versions of p85α fully restored the cdc42 response, despite their dichotomous effects on PI3K activity. On the other hand, the RARA mutant had neutral effects on both PI3K and cdc42 activities. These data are in agreement with the in vivo JNK data (Fig. 5B) that indicate that full-length p85α with functional SH2 domains is necessary for the insulin-dependent activation of cdc42/JNK while PI3K activity is not.
These molecular changes in cdc42/JNK corresponded to physiologic changes, where increased JNK activity led to decreased insulin sensitivity. For instance, the expression of either WT p85α and ΔiSH2, which increased cdc42 and JNK activation, led to worsened glucose tolerance compared to that of L-Pik3r1KO mice treated with control LacZ adenovirus (Fig. 5E), which normally possess heightened insulin sensitivity (46). The ΔiSH2 mutant caused significant glucose intolerance consistent with diabetes, probably because of the inhibition of the positive effects of PI3K in addition to the negative effects of JNK activation. Consistent with the cdc42/JNK data, the RARA p85α mutant had negligible effects on insulin sensitivity.
The negative effects on insulin signaling are specific to p85α.
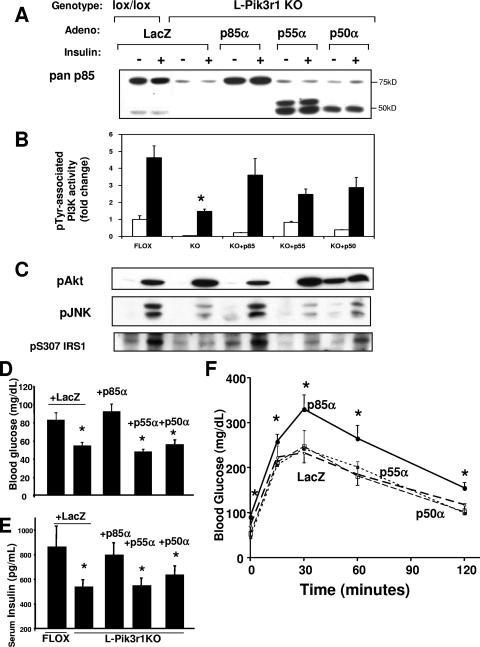
Since Pik3r1 encodes three different regulatory subunit isoforms, the possibility emerged that the negative regulation of insulin signaling could be isoform specific. To address this possibility, we used adenovirus-mediated gene transfer to reconstitute the livers of L-Pik3r1KO mice with each of the three Pik3r1 gene products, p85α, p55α, or p50α. Consistent with our observations of other cell types (51), the hepatic reconstitution of L-Pik3r1KO mice with each of the three Pik3r1 isoforms restored pTyr-associated PI3K activity to a similar extent (Fig. 6B).
FIG. 6.
The suppression of insulin action by Pik3r1 is specific to the p85α isoform. (A) Recombinant adenoviruses (Adeno) were injected via tail vein into 10- to 12-week-old male mice of the indicated genotypes. Mice were injected with adenoviruses encoding control LacZ or one of the Pik3r1 gene products, p85α, p55α, or p50α. Immunoblot assays were then performed to detect the expression of the p85α isoforms. An extra band of approximately 50 kDa appears in the livers treated with p55α adenovirus, and this likely represents a proteolytic breakdown product of p55α. (B) PI3K activity from mice injected with the indicated adenoviruses. (C) Western blot assays were then performed against liver lysates of mice treated with adenovirus, with phospho-JNK and phosphoserine 307 antibodies. The phosphospecific-antibody blots were stripped and reprobed with the appropriate antibodies to determine the total levels of the corresponding proteins (data not shown). Fasting blood glucose (D) and fasting serum insulin (E) levels of mice treated with the indicated adenoviruses are shown. (F) GTT of mice whose livers were reconstituted with one of the Pik3r1 gene products. The bars represent the SEM (n = 6 to 8).
Despite the fact the PI3K activity enabled by each of these isoforms was nearly the same, Akt activation was differentially affected by the expression of p85α. Thus, while the expression of control LacZ, p55α, or p50α maintained the elevated Akt activation observed in L-Pik3r1KO mice, the expression of p85α caused a relative decrease in Akt phosphorylation to a level similar to that of lox/lox controls. In addition, reexpression of p85α specifically restored several mechanisms of negative regulation to L-Pik3r1KO animals as p85α restored insulin-stimulated JNK activation and IRS-1 serine phosphorylation to levels comparable to those of lox/lox controls (Fig. 6C).
These changes at the molecular level correlated with changes at the physiologic level. The reconstitution of L-Pik3r1KO mice with p85α, but not its short isoforms, reversed the improvements in fasting blood glucose and fasting serum insulin levels and restored them to the levels of lox/lox controls (Fig. 6D). Moreover, reexpression of p85α produced a relative glucose intolerance in L-Pik3r1KO mice compared to LacZ-treated KO mice (Fig. 6E), such that the glucose excursion curves of p85α-expressing mice were indistinguishable from those of lox/lox controls.
An intact N terminus of p85 is required for the activation of cdc42.
The fact that only the full-length forms of p85α are able to activate JNK and suppress insulin action suggested that unique structural features of p85α may account for the functional differences. All regulatory subunits of class IA PI3K have the same C terminus but diverge greatly in the length and composition of their N termini (10). While the N termini of short isoforms p55α and p50α are only 34 and 6 amino acids long, respectively (1, 24), the N terminus of p85α is 339 amino acids long and contains an SH3 domain, two proline-rich regions, and a domain homologous to a portion of the breakpoint cluster region (bcr) gene product (BH domain).
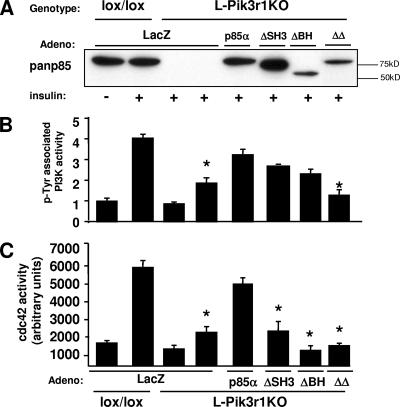
To investigate whether the negative effects of p85α are specific to one of the domains in the N terminus, we created an adenoviral p85 construct which substitutes a FLAG tag for either the ∼80-amino-acid SH3 domain (ΔSH3) or the ∼170-amino-acid BH domain (ΔBH), which effectively deleted the domain while providing an epitope tag for easy detection by Western blotting. We also created one construct with a combined deletion of both the SH3 domain and the inter-SH2 domain (ΔΔp85) to serve as a control for PI3K activity (Fig. 7A). Primary hepatocytes from L-Pik3r1KO mice were infected with ΔSH3, ΔBH, and ΔΔp85 adenoviruses, and total insulin-stimulated (pTyr-associated) PI3K and cdc42 activities were measured. The loss of either the SH3 or the BH domain from the N terminus of p85α did not affect the ability of p85 to rescue PI3K activity in L-Pik3r1KO hepatocytes (Fig. 7B). On the other hand, the control ΔΔp85 adenovirus, which lacks the p110 binding region in the C terminus fully inhibited PI3K activation (Fig. 8B). However, despite normal PI3K activity in the cells infected with ΔSH3 and ΔBH, cdc42 activity was significantly ablated (Fig. 7C). This abrogation of cdc42 activity also occurred in the ΔΔp85 adenovirus cells, which indicates that a fully intact N terminus of p85 is also necessary for the activation of cdc42. These data support the notion that the expression or function of some part of the N terminus of p85, and not PI3K activity, is required for the insulin-stimulated activation of cdc42/JNK.
FIG. 7.
The N terminus of p85α is required for cdc42 activation. Primary hepatocytes were isolated from lox/lox or L-Pik3r1KO mice and then infected with control LacZ, WT p85α, ΔSH3, ΔΒΗ, or ΔΔp85α adenovirus (Adeno). (A) Western blot assay with the pan-p85 antibody confirming expression levels of the construct. PI3K activity (B) and cdc42 activity (C) were measured in insulin-stimulated lysates of the infected primary hepatocytes. The bars represent the SEM (n = 3 to 4).
FIG. 8.
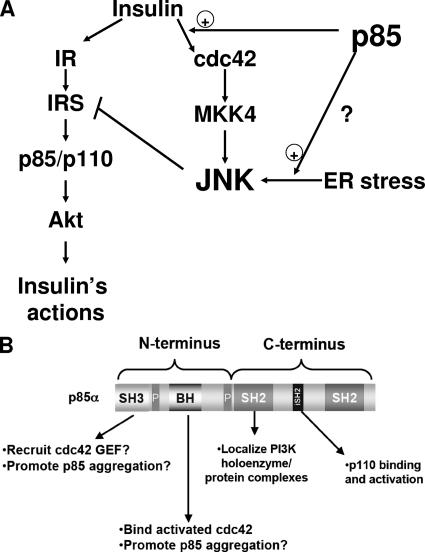
Molecular mechanisms of JNK activation by p85. (A) Schematic depiction of the role that p85 plays in activating JNK. The full-length p85 regulatory subunit potentiates the activation of JNK through the insulin/cdc42/MKK4 pathway and also facilitates the activation of JNK via the ER stress pathway. Activation of JNK through either pathway results in negative feedback on the insulin signaling pathway. Thus, the loss of p85 improves insulin sensitivity, in part by diminishing this JNK-mediated negative regulation. (B) Schematic of the structural features of the p85 regulatory subunit of PI3K showing how they may impact insulin action. The N terminus contains an SH3 domain, which may mediate intermolecular interactions with possible cdc42 guanine nucleotide exchange factors, while the BH domain binds activated cdc42. The SH2 domains in the C terminus are critical for proper function and localization of PI3K and may also mediate the recruitment of p85α to various cellular complexes. The proline-rich domains (P) are diagrammed, but we have not investigated the roles of these domains in p85α function.
DISCUSSION
The stress kinase JNK is a critical component of the pathobiology of insulin resistance and obesity through its negative regulation of insulin signaling. Here we show that an integral component of the insulin signaling cascade, the p85 regulatory subunit of PI3K, is a novel positive regulator of JNK activity independently of its role as a component of the PI3K heterodimer. Furthermore, the p85 regulatory subunit is required for the activation of JNK by both insulin and stimuli that induce ER stress (obesity and chemical agents). Thus, in addition to its traditional positive functions in the PI3K pathway, p85 acts as a negative regulator of insulin signaling as PI3K activity via a JNK-mediated negative feedback loop (Fig. 8A).
Obesity is a pathophysiologic state where there is enhanced cytokine production and increased ER stress, both conditions which strongly activate JNK in the liver and other tissues (37). When mice lacking p85 in the liver (L-Pik3r1KO) were challenged with an HFD, they became obese but remained insulin sensitive. L-Pik3r1KO mice exhibited diminished hepatic JNK activation and enhanced Akt activation even in the face of massive obesity. The mechanism of this effect was further characterized in Pik3r1−/− fibroblasts, where it was shown that p85 is required for full JNK activation in response to ER stress. This regulation of the ER stress response by p85 may be a dominant mechanism by which p85 attenuates the insulin signal in states of obesity-related insulin resistance, as suggested by the finding that insulin is a 10- to 20-fold weaker JNK agonist than stronger inducers of ER stress such as tunicamycin. Thus, p85 may regulate insulin sensitivity in vivo through multilayered positive regulation of JNK.
The p85 regulatory subunit also regulates the activation of JNK by insulin in nonpathophysiologic situations. Insulin has been known to activate JNK in cell lines (32, 52), and in this study we found that JNK is also activated by insulin in mouse tissues in vivo. Furthermore, p85α is an important positive regulator of this process, since L-Pik3r1KO mice exhibit a 75% decrease in JNK activation. The mechanism of this regulation occurs through the positive action of p85 on the small GTPase cdc42, which activates MKK4, a kinase that lies directly upstream of JNK. Indeed, L-Pik3r1KO mice also had a threefold decrease in insulin-stimulated cdc42 activity and MKK4 phosphorylation. In addition, the expression of constitutively active forms of cdc42 or MKK4 increased JNK activity twofold, while dominant negative cdc42 decreased JNK activation by 50%. We cannot exclude the possibility that MKK7, which is similar in structure to MKK4 and also activates JNK (15), may also contribute to the effects of p85, since no good antibodies to activated forms of MKK7 exist.
While the exact mechanism by which p85α activates cdc42 and JNK needs further study, the present work has revealed several interesting aspects of this regulation. First, the activation of cdc42 and JNK by insulin occurs independently of PI3K activity, since the PI3K inhibitor LY294002 does not alter the magnitude of insulin-mediated JNK activation. Consistent with our findings, two other studies with different cell lines have found that p85 can potentiate small GTPases like cdc42 even in the presence of wortmannin (26, 56). The strongest evidence in support of a p85-dependent but PI3K-independent mechanism for the activation of cdc42/JNK by insulin comes from the experiments with the ΔiSH2 mutant of p85, which is a dominant negative mutant that cannot bind the catalytic subunit but can activate cdc42/JNK (Fig. 5B and 7B and C). A recent study by Garcia et al. also found that dominant negative forms of p85 that cannot activate PI3K are able to fully activate cdc42 in certain cell lines (15a).
Several lines of evidence indicate that the unique N terminus of p85α is required for activation of the cdc42/JNK pathway by insulin. For instance, the reconstitution of L-Pik3r1KO livers with p85α (which has a full-length and complex N terminus) restores the ability of insulin to activate JNK, while reexpression of the shorter p55α and p50α isoforms (which have N termini of only 34 and 6 amino acids, respectively) does not. We also found that deletion of either the SH3 or the BH domain ablated the ability of p85 to activate cdc42 but did not alter its ability to activate PI3K (Fig. 7B and C and reference 5), which suggests that the decreased cdc42 activation is not due to the a general malfunction of p85 but is rather the disruption of specific functions mediated by both the SH3 and BH domains.
The mechanism by which the N terminus of p85 activates cdc42, while still unknown, likely involves specific roles for each of the N-terminal domains (Fig. 8B) (35). The BH domain is similar in structure to the Rho GTPase-activating protein domain of the breakpoint cluster region (bcr) protein (35) and can bind activated cdc42 but has no intrinsic GTPase-activating protein activity because it lacks certain conserved residues in the switch domains (12). Thus, to promote cdc42 activation, the N-terminal domains of p85 might facilitate the formation of a complex with a cdc42 guanine nucleotide exchange factor, thereby providing a positive feedback loop for further activation of cdc42 protein and, ultimately, JNK (5). Interestingly, Hill et al. (17) reported that while wild-type p85 can activate JNK, deletions in either the N terminus or SH2 domain rendered p85 ineffective in activating JNK.
Lastly, while our data strongly implicate the cdc42/JNK pathway as the major arbiter of p85's negative effects, we cannot rule out the contribution of other negative effectors. For instance, we have recently demonstrated that p85 positively regulates PTEN activity, such that mice with a liver-specific KO of Pik3r1 exhibit enhanced PI3K signaling due to decreased turnover of the lipid second messenger phosphatidylinositol-3,4,5-triphosphate (47). It is unknown whether the regulation of PTEN by p85 occurs through the cdc42/JNK pathway or through some other unidentified pathway.
Taken together, our data demonstrate a unique role for the p85α regulatory subunit in insulin signaling and the physiologic regulation of glucose homeostasis. Although p85 is an essential part of the PI3K heterodimer, it also plays a novel role in regulating a cdc42/MKK4/JNK pathway that suppresses insulin action in both lean and obese mice. These mechanisms not only provide a level of internal negative feedback on this critical node (45) in insulin and growth factor signaling but also allow cross talk between the PI3K signaling pathway and the stress or inflammatory response, thus creating an important connection that could have a broad impact on the basic understanding of cell growth and metabolism. This powerful link between p85α and JNK activation might also represent an exciting new opportunity for therapeutic intervention against type 2 diabetes.
Supplementary Material
Acknowledgments
We greatly appreciate the technical assistance of Laureen Mazzola, Will Wisdom, and Michael Rourk.
This work was supported by National Institutes of Health grants DK33201 and DK55545, Joslin Diabetes and Endocrinology Research Center grant DK34834 (to C.R.K.), and grants GM41890 and CA089021 to L.C.C. C.M.T. acknowledges support from the American Diabetes Association Medical Scholars Award and a Medical Scientist Training Program scholarship (Harvard Medical School). J.L. acknowledges support from an HHMI predoctoral fellowship.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Antonetti, D. A., P. Algenstaedt, and C. R. Kahn. 1996. Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunit of phosphatidylinositol 3-kinase in muscle and brain. Mol. Cell. Biol. 16:2195-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backer, J. M., M. G. Myers, Jr., S. E. Shoelson, D. J. Chin, X. J. Sun, M. Miralpeix, P. Hu, B. Margolis, E. Y. Skolnik, J. Schlessinger, and M. F. White. 1992. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11:3469-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay, G. K., J. G. Yu, J. Ofrecio, and J. M. Olefsky. 2005. Increased p85/55/50 expression and decreased phosphatidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54:2351-2359. [DOI] [PubMed] [Google Scholar]
- 4.Baud, V., Z. G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 13:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeton, C. A., P. Das, M. D. Waterfield, and P. R. Shepherd. 1999. The SH3 and BH domains of the p85α adapter subunit play a critical role in regulating class Ia phosphoinositide 3-kinase function. Mol. Cell. Biol. Res. Commun. 1:153-157. [DOI] [PubMed] [Google Scholar]
- 6.Bertagnolo, V., F. Brugnoli, M. Marchisio, C. Celeghini, C. Carini, and S. Capitani. 2004. Association of PI 3-K with tyrosine phosphorylated Vav is essential for its activity in neutrophil-like maturation of myeloid cells. Cell Signal. 16:423-433. [DOI] [PubMed] [Google Scholar]
- 7.Block, G. D., J. Locker, W. C. Bowen, B. E. Petersen, S. Katyal, S. C. Strom, T. Riley, T. A. Howard, and G. K. Michalopoulos. 1996. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J. Cell Biol. 132:1133-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brachmann, S. M., C. M. Yballe, M. Innocenti, J. A. Deane, D. A. Fruman, S. M. Thomas, and L. C. Cantley. 2005. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol. Cell. Biol. 25:2593-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8:153-158. [DOI] [PubMed] [Google Scholar]
- 11.Dhand, R., K. Hara, I. Hiles, B. Bax, I. Gout, G. Panayotou, M. J. Fry, K. Yonezawa, M. Kasuga, and M. D. Waterfield. 1994. PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J. 13:511-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidyk, N. J., and R. A. Cerione. 2002. Understanding the catalytic mechanism of GTPase-activating proteins: demonstration of the importance of switch domain stabilization in the stimulation of GTP hydrolysis. Biochemistry 41:15644-15653. [DOI] [PubMed] [Google Scholar]
- 13.Fruman, D. A., L. C. Cantley, and C. L. Carpenter. 1996. Structural organization and alternative splicing of the murine phosphoinositide 3-kinase p85α gene. Genomics 37:113-121. [DOI] [PubMed] [Google Scholar]
- 14.Fruman, D. A., F. Mauvais-Jarvis, D. A. Pollard, C. M. Yballe, D. Brazil, R. T. Bronson, C. R. Kahn, and L. C. Cantley. 2000. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85α. Nat. Genet. 26:379-382. [DOI] [PubMed] [Google Scholar]
- 15.Gallo, K. A., and G. L. Johnson. 2002. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell. Biol. 3:663-672. [DOI] [PubMed] [Google Scholar]
- 15a.Garcia, Z., V. Silio, M. Marques, I. Cortes, A. Kumar, C. Hernandez, A. I. Checa, A. Serrano, and A. C. Carrera. 2006. A P13K activity-independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 25:4740-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, K. M., Y. Huang, S. C. Yip, J. Yu, J. E. Segall, and J. M. Backer. 2001. N-terminal domains of the class Ia phosphoinositide 3-kinase regulatory subunit play a role in cytoskeletal but not mitogenic signaling. J. Biol. Chem. 276:16374-16378. [DOI] [PubMed] [Google Scholar]
- 18.Hirashima, Y., K. Tsuruzoe, S. Kodama, M. Igata, T. Toyonaga, K. Ueki, C. R. Kahn, and E. Araki. 2003. Insulin down-regulates insulin receptor substrate-2 expression through the phosphatidylinositol 3-kinase/Akt pathway. J. Endocrinol. 179:253-266. [DOI] [PubMed] [Google Scholar]
- 19.Hirosumi, J., G. Tuncman, L. Chang, C. Z. Gorgun, K. T. Uysal, K. Maeda, M. Karin, and G. S. Hotamisligil. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333-336. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil, G. S., P. Peraldi, A. Budavari, R. Ellis, M. F. White, and B. M. Spiegelman. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271:665-668. [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted.
- 22.Hu, F. B., J. E. Manson, M. J. Stampfer, G. Colditz, S. Liu, C. G. Solomon, and W. C. Willett. 2001. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345:790-797. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Inukai, K., M. Anai, E. Van Breda, T. Hosaka, H. Katagiri, M. Funaki, Y. Fukushima, T. Ogihara, Y. Yazaki, Kikuchi, Y. Oka, and T. Asano. 1996. A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55PIK Is generated by alternative splicing of the p85α gene. J. Biol. Chem. 271:5317-5320. [DOI] [PubMed] [Google Scholar]
- 25.Iordanov, M. S., D. Pribnow, J. L. Magun, T. H. Dinh, J. A. Pearson, S. L. Chen, and B. E. Magun. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17:3373-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiménez, C., R. A. Portela, M. Mellado, J. M. Rodriguez-Frade, J. Collard, A. Serrano, A. C. Martinez, J. Avila, and A. C. Carrera. 2000. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol. 151:249-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang, H., H. Schneider, and C. E. Rudd. 2002. Phosphatidylinositol 3-kinase p85 adaptor function in T-cells. Co-stimulation and regulation of cytokine transcription independent of associated p110. J. Biol. Chem. 277:912-921. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn, T. B., P. J. Meberg, M. D. Brown, B. W. Bernstein, L. S. Minamide, J. R. Jensen, K. Okada, E. A. Soda, and J. R. Bamburg. 2000. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J. Neurobiol. 44:126-144. [PubMed] [Google Scholar]
- 29.Luo, J., S. J. Field, J. Y. Lee, J. A. Engelman, and L. C. Cantley. 2005. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J. Cell Biol. 170:455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo, J., J. R. McMullen, C. L. Sobkiw, L. Zhang, A. L. Dorfman, M. C. Sherwood, M. N. Logsdon, J. W. Horner, R. A. Depinho, S. Izumo, and L. C. Cantley. 2005. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol. Cell. Biol. 25:9491-9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauvais-Jarvis, F., K. Ueki, D. A. Fruman, M. F. Hirshman, K. Sakamoto, L. J. Goodyear, M. Iannacone, D. Accili, L. C. Cantley, and C. R. Kahn. 2002. Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J. Clin. Investig. 109:141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, B. S., U. T. Shankavaram, M. J. Horney, A. C. Gore, D. T. Kurtz, and S. A. Rosenzweig. 1996. Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin. Biochemistry 35:8769-8775. [DOI] [PubMed] [Google Scholar]
- 33.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 34.Miyake, K., W. Ogawa, M. Matsumoto, T. Nakamura, H. Sakaue, and M. Kasuga. 2002. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J. Clin. Investig. 110:1483-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokdad, A. H., E. S. Ford, B. A. Bowman, W. H. Dietz, F. Vinicor, V. S. Bales, and J. S. Marks. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. J. Am. Med. Assoc. 289:76-79. [DOI] [PubMed] [Google Scholar]
- 36.Musacchio, A., L. C. Cantley, and S. C. Harrison. 1996. Crystal structure of the breakpoint cluster region-homology domain from phosphoinositide 3-kinase p85α subunit. Proc. Natl. Acad. Sci. USA 93:14373-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okkenhaug, K., and B. Vanhaesebroeck. 2001. New responsibilities for the PI3K regulatory subunit p85α. Sci. STKE 2001:PE1. doi: 10.1126/stke.2001.65.pe1. [DOI] [PubMed] [Google Scholar]
- 38.Ozcan, U., Q. Cao, E. Yilmaz, A. H. Lee, N. N. Iwakoshi, E. Ozdelen, G. Tuncman, C. Gorgun, L. H. Glimcher, and G. S. Hotamisligil. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457-461. [DOI] [PubMed] [Google Scholar]
- 39.Pirola, L., A. M. Johnston, and E. Van Obberghen. 2004. Modulation of insulin action. Diabetologia 47:170-184. [DOI] [PubMed] [Google Scholar]
- 40.Postic, C., and M. A. Magnuson. 2000. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26:149-150. [DOI] [PubMed] [Google Scholar]
- 41.Reaven, G. M. 2005. Why syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 1:9-14. [DOI] [PubMed] [Google Scholar]
- 42.Shaw, M., P. Cohen, and D. R. Alessi. 1998. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem. J. 336(Pt. 1):241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha, R., G. Fisch, B. Teague, W. V. Tamborlane, B. Banyas, K. Allen, M. Savoye, V. Rieger, S. Taksali, G. Barbetta, R. S. Sherwin, and S. Caprio. 2002. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 346:802-810. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan, S., M. Ohsugi, Z. Liu, S. Fatrai, E. Bernal-Mizrachi, and M. A. Permutt. 2005. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3beta in mouse insulinoma cells. Diabetes 54:968-975. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi, C. M., B. Emanuelli, and C. R. Kahn. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell. Biol. 7:85-96. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi, C. M., T. Kondo, M. Sajan, J. Luo, R. Bronson, T. Asano, R. Farese, L. C. Cantley, and C. R. Kahn. 2006. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metab. 3:343-353. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi, C. M., T. T. Tran, T. Kondo, J. Luo, K. Ueki, L. C. Cantley, and C. R. Kahn. 2006. Phosphoinositide 3-kinase regulatory subunit p85α suppresses insulin action via positive regulation of PTEN. Proc. Natl. Acad. Sci. USA 103:12093-12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi, C. M., K. Ueki, and R. Kahn. 2005. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J. Clin. Investig. 115:718-727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Terauchi, Y., J. Matsui, J. Kamon, T. Yamauchi, N. Kubota, K. Komeda, S. Aizawa, Y. Akanuma, M. Tomita, and T. Kadowaki. 2004. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85α phosphoinositide 3-kinase. Diabetes 53:2261-2270. [DOI] [PubMed] [Google Scholar]
- 50.Tolias, K. F., L. C. Cantley, and C. L. Carpenter. 1995. Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 270:17656-17659. [DOI] [PubMed] [Google Scholar]
- 51.Ueki, K., P. Algenstaedt, F. Mauvais-Jarvis, and C. R. Kahn. 2000. Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85α regulatory subunit. Mol. Cell. Biol. 20:8035-8046.11027274 [Google Scholar]
- 52.Ueki, K., D. A. Fruman, C. M. Yballe, M. Fassaur, J. Klein, T. Asano, L. C. Cantley, and C. R. Kahn. 2003. Positive and negative roles of p85α and p85β regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 278:48453-48466. [DOI] [PubMed] [Google Scholar]
- 53.Ueki, K., C. M. Yballe, S. M. Brachmann, D. Vicent, J. M. Watt, C. R. Kahn, and L. C. Cantley. 2002. Increased insulin sensitivity in mice lacking p85β subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 99:419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Usui, I., T. Imamura, J. Huang, H. Satoh, and J. M. Olefsky. 2003. Cdc42 is a Rho GTPase family member that can mediate insulin signaling to glucose transport in 3T3-L1 adipocytes. J. Biol. Chem. 278:13765-13774. [DOI] [PubMed] [Google Scholar]
- 55.Virkamäki, A., K. Ueki, and C. R. Kahn. 1999. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Investig. 103:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng, Y., S. Bagrodia, and R. A. Cerione. 1994. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 269:18727-18730. [PubMed] [Google Scholar]
- 57.Zhu, T., E. L. Goh, D. LeRoith, and P. E. Lobie. 1998. Growth hormone stimulates the formation of a multiprotein signaling complex involving p130(Cas) and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J. Biol. Chem. 273:33864-33875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.