Abstract
As a subunit of a ubiquitin ligase, Skp2 is implicated in facilitating cell cycle progression via degradation of various protein targets. We report here that Skp2 is rapidly degraded following cellular stimulation by the cytokine transforming growth factor β (TGF-β) and that this degradation stabilizes the cell cycle arrest protein p27. The Skp2 degradation is mediated by Cdh1-anaphase-promoting complex (APC), as shown by depletion of Cdh1 with small interfering RNA, and by reconstitution of ubiquitylation reactions in a purified system. Blockage of Skp2 degradation greatly reduces TGF-β-induced cell cycle arrest, as does expression of a nondegradable Skp2 mutant. Furthermore, we demonstrate that TGF-β-induced Skp2 degradation is mediated by the Smad cascade. The degradation of Skp2 stabilizes p27, thereby ensuring TGF-β-induced cell cycle arrest. These results identify a novel mechanism for tumor suppression by TGF-β and explain why dysfunction of APC in the TGF-β pathway in responsive cells is associated with cancer.
Transforming growth factor β (TGF-β) is a pluripotent cytokine that regulates a variety of biological effects, including cell growth inhibition, differentiation, matrix production, and apoptosis (24). Loss of its regulatory function has been implicated in enhancing oncogenic growth (8, 35). Transduction of intracellular TGF-β signals from the cell surface to the nucleus is accomplished by the ordered association of type II and type I receptors and a cascade of intracellular signal transducing proteins, called Smads (14). Binding of ligands to the type II receptor results in phosphorylation of the GS domain of the type I receptor, leading to phosphorylation of Smad2 or Smad3 (R-Smads) on the carboxy-terminal SSXS residues (2, 49). Upon phosphorylation, Smad2 or Smad3 forms oligomers with Smad4 (Co-Smad), translocates to the nucleus, and regulates gene transcription, usually through additional coordination with coactivators such as p300/CBP, cosuppressors such as c-Ski/SnoN, or other transcription factors such as AP-1 (49). Currently, an important area of research in TGF-β focuses on how ubiquitin-dependent proteolysis modulates TGF-β signaling. The significance of the ubiquitin-proteasome system (UPS) in TGF-β signaling is reflected by two aspects. First, after each step of signaling, phosphorylated signaling components are removed via proteolysis. Second, proteins suppressing TGF-β-induced transactivation are degraded. These notions are based on recent proteomic studies suggesting that proteolytic regulation is more complex than previously believed (6). Thus, a thorough investigation of the UPS in modulating TGF-β signaling could provide novel insights into the mechanism of TGF-β function.
UPS modulates TGF-β signaling through targeting TGF-β receptor, various Smad proteins, accumulated nuclear proteins, and certain transcriptional cofactors such as SnoN/Ski (7). Smad proteins in both unstimulated cells and TGF-β-stimulated cells are degraded by proteolysis. Smad ubiquitin regulatory factors (Smurfs), ubiquitin ligases containing C2-WW-HECT domains, have been implicated in the destruction of Smads and phosphorylated TGF-β receptors through an interaction between their WW domains and PPXY motifs present on the substrates. Smad1 and Smad5 are degraded by Smurf1 (55), whereas activated TGF-β receptors are degraded by Smurf2 in association with Smad7 (11). Destruction of nuclear accumulated Smad1 and Smad2 is also mediated by Smurf2 (17, 53). Recently, the SCF complex and ectodermin have also been demonstrated to govern Smad4 degradation (10). Besides the proteolytic regulation of Smads and receptors, protein degradation is also required for transcriptional initiation. SnoN and Ski, two corepressors of transcription, are rapidly removed upon stimulation with TGF-β in order to initiate TGF-β-induced transactivation. In particular, degradation of SnoN has been reported to require the involvement of anaphase-promoting complex/cyclosome (APC/C), whereas destruction of Ski is suggested to be through the SCF (Skp1-Cul1-F-box) complex (21, 38, 43).
Our recent studies systematically measuring protein profiles in response to TGF-β stimulation show that several proteins are significantly altered in a rapid manner that suggests protein degradation (unpublished data). Among these proteins, the most intriguing protein whose levels were altered is Skp2. Skp2, an F-box protein, is the substrate-recognition subunit of the SCF ubiquitin ligase complex that has been implicated in targeting various proteins for degradation. Dysfunction of Skp2 has been detected in various types of cancer (36). The major role of Skp2 is to target p27 for degradation (4, 41). Both transcriptional and posttranslational changes are thought to underlie the cell cycle-dependent oscillation of Skp2 levels (3, 46-48). The SCF complex has been suggested to be the E3 ligase ubiquitylating and degrading Skp2 in G1/G0 quiescent cells, thereby leading to a high level of p27 (48). During the cell cycle, Skp2 protein levels drop in late mitosis and G1 and accumulate once cells progress to S phase (3). It is now known that ubiquitin-dependent Skp2 turnover in G1 is governed by APC/C (3, 47), where degradation of Skp2 is required to orchestrate p27 levels, therefore preventing unscheduled entry into S phase.
We have identified a novel mechanism for TGF-β-induced tumor suppression. Among numerous TGF-β-responsive genes, cyclin-dependent kinase (CDK) inhibitors are key in facilitating TGF-β-induced growth inhibition. Major attention has been focused on the activation of transcription mediated by activated Smads, resulting in induction of CDK inhibitors. Nevertheless, the mechanism of maintaining the expression of CDK inhibitors and thereby acquiring more sustained growth inhibition has been less well investigated. We show here that Cdh1-APC targets Skp2SCF for degradation in response to TGF-β, resulting in an increased half-life of p27. Accumulation of p27 induced by TGF-β contributes to inhibition of Cdk2/cyclin E, a major driver for the G1/S transition, and leads to cell cycle arrest. Impairment of APC is frequently detected in various human cancers (27, 45, 54), and our results further explain why a dysfunction of APC in the TGF-β pathway is associated with cancer.
MATERIALS AND METHODS
Plasmids and constructs.
Skp2 was engineered by PCR using the primers 5′-AAAATCGATATGCACAGGAAGCACCTCAG-3′ and 5′-TTGGCGCGCCTAGACAACTGGGCTTTTGCAGTGTC-3′ and then cloned into pCS2-HA (where HA is hemagglutinin), a mammalian expression vector. Skp2Δdb (Skp2 with a deletion of the D box) was generated by deleting amino acids 1 to 11 using a PCR-based approach. The primers used for constructing this mutant were the following: 5′-AAAAATCGATATGCTGAGTAGCAACGTTGCCACC-3′ and 5′-TTGGCGCGCCTAGACAACTGGGCTTTTGCAGTGTC-3′. pREX-IRES-GFP (where IRES is internal ribosome entry site and GFP is green fluorescent protein) and pREX-IRES-CD2 were gifts from Xuedong Liu (University of Colorado-Boulder). pREX-HA-Skp2-IRES-CD2 and pREX-HA-Skp2Δdb-IRES-CD2 were generated by PCR using the following primers: 5′-AAAAGTCGACCCACAGGAAGCACCTCCAGGAG-3′ and 5′-TTGCGGCCGCTCATAGACAACTGGGCTTTTG-3′. pREX-HA-SnoN-IRES-GFP and pREX-HA-SnoNΔdb-IRES-GFP were constructed by PCR using primers described previously (43).
Antibodies.
Western blot analysis was performed using the anti-Skp2 (Santa Cruz), anti-p27 (Santa Cruz), anti-Cdh1 (Oncogen), anti-Smad2 (Santa Cruz), anti-Smad3 (Santa Cruz), anti-Smad4 (Santa Cruz), anti-PCNA (Santa Cruz), anti-HA (Santa Cruz), anti-Myc (Santa Cruz), anti-V5 (Santa Cruz), anti-Flag (Sigma), anti-SnoN (Cascade BioScience), and horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibody (Santa Cruz) with an ECL detection kit (Amersham). Semiquantification of data was performed using NIH Image.
Construction of Cdh1-siRNA stable expressing cell lines.
The following three constructs were engineered: (i) pSUPER-Cdh1-N (amino acids 266 to 286), (ii) pSUPER-Cdh1-C (amino acids 566 to 586), and (iii) pSuper-Control (firefly luciferase small interfering RNA [siRNA]) (47). siRNA-Cdh1 retrovirus was packaged by transfecting siRNA-Cdh1 constructs into Phoenix cells using Lipofectamine 2000 (Invitrogen). Mv1Lu cells were infected with the virus, and positive clones were selected in the presence of puromycin (4 μM)-containing medium.
In vitro protein degradation assay and ubiquitylation assay.
35S-labeled HA-tagged human Skp2 protein was synthesized in the TNT expression system (Promega). Approximately 10 ng of in vitro translated Skp2 was added to 20-μl extracts supplemented with degradation cocktail (1.25 mg/ml ubiquitin, 1× energy regeneration, 0.1 mg/ml cycloheximide). Aliquots were removed at different times and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography (43). The method for the Skp2 in vitro ubiquitylation assay was previously described (42).
Coimmunoprecipitation assays for Skp2 and Cdh1.
Mv1Lu cells were cotransfected with expression vectors encoding HA-Skp2, Myc-Cdh1, Flag-Smad3, and V5-Cdc27 using Lipofectamine 2000 (Invitrogen). The transfected cells were preincubated with the proteasome inhibitor MG-132 (50 μM) or vehicle (dimethyl sulfoxide [DMSO]) for 1 h before addition of TGF-β. HA-Skp2 complexes were pulled down by anti-HA matrix (Roche). Interaction among Skp2, Cdh1, Smad3, and Cdc27 was judged by protein immunoblotting with anti-HA, anti-Myc, anti-Flag, and anti-V5 antibodies.
Knock-down of Smad3 and Skp2.
siRNAs were custom synthesized by Dharmaco. The sequence for anti-Smad3 siRNA was 5′-AAUGGUGCGAGAAGGCGGUCAdTdT-3′ (dT is deoxyribosylthymine) (30). The sequence for anti-Skp2 siRNA was 5′-AUUCAGCUGGGUGAUGGUCUCdTdT-3′ (3). Cells were transfected with ∼0.4 mM specific siRNAs or randomized cocktails of double-stranded RNA as a control, with the use of the Oligofectamine reagent (Gibco/Life Technologies). At 24 h posttransfection, cells were transfected again with the same siRNA preparations to ensure efficient depletion.
RNA extraction and reverse transcription-PCR (RT-PCR).
The SV Total RNA Isolation System (Promega) was used to extract RNA. The following primers were used: for β-Actin (291 bp), 5′-CCACACTGTGCCCATCTACG-3′ (5′ primer) and 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (3′ primer); for Skp2 (122 bp), 5′-CTGTCTCAGTGTTCCAAGTTGCA-3′ (5′ primer) and 5′-CAGAACACCCAGAAAGGTTAAGT-3′ (3′ primer).
Growth inhibition assay.
For the growth inhibition assay, 5 × 103 Mv1Lu cells were incubated with various concentrations of TGF-β1 for 3 to 4 days. The growth of cells was determined by counting and comparing the results with those for unstimulated cells (38).
RESULTS
TGF-β induces the fast turnover of Skp2 resulting in p27 accumulation.
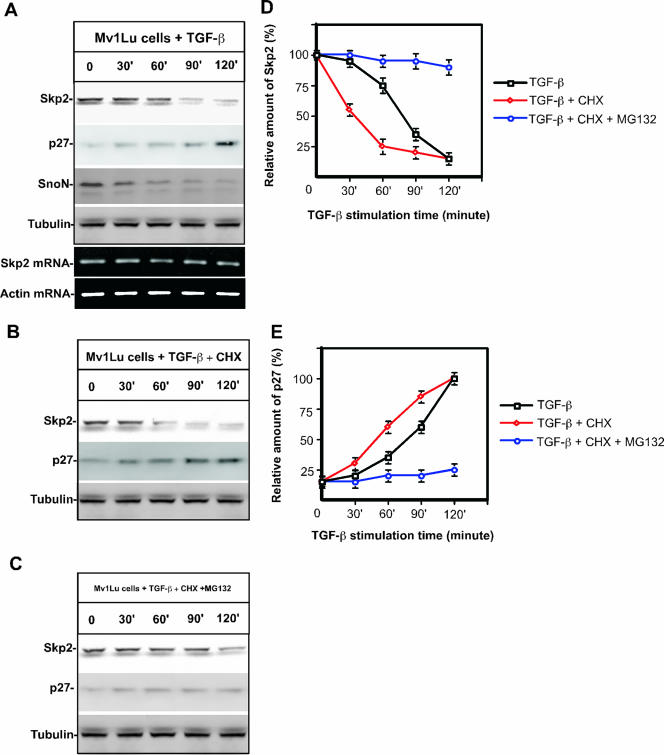
The TGF-β-induced physiological response is tightly regulated by the UPS (7). To thoroughly probe the biological significance of TGF-β-induced protein turnover, we systematically monitored the alteration in the protein profiles of a number of proteins (data not shown). This work has resulted in identification of several previously unreported proteins that are degraded in response to TGF-β, including Skp2 (Fig. 1A). Our result shows that Skp2 is degraded in response to TGF-β stimulation, resulting in the accumulation of p27 (Fig. 1A, D, and E). The half-life of Skp2 in response to TGF-β signaling is approximately 60 min (Fig. 1A and B), whereas the half-life of SnoN turnover in response to TGF-β demonstrated previously is about 30 min (Fig. 1A) (43). Furthermore, Skp2 degradation is blocked by incubating cells with a 50 μM concentration of proteasome inhibitor, MG-132 (Fig. 1C and D). To assess the effect of TGF-β on Skp2 and p27 beyond 2 h, we have monitored the alteration of protein levels for Skp2 and p27 for 18 h after stimulation with TGF-β. As shown in Fig. S1A in the supplemental material, Skp2 is dramatically degraded in the first 90 min, and low levels of Skp2 were maintained up to 18 h, while the protein levels of p27 were gradually accumulated after 90 min in response to TGF-β stimulation. A similar result was repeated for BaF3 cells (see Fig. S1B in the supplemental material). To exclude the possibility that the observed change in Skp2 protein levels is due to altered transcriptional regulation, we measured Skp2 mRNA levels after stimulation with TGF-β by RT-PCR. As shown in Fig. 1A, Skp2 mRNA is constant, suggesting that the drop in Skp2 protein levels is due to protein degradation. It appears that TGF-β-induced Skp2 degradation stabilizes p27, thereby facilitating cell cycle arrest at the G1/S transition by inhibiting activity of Cdk2/cyclin E (12, 32).
FIG. 1.
Skp2 is rapidly degraded in response to TGF-β signaling. (A) Skp2 protein levels drop drastically in response to TGF-β stimulation. The half-life of Skp2 in response to TGF-β signaling is about 60 min while the half-life for SnoN is about 30 min. p27 protein levels gradually increase while Skp2 protein levels decrease. Mink lung epithelia cells (Mv1Lu) were treated with TGF-β (100 pM). Protein levels were measured by immunoblotting. Equal amounts of total protein were subjected to immunoblot analysis, as evidenced by the equal concentration of tubulin. Skp2 and actin (control) mRNA levels were monitored by RT-PCR analysis. (B) Skp2 protein chase analysis in response to TGF-β stimulation. Mv1Lu cells were treated with 20 μM cycloheximide. Skp2 protein turnover was measured by immunoblotting. (C) Skp2 degradation is blocked by the proteasomal inhibitor MG-132 (50 μM). (D) Quantification of Skp2 protein levels in response to TGF-β in the presence or absence of cycloheximide or MG-132. (E) Quantification of p27 protein levels in response to TGF-β in the presence or absence of cycloheximide or MG-132. CHX, cycloheximide.
Cdh1 silencing attenuates TGF-β-induced Skp2 degradation and abrogates TGF-β-induced growth inhibition.
Cdh1-APC is activated by TGF-β-induced signaling to facilitate the destruction of SnoN, a transcriptional corepressor, which permits transactivation of TGF-β-responsive genes (43). Cdh1-APC has been implicated in targeting Skp2 for ubiquitylation and degradation during the G1/S transition (3, 47). Given our suggestion that Skp2 is degraded in response to TGF-β signaling (Fig. 1) and that Cdh1-APC targets Skp2 for degradation during cell cycle control, we asked whether TGF-β-induced Skp2 degradation is mediated by Cdh1-APC. To test this hypothesis, we depleted Cdh1 by RNA interference in Mv1Lu cells and then examined Skp2 protein levels.
To silence Cdh1 in Mv1Lu cells, we designed several targeting oligonucleotides using the Oligoengine program. Our previous Cdh1 knock-down experiment using transient transfection of duplex oligonucleotides has revealed that Cdh1-N and Cdh1-C provide the best targeting efficiency in HeLa cells (47). Cdh1 is very conserved in vertebrates, sharing over 98% identity among human, mouse, and frog (42). To engineer Cdh1 siRNA targeting constructs for establishing stable cell lines, we confirmed that the DNA sequences of the regions targeted by the siRNA oligonucleotides (Cdh1-N and Cdh1-C) between human and mink are identical by RT-PCR and DNA sequencing. We cloned the oligonucleotides into a pSuper vector (Invitrogen). Using retroviral infection, we established two Cdh1 siRNA stable clones and an siRNA control clone (firefly luciferase siRNA). As shown in Fig. 2A, Cdh1 levels were reduced by 90% in these clones. To verify whether Cdh1-APC mediates Skp2 degradation in response to TGF-β signaling, we measured Skp2 protein levels in response to TGF-β stimulation by immunoblotting in both Mv1Lu wild-type and Cdh1 siRNA cells. As shown in Fig. 2B and C, silencing of Cdh1 significantly blocks Skp2 degradation while Skp2 protein levels drop in response to TGF-β in wild-type cells as well as in siRNA control cells. Furthermore, we demonstrated that Cdh1 protein levels are not affected by the stimulation with TGF-β (see Fig. S2A in the supplemental material). In addition, the degradation of Skp2 is rescued in Cdh1-depleted cells by supplementation of purified recombinant Cdh1 (see Fig. S2C in the supplemental material) (42). Taken together, these data suggest that Cdh1-APC is required to mediate TGF-β-induced Skp2 degradation.
FIG. 2.
Cdh1 is necessary and sufficient for downregulation of Skp2 in response to TGF-β stimulation. (A) Creation of Cdh1 siRNA stable clones. Based on Cdh1 knock-down results using transient transfection of siRNA duplex, two oligonucleotides (N terminus, residues 338 to 358; C terminus, residues 566 to 586) were chosen for construction of Cdh1 siRNA stable cells. Cell extracts (100 μg) were blotted with Cdh1 and PCNA (control) antibodies. Cdh1 levels in clone 3 (using an N-terminal oligonucleotide) and clone 6 (using a C-terminal oligonucleotide) were reduced approximately 90%, while Cdh1 levels did not fall in clone 1 (a control clone generated using firefly luciferase siRNA). (B) Depletion of Cdh1 blocks Skp2 degradation in response to TGF-β stimulation. (C) Quantification of Skp2 and p27 protein levels in response to TGF-β signaling in wild-type and Cdh1 siRNA cells. (D) Knock-down of Cdh1 antagonizes TGF-β-induced G1 arrest. Wild-type and Cdh1 siRNA cells were treated with TGF-β. The cell cycle profile was measured by fluorescence-activated cell sorting analysis 20 h after the stimulation with TGF-β. (E) Depletion of Cdh1 blocks the TGF-β-induced cell growth inhibition. Wild-type and Cdh1 siRNA cells were treated with TGF-β at different concentrations. Cell numbers were counted 72 h after the stimulation with TGF-β. WT, wild type.
To confirm the physiological role of Cdh1-APC in the TGF-β signaling pathway, we tested the effect of Cdh1 depletion on the physiological response of cells to TGF-β stimulation. As shown in Fig. 2D, depletion of Cdh1 abrogated G1 arrest induced by TGF-β signaling. Moreover, knockdown of Cdh1 significantly antagonized TGF-β-induced growth inhibition (Fig. 2E). These data confirm the role of Cdh1-APC in mediating TGF-β signaling by targeting Skp2 as well as SnoN for degradation.
Cdh1-APC targets Skp2 for degradation in response to TGF-β stimulation.
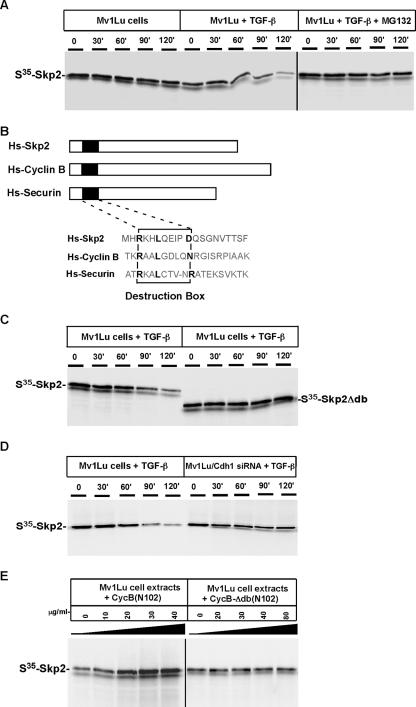
To directly test whether Skp2 is a substrate for Cdh1-APC, we analyzed Skp2 degradation in a Mv1Lu cell-free system, as previously described (43). 35S-labeled in vitro translated Skp2 was added to an extract prepared from Mv1Lu cells exposed to TGF-β. Aliquots were removed and resolved by SDS-PAGE, and the presence of Skp2 was detected by autoradiography. As shown in Fig. 3A, Skp2 was degraded in extracts from cells previously stimulated by exposure to TGF-β. Under this condition, Skp2 has a half-life of approximately 60 min, which is consistent with the data presented in Fig. 1A. Skp2 degradation in TGF-β-stimulated extracts was blocked by MG 132 (Fig. 3A).
FIG. 3.
Degradation of Skp2 induced by TGF-β is mediated by Cdh1-APC. (A) Skp2 degradation is induced by TGF-β stimulation. Mv1Lu cells were treated (or not treated) with 100 pM TGF-β for 60 min, and extracts were prepared. 35S-labeled in vitro translated Skp2 was added to the extracts and supplemented with protein degradation cocktail. Aliquots were removed at the indicated times and resolved by SDS-PAGE. Skp2 degradation was measured by autoradiography. (B) Alignment of Skp2 with known APC substrates, cyclin B, and securin. Similar to other substrates of APC, Skp2 contains a conserved destruction box [RXXLXXX(N/D)] at its NH2 terminus. (C) Destruction box is required to mediate TGF-β-induced Skp2 degradation. Wild-type and mutant Skp2 proteins lacking the destruction box were incubated in TGF-β-stimulated extracts. (D) Depletion of Cdh1 blocks Skp2 degradation in TGF-β-stimulated extracts. Skp2 was incubated with the extracts prepared from wild-type Mv1Lu or Cdh1 siRNA cells. (E) Degradation of Skp2 is blocked by the addition of a destruction box-containing peptide. NH2-terminal fragments (amino acids 1 to 102) of Xenopus cyclin B or a control fragment lacking the destruction box [CycB-Δdb (N102)] were added to the TGF-β-stimulated extract and 35S-labeled in vitro translated Skp2 at the indicated concentrations. After incubation for 90 min at room temperature, samples were analyzed by SDS-PAGE and autoradiography.
Most APC substrates are ubiquitylated in a destruction box [RXXLXXXX(D/N/E)]-dependent manner (51). To ask whether the putative D box at the NH2 terminus of Skp2 regulates degradation, we deleted the D box (Fig. 3B). In Fig. 3C, D-box mutant Skp2 was stable in TGF-β-stimulated extracts, showing that the D box is required for Skp2 degradation. Furthermore, Skp2 was stabilized in TGF-β-stimulated extracts prepared from Cdh1 siRNA cells (Fig. 3D). Skp2 was also stabilized in the presence of excess NH2-terminal fragments of cyclin B containing a D box, serving as a competitive inhibitor for APC-dependent degradation (Fig. 3E) (25). In summary, these results demonstrate that Skp2 is an APC substrate and that recognition of Skp2 by Cdh1-APC is mediated by the D box at its NH2 terminus.
Recapitulation of Skp2 ubiquitylation in vivo and in a purified system.
To elucidate the biochemical mechanism by which Skp2 is ubiquitylated in response to TGF-β signaling, we initially measured the activation of APC in response to stimulation with TGF-β.
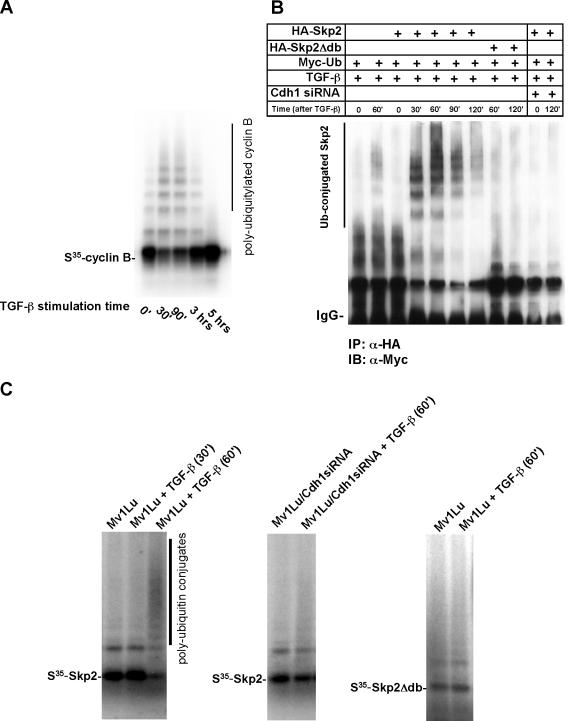
Using an in vitro ubiquitylation assay, we assayed the activation of APC in response to TGF-β stimulation, as previously described (43). Based on extracts prepared from cells stimulated with TGF-β at various times, APC was purified using anti-Cdc27 antibodies coupled with protein A beads. The purified APC was then mixed with E1, Ubcx, and other supplements in an ubiquitylation assay to target a known substrate, cyclin B. As shown in Fig. 4A, the capacity of APC activated by TGF-β to catalyze ubiquitylation of cyclin B is dramatically increased in response to TGF-β stimulation, with gradual quenching occurring 2 h after stimulation. This result demonstrates that Cdh1-APC is activated approximately 30 min after TGF-β stimulation. The potency of APC activation stimulated by TGF-β lasts for about 2 h.
FIG. 4.
Skp2 is ubiquitylated in vivo and in a purified system. (A) Activity of APC is enhanced following stimulation by TGF-β. Mv1Lu cells were stimulated with TGF-β and harvested at different times as indicated. APC was subsequently purified from the cell lysate using anti-Cdc27 antibody coupled to protein A beads and was subjected to ubiquitylation mixture for a ubiquitylation assay. 35S-labeled in vitro translated cyclin B was used as a putative substrate for APC activity analysis. Polyubiquitin conjugates of cyclin B were measured by autoradiography. (B) Skp2 is polyubiquitylated in vivo. Myc-tagged ubiquitin and HA-tagged wild-type Skp2 or mutant Skp2 lacking the destruction box were cotransfected into wild-type or Cdh1 siRNA Mv1Lu cells. Cells were treated with TGF-β and harvested at the indicated times. Skp2 complex was immuno-purified by anti-HA antibody. The polyubiquitin-conjugated Skp2 was detected by immunoblotting using anti-Myc antibody. (C) Recapitulation of Skp2 ubiquitylation in a purified system. Mv1Lu cells were stimulated with TGF-β and harvested at different times as indicated. APC was purified from the cell lysate using anti-Cdc27 antibody coupled to protein A beads and was subjected to a ubiquitylation mixture for the ubiquitylation assay. Polyubiquitin conjugates of Skp2 were measured by autoradiography. Depletion of Cdh1 attenuates TGF-β induced Skp2 ubiquitylation. In addition, disruption of the destruction box blocks TGF-β-induced Skp2 ubiquitylation. IP, immunoprecipitation; IB, immunoblotting; α, anti.
To recapitulate TGF-β-induced Skp2 ubiquitylation and further examine the components that contribute to this reaction, we next performed Skp2 ubiquitylation assays both in cultured Mv1Lu cells and in a purified system. To demonstrate that TGF-β could induce polyubiquitin conjugation of Skp2, we cotransfected HA-tagged wild-type Skp2 and D-box mutated Skp2 with Myc-tagged ubiquitin into either wild-type Mv1Lu or Cdh1 siRNA Mv1Lu cells. Cells were subsequently stimulated with TGF-β and harvested at various times; the Skp2 immunocomplex was pulled down using an anti-HA antibody coupled with protein A/G beads. Polyubiquitylated Skp2 was then detected by immunoblotting using an anti-Myc antibody. As shown in Fig. 4B, Skp2 is ubiquitylated 30 min after TGF-β stimulation, with polyubiquitin conjugation peaking in 60 min. Mutation of the D box in Skp2 blocked the formation of a polyubiquitin chain on Skp2. Furthermore, depletion of Cdh1 by siRNA abolished attachment of ubiquitin to Skp2 induced by TGF-β. These results were further validated by an in vivo Skp2 ubiquitylation assay based on cell extraction under denaturing conditions (see Fig. S2B in the supplemental material) (18, 23). Thus, in vivo, ubiquitylation of Skp2 is catalyzed by Cdh1-APC upon TGF-β stimulation.
We also examined which E2 associates with the purified APC by incubating different E2s, including Ubcx, Ubc4, Ubc5, UbcH10, and Cdc34 with purified APC and 35S-labeled Skp2 as well as supplements (data not shown). The E2 Ubc5 most potently catalyzed TGF-β-induced Skp2 ubiquitylation in association with APC. To recapitulate Skp2 ubiquitylation, we purified activated APC by using anti-Cdc27 antibody coupled with protein A beads from extracts prepared from cells exposed to TGF-β for 60 min. At this point, APC activity stimulated by TGF-β is highest (Fig. 4A). As described previously, we added the purified APC together with E2 (Ubc5) and 35S-labeled Skp2 and subsequently observed Skp2 ubiquitylation (Fig. 4C) (42). However, deletion of the Skp2 D box or depletion of Cdh1 by siRNA in Mv1Lu cells failed to recapitulate Skp2 ubiquitylation in the presence of Ubc5 and APC (Fig. 4C). In summary, these data confirm that TGF-β-induced Skp2 degradation occurs via its ubiquitylation, catalyzed by Cdh1-APC, and the D box in Skp2 is critical for its ubiquitylation.
TGF-β-induced Skp2 degradation is mediated by the Smad cascade.
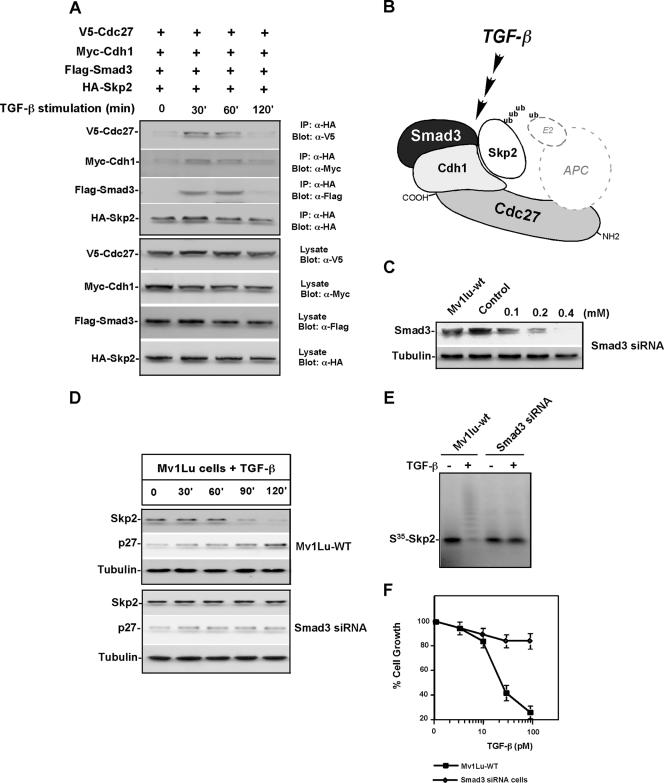
Cdh1 binds directly to APC substrates through recognition of a degron in the substrate (29, 43). Smad3 is a major signal transducer that interacts with various downstream proteins in the TGF-β pathway (33, 34, 40). To ask whether Cdh1 interacts with Skp2 and whether this interaction depends on the presence of Smad3 and/or TGF-β stimulation in Mv1Lu cells, we examined proteins associated with Cdh1 in the presence of TGF-β activation. We first cotransfected Mv1Lu cells with a combination of expression vectors encoding differently tagged versions of Cdh1, Smad3, Skp2, and Cdc27 (a core subunit of the APC). The transfected cells were incubated with the proteasome inhibitor, MG-132 (50 μM), or vehicle (DMSO) for 1 h before TGF-β addition. After 60 min, the interaction among Cdh1, Smad3, Skp2, and Cdc27 was examined by immunoprecipitation coupled with immunoblotting. In the absence of TGF-β stimulation, the association of Cdh1 with other transfected proteins, including Skp2, Smad3, and Cdc27, was barely detectable. In contrast, the formation of a transient quaternary complex of Cdh1-Skp2-Smad3-Cdc27 was detected 30 min after TGF-β stimulation when Cdh1, Skp2, Smad3, and Cdc27 were expressed at similar levels (Fig. 5A and B). Interaction of endogenous Cdh1, Cdc27, Skp2, and Smad3 was further detected in TGF-β-stimulated Mv1Lu cells (see Fig. S3A and B in the supplemental material). In addition, a moderate abundance of Smad2 and Smad4 was also measured in Cdc27 immunoprecipitation complex in the presence of TGF-β signaling, suggesting that TGF-β-induced Skp2 degradation is mediated by the Smad cascade (see Fig. S3C and D in the supplemental material).
FIG. 5.
TGF-β-induced Skp2 degradation is mediated by a Smad cascade. (A) Skp2 forms a complex with Cdh1 in response to TGF-β signaling. Mv1Lu cells were cotransfected with expression vectors encoding HA-Skp2, Myc-Cdh1, Flag-Smad3, and V5-Cdc27. Transfected cells were preincubated with the proteasomal inhibitor, MG-132, or vehicle (DMSO) for 1 h before stimulation with TGF-β. HA-Skp2 complexes were recovered using an anti-HA matrix. The interaction among Skp2, Cdh1, Smad3, and Cdc27 was analyzed by protein immunoblotting with anti-HA, anti-Myc, anti-Flag, and anti-V5. Expression levels of HA-Skp2, Myc-Cdh1, Flag-Smad3, and V5-Cdc27 were determined by immunoblotting. (B) Diagram for TGF-β-induced quaternary complex of Skp2, Cdh1, Smad3, and Cdc27. (C) Depletion of Smad3 by transfection of Smad3 siRNA oligonucleotides. (D) Depletion of Smad3 protein attenuated TGF-β-induced Skp2 degradation. (E) Knock-down of Smad3 blocked TGF-β-induced Skp2 ubiquitylation. (F) Depletion of Smad3 antagonized TGF-β-induced growth inhibition. IP, immunoprecipitation.
To validate the role of Smads in facilitating Skp2 degradation in response to TGF-β signaling, we performed a Smad3 depletion experiment using transfection of Smad3 siRNA oligonucleotides (Fig. 5C) (30). As shown in Fig. 5D, knock-down of Smad3 significantly attenuated TGF-β-induced Skp2 degradation, while Skp2 was degraded in response to TGF-β stimulation in wild-type Mv1Lu cells. Furthermore, TGF-β-induced Skp2 ubiquitylation was blocked in response to Smad3 depletion (Fig. 5E). In addition, depletion of Smad3 attenuated TGF-β-induced growth inhibition (Fig. 5F), and knock-down of Smad3 significantly reduced TGF-β-induced interaction of Cdh1 and Skp2 (see Fig. S4A in the supplemental material).
Expression of a nondegradable Skp2 antagonizes TGF-β-induced growth inhibition.
Stabilization of Skp2 should promote the proteolysis of p27 and therefore antagonize the TGF-β response. To test this hypothesis, wild-type Skp2 or Skp2 with a deletion of the D box was introduced stably into Mv1Lu cells by retroviral infection (Fig. 6A) (19). Subsequently, the pools of infected cells were measured for their ability to respond to TGF-β-induced growth inhibition. As shown in Fig. 6B (panel a), stably expressed Skp2 was degraded in response to TGF-β stimulation while D-box-mutated Skp2 was quite stable (Fig. 6B, panel b). To know if the expressed nondegradable Skp2 is functional for p27 degradation and whether nondegradable Skp2 affects APC activity, we monitored the alteration of endogenous protein levels of Skp2 and p27 in response to TGF-β stimulation in the presence of nondegradable Skp2. As shown Fig. 6B (panel b), expression of nondegradable Skp2 did not affect Skp2 (endogenous) degradation induced by TGF-β stimulation, while TGF-β-induced p27 accumulation was blocked by expression of nondegradable Skp2. Furthermore, we tested the potential effect of nondegradable Skp2 on p27 transcripts in response to TGF-β stimulation. As shown in Fig. S4B in the supplemental material, no change in p27 transcripts was detected in response to overexpression of wild-type or nondegradable Skp2 using RT-PCR. These results suggest that nondegradable Skp2 is functional for p27 degradation and that it does not alter APC activity and p27 transcription.
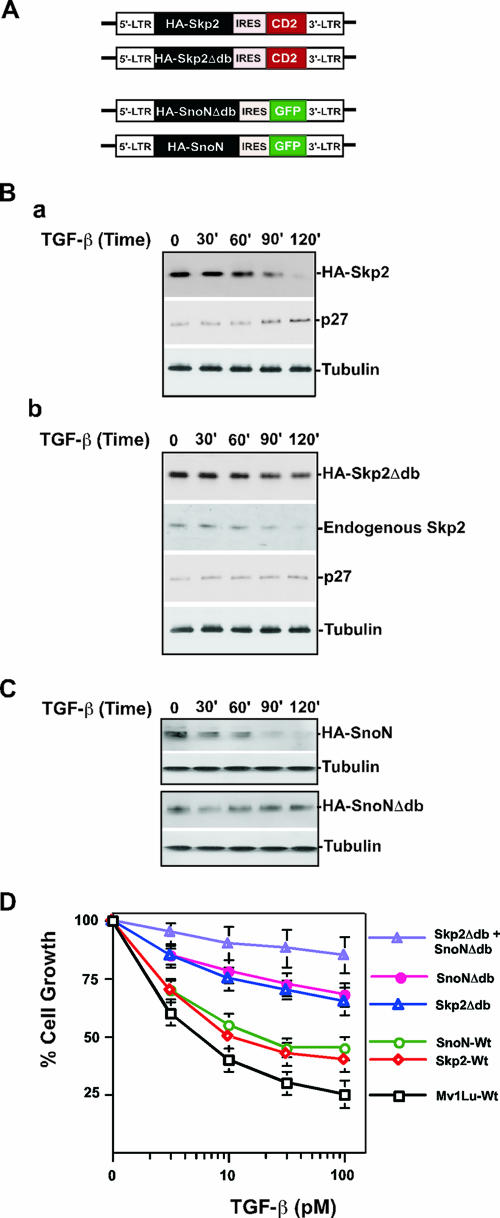
FIG. 6.
Expression of nondegradable Skp2 and SnoN antagonizes TGF-β signaling. (A) Diagram of retroviral vector expressing nondegradable Skp2 and SnoN. (B) Protein levels of stably expressed wild-type Skp2 (a) and nondegradable Skp2 (b) in response to TGF-β signaling. (C) Protein levels of stably expressed wild-type SnoN and nondegradable SnoN in response to TGF-β signaling. (D) Growth inhibition assay. Mv1Lu cells stably expressing Skp2, Skp2Δdb, SnoN, and SnoNΔdb, respectively, and coexpressing Skp2Δdb and SnoNΔdb were incubated for 4 days with various concentrations of TGF-β1 as indicated. The growth of cells was quantified by cell counting and compared with the growth of unstimulated cells. WT, wild type.
As indicated in Fig. 6D, the growth inhibition analysis showed that expression of wild-type Skp2 only moderately blocked the ability of cells to undergo TGF-β-induced cell cycle arrest. The reason for this may be because the wild-type Skp2 was unstable in response to TGF-β stimulation and thus failed to accumulate (Fig. 6B, panel a). In contrast, D-box-mutated Skp2 markedly attenuated TGF-β-induced growth inhibition (Fig. 6D). Therefore, Skp2 stabilization impairs the TGF-β-induced growth inhibitory effect. These results support the hypothesis that degradation of Skp2 induced by TGF-β signaling helps to maintain p27 protein levels, which results in Cdk2/cyclin E inhibition.
Previous studies have shown that TGF-β targets SnoN for degradation mediated by Cdh1-APC (38, 43). Removal of SnoN by proteolysis is required for initiation of TGF-β transcriptional regulation. Degradation of SnoN and Skp2 occurs at different time points after TGF-β stimulation (Fig. 1A and D and 3A; see Fig. S1C in the supplemental material), suggesting that the substrate specificity for Cdh1-APC catalyzing SnoN and Skp2 ubiquitylation is under temporal control. To test whether stabilization of both SnoN and Skp2 could more potently antagonize TGF-β signaling, we engineered Mv1Lu cells that stably express both nondegradable SnoN and Skp2 (Fig. 6A). As shown in Fig. 6C, D-box-mutated SnoN is stable in response to TGF-β stimulation. Expression of D-box-mutated SnoN abolished approximately 60% of TGF-β-induced growth inhibition. In contrast, simultaneous expression of both nondegradable SnoN and Skp2 abrogated approximately 80% of TGF-β-induced growth inhibition, which is more than the expression of either SnoNΔdb or the Skp2Δdb mutant alone (Fig. 6D). Together, these results support the finding that Cdh1-APC regulates TGF-β signaling. Removal of SnoN by Cdh1-APC results in initiation of TGF-β-induced transactivation, while degradation of Skp2 mediated by Cdh1-APC leads to accumulation of p27, thereby inhibiting Cdk2/cyclin E (Fig. 7) (40, 43, 52).
FIG. 7.

Model for the role of Cdh1-APC in TGF-β-induced degradation of Skp2 and SnoN. APC is activated by TGF-β stimulation. Activated APC then targets Skp2 for degradation, thereby stabilizing p27. In addition, activated APC is required for SnoN degradation, hence permitting transactivation of TGF-β-responsive tumor suppressor genes. Thus, both induction of tumor suppressors and stabilization of p27 are necessary to achieve cell cycle arrest.
DISCUSSION
A new mechanism for growth inhibition by TGF-β.
The TGF-β signaling pathway has attracted substantial interest for its duality in tumor suppression and metastasis based on tumor type and stage (9, 26, 35). In this work, we have identified a new mechanism for TGF-β-induced growth inhibition via destruction of Skp2, an F-box protein, that, if overexpressed, could lead to upregulated cell cycle progression and poor prognosis in terms of cancer treatment (36).
The effect of decreased Skp2 expression in response to TGF-β signaling is to achieve a longer half-life for p27, a major cell cycle inhibitor. Thus, induction of certain tumor suppressors such as p21 and p15 together with stabilization of p27 orchestrates a concerted effect of TGF-β signaling, thereby providing an efficient TGF-β-induced inhibitory response. We have here addressed the mechanism governing TGF-β-induced Skp2 degradation, which involves Cdh1-APC activation by TGF-β. Cdh1-APC-facilitated Skp2 degradation in response to TGF-β signaling is mediated by the Smad cascade.
In normal cells, Skp2 protein levels oscillate during the cell cycle, falling in mitosis and early G1 and rising after entry into S phase. It is now known that Skp2 is an important ubiquitin E3 ligase whose accumulation is required for ubiquitylation and degradation of p27, which in turn liberates Cdk2/cyclin E, a major driver for the G1/S transition. Abnormal expression of Skp2 is often observed in various human cancers, where p27 expression is usually inversely correlated with Skp2 accumulation (36). TGF-β signaling orchestrates the activity of Cdk2/cyclin E via an Skp2-p27 cascade with TGF-β activating the UPS to degrade Skp2, thereby slowing the turnover of p27 and prolonging the inhibition of Cdk2/cyclin E. Previously, we demonstrated that the E3 ligase, APC, is activated in response to TGF-β stimulation (43). Our other studies have shown that in the normal cell cycle Cdh1-APC targets Skp2 for degradation, thereby preventing premature entry into S phase (3, 47). Here, we have identified Cdh1-APC as the E3 ligase that is activated by TGF-β-induced signaling for degradation of Skp2.
Skp2 as a novel substrate for APC in TGF-β signaling.
Other than facilitating chromatid separation during mitosis, the critical role of APC has also been shown in the control of G1 progression, genomic integrity, and signal transduction, as well as multiple developmental events (3, 13, 37, 39, 43, 47). Malfunction of APC has been implicated in a variety of carcinomas including breast cancer, colorectal cancer, and hematopoietic cancer (27, 44, 45, 54). Given the pivotal role of TGF-β signaling in tumorigenesis and the notion that Cdh1-APC mediates TGF-β-induced cell cycle inhibition, abrogation of APC function in the TGF-β signaling pathway could be one means of initiating cancer formation. Identification of Skp2, a novel substrate for Cdh1-APC in the TGF-β signaling pathway, has further filled the gap in our understanding of TGF-β-mediated physiology. To fully address the mechanism by which Skp2 is degraded by Cdh1-APC in the presence of TGF-β, we utilized our previously developed cell-free-based degradation assay in association with an in vitro ubiquitylation assay (42, 43).
APC is known to target its substrates for degradation through recognizing molecular motifs including the D box, KEN box, and the A box (28). Using degradation and ubiquitylation assays, we demonstrated that mutation of the D box at the NH2 terminus of Skp2 significantly blocked TGF-β-induced Skp2 ubiquitylation and destruction. This D-box-dependent Skp2 degradation is supported further by the competitive inhibition of Skp2 degradation with an NH2-terminal fragment of cyclin B that contains the D box. These data implicate Cdh1-APC as the E3 ligase that mediates Skp2 degradation in TGF-β-governed physiological responses. To verify whether Cdh1 is the substrate-specific activator directing APC for targeting Skp2, we also engineered an siRNA Cdh1 cell line. Depletion of Cdh1 attenuates the fast turnover of Skp2 induced by TGF-β. To complement these genetic analyses, we examined the complex formation among Smad3, Cdh1, Skp2, and APC in the presence of TGF-β signaling. The coimmunoprecipitation analysis shows that Smad3-Cdh1-Skp2-Cdc27 forms a stable quaternary complex in response to TGF-β stimulation. Therefore, both genetic and biochemical experiments support the hypothesis that Cdh1 is the substrate-specific activator mediating TGF-β signaling for activation of APC in TGF-β-induced Skp2 degradation.
Management of substrate degradation after TGF-β stimulation.
The elevated rate of degradation via APC in response to TGF-β stimulation is not limited to Skp2. Previous studies suggested that acceleration of SnoN degradation is mediated by APC (38, 43). We do not know at present how APC is coordinated to catalyze these two substrates in the TGF-β signaling pathway. We have noticed that APC activity is potently increased in the first 2 h in response to TGF-β stimulation. In the presence of TGF-β, the half-life of Skp2 is approximately 1 h, whereas the half-life of SnoN is about 30 min. Therefore, we propose that the mechanism of substrate specificity for APC targeting Skp2 and SnoN in the TGF-β pathway is potentially through temporal management.
Smad3 plays a critical role in mediating TGF-β signaling to activate transcription and to transduce the TGF-β signal to other effectors (35). Detection of Smad3 as a component of a Cdh1 complex suggests that Smad3 is critical for connecting the TGF-β cascade to the ubiquitin machinery for Skp2 degradation. This result fits well with our current understanding that Cdh1 recognizes the substrate (Skp2) and brings it to the APC and Smad3. This conclusion has been further confirmed by a Smad3 knock-down experiment with depletion of Smad3 abolishing TGF-β-induced Skp2 ubiquitylation and degradation (Fig. 5D and E). Although we have demonstrated that Smad3 mediates TGF-β signaling to activate APC for Skp2 degradation, the precise mechanism by which Cdh1-APC is activated by Smad3-TGF-β still remains unknown. Possibly, TGF-β targets Cdc27 and therefore increases the affinity of Cdh1 for APC or Skp2 or both. It may also have some effects on polyubiquitylation and deubiquitylation in addition to its effects on binding. In accordance with the findings of previous studies (38, 43), Smad3 may serve to coordinate APC-mediated time-dependent degradation of SnoN and Skp2. These ubiquitylation events ensure the activation of TGF-β-induced gene expression and stabilization of p27, both of which are required for inhibition of cell growth as shown in Fig. 6D and 7. To better understand the mechanism by which APC is activated by TGF-β signaling, further experiments should be carried out to examine if Cdh1 or components of the APC become posttranslationally modified by TGF-β signaling.
Integration of our findings with the current paradigm.
Regulation of p27 expression is thought to be important for achieving TGF-β-induced growth inhibition (31). Previous reports have demonstrated that the up-regulation of p15 by TGF-β results in the release of p27 from CDK4/6 complexes with a corresponding increase of p27 in complex with Cdk2-cyclin E (12, 32). A reasonable synthesis of our findings and this established model could be that p27, a short-lived regulatory protein, is consistently targeted by Skp2/SCF for turnover, no matter whether p27 is associated with CDK4/6, bound to Cdk2-cyclin E, or exists singularly. Thus, a mechanism to stabilize p27 through TGF-β is needed to effectuate complete and successful redistribution of p27 from CDK4/6 to CDK2-cyclin E to achieve arrest of cell growth. Observations of changes in p27 protein levels induced by TGF-β have been variable, dependent upon cell types, where p27 dramatically accumulated in some cases while alteration of p27 was subtle in other cases. In addition, between different cell types that exhibited increases in p27 following TGF-β induction, the kinetics of TGF-β-induced p27 accumulation also differed, with a significant increase of p27 seen from 1 h to 72 h (22, 50).
We have found that APC is activated by TGF-β, with its activation being maintained for approximately 3 h in response to TGF-β stimulation. Given that complete arrest of the cell cycle by TGF-β usually requires about 20 h, there is still a gap between the TGF-β-induced transient activation of APC and timing of TGF-β-induced cell cycle arrest. This inconsistency could be explained by the theory that TGF-β may induce two mechanisms to deplete Skp2, thereby achieving elevation of p27 protein levels, including Skp2 degradation and inhibition of Skp2 protein translation. If this is the case, then 3 h of activation of APC could be enough to remove Skp2 and maintain a long-term increase of p27 if no novel Skp2 is synthesized. Accumulation of p27 starts once Skp2 is degraded by TGF-β-activated APC, and high levels of p27 are maintained until cell growth is completely arrested. This interpretation is supported by the measurement of bromodeoxyuridine (BrdU) incorporation during the 24-h time course in response to TGF-β stimulation (see Fig. S5A to C in the supplemental material), where the number of BrdU-positive cells gradually drops in response to TGF-β treatment. The effect of Cdh1 depletion on BrdU incorporation was detected in a window of time soon after stimulation with TGF-β (see Fig. S5B in the supplemental material). Although our work suggests the important role of Cdh1-APC in facilitating TGF-β-induced growth inhibition via Skp2 degradation and therefore stabilizing p27, more careful work is needed to address the timely coordination between the Cdh1-APC-Skp2-p27 cascade and the TGF-β-induced physiological response.
Cancer and dysfunction of the APC in the TGF-β signaling pathway.
Malfunction of the ubiquitin-proteasome pathway can result in carcinogenesis by disrupting the balance between oncoproteins and tumor suppressor proteins (1, 7, 36). Abnormal expression of Skp2 is often detected in various types of human cancer including breast cancer, hematopoietic tumors, and colorectal cancer (15, 16, 36). Conversely, decreased p27 protein levels are usually measured in these cancer types (5, 16, 20). We have demonstrated that the ubiquitin protein ligase, Cdh1-APC, targets Skp2 for degradation in the TGF-β pathway, contributing to a new paradigm of how a ubiquitylation cascade could modulate the normal physiological effect of TGF-β and the potential dysfunction of this mechanism on tumorigenesis. Our current study shows that Cdh1-APC is recruited by TGF-β signaling, leading to cell growth inhibition through a Cdh1-APC-Skp2-p27-Cdk2/cyclin E axis. These results provide a potential mechanism for the induction of cancer formation caused by failure in Skp2 and p27 regulation. Given the notion that the APC is impaired in a variety of human cancers (27, 44, 45, 54), our studies have opened a new avenue for exploring the mechanism of tumorigenesis induced by dysfunction of the APC in the TGF-β signaling pathway.
Supplementary Material
Acknowledgments
We thank X. Liu, W. Malcolm, W. Kaelin, A. Weissman, and D. Zhang for cDNA clones. We are grateful to R. Wood and J. Brodsky and the members of our laboratory for critical reading of the manuscript. We thank D. Finley, R. Deshaies and J. Massague for discussions. This project was initiated from Marc W. Kirschner's laboratory.
This work is supported by NIH grants CA115943 and GM070681. Y.W. is a scholar of the American Cancer Society and V Cancer Research Foundation.
Footnotes
Published ahead of print on 5 February 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ang, X. L., and J. W. Harper. 2004. Interwoven ubiquitination oscillators and control of cell cycle transitions. Sci. STKE 2004:e31. [DOI] [PubMed] [Google Scholar]
- 2.Attisano, L., J. L. Wrana, E. Montalvo, and J. Massague. 1996. Activation of signalling by the activin receptor complex. Mol. Cell. Biol. 16:1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF (Skp2-Cks1) ubiquitin ligase by the APC/C (Cdh1) ubiquitin ligase. Nature 428:190-193. [DOI] [PubMed] [Google Scholar]
- 4.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 5.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3:227-230. [DOI] [PubMed] [Google Scholar]
- 6.Colland, F., X. Jacq, V. Trouplin, C. Mougin, C. Groizeleau, A. Hamburger, A. Meil, J. Wojcik, P. Legrain, and J. M. Gauthier. 2004. Functional proteomics mapping of a human signaling pathway. Genome Res. 14:1324-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datto, M., and X. F. Wang. 2005. Ubiquitin-mediated degradation: a mechanism for fine-tuning TGF-beta signaling. Cell 121:2-4. [DOI] [PubMed] [Google Scholar]
- 8.Derynck, R., R. J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29:117-129. [DOI] [PubMed] [Google Scholar]
- 9.Derynck, R., and Y. E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577-584. [DOI] [PubMed] [Google Scholar]
- 10.Dupont, S., L. Zacchigna, M. Cordenonsi, S. Soligo, M. Adorno, M. Rugge, and S. Piccolo. 2005. Germ-layer specification and control of cell growth by ectodermin, a Smad4 ubiquitin ligase. Cell 121:87-99. [DOI] [PubMed] [Google Scholar]
- 11.Kavsak, P., R. K. Rasmussen, C. G. Causing, S. Bonni, H. Zhu, G. H. Thomsen, and J. L. Wrana. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell 6:1365-1375. [DOI] [PubMed] [Google Scholar]
- 12.Koff, A., M. Ohtsuki, K. Polyak, J. M. Roberts, and J. Massague. 1993. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science 260:536-539. [DOI] [PubMed] [Google Scholar]
- 13.Konishi, Y., J. Stegmuller, T. Matsuda, S. Bonni, and A. Bonni. 2004. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303:1026-1030. [DOI] [PubMed] [Google Scholar]
- 14.Kretzschmar, M., and J. Massague. 1998. SMADs: mediators and regulators of TGF-beta signaling. Curr. Opin. Genet. Dev. 8:103-111. [DOI] [PubMed] [Google Scholar]
- 15.Li, J. Q., F. Wu, T. Masaki, A. Kubo, J. Fujita, D. A. Dixon, R. D. Beauchamp, T. Ishida, S. Kuriyama, and K. Imaida. 2004. Correlation of Skp2 with carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. Int. J. Oncol. 25:87-95. [PubMed] [Google Scholar]
- 16.Lim, M. S., A. Adamson, Z. Lin, B. Perez-Ordonez, R. C. Jordan, S. Tripp, S. L. Perkins, and K. S. Elenitoba-Johnson. 2002. Expression of Skp2, a p27 (Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27 (Kip1) and proliferation index. Blood 100:2950-2956. [DOI] [PubMed] [Google Scholar]
- 17.Lin, X., M. Liang, and X. H. Feng. 2000. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J. Biol. Chem. 275:36818-36822. [DOI] [PubMed] [Google Scholar]
- 18.Lisztwan, J., A. Marti, H. Sutterluty, M. Gstaiger, C. Wirbelauer, and W. Krek. 1998. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45 (SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 17:368-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, X., S. N. Constantinescu, Y. Sun, J. S. Bogan, D. Hirsch, R. A. Weinberg, and H. F. Lodish. 2000. Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal. Biochem. 280:20-28. [DOI] [PubMed] [Google Scholar]
- 20.Loda, M., B. Cukor, S. W. Tam, P. Lavin, M. Fiorentino, G. F. Draetta, J. M. Jessup, and M. Pagano. 1997. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3:231-234. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald, M., Y. Wan, W. Wang, E. Roberts, T. H. Cheung, R. Erickson, M. T. Knuesel, and X. Liu. 2004. Control of cell cycle-dependent degradation of c-Ski proto-oncoprotein by Cdc34. Oncogene 23:5643-5653. [DOI] [PubMed] [Google Scholar]
- 22.Mahmud, N., N. Katayama, K. Nishii, T. Sugawara, Y. Komada, H. Mitani, H. Araki, K. Ohishi, M. Watanabe, M. Masuya, M. Nishikawa, N. Minami, H. Ohashi, and H. Shiku. 1999. Possible involvement of bcl-2 in regulation of cell-cycle progression of haemopoietic cells by transforming growth factor-beta1. Br. J. Haematol. 105:470-477. [PubMed] [Google Scholar]
- 23.Marti, A., C. Wirbelauer, M. Scheffner, and W. Krek. 1999. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat. Cell Biol. 1:14-19. [DOI] [PubMed] [Google Scholar]
- 24.Massague, J., and D. Wotton. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarry, T. J., and M. W. Kirschner. 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93:1043-1053. [DOI] [PubMed] [Google Scholar]
- 26.Minn, A. J., G. P. Gupta, P. M. Siegel, P. D. Bos, W. Shu, D. D. Giri, A. Viale, A. B. Olshen, W. L. Gerald, and J. Massague. 2005. Genes that mediate breast cancer metastasis to lung. Nature 436:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, K. H., S. E. Choi, M. Eom, and Y. Kang. 2005. Downregulation of the anaphase-promoting complex (APC)7 in invasive ductal carcinomas of the breast and its clinicopathologic relationships. Breast Cancer Res. 7:R238-R247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9:931-943. [DOI] [PubMed] [Google Scholar]
- 29.Pfleger, C. M., and M. W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655-665. [PMC free article] [PubMed] [Google Scholar]
- 30.Ray, D., Y. Terao, D. Nimbalkar, L. H. Chu, M. Donzelli, T. Tsutsui, X. Zou, A. K. Ghosh, J. Varga, G. F. Draetta, and H. Kiyokawa. 2005. Transforming growth factor β facilitates β-TrCP-mediated degradation of Cdc25A in a Smad3-dependent manner. Mol. Cell. Biol. 25:3338-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynisdottir, I., and J. Massague. 1997. The subcellular locations of p15 (Ink4b) and p27 (Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11:492-503. [DOI] [PubMed] [Google Scholar]
- 32.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, A. B., A. Russo, A. Felici, and K. C. Flanders. 2003. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann. N. Y. Acad. Sci. 995:1-10. [DOI] [PubMed] [Google Scholar]
- 34.Shen, X., P. P. Hu, N. T. Liberati, M. B. Datto, J. P. Frederick, and X. F. Wang. 1998. TGF-beta-induced phosphorylation of Smad3 regulates its interaction with coactivator p300/CREB-binding protein. Mol. Biol. Cell 9:3309-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel, P. M., and J. Massague. 2003. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 3:807-821. [DOI] [PubMed] [Google Scholar]
- 36.Signoretti, S., M. L. Di, A. Richardson, S. Ramaswamy, B. Isaac, M. Rue, F. Monti, M. Loda, and M. Pagano. 2002. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Investig. 110:633-641. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Sigrist, S. J., and C. F. Lehner. 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90:671-681. [DOI] [PubMed] [Google Scholar]
- 38.Stroschein, S. L., S. Bonni, J. L. Wrana, and K. Luo. 2001. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 15:2822-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudo, T., Y. Ota, S. Kotani, M. Nakao, Y. Takami, S. Takeda, and H. Saya. 2001. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 20:6499-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun, Y., X. Liu, E. Ng-Eaton, H. F. Lodish, and R. A. Weinberg. 1999. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor β signaling. Proc. Natl. Acad. Sci. USA 96:12442-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 42.Wan, Y., and M. W. Kirschner. 2001. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc. Natl. Acad. Sci. USA 98:13066-13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan, Y., X. Liu, and M. W. Kirschner. 2001. The anaphase-promoting complex mediates TGF-β signaling by targeting SnoN for destruction. Mol. Cell 8:1027-1039. [DOI] [PubMed] [Google Scholar]
- 44.Wang, C. X., B. C. Fisk, M. Wadehra, H. Su, and J. Braun. 2000. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood 96:259-263. [PubMed] [Google Scholar]
- 45.Wang, Q., C. Moyret-Lalle, F. Couzon, C. Surbiguet-Clippe, J. C. Saurin, T. Lorca, C. Navarro, and A. Puisieux. 2003. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 22:1486-1490. [DOI] [PubMed] [Google Scholar]
- 46.Wang, W., D. Ungermannova, J. Jin, J. W. Harper, and X. Liu. 2004. Negative regulation of SCFSkp2 ubiquitin ligase by TGF-β signaling. Oncogene 23:1064-1075. [DOI] [PubMed] [Google Scholar]
- 47.Wei, W., N. G. Ayad, Y. Wan, G. J. Zhang, M. W. Kirschner, and W. G. Kaelin, Jr. 2004. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428:194-198. [DOI] [PubMed] [Google Scholar]
- 48.Wirbelauer, C., H. Sutterluty, M. Blondel, M. Gstaiger, M. Peter, F. Reymond, and W. Krek. 2000. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 19:5362-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wotton, D., and J. Massague. 2001. Smad transcriptional corepressors in TGF beta family signaling. Curr. Top. Microbiol. Immunol. 254:145-164. [PubMed] [Google Scholar]
- 50.Yue, J., A. Buard, and K. M. Mulder. 1998. Blockade of TGFβ3 up-regulation of p27Kip1 and p21Cip1 by expression of RasN17 in epithelial cells. Oncogene 17:47-55. [DOI] [PubMed] [Google Scholar]
- 51.Zachariae, W., and K. Nasmyth. 1999. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13:2039-2058. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, F., M. Monkkonen, S. Roth, and M. Laiho. 2002. Proteasomal activity modulates TGF-ss signaling in a gene-specific manner. FEBS Lett. 527:58-62. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., C. Chang, D. J. Gehling, A. Hemmati-Brivanlou, and R. Derynck. 2001. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 98:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, N., F. Lai, A. A. Fernald, J. D. Eisenbart, R. Espinosa, P. W. Wang, and M. M. Le Beau. 1998. Human CDC23: cDNA cloning, mapping to 5q31, genomic structure, and evaluation as a candidate tumor suppressor gene in myeloid leukemias. Genomics 53:184-190. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, H., P. Kavsak, S. Abdollah, J. L. Wrana, and G. H. Thomsen. 1999. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400:687-693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.