Abstract
Defects in the yeast cysteine desulfurase Nfs1 cause a severe impairment in the 2-thio modification of uridine of mitochondrial tRNAs (mt-tRNAs) and cytosolic tRNAs (cy-tRNAs). Nfs1 can also provide the sulfur atoms of the iron-sulfur (Fe/S) clusters generated by the mitochondrial and cytosolic Fe/S cluster assembly machineries, termed ISC and CIA, respectively. Therefore, a key question remains as to whether the biosynthesis of Fe/S clusters is a prerequisite for the 2-thio modification of the tRNAs in both of the subcellular compartments of yeast cells. To elucidate this question, we asked whether mitochondrial ISC and/or cytosolic CIA components besides Nfs1 were involved in the 2-thio modification of these tRNAs. We demonstrate here that the three CIA components, Cfd1, Nbp35, and Cia1, are required for the 2-thio modification of cy-tRNAs but not of mt-tRNAs. Interestingly, the mitochondrial scaffold proteins Isu1 and Isu2 are required for the 2-thio modification of the cy-tRNAs but not of the mt-tRNAs, while mitochondrial Nfs1 is required for both 2-thio modifications. These results clearly indicate that the 2-thio modification of cy-tRNAs is Fe/S protein dependent and thus requires both CIA and ISC machineries but that of mt-tRNAs is Fe/S cluster independent and does not require key mitochondrial ISC components except for Nfs1.
The IscS/NifS proteins function as cysteine desulfurases, and similar proteins in numerous organisms have been identified (12, 22). Biochemical analyses of IscS/NifS proteins have revealed their unique desulfuration mechanism: a sulfur atom of the sulfide group of cysteine is eliminated in a pyridoxal phosphate-catalyzed reaction and binds transiently to the active-site cysteine residue of the protein to form an enzyme-bound persulfide and then is transferred as sulfane sulfur to various acceptor proteins (41, 42). One important physiological role of IscS/NifS is the involvement in Fe/S cluster biogenesis by providing the sulfur atoms of the cluster (34, 37, 40). The yeast orthologue of IscS, Nfs1, is located mainly in the mitochondrial matrix (15, 29), but trace amounts of this protein in the nucleus are essential for cell survival (28). In yeast and human cells, the mitochondrial form of Nfs1 (together with other members of the ISC assembly machinery) was shown to be responsible for biogenesis of both mitochondrial and cytosolic Fe/S proteins (4, 6, 15).
The IscS proteins also participate in other physiologically important thio modification reactions, such as thiamine biogenesis and posttranscriptional thio modification of tRNA (13, 14, 18, 19, 31). We found previously that Nfs1 was required for the 2-thio modification of both mitochondrial tRNAs (mt-tRNAs) and cytosolic tRNAs (cy-tRNAs) (26, 30). Another mitochondrial protein, Mtu1, the yeast homologue of the bacterial MnmA, has been shown to be responsible for the 2-thio modification of mt-tRNAUUULys(36). The process of 2-thio modification of the wobble uridine of cy-tRNAs in yeast is poorly understood, except for the finding that the depletion of Nfs1 causes a severe defect in the 2-thio modification of cy-tRNAUUULys2and cy-tRNAUUCGlu3, as well as that of mt-tRNAUUULysand mt-tRNAUUGGln(30).
Bacterial tRNA thio modification has been proposed to occur by two distinct mechanisms; one is Fe/S protein dependent and the other is Fe/S protein independent, although both pathways require IscS as a sulfur donor (20, 24). For the eukaryotic system, it remains unclear whether the mt-tRNA and/or cy-tRNA thio modifications require the participation of any Fe/S proteins.
In eukaryotes, Fe/S proteins are found in mitochondria, the cytosol, and the nucleus. Their maturation requires three complex proteinaceous machineries in mitochondria (termed the ISC assembly and export systems) and in the cytosol (designated the CIA machinery [22]). Several mitochondrial ISC assembly proteins, including Nfs1, Isu1, Isu2, Yfh1, and Yah1, are required for maturation of extramitochondrial Fe/S proteins. They produce a still-unknown compound that is exported to the cytosol by the ABC transporter Atm1 to support biogenesis in the cytosol (15). The CIA machinery contains two cytosolic P-loop nucleoside triphosphatases (NTPases), Cfd1 and Nbp35; the iron-only hydrogenase-like protein, Nar1; and a WD40 protein, Cia1 (22, 23). All of them are essential for cell viability, but their precise functions remain unclear.
To obtain further insights into the molecular mechanisms of 2-thio modification of tRNA in the mitochondria and cytosol of yeast cells, we examined the possible participation of ISC and CIA machinery members other than Nfs1 in these pathways.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Saccharomyces cerevisiae W303-1B (MATα ade2-1 his3-11,15 ura3-1 leu2-3,112 trp1-1 can1-100) was used as the wild-type strain. Gal-NFS1/YN was formerly named YN101 (28), and Gal-NBP35/YN, in which the NBP35 gene was expressed under the GAL1 promoter, was constructed as described previously (28). Other GAL1 promoter-regulatable strains, Gal-ISU1/Δisu2 (7) and Gal-CIA1 (2), were also used. The yeast Tet-promoters Hughes strain (Open Biosystems) of CFD1 (TH_3296, designated TH-CFD1) and that of RLI1 (TH_5586, called TH-RLI1) were also used to control their expression. Growth media and conditions of the GAL1-10-regulatable strains were as described previously (30). In brief, these strains were grown in galactose-containing medium to induce the GAL1 promoter. Then, the medium was changed to yeast extract-peptone-dextrose or yeast extract-peptone-glycerol to repress the promoter and cells were cultivated until growth arrest occurred. To repress the Tet promoter of TH-CFD1 or TH-RLI1 cells, doxycycline (10 μg/ml) was added and cells were further cultivated until growth arrest occurred.
Preparation of tRNA and detection of thio-modified tRNA by the APM-containing gel retardation assay and Northern hybridization.
Yeast cells were suspended with 20 mM Tris-Cl, pH 7.5, with 10 mM MgCl2, and then the total tRNAs were extracted by mixing with an equal volume of phenol and precipitated with 2-propanol. Each unit of optical density at 260 nm (OD260) equal to 0.05 (2 μg) of total tRNA was applied to a 7 M urea gel containing [(N-acryloylamino)-phenyl]mercuric chloride (APM), and the gel retardation assay coupled with hybridization analysis (APM-Northern analysis) was performed with a specific DNA probe against cy-tRNAUUULys2, cy-tRNAUUCGlu3, mt-tRNAUUULys, or mt-tRNAUUGGln, as described previously (30). Another oligonucleotide probe, against cy-tRNAUCUArg(5′-CACTCACGATGGGGGTCGAACCCA-3′), was used as a control. To estimate the proportion of 2-thio-modified tRNAs in each tRNA sample, the radioactive signal intensity was quantitated with a BAS2500 imaging analyzer, followed by calculation with Image Gauge, version 3.45, software (Fuji Photo Film Co. Ltd. and Kohshin Graphic Systems).
In vitro 2-thio modification assay with non-thio-modified cy-tRNAUUULys2prepared from Cfd1-depleted cells and the cytosol fraction of wild-type cells.
Total tRNAs of 1 OD260 unit prepared from Cfd1-depleted cells were incubated with the yeast cytosol fraction (5 μg for lane 2 and 50 μg for lane 4 [see Fig. 2]) prepared from wild-type cells. After incubation for 10 min at 30°C, total tRNAs were purified from the reaction mixture and examined by APM-Northern analysis with the DNA probe for cy-tRNAUUULys2.
FIG. 2.
Unmodified cy-tRNAUUULys2accumulated in Cfd1-depleted cells can be thio modified by Cfd1-replete cytosol in vitro. Total tRNAs prepared from Cfd1-depleted cells (lane 1) were examined for 2-thio modification by the addition of yeast cytosolic fraction prepared from wild-type cells (lane 3). A 10-fold-higher amount of the cytosolic fraction of the wild-type cells is shown in lane 4 (+++). Total tRNAs prepared from the wild-type cells (lane 2) were also analyzed as controls. The 2-thio-modified and unmodified cy-tRNAUUULys2was detected and shown as in Fig. 1.
RESULTS
Cfd1 is involved in the 2-thio modification of cy-tRNAs but not of mt-tRNAs.
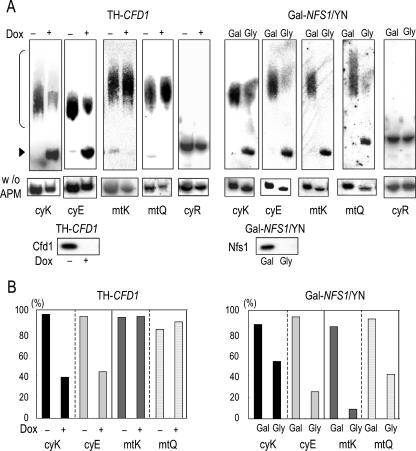
In bacterial 2-thio modification of tRNAUUULys, the sulfur atom delivered from IscS is finally attached to the tRNAUUULysby the function of a P-loop ATPase termed MnmA (10). We therefore examined whether the yeast P-loop NTPase Cfd1 would be involved in the 2-thio modification of cy-tRNAUUULys2and/or cy-tRNAUUCGlu3. To this end, TH-CFD1 cells in which expression of Cfd1 can be strongly decreased by the addition of doxycycline were used (Fig. 1A, bottom panel). Total cellular tRNAs were subjected to polyacrylamide gel electrophoresis containing APM and combined with Northern hybridization for detection of these cy-tRNAs (see Materials and Methods) (Fig. 1A [top panel] and B). The cy-tRNAUUULys2 and cy-tRNAUUCGlu3in TH-CFD1 cells that expressed Cfd1 showed specific mobility retardation in the APM-containing gel due to the strong affinity of the 2-thio group of uridine in tRNAs for the mercuric compound in the gel. In contrast, these cytosolic tRNAs of doxycycline-treated TH-CFD1 cells whose expression of Cfd1 was fully repressed showed no mobility retardation of cy-tRNAUUULys2or of cy-tRNAUUCGlu3in the APM gel (Fig. 1A and B, left panels).
FIG. 1.
Cfd1 is required for 2-thio modification of cy-tRNAs but not of mt-tRNAs. (A) Total tRNAs prepared from TH-CFD1 cells grown without (−) or with (+) doxycycline (Dox) for 24 h (left panels) and from Gal-NFS1/YN cells grown with galactose for 20 h (Gal) or glycerol for 48 h (Gly) (right panels) were used. Isolated tRNAs (0.05 OD260 unit [2 μg]) were loaded onto APM-containing (top panels) and APM-lacking (middle panels) denaturing gels, and after electrophoresis they were blotted to nylon membranes. These blots were hybridized with oligonucleotide probes specific to cy-tRNAUUULys2(cyK), cy-tRNAUUCGlu3(cyE), mt-tRNAUCUArg(cyR), mt-tRNAUUULys(mtK), and mt-tRNAUUGGln(mtQ). Positions of 2-thio-modified tRNAs that exhibited mobility retardation are indicated by a bracket, and positions of non-thio-modified tRNAs are indicated by an arrowhead. The protein levels of Cfd1 and Nfs1 were examined at time points similar to those described above by immunoblotting (bottom panels). (B) The relative proportions of 2-thio-modified tRNA in each tRNA were estimated. After the APM-containing gel electrophoresis shown in panel A, radioactivity bound to either the 2-thio-modified form or the unmodified form was measured as described in Materials and Methods. Values are represented as percentages of the amount of 2-thio-modified tRNA and the total amount of each tRNA (i.e., both the 2-thio modified and unmodified tRNA).
Another cy-tRNA, cy-tRNAUCUArg, was analyzed as a negative control; the cy-tRNAUCUArgpossesses the same methoxycarbonylmethyl modification at the fifth position in the uridine as cy-tRNAUUULys2and cy-tRNAUUCGlu3but is not 2-thio modified. In the total tRNAs prepared from Cfd1-depleted cells, cy-tRNAUCUArgwas detected at exactly the same position in the APM-containing gel as in the total tRNAs prepared from the Cfd1-replete cells. We also carried out Northern analysis following electrophoresis without APM to compare the amounts of each cy-tRNA in the total tRNA samples prepared from the Cfd1-depleted cells with those from the Cfd1-replete cells (Fig. 1A, middle panel). In the tRNAs prepared from Cfd1-depleted cells, only slightly reduced amounts of cy-tRNAs were detected in similar positions of the gel compared with those detected in the tRNAs prepared from Cfd1-replete cells.
These results indicate that Cfd1 depletion causes a severe impairment of 2-thio modification of cy-tRNAUUULys2and cy-tRNAUUCGlu3. It seemed to be comparable to or even more severe than the defect observed in Nfs1-depleted cells (Fig. 1A and B, right panels), suggesting a closer involvement of Cfd1 in the 2-thio modification of these cy-tRNAs than of Nfs1. On the other hand, we observed a specific mobility retardation of mt-tRNAUUULysand of mt-tRNAUUGGlnin the Cfd1-depleted yeast cells described above (Fig. 1A and B, right panels), indicating that Cfd1 is not required for the 2-thio modification of mt-tRNAs. In contrast, as reported previously (30), the 2-thio modification of mt-tRNAUUULysand mt-tRNAUUGGlnwas largely affected by Nfs1 depletion (Fig. 1A, right panels). Thus, Cfd1 is specifically required for 2-thio modification of cy-tRNAs, whereas Nfs1 is essential for 2-thio modification of both mt-tRNA and cy-tRNA molecules.
Next, we examined whether non-2-thio-modified cy-tRNAUUULys2prepared from Cfd1-depleted cells could be 2-thio modified in the presence of the Cfd1-replete cytosol in vitro (Fig. 2). Using the purified total tRNAs that contained a considerable amount of non-thio-modified cy-tRNAUUULys2(Fig. 2, lane 1), we observed a significant conversion of the unmodified cy-tRNAUUULys2to the modified form upon incubation with a concentrated cytosol fraction prepared from Cfd1-replete cells (Fig. 2, lane 3). Such conversion was not observed with the Cfd1-depleted cytosol (data not shown). The concentrated cytosol fraction of the Cfd1-replete cells itself contained undetectable levels of thio-modified and unmodified cy-tRNAUUULys2, probably due to its instability during the conventional preparation of the cytosolic protein fraction (Fig. 2, lane 4). These results indicate that the non-2-thio-modified form of cy-tRNAUUULys2that accumulated in the Cfd1-depleted cells is competent to be 2-thio modified by the addition of the Cfd1-replete cytosol.
The CIA members Nbp35 and Cia1 are required for 2-thio modification of cy-tRNAs, while the essential cytosolic Fe/S protein Rli1 is not.
We next analyzed whether other CIA components may participate in 2-thio modification of cy-tRNAs. Nbp35, a second cytosolic P-loop NTPase protein that can bind an Fe/S cluster, is essential for viability and genetically interacts with Cfd1 and Nar1 (3, 8). Nbp35 was depleted by incubation of regulatable Gal-NBP35/YN cells with glycerol-containing medium (Fig. 3A and B; see also Fig. S1 in the supplemental material). Compared to Nbp35-expressing cells, a considerable amount of cy-tRNAUUULys2and cy-tRNAUUCGlu3in Nbp35-depleted cells was found to be unmodified. However, as was seen with Cfd1-depleted cells, Nbp35 depletion did not cause any impairment of 2-thio modification of mt-tRNAUUULysor mt-tRNAUUGGln, indicating that Nbp35 is required for the 2-thio modification of cy-tRNAs but not of mt-tRNAs.
FIG. 3.
Both Nbp35 and Cia1 are required for the 2-thio modification of cy-tRNAUUULys2, while the essential cytosolic Fe/S protein Rli1 is not. (A) The 2-thio modifications in Gal-NBP35/YN cells grown with galactose for 16 h (Gal) or with glycerol for 60 h (Gly) were examined by APM-Northern analysis with specific probes against cy-tRNAUUULys2(cyK) and mt-tRNAUUULys(mtK). An oligonucleotide probe against mt-tRNAUCUArg(cyR) was also used. The bottom panel represents the protein level of Nbp35 in the corresponding cells analyzed by immunological methods. The relative proportions of 2-thio-modified tRNAs are depicted in panel B as described in the legend to Fig. 1B. (C) The 2-thio modifications of cy-tRNAUUULys2in Gal-CIA1 cells grown with galactose for 16 h (Gal) and with glycerol for 60 h (Gly) and in TH-RLI1 cells grown without doxycycline (Dox) for 23 h (−) and with Dox for 50 h (+) were monitored as in panel A. The protein levels of Cia1 and Rli1 (bottom panels) and the relative proportions of the 2-thio-modified tRNAs (D) are also shown.
Cia1 is another CIA machinery component that is involved late in the maturation pathway, as it is required for biogenesis of true target Fe/S proteins such as Rli1 and Leu1 but is dispensable for Fe/S cluster incorporation into the two Fe/S cluster-binding proteins of the CIA machinery, i.e., Nbp35 and Nar1 (2). When the 2-thio modification of cy-tRNAUUULys2was examined with the technique described above, we found a considerable impairment of 2-thio modification in cy-tRNAUUULys2of Gal-CIA1 cells whose growth was arrested due to depletion of Cia1 (Fig. 3C and D), indicating that Cia1 is also required for the 2-thio modification of cy-tRNAUUULys2.
The requirement of three CIA components for the 2-thio modification of cy-tRNAs suggests that a cytosolic Fe/S protein whose Fe/S cluster is assembled with the aid of the CIA machinery may be essential for the 2-thio modification of cy-tRNAs. One such candidate may be Rli1, an essential Fe/S cluster-binding protein whose depletion affects pre-rRNA processing and thereby ribosome biogenesis and translation (5, 16, 39). Its precise physiological role remains unclear. Therefore, we examined whether depletion of Rli1 would elicit a phenotype in the 2-thio modification of cy-tRNAUUULys2comparable to that shown for the depletion of Cfd1, Nbp35, or Cia1. However, Rli1 depletion did not cause any defect in the 2-thio modification of cy-tRNAUUULys2(Fig. 3C and D), even when cell growth fully ceased after doxycycline treatment for 50 h. In summary, the 2-thio modification of cy-tRNAUUULys2strictly depends on the cytosolic Fe/S protein assembly process involving the CIA machinery but does not require the Fe/S protein Rli1. This suggests that another cytosolic or nuclear Fe/S protein might be involved in the 2-thio modification of cy-tRNAs such as cy-tRNAUUULys2.
Extramitochondrial Nfs1 is not sufficient for the 2-thio modification of cy-tRNAUUULys2.
We previously showed that, upon depletion of Nfs1, the 2-thio modification of cy-tRNAUUULys2and cy-tRNAUUCGlu3was decreased, but the defect was slightly delayed compared to the defect in the 2-thio modification of mt-tRNAUUULysand mt-tRNAUUGGln(30). We also found that trace amounts of nuclear Nfs1 appeared to be essential for yeast viability, although the vast majority of Nfs1 was located in the mitochondria (28). Therefore, if such extramitochondrial Nfs1 can act as a sulfur donor for the 2-thio modification of cy-tRNAUUULys2in the cytosol, the ectopic expression of the mature form of Nfs1 outside the mitochondria would be expected to restore the cytosolic 2-thio modification activity. To elucidate whether extramitochondrial Nfs1 can support the 2-thio modification of cy-tRNAUUULys2, a plasmid-borne Nfs1 lacking its mitochondrial presequence was produced in Gal-NFS1/YN cells under Nfs1 depletion conditions. Total tRNA, including the cy-tRNAUUULys2, was prepared from the cells and subjected to APM-Northern analysis. As shown in Fig. 4, neither cy-tRNAUUULys2nor mt-tRNAUUULys showed any mobility retardation when Nfs1 was present in the cytosol/nucleus but lacking in the mitochondria, indicating that cytosolic/nuclear Nfs1 was not sufficient to complement the defects in the 2-thio modifications of these tRNAs. On the other hand, when the plasmid-borne, full-length Nfs1 was expressed in the cells, efficient 2-thio modifications of both cy-tRNAUUULys2and mt-tRNAUUULyswere recovered. These results indicate that mitochondrial Nfs1 is essential for the 2-thio modification of both cy-tRNAUUULys2and mt-tRNAUUULys.
FIG. 4.
Extramitochondrial Nfs1 is not sufficient for 2-thio modification of cy-tRNAUUULys2. Gal-NFS1/YN cells without plasmid-borne Nfs1 (lane 1), with plasmid-borne Nfs1 lacking mitochondrial presequence (lane 2), or with plasmid-borne full-length Nfs1 precursor to be localized in the mitochondria (lane 3) were grown with glucose for 50 h, and their total tRNAs were subjected to APM-Northern analysis with cy-tRNAUUULys2-specific oligonucleotide probe (cyK) and with mt-tRNAUUULys-specific oligonucleotide probe (mtK). The 2-thio-modified and unmodified tRNAs are indicated as in Fig. 1. The bottom panel shows the levels of Nfs1 analyzed by immunological detection.
The mitochondrial scaffold proteins Isu1 and Isu2 are required for the 2-thio modification of cy-tRNAUUULys2.
The requirement of both mitochondrial Nfs1 and the cytosolic CIA machinery for the 2-thio modification of cy-tRNAUUULys2and cy-tRNAUUCGlu3reminded us of a similar situation for Fe/S protein biogenesis. The requirement of mitochondrial Nfs1 in the 2-thio modification of cy-tRNAs could be explained by the facts that mitochondrial Fe/S protein biosynthesis strictly requires the function of mitochondrial Nfs1 (15, 21, 26) and that cytosolic CIA function depends on mitochondrial Fe/S protein biogenesis (22, 23). In mitochondria, the sulfur atom generated by Nfs1 is transferred to the scaffold proteins Isu1 and Isu2, on which the Fe/S cluster is assembled (27). Therefore, if the 2-thio modification of these cy-tRNAs depends on Fe/S protein biogenesis in mitochondria, then depletion of the Isu scaffold proteins should cause an impairment of the 2-thio modification of these cy-tRNAs.
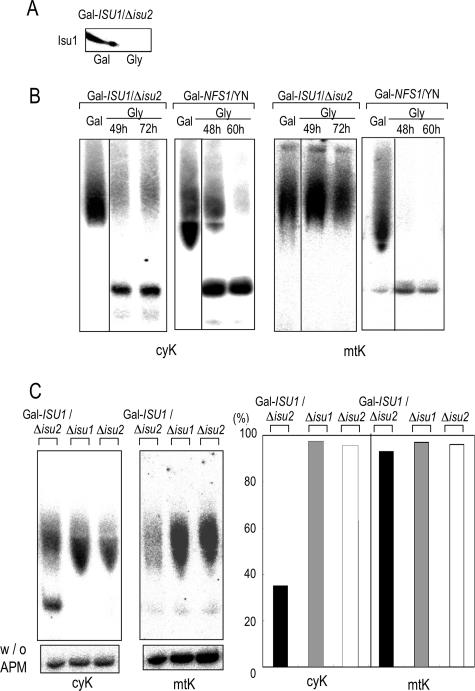
To address this question, we analyzed 2-thio modification in the Gal-ISU1/Δisu2 yeast strain; depletion of Isu1 in the absence of Isu2 was shown to cause severe growth and Fe/S protein biogenesis defects (7). Upon depletion of the Isu proteins, a significant impairment in the 2-thio modification of the cy-tRNAUUULys2was observed (Fig. 5A and B). Such impairment was not observed in the Δisu1 or Δisu2 single-disruptant strains, indicating that one of the Isu proteins is sufficient for the 2-thio modification of the cy-tRNAUUULys2. (Fig. 5C). A small amount of the 2-thio-modified cy-tRNAUUULys2remained even after prolonged incubation of Gal-ISU1/Δisu2 cells. In contrast, Gal-NFS1/YN cells whose growth ceased by 48 h of incubation under Nfs1 depletion conditions accumulated a considerable amount of 2-thio-modified cy-tRNAUUULys2, but this amount of 2-thio-modified cy-tRNAUUULys2drastically decreased upon prolonged Nfs1 depletion (Fig. 5B). We conclude that an effective depletion of Nfs1 or Isu proteins is needed for a strong defect in the 2-thio modification of cy-tRNAs. The much weaker depletion of Yah1 and Atm1 in the regulated yeast strains Gal-YAH1 and Gal-ATM1, respectively, resulted in only a weak phenotype in thio modification (26; also data not shown). The incomplete disappearance of 2-thio-modified cy-tRNAUUULys2observed even after extended depletion in Gal-ISU1/Δ isu2 cells was presumably caused by cell death before complete exhaustion of preexisting 2-thio-modified cy-tRNAUUULys2. Interestingly, the 2-thio modification of mt-tRNAUUULyswas virtually unaffected upon depletion of Isu1 and Isu2 (Fig. 5B and C), excluding the possibility that the effect on cy-tRNA is caused by dying cells. Notably, the phenotype of Isu protein-depleted cells is in clear contrast to that found for depletion of Nfs1. Complete impairment of the 2-thio modification of mt-tRNAUUULyswas observed even after depletion of Nfs1 for 48 h (Fig. 5B). Taken together, 2-thio modification of cy-tRNAUUULys2requires functional mitochondrial ISC assembly machinery, including the Isu proteins and Nfs1, whereas that of mt-tRNAUUULysexclusively depends on Nfs1 function, suggesting that it can take place without functional mitochondrial Fe/S proteins.
FIG. 5.
Depletion of the essential mitochondrial Isu scaffold proteins causes impairment in the 2-thio modification of cy-tRNAUUULys2but not of mt-tRNAUUULys. (A) Isu1 levels were determined by immunological analysis with anti-Isu1 antibody in Gal-ISU1/Δisu2 cells grown with galactose (Gal) or glycerol (Gly) for 48 h. (B) Total tRNAs of Gal-ISU1/Δisu2 cells grown with galactose (Gal) for 12 h or glycerol (Gly) for 49 h and 72 h were examined by APM-Northern analysis. Total tRNAs of Gal-NFS1/YN cells were tested in a similar fashion. The 2-thio modifications of cy-tRNAUUULys2(left) andmt-tRNAUUULys(right) were analyzed by the APM-Northern method. The 2-thio-modified and unmodified tRNAs are shown as in Fig. 1C. (C) The 2-thio modifications of cy-tRNAUUULys2and mt-tRNAUUULyswere examined with the single deletion mutants Δisu1 and Δisu2. These cells, both of which showed normal growth rates, were grown with glucose for 20 h. Gal-ISU1/Δisu2 cells grown with glucose medium for 48 h to repress the expression of Isu1 in the absence of Isu2 were analyzed in parallel. The same amounts of tRNA samples were examined in a denaturing gel with APM (upper left panel) and without APM (w/o APM). The oligonucleotide probes used were the same as shown in Fig. 1. The right panel indicates the relative proportions of 2-thio-modified tRNAs estimated from APM-Northern analysis shown in the upper left panel, as described in the legend to Fig. 1B.
DISCUSSION
We show here that two yeast cytosolic P-loop NTPases, Cfd1 and Nbp35, and a WD40 protein, Cia1, all of which belong to the CIA machinery, are required for the 2-thio modification of cy-tRNAs but not of mt-tRNAs (Fig. 1 and 3). These findings strongly suggest that 2-thio modification of cy-tRNAs crucially depends on a cytosolic/nuclear Fe/S protein. Our in vivo findings are further supported by the efficient 2-thio modification of isolated, unmodified tRNA by a cytosolic extract in vitro.
We also demonstrate here that the mitochondrial form of Nfs1 is involved in cy-tRNAUUULys2modification (Fig. 4). Sulfur to be used for Fe/S cluster synthesis is transferred from mitochondrial Nfs1 to the scaffold proteins Isu1 and Isu2 (7, 27). Moreover, our finding that the depletion of both Isu1 and Isu2 led to impairment in the 2-thio modification of cy-tRNAUUULys2(Fig. 5B and C) indicates that the 2-thio modification of cy-tRNA requires mitochondrial Fe/S protein biogenesis involving the ISC machinery.
We previously showed that nuclear localization of Nfs1 is essential for yeast cell viability in addition to its indispensable role in the mitochondria (28). We therefore examined whether 2-thio modification of cy-tRNAs would be affected by the mutation of Nfs1, whose nuclear localization signal was disrupted while its desulfurase activity was retained. No remarkable impairment of 2-thio modification of cy-tRNAUUULys2was observed, although the yeast cells ceased growth, suggesting that the nuclear version of Nfs1 is not directly involved in the thio modification of cy-tRNAUUULys2(data not shown). These findings suggest another essential role of Nfs1 in the nucleus in a pathway other than Fe/S protein biogenesis and 2-thio modification of tRNA.
The most likely explanation for why 2-thio modification of cy-tRNAUUULys2requires the ISC and CIA machineries is the essential assistance of at least one extramitochondrial Fe/S protein in this process. One such candidate for an essential Fe/S protein is Rli1, but our results provide evidence that Rli1 is not involved in the 2-thio modification of cy-tRNAUUULys2(Fig. 3B). A component of the yeast Elongator complex, Elp3, has been reported recently to be an Fe/S cluster-containing, radical-SAM protein (32) that associates with certain cy-tRNAs during an early step of modification at the wobble uridine (9). However, Elp3 itself is not essential and does not appear to be related to the 2-thio modification step, because the wobble uridine of cy-tRNAUUCGluin the Δelp3 mutant still possesses 2-thio modification although it lacks the 5-methoxycarbonylmethyl group (9). Thus, the proposed Fe/S protein required for 2-thio modification of cy-tRNAs remains to be identified. It should be noted that we cannot exclude the possibility that the CIA machinery itself might suffice to support the 2-thio modification of cy-tRNAUUULys2, the more so as Nbp35 and Nar1 have been shown to contain Fe/S clusters (3, 8). The in vitro assay described here (Fig. 2) will be useful for addressing this question directly.
Bacterial IscS has been shown to participate in the production of all of the thio-modified species found in tRNAs (18, 31), and these IscS-mediated thio modifications of tRNAs are classified into two groups: Fe/S protein-independent and Fe/S protein-dependent thio modifications. In the case of the 2-thio and 4-thio modifications of uridine in Escherichia coli, the cysteine sulfur is firstly bound to IscS and then transferred via a sulfur transfer with disulfide formation within TusABCDE and MnmA and within ThiI, respectively (10, 25). These processes seem to proceed independently of any Fe/S protein (20, 24). On the other hand, the last step of the biosynthesis of 2-methylthio-N6-isopentenyl-adenosine and the 2-thio modification of cytidine involve Fe/S proteins, namely, MiaB and TtcA, respectively (11, 33). In these cases, sulfur atoms assembled into the Fe/S cluster on MiaB or TtcA have been proposed to be used for 2-thio modification, and in such a case, the sulfur atoms might be regenerated on MiaB or TtcA with the aid of IscS, resulting in repair of the Fe/S cluster. However, no MiaB or TtcA homologues have been yet found in yeast. Nevertheless, by analogy, we propose that the Fe/S cluster-dependent 2-thio modification of yeast cy-tRNAUUULys2may involve a similar mechanism in which a constituent sulfur atom of the Fe/S cluster of an essential extramitochondrial Fe/S protein might be utilized directly for 2-thio modification. On the other hand, mitochondrial 2-thio modification of the wobble uridine in tRNAUUULysappears to be Fe/S protein independent, because the Isu proteins, the key mitochondrial scaffold proteins for Fe/S cluster synthesis, were not required in this process. Hence, the mitochondrial 2-thio modification system seems to be similar to the bacterial, Fe/S protein-independent 2-thio modification system described above (14, 20, 31). This notion is based on our finding that mitochondrial Nfs1 is strictly required in that process (Fig. 5B) (30) and on a recent study of yeast mitochondrial Mtu1, a protein homologous to bacterial MnmA, demonstrating its involvement in the 2-thio modification of mt-tRNAUUULys(36). However, in contrast to the bacterial system, a complex corresponding to the sulfur relay complex termed TusABCDE does not exist in eukaryotic mitochondria. Since this complex operates between bacterial IscS and MnmA, other mitochondrial proteins may replace its function. Recently, a yeast protein named Isd11 that forms a tight complex with mitochondrial Nfs1 has been identified and shown to participate in mitochondrial Fe/S cluster biogenesis (1, 38). It will be interesting to elucidate whether Nfs1 alone or the stable complex Nfs1-Isd11 is responsible for mediating the transfer of sulfur from Nfs1 to Mtu1.
Yeast NFS1 was first identified as a locus related to deleted tRNA splicing (therefore, its alternative name is SPL1) (17). We report here that the mitochondrial and extramitochondrial Fe/S protein maturation processes are involved in the 2-thio modification of cy-tRNA. It is reasonable to expect that such an Nfs1-involving tRNA modification might be intimately connected to the tRNA splicing process, the more so as tRNA splicing in yeast seems to be more complicated than previously thought (35).
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (17570121) to Y.N., and R.L. acknowledges support from Deutsche Forschungsgemeinschaft (SFB 593 and TR1, Gottfried-Wilhelm Leibniz program, and GRK 1216) and Fonds der chemischen Industrie.
Footnotes
Published ahead of print on 5 February 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adam, A. C., C. Bornhovd, H. Prokisch, W. Neupert, and K. Hell. 2006. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 25:174-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balk, J., D. J. Aguilar Netz, K. Tepper, A. J. Pierik, and R. Lill. 2005. The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly. Mol. Cell. Biol. 25:10833-10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balk, J., A. J. Pierik, D. J. Netz, U. Mühlenhoff, and R. Lill. 2004. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins. EMBO J. 23:2105-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biederbick, A., O. Stehling, R. Rösser, B. Niggemeyer, Y. Nakai, H. P. Elsässer, and R. Lill. 2006. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol. Cell. Biol. 26:5675-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, J., R. Lai, K. Nielsen, C. A. Fekete, H. Qiu, and A. G. Hinnebusch. 2004. The essential ATP-binding cassette protein RLI1 functions in translation by promoting preinitiation complex assembly. J. Biol. Chem. 279:42157-42168. [DOI] [PubMed] [Google Scholar]
- 6.Fosset, C., M.-J. Chauveau, B. Guillon, F. Canal, J.-C. Drapier, and C. Bouton. 2006. RNA silencing of mitochondrial m-Nfs1 reduces Fe-S enzyme activity both in mitochondria and cytosol of mammalian cells. J. Biol. Chem. 281:25398-25406. [DOI] [PubMed] [Google Scholar]
- 7.Gerber, J., K. Neuman, C. Prohl, U. Mühlenhoff, and R. Lill. 2004. The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins. Mol. Cell. Biol. 24:4848-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausmann, A., D. J. Aguilar Netz, J. Balk, A. J. Pierik, U. Mühlenhoff, and R. Lill. 2005. The eukaryotic P loop NTPase Nbp35: an essential component of the cytosolic and nuclear iron-sulfur protein assembly machinery. Proc. Natl. Acad. Sci. USA 102:3266-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, B., M. J. Johansson, and A. S. Byström. 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11:424-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeuchi, Y., N. Shigi, J. Kato, A. Nishimura, and T. Suzuki. 2006. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 21:97-108. [DOI] [PubMed] [Google Scholar]
- 11.Jäger, G., R. Leipuviene, M. G. Pollard, Q. Qian, and G. R. Björk. 2004. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 186:750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, D. C., D. R. Dean, A. D. Smith, and M. K. Johnson. 2005. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 74:247-281. [DOI] [PubMed] [Google Scholar]
- 13.Kambampati, R., and C. T. Lauhon. 1999. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry 38:16561-16568. [DOI] [PubMed] [Google Scholar]
- 14.Kambampati, R. and C. T. Lauhon. 2003. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 42:1109-1117. [DOI] [PubMed] [Google Scholar]
- 15.Kispal, G., P. Csere, C. Prohl, and R. Lill. 1999. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 18:3981-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kispal, G., K. Sipos, H. Lange, Z. Fekete, T. Bedekovics, T. Janaky, J. Bassler, D. J. Aguilar Netz, J. Balk, C. Rotte, and R. Lill. 2005. Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J. 24:589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolman, C. and D. Söll. 1993. SPL1-1, a Saccharomyces cerevisiae mutation affecting tRNA splicing. J. Bacteriol. 175:1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauhon, C. T. 2002. Requirement for IscS in biosynthesis of all thionucleosides in Escherichia coli. J. Bacteriol. 184:6820-6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauhon, C. T., and R. Kambampati. 2000. The iscS gene in Escherichia coli is required for the biosynthesis of 4-thiouridine, thiamin, and NAD. J. Biol. Chem. 275:20096-20103. [DOI] [PubMed] [Google Scholar]
- 20.Leipuviene, R., Q. Qian, and G. R. Björk. 2004. Formation of thiolated nucleosides present in tRNA from Salmonella enterica serovar Typhimurium occurs in two principally distinct pathways. J. Bacteriol. 186:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J., M. Kogan, S. A. Knight, D. Pain, and A. Dancis. 1999. Yeast mitochondrial protein, Nfs1p, coordinately regulates iron-sulfur cluster proteins, cellular iron uptake, and iron distribution. J. Biol. Chem. 274:33025-33034. [DOI] [PubMed] [Google Scholar]
- 22.Lill, R. and U. Mühlenhoff. 2005. Iron-sulfur-protein biogenesis in eukaryotes. Trends Biochem. Sci. 30:133-141. [DOI] [PubMed] [Google Scholar]
- 23.Lill, R. and U. Mühlenhoff. 2006. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu. Rev. Cell Dev. Biol. 22:457-486. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren, H. K., and G. R. Björk. 2006. Structural alterations of the cysteine desulfurase IscS of Salmonella enterica serovar Typhimurium reveal substrate specificity of IscS in tRNA thiolation. J. Bacteriol. 188:3052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller, E. G., P. M. Palenchar, and C. J. Buck. 2001. The role of the cysteine residues of ThiI in the generation of 4-thiouridine in tRNA. J. Biol. Chem. 276:33588-33595. [DOI] [PubMed] [Google Scholar]
- 26.Mühlenhoff, U., J. Balk, N. Richhardt, J. T. Kaiser, K. Sipos, G. Kispal, and R. Lill. 2004. Functional characterization of the eukaryotic cysteine desulfurase Nfs1p from Saccharomyces cerevisiae. J. Biol. Chem. 279:36906-36915. [DOI] [PubMed] [Google Scholar]
- 27.Mühlenhoff, U., J. Gerber, N. Richhardt, and R. Lill. 2003. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 22:4815-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai, Y., M. Nakai, H. Hayashi, and H. Kagamiyama. 2001. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 276:8314-8320. [DOI] [PubMed] [Google Scholar]
- 29.Nakai, Y., Y. Yoshihara, H. Hayashi, and H. Kagamiyama. 1998. cDNA cloning and characterization of mouse nifS-like protein, m-Nfs1: mitochondrial localization of eukaryotic NifS-like proteins. FEBS Lett. 433:143-148. [DOI] [PubMed] [Google Scholar]
- 30.Nakai, Y., N. Umeda, T. Suzuki, M. Nakai, H. Hayashi, K. Watanabe, and H. Kagamiyama. 2004. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 279:12363-12368. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson, K., H. K. Lundgren, T. G. Hagervall, and G. R. Björk. 2002. The cysteine desulfurase IscS is required for synthesis of all five thiolated nucleosides present in tRNA from Salmonella enterica serovar typhimurium. J. Bacteriol. 184:6830-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paraskevopoulou, C., S. A. Fairhurst, D. J. Lowe, P. Brick, and S. Onesti. 2006. The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 59:795-806. [DOI] [PubMed] [Google Scholar]
- 33.Pierrel, F., G. R. Björk, M. Fontecave, and M. Atta. 2002. Enzymatic modification of tRNAs: MiaB is an iron-sulfur protein. J. Biol. Chem. 277:13367-13370. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz, C. J., O. Djaman, J. A. Imlay, and P. J. Kiley. 2000. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takano, A., T. Endo, and T. Yoshihisa. 2005. tRNA actively shuttles between the nucleus and cytosol in yeast. Science 309:140-142. [DOI] [PubMed] [Google Scholar]
- 36.Umeda, N., T. Suzuki, M. Yukawa, Y. Ohya, H. Shindo, K. Watanabe, and T. Suzuki. 2005. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J. Biol. Chem. 280:1613-1624. [DOI] [PubMed] [Google Scholar]
- 37.Urbina, H. D., J. J. Silberg, K. G. Hoff, and L. E. Vickery. 2001. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 276:44521-44526. [DOI] [PubMed] [Google Scholar]
- 38.Wiedemann, N., E. Urzica, B. Guiard, H. Muller, C. Lohaus, H. E. Meyer, M. T. Ryan, C. Meisinger, U. Mühlenhoff, R. Lill, and N. Pfanner. 2006. Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 25:184-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarunin, A., V. G. Panse, E. Petfalski, C. Dez, D. Tollervey, and E. C. Hurt. 2005. Functional link between ribosome formation and biogenesis of iron-sulfur proteins. EMBO J. 24:580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuvaniyama, P., J. N. Agar, V. L. Cash, M. K. Johnson, and D. R. Dean. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. USA 97:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng, L., V. L. Cash, D. H. Flint, and D. R. Dean. 1998. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273:13264-13272. [DOI] [PubMed] [Google Scholar]
- 42.Zheng, L., R. H. White, V. L. Cash, and D. R. Dean. 1994. Mechanism for the desulfurization of l-cysteine catalyzed by the nifS gene product. Biochemistry 33:4714-4720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.