Abstract
Neutral sphingomyelinase (nSMase), the initial enzyme of the sphingolipid signaling pathway, is thought to play a key role in cellular responses to tumor necrosis factor alpha (TNF-α), such as inflammation, proliferation, and apoptosis. The mechanism of TNF-α-induced nSMase activation is only partly understood. Using biochemical, molecular, and pharmacological approaches, we found that nSMase activation triggered by TNF-α is required for TNF-α-induced proliferation and in turn requires a proteolytic cascade involving furin, membrane type 1 matrix metalloproteinase (MT1-MMP), and MMP2, and leading finally to extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation and DNA synthesis, in smooth muscle cells (SMC) and fibroblasts. Pharmacological and molecular inhibitors of MMPs (batimastat), furin (α1-PDX inhibitor-transfected SMC), MT1-MMP (SMC overexpressing a catalytically inactive MT1-MMP), MMP2 (fibroblasts from MMP2−/− mice), and small interfering RNA (siRNA) strategies (siRNAs targeting furin, MT1-MMP, MMP2, and nSMase) resulted in near-complete inhibition of the activation of nSMase, sphingosine kinase-1, and ERK1/2 and of subsequent DNA synthesis. Exogenous MT1-MMP activated nSMase and SMC proliferation in normal but not in MMP2−/− fibroblasts, whereas exogenous MMP2 was active on both normal and MMP2−/− fibroblasts. Altogether these findings highlight a pivotal role for furin, MT1-MMP, and MMP2 in TNF-α-induced sphingolipid signaling, and they identify this system as a possible target to inhibit SMC proliferation in vascular diseases.
Tumor necrosis factor alpha (TNF-α) is the prototype of proinflammatory cytokines that signal through cell surface receptors (20, 31, 52). Members of the TNF/TNF receptor (TNFR) superfamily are cellular organizers of multicellular structures, including lymphoid organs, hair follicles, bone, and lactating mammary gland. They also coordinate the complex intercellular cross talk involved in immune and inflammatory responses (20, 31, 52). The TNF/TNFR superfamily system has been implicated in a variety of diseases, such as bone diseases, ectodermal defects, impaired immune response, immunoinflammatory diseases, septic shock, lymphoproliferation, tumorigenesis, cachexia, and atherosclerosis (11, 20, 31, 52).
TNF-α signals through TNFR1, an ubiquitously expressed receptor for soluble TNF, and TNFR2, with restricted tissue-specific expression and preferential affinity for membrane bound TNF (20, 52). Binding of homotrimeric TNF-α to preassembled receptor homotrimer triggers conformational changes that enable the cytoplasmic domains to bind cognate adaptors. These adaptors regulate several signaling pathways, including nuclear factor κB (NF-κB), Jun N-terminal protein kinases, and reactive oxygen species, as well as expression of genes involved in cell survival, proliferation, and apoptosis (2, 20, 31, 52). TNF-α activates the sphingomyelin pathway, which is characterized by the hydrolysis of sphingomyelin at the plasma membrane by acid sphingomyelinase and neutral sphingomyelinase (nSMase) isoforms, through different cytoplasmic domains of TNFR (28). Sphingomyelin hydrolysis generates the second messenger ceramide, which exhibits different properties depending on the topological location of its generation and the nature of the SMase involved (19, 28). Subsequent metabolism of ceramide involves acid or neutral ceramidases and sphingosine kinases (SK) that may influence the balance between proapoptotic (ceramide and sphingosine) and antiapoptotic (sphingosine-1-phosphate) metabolites (9, 40, 46, 50). So far, the precise links between TNFR and nSMase activation remain largely unknown (6), but they could involve metalloproteinases, as reported recently (5).
Matrix metalloproteinases (MMPs) are implicated in extracellular matrix degradation, cell migration, proliferation, and tissue remodeling and thereby may play a role in growth and development, angiogenesis, tumor invasion, and atherosclerosis (37, 53). MMPs are synthesized as latent proenzymes which are converted into mature, catalytically active forms by proteolytic cleavage of the N-terminal propeptide mediated by serine proteases, such as plasmin or thrombin, or by membrane-type MMPs (MT-MMPs) (25, 34). Activation of pro-MMP2 takes place at the cell surface and involves interactions with active MT1-MMP, which is itself activated through rapid trafficking to the cell surface and proteolytic processing (34, 48). MT1-MMP is cleaved at a 108RRKR cleavage site of its prodomain sequence by proprotein convertases such as furin (42, 44). Furin is a Golgi-associated serine proteinase which is synthesized as an inactive enzyme whose activation is spatially and temporally regulated through a multistep autocatalytic processing of the N-terminal prosegment occurring in the endoplasmic reticulum (ER) and in the acidic trans-Golgi network (TGN) (58).
We report here that the mitogenic effect of TNF-α on mesenchymal cells is mediated by the sphingolipid pathway and that nSMase2 (Smpd3), the initial step of the sphingolipid pathway, is regulated by a proteinase cascade involving furin, MT1-MMP, and MMP2.
MATERIALS AND METHODS
Chemicals.
[3H]thymidine (5 Ci/mmol) and [γ-33P]ATP were from Perkin-Elmer. Rabbit anti-MT1-MMP and rabbit antifurin were from Santa Cruz Biotechnologies (Santa Cruz, CA), and rabbit anti-(activated) phospho-mitogen-activated protein kinase was from Promega (Madison, WI). RPMI 1640 containing Glutamax and fetal calf serum (FCS) was from Invitrogen (France). Pure recombinant MT1-MMP and MMP2 proenzymes were from VWR and were activated by 2 h of incubation with 10 μmol/liter p-aminophenylmercuric acetate (APMA), as reported previously (5). Batimastat was a generous gift of H.-W. Krell (Roche Diagnostics, Penzberg, Germany). MMP substrates [dansyl-Pro-Leu-Ala-Cys(p-OMeBz)-Trp-Ala-Arg-NH2 for MT1-MMP and MCA-Pro-Leu-Ala-Nva-Dpa-Ala-Arg-NH2 for MMP2) and the fluorogenic furin substrate (Pyr-Arg-Thr-Lys-Arg-AMC) were from VWR, and human TNF-α and the furin inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethylketone (FI) were from Abcys. Brefeldin A, monensin, and other reagents were obtained from Sigma.
Cell culture.
Rabbit femoral artery smooth muscle cells (SMC) and human aortic SMC (CRL1999) were obtained from ATCC and were grown in RPMI 1640 supplemented with 10% FCS.
Mutant SMC/PDX were obtained by transfection with a plasmid containing the furin inhibitor cDNA α1-PDX and a gene coding for resistance to Geneticin (55), using Lipofectamine solution (Invitrogen). Cells were grown in Geneticin (0.4 mg/ml)-supplemented medium to obtain a stable cell line expressing PDX (SMC/PDX). SMC expressing mutant catalytically inactive MT1-MMP (iMT1/SMC) were obtained by transfection of a pcDNA3.1 vector containing a zeocin resistance gene and the cDNA of MT1-MMP with the substitution E240A in the active site (a generous gift from Alex Strongin, La Jolla, CA) (42). MMP2-deficient fibroblasts from MMP2 −/− mice (26) were grown in Dulbecco modified Eagle medium supplemented with 10% FCS. At 24 h before an experiment, the medium was removed and replaced by serum-free RPMI or Dulbecco modified Eagle medium.
A small interfering RNA (siRNA) targeting human nSMase2 (sequence, AAtgctactggctggtggacc) was from Eurogentec, Belgium, and was used under the conditions described by Marchesini et al. (32). siRNAs targeting furin (SMARTPool furin; catalog number L-005882), MT1-MMP (SMARTPool MT1-MMP; catalog number L-062241), and MMP2 (SMARTPool MMP2; catalog number L-005959) and scrambled siRNA were purchased from Dharmacon (Lafayette, CO). Murine fibroblasts and human SMC were transfected with 20 μM double-stranded siRNA in Optimem medium (Gibco) mixed with Oligofectamine according to the supplier's instructions. At 3 hours after transfection, cells were incubated in RPMI containing 10% FCS for 24 h. Fibroblasts were then incubated for 12 h in RPMI containing 0.5% FCS before experiments. Rescue experiments for nSMase2 and MT1-MMP were done by cotransfecting either a plasmid coding for the murin form of nSMase2 in nSMase2/siRNA-treated-SMC (32) or a plasmid coding for the human form of MT1-MMP (42) in MT1-MMP/siRNA-silenced murin fibroblasts according to the manufacturer instructions.
DNA synthesis.
DNA synthesis was evaluated by [3H]thymidine incorporation under previously described conditions (7).
nSMase determination.
Cells were harvested and homogenized by sonication in 0.1% Triton X-100, 10 mM MgCl2, 5 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM glycerophosphate, 750 μM ATP, 1 mM phenylmethylsulfonyl fluoride, 2 mM EDTA, 10 μM leupeptin, and 10 μM pepstatin. nSMase activity was determined as reported previously (4). Briefly, 100 μl of substrate [choline-methyl-14C]sphingomyelin (120,000 dpm/assay) in 0.1% Triton X-100-20 mM HEPES buffer (pH 7.4) containing 1 mM MgCl2 was added to 100 μl of cell homogenate. After 2 h of incubation at 37°C, the liberated [methyl-14C]choline was partitioned under the previously used conditions (4) and quantitated by liquid scintillation counting.
SK-1 activity.
SK-1 activity was determined as described previously with minor modifications (7). Briefly, after incubation with or without TNF-α, cells were washed with ice-cold phosphate-buffered saline (PBS), harvested, and sedimented by centrifugation, and cell pellets were solubilized in kinase buffer (7). The SK-1 assay was performed with cell extracts supplemented with 50 μM sphingosine and 0.25% Triton X-100 (10 μl of substrate stock solution containing 1 mM sphingosine in 5% Triton X-100), [33P]ATP (0.5 μCi, 20 mM), unlabeled ATP (1 mM), and 10 mM MgCl2 in a final volume of 200 μl. After incubation for 30 min at 37°C, the reaction was stopped by the addition of 20 μl of 1 N HCl, and the [33P]ATP-labeled sphingosine 1-phosphate (S1P) was extracted, isolated by thin-layer chromatography, and quantified as described previously (7).
MMP activity.
MMP2 activity in concentrated SMC medium or cell pellet was determined by use of the fluorogenic substrates MCA-Pro-Leu-Ala-Nva-Dpa-Ala-Arg-NH2 for MMP2 and dansyl-Pro-Leu-Ala-Cys(p-OMeBz)-Trp-Ala-Arg-NH2 for MT1-MMP (Calbiochem-WWR) as described previously (35). The experiment was performed in the presence and absence of EDTA (5 μM), and two blanks (without cell and without substrate) were used. After 3 h of incubation (37°C), 1 ml Tris-HCl buffer (pH 7) was added and the fluorescence was read (excitation and emission wavelengths, 325 to 395 and 260 to 340 nm, respectively).
Furin activity.
Furin activity was determined under the conditions recommended by the supplier. Cells were washed twice in cold PBS, scraped in 3 ml PBS, and suspended in 300 μl of reaction buffer (100 mM MES [morpholineethanesulfonic acid]-NaOH, 1 mM CaCl2, pH 7.0). The fluorogenic substrate for furin (Pyr-Arg-Thr-Lys-Arg-AMC; Calbiochem) was added at a 20 nM final concentration. The cell suspension was incubated 4 h at 37°C under gentle agitation. The reaction was stopped with 1 ml of 5 mM EDTA. The fluorescence of AMC released was measured (excitation and emission wavelengths, 370 and 450 nm, respectively).
Western blotting.
Western blotting was performed as described previously (5). The protein concentration was determined using the Bradford reagent (Bio-Rad).
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Estimates of statistical significance were performed by analysis of variance (Tukey test; SigmaStat software). P values of <0.05 were considered significant.
RESULTS
TNF-α-induced proliferation of SMC requires the sphingomyelin/ceramide/S1P pathway.
TNF-α (2 ng/ml) stimulates DNA biosynthesis in vascular SMC, as assessed by TNF-α-stimulated [3H]thymidine incorporation (Fig. 1A). The mitogenic effect of TNF-α (50% increase) was in agreement with previous observations for SMC with TNF-α (49, 54) and other inflammatory cytokines and oxidative stress (36), all of which are found within the atherosclerosis plaque. It may be noted that, under these conditions (0% FCS and absence of protein synthesis inhibitors), TNF-α was not found to be toxic for SMC and fibroblasts (data not shown). TNF-α triggered extracellular signal-regulated kinase 1/2 (ERK1/2) activation, which started 60 min following TNF-α addition and was sustained for at least 1 h (Fig. 1B).
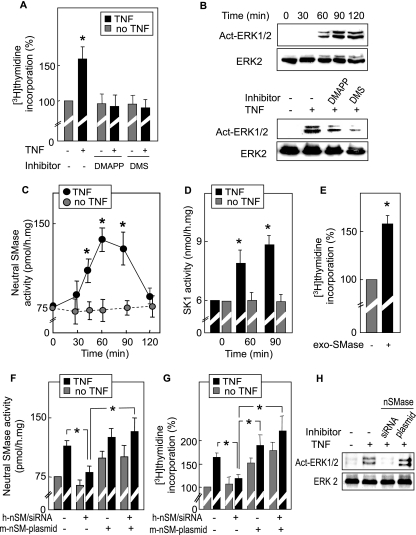
FIG. 1.
The sphingomyelin/ceramide pathway is required for TNF-α-induced SMC and fibroblast proliferation. (A) TNF-α-induced DNA synthesis was quantified using [3H]thymidine incorporation, as indicated in Materials and Methods. SMC were seeded in RPMI containing 10% FCS and then were starved in RPMI without FCS for 24 h and stimulated by TNF-α (2 ng/ml) after pretreatment with DMAPP (10 μmol/liter, 12 h) or DMS (2 μmol/liter, 1 h) as indicated. (B) Western blot of ERK1/2 kinase activation by TNF-α, showing time course and effect of DMAPP or DMS (used as for panel A). Western blots were revealed by anti-activated (phospho)-ERK1/2 and by anti-ERK2 antibodies. (C and D) Time course of nSMase (C) and SK-1 (D) activation in SMC treated with TNF-α. (E) Effect of exogenous bacterial SMase (100 mU/ml, 1 h) on DNA synthesis in SMC. (F to H) Effect of siRNA targeting human nSMase2 (h-nSM/siRNA) and/or cotransfection of a plasmid coding for the murine nSMase2 (m-nSM-plasmid for rescue experiments, with m-nSM-plasmid being resistant to h-nSM/siRNA) on nSMase activation (F), DNA synthesis (G), and ERK1/2 phosphorylation (H) triggered by TNF-α in human SMC. Results are means ± SEM form three to five separate experiments. *, P < 0.05 (comparison between cells treated with or without TNF-α, as indicated).
TNF-α-induced DNA synthesis and ERK1/2 activation were strongly reduced by inhibitors of the sphingolipid signaling pathway, i.e., d-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol (DMAPP), an inhibitor of ceramidases, and N,N-dimethylsphingosine (DMS), an inhibitor of SK (utilized under nontoxic conditions) (7) (Fig. 1A and B). These data, together with the TNF-α-induced activation of nSMase and SK1 (Fig. 1C and D), strongly suggest that the sphingolipid signaling pathway is required for the mitogenic effect of TNF-α. The role of sphingomyelin hydrolysis was also supported by experiments with exogenous bacterial SMase, which hydrolyzes sphingomyelin of the outer leaflet of the plasma membrane and generates subsequent mediators of the sphingolipid pathway (4, 50). Preincubation of SMC with exogenous bacterial SMase under nontoxic conditions triggered DNA synthesis, thus mimicking the mitogenic effect of TNF-α and supporting the hypothesis of the mitogenic role of the SMase (Fig. 1E).
In order to further support this conclusion and to identify the specific nSMase involved in the sphingolipid pathway implicated in TNF-α-induced mitogenic signaling, we performed experiments on human SMC with a specific siRNA targeting human nSMase2 (anti-human SMPD3 siRNA), associated with rescue experiments by transfection of a mouse nSMase2 cDNA (smpd3, insensitive to the anti-human SMPD3 siRNA). As expected, the anti-human SMPD3 siRNA inhibited the TNF-α-stimulated nSMase activity in human SMC (Fig. 1F). Transfection of SMC with mouse nSMase2 cDNA induced a rise of both basal and TNF-α-stimulated nSMase activity that was resistant to the anti-human SMPD3 siRNA (Fig. 1F). In the same way, TNF-α-induced DNA synthesis and ERK1/2 activation were inhibited by the anti-human SMPD3 siRNA in human SMC and were rescued in human SMC transfected with the mouse nSMase cDNA (Fig. 1G and H).
Altogether, these data suggest that nSMase2 (SMPD3) and the sphingolipid signaling pathway are required for the mitogenic effect of TNF-α in SMC.
MMP2 is required for TNF-α-induced activation of the nSMase and mitogenic effect.
We recently reported that MMP2 mediates both activation of nSMase and subsequent proliferation induced by oxidized low-density lipoprotein (LDL) in SMC and fibroblasts (5). This led us to investigate whether MMP2 plays a role in TNF-α-induced nSMase activation and mitogenic effect.
Consistent with this hypothesis, the MMP inhibitor batimastat (10 nmol/liter) inhibited TNF-α-induced DNA synthesis in SMC (Fig. 2A and B). Moreover, as shown in Fig. 2A, batimastat (like DMS [data not shown]) did not inhibit DNA synthesis triggered by FCS, suggesting that TNF-α and growth factors activate distinct mitogenic signaling pathways.
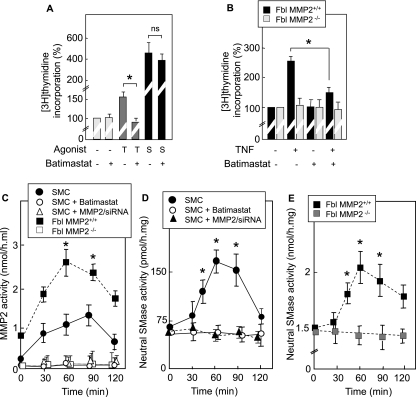
FIG. 2.
MMP2 is required for TNF-α-induced proliferation via nSMase activation. (A and B) SMC (A) or MMP2+/+ or MMP2−/− fibroblasts (Fbl) (obtained from MMP2+/+ and MMP2−/− mice, respectively) (B) were serum starved for 24 h in RPMI and stimulated with TNF-α (T) or 10% FCS (S) in the presence or absence of batimastat (10 nM). DNA synthesis was evaluated by [3H]thymidine incorporation. (C to E) Enzyme activities were measured in culture media (MMP2) and cell lysates (nSMase) from SMC or fibroblasts treated with or without TNF-α (2 ng/ml), batimastat, and MMP2/siRNA. Results are means ± SEM from three separate experiments. *, P < 0.05 (comparison with controls). ns, not significant.
The involvement of MMP2 was assessed on the basis of several approaches. (i) In wild-type (wt) fibroblasts expressing MMP2, TNF-α induced the activation of MMP2 and nSMase, as well as DNA synthesis (Fig. 2B to E). (ii) In contrast, in MMP2-deficient fibroblasts (isolated from MMP2−/− mice), TNF-α failed to activate DNA synthesis and nSMase (Fig. 2B and E). (iii) In SMC, TNF-α triggered the activation of MMP2 and nSMase, which was abrogated by the MMP inhibitor batimastat and by anti-MMP2 siRNA (Fig. 2C and D). These data strongly suggest that MMP2 is required for the TNF-α-induced activation of nSMase.
Complementing these data, addition of exogenous activated MMP2 induced the activation of both nSMase and SK-1 (Fig. 3A and B), as well as DNA synthesis in SMC (Fig. 3C). As expected, MMP2 silencing by siRNA did not inhibit the activation of nSMase and DNA synthesis induced by exogenous MMP2 in SMC (Fig. 3A and C). Moreover, exogenous MMP2 was able to induce nSMase activation in MMP2−/− fibroblasts (Fig. 3D).
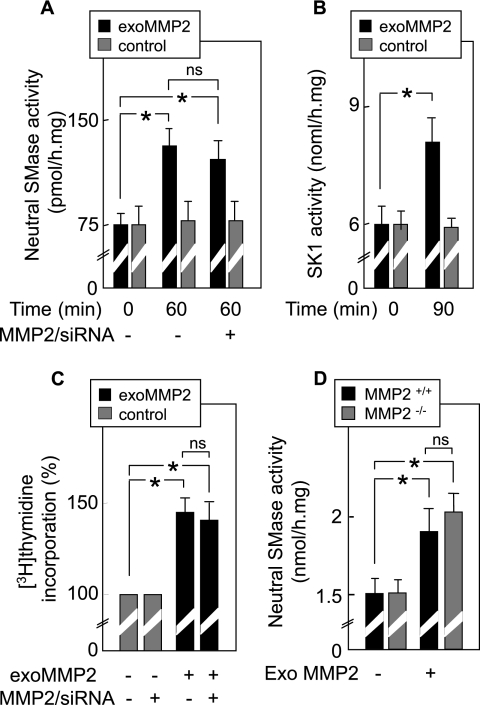
FIG. 3.
Exogenous MMP2 mimics cell signaling induced by TNF-α. (A to C) nSMase and SK-1 activities and DNA synthesis were measured in SMC treated or not with MMP2/siRNA and stimulated for 40 min by exogenous MMP2 (exoMMP2) (1 nM, activated for 2 h by 10 μM APMA) (2). (D) MMP2−/− and MMP2+/+ fibroblasts were stimulated by exogenous MMP2 for 40 min as described above, and then nSMase activities were determined. Control experiments for toxicity using activated MMP2 were done simultaneously and exhibited no toxicity for the cells during the time course of the experiment (not shown). Results are means ± SEM from three separate experiments. *, P < 0.01 (comparison between MMP2-treated and untreated cells). ns, not significant.
Altogether, these data strongly support the hypothesis that MMP2 is required to mediate the TNF-α-induced activation of nSMase and subsequent mitogenic effect.
MT1-MMP acts upstream from MMP2 to mediate the TNF-α-induced nSMase activation.
Next, we investigated whether MT1-MMP was involved in TNF-α-induced MMP2 activation, since MT1-MMP is known to activate MMP2 through the cleavage of the N-terminal prosegment (34, 48). Serum proteases that may also activate MMP2, such as plasmin or thrombin (25), were probably not implicated in our system, since cells were treated with TNF-α in serum-free medium.
To investigate the potential role of MT1-MMP, we used SMC transfected with a pcDNA3-zeo vector coding for a catalytically inactive form of MT1-MMP (iMT1-SMC) and SMC transfected with an empty pcDNA3-zeo vector (zv-SMC) as a control. In wt SMC and in zv-SMC, TNF-α triggered MT1-MMP activation (with activity peaking at 60 min), concomitant with the cleavage of pro-MT1-MMP into active MT1-MMP (Fig. 4A and B, upper panels). In contrast, in iMT1-SMC, TNF-α triggered no activation of MT1-MMP (Fig. 4A), whereas overexpressed pro-iMT1-MMP (and possibly pro-MT1-MMP) was cleaved (with a delayed time course) (Fig. 4B, lower panels). This is consistent with the hypothesis of cleavage of iMT1-MMP by furin, since the furin cleavage site is intact in iMT1-MMP (E240A). It may be noted that self-proteolysis did not occur (or was very low), since this mutant is catalytically inactive (42) and since the whole MT1-MMP activity is very low in iMT1-SMC (Fig. 4A). This dominant negative effect of iMT1-MMP could be explained by inhibition of pro-MT1-MMP processing resulting from competition between the overexpressed pro-iMT1-MMP and pro-MT1-MMP. In addition, mutants devoid of the catalytic domain exert a dominant negative effect by interfering with the oligomerization of MT1-MMP (29).
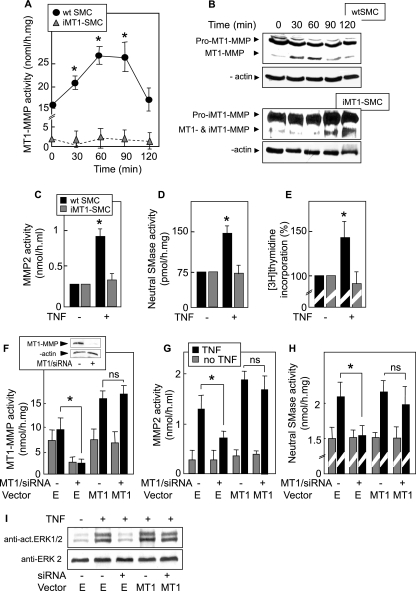
FIG. 4.
Role of MT1-MMP in TNF-α-induced proliferation via MMP/SMase. (A) Time course of MT1-MMP enzymatic activity in wt SMC, zv-SMC (SMC transfected with an empty pcDNA3-zeo vector), and iMT1-SMC (SMC overexpressing pro-iMT1-MMP, a mutated form of MT1-MMP [MMP-E240A], which is catalytically inactive but cleavable by furin to form iMT1-MMP). Cells were treated with TNF-α (2 ng/ml). MT1-MMP activity in cell lysate was determined using a fluorogenic substrate, as described in Materials and Methods. (B) Western blot of MT1-MMP in SMC and iMT1-SMC treated or not with TNF-α (0 to 120 min) and revealed by anti-MT1-MMP and anti-β-actin (upper and lower panels, respectively). (C to E) Evaluation of MMP2 and nSMase activities and DNA synthesis in wt SMC and iMT1-SMC treated (when indicated) by TNF-α. (F to H) Rescue experiment using mouse fibroblasts transfected with MT1-MMP/siRNA and cotransfected with a plasmid coding for the human form of MT1-MMP (MT1) or with the empty vector (E). After 24 h of transfection, siRNA and plasmid were removed and MT1-MMP (F), MMP2 (G), and nSMase (H) activities were quantified. Inset in panel F, silencing effect of MT1-MMP siRNA on MT1-MMP expression in SMC. (I) Western blot showing ERK1/2 activation in the presence of TNF-α in cells transfected or not with MT1-MMP/siRNA in the presence or absence of MT1-MMP plasmid. Results are means ± SEM from three separate experiments. *, P < 0.05 (comparison between cells treated or not with TNF-α, or as indicated). ns, not significant.
TNF-α triggered the activation of MMP2 and nSMase and stimulated DNA synthesis in wt SMC (Fig. 4C to E) (as well as in zv-SMC [data not shown]). In contrast, in iMT1-SMC, TNF-α failed to activate MMP2 and nSMase (Fig. 4C and D) and DNA synthesis (Fig. 4E).
In an alternative approach, MT1-MMP was silenced in murine fibroblasts with siRNA specific for the murine form of MT1-MMP (Fig. 4F, inset). This induced the inhibition of the TNF-α-induced activation of MT1-MMP, MMP2, nSMase, and ERK1/2 (Fig. 4F, G, H, and I). This inhibitory effect was prevented by cotransfecting the siRNA-treated cells with a plasmid coding for human MT1-MMP, which rescued both MMP and nSMase activation and ERK1/2 phosphorylation induced by TNF-α (Fig. 4F, G, H, and I).
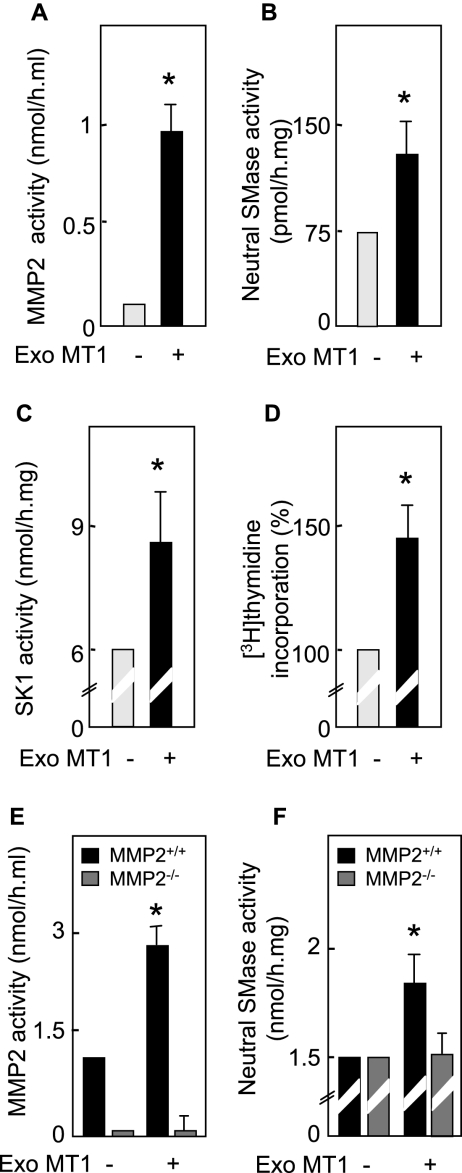
In order to confirm the role of MT1-MMP in MMP2-mediated nSMase activation, SMC were treated with exogenous recombinant MT1-MMP. Exogenous MT1-MMP triggered the activation of MMP2, nSMase, and SK-1 (peaking at 60 [MMP2 and nSMase] and 90 [SK-1] min) and stimulated DNA synthesis in SMC, thereby mimicking the effect of TNF-α (Fig. 5A to D). Exogenous MT1-MMP was able to activate MMP2 (Fig. 5E), thus indicating that MMP2 is downstream of MT1-MMP. Importantly, exogenous MT1-MMP was unable to induce nSMase activation in MMP2−/− fibroblasts (Fig. 5F), thus demonstrating that MMP2 mediates MT1-MMP-induced activation of nSMase.
FIG. 5.
Exogenous MT1-MMP triggers cell signaling (activation of MMP2, nSMase, and SK-1) and DNA synthesis. (A to D) Effect of exogenous MT1-MMP (Exo MT1, 1 nM) activated by 10 μM APMA. The MMP2, nSMase, and SK-1 activities and DNA synthesis were evaluated in SMC treated or not with activated MT1-MMP (Exo MT1) for 40 min, as indicated in Materials and Methods. (E and F) MMP2−/− and MMP2+/+ fibroblasts were stimulated by exogenous active MT1-MMP for 40 min as described above, and then MMP2 and nSMase activities were determined. Control experiments for toxicity using activated MT1-MMP were done simultaneously and exhibited no toxicity for the cells during the time course of the experiment (not shown). Results are means ± SEM from three separate experiments. *, P < 0.01 (comparison between cells treated or not with exo MT1-MMP).
Altogether, these data suggest that in SMC and fibroblasts, TNF-α-induced activation of nSMase requires MT1-MMP (upstream MMP2), and is involved in cell proliferation.
Furin is required for TNF-α-induced MMP/sphingolipid pathway activation and cell proliferation.
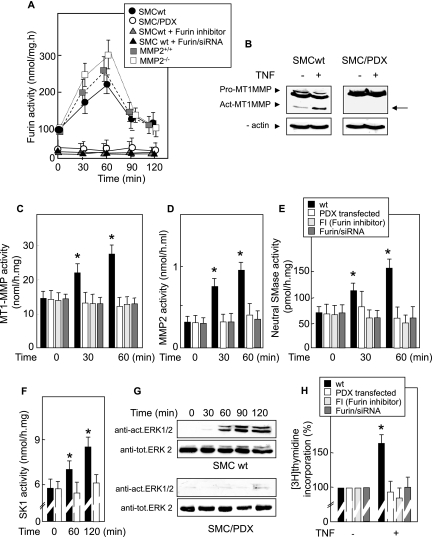
As the inactive pro-MT1-MMP is generally converted into active MT1-MMP by proteolysis mediated by the ubiquitously expressed proconvertase furin (12, 38, 51, 59), we investigated whether furin is implicated in the TNF-α-induced signaling MMP/sphingolipid pathway. For this purpose, we used several molecular and pharmacological approaches, i.e., specific siRNA, pRc/CMV vector coding for the furin inhibitor α1-PDX (SMC/PDX) (55), and the specific, potent, and cell-permeative furin inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethylketone (FI). TNF-α triggered furin activation peaking at 30 to 60 min in wt SMC (Fig. 6A) (and in SMC transfected with an empty pRc/CMV vector [data not shown]). In contrast, TNF-α did not activate furin in SMC/PDX, in FI-treated SMC, or in SMC with furin silenced by siRNA (Fig. 6A).
FIG. 6.
Furin is required for TNF-α-induced MMP/nSMase/SK-1 activation. (A) Time course of furin activation in wt SMC, furin-silenced SMC (pretreated with specific siRNA for 24 h), SMC/PDX (SMC transfected with pCR/CMV-PDX plasmid), and SMC treated with the furin inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethyl-ketone (FI) at 25 μM for 16 h and then treated with TNF-α (2 ng/ml) and time course of furin in MMP2+/+ or MMP2−/− fibroblasts treated with TNF-α (2 ng/ml). (B) Western blot of MT1-MMP activation induced by TNF-α in wt SMC or SMC/PDX. Membranes were blotted with anti-MT1-MMP and anti-β-actin antibodies (the arrow indicates the active form of MT1-MMP). (C to F) Determination of MT1-MMP, MMP2, nSMase, and SK-1 activities in wt SMC, SMC/PDX, FI-treated SMC, and furin-silenced SMC stimulated with TNF-α (2 ng/ml). (G) TNF-α-induced ERK1/2 phosphorylation in wt SMC and SMC/PDX. Western blots were labeled with anti-activated-ERK1/2 and ERK2 antibodies. Results are representative of three separate experiments. (H) DNA synthesis induced by TNF-α in wt SMC, furin-silenced SMC, SMC/PDX, and FI-treated SMC. Results are means ± SEM from three or four separate experiments. *, P < 0.05 (comparison between cells treated or not with TNF-α).
TNF-α induced the proteolytic processing and activation of MT1-MMP in SMC but not in furin-silenced SMC and in SMC/PDX (Fig. 6B and C), thus indicating that MT1-MMP processing and activation induced by TNF-α are dependent on furin. Similarly, TNF-α did not activate the MT1-MMP-mediated proteolytic cascade leading to the activation of MMP2, nSMase, and SK-1 in SMC/PDX and in cells treated with furin-directed siRNA or with FI (Fig. 6D to F). In addition, TNF-α induced no ERK1/2 activation and DNA synthesis in furin-inhibited cells (Fig. 6G and H).
Altogether, these data demonstrate that furin is involved in TNF-α-induced mitogenic signaling by inducing proteolysis and activation of MT1-MMP that in turn activates MMP2 and the MMP2/sphingolipid pathway.
Brefeldin A and monensin inhibit TNF-α-induced mitogenic signaling in SMC.
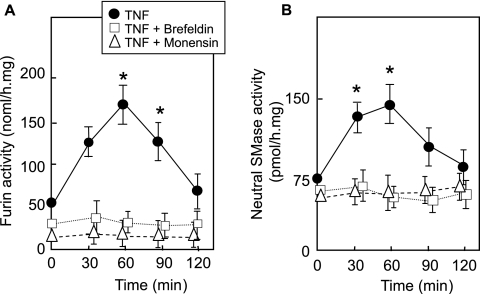
The activation of furin is mediated by a multistep autocatalytic processing that is spatially regulated during its translocation from the ER towards the TGN and plasma membrane (51). This led us to speculate that TNF-α-induced furin-dependent signaling should be inhibited by brefeldin A and monensin, two inhibitors that induce the disassembly of the Golgi complex and block ER-Golgi-cell surface vesicular transport of proteins, respectively. Under the conditions used, these inhibitors blocked the TNF-α-induced activation of furin and subsequent nSMase activation (Fig. 7), without altering the basal level of furin.
FIG. 7.
Role of TGN and vesicular transport in furin and nSMase activation by TNF-α. Brefeldin A (10 μM) or monensin (10 μM) was incubated with SMC for 6 h before stimulation with TNF-α and furin (A) or nSMase (B) activity determination. Brefeldin and monensin controls were done simultaneously and exhibited no toxicity for the cells during the time course of the experiment (not shown). Results are means ± SEM from three separate experiments. *, P < 0.05 (comparison between treated and untreated cells at time zero).
DISCUSSION
TNF-α is a pleiotropic cytokine involved in the regulation of multiple cellular functions such as cell growth, differentiation, or apoptosis, and it is associated with the development of various inflammatory diseases, atherosclerosis, cardiovascular dysfunction, and ischemia-reperfusion injuries (43). TNF-α is expressed in vascular SMC within atherosclerotic plaques and, like the other proinflammatory cytokines, may play a role in the formation and development in neointima by promoting cell migration and proliferation, which are critical for the progression of vascular lesions (21). The sphingomyelin/ceramide pathway is thought to play a key role in TNF-α signaling and subsequent biological responses, including endothelium vasoconstriction, endothelial nitric oxide synthase expression, cell proliferation, and apoptosis (20, 23, 27, 28). So far, little is known about the precise mechanisms linking TNF-α to nSMase, a key enzyme involved in sphingomyelin hydrolysis, ceramide generation, and further signaling (23). This is, to our knowledge, the first report of a mechanism of nSMase activation by TNF-α that involves complex regulation by a proteolytic cascade (furin, MT1-MMP, and MMP2) which plays a role in the mitogenic effect of TNF-α in SMC.
Our data show that activation of nSMase (and SK-1) is a prerequisite for TNF-α-induced proliferation of SMC and fibroblasts. The involvement of nSMase, and more precisely nSMase type 2 (SMPD3), was clearly demonstrated by the use of an siRNA specific for human nSMase2, which completely inhibited the TNF-α-induced activation of the enzyme (in agreement with previous results [32]) and subsequent SMC proliferation, whereas rescue experiments using cotransfection of a plasmid coding for the murine form of nSMase2 (smpd3) allowed restoration of both parameters. These data confirm the role of nSMase2 in the mitogenic effect of TNF-α, in this cell type, and are in agreement with the role of nSMase in TNF-α-induced signaling as reported previously (28). It is to be noted that nSMase2 transfection in MCF7 cells induces the opposite effect (growth arrest) in response to TNF-α (32). This may be attributed to the cell type, the tumoral state of these cells, the lack of MT1-MMP expression in MCF7 cells (41, 42), and/or the lack of coupling of sphingomyelin hydrolysis to activation of SK-1 in MCF7 cells, all of which appear to be necessary for the mitogenic effect of TNF-α, via MMP2 and nSMase activation, as reported in this work. This is consistent with the effect of DMS (an SK-1 inhibitor), which inhibited both ERK1/2 phosphorylation and cell proliferation elicited by TNF-α, indicating that SK-1 activation and subsequent S1P generation are required for SMC proliferation triggered by TNF-α. This is also in agreement with the role of the sphingolipid pathway in the mitogenic effect of oxidized LDL or plasminogen activators (7, 33). Finally, the early activation of nSMase2 by TNF-α was clearly involved in increased DNA synthesis, as assessed by the use of an siRNA specific to nSMase2. Beside nSMase2, the acid SMase has been implicated in sphingolipid metabolism triggered by TNF-α (1, 22, 23, 28). The membrane-proximal region of the cytoplasmic portion of TNFR is required for nSMase activation, whereas the membrane-distal region is implicated in acidic SMase activation (28). Ceramide generated from sphingomyelin hydrolysis by acidic SMase is apparently proapoptotic (39), whereas the apoptotic role of nSMase is still controversial.
Therefore, the specific roles of various SMases (and of nSMase2) in determining the cellular fate may depend on stimulus intensity, cell type, concomitant signaling pathways, and gene expression, including SK-1 and the balance between pro- and antiapoptotic factors (7, 24, 33, 46, 56, 57). It may be noted that, under the conditions used here (absence of an inhibitor of protein synthesis), nSMase activation by TNF-α was not associated with appreciable apoptosis (data not shown), in agreement with the recent observation that mice deficient in nSMase2 (smpd3) do not exhibit any apparent defect in apoptosis (3, 47). This is also in agreement with the role of the sphingolipid pathway in the mitogenic effect of oxidized LDL and plasminogen activators (7, 33).
Another major point of this work is the precise mechanism by which nSMase2 (and consequently the sphingolipid pathway) is activated by TNF-α. Our data point out the role of MMPs in the TNF-α-induced activation of the sphingolipid pathway, as assessed by the use of the MMP inhibitor batimastat, of mutant iMT1-SMC overexpressing an inactive form of MT1-MMP (which acts in our system as a dominant negative form), of MMP2−/− fibroblasts, and of siRNA strategies, which led to a complete inhibition of nSMase, ERK1/2 phosphorylation, and DNA synthesis. Moreover, exogenous MMP2 and MT1-MMP triggered per se nSMase activation and DNA synthesis in SMC. MT1-MMP and MMP2 are required for nSMase activation, with MMP2 being involved in the activation of nSMase, and MT1-MMP being necessary for MMP2 processing and activation, since neither iMT1-SMC nor MMP2−/− cells exhibited nSMase activation in response to TNF-α (these data were further confirmed in SMC and fibroblasts silenced for MMP2 and MT1-MMP by specific siRNA). This crucial role of MMPs in the TNF-α-induced nSMase activation and subsequent mitogenic effect is similar to that triggered by oxidized LDL (5). The precise molecular mechanism by which MMP2 induces nSMase activation remains to be elucidated. One hypothesis is that TNF-α may trigger the formation of a complex involving MT1-MMP, MMP2, and integrins (as reported previously [13]) but also nSMase, which could be indirectly activated by MMP2 within this complex, perhaps after the degradation of an inhibitory extracellular matrix component or of a more specific nSMase inhibitor specifically degraded by MMP2. It is likely that TIMP-2 also interacts with this signaling complex, since it is essential for cell-mediated activation of MMP2, by forming a complex with MT1-MMP and pro-MMP2 that allows pro-MMP2 cleavage by a second MT1-MMP molecule (18, 29, 48). However, the activation of MMP2 by exogenous APMA-activated MT1-MMP suggests that the role of TIMP-2 could not be essential in this system.
Finally, an important message is the mechanism by which MT1-MMP is activated by TNF-α. Previous results have shown that nSMase is inhibited by serine protease inhibitors, suggesting an involvement of such proteases in the activation of nSMase (5). Furin is a serine protease (proprotein convertase) involved in the activation of newly synthesized MT1-MMP during its translocation from the Golgi compartment to the cell surface (41, 48). In our experiments, the involvement of furin in MT1-MMP activation by TNF-α was clearly demonstrated by the lack of MT1-MMP and nSMase activation in SMC in which furin was silenced by siRNA treatment and in α1-PDX-transfected SMC, in which furin is constitutively inhibited with α1-antitrypsin Portland, a potent furin inhibitor (51). The TNF-α-induced furin activation was abrogated by brefeldin A and monensin, two agents that block ER-Golgi transport and Golgi-cell surface vesicular transport of proteins, respectively (14), in agreement with the proposal that furin is activated during its transport from the TGN to the cell surface (16, 51). Moreover, as expected, TNF-α-induced nSMase activation was also blocked by these inhibitors, thus confirming that TNF-α-induced activation of nSMase requires furin that acts upstream from the MMPs. The mechanism of activation of the furin propeptide is a multistep process with compartment- and pH-specific proteolytic cleavages leading to active enzyme, as reported previously (16, 51). This mechanism remains unknown for TNF-α (release of the excised propeptide in late secretory compartments, activation of furin autocleavage) and is currently under investigation.
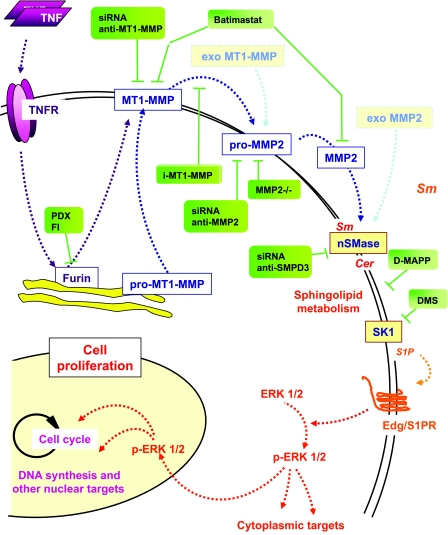
In summary, TNF-α triggers a signaling cascade (summarized in Fig. 8), including the transport of furin from the TGN network to the cellular membrane and sequential processing and activation of MT1-MMP and MMP2, which in turn activates nSMase2, the sphingolipid pathway, and cell proliferation (Fig. 7). So far the links between the TNF-α receptor and furin activation are not known. An involvement of oxidative stress could be hypothesized, since N-acetylcysteine totally inhibited this signaling cascade, including furin, MMPs, and the sphingolipid pathway, as well as SMC proliferation (data not shown), in agreement with reference 45 and previous reports showing an inhibition of nSMase by glutathione depletion and N-acetylcysteine (30).
FIG. 8.
Schematic diagram of the signaling pathways activated by mitogenic concentrations of TNF-α and roles of furin, MT1-MMP, and MMP2 in the activation of the nSMase in mesenchymal cells. nSMase is the first step of the sphingomyelin/ceramide pathway, which leads to S1P generation (when the intermediate steps, including ceramidase and SK, are working). S1P can in turn interact with Edg/S1P receptors, which trigger a signaling mitogenic cascade involving ERK1/2 activation. This effect is mimicked by adding exogenous activated MT1-MMP and MMP2 (light blue). The sites of action of the molecular and pharmacological inhibitors used in the paper are indicated by green labels. Sm, sphingomyelinase.
Recent evidence indicates that MT1-MMP promotes arterial wall invasion by SMC and could thereby play a major role in vascular wall remodeling and neointima formation (15). MMP2 expression is also associated with extracellular matrix proteolysis and migration and proliferation of SMC (8, 10, 15, 17). These events play a potential crucial role in atherosclerotic intima thickening, plaque formation, vascular wall remodeling, and restenosis (10). Moreover, MMPs are also thought to be involved in tumor spreading, metastasis invasion, and angiogenesis (15). Our results suggest a new cellular signaling network including furin, MT1-MMP, and MMP2, leading to the activation of nSMase2. Interestingly, this pathway is not activated by classical growth factors such as platelet-derived growth factor (data not shown) or FCS and thus could represent a general mechanism shared by various stress-inducing agents (oxidized LDL, oxidative stress, and cytokines) which are involved in vascular diseases and cancers.
Acknowledgments
We thank Alex Strongin (Cancer Research Center, The Burnham Institute, La Jolla, CA) for the plasmids encoding inactive and fully active human MT1-MMP and Hans-Willi Krell (Roche Diagnostics GmbH, Penzberg, Germany) for providing batimastat. We thank Gary Thomas (Vollum Institute, Oregon Health Sciences University, Portland) for providing the PDX-1-encoding vector and fruitful discussion.
This work was supported by INSERM, Paul Sabatier University, Agence Nationale pour la Recherche (ANR-05-PCOD-D019-01 Lisa), and Groupement Lipids et Nutrition. E.T. is the recipient of support from the Ministère de l'Enseignement Supérieur et de la Recherche.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Adam, D., K. Wiegmann, S. Adam-Klages, A. Ruff, and M. Kronke. 1996. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. J. Biol. Chem. 271:14617-14622. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal, B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. [DOI] [PubMed] [Google Scholar]
- 3.Aubin, I., C. P. Adams, S. Opsahl, D. Septier, C. E. Bishop, N. Auge, R. Salvayre, A. Negre-Salvayre, M. Goldberg, J. L. Guenet, and C. Poirier. 2005. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat. Genet. 37:803-805. [DOI] [PubMed] [Google Scholar]
- 4.Auge, N., N. Andrieu, A. Negre-Salvayre, J. C. Thiers, T. Levade, and R. Salvayre. 1996. The sphingomyelin-ceramide signaling pathway is involved in oxidized low density lipoprotein-induced cell proliferation. J. Biol. Chem. 271:19251-19255. [DOI] [PubMed] [Google Scholar]
- 5.Auge, N., F. Maupas-Schwalm, M. Elbaz, J. C. Thiers, A. Waysbort, S. Itohara, H. W. Krell, R. Salvayre, and A. Negre-Salvayre. 2004. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 110:571-578. [DOI] [PubMed] [Google Scholar]
- 6.Auge, N., A. Negre-Salvayre, R. Salvayre, and T. Levade. 2000. Sphingomyelin metabolites in vascular cell signaling and atherogenesis. Prog. Lipid Res. 39:207-229. [DOI] [PubMed] [Google Scholar]
- 7.Auge, N., M. Nikolova-Karakashian, S. Carpentier, S. Parthasarathy, A. Negre-Salvayre, R. Salvayre, A. H. Merrill, Jr., and T. Levade. 1999. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J. Biol. Chem. 274:21533-21538. [DOI] [PubMed] [Google Scholar]
- 8.Belien, A. T., P. A. Paganetti, and M. E. Schwab. 1999. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J. Cell Biol. 144:373-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolick, D. T., S. Srinivasan, K. W. Kim, M. E. Hatley, J. J. Clemens, A. Whetzel, N. Ferger, T. L. Macdonald, M. D. Davis, P. S. Tao, K. R. Lynch, and C. C. Hedrick. 2005. Sphingosine-1-phosphate prevents tumor necrosis factor-α-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler. Thromb. Vasc. Biol. 25:976-981. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein, P., A. Agah, and T. R. Kyriakides. 2004. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int. J. Biochem. Cell. Biol. 36:1115-1125. [DOI] [PubMed] [Google Scholar]
- 11.Canault, M., F. Peiretti, C. Mueller, F. Kopp, P. Morange, S. Rihs, H. Portugal, I. Juhan-Vague, and G. Nalbone. 2004. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis 172:211-218. [DOI] [PubMed] [Google Scholar]
- 12.Cao, J., P. Kozarekar, M. Pavlaki, C. Chiarelli, W. F. Bahou, and S. Zucker. 2004. Distinct roles for the catalytic and hemopexin domains of membrane type 1-matrix metalloproteinase in substrate degradation and cell migration. J. Biol. Chem. 279:14129-14139. [DOI] [PubMed] [Google Scholar]
- 13.Deryugina, E. I., B. Ratnikov, E. Monosov, T. I. Postnova, R. DiScipio, J. W. Smith, and A. Y. Strongin. 2001. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp. Cell Res. 263:209-223. [DOI] [PubMed] [Google Scholar]
- 14.Dinter, A., and E. G. Berger. 1998. Golgi-disturbing agents. Histochem. Cell Biol. 109:571-590. [DOI] [PubMed] [Google Scholar]
- 15.Egeblad, M., and Z. Werb. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2:161-174. [DOI] [PubMed] [Google Scholar]
- 16.Feliciangeli, S. F., L. Thomas, G. K. Scott, E. Subbian, C. H. Hung, S. S. Molloy, F. Jean, U. Shinde, and G. Thomas. 2006. Identification of a pH sensor in the furin propeptide that regulates enzyme activation. J. Biol. Chem. 281:16108-16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippov, S., G. C. Koenig, T. H. Chun, K. B. Hotary, I. Ota, T. H. Bugge, J. D. Roberts, W. P. Fay, H. Birkedal-Hansen, K. Holmbeck, F. Sabeh, E. D. Allen, and S. J. Weiss. 2005. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J. Exp. Med. 202:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillmore, H. L., T. E. VanMeter, and W. C. Broaddus. 2001. Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion. J. Neurooncol. 53:187-202. [DOI] [PubMed] [Google Scholar]
- 19.Futerman, A. H., and Y. A. Hannun. 2004. The complex life of simple phospholipids. EMBO Rep. 5:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaur, U., and B. B. Aggarwal. 2003. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 66:1403-1408. [DOI] [PubMed] [Google Scholar]
- 21.Goetze, S., U. Kintscher, K. Kaneshiro, W. P. Meehan, A. Collins, E. Fleck, W. A. Hsueh, and R. E. Law. 2001. TNF induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis 159:93-101. [DOI] [PubMed] [Google Scholar]
- 22.Goni, F. M., and A. Alonso. 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531:38-46. [DOI] [PubMed] [Google Scholar]
- 23.Hannun, Y. A. 1996. Functions of ceramide in coordinating cellular responses to stress. Science 274:1855-1859. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka, E., S. Kawashima, T. Takahashi, Y. Rikitake, T. Kitamura, W. Ogawa, and M. Yokoyama. 2001. TNF-alpha induces protein synthesis through PI3-kinase-Akt/PKB pathway in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 280:H1861-H1868. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda, U., and K. Shimada. 2003. Matrix metalloproteinases and coronary artery diseases. Clin. Cardiol. 26:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh, T., T. Ikeda, H. Gomi, S. Nakao, T. Suzuki, and S. Itohara. 1997. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 272:22389-22392. [DOI] [PubMed] [Google Scholar]
- 27.Kolesnick, R. 1994. Signal transduction through the sphingomyelin pathway. Mol. Chem. Neuropathol. 21:287-297. [DOI] [PubMed] [Google Scholar]
- 28.Kronke, M. 1999. Involvement of sphingomyelinases in TNF signaling pathways. Chem. Phys. Lipids 102:157-166. [DOI] [PubMed] [Google Scholar]
- 29.Lehti, K., J. Lohi, M. M. Juntunen, D. Pei, and J. Keski-Oja. 2002. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 277:8440-8448. [DOI] [PubMed] [Google Scholar]
- 30.Liu, B., N. Andrieu-Abadie, T. Levade, P. Zhang, L. M. Obeid, and Y. A. Hannun. 1998. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J. Biol. Chem. 273:11313-11320. [DOI] [PubMed] [Google Scholar]
- 31.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 32.Marchesini, N., W. Osta, J. Bielawski, C. Luberto, L. M. Obeid, and Y. A. Hannun. 2004. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J. Biol. Chem. 279:25101-25111. [DOI] [PubMed] [Google Scholar]
- 33.Maupas-Schwalm, F., N. Auge, C. Robinet, J. P. Cambus, S. J. Parsons, R. Salvayre, and A. Negre-Salvayre. 2004. The sphingomyelin/ceramide pathway is involved in ERK1/2 phosphorylation, cell proliferation, and uPAR overexpression induced by tissue-type plasminogen activator. FASEB J. 18:1398-1400. [DOI] [PubMed] [Google Scholar]
- 34.Nagase, H., and J. F. Woessner, Jr. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491-21494. [DOI] [PubMed] [Google Scholar]
- 35.Netzel-Arnett, S., S. K. Mallya, H. Nagase, H. Birkedal-Hansen, and H. E. Van Wart. 1991. Continuously recording fluorescent assays optimized for five human matrix metalloproteinases. Anal. Biochem. 195:86-92. [DOI] [PubMed] [Google Scholar]
- 36.Newby, A. C., and A. B. Zaltsman. 1999. Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc. Res. 41:345-360. [PubMed] [Google Scholar]
- 37.Newby, A. C. 2005. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 85:1-31. [DOI] [PubMed] [Google Scholar]
- 38.Pei, D., and S. J. Weiss. 1996. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J. Biol. Chem. 271:9135-9140. [DOI] [PubMed] [Google Scholar]
- 39.Pena, L. A., Z. Fuks, and R. Kolesnick. 1997. Stress-induced apoptosis and the sphingomyelin pathway. Biochem. Pharmacol. 53:615-621. [DOI] [PubMed] [Google Scholar]
- 40.Pettus, B. J., C. E. Chalfant, and Y. A. Hannun. 2002. Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta 1585:114-125. [DOI] [PubMed] [Google Scholar]
- 41.Remacle, A. G., D. V. Rozanov, M. Fugere, R. Day, and A. Y. Strongin. 2006. Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene 25:5648-5655. [DOI] [PubMed] [Google Scholar]
- 42.Rozanov, D. V., E. I. Deryugina, B. I. Ratnikov, E. Z. Monosov, G. N. Marchenko, J. P. Quigley, and A. Y. Strongin. 2001. Mutation analysis of membrane type-1 matrix metalloproteinase (MT1-MMP). The role of the cytoplasmic tail Cys(574), the active site Glu(240), and furin cleavage motifs in oligomerization, processing, and self-proteolysis of MT1-MMP expressed in breast carcinoma cells. J. Biol. Chem. 276:25705-25714. [DOI] [PubMed] [Google Scholar]
- 43.Sack, M. 2002. Tumor necrosis factor-alpha in cardiovascular biology and the potential role for anti-tumor necrosis factor-alpha therapy in heart disease. Pharmacol. Ther. 94:123-135. [DOI] [PubMed] [Google Scholar]
- 44.Sato, T., T. Kondo, T. Fujisawa, M. Seiki, and A. Ito. 1999. Furin-independent pathway of membrane type 1-matrix metalloproteinase activation in rabbit dermal fibroblasts. J. Biol. Chem. 274:37280-37284. [DOI] [PubMed] [Google Scholar]
- 45.Singh, I., K. Pahan, M. Khan, and A. K. Singh. 1998. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J. Biol. Chem. 273:20354-20362. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel, S., and S. Milstein. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 4:397-407. [DOI] [PubMed] [Google Scholar]
- 47.Stoffel, W., B. Jenke, B. Block, M. Zumbansen, and J. Koebke. 2005. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl. Acad. Sci. USA 102:4554-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strongin, A. Y., I. Collier, G. Bannikov, B. L. Marmer, G. A. Grant, and G. I. Goldberg. 1995. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 270:5331-5338. [DOI] [PubMed] [Google Scholar]
- 49.Suh, S. J., U. H. Jin, S. H. Kim, H. W. Chang, J. K. Son, S. H. Lee, K. H. Son, and C. H. Kim. 2006. Ochnaflavone inhibits TNF-alpha-induced human VSMC proliferation via regulation of cell cycle, ERK1/2, and MMP-9. J. Cell Biochem. 99:1298-1307. [DOI] [PubMed] [Google Scholar]
- 50.Tani, M., Y. Igarashi, and M. Ito. 2005. Involvement of neutral ceramidase in ceramide metabolism at the plasma membrane and in extracellular milieu. J. Biol. Chem. 280:36592-36600. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 3:753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varfolomeev, E. E., and A. Ashkenazi. 2004. Tumor necrosis factor: an apoptosis JuNKie? Cell 116:491-497. [DOI] [PubMed] [Google Scholar]
- 53.Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92:827-839. [DOI] [PubMed] [Google Scholar]
- 54.Wang, Z., P. J. Rao, M. R. Castresana, and W. H. Newman. 2005. TNF-alpha induces proliferation or apoptosis in human saphenous vein smooth muscle cells depending on phenotype. Am. J. Physiol. Heart Circ. Physiol. 288:H293-301. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe, M., A. Hirano, S. Stenglein, J. Nelson, G. Thomas, and T. C. Wong. 1995. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J. Virol. 69:3206-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiegmann, K., S. Schutze, T. Machleidt, D. Witte, and M. Kronke. 1994. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 78:1005-1015. [DOI] [PubMed] [Google Scholar]
- 57.Xia, P., L. Wang, J. R. Gamble, and M. A. Vadas. 1999. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J. Biol. Chem. 274:34499-34505. [DOI] [PubMed] [Google Scholar]
- 58.Yana, I., and S. J. Weiss. 2000. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell 11:2387-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zucker, S., M. Hymowitz, C. E. Conner, E. A. DiYanni, and J. Cao. 2002. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab. Investig. 82:1673-1684. [DOI] [PubMed] [Google Scholar]