Abstract
Proper transcription by RNA polymerase II is dependent on the modification state of the chromatin template. The Paf1 complex is associated with RNA polymerase II during transcription elongation and is required for several histone modifications that mark active genes. To uncover additional factors that regulate chromatin or transcription, we performed a genetic screen for mutations that cause lethality in the absence of the Paf1 complex component Rtf1. Our results have led to the discovery of a previously unstudied gene, RKR1. Strains lacking RKR1 exhibit phenotypes associated with defects in transcription and chromatin function. These phenotypes include inositol auxotrophy, impaired telomeric silencing, and synthetic lethality with mutations in SPT10, a gene that encodes a putative histone acetyltransferase. In addition, deletion of RKR1 causes severe genetic interactions with mutations that prevent histone H2B lysine 123 ubiquitylation or histone H3 lysine 4 methylation. RKR1 encodes a conserved nuclear protein with a functionally important RING domain at its carboxy terminus. In vitro experiments indicate that Rkr1 possesses ubiquitin-protein ligase activity. Taken together, our results identify a new participant in a protein ubiquitylation pathway within the nucleus that acts to modulate chromatin function and transcription.
Progression of the RNA polymerase II (Pol II) transcription cycle involves the coordinated functions of a large number of regulatory proteins. During transcription elongation, proteins that associate with Pol II assist it in overcoming obstacles to transcription, including DNA damage and condensed chromatin structure. Transcription elongation factors use a variety of mechanisms to facilitate movement of Pol II through a nucleosomal template and coordinate transcription with RNA processing. The Paf1 complex is a Pol II-associated factor that alters the state of the chromatin template during transcription elongation. Biochemical purification of the Saccharomyces cerevisiae Paf1 complex showed that it minimally contains five proteins: Paf1, Ctr9, Rtf1, Cdc73, and Leo1 (37, 44, 76). Chromatin immunoprecipitation experiments demonstrated that this complex is associated with the open reading frames (ORFs) of actively transcribed genes (37, 59, 72). Strains lacking members of the Paf1 complex are sensitive to the base analogs 6-azauracil and mycophenolic acid and exhibit altered RNA levels for a large number of genes (10, 44, 57, 76). These results, combined with genetic and physical interactions with the elongation factors Spt4-Spt5 and Spt16-Pob3 (yFACT complex) (76), suggest that the Paf1 complex is important for transcription elongation. Members of the Paf1 complex are also required for proper 3′ end formation of both polyadenylated and nonpolyadenylated transcripts (57, 68).
It is now well established that the arrangements of posttranslational modifications on nucleosomal histones, along with interactions between histones and nonhistone proteins, coordinately affect chromatin structure. Histones can be methylated, acetylated, phosphorylated, sumoylated, and ubiquitylated (3, 49, 69). The dynamic state of posttranslational chromatin modifications affects many processes, including transcription. A primary function of the Paf1 complex is to mediate several histone modifications during transcription elongation. The Paf1 complex component Rtf1 is required for the recruitment and activity of Rad6 and Bre1, the ubiquitin-conjugating enzyme and ubiquitin-protein ligase that monoubiquitylate histone H2B at lysine 123 (K123) (52, 84, 85). In contrast to protein polyubiquitylation, monoubiquitylation of histone H2B K123 does not lead to proteolysis. Instead, this modification promotes methylation of histone H3 at K4 and K79 by the Set1 and Dot1 methyltransferases, respectively (7, 54, 79). Therefore, Rtf1 is also required for these methylation events. Like the Paf1 complex, histone H3 K4 trimethylation is enriched at actively transcribed regions of the genome (66).
In addition to histone H2B, several other proteins important for Pol II transcription are modified by the protein ubiquitylation machinery within the nucleus (reviewed in references 40 and 47). When arrested in transcription elongation by DNA damage, Pol II is polyubiquitylated and degraded as part of the repair process (75). Ubiquitylation of numerous transcriptional activators has been observed, and for several of these, including the well-studied yeast activators Gcn4 and Gal4, ubiquitylation and proteasome-dependent proteolysis correlate with gene activation (41, 46). However, whether proteolytic turnover of activator proteins is obligatory for activation remains a topic of debate (48). The proteasome itself has been shown to reside within the nucleus and regulate gene expression through both proteolytic and nonproteolytic mechanisms (reviewed in reference 9). Components of the proteasome 19S regulatory complex facilitate interactions between the SAGA histone acetyltransferase complex and transcriptional activators (39), couple H3 K4 and K79 methylation to H2B ubiquitylation (15), and localize to genes in a transcription-dependent manner (21).
Covalent attachment of ubiquitin to a substrate protein requires the coordinated functions of a ubiquitin-activating enzyme, a ubiquitin-conjugating enzyme, and a ubiquitin-protein ligase (reviewed in reference 16). While yeast contains only 1 ubiquitin-activating enzyme and 11 ubiquitin-conjugating enzymes, there are many predicted ubiquitin-protein ligases, consistent with their proposed roles in determining substrate specificity (reviewed in reference 58). Ubiquitin-protein ligases contain functional domains known as RING or HECT domains that interact with ubiquitin-conjugating enzymes (30, 42). Several nuclear RING domain-containing ubiquitin-protein ligases have been characterized in yeast. These include Rad5 and Rad18, which direct the ubiquitylation of PCNA during DNA damage repair (27); San1, which is required for the degradation of certain misfolded nuclear proteins (11, 19); Bre1, which ubiquitylates histone H2B K123 (31, 83); and Not4, which is a component of the multifunctional Ccr4-Not complex (56).
Within the nucleus, many regulatory pathways act coordinately to promote transcription. The identification of all proteins involved in these pathways, as well as the elucidation of their functions and regulation, is needed to fully appreciate how transcription proceeds despite barriers imposed by the chromatin template. In this paper, we report the discovery of a previously unstudied nuclear protein, Rkr1, with strong functional links to chromatin and transcription. Our genetic studies suggest that Rkr1 operates in parallel with histone H2B ubiquitylation and histone H3 K4 methylation to regulate an important nuclear process. Consistent with a role in chromatin regulation, deletion of RKR1 causes defects in telomeric silencing. A highly conserved RING domain at the carboxy terminus of Rkr1 is essential for its in vivo functions, and in vitro experiments demonstrate that this RING domain has ubiquitin-protein ligase activity. Together, our data indicate that Rkr1 likely functions as a nuclear ubiquitin-protein ligase that targets proteins important for proper chromatin structure and transcription.
MATERIALS AND METHODS
Media.
Rich (yeast extract-peptone-dextrose [YPD]), YP-glycerol (YPG), synthetic complete (SC), synthetic dextrose (SD), 5-fluoro-orotic acid (5-FOA), and sporulation media were prepared as described previously (62). Media lacking or containing 200 μM inositol were prepared with yeast nitrogen base that contained ammonium sulfate but lacked inositol (Q-Bio Systems). NaCl, LiCl, caffeine, and hydroxyurea were added to YPD medium to final concentrations of 1.4 M, 0.3 M, 15 mM, and 100 mM, respectively. 6-Azauracil (6AU) was added to SC-Ura medium to a final concentration of 50 μg/ml. G418 medium, for selection of yeast strains expressing the kanMX4 gene, was prepared as described previously (33).
Genetic methods.
With the exception of O660 and KA102 to KA106, all strains (Table 1) are isogenic to FY2, a GAL2+ derivative of S288C (82). RKR1, BRE1, LGE1, DOT1, SET1, and SET2 disruptions were created by a PCR-based method using the HIS3-marked plasmid pRS303 and the kanMX4-marked plasmid pRS400 (2). In each case, disruptions were made in diploid strains and confirmed by PCR or Southern analysis. Haploid mutant progeny were obtained by tetrad dissection. All disruptions remove the entire ORF and replace it with the indicated selectable marker. Genetic crosses, tetrad analyses, and yeast transformations were performed by standard methods (20, 62).
TABLE 1.
Saccharomyces cerevisiae strains
| Straina | Genotype |
|---|---|
| FY14 | MATaura3-52 trp1Δ63 |
| FY245 | MATaspt4Δ::URA3 ura3-52 trp1Δ63 |
| FY406 | MATa (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pSAB6 = HTA1-HTB1 URA3 CEN/ARS] |
| FY623 | MATα rad6Δ::URA3 his4-912δ lys2-128δ leu2Δ1 ura3-52 suc2ΔUAS (−1,900/−390) |
| FY632 | MATa/α his4-917δ/his4-917δ lys2-173R2/lys2-173R2 leu2Δ1/leu2Δ1 ura3-52/ura3-52 trp1Δ63/trp1Δ63 |
| FY896 | MATaspt10Δ::TRP1 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 suc2ΔUAS (−1,900/−390) |
| FY1990 | MATα (hht1-hhf1)Δ::LEU2 (hht2-hhf2)Δ::kanMX4 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pDM1 = HHT2-HHF2 URA3 CEN/ARS] |
| FY2199 | MATaspt21Δ::HIS3 his3Δ200 lys2-128δ leu2Δ0 ura3Δ0 |
| GHY1094 | MATα ctr9Δ::kanMX4 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| O660 | MATα TELVR::URA3 his3−ura3-52 trp1− |
| KA102 | MATα rkr1Δ::kanMX4 TELVR::URA3 lys2-128δ ura3-52 trp1− |
| KA103 | MATarkr1Δ::kanMX4 TELVR::URA3 lys2-128δ ura3-52 |
| KA104 | MATα TELVR::URA3 lys2-128δ ura3-52 |
| KA105 | MATα spt10Δ201::HIS3 TELVR::URA3 his3Δ200 lys2-128δ ura3(−52 or Δ0) trp(1Δ63 or 1−) [pMB27 = SPT10 TRP1 CEN/ARS] |
| KA106 | MATα spt10Δ201::HIS3 TELVR::URA3 his3Δ200 lys2-128δ ura3(−52 or Δ0) trp(1Δ63 or 1−) [pMB27 = SPT10 TRP1 CEN/ARS] |
| KY714 | MATappr2Δ::HISG-URA3 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY715 | MATaspt5-194 his3Δ200 leu2Δ1 ura3-52 |
| KY802 | MATapaf1Δ::URA3 his3Δ200 lys2-173R2 ura3(-52 or Δ0) |
| KY806 | MATaleo1Δ::URA3 his3Δ200 lys2-173R2 ura3-52 |
| KY903 | MATadot1Δ::HIS3 his3Δ200 his4-912δ lys2-128δ leu2Δ1 ura3-52 |
| KY907 | MATaset1Δ::HIS3 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY912 | MATaset2Δ::HIS3 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY957 | MATartf1Δ101::LEU2 his3Δ200 leu2Δ1 ura3-52 trp1Δ63 |
| KY960 | MATartf1Δ::LEU2 rkr1Δ::kanMX4 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pKA69 = RTF1 URA3 CEN/ARS] |
| KY968 | MATabre1Δ::kanMX4 his3Δ200 ura3-52 |
| KY981 | MATarkr1Δ::kanMX4 (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pSAB6 = HTA1-HTB1 URA3 CEN/ARS] |
| KY982 | MATartf1Δ::kanMX4 (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pSAB6 = HTA1-HTB1 URA3 CEN/ARS] |
| KY1064 | MATα rkr1Δ::HIS3 (hht1-hhf1)Δ::LEU2 (hht2-hhf2)Δ::kanMX4 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pDM1 = HHT2-HHF2 URA3 CEN/ARS] |
| KY1162 | MATα cdc73Δ::kanMX4 his3Δ200 leu2Δ0 ura3-52 |
| KY1163 | MATα spt5-242 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY1164 | MATα spt6-14 his3Δ200 lys2-128δ leu2Δ1 |
| KY1165 | MATaspt16-197 his3Δ200 his4-912δ leu2Δ1 ura3-52 |
| KY1166 | MATarkr1Δ::kanMX4 his3Δ200 leu2Δ1 trp1Δ63 |
| KY1167 | MATarkr1Δ::kanMX4 ura3-52 |
| KY1168 | MATα rkr1Δ::kanMX4 his3Δ200 trp1Δ63 |
| KY1169 | MATα rkr1Δ::kanMX4 his3Δ200 lys2-128δ ura3-52 |
| KY1170 | MATarkr1Δ::kanMX4 ura3-52 trp1Δ63 |
| KY1171 | MATα rkr1Δ::HIS3 his3Δ200 his4-912δ leu2Δ1 |
| KY1172 | MATα rkr1Δ::kanMX4 leu2Δ1 ura3-52 trp1Δ63 |
| KY1173 | MATa/α RKR1/rkr1Δ::HIS3 his3Δ200/his3Δ200 LEU2/leu2Δ1 URA3/ura3-52 TRP1/trp1Δ63 |
| KY1174 | MATα rtf1Δ3 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 arg4-12 |
| KY1175 | MATα rtf1Δ4 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 arg4-12 |
| KY1176 | MATα rkr1Δ::HIS3 his3Δ200 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 |
| KY1177 | MATα rkr1Δ::HIS3 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY1178 | MATarkr1Δ::HIS3 his3Δ200 lys2-128δ leu2Δ1 ura3-52 |
| KY1179 | MATα rkr1Δ::HIS3 his3Δ200 his4-912δ lys2-128δ leu2Δ1 trp1Δ63 |
| KY1180 | MATarkr1Δ::HIS3 his3Δ200 lys2-128δ leu2Δ1 trp1Δ63 |
| KY1222 | MATα rkr1Δ::kanMX4 set1Δ::HIS3 his3Δ200 leu2Δ1 ura3-52 [pMB77 = rkr1-C1508A LEU2 CEN/ARS] |
FY, GHY, and KA/KY strains were generated in the laboratories of F. Winston, G. A. Hartzog, and K. M. Arndt, respectively. Strain O660 was obtained from F. Winston.
Plasmids.
Standard cloning techniques were used to construct all plasmids (2). pPC65, a pRS314 (71) derivative of a library plasmid (pPC62) that was obtained through complementation of the SL505 mutation, contains a 7,447-bp insert that includes tA(AGC)M2 (alanine tRNA), RKR1, and part of FAA4. pMB11 is identical to pPC65, except for the addition of the triple hemagglutinin (HA) epitope sequence (3XHA) at the amino terminus of Rkr1. To introduce the epitope tag, pSH3 was created by subcloning the XhoI/AatII fragment of pPC65 (containing the promoter/5′ end of RKR1) into pRS406 (71). Site-directed mutagenesis (Quikchange; Stratagene) was used to introduce an NdeI site at the ATG of RKR1 in pSH3, and a PCR fragment encoding the triple HA tag flanked by NdeI sites was inserted at this site. The XhoI/AatII fragment was then subcloned back into pPC65 to create pMB11. pMB66 was created by site-directed mutagenesis using pMB11 as a template to replace cysteine 1508 of Rkr1 with alanine. pMB11 and pMB66 were sequenced over the entire RKR1 ORF to ensure that no undesired mutations were introduced. pMB26, which contains HTA1, FLAG-htb1-K123R, and HIS3, was created by replacing a 737-bp XhoI/SphI fragment from a plasmid (54) containing FLAG-HTB1 with a 737-bp fragment containing htb1-K123R (54). The presence of FLAG-htb1-K123R in pMB26 was confirmed by DNA sequencing. Derivatives of pRS314 that contain HHT2 and myc-HHF2 (pNOY436) (35), wild-type HHT2 and HHF2 (pJH18) (28), or hht2-K4R and HHF2 (6) were described previously. pMB27, which contains SPT10 and TRP1, was created by ligating a 2,503-bp SpeI/XhoI fragment from pGN1101 (50) to SpeI/XhoI-digested pRS314 (71). pMB30 was created by ligating a 1,137-bp blunt-ended BanI/StuI fragment from pPC65 to SmaI-digested pGEX-3X (74) to generate a glutathione S-transferase (GST) fusion protein containing amino acids 1251 to 1562 of Rkr1. pMB81 was created by site-directed mutagenesis of pMB30, replacing cysteine 1508 of Rkr1 with an alanine. pMB81 was sequenced throughout the RKR1 ORF to ensure that no secondary mutations were created.
Identification of RKR1.
RKR1 was identified in a previously described synthetic lethal screen with rtf1Δ (10). Determination of the identity of RKR1 was performed in the same manner as that of other genes identified in this screen. Briefly, RKR1 was cloned by complementation of the SL505 mutation identified in the synthetic lethal screen. Subcloning, linkage, and DNA sequencing analyses were performed to confirm that RKR1 was allelic to SL505. To determine the nature of the SL505 mutation, pPC62 was digested with BsrGI to delete nucleotides 1086 to 3468 of the RKR1 ORF. The unligated plasmid was transformed into a strain containing the SL505 mutation for gap repair (63). DNA sequencing showed that the SL505 mutation introduced a premature stop codon at position 1133 of the Rkr1 protein.
Sequence analysis.
PROSITE (http://ca.expasy.org/prosite) sequence analysis tools were used to predict the presence of any functional motifs or domains within the primary amino acid sequence of Rkr1. A RING domain is predicted at the carboxy terminus of Rkr1, consisting of amino acids 1508 to 1554. The sequence alignment shown in Fig. 2 was obtained from BLAST (http://www.ncbi.nlm.nih.gov/BLAST) searches, with the S. cerevisiae Rkr1 protein sequence in its entirety as the query. Sequences of the most similar proteins from Homo sapiens (accession no. NP_056380.1; E value, 4e−41), Mus musculus (accession no. XP_982690.1; E value, 7e−42), Drosophila melanogaster (accession no. NP_730427; E value, 1e−21), Arabidopsis thaliana (accession no. NP_200649.1; E value, 2e−36), and Schizosaccharomyces pombe (accession no. CAA20765.1; E value, 3e−51) were aligned with the S. cerevisiae Rkr1 sequence by using the ClustalW program (http://www.ebi.ac.uk/clustalw). The alignment of the last 150 amino acids of each protein was copied into Jalview (http://www.jalview.org), and grayscale shading was added with a threshold of 50% sequence identity.
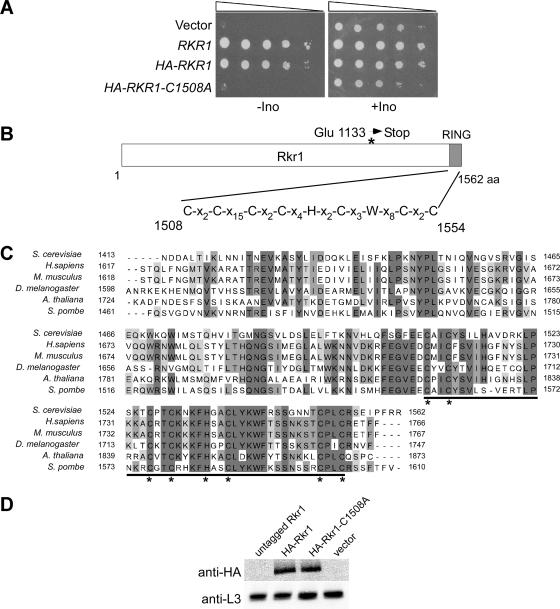
FIG. 2.
Rkr1 contains a conserved RING domain that is essential for in vivo function. (A) An rkr1Δ strain (KY1168) was transformed separately with the TRP1-marked CEN/ARS plasmids pPC65 (untagged Rkr1), pMB11 (HA-Rkr1), pMB66 (HA-Rkr1-C1508A), and pRS314 (empty vector). Cultures were spotted as 10-fold serial dilutions onto media lacking tryptophan in the presence or absence of inositol (Ino) and grown at 30°C for 3 days. (B) Schematic representation of the Rkr1 protein sequence. The conserved RING domain is highlighted in gray. The position of the amino acid change encoded by the SL505 mutation is indicated by an asterisk. (C) Alignment of primary amino acid sequences of the carboxy-terminal 150 amino acids of Rkr1 homologs as determined by BLAST searches. The degree of shading correlates with amino acid identity among the six sequences listed. The residues that make up the RING domain are underlined. The critical cysteine and histidine residues within the RING domain are marked with asterisks. (D) Immunoblotting analysis of strains used in panel A shows that the wild-type and C1508A forms of Rkr1 are expressed equally. L3 levels served as a loading control.
Growth assays.
Saturated cultures of each strain were grown in the appropriate media. Cells were collected by centrifugation, and cell pellets were washed two times with sterile water. Tenfold serial dilutions (1 × 108 cells/ml to 1 × 104 cells/ml) were made in sterile water, and 2 or 3 μl of each dilution was spotted onto the appropriate media. Spots were allowed to dry and plates were incubated at 30°C for 3 to 5 days. YPD and YPG plates were used in every assay to ensure even spotting and to assay for petite cells, respectively.
Immunoblotting analysis.
Transformed cells were grown under selective conditions to a density of approximately 4 × 107 cells/ml. Whole-cell extracts were prepared by glass bead lysis in lysis buffer (100 mM sodium acetate, 20 mM HEPES [pH 7.4], 2 mM magnesium acetate, 10 mM EDTA, 10% glycerol, and 1 mM dithiothreitol DTT, plus protease inhibitors), as previously described (70). Proteins (20 or 25 μg extract per lane) were separated on 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels for analysis of Rkr1 levels or on 15% SDS-polyacrylamide gels for analysis of histone levels and were transferred to nitrocellulose membranes. The following antibodies were used: anti-HA (1:3,000 dilution; Roche), anti-L3 (1:5,000 dilution) (81), anti-FLAG M2 (1:1,000; Sigma), anti-myc (1:500; Covance), and anti-H3 (1:2,000; Upstate). Horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Biosciences) were used at a 1:5,000 dilution. Immunoreactive proteins were detected by chemiluminescence (Perkin Elmer) and visualized with a Kodak 440CF digital imaging station.
Indirect immunofluorescence.
Yeast strain FY632 was transformed with pPC65 (untagged RKR1) or pMB11 (3XHA-RKR1), and transformants were grown in SC medium lacking tryptophan to a density of approximately 1 × 107 cells/ml. A total of 1.4 × 108 cells were processed for indirect immunofluorescence, as described previously (77). The primary antibody, anti-HA (Roche), was added at a 1:3,000 dilution, and the secondary antibody, Cy3-conjugated goat anti-mouse immunoglobulin G (Alexa Fluor 488; Molecular Probes), was added at a 1:250 dilution. DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride) was used to stain the DNA, as described previously (60). Cells were visualized with an Olympus BX60 epifluorescence microscope and photographed with QED in vivo software.
RT-PCR analysis.
Total RNA was prepared from yeast strains FY14, KY1170, and FY896 grown in YPD to approximately 1 × 107 cell/ml and used for reverse transcription (RT)-PCR analysis, as described previously (26). RNA was DNase treated with the Turbo DNA-free kit (Ambion), and cDNA was prepared with 1 μg of RNA, 500 ng oligo(dT20), and 200 units of SuperScript II reverse transcriptase (Invitrogen) in a 20-μl reaction. Two different amounts of cDNA (0.05 and 0.15 μg) were used in separate PCRs to ensure linearity. Experiments were performed in duplicate and analyzed by ethidium bromide staining of agarose gels.
Purification of recombinant proteins and in vitro ubiquitylation assays.
BL21(DE3) cells were transformed separately with plasmids expressing wild-type (pMB30) or mutant (pMB81) GST-Rkr1 fusion proteins or GST alone (pGEX-3X). Cells were grown at 37°C and induced for 1.5 h at an optical density at 600 nm of 0.6 to 0.7 with 0.3 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Cell lysates were made by sonication in a buffer containing 50 mM Tris-Cl, pH 8.0, and 1 mM EDTA, as described previously (11). Lysate (10 ml) was incubated for 1 h at 4°C with 1 ml prewashed GST-Sepharose beads in the presence of 1% Triton X-100 and protease inhibitors. Sepharose beads were pelleted and washed three times with phosphate-buffered saline (PBS) (pH 7.3) (2) plus 10% glycerol, 1% Triton X-100, and protease inhibitors. Fusion proteins were eluted in PBS plus 1% Triton X-100 and 50 mM glutathione for 10 min at room temperature.
Ubiquitylation assays were performed as previously described (19), with some modifications. Ubiquitin-activating enzyme (yeast recombinant Uba1), ubiquitin-conjugating enzyme (human recombinant UbcH5a), and ubiquitin (human recombinant) were purchased from Boston Biochem. One hundred nanograms of Uba1, 200 ng of UbcH5a, and ∼500 ng of GST fusion proteins were combined in reaction buffer (50 mM Tris-Cl [pH 7.5], 2 mM ATP, 2.5 mM MgCl2, and 0.5 mM dithiothreitol) with 2.5 μg of ubiquitin in 30-μl reaction volumes. Reaction mixtures were incubated for 1.5 h at 30°C. Proteins were separated on a 7 to 20% gradient SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Immunoblotting analysis was performed with antiubiquitin antibody (34) at a 1:50 dilution to detect ubiquitin-conjugated substrates and anti-GST antibody (Invitrogen) at a 1:500 dilution to detect and normalize the levels of GST fusion proteins. HRP-conjugated secondary antibodies (Amersham Biosciences) were used at 1:5,000 dilutions. Chemiluminescent signals (Perkin Elmer) were detected with a Kodak 440CF digital imaging station.
RESULTS
Identification of Rkr1.
The Paf1 complex functions in transcription elongation, RNA 3′ end formation, and histone modification. Original support for a role of the Paf1 complex in transcription elongation came from a genetic screen for mutations that cause lethality in combination with an rtf1Δ mutation (10). This screen identified synthetic lethal mutations in CTK1, FCP1, and POB3, which encode an RNA Pol II CTD kinase, an RNA Pol II CTD phosphatase, and a component of the FACT transcription elongation complex, respectively. In addition to these well-characterized RNA Pol II transcription factors, this screen also identified a synthetic lethal mutation, the SL505 mutation, in a previously uncharacterized gene, YMR247c. YMR247c is a 4,686-bp ORF that is predicted to encode a protein of 1,562 amino acids. Linkage analysis confirmed that YMR247c contained the SL505 mutation, and DNA sequence analysis of a gap-repaired plasmid containing the YMR247c SL505 mutation showed that it led to a premature stop codon at amino acid 1133. Further analysis, which is described below, showed that truncation of this nonessential protein results in the loss of a functionally important RING domain at the carboxy terminus. We therefore decided to rename the gene RKR1, for “RING domain mutant killed by rtf1Δ.”
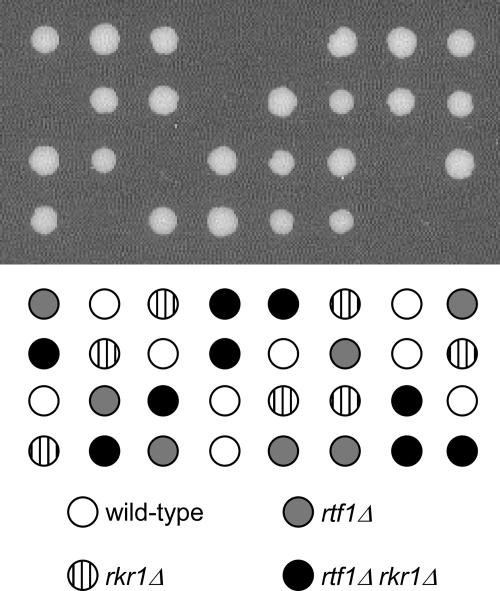
The results of the plasmid-based synthetic lethal screen were confirmed by tetrad analysis of spores derived from diploid strains that were doubly heterozygous for complete deletions of RTF1 and RKR1. No viable haploid rtf1Δ rkr1Δ strains were obtained after tetrad dissection (Fig. 1). This synthetic lethal interaction indicates that Rkr1 and Rtf1 regulate similar processes in yeast.
FIG. 1.
Loss of RKR1 and RTF1 leads to synthetic lethality. Yeast strains of opposite mating types containing deletions of RKR1 (KY1172) or RTF1 (KY957) were mated and sporulated. Tetrads were dissected and incubated for 3 days at 30°C.
Rkr1 contains a conserved, functionally important RING domain.
In an attempt to identify a cellular process that requires Rkr1, rkr1Δ strains were exposed to a wide range of phenotypic tests. We tested rkr1Δ mutants for the ability to grow on media lacking inositol or on media containing 6AU, caffeine, formamide, hydroxyurea, benomyl, sucrose, raffinose, glycerol, or high concentrations of salt (sodium chloride or lithium chloride). We also assayed for growth defects at 37° and 15°C on YPD. For most of these phenotypes, the rkr1Δ strains appeared to be similar to the wild type. However, we observed that rkr1Δ strains grow poorly on media lacking inositol, a phenotype associated with general defects in transcription (Fig. 2A) (22). This phenotype correlates well with the genetic interaction with RTF1, as defects in the Paf1 complex also cause inositol auxotrophy (4).
In parallel with the phenotypic analyses, we performed several database searches in an effort to identify a cellular role for Rkr1. Blast analysis showed that the protein is conserved with other uncharacterized proteins of similar size in many eukaryotes, including humans. There is significant homology throughout the protein, but a region at the carboxy terminus is the most highly conserved. Sequence analysis indicated that a RING domain exists in this region between amino acids 1508 and 1554 (Fig. 2B and C). RING domains consist of eight critical amino acids, often seven cysteine residues and one histidine residue, which bind two zinc ions to form a cross-brace structure (reviewed in reference 32). The RING domain of Rkr1 is of the C4HC3 type and is the only recognizable domain or motif within the protein. To determine whether the RING domain is important for the in vivo function of Rkr1, we mutated the first cysteine of the RING domain, changing it to an alanine (Rkr1-C1508A). Similar substitutions have been shown to disrupt the functions of other RING domain-containing proteins (11, 80). Strains lacking the genomic copy of RKR1 were transformed with plasmids expressing wild-type and C1508A forms of Rkr1. Growth assays showed that strains expressing wild-type Rkr1 grow on media lacking inositol, while strains expressing Rkr1-C1508A grow as poorly as rkr1Δ strains (Fig. 2A). Furthermore, the Rkr1-C1508A derivative failed to complement the synthetic lethality between rtf1Δ and rkr1Δ (Table 2). Immunoblotting analysis demonstrated that the inability of Rkr1-C1508A to complement the rkr1Δ allele is not due to instability of the Rkr1 mutant protein (Fig. 2D). Together, these data demonstrate that the conserved RING domain of Rkr1 is required for the function of the protein in vivo.
TABLE 2.
Genetic interactions between RKR1 and genes encoding transcription elongation factors
| Relevant genotypea | Phenotypeb |
|---|---|
| rtf1Δ rkr1Δ | Dead |
| rtf1Δ rkr1-C1508A | Deadc |
| paf1Δ rkr1Δ | Slow growth |
| ctr9Δ rkr1Δ | Slow growth |
| cdc73Δ rkr1Δ | NaCl−, Caff− |
| leo1Δ rkr1Δ | None |
| spt4Δ rkr1Δ | LiCl− |
| spt5-194 rkr1Δ | LiCl−, NaCl− |
| spt5-242 rkr1Δ | LiCl−, NaCl− |
| spt6-14 rkr1Δ | Increased 6AUS, NaCl−, LiCl− |
| spt16-197 rkr1Δ | LiCl−, NaCl−, enhanced Ino− |
| ppr2Δ rkr1Δ | Caff− |
The parents for the crosses, in the order listed, are KY1172 and KY957, KY1177 and KY802, KY1180 and GHY1094, KY1180 and KY1162, KY1177 and KY806, KY1178 and a strain derived from FY245, KY1179 and KY715, KY1180 and KY1163, KY1180 and KY1164, KY1179 and KY1165, and KY1176 and KY714.
All phenotypes listed correspond to the synthetic phenotypes observed for the double mutant strains. The 6AUS phenotype caused by spt6-14 and the Ino− phenotype caused by rkr1Δ are enhanced in the double mutants, as indicated. Definitions of other phenotypes are as follows: slow growth, small colonies after 3 to 5 days of growth at 30°C; NaCl−, sensitive to 1.4 M NaCl; LiCl−, sensitive to 0.3 M LiCl; Caff−, sensitive to 15 mM caffeine.
Complementation of the rtf1Δ rkr1Δ lethality by rkr1-C1508A was tested by a plasmid shuffle experiment with strain KY960 and plasmid pMB66.
Rkr1 is a nuclear protein.
A large-scale study has been performed to determine the subcellular localization of all yeast proteins (29). This study involved the construction of carboxy-terminal green fluorescent protein (GFP) fusions for the majority of ORFs in yeast and microscopy to detect localization of the GFP signal. Rkr1 was not localized to any cellular compartment in this study, possibly because incorporation of the GFP tag at the carboxy terminus disrupted the structure and/or function of the RING domain. Database analyses did not reveal any cellular localization signals within the primary amino acid sequence of Rkr1. Therefore, we used indirect immunofluorescence to determine the cellular localization of Rkr1. To detect Rkr1, we constructed an amino-terminal HA epitope-tagged version of the protein. This tag does not appear to disrupt activity, as determined by complementation of the Ino− phenotype of an rkr1Δ strain (Fig. 2A), and immunoblotting analysis showed that HA-Rkr1 migrates in denaturing gels at its predicted molecular mass of approximately 180 kDa (Fig. 2D). Indirect immunofluorescence experiments showed that HA-Rkr1 is localized to the nucleus (Fig. 3).
FIG. 3.
HA-Rkr1 localizes to the nucleus. Indirect immunofluorescence experiments were performed with transformants of a wild-type strain (FY632) expressing HA-Rkr1 (A and B) or untagged Rkr1 (C and D). Localization of the triple HA epitope was detected with Cy3-coupled secondary antibody (A and C), and nuclear and mitochondrial DNAs were detected with DAPI stain (B and D). HA-Rkr1 and untagged Rkr1 were expressed from the CEN/ARS plasmids pMB11 and pPC65, respectively.
Genetic interactions suggest that Rkr1 is functionally connected to chromatin modification.
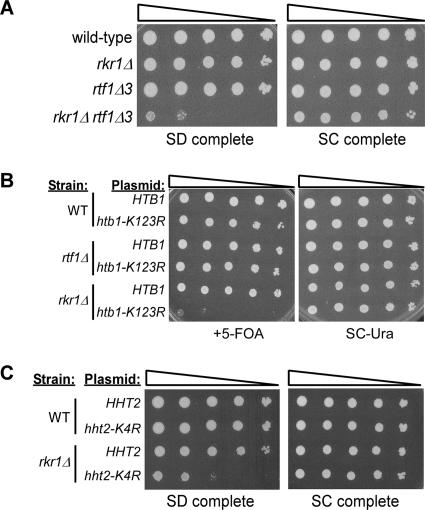
The synthetic lethal relationship between rkr1Δ and rtf1Δ suggests that Rkr1 and Rtf1 regulate a common process within the cell. We undertook a genetic approach to begin to identify this process. We first wanted to learn whether the genetic interaction between RKR1 and RTF1 extended to other members of the Paf1 complex, Paf1, Ctr9, Cdc73, and Leo1. Interestingly, although rkr1Δ paf1Δ and rkr1Δ ctr9Δ double mutants grow very slowly, only rkr1Δ rtf1Δ double mutants are unviable (Table 2). This result is particularly striking because deletion of PAF1 or CTR9 generally causes stronger mutant phenotypes than deletion of any other member of the Paf1 complex, including RTF1 (4, 76). Therefore, the synthetic lethality between RKR1 and RTF1 most likely relates to a function of the Paf1 complex that is primarily carried out by Rtf1, potentially histone H2B ubiquitylation or histone H3 K4 or K79 methylation. The Paf1 complex physically and genetically interacts with several transcription elongation factors, including Spt4-Spt5 and Spt16-Pob3 (yFACT) (76). To determine whether Rkr1 is involved in transcription elongation, we performed genetic crosses with rkr1Δ strains and strains containing mutations in genes that encode transcription elongation factors, including SPT4, SPT5, SPT6, SPT16, and PPR2, which encodes TFIIS. While we observed several enhanced mutant phenotypes in the double mutant strains, we found no severe genetic interactions to indicate that Rkr1 is solely or primarily involved in transcription elongation (Table 2).
To better define why Rtf1 is required for viability when Rkr1 is absent, we took advantage of a set of rtf1 internal deletion mutations that disrupt specific functions of the protein (M. H. Warner, K. L. Roinick, and K. M. Arndt, submitted for publication). Discrete regions of Rtf1 are important for its association with actively transcribed genes, interactions with other members of the Paf1 complex, and posttranslational histone modifications. We crossed strains lacking RKR1 to the different rtf1 deletion mutants and found that the rtf1Δ3 and rtf1Δ4 mutations, which together eliminate amino acids 62 to 152 from the Rtf1 protein, show a strong genetic interaction with rkr1Δ (Table 3 and Fig. 4A). Specifically, the rkr1Δ rtf1Δ3 and rkr1Δ rtf1Δ4 double mutants grow very poorly on minimal synthetic dextrose medium. Notably, deletion of amino acids 62 to 152 in Rtf1 eliminates histone H2B ubiquitylation and histone H3 K4 and K79 methylation (Warner et al., submitted).
TABLE 3.
Genetic interactions between RKR1 and genes encoding proteins involved in histone modification
| Relevant genotypea | Phenotypeb |
|---|---|
| rtf1Δ3 rkr1Δ | SD− |
| rtf1Δ4 rkr1Δ | SD− |
| rad6Δ rkr1Δ | Slow growth, SD−, Gly− |
| bre1Δ rkr1Δ | SD−, Gly− |
| lge1Δ rkr1Δ | SD− |
| set1Δ rkr1Δ | SD−, HUS, NaCl− |
| set1Δ rkr1-C1508A | SD−c |
| dot1Δ rkr1Δ | None |
| set2Δ rkr1Δ | None |
| spt10Δ rkr1Δ | Dead |
| spt21Δ rkr1Δ | Slow growth |
Double mutants, in the order listed, were generated from the following genetic crosses (unless stated otherwise): KY1166 and KY1174, KY1166 and KY1175, KY1167 and FY623, KY1171 and KY968, none (lge1Δ was created in an RKR1/rkr1Δ diploid [KY1173] prior to sporulation and tetrad dissection), KY1168 and KY907, none (the set1Δ rkr1-C1508A double mutant [KY1222] was generated by transformation), KY1168 and KY903, KY1168 and KY912, FY896 and KY1168, and FY2199 and KY1168.
All phenotypes listed correspond to the synthetic phenotypes observed for the double mutant strains. Definitions of phenotypes are as follows: SD−, poor growth on SD medium; slow growth, small colonies after 7 days of growth at 30°C on YPD; Gly−, inviable on YPG medium; HUS, sensitive to 100 mM hydroxyurea; NaCl−, sensitive to 1.4 M NaCl.
Growth on SD medium was the only phenotype tested for this double mutant.
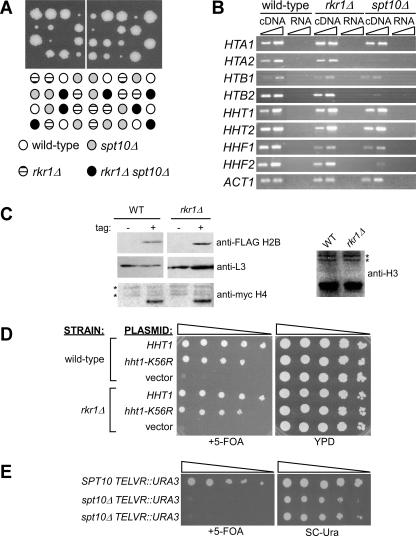
FIG. 4.
rkr1Δ exhibits synthetic growth defects with mutations that eliminate histone H2B K123 ubiquitylation and histone H3 K4 methylation. (A) rkr1Δ rtf1Δ3 strains exhibit the SD− phenotype seen with many double mutants. Cultures were spotted as 10-fold serial dilutions onto SC or SD medium and grown at 30°C for 3 days. (B) Plasmids expressing FLAG-HTB1 or FLAG-htb1-K123R were introduced by plasmid shuffle into wild-type (FY406), rtf1Δ (KY982), and rkr1Δ (KY981) strains lacking both genomic copies of HTB. Tenfold serial dilutions of cultures were spotted onto medium lacking or containing 5-FOA and incubated at 30°C for 3 days. (C) Plasmids expressing HHT2 or hht2-K4R were introduced by plasmid shuffle into wild-type (FY1990) or rkr1Δ (KY1064) strains. 5-FOAR colonies were obtained and analyzed for growth on SD or SC complete medium as described for panel A.
To investigate further a potential connection between RKR1 and posttranslational histone modifications, we crossed rkr1Δ strains with strains lacking specific histone-modifying enzymes. Rad6 is the ubiquitin-conjugating enzyme and Bre1, in association with Lge1, is the ubiquitin-protein ligase required for histone H2B K123 ubiquitylation (31, 61, 83). Set1, Set2, and Dot1 are the methyltransferases responsible for methylating K4, K36, and K79 of histone H3, respectively (18, 66, 78). The Paf1 complex is required for each of these modifications (36, 38, 52, 53, 84). Interestingly, rkr1Δ rad6Δ double mutants exhibit a strong synthetic growth defect (Table 3). Further genetic analysis using strains lacking both RKR1 and the H2B ubiquitylation site (htb1-K123R) indicates that Rkr1 is important for cell growth in the absence of H2B ubiquitylation (Fig. 4B). Moreover, rkr1Δ strains lacking BRE1, LGE1, or SET1 grow very poorly on SD medium, similar to the rtf1Δ3 or rtf1Δ4 genetic interactions (Table 3). No strong genetic interactions were observed with dot1Δ and set2Δ strains. Defective growth on SD medium was also observed for an rkr1-C1508A set1Δ double mutant strain, indicating a requirement for the Rkr1 RING domain when Set1 is absent (Table 3). Because Set1 has been shown to methylate substrates other than histone H3 K4 (87), we examined the phenotype of an rkr1Δ strain in which histone H3 K4 could not be methylated (hht2-K4R). Like an rkr1Δ set1Δ strain, the rkr1Δ hht2-K4R strain grows poorly on SD medium (Fig. 4C), suggesting that the synthetic phenotype between rkr1Δ and set1Δ is due to a lack of histone H3 K4 methylation. While deletion of RKR1 causes significant growth defects in strains defective for histone H2B K123 ubiquitylation or H3 K4 methylation, Rkr1 itself does not appear to affect these modifications. Histone H3 K4 trimethylation and K79 dimethylation as well as histone H2B K123 ubiquitylation occur at wild-type levels in strains lacking RKR1 (data not shown). Taken together, our findings suggest that Rkr1 acts in parallel with Rtf1-dependent histone modifications.
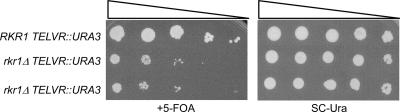
Strains lacking RKR1 have defects in telomeric silencing.
Transcriptional silencing of genes that are positioned near telomeres requires the proper modification of histones. Telomeric chromatin is enriched in hypoacetylated and hypomethylated histones, which provide interaction sites for the Sir proteins (reviewed in reference 64). Strains lacking Paf1 complex members exhibit defects in telomeric silencing (36, 52), most likely because the genome-wide loss of histone H3 methylation leads to the redistribution of Sir proteins away from their normal sites of action (65). To determine whether Rkr1 is important for telomeric silencing, wild-type and rkr1Δ strains, both containing a telomeric URA3 reporter gene, were plated to medium containing 5-FOA. Wild-type strains grow robustly on this medium, indicating silencing of the telomeric URA3 gene (Fig. 5). However, strains lacking RKR1 grow poorly on the 5-FOA medium, suggesting that telomeric silencing is disrupted in the rkr1Δ strains (Fig. 5). In contrast, transcriptional silencing at the ribosomal DNA and silent mating-type loci occur normally in rkr1Δ strains (data not shown). A role in telomeric silencing is consistent with the idea that Rkr1 modulates chromatin structure or function.
FIG. 5.
Strains lacking RKR1 have defects in telomeric silencing. RKR1 (O660) and rkr1Δ (KA102 and KA103) strains were spotted in 10-fold serial dilutions onto medium containing or lacking 5-FOA. KA102 and KA103 are two independent rkr1Δ strains that contain the TELVR::URA3 telomeric silencing marker.
RKR1 genetically interacts with SPT10, a gene encoding a potential histone acetyltransferase.
SPT10 is one of many SPT genes that were originally identified in a screen for suppressors of transposable element insertion mutations in yeast (17, 50). Strains lacking Spt10 exhibit global changes in chromatin structure and gene expression (14, 86). Spt10 and its interacting partner Spt21 bind to the promoters of histone genes and activate their transcription, providing a potential explanation for the broad transcriptional effects of spt10 mutations (13, 14, 25, 86). Interestingly, Spt10 contains a predicted acetyltransferase domain (51) that is required for its transcription activation activity (25), and spt10 mutants have reduced histone H3 K56 acetylation at histone gene promoters (86). However, direct acetylation of histones by Spt10 has yet to be demonstrated (25, 86), and a recent study indicates that global levels of histone H3 K56 acetylation are greatly reduced in strains lacking Rtt109 or Asf1, but not Spt10 (67). A synthetic lethal screen involving spt10Δ identified mutations in RKR1 (D. Hess and F. Winston, personal communication). We confirmed this result by tetrad analysis using complete deletions of RKR1 and SPT10 (Fig. 6A).
FIG. 6.
RKR1 genetically interacts with SPT10. (A) Loss of RKR1 and SPT10 results in synthetic lethality. Strains of opposite mating types containing deletions of RKR1 (KY1169) or SPT10 (FY896) were mated and sporulated. Tetrads were dissected and incubated at 30°C for 5 days. Growth differences among spt10Δ single mutants are most likely due to spontaneous secondary mutations that suppress the growth defects of the spt10Δ strain (J. Chang and F. Winston, personal communication). (B) RT-PCR analysis of histone gene transcription. Total RNA was isolated from wild-type (FY14), rkr1Δ (KY1170), or spt10Δ (FY896) strains and used for RT-PCR analysis. Two different amounts of cDNA (1× and 3×) were used in the reactions to demonstrate linearity of the PCR. Reactions programmed with RNA confirmed that the RNA preparations used for cDNA synthesis were free of genomic DNA. Results shown are representative of those obtained in two independent experiments. (C) Immunoblot analysis of histone protein levels. For detection of histones H2B and H3, wild-type (FY406) and rkr1Δ (KY981) strains were transformed with plasmids that express untagged or FLAG-tagged H2B (54). For detection of histone H4, wild-type (FY1990) and rkr1Δ (KY1064) strains were transformed with plasmids that express untagged (pJH18) or myc-tagged H4 (pNOY436). L3 served as a loading control for the FLAG-H2B immunoblot, and cross-reacting bands, marked by asterisks, served as loading controls for the myc-H4 and total H3 immunoblots. (D) rkr1Δ is not synthetically lethal with a mutation that alters K56 of histone H3. TRP1-marked CEN/ARS plasmids expressing HHT1 or hht1-K56R were introduced by plasmid shuffle into RKR1 (FY1990) or rkr1Δ (KY1064) strains that lacked both genomic copies of HHT. Cultures were serially diluted and spotted onto YPD and synthetic medium containing 5-FOA. Plates were incubated at 30°C for 3 days. (E) Strains lacking SPT10 have defects in telomeric silencing. Tenfold serial dilutions of SPT10 (KA104) or spt10Δ (KA106 and KA105) cultures were spotted onto medium containing or lacking 5-FOA. Plates were incubated at 30°C for 5 days. KA106 and KA105 are independent strains that contain the TELVR::URA3 telomeric silencing marker and spt10Δ.
Because Spt10 has a well-studied role in histone gene transcription, we measured histone mRNA levels in an rkr1Δ strain using RT-PCR analysis and oligonucleotide primers that distinguish among the highly related histone genes (25). We found that all of the histone genes are expressed at nearly wild-type levels in the rkr1Δ strain (Fig. 6B), while HTA2, HTB2, and HHF2 mRNA levels are significantly decreased in the spt10Δ strain, as expected from earlier studies (14, 25). We confirmed by immunoblotting analysis that histone H2B, H3, and H4 levels are unaffected by the rkr1Δ mutation (Fig. 6C) (H2A levels were not tested). Therefore, the inviability of rkr1Δ spt10Δ double mutant strains is most likely not due to insufficient histone gene expression. Consistent with this idea, rkr1Δ spt21Δ double mutants are viable (Table 3), even though spt21Δ and spt10Δ mutations both reduce histone mRNA levels (13, 25).
To determine whether Rkr1 is essential in the absence of Spt10 because histone H3 K56 acetylation is defective, we performed a plasmid shuffle experiment with RKR1+ and rkr1Δ strains that lacked both chromosomal copies of the genes for histones H3 and H4 and carried a URA3-marked HHT2-HHF2 CEN/ARS plasmid. Cells were transformed with a TRP1-marked plasmid that expressed a histone H3 derivative in which K56 was replaced with arginine (43). Following growth on synthetic medium containing 5-FOA, the histone H3 K56R derivative was the only version of histone H3 available in the cell. The results of the plasmid shuffle revealed that rkr1Δ strains grow as well as RKR1+ strains in the presence of the histone H3 K56R derivative (Fig. 6D). In addition, histone H3 K56 acetylation occurs at wild-type levels in rkr1Δ cells (data not shown). Therefore, the basis for the synthetic lethality between rkr1Δ and spt10Δ remains unclear but appears not to be related to the proposed role of Spt10 in histone H3 K56 acetylation (86).
Since the loss of Rkr1 or Rtf1 alleviates telomeric silencing, we wanted to determine whether the loss of SPT10 also causes this phenotype. Strains lacking SPT10 and containing the telomeric URA3 reporter gene were constructed and plated on 5-FOA medium to monitor URA3 expression. Consistent with a role for Spt10 in chromatin structure or function, the spt10Δ strains grow extremely poorly in the presence of 5-FOA, indicating a strong defect in telomeric silencing (Fig. 6E).
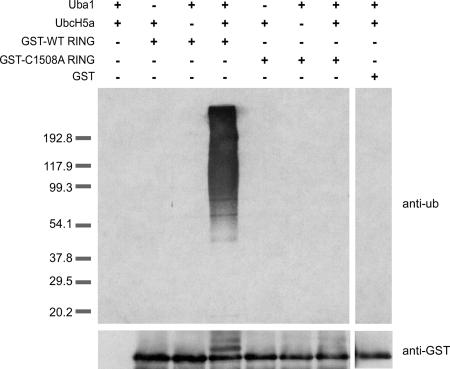
The RING domain of Rkr1 possesses ubiquitin-protein ligase activity.
Proteins that contain a RING domain often possess ubiquitin-protein ligase activity (16). Within the ubiquitylation pathway, RING domain ubiquitin-protein ligases are thought to bring the ubiquitin-conjugating enzyme and substrate together to facilitate the transfer of ubiquitin to the substrate (16). Since the only identified domain or motif in Rkr1 is the carboxy-terminal RING domain, we determined whether Rkr1 possesses ubiquitin-protein ligase activity. Because attempts to express full-length, recombinant Rkr1 were unsuccessful, GST fusions to the carboxy terminus of Rkr1 (amino acids 1251 to 1562) were constructed. Wild-type Rkr1 and Rkr1-C1508A GST fusions, as well as GST alone, were purified from bacteria. In vitro ubiquitin-protein ligase assays were performed with recombinant ubiquitin, ubiquitin-activating enzyme (yeast Uba1), ubiquitin-conjugating enzyme (human UbcH5a), and GST-RING proteins. These conditions have been used previously to show that other RING domain-containing proteins can facilitate polyubiquitylation (19). Reactions lacking individual components of the ubiquitylation pathway were performed to show that all components of the pathway are required for efficient ubiquitylation. The results show that the RING domain of Rkr1 has ubiquitin-protein ligase activity that is dependent on the presence of the first cysteine residue in the RING motif (Fig. 7). Neither the Rkr1-C1508A fusion protein nor GST yielded detectable levels of protein ubiquitylation. These data suggest that Rkr1 may also act as a ubiquitin-protein ligase in vivo. Combined with previous data, we propose a model in which the ubiquitin-protein ligase activity of Rkr1 targets proteins important for transcription and/or chromatin function.
FIG. 7.
The RING domain of Rkr1 possesses ubiquitin-protein ligase activity in vitro. Purified recombinant ubiquitin-activating enzyme (Uba1), ubiquitin-conjugating enzyme (UbcH5a), or GST-RING fusion proteins were combined with recombinant ubiquitin. Reactions contained GST fusions to the wild-type Rkr1 RING domain (lanes 2 to 4), the Rkr1-C1508A RING domain (lanes 5 to 7), or GST alone (lane 8). Proteins were separated by SDS-polyacrylamide gel electrophoresis analysis, and immunoblotting was performed with antiubiquitin antibody to detect ubiquitylated substrates or anti-GST antibody to detect GST-RING fusions. Numbers on the left indicate protein molecular mass standards, in kilodaltons. All GST proteins migrated at predicted molecular masses. The GST-only reaction lane originated from the same gel but was aligned with reactions containing GST fusion products for presentation purposes.
DISCUSSION
Chromatin structure controls many nuclear processes, including transcription and DNA repair. In this paper, we report the identification of a previously unstudied nuclear RING domain protein, Rkr1, and provide evidence to suggest a role for this protein in transcription and chromatin function. We identified Rkr1 in a genetic screen for factors that become essential in the absence of the Paf1 complex component Rtf1, a protein required for several histone modifications that mark active genes. In addition to Rtf1, Rkr1 exhibits genetic interactions with several other factors involved in chromatin modification. These interactions indicate that the function of Rkr1 overlaps with the functions of histone modifications that are important for transcription. We also demonstrate that Rkr1 contains an important RING domain. This domain possesses ubiquitin-protein ligase activity in vitro, suggesting that Rkr1 mediates its in vivo effects through protein ubiquitylation.
Several lines of evidence implicate Rkr1 in the regulation of chromatin structure or function. Rkr1 exhibits strong genetic interactions with factors required for histone H2B ubiquitylation and histone H3 K4 methylation. Specifically, rkr1Δ strains containing an amino acid replacement for lysine 123 on histone H2B are severely sick. In addition, rkr1Δ set1Δ and rkr1Δ hht2-K4R double mutants grow very poorly on minimal media. In contrast, no synthetic phenotypes were observed between rkr1Δ and dot1Δ. This is surprising because both histone H3 K4 and K79 methylation depend on histone H2B K123 ubiquitylation. Therefore, our data suggest that these modifications have distinct functions in vivo.
Interestingly, strains lacking Rtf1, but not other members of the Paf1 complex, are inviable when RKR1 is deleted. This observation is noteworthy because mutant phenotypes and protein stabilities suggest that Paf1 and Ctr9 are integral members of the Paf1 complex, while Rtf1 appears to be a more peripheral member of the complex (4, 45, 76). Interactions between rtf1Δ and rkr1Δ indicate that Rtf1 performs a function for which Paf1 and Ctr9 are not sufficient. Importantly, Rtf1 appears to be the only member of the complex that is essential for normal levels of H3 K4 and K79 methylation (M. H. Warner and K. M. Arndt, unpublished observations). Although Paf1 has been shown to be important for these modifications (36, 52, 53), paf1Δ strains have significantly reduced levels of Rtf1 protein (45, 76), which may explain the decreased levels of H3 K4 and K79 methylation in the absence of Paf1. The synthetic lethal interaction between Rtf1 and Rkr1 is also specific to Rkr1; deletion of SAN1, a gene encoding another nuclear RING protein, exhibits no synthetic interactions with rtf1Δ (data not shown). Consistent with the conclusion that synthetic lethality often identifies pathways that operate in parallel to control an essential process (55), we have not detected a physical interaction between Rkr1 and Rtf1 or Paf1 (data not shown).
We have also observed that removal of residues 62 to 152 of Rtf1 (rtf1Δ3 and rtf1Δ4), which are responsible for histone H2B ubiquitylation and histone H3 K4 and K79 methylation, causes severe growth defects on minimal medium when combined with the loss of RKR1. Our genetic results indicate that removal of amino acids 62 to 152 of Rtf1 interferes with Rad6/Bre1 activity, as similar phenotypes are observed with rad6Δ rkr1Δ and bre1Δ rkr1Δ strains. However, it is not clear why rtf1Δ3 rkr1Δ and rtf1Δ4 rkr1Δ double mutants are viable while rkr1Δ cells containing a complete deletion of RTF1 are not. Presumably the synthetic lethality between rtf1Δ and rkr1Δ is due to the absence of more than one activity of Rtf1.
Additional evidence linking RKR1 to chromatin modification is its synthetic lethal relationship with SPT10. Spt10 regulates the transcription of many genes in yeast, particularly histone genes, and is important for histone H3 K56 acetylation at histone gene promoters in vivo (13, 25, 86). However, a recent report indicates that Spt10 is not required for global histone H3 K56 acetylation (67). Here, we show that Rkr1 does not affect histone gene expression, nor does it regulate a process that overlaps with histone H3 K56 acetylation, supporting the idea that Spt10 has functions independent of histone H3 K56 acetylation. Consistent with a role in chromatin structure and histone modification, we show that strains lacking either RKR1 or SPT10 have defects in telomeric silencing.
Although the mechanism that links Rkr1 to chromatin is unknown, the presence of a RING domain suggests that Rkr1 may posttranslationally modify transcription factors or chromatin components to alter their activity or stability. Several nuclear RING domain proteins have been described, and they control many different processes, including transcription. In most RING domains, the cysteine and histidine residues are arranged in either a C3HC4 or C3H2C3 sequence (reviewed in reference 58). The C4HC3 pattern of the Rkr1 RING domain appears to be less common (12, 23). However, the solution structure of the C4HC3 RING domain of the Kaposi's sarcoma-associated herpesvirus K3 protein has been solved (12). Chemical mutagenesis and two-hybrid analysis showed that this noncanonical RING domain interacts with ubiquitin-conjugating enzymes on the same face of the RING domain as classical RING domains (12).
RING domains bear sequence and structural similarity to another protein interaction domain, the plant homeodomain (PHD) finger (reviewed in reference 5). Interestingly, PHD fingers have recently been shown to interact with methylated lysine residues on histones (reviewed in reference 73). However, amino acids outside of the eight critical cysteine and histidine residues indicate that Rkr1 contains a RING domain and not a PHD finger. The RING domain of Rkr1 contains a tryptophan four residues after the cysteine in the sixth position of the Cys/His sequence, and this amino acid is highly conserved among RING domain proteins (12). In contrast, PHD fingers contain an invariant tryptophan two positions amino-terminal to the seventh cysteine, a residue not found in Rkr1 (1).
From our in vitro experiments, we suggest that the RING domain of Rkr1 is required for the ubiquitylation of one or more nuclear proteins. Protein sumoylation, a ubiquitin-like modification, is also facilitated by RING domain-containing proteins (reviewed in reference 24). While it remains possible that Rkr1 could act as a SUMO-protein ligase in vivo, our current genetic and biochemical data do not support this idea (M. A. Braun and K. M. Arndt, unpublished observations). For example, we do not see any genetic interactions in strains containing mutations in RTF1 and UBC9, the gene encoding the sole SUMO-conjugating enzyme in yeast. Moreover, Cheng et al. recently reported that SUMO-protein ligases, such as Siz1, Siz2, and Zip3, have their own variant RING sequence, C3HCHC2 (8).
In summary, our results indicate the existence of a new nuclear protein ubiquitylation pathway that is functionally connected to chromatin and transcription. Based on the findings in this paper, we hypothesize that the ubiquitin-protein ligase activity of Rkr1 operates in parallel with Rtf1 and several histone modifications to regulate a common, essential process. Although the best-characterized functions for Rtf1 relate to transcription, our data do not exclude the possibility that the synthetic lethal relationship between Rtf1 and Rkr1 is due to their involvement in other essential processes, which remain to be identified. Future experiments will aim to expose the in vivo target(s) of the ubiquitin-protein ligase activity of Rkr1 and explain how the target(s) relates to chromatin structure or function. Finally, because Rkr1 is a conserved protein, further studies with yeast should illuminate the role of homologous proteins in humans and other eukaryotes.
Acknowledgments
We thank David Hess, Jennifer Chang, and Fred Winston for communicating unpublished results on the SPT10-RKR1 genetic interaction and for yeast strains, plasmids, and technical advice. We are grateful to Stephen Hancock for performing the rkr1-SL505 gap repair; Alain Verreault for plasmids expressing histone H3 K56 derivatives and antibody against acetylated histone H3 K56; Mary Bryk, Scott Briggs, and Kevin Struhl for wild-type, mutant, and epitope-tagged histone plasmids; William Saunders for assistance with the indirect immunofluorescence experiments; and Richard Gardner, Arindam Dasgupta, and David Auble for reagents and assistance with in vitro ubiquitylation assays. We also thank Greg Prelich, Richard Gardner, Jeff Brodsky, Fred Winston, and members of the Arndt laboratory for insightful discussions and critical reading of the manuscript.
This work was supported by NIH grant GM52593 to K.M.A.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Aasland, R., T. J. Gibson, and A. F. Stewart. 1995. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20:56-59. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1988. Current protocols in molecular biology. Wiley-Interscience, New York, NY.
- 3.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 4.Betz, J. L., M. Chang, T. M. Washburn, S. E. Porter, C. L. Mueller, and J. A. Jaehning. 2002. Phenotypic analysis of Paf1/RNA polymerase II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics 268:272-285. [DOI] [PubMed] [Google Scholar]
- 5.Bienz, M. 2006. The PHD finger, a nuclear protein-interaction domain. Trends Biochem. Sci. 31:35-40. [DOI] [PubMed] [Google Scholar]
- 6.Briggs, S. D., M. Bryk, B. D. Strahl, W. L. Cheung, J. K. Davie, S. Y. Dent, F. Winston, and C. D. Allis. 2001. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15:3286-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs, S. D., T. Xiao, Z. W. Sun, J. A. Caldwell, J. Shabanowitz, D. F. Hunt, C. D. Allis, and B. D. Strahl. 2002. Gene silencing: trans-histone regulatory pathway in chromatin. Nature 418:498. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, C. H., Y. H. Lo, S. S. Liang, S. C. Ti, F. M. Lin, C. H. Yeh, H. Y. Huang, and T. F. Wang. 2006. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 20:2067-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, G. A., and W. P. Tansey. 2006. The proteasome: a utility tool for transcription? Curr. Opin. Genet. Dev. 16:197-202. [DOI] [PubMed] [Google Scholar]
- 10.Costa, P. J., and K. M. Arndt. 2000. Synthetic lethal interactions suggest a role for the Saccharomyces cerevisiae Rtf1 protein in transcription elongation. Genetics 156:535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta, A., K. L. Ramsey, J. S. Smith, and D. T. Auble. 2004. Sir antagonist 1 (San1) is a ubiquitin ligase. J. Biol. Chem. 279:26830-26838. [DOI] [PubMed] [Google Scholar]
- 12.Dodd, R. B., M. D. Allen, S. E. Brown, C. M. Sanderson, L. M. Duncan, P. J. Lehner, M. Bycroft, and R. J. Read. 2004. Solution structure of the Kaposi's sarcoma-associated herpesvirus K3 N-terminal domain reveals a novel E2-binding C4HC3-type RING domain. J. Biol. Chem. 279:53840-53847. [DOI] [PubMed] [Google Scholar]
- 13.Dollard, C., S. L. Ricupero-Hovasse, G. Natsoulis, J. D. Boeke, and F. Winston. 1994. SPT10 and SPT21 are required for transcription of particular histone genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:5223-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson, P. R., G. Mendiratta, N. B. McLaughlin, T. G. Wolfsberg, L. Marino-Ramirez, T. A. Pompa, M. Jainerin, D. Landsman, C. H. Shen, and D. J. Clark. 2005. Global regulation by the yeast Spt10 protein is mediated through chromatin structure and the histone upstream activating sequence elements. Mol. Cell. Biol. 25:9127-9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezhkova, E., and W. P. Tansey. 2004. Proteasomal ATPases link ubiquitylation of histone H2B to methylation of histone H3. Mol. Cell 13:435-442. [DOI] [PubMed] [Google Scholar]
- 16.Fang, S., and A. M. Weissman. 2004. A field guide to ubiquitylation. Cell. Mol. Life Sci. 61:1546-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fassler, J. S., and F. Winston. 1988. Isolation and analysis of a novel class of suppressor of Ty insertion mutations in Saccharomyces cerevisiae. Genetics 118:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, Q., H. Wang, H. H. Ng, H. Erdjument-Bromage, P. Tempst, K. Struhl, and Y. Zhang. 2002. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr. Biol. 12:1052-1058. [DOI] [PubMed] [Google Scholar]
- 19.Gardner, R. G., Z. W. Nelson, and D. E. Gottschling. 2005. Degradation-mediated protein quality control in the nucleus. Cell 120:803-815. [DOI] [PubMed] [Google Scholar]
- 20.Gietz, R. D., and R. A. Woods. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350:87-96. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez, F., A. Delahodde, T. Kodadek, and S. A. Johnston. 2002. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science 296:548-550. [DOI] [PubMed] [Google Scholar]
- 22.Hampsey, M. 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast 13:1099-1133. [DOI] [PubMed] [Google Scholar]
- 23.Hassink, G., M. Kikkert, S. van Voorden, S. J. Lee, R. Spaapen, T. van Laar, C. S. Coleman, E. Bartee, K. Fruh, V. Chau, and E. Wiertz. 2005. TEB4 is a C4HC3 RING finger-containing ubiquitin ligase of the endoplasmic reticulum. Biochem. J. 388:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay, R. T. 2005. SUMO: a history of modification. Mol. Cell 18:1-12. [DOI] [PubMed] [Google Scholar]
- 25.Hess, D., B. Liu, N. R. Roan, R. Sternglanz, and F. Winston. 2004. Spt10-dependent transcriptional activation in Saccharomyces cerevisiae requires both the Spt10 acetyltransferase domain and Spt21. Mol. Cell. Biol. 24:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess, D., and F. Winston. 2005. Evidence that Spt10 and Spt21 of Saccharomyces cerevisiae play distinct roles in vivo and functionally interact with MCB-binding factor, SCB-binding factor and Snf1. Genetics 170:87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoege, C., B. Pfander, G. L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135-141. [DOI] [PubMed] [Google Scholar]
- 28.Hsu, J. Y., Z. W. Sun, X. Li, M. Reuben, K. Tatchell, D. K. Bishop, J. M. Grushcow, C. J. Brame, J. A. Caldwell, D. F. Hunt, R. Lin, M. M. Smith, and C. D. Allis. 2000. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102:279-291. [DOI] [PubMed] [Google Scholar]
- 29.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 30.Huibregtse, J. M., M. Scheffner, S. Beaudenon, and P. M. Howley. 1995. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA 92:2563-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang, W. W., S. Venkatasubrahmanyam, A. G. Ianculescu, A. Tong, C. Boone, and H. D. Madhani. 2003. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol. Cell 11:261-266. [DOI] [PubMed] [Google Scholar]
- 32.Jackson, P. K., A. G. Eldridge, E. Freed, L. Furstenthal, J. Y. Hsu, B. K. Kaiser, and J. D. Reimann. 2000. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429-439. [DOI] [PubMed] [Google Scholar]
- 33.Jauert, P. A., L. E. Jensen, and D. T. Kirkpatrick. 2005. A novel yeast genomic DNA library on a geneticin-resistance vector. Yeast 22:653-657. [DOI] [PubMed] [Google Scholar]
- 34.Kahana, A., and D. E. Gottschling. 1999. DOT4 links silencing and cell growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6608-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keener, J., J. A. Dodd, D. Lalo, and M. Nomura. 1997. Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc. Natl. Acad. Sci. USA 94:13458-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, O. W. Ryan, A. Golshani, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11:721-729. [DOI] [PubMed] [Google Scholar]
- 37.Krogan, N. J., M. Kim, S. H. Ahn, G. Zhong, M. S. Kobor, G. Cagney, A. Emili, A. Shilatifard, S. Buratowski, and J. F. Greenblatt. 2002. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol. Cell. Biol. 22:6979-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 23:4207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, D., E. Ezhkova, B. Li, S. G. Pattenden, W. P. Tansey, and J. L. Workman. 2005. The proteasome regulatory particle alters the SAGA coactivator to enhance its interactions with transcriptional activators. Cell 123:423-436. [DOI] [PubMed] [Google Scholar]
- 40.Lipford, J. R., and R. J. Deshaies. 2003. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat. Cell Biol. 5:845-850. [DOI] [PubMed] [Google Scholar]
- 41.Lipford, J. R., G. T. Smith, Y. Chi, and R. J. Deshaies. 2005. A putative stimulatory role for activator turnover in gene expression. Nature 438:113-116. [DOI] [PubMed] [Google Scholar]
- 42.Lorick, K. L., J. P. Jensen, S. Fang, A. M. Ong, S. Hatakeyama, and A. M. Weissman. 1999. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl. Acad. Sci. USA 96:11364-11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masumoto, H., D. Hawke, R. Kobayashi, and A. Verreault. 2005. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 436:294-298. [DOI] [PubMed] [Google Scholar]
- 44.Mueller, C. L., and J. A. Jaehning. 2002. Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol. Cell. Biol. 22:1971-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller, C. L., S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2004. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol. Cell 14:447-456. [DOI] [PubMed] [Google Scholar]
- 46.Muratani, M., C. Kung, K. M. Shokat, and W. P. Tansey. 2005. The F box protein Dsg1/Mdm30 is a transcriptional coactivator that stimulates Gal4 turnover and cotranscriptional mRNA processing. Cell 120:887-899. [DOI] [PubMed] [Google Scholar]
- 47.Muratani, M., and W. P. Tansey. 2003. How the ubiquitin-proteasome system controls transcription. Nat. Rev. Mol. Cell Biol. 4:192-201. [DOI] [PubMed] [Google Scholar]
- 48.Nalley, K., S. A. Johnston, and T. Kodadek. 2006. Proteolytic turnover of the Gal4 transcription factor is not required for function in vivo. Nature 442:1054-1057. [DOI] [PubMed] [Google Scholar]
- 49.Nathan, D., K. Ingvarsdottir, D. E. Sterner, G. R. Bylebyl, M. Dokmanovic, J. A. Dorsey, K. A. Whelan, M. Krsmanovic, W. S. Lane, P. B. Meluh, E. S. Johnson, and S. L. Berger. 2006. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 20:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natsoulis, G., F. Winston, and J. D. Boeke. 1994. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics 136:93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuwald, A. F., and D. Landsman. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154-155. [DOI] [PubMed] [Google Scholar]
- 52.Ng, H. H., S. Dole, and K. Struhl. 2003. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 278:33625-33628. [DOI] [PubMed] [Google Scholar]
- 53.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11:709-719. [DOI] [PubMed] [Google Scholar]
- 54.Ng, H. H., R. M. Xu, Y. Zhang, and K. Struhl. 2002. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J. Biol. Chem. 277:34655-34657. [DOI] [PubMed] [Google Scholar]
- 55.Ooi, S. L., X. Pan, B. D. Peyser, P. Ye, P. B. Meluh, D. S. Yuan, R. A. Irizarry, J. S. Bader, F. A. Spencer, and J. D. Boeke. 2006. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 22:56-63. [DOI] [PubMed] [Google Scholar]
- 56.Panasenko, O., E. Landrieux, M. Feuermann, A. Finka, N. Paquet, and M. A. Collart. 2006. The yeast Ccr4-Not complex controls ubiquitination of the nascent-associated polypeptide (NAC-EGD) complex. J. Biol. Chem. 281:31389-31398. [DOI] [PubMed] [Google Scholar]
- 57.Penheiter, K. L., T. M. Washburn, S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2005. A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20:213-223. [DOI] [PubMed] [Google Scholar]
- 58.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 59.Pokholok, D. K., N. M. Hannett, and R. A. Young. 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9:799-809. [DOI] [PubMed] [Google Scholar]
- 60.Pringle, J. R., R. A. Preston, A. E. Adams, T. Stearns, D. G. Drubin, B. K. Haarer, and E. W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 61.Robzyk, K., J. Recht, and M. A. Osley. 2000. Rad6-dependent ubiquitination of histone H2B in yeast. Science 287:501-504. [DOI] [PubMed] [Google Scholar]
- 62.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 63.Rothstein, R. 1991. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194:281-301. [DOI] [PubMed] [Google Scholar]
- 64.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481-516. [DOI] [PubMed] [Google Scholar]
- 65.Santos-Rosa, H., A. J. Bannister, P. M. Dehe, V. Geli, and T. Kouzarides. 2004. Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J. Biol. Chem. 279:47506-47512. [DOI] [PubMed] [Google Scholar]
- 66.Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein, N. C. Emre, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419:407-411. [DOI] [PubMed] [Google Scholar]
- 67.Schneider, J., P. Bajwa, F. C. Johnson, S. R. Bhaumik, and A. Shilatifard. 2006. Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. J. Biol. Chem. 281:37270-37274. [DOI] [PubMed] [Google Scholar]
- 68.Sheldon, K. E., D. M. Mauger, and K. M. Arndt. 2005. A requirement for the Saccharomyces cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol. Cell 20:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shilatifard, A. 2006. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem. 75:243-269. [DOI] [PubMed] [Google Scholar]
- 70.Shirra, M. K., S. E. Rogers, D. E. Alexander, and K. M. Arndt. 2005. The Snf1 protein kinase and Sit4 protein phosphatase have opposing functions in regulating TATA-binding protein association with the Saccharomyces cerevisiae INO1 promoter. Genetics 169:1957-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simic, R., D. L. Lindstrom, H. G. Tran, K. L. Roinick, P. J. Costa, A. D. Johnson, G. A. Hartzog, and K. M. Arndt. 2003. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 22:1846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sims, R. J., 3rd, and D. Reinberg. 2006. Histone H3 Lys 4 methylation: caught in a bind? Genes Dev. 20:2779-2786. [DOI] [PubMed] [Google Scholar]
- 74.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 75.Somesh, B. P., J. Reid, W. F. Liu, T. M. Sogaard, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 2005. Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell 121:913-923. [DOI] [PubMed] [Google Scholar]
- 76.Squazzo, S. L., P. J. Costa, D. L. Lindstrom, K. E. Kumer, R. Simic, J. L. Jennings, A. J. Link, K. M. Arndt, and G. A. Hartzog. 2002. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 21:1764-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stolinski, L. A., D. M. Eisenmann, and K. M. Arndt. 1997. Identification of RTF1, a novel gene important for TATA site selection by TATA box-binding protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4490-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strahl, B. D., P. A. Grant, S. D. Briggs, Z. W. Sun, J. R. Bone, J. A. Caldwell, S. Mollah, R. G. Cook, J. Shabanowitz, D. F. Hunt, and C. D. Allis. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol. Cell. Biol. 22:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun, Z. W., and C. D. Allis. 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418:104-108. [DOI] [PubMed] [Google Scholar]
- 80.Takagi, Y., C. A. Masuda, W. H. Chang, H. Komori, D. Wang, T. Hunter, C. A. Joazeiro, and R. D. Kornberg. 2005. Ubiquitin ligase activity of TFIIH and the transcriptional response to DNA damage. Mol. Cell 18:237-243. [DOI] [PubMed] [Google Scholar]
- 81.Vilardell, J., and J. R. Warner. 1997. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell. Biol. 17:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 83.Wood, A., N. J. Krogan, J. Dover, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, A. Golshani, Y. Zhang, J. F. Greenblatt, M. Johnston, and A. Shilatifard. 2003. Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol. Cell 11:267-274. [DOI] [PubMed] [Google Scholar]
- 84.Wood, A., J. Schneider, J. Dover, M. Johnston, and A. Shilatifard. 2003. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 278:34739-34742. [DOI] [PubMed] [Google Scholar]
- 85.Xiao, T., C. F. Kao, N. J. Krogan, Z. W. Sun, J. F. Greenblatt, M. A. Osley, and B. D. Strahl. 2005. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol. Cell. Biol. 25:637-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu, F., K. Zhang, and M. Grunstein. 2005. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell 121:375-385. [DOI] [PubMed] [Google Scholar]
- 87.Zhang, K., W. Lin, J. A. Latham, G. M. Riefler, J. M. Schumacher, C. Chan, K. Tatchell, D. H. Hawke, R. Kobayashi, and S. Y. Dent. 2005. The Set1 methyltransferase opposes Ipl1 aurora kinase functions in chromosome segregation. Cell 122:723-734. [DOI] [PMC free article] [PubMed] [Google Scholar]