Abstract
Forkhead (Fkh) transcription factors influence cell death, proliferation, and differentiation and the cell cycle. In Saccharomyces cerevisiae, Fkh2 both activates and represses transcription of CLB2, encoding a B-type cyclin. CLB2 is expressed during G2/M phase and repressed during G1. Fkh2 recruits the coactivator Ndd1, an interaction which is promoted by Clb2/Cdk1-dependent phosphorylation of Ndd1, suggesting that CLB2 is autoregulated. Ndd1 is proposed to function by antagonizing Fkh2-mediated repression, but nothing is known about the mechanism. Here we ask how Fkh2 represses CLB2. We show that Fkh2 controls a repressive chromatin structure that initiates in the early coding region of CLB2 and spreads up the promoter during the M and G1 phases. The Isw2 chromatin-remodeling ATPase cooperates with Fkh2 to remodel the chromatin and repress CLB2 expression throughout the cell cycle. In addition, the related factors Isw1 and Fkh1 configure the chromatin at the early coding region and negatively regulate CLB2 expression but only during G2/M phase. Thus, the cooperative actions of two forkhead transcription factors and two chromatin-remodeling ATPases combine to regulate CLB2. We propose that chromatin-mediated repression by Isw1 and Isw2 may serve to limit activation of CLB2 expression by the Clb2/Cdk1 kinase during G2/M and to fully repress expression during G1.
The forkhead (Fkh) family of transcription factors is highly conserved in eukaryotes, with roles in cell cycle control, cell death, proliferative responses, and differentiation (1, 3, 4, 27). All four forkhead factors have been characterized in Saccharomyces cerevisiae. Fhl1 and its coregulator Ihf1 regulate cell proliferation primarily through control of ribosomal protein gene expression (5, 18, 21, 35, 50, 52, 59). Different phases of the cell cycle are regulated by two forkhead factors, Hcm1 and Fkh2. Hcm1 regulates chromosome segregation genes and controls the S-phase transition (46). Fkh2 and its coactivator Ndd1 influence the expression of a wide range of genes (56), including the genes of the CLB2 cluster that control the G2/M and M/G1 phases of the cell cycle (15, 23, 62) which are the best-characterized targets of Fkh2/Ndd1 regulation. Genes belonging to the CLB2 cluster, including CLB2 itself, contain one or more SFF binding sites at which Fkh2, Ndd1, and the MADS box protein Mcm1 bind. Both Fkh2 and Ndd1 are subject to extensive phosphorylation during the cell cycle. The Clb5/Cdc28 complex phosphorylates residues on the C-terminal region of Fkh2 between the S phase and G2 (44). The interaction between Fkh2 and Ndd1 is optimal when both proteins are phosphorylated. Ndd1 is a substrate for the Clb2/Cdc28 G2/M kinase, and phosphorylation promotes the interaction between Ndd1 and the FHA domain of Fkh2 (11, 49). Thus, CLB2 expression is likely to be subjected to a positive-feedback loop (11, 23, 44, 49) and may be regulated differently from other genes in the CLB2 cluster. Very little is understood about how this feedback loop is broken to repress expression during G1 and S phase.
Fkh1, like Fkh2, contains an FHA domain and can function redundantly with Fkh2 to maintain cell cycle-regulated gene expression through an interaction with Ndd1 at the SFF site (11, 20, 23, 49). Despite this redundancy, Fkh1 and Fkh2 do not have equivalent functions, although precisely how Fkh1 functions remains to be defined. Fkh1 is unlikely to form a stable ternary complex with Mcm1 (20), as it lacks the Mcm1 interaction domain present on Fkh2 (2). Ablation of each individual Fkh factor has a different effect on the steady-state levels of CLB2 mRNA and the time spent in each phase of the cell cycle (19, 25, 43). Genetic data support opposing actions for FKH1 and FKH2 in silencing the mating type locus HMR (19) and, more generally, in transcription (42). A strain lacking FKH2 shows synthetic phenotypes when SPT4 (fkh2Δ spt4Δ is temperature sensitive) or DST1 (fkh2Δ dst1Δ shows poor viability), encoding factors required to maintain processivity of elongating RNA polymerase (36), are deleted. These phenotypes are suppressed by the additional deletion of FKH1 (42). Thus, Fkh1 and Fkh2 are likely to influence transcription in opposite ways. In addition, global cluster and periodicity analysis of cell cycle-regulated transcription factor activities suggests that Fkh1 forms a distinct hierarchical group of regulators with Cbf1, Msn4, Met4, Mth1, and Rfx1 that can be distinguished from Fkh2, Fhl1, Mcm1, and Ndd1 (60). Both Fkh1 and Fkh2 contain the forkhead or winged helix DNA binding domain. The forkhead domain has structural homology with the linker histones (6, 9), raising the possibility of an additional mode by which these factors might associate with nucleosomal DNA (16).
Fkh1 also differs from Fkh2 in that it lacks the C-terminal extension on which Fkh2 is phosphorylated by Clb5-Cdk1. This domain of Fkh2 is likely to play an important role in overcoming some mechanism for transcriptional repression. The reasoning behind this is that deletion of NDD1 encoding the activator is lethal but when the C-terminal domain of Fhk2 is removed, viability is restored. This supports the idea of a role for Fkh2 as a platform to control the recruitment or activity of factors that activate transcription (e.g., Ndd1) and repress transcription. Thus, ablation of factors that repress expression of CLB2 by acting through Fkh2 might also suppress the lethality of ndd1Δ.
Here we address the question of how Fkh2 represses expression of CLB2. We identify Isw1 and Isw2, chromatin-remodeling ATPases (38, 57), as potential candidate repressors of CLB2 that act through Fkh2. We show that CLB2 undergoes a cell cycle-dependent reorganization of nucleosomes over the promoter and early coding region. This reorganization is dependent on the Isw2 ATPase activity, the integrity but not the ATPase activity of Isw1, and the redundant function of Fkh1 and Fkh2. The ATPase activity of Isw1, in collaboration with Fkh1, remodels a region at the beginning of the CLB2 ORF to repress CLB2 expression. Having analyzed the dynamics of chromatin remodeling at CLB2 in real time, we suggest that gene repression is initiated in the early coding region of the active gene and that repressive chromatin remodeling spreads up the promoter to the regulatory sequences. This is reversed on gene activation. We conclude that Fkh2, Fkh1, Isw1, and Isw2 cooperate to attenuate the activity of the CLB2 promoter and that Fkh2 also antagonizes this repression.
MATERIALS AND METHODS
Strains.
Strains were constructed using PCR-mediated deletion and modification, using KanMX or other selectable markers exactly as previously described (33) in the W303-1a background for both isw strains (57) and fkh strains (Table 1) (43). Fluorescence-activated cell sorting (FACS) analysis reveals that MATa cells containing isw1Δ or expressing a catalytically inactive version of Isw2 (isw2K215R) failed to arrest in G1 when treated with α-factor (54). This arrest could be restored by deletion of the HMLα silent mating type locus, strongly supporting a role for the integrity of Isw1 and the catalytic activity of Isw2 in efficient silencing of HML. Thus, all experiments using these strains were conducted or repeated in an hmlΔ background. Both the mutations leading to loss of ATPase activity result in a lysine-to-arginine substitution in the ATPase domain of the ISWI protein and abolish nucleosome sliding in vitro (13) (T. Tsukiyama, personal communication).
TABLE 1.
Yeast strains
| Strain | Genotype | Source and/or reference |
|---|---|---|
| W303-1a wild type | MATaade2-1 his3-11-13 leu2-3-112 trp1-1 ura3-1 can1-100 | |
| YTT166 wild type | W303-1a; RAD5 | T. Tsukiyama |
| YTT186 Δisw1 | YTT166; isw1::ADE2 | T. Tsukiyama |
| YTT196 Δisw2 | YTT166 except MATα isw2::LEU2 | T. Tsukiyama |
| YTT199 Δisw1 Δisw2 | YTT166; isw1::ADE2 isw2::LEU2 | T. Tsukiyama |
| YTT1223 isw1K227R | YTT166; isw1 K227R (ATPase activity abolished) | T. Tsukiyama |
| YTT581 isw2K215R | YTT166; isw2 K215R (ATPase activity abolished) | T. Tsukiyama |
| YTT166 wild-type Δhml | W303-1a; RAD5 hml::KanMX | This study |
| YTT186 Δisw1 Δhml | YTT166; hml::KanMX isw1::ADE2 | This study |
| YTT196 Δisw2 Δhml | YTT166 except MATα hml::KanMX isw2::LEU2 | This study |
| YTT199 Δisw1Δisw2 Δhml | YTT166; hml::KanMX isw1::ADE2 isw2::LEU2 | This study |
| YTT1223 isw1K227R Δhml | YTT166; hml::KanMX ISW1 K227R (ATPase activity abolished) | This study |
| YTT581 isw2K215R Δhml | YTT166; hml::KanMX ISW2 K215R (ATPase activity abolished) | This study |
| Δfkh1 AP3 | W303-1a; fkh1::HIS3 | B. Morgan (43) |
| Δfkh2 AP5 | W303-1a; fkh2::URA3 | B. Morgan (43) |
| Δfkh1Δfkh2 AP11 | W303-1a; fkh1::HIS3 fkh2::URA3 | B. Morgan (43) |
| US262 | W303-1a; ndd1Δ::LEU2 pGAL1-NDD1 | 34 |
| US262 isw2Δ | W303-1a; ndd1Δ::LEU2 pGAL1-NDD1 isw2::KanMX | This study |
| US262 isw1Δ | W303-1a; ndd1Δ::LEU2 pGAL1-NDD1 isw1::KanMX | This study |
Growth conditions.
All strains were grown in YPD (1% Bacto peptone, 1% yeast extract, 2% d-glucose) or YPGAL (1% Bacto peptone, 1% yeast extract, 2% d-galactose) at 29°C. Plates were made with 2% agar. Cell cycle blocking was carried out by incubation for approximately 2 h using 10 μg/ml alpha mating factors (Sigma) or 10 μg/ml nocodazole in dimethyl sulfoxide (Sigma) until the budding index was >90% by light microscopy. Samples were also fixed for FACS to confirm cell cycle blocks. For analysis of the chromatin structures during the cell cycle, 500 ml of the diploid strain J2, containing a temperature-sensitive mutation in DBF2, was grown at 25°C to a density of 7 × 106/ml and synchronized for 110 min at 37°C until the budding index was >90%. The cells were harvested in a prewarmed rotor and resuspended at 1.1 × 107/ml in fresh YPD at 25°C. Samples containing identical numbers of cells were taken every 20 min. The position in the cell cycle was assessed by the budding index and by FACS analysis. The cells remained in good synchrony for three cell cycles. As the biochemistry of cells changes as they proceed through the cell cycle, it was necessary to show that the changes in micrococcal (MNase) cleavage patterns did not result from differential susceptibilities to MNase nuclease. To show this, cells were synchronized and samples were taken for analysis at 80 min and 120 min after release from the block and subjected to digestion with increasing concentrations of MNase nuclease. The cleavage patterns remained distinct for the two time points, indicating that any changes that occurred did not result from differential susceptibility to MNase.
In vivo chromatin analysis.
Experiments were conducted exactly as described in reference 22 and were repeated at least twice. Briefly, the cell walls were removed and cells stabilized in sorbitol. Membranes were permeabilized with NP-40 prior to treatment with up to 600 units/ml of micrococcal nuclease. The isolated DNA was restricted with BglII and separated on 1.2% gels. The probe was prepared by PCR amplification of a 1.2-kb fragment that was subsequently restricted with BglII to generate a 400-bp fragment for the end label extending from position +704 to +304. This allowed good resolution of the promoter nucleosomes and nucleosome +1. Molecular weights of MNase cleavage products in chromatin and naked DNA were assessed using One-D scan (Scanalytics). Profiles of the MNase cleavage products (band intensities) were generated in Excel.
Northern analysis.
RNA was isolated from 2 × 108 cells by use of RNeasy Midi and Oligotex mRNA isolation kits (QIAGEN). One-quarter of each mRNA-enriched preparation was processed under conditions specified by the manufacturers and was hybridized with a CLB2 end label probe followed by a 400-bp fragment from the ACT1 actin gene or a 1.8-kb EcoRI fragment specific for the 1.8-kb rRNA.
RESULTS
Isw1 and Isw2 repress CLB2 expression.
CLB2 was identified as a potential substrate for the chromatin-remodeling Isw1 and Isw2 ATPases during a genome-wide screening (22). In asynchronously growing isw1Δ isw2Δ cells, there is a distinctive chromatin structure upstream of the activator binding site beginning at −675 and extending into the coding region of CLB2 that is different from that of the wild type (WT) (Fig. 1A; compare lanes 2 and 3 and lanes 4 and 5). This was revealed using micrococcal nuclease (MNase) and indirect end labeling to detect intranucleosomal cleavage sites in vivo (22). The presence of positioned nucleosomes within this region of CLB2 can be inferred from the protection in chromatin of MNase cleavage sites in naked DNA (Fig. 1A, lane 1). In the profile of band intensities shown in Fig. 1B, the changes are evident. These include increased sensitivity to MNase and additional cleavage products (regions B and C) and missing cleavage sites (the higher band of the double-cleavage sites at region D and both the doublet sites at region F are missing). We asked how the ISWI factors influence expression of CLB2 by comparing mRNA prepared from a WT strain or an isw1Δ isw2Δ strain (Fig. 1C). mRNA was prepared from cells cycling asynchronously (lanes 1 and 4), arrested at G2/M phase (lanes 2 and 5), or arrested at G1 phase (lanes 3 and 6). The isw1Δ isw2Δ strain is defective in repression of CLB2 mRNA at all phases of the cell cycle, as levels are three- to fivefold higher than in the equivalent in wild-type cells. In addition, the ISWI factors are required to repress expression at the G1 phase of the cell cycle, when CLB2 is normally repressed.
FIG. 1.
Isw1p and Isw2p repress CLB2 expression. (A) Southern blot showing an indirect end label analysis of MNase cleavage sites in DNA prepared from strains YTT166 (WT; lanes 3 and 4), YTT199 (isw1Δ isw2Δ; lanes 5 and 6), and AP11 (fkh1Δ fkh2Δ; lanes 7 and 8). Chromatin was digested with 150 or 300 units/ml of MNase (open triangle). Total DNA was restricted with BglII, separated on a 1.2% gel, and detected using a probe extending 400 bp from the BglII site at +704. The results seen with naked DNA digested with MNase are shown in lane 2. A schematic showing the position of XhoI sites at CLB2 (MK; lane 1) relative to the BglII cleavage site at +704 is shown. The XhoI site at −630 resides next to the Mcm/SFF site and provides a reference point for this and all subsequent autoradiographs. Labels A to H represent the approximate positions of eight MNase cleavage sites referred to in the text. (B) Scans of band intensity for each strain digested with 300 U/ml MNase. The positions of the major cleavage products in chromatin and in naked DNA, averaged from the results of a number of experiments, are shown below the scan. Alternative cleavage sites are indicated with a subscript 1 or 2. Given the specificity of MNase for nucleosomal linker DNA, protection of a MNase cleavage site normally observed in naked DNA and its replacement by two flanking chromatin-specific cleavage sites 150 to 200 bp apart are taken to imply the presence of a positioned nucleosome. The band intensities are shown relative to the key features of the CLB2 gene, including the Mcm1/SFF binding site (box; −687 to −665), the putative TATA sequence (T at −477), and the major mRNA initiation site at −361 (arrow). The open arrow represents a nuclease hypersensitive site in the vicinity of the complex binding site. The open rectangle represents the open reading frame. (C) Autoradiograph of a Northern blot showing mRNA isolated from strains YTT166 (WT; lanes 1 to 3) and YTT199 (isw1Δ isw2Δ; lanes 4 to 6) growing asynchronously (labels A) in YPD (lanes 1 and 4) or after treatment for 2 h with 10 μg/ml α-factor (cells arrested at G1 phase; lanes 3 and 6) or nocodazole (cells arrested at G2/M phase; lanes 2 and 5).
isw1Δ or isw2Δ suppresses lethality associated with ndd1Δ.
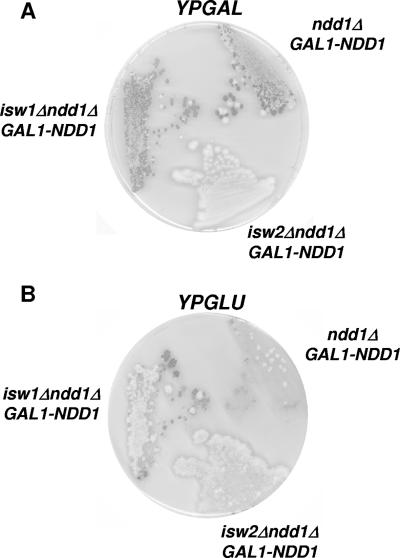
Cells cannot grow when the Ndd1 activator is absent. An ndd1Δ fkh2Δ strain is viable, however, suggesting that Ndd1 is required to overcome repression mediated via Fkh2. Thus, loss of repressors that function via Fkh2 should also restore viability to an ndd1Δ strain. To explore whether Isw1 and Isw2 act through Fkh2 to repress CLB2 expression, we constructed an isw1Δ ndd1Δ strain, an isw2Δ ndd1Δ strain, and an ndd1Δ strain containing the NDD1 gene under the control of the galactose-inducible GAL1 promoter. All three strains are viable on galactose medium (Fig. 2A). As expected, the ndd1Δ [pGAL1-NDD1] strain grows very poorly on glucose medium (Fig. 2B). In contrast, growth of the isw2Δ ndd1Δ [pGAL1-NDD1] and the isw1Δ ndd1Δ [pGAL1-NDD1] strains was observed on glucose (Fig. 2B). Best growth was observed at room temperature. This suggests that Isw1 and Isw2 repress CLB2 expression through Fkh2. This observation, however, could also result from a failure of Isw1 or Isw2 to repress the GAL1 promoter in glucose. We did not observe any detectable transcript arising from the GAL1 promoter in isw1Δ or isw2Δ strains (data not shown) but cannot rule out very low levels of Ndd1 leading to the suppression observed. If the ISWI factors are repressing through Fkh2 then we would expect to see an effect of loss of Fkh2 on the chromatin structure at CLB2.
FIG. 2.
isw1Δ and isw2Δ suppress lethality associated with ndd1Δ. (A) The three strains indicated were plated on rich medium containing galactose (YPGAL) and cultured for 2 days at 29°C. (B) A replica was stamped onto rich medium containing glucose (YPGLU) and incubated for 4 days at 21°C.
The chromatin structure in the fkh1Δ fkh2Δ strain resembles that in the isw1Δ isw2Δ strain.
To distinguish whether Isw1 and Isw2 are acting via Fkh2 or indirectly on expression of GAL-NDD1, to suppress the lethality of the ndd1Δ, we examined the chromatin organization at CLB2 in the fkh1Δ fkh2Δ strain. The double deletion was used because Fkh1 can suppress loss of Fkh2. The structure was disrupted in a way similar, but not identical, to that observed in the isw1Δ isw2Δ strain (Fig. 1A; compare lanes 4 and 5 and lanes 6 and 7). In the profile of band intensities, the changes that are similar to those seen with the isw1Δ isw2Δ strain are evident and include addition cleavage sites around regions C and B and a missing cleavage site (one of the double cleavage sites at region D) (Fig. 1B). There are some changes that are specific to the fkh1Δ fkh2Δ strain; these include an additional band below position E, loss of cleavage at position G, and loss of only one of the two cleavage sites at position F. These data suggest that Isw1 and Isw2 are likely to be acting to repress CLB2 expression via Fkh2 and that nucleosome positioning might be part of the mechanism. Next we asked whether nucleosome positioning at CLB2 is related to gene expression (G2/M phase) or gene repression (G1 phase) during the cell cycle.
Dynamic nucleosome positioning at CLB2 during the cell cycle.
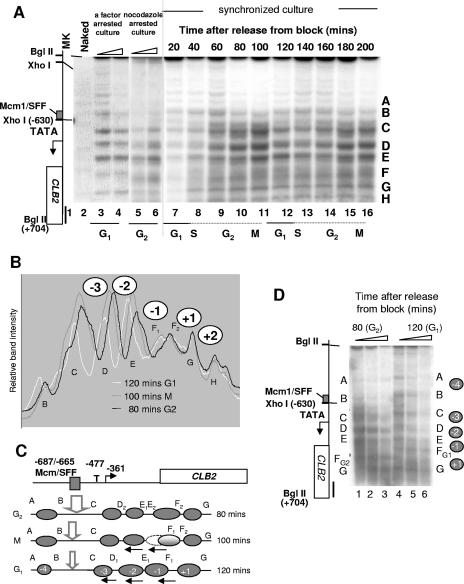
A synchronous population of cells was prepared and the chromatin structure at CLB2 examined at 20-min intervals throughout two cell cycles (Fig. 3A, lanes 7 to 16). The experiment was done twice, and the data were reproducible. Synchrony was judged to be over 80%, as monitored visually by counting, by assessing the budding index, and by 4′,6′-diamidino-2-phenylindole (DAPI) staining. The analysis of the eight major intranucleosomal cleavage positions (labeled A to H) at three time points revealed five major changes in the CLB2 promoter spanning −1020 to +300. The presence of positioned nucleosomes over this region of CLB2 can be inferred from the protection in chromatin of MNase cleavage sites in naked DNA (lane 2). In addition, the distances between bands C, D, E, F, and G range between approximately 160 bp and 260 bp, as is consistent with nucleosome occupancy (see Fig. 1B). During the G1 and G2 phases of the cell cycle, nucleosomes +1, −1, −2, −3, and −4 are positioned differently (compare the results seen at 80 and 120 min) (Fig. 3B and C). Synchronized cells were prepared at these times and subjected to digestion with increasing concentrations of MNase (Fig. 3D). This side-by-side comparison shows that the chromatin structures are stable and reveals the nucleosome changes. An interpretation of this data is that in G1 phase (120 min), the nucleosomes tend to shift in the 5′ direction on the promoter (arrows), whereas in G2 phase, they are shifted in the 3′ direction towards the coding region.
FIG. 3.
Cell-cycle-dependent chromatin dynamic at CLB2. (A) Autoradiograph of a southern blot showing indirect end label analysis of MNase cleavage sites in DNA prepared from a synchronized culture of J2 (dbf2ts). Samples were taken at 20-min intervals after shifting to the permissive temperature and were treated with 600 U/ml MNase (lanes 7 to 16). At each time point, the budding index and the number of cells in the population were counted using a hemocytometer. DNAs prepared from equal numbers of cells were used at each time point. Also shown are the results obtained with chromatin prepared from WT cells arrested at G1 phase (lanes 3 to 4) or G2/M phase (lanes 5 to 6), a naked DNA control (Naked; lane 2), and markers (lane 1). (B and C) Scans of band intensities at three time points (B); an interpretation of nucleosome positions (ovals) at three time points (C). Nucleosomes are labeled −4 to +1. The major MNase cleavage sites discussed in the text are marked A to G. Alternate cleavage sites observed during the cell cycle are indicated by subscript 1 or 2. The arrows indicate shifted nucleosomes (ovals). (D) Southern blot showing indirect end label analysis of MNase cleavage sites in DNA prepared from a synchronized culture of J2 at 80 min (G2; lanes 1 to 3) or 120 min (G1; lanes 4 to 6) after release from the blocking. Chromatin was digested with 150, 300, or 600 units/ml of MNase. See Fig. 1 for details.
The relative band intensities reveal other notable changes to the chromatin, including an increase in nuclease sensitivity at regions C and D when the gene is expressed in G2/M phase and differences in nuclease sensitivity around the coding region nucleosomes (+1) that begin in M phase and precede the major shift up the promoter for nucleosomes −1 to −4 that occurs in G1 phase (Fig. 3B). The results obtained in an analysis of chromatin from a strain arrested in the G1 and G2/M phases of the cell cycle prepared in a different experiment are included for comparison (Fig. 3A, lanes 3 to 6). We note that although the intranucleosomal cleavage sites reflecting the positions of the promoter nucleosomes (−1 to −4) in the synchronized population are similar to those seen with the α-factor-arrested cells (shifted up; compare lanes 3, 7, and 12), the structures around nucleosome +1 are different. In the α-factor-arrested cells, there is a distinct band suggesting that nucleosomes +1 and +2 are positioned in the population, whereas in the synchronized population of cells, the cleavage pattern suggests a more dynamic structure. Similarly for the cells arrested in G2/M phase, aspects of the structure are similar to those seen with the synchronized cells in G2 phase, particularly the predominant lower band at positions D, E, and F (lanes 5 and 6). However, other changes, such as the extra cleavage sites at position C, are not evident, again supporting the idea of a dynamic structure in the synchronized cells that is lost when cells are arrested for growth. Note that MNase digestion in nocodazole-arrested cells is inhibited by the dimethyl sulfoxide solvent. No significant cell cycle-dependent changes were observed in the main body of the coding region (data not shown). We conclude that nucleosome positioning at CLB2 is dynamic and changes throughout the cell cycle at both the promoter and early coding regions. Next we examined how Isw1 and Isw2 influence this chromatin structure during the cell cycle.
Isw1 and Isw2 are required for cell cycle-dependent changes at CLB2.
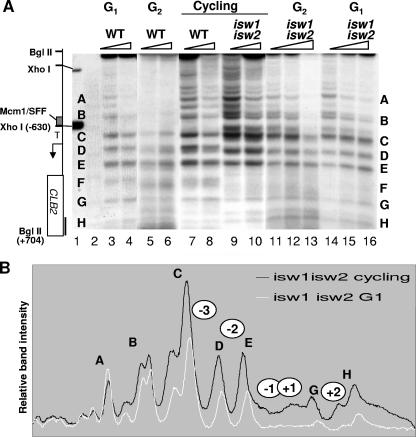
The changes in the chromatin structure at CLB2 during the cell cycle are consistent with sliding of nucleosomes rather than nucleosome displacement or loss, supporting the idea of a role for the ISWI family of ATPase known to be associated primarily with nucleosome sliding in vivo (14, 22, 26). We asked whether Isw1 and Isw2 are required for the cell cycle-dependent changes at CLB2. Strains lacking both Isw1 and Isw2 were grown asynchronously and arrested at the G2/M transition or in the G1 phase of the cell cycle, and the chromatin structure at CLB2 was compared to those of an asynchronously cultured WT strain and a WT strain arrested in G1 or G2/M phase (Fig. 4A; compare lanes 3 to 6 with 11 to 16). We observed no significant difference in chromatin organization at either phase of the cell cycle in the isw1Δ isw2Δ strain. The structure resembled that seen in the asynchronous isw1Δ isw2Δ strain (Fig. 4A, lanes 9 to 10, and Fig. 4B). None of the changes associated with progression through the cell cycle were evident in the isw1Δ isw2Δ strain. We conclude that the Isw factors are required for the cell cycle-dependent chromatin shift at CLB2. Furthermore, this may be linked to the repression of CLB2 expression during the cell cycle, as the Isw factors are also required for this (see Fig. 1C). Next we asked whether the repression of CLB2 expression requires either Isw1 or Isw2 or both factors.
FIG. 4.
Isw1 and Isw2 are required for cell cycle-dependent chromatin remodeling at CLB2. (A) Autoradiographs of Southern blots showing indirect end label analysis of MNase cleavage sites in chromatin prepared from the indicated strains, growing asynchronously (cycling) or blocked at G1 or G2/M phase and treated with 150 (lanes 3, 5, 7, 9, 11, and 14) or 300 U/ml MNase. See Fig. 1 for details. (B) Scans of band intensities comparing the results seen with the isw1Δ isw2Δ strain arrested at G1 to those seen with asynchronous growth (cycling). Just two profiles are show for clarity. No significant difference was observed between the results obtained with the isw1Δ isw2Δ strain arrested at G1 and those seen with the strain in G2/M phase.
The ATPase activities of either Isw1 or Isw2 are required to repress CLB2 expression.
We used strains resulting from complete deletions of either ISW1 or ISW2 and strains expressing catalytically inactive versions of Isw1 and Isw2 to distinguish which factor is required to repress CLB2 expression during the cell cycle.
The strains lacking Isw1 or Isw2 protein lose the ability to repress CLB2 expression to wild-type levels, supporting roles for both Isw1 and Isw2 (Fig. 5A, lanes 1 and 2). Significantly, the mRNA levels are always slightly higher in either of the single-mutant strains compared to the results seen with the strain lacking both Isw1 and Isw2 (Fig. 5A; compare lanes 1 and 2 with lane 3). The catalytic activities of either Isw1p or Isw2p are required to repress CLB2 mRNA levels to normal in asynchronously grown cultures (Fig. 5A, lanes 5 and 6). The levels of mRNA in these strains are not as high as in the single isw1Δ or isw2Δ strains, supporting the idea of roles for both the ATPase activities and the integrity of the proteins in gene repression.
FIG. 5.
Distinct functions for Isw1 and Isw2 in chromatin remodeling at CLB2. (A and B) Autoradiographs of Northern blots (probed sequentially for CLB2 and then for ACT1) showing mRNA isolated from strains YTT186 (isw1Δ; A, lane 1, and B, lane 3), YTT192 (isw2Δ; A, lane 2, and B, lane 4), YTT199 (isw1Δ isw2Δ; A, lane 3, and B, lane 6), YTT166 (WT; A, lane 4, and B, lane 1), YTT1223 (isw1K227R; A, lane 5, and B, lane 2), and YTT581 (isw2K215R; A, lane 6, and B, lane 5). Cells in panel B also contained hmlΔ to facilitate arrest in the G1 phase of the cell cycle with α-factor. (C) Southern blots showing indirect end label analysis of MNase cleavage sites in chromatin prepared from the indicated strains growing asynchronously (cycling) or blocked at G1 or G2/M phase and treated with 25 U/ml (lanes 1, 3, and 5), 50 U/ml (lanes 2, 4, and 6), 150 U/ml (lanes 7, 9, 11, 13, and 15), or 300 U/ml (lanes 8, 10, 12, 14, and 16) of MNase. See Fig. 1 for details. Scans of band intensities comparing the isw1K227R and the isw2K215R mutant strain results with the results seen with the WT strain growing asynchronously (cycling) are shown below the gel. (D) Southern blots showing indirect end label analysis of MNase cleavage sites in chromatin prepared from the indicated strains growing asynchronously (cycling) or blocked in G2/M phase and treated with 150 U/ml (lanes 3, 5, 7, 9, and 11) or 300 U/ml (lanes 4, 6, 8, 10, and 12) of MNase. See Fig. 1 for details. Scans of band intensities comparing the isw1Δ and isw2Δ strain results with the results obtained with the WT and isw1Δisw2Δ strains growing asynchronously (cycling) are shown above the gel.
Next we examined the effect of these mutations on repression of CLB2 expression during the G1 phase of the cell cycle (Fig. 5B). The integrity of both Isw1 and Isw2 and the ATPase activity of Isw2 are required to repress CLB2 expression in G1 phase. However, the Isw1 ATPase activity is dispensable for repression in G1 phase. This suggests distinct roles for the integrity of Isw1 and its ATPase activity in cell cycle control of CLB2.
Isw1 and Isw2 remodel distinct regions of CLB2 chromatin.
To assess the contribution of Isw1 and Isw2 to nucleosome positioning at CLB2, we compared strains expressing the catalytically inactive versions of Isw1 or Isw2 in asynchronous cultures (cycling) or in phase G1-arrested cells (Fig. 5C).
There was no mRNA detectable above the background level in phase G1-arrested cells in the isw1K227R strain (Fig. 5B; compare lanes 1 and 2), suggesting that repression is normal during this phase of the cell cycle. Supporting this observation, the promoter nucleosomes (−2 and −3 flanking cleavage site D) are positioned like those of the wild-type strain in the isw1K227R mutant in phase G1-arrested cells (compare lanes 9 and 10 and lanes 11 and 12) and show cell cycle-dependent changes in position (evident from both cleavage sites at D being present in asynchronous cultures) (Fig. 5C; compare lanes 1 and 2 with lanes 3 and 4).
The chromatin structure within the transcribed-coding region structure is defective when cells lack the Isw1 ATPase activity. Band F is missing between nucleosomes −1 and +1 (Fig. 5C, lanes 3 to 4). This defect is also present in the isw1Δ isw2Δ strain (Fig. 1A and Fig. 4A), supporting the idea of a specific function for the Isw1 ATPase in configuring this structure and repressing CLB2 expression during only the G2 phase of the cell cycle. We conclude that the ATPase activity of Isw1 remodels nucleosomes −1 and +1 flanking cleavage site F at CLB2.
By contrast, the Isw2 ATPase is required for the cell cycle-dependent shift in both the promoter and coding region nucleosomes. The lower positions for bands D and F, and the extra band below E, all characteristic of cells in the G2 phase of the cell cycle (compare lanes 5 and 6 with lanes 7 and 8), are observed in ISW2K215R cells even when cells are arrested with α-factor in G1 phase (Fig. 5C, lanes 13 and 14). In addition, there is a shift down in the position of the hypersensitive region at the Mcm/SFF binding site in the ISW2K215R strain (Fig. 5C, lanes 5 and 6). These differences at the SFF site are exacerbated by the use of low concentrations of MNase (25 and 50 U/ml; Fig. 5C, lanes 1 to 6). At higher concentrations (150 and 300 U/ml; Fig. 5C, lanes 7 to 14), this hypersensitive region cleavage is lost but the MNase cleavage pattern at the remaining sites is constant, indicating stable structures. The Isw2 ATPase is different from the Isw1 ATPase in that it is required to produce a structure leading to cleavage at band G (between nucleosomes +1 and +2) but not band F (Isw1 dependent and between nucleosomes −1 and +1) (Fig. 5C). We conclude that the structures present in strains expressing catalytically inactive versions of Isw1p or Isw2p are distinct and that the ATPase activities of Isw1 or Isw2 remodel distinct but overlapping regions of CLB2 to repress expression at different phases of the cell cycle.
Chromatin remodeling by Isw2 requires Isw1p integrity but not Isw1 ATPase activity.
To see whether the actions of Isw1 and Isw2 are independent or cooperative, we examined the chromatin structures in strains resulting from a complete deletion of ISW1 or ISW2 (isw1Δ or isw2Δ) and compared them to the those of the isw1Δ isw2Δ double-deleted strain (Fig. 5D). In the isw1Δ and the isw2Δ strains the nucleosomes are identically positioned in a pattern resembling that seen in the G2 phase of the cell cycle (Fig. 5D; compare lanes 7 to 10 with lanes 11 and 12). The MNase cleavage patterns in the isw1Δ or isw2Δ strains are different from those seen with the isw1Δ isw2Δ strain (Fig. 5D) or either the isw2K215R or the isw1K227R strains (Fig. 5C). Over the promoter region, the single and double ISWI mutants have identical chromatin structures. This suggests that the integrity of Isw1, but not the ATPase activity, is required for Isw2 functional control over the promoter of CLB2. A difference between the single and double mutants is observed in the region of nucleosomes −1 and +1 and is reflected in the presence or absence of band F and the lower band at E (Fig. 5D). This region has no definitive cleavage site in the isw1Δ isw2Δ strain and lacks E2, while the cleavage pattern (lower bands at E and F) is consistent with a positioned nucleosome in the G2 configuration in the single mutants (compare lanes 7 to 10 with lanes 11 and 12). In all three strains, band G is positioned like that of the WT. The presence of band G is similar to that seen with the ISW1K227R strain but different from that seen with the ISW2K215R strain, which lacks this band (Fig. 5C). Taken together, these data support the idea that in the absence of one ISWI enzyme the second is able to position the early coding region nucleosomes. Thus, in this region Isw1 and Isw2 act redundantly. Loss of function through the dominant-negative effect of the catalytically inactive forms of ISWI enzymes would explain the different structures in this region in these strains.
We conclude that Isw1 and Isw2 may act redundantly in the early coding region and cooperatively at the CLB2 promoter to produce these distinct chromatin structures, suggesting at least two distinct forms of the enzymes.
Redundant functions for Fkh1p and Fkh2p at the CLB2 promoter.
We looked at the effect of individual fkh1Δ and fkh2Δ deletions and compared their influence on nucleosome positioning at CLB2 with the double fkh1Δ fkh2Δ strain results (Fig. 6). In the fkh1Δ strain, nucleosome positioning at the CLB2 promoter resembles that of the wild-type strain except that subtle changes are evident in the early coding region (Fig. 6B; compare lanes 1 and 2 with lanes 6 and 7). There is no clear positioning of nucleosomes −1 and +1, and cleavage at position F is lost. This defect in nucleosome positioning in the fkh1Δ strain is very similar to that in the isw1K227R strain and suggests that Isw1 and Fkh1 may influence CLB2 expression is similar ways. Both Isw1 and Fkh1 (19) are required to repress CLB2 expression levels to the level observed in WT cells (Fig. 5A; note that the WT control [lane 1] is overloaded).
FIG. 6.
Fkh1 and Fkh2 remodel distinct regions of CLB2. (A) Autoradiograph of a Northern blot showing mRNA isolated from strains YTT1223 (isw1K227R; lane 5), AP5 (fkh2; lane 4), AP3 (fkh1; lane 3), and AP11 (fkh1 fkh2; lane 2) and the WT strain (lane 1). (B and C) Autoradiographs of Southern blots showing indirect end label analysis of MNase cleavage sites in chromatin prepared from the indicated strains by use of 25 U/ml (C, lanes 1, 2, 3, and 5), 50 U/ml (C, lane 4), 150/ml (B, lanes 1, 3, 5, 7, 9, and 11) or 300 U/ml (B, lanes 2, 4, 6, 8, and 10) of MNase. (D) Band intensities for the data obtained in panel B (150 U/ml). See Fig. 1 for details.
By contrast, in the fkh2Δ strain the structure resembles that seen with wild-type cells blocked in the G1 phase of the cell cycle (Fig. 6B; compare lanes 8 and 9 with lanes 10 and 11). A higher proportion of the G1 bands are evident at the alternative cleavage sites in regions D and F. A predominantly G1-like structure would explain the lower levels of CLB2 transcript observed in the fkh2Δ strains (Fig. 5A, lane 4) and the slower cell cycling time (19, 20, 23, 25, 43, 62). This structure also suggests that Fkh1 is not able to overcome the repressing chromatin structure mediated by the Isw2 ATPase. Neither of the structures in the individual mutants resembles that in the double fkh1Δ fkh2Δ deletion strain (Fig. 6B; compare lanes 3 to 5 and lanes 6 to 9), supporting the idea of redundant and distinct functions for Fkh1 and Fkh2 in chromatin remodeling at CLB2. The structure in the strain expressing the catalytically dead Isw2 is very similar to that in the fkh1Δ fkh2Δ strain (Fig. 6C). This supports a role for Fkh2 (or Fkh1 when Fkh2 is absent) in influencing the Isw2-dependent remodeling of CLB2 chromatin during the cell cycle.
DISCUSSION
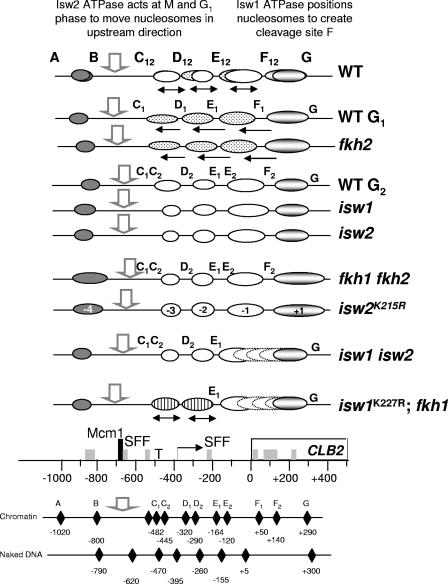
The data presented support the idea of functional links between two forkhead transcription factors and the imitation switch chromatin-remodeling ATPases in regulating the expression of CLB2. A schematic interpretation of the influence of each of the mutant strains studied on the chromatin structure at CLB2 is shown in Fig. 7. This reveals related functions for (i) Fkh1 and the Isw1ATPase and (ii) the Isw2 ATPase and Fkh2 (with the redundant action of Fkh1). Furthermore, the activities of all four proteins are likely to be coordinated through the requirement for intact Isw1 for Isw2-dependent remodeling.
FIG. 7.
Nucleosome positions at CLB2. A summary of nucleosome positions at CLB2 in the various mutant strains indicated based on the data presented in Fig. 1 to 6, including relative band intensities. Putative nucleosomes (−4 to +1) are represented by ovals. White ovals represent nucleosomes at G2 phase, stippled ovals represent nucleosomes at G1 phase (solid arrows pointing upstream), and lined ovals represent nucleosomes that shift from G1 to G2 phase (solid doubled-headed arrows) during the cell cycle in some mutant strains.The open arrow shows the hypersensitive site at the Mcm1/SFF binding site. The specialized nucleosome (+1), remodeled by Fkh1, Fkh2, Isw1, and Isw2, is shaded. Nucleosomes −1 and +1 that appear not to be positioned in some strains are represented by broken lines. The positions of the nucleosomes are shown relative to the key elements at CLB2. T, TATA block; right-angled arrow, RNA initiation site; empty rectangle, open reading frame. SFF sites are represented by rectangles and the Mcm1 site by the tall rectangle.
We chose to assess the function of the ISWI factors directly by assessing chromatin structures rather than using chromatin immunoprecipitation (ChIP), often a preferred way of exploring where proteins function on chromatin in vivo. We found that for both the Isw1 and Isw2 enzymes, the ChIP signals extend far beyond the region of remodeled chromatin at a number of known targets (data not shown). This suggests that for these enzymes, a ChIP signal does not equate with ATPase activity, at least as far as leading to nucleosome changes detectable by indirect end labels is concerned. This idea is supported by the observation that Isw2 associates relatively nonspecifically with chromatin (17).
Isw1 and Isw2 have been isolated in biochemically distinct complexes (37, 57, 58) and are generally considered to remodel distinct regions of chromatin in vivo (22). The results of this study support this idea, as Isw1 and Isw2 remodel overlapping but distinct nucleosomes at CLB2. Isw1 is not required for the expression of Isw2 and yet is clearly required for Isw2 function at CLB2. Isw1 might function as part of a complex that modifies chromatin, making it a suitable substrate for Isw2-dependent remodeling. For example, a basic patch on the histone H4 N-terminal region is critical for targeted Isw2 function (12). Alternatively, our observations may reflect a closer association of Isw1 and Isw2 within one or more protein complexes, involved in chromatin maintenance, that have been described previously (24).
Our study revealed related functions for Isw1 and Fkh1 in attenuating CLB2 expression during G2/M phase and in remodeling a highly localized region of chromatin within the transcribed-early coding region of the gene. Such Isw1-dependent structures have been observed at a number of other genes, including MET16 and SWI5 (22, 40, 41) (our unpublished data), and, as seen here, involve a small number of nucleosomes at the beginning of the transcribed-coding regions. This region is the site of enrichment for nucleosomes containing the variant histone Htz1 (28, 47, 61). Significantly, genes regulated by Fkh1 are particularly enriched with Htz1 (61). In addition, strains lacking both Isw1 and Htz1 are not viable (30), suggesting that the two factors cooperate to regulate gene expression. Our data suggest that Htz1 and Isw1 are likely to act at the same highly distinct region of a promoter. The chromatin in this region of active genes is often subject to extensive posttranscriptional covalent modification (31, 45). One such modification is trimethylation of lysine 4 on histone H3, and we have shown that a peptide methylated at K4 is able to interact with protein complexes containing Isw1 (51). Although H3K4me3 is generally considered to be associated with active genes, it is known to recruit factors involved in both maintaining and repressing gene expression (29, 55), providing a rationale for why an “active” modification might recruit a repressive factor such as Isw1. Such an association does help to explain why chromatin remodeling by Isw1 is so highly localized. However, the Isw1 ATPase is not required for repression of CLB2 in G1 phase, suggesting that it attenuates expression only from the active gene. Genetic data support repressive roles for both Isw1 and Fkh1 at some stage after the initiation of transcription (40-42), as is consistent with their site of action at CLB2. It is not clear how Isw1 or Fkh1 functions. There are a cluster of potential Fkh1 binding sites (the SFF site) over the region of CLB2 at which the Isw1 ATPase and Fkh1-dependent chromatin structures are observed (see Fig. 7). Furthermore, the association of Fkh1 with chromatin peaks at the early coding region of CLB2 (42). Fkh1 might function like a linker histone (6, 9, 48) to stabilize the nucleosomes remodeled by Isw1 or might bring other repressing activities, such as that of histone deacetylase, to this region of the gene.
The region remodeled by the Isw1 ATPase, containing nucleosome −1 and +1, appears highly dynamic and undergoes cell cycle-dependent changes. The Isw1 ATPase, however, is not required to repress CLB2 during the G1 phase of the cell cycle. A likely candidate for the cell cycle-dependent changes in this region is the Isw2 ATPase, as remodeling defects extend from nucleosome −3 to nucleosome +2 in the mutant. By analyzing the chromatin structure changes at CLB2 throughout the cell cycle, it is evident that cell cycle-dependent repressive changes to the structure start in this region during M phase and precede the apparent sliding of nucleosomes −1 to −3 in an upstream direction on the promoter. We suggest that nucleosomes −1 and +1, and their associated factors, play a key role in switching expression off and on during the cell cycle. How this is coordinated is less clear but is likely to involve the forkhead factors, as the chromatin structure from nucleosome −4 to nucleosome +2, and maybe further into the coding region, is also disrupted in the fkh1Δ fkh2Δ strain. Both Fkh1 and Fkh2 can be isolated with Sin3, a component of the Rpd3 histone deacetylase complex. Deacetylated chromatin is associated with repressed genes. Moreover, Isw2 functions in a parallel pathway with the Rpd3/Sin3 complex (13), raising the possibility that specific deacetylated residues, e.g., those on the H4 tail (7, 8, 10, 37, 53), may be essential for remodeling by Isw2.
How does Isw2 contribute to repression of CLB2? A major Isw2-dependent nucleosome (−3) movement during the cell cycle occurs in the vicinity of the TATA box and RNA initiation site. A simple explanation would be that this movement might be a cause or a consequence of displacement of components of the basal transcription machinery, such as the TATA-binding protein (TBP). The chromatin shifts may be a direct consequence of a change in the conformation of the basal transcription machinery on gene activation. At some promoters, a bend induced by the TBP on DNA causes repositioning of the nucleosome (32). We have previously described a role for the related ATPase, Isw1, in regulated displacement of the TBP from promoters (39), but it is not known whether Isw2 has a related function.
It is likely that Isw1 and Isw2 are not the only repressors of CLB2 that act through Fkh2, as strains lacking these factors show only weak suppression of the ndd1Δ lethality and the resulting yeasts are not normal. Given the requirements for Isw2-mediated repression at other loci, a histone deacetylase activity would also be a good candidate repressor for CLB2.
Although Fkh1 and Fkh2 have redundant functions, Fkh1 is required to represses CLB2 expression whereas Fkh2 activates the gene (19, 20, 23, 25, 43, 62). This work goes some way towards resolving the dilemma of why when both factors are able to recruit Ndd1p their effects on expression are distinct. The chromatin is constitutively remodeled into a repressive structure in the fkh2Δ strain. We propose that the reason for the very low expression of CLB2 in the fkh2Δ strain is that Fkh2 plays a unique role, via the C-terminal extension not shared by Fkh1, in overcoming Isw2-mediated repression and that the ability to recruit Ndd1p (by Fkh1p) is not sufficient. We propose that Fkh1, on the other hand, cooperates with Isw1, and possibly Isw2, to repress CLB2 expression.
As CLB2 is not an essential gene, the lethality of ndd1Δ strains cannot result simply from a failure to induce this gene. This strongly suggests that there are other targets at which Ndd1 is required to overcome ISWI-mediated repression. CLB2 is one of the members of a coregulated family of genes known as the CLB2 cluster that have Mcm/SFF binding sites and require the forkhead factors and Ndd1 for cell cycle-regulated expression. CLB2 is unique in this family, as it encodes the cyclin component of the kinase that phosphorylates Ndd1 to promote its interaction with Fkh2 and thus is likely to be autoregulated. In addition, CLB2 expression is required to activate the rest of the CLB2 cluster, including genes such as CLB1 (which acts redundantly with CLB2 to maintain viability), SWI5, and ACE2. Analysis of the role of the forkhead and ISWI factors at the genes of the CLB2 cluster is complicated by the effect of mutations in their genes on CLB2 expression and thus on the amount of Clb2 cyclin protein produced. Nevertheless, our analysis of SWI5, ACE2, and CLB1 reveals that their regulation is different from that of CLB2 (reference 54 and our unpublished data), suggesting that there is unlikely to be one common mechanism by which the all the genes of the CLB2 cluster are controlled. There are more than 30 genes in the CLB2 cluster, however, and other Ndd1 targets, some of which may be regulated like CLB2, requiring activated Ndd1 to overcome ISWI-mediated repression.
Regulation of CLB2 expression is required to coordinate progression through the cell cycle with environmental signals. Down-regulating expression during the G2/M phase, for example, facilitates the switch into pseudohyphal growth. This study suggests that Isw1, acting with the Fkh1 transcription factor, may be a key modulator of G2/M transcription. On the other hand, Fkh2 and the Isw2 ATPase may coordinate expression during the cell cycle, and particularly repression in G1 phase. At CLB2, these two processes may be coordinated through the unique region of chromatin (nucleosomes −1 and +1) at the beginning of the coding region at which all four factors function.
Acknowledgments
We thank Anitha Nair for excellent technical assistance, Antonin Morillon for constructing the hmlΔ strains, Brian Morgan for fkhΔ strains, Toshio Tsukiyama for iswΔ strains and helpful discussion, and Gustav Ammerer for the ndd1Δ strain and for communicating unpublished results.
This work was funded by the Wellcome Trust. J.S. acknowledges the support of a BBRSC studentship and a scholarship from Corpus Christi College, Oxford, United Kingdom.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. [DOI] [PubMed] [Google Scholar]
- 2.Boros, J., F.-L. Lim, Z. Darieva, A. Pic-Taylor, R. Harman, B. A. Morgan, and A. D. Sharrocks. 2003. Molecular determinants of the cell-cycle regulated Mcm1p-Fkh2p transcription factor complex. Nucleic Acids Res. 31:2279-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulmer, R., A. Pic-Taylor, S. K. Whitehall, K. A. Martin, J. B. A. Millar, J. Quinn, and B. A. Morgan. 2004. The forkhead transcription factor Fkh2 regulates the cell division cycle of Schizosaccharomyces pombe. Eukaryot. Cell 3:944-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson, P., and M. Mahlapuu. 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250:1-23. [DOI] [PubMed] [Google Scholar]
- 5.Cherel, I., and P. Thuriaux. 1995. The IFH1 gene product interacts with a fork head protein in Saccharomyces cerevisiae. Yeast 11:261-270. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo, L. A., C. E. McPherson, P. Bossard, K. Stevens, S. Cherian, E. Y. Shim, K. L. Clark, S. K. Burley, and K. S. Zaret. 1998. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 17:244-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapier, C. R., G. Längst, D. F. Corona, P. B. Becker, and K. P. Nightingale. 2001. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol. Cell. Biol. 21:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapier, C. R., K. P. Nightingale, and P. B. Becker. 2002. A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res. 30:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, K., E. Halay, E. Lai, and S. Burley. 1993. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364:412-420. [DOI] [PubMed] [Google Scholar]
- 10.Corona, D. F., C. R. Clapier, P. B. Becker, and J. W. Tamkun. 2002. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 3:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darieva, Z., A. Pic-Taylor, J. Boros, A. Spanos, M. Geymonat, R. J. Reece, S. G. Sedgwick, A. D. Sharrocks, and B. A. Morgan. 2003. Cell cycle-regulated transcription through the FHA domain of Fkh2p and the coactivator Ndd1p. Curr. Biol. 13:1740-1745. [DOI] [PubMed] [Google Scholar]
- 12.Fazzio, T. G., M. E. Gelbart, and T. Tsukiyama. 2005. Two distinct mechanisms of chromatin interaction by the Isw2 chromatin remodeling complex in vivo. Mol. Cell. Biol. 25:9165-9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazzio, T. G., C. Kooperberg, J. P. Goldmark, C. Neal, R. Basom, J. Delrow, and T. Tsukiyama. 2001. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol. Cell. Biol. 21:6450-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazzio, T. G., and T. Tsukiyama. 2003. Chromatin remodeling in vivo: evidence for a nucleosome sliding mechanism. Mol. Cell 12:1333-1340. [DOI] [PubMed] [Google Scholar]
- 15.Futcher, B. 2002. Transcriptional regulatory networks and the yeast cell cycle. Curr. Opin. Cell Biol. 14:676-683. [DOI] [PubMed] [Google Scholar]
- 16.Gajiwala, K. S., H. Chen, F. Cornille, B. P. Roques, W. Reith, B. Mach, and S. K. Burley. 2000. Structure of the winged-helix protein hRFX1 reveals a new mode of DNA binding. Nature 403:916-921. [DOI] [PubMed] [Google Scholar]
- 17.Gelbart, M. E., N. Bachman, J. Delrow, J. D. Boeke, and T. Tsukiyama. 2005. Genome-wide identification of Isw2 chromatin-remodeling targets by localization of a catalytically inactive mutant. Genes Dev. 19:942-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann-Le Denmat, S., M. Werner, A. Sentenac, and P. Thuriaux. 1994. Suppression of yeast RNA polymerase III mutations by FHL1, a gene coding for a fork head protein involved in rRNA processing. Mol. Cell. Biol. 14:2905-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollenhorst, P. C., M. E. Bose, M. R. Mielke, U. Muller, and C. A. Fox. 2000. Forkhead genes in transcriptional silencing, cell morphology and the cell cycle. Overlapping and distinct functions for FKH1 and FKH2 in Saccharomyces cerevisiae. Genetics 154:1533-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenhorst, P. C., G. Pietz, and C. A. Fox. 2001. Mechanisms controlling differential promoter-occupancy by the yeast forkhead proteins Fkh1p and Fkh2p: implications for regulating the cell cycle and differentiation. Genes Dev. 15:2445-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen, P., I. Rupes, J. R. Sharom, L. Schneper, J. R. Broach, and M. Tyers. 2004. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 18:2491-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent, N. A., N. Karabetsou, P. K. Politis, and J. Mellor. 2001. In vivo chromatin remodeling by yeast ISWI homologs Isw1p and Isw2p. Genes Dev. 15:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koranda, M., A. Schleiffer, L. Endler, and G. Ammerer. 2000. Forkhead-like transcription factors recruit Ndd1 to the chromatin of G2/M-specific promoters. Nature 406:94-98. [DOI] [PubMed] [Google Scholar]
- 24.Krogan, N. J., G. Cagney, H. Yu, G. Zhong, X. Guo, A. Ignatchenko, J. Li, S. Pu, N. Datta, A. P. Tikuisis, T. Punna, J. M. Peregrin-Alvarez, M. Shales, X. Zhang, M. Davey, M. D. Robinson, A. Paccanaro, J. E. Bray, A. Sheung, B. Beattie, D. P. Richards, V. Canadien, A. Lalev, F. Mena, P. Wong, A. Starostine, M. M. Canete, J. Vlasblom, S. Wu, C. Orsi, S. R. Collins, S. Chandran, R. Haw, J. J. Rilstone, K. Gandi, N. J. Thompson, G. Musso, P. St. Onge, S. Ghanny, M. H. Lam, G. Butland, A. M. Altaf-Ul, S. Kanaya, A. Shilatifard, E. O'Shea, J. S. Weissman, C. J. Ingles, T. R. Hughes, J. Parkinson, M. Gerstein, S. J. Wodak, A. Emili, and J. F. Greenblatt. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440:637-643. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, R., D. M. Reynolds, A. Shevchenko, S. D. Goldstone, and S. Dalton. 2000. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr. Biol. 10:896-906. [DOI] [PubMed] [Google Scholar]
- 26.Längst, G., E. Bonte, D. Corona, and P. Becker. 1999. Nucleosome movement by CHRAC and ISWI without disruption or trans-displacement of the histone octamer. Cell 97:843-852. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann, O. J., J. C. Sowden, P. Carlsson, T. Jordan, and S. S. Bhattacharya. 2003. Fox's in development and disease. Trends Genet. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 28.Li, B., S. G. Pattenden, D. Lee, J. Gutierrez, J. Chen, C. Seidel, J. Gerton, and J. L. Workman. 2005. Preferential occupancy of histone variant H2AZ at inactive promoters influences local histone modifications and chromatin remodeling. Proc. Natl. Acad. Sci. USA 102:18385-18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, H., S. Ilin, W. Wang, E. M. Duncan, J. Wysocka, C. D. Allis, and D. J. Patel. 2006. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 442:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindstrom, K. C., J. C. Vary, Jr., M. R. Parthun, J. Delrow, and T. Tsukiyama. 2006. Isw1 functions in parallel with the NuA4 and Swr1 complexes in stress-induced gene repression. Mol. Cell. Biol. 26:6117-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, C. L., T. Kaplan, M. Kim, S. Buratowski, S. L. Schreiber, N. Friedman, and O. J. Rando. 2005. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 3:e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomvardas, S., and D. Thanos. 2001. Nucleosome sliding via TBP DNA binding in vivo. Cell 106:685-696. [DOI] [PubMed] [Google Scholar]
- 33.Longtine, M. S., A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 34.Loy, C. J., D. Lydall, and U. Surana. 1999. NDD1, a high-dosage suppressor of cdc28-1N, is essential for expression of a subset of late-S-phase-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3312-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, D. E., A. Soulard, and M. N. Hall. 2004. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell 119:969-979. [DOI] [PubMed] [Google Scholar]
- 36.Mason, P. B., and K. Struhl. 2005. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol. Cell 17:831-840. [DOI] [PubMed] [Google Scholar]
- 37.McConnell, A. D., M. E. Gelbart, and T. Tsukiyama. 2004. Histone fold protein Dls1p is required for Isw2-dependent chromatin remodeling in vivo. Mol. Cell. Biol. 24:2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellor, J., and A. Morillon. 2004. ISWI complexes in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1677:100-112. [DOI] [PubMed] [Google Scholar]
- 39.Moreau, J. L., M. Lee, N. Mahachi, J. Vary, J. Mellor, T. Tsukiyama, and C. R. Goding. 2003. Regulated displacement of TBP from the PHO8 promoter in vivo requires Cbf1 and the Isw1 chromatin remodeling complex. Mol. Cell 11:1609-1620. [DOI] [PubMed] [Google Scholar]
- 40.Morillon, A., N. Karabetsou, A. Nair, and J. Mellor. 2005. Dynamic lysine methylation on histone H3 defines the regulatory phase of gene transcription. Mol. Cell 18:723-734. [DOI] [PubMed] [Google Scholar]
- 41.Morillon, A., N. Karabetsou, J. O'Sullivan, N. Kent, N. Proudfoot, and J. Mellor. 2003. Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell 115:425-435. [DOI] [PubMed] [Google Scholar]
- 42.Morillon, A., J. O'Sullivan, A. Azad, N. Proudfoot, and J. Mellor. 2003. Regulation of elongating RNA polymerase II by forkhead transcription factors in yeast. Science 300:492-495. [DOI] [PubMed] [Google Scholar]
- 43.Pic, A., F.-L. Lim, S. J. Ross, E. A. Veal, A. L. Johnson, M. R. A. Sulton, A. G. West, L. H. Johnson, A. D. Sharrocks, and B. A. Morgan. 2000. The forkhead protein Fkh2 is a component of the yeast cell cycle transcription factor SFF. EMBO J. 19:3750-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pic-Taylor, A., Z. Darieva, B. A. Morgan, and A. D. Sharrocks. 2004. Regulation of cell cycle-specific gene expression through cyclin-dependent kinase-mediated phosphorylation of the forkhead transcription factor Fkh2p. Mol. Cell. Biol. 24:10036-10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pokholok, D. K., C. T. Harbison, S. Levine, M. Cole, N. M. Hannett, T. I. Lee, G. W. Bell, K. Walker, P. A. Rolfe, and E. Herbolsheimer. 2005. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122:517-527. [DOI] [PubMed] [Google Scholar]
- 46.Pramila, T., W. Wu, S. Miles, W. S. Noble, and L. L. Breeden. 2006. The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the cell cycle. Genes Dev. 20:2266-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raisner, R. M., P. D. Hartley, M. D. Meneghini, M. Z. Bao, C. L. Liu, S. L. Schreiber, O. J. Rando, and H. D. Madhani. 2005. Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123:233-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramakrishnan, V., J. Finch, V. Graziano, P. Lee, and R. Sweet. 1993. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature 362:219-223. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds, D., B. J. Shi, C. McLean, F. Katsis, B. Kemp, and S. Dalton. 2003. Recruitment of Thr 319-phosphorylated Ndd1p to the FHA domain of Fkh2p requires Clb kinase activity: a mechanism for CLB cluster gene activation. Genes Dev. 17:1789-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudra, D., Y. Zhao, and J. R. Warner. 2005. Central role of Ifh1p-Fhl1p interaction in the synthesis of yeast ribosomal proteins. EMBO J. 24:533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos-Rosa, H., R. Schneider, B. E. Bernstein, N. Karabetsou, A. Morillon, C. Weise, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2003. Methylation of histone H3K4 mediates association of the Isw1p ATPase with chromatin. Mol. Cell 12:1325-1332. [DOI] [PubMed] [Google Scholar]
- 52.Schawalder, S. B., M. Kabani, I. Howald, U. Choudhury, M. Werner, and D. Shore. 2004. Growth-regulated recruitment of the essential yeast ribosomal protein gene activator Ifh1. Nature 432:1058-1061. [DOI] [PubMed] [Google Scholar]
- 53.Schwanbeck, R., H. Xiao, and C. Wu. 2004. Spatial contacts and nucleosome step movements induced by the NURF chromatin remodeling complex. J. Biol. Chem. 279:39933-39941. [DOI] [PubMed] [Google Scholar]
- 54.Sherriff, J. 2004. ISWI-dependent chromatin remodeling at cell cycle-regulated promoters. Ph.D. dissertation. University of Oxford, Oxford, United Kingdom.
- 55.Shi, X., T. Hong, K. L. Walter, M. Ewalt, E. Michishita, T. Hung, D. Carney, P. Pena, F. Lan, M. R. Kaadige, N. Lacoste, C. Cayrou, F. Davrazou, A. Saha, B. R. Cairns, D. E. Ayer, T. G. Kutateladze, Y. Shi, J. Cote, K. F. Chua, and O. Gozani. 2006. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442:96-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon, I., J. Barnett, N. Hannett, C. T. Harbison, N. J. Rinaldi, T. L. Volkert, J. J. Wyrick, J. Zeitlinger, D. K. Gifford, T. S. Jaakkola, and R. A. Young. 2001. Serial regulation of transcriptional regulators in the yeast cell cycle. Cell 106:697-708. [DOI] [PubMed] [Google Scholar]
- 57.Tsukiyama, T., J. Palmer, C. C. Landel, J. Shiloach, and C. Wu. 1999. Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev. 13:686-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vary, J. C., Jr., V. K. Gangaraju, J. Qin, C. C. Landel, C. Kooperberg, B. Bartholomew, and T. Tsukiyama. 2003. Yeast Isw1p forms two separable complexes in vivo. Mol. Cell. Biol. 23:80-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wade, J. T., D. B. Hall, and K. Struhl. 2004. The transcription factor Ifh1 is a key regulator of yeast ribosomal protein genes. Nature 432:1054-1058. [DOI] [PubMed] [Google Scholar]
- 60.Yang, Y. L., J. Suen, M. P. Brynildsen, S. J. Galbraith, and J. C. Liao. 2005. Inferring yeast cell cycle regulators and interactions using transcription factor activities. BMC Genomics 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, H., D. N. Roberts, and B. R. Cairns. 2005. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123:219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, G., P. T. Spellman, T. Volpe, P. O. Brown, D. Botstein, T. N. Davis, and B. Futcher. 2000. Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature 406:90-94. [DOI] [PubMed] [Google Scholar]