Abstract
Fission yeast has two TOR (target of rapamycin) kinases, namely Tor1 and Tor2. Tor1 is required for survival under stressed conditions, proper G1 arrest, and sexual development. In contrast, Tor2 is essential for growth. To analyze the functions of Tor2, we constructed two temperature-sensitive tor2 mutants. Interestingly, at the restrictive temperature, these mutants mimicked nitrogen starvation by arresting the cell cycle in G1 phase and initiating sexual development. Microarray analysis indicated that expression of nitrogen starvation-responsive genes was induced extensively when Tor2 function was suppressed, suggesting that Tor2 normally mediates a signal from the nitrogen source. As with mammalian and budding yeast TOR, we find that fission yeast TOR also forms multiprotein complexes analogous to TORC1 and TORC2. The raptor homologue, Mip1, likely forms a complex predominantly with Tor2, producing TORC1. The rictor/Avo3 homologue, Ste20, and the Avo1 homologue, Sin1, appear to form TORC2 mainly with Tor1 but may also bind Tor2. The Lst8 homologue, Wat1, binds to both Tor1 and Tor2. Our analysis shows, with respect to promotion of G1 arrest and sexual development, that the loss of Tor1 (TORC2) and the loss of Tor2 (TORC1) exhibit opposite effects. This highlights an intriguing functional relationship among TOR kinase complexes in the fission yeast Schizosaccharomyces pombe.
TOR (target of rapamycin) is a serine/threonine kinase of the phosphatidylinositol kinase-related kinase family, which is structurally and functionally conserved from yeast to mammals. Members of this family include ATM, ATR, and DNA-dependent protein kinase. They share conserved structural motifs such as an extended region of HEAT repeats as well as FAT and FATC domains (1). In mammalian cells, mTOR is a critical player in the tuberous sclerosis complex (TSC)/Rheb/mTOR signaling pathway that regulates protein synthesis in response to growth factors and nutrient and energy conditions. The regulation of protein synthesis is mediated by the phosphorylation of S6K, a kinase responsible for the phosphorylation of the ribosomal protein S6. In addition, mTOR phosphorylates 4EBP1, an inhibitor of protein synthesis (reviewed in references 39 and 54). TOR is activated by Rheb, a unique family of the Ras superfamily GTPase (2, 20, 41). Rheb is negatively regulated by the TSC1/TSC2 complex that acts as a GTPase activating protein for Rheb. Mutations in the TSC1 or TSC2 gene cause tuberous sclerosis, a genetic disorder associated with the appearance of benign tumors in various organs (reviewed in reference 22).
Fission yeast Schizosaccharomyces pombe has recently emerged as an ideal system to investigate the function of TOR. Homologues of TSC, Rheb, and TOR have been identified in fission yeast. Like the mammalian system, fission yeast TSC proteins (Tsc1 and Tsc2) form a complex that acts to downregulate Rheb (Rhb1) (24, 45). This contrasts with budding yeast that does not have TSC genes. Fission yeast has two TOR genes, namely tor1 and tor2 (15, 48). Fission yeast tor1 is not essential; however, tor1Δ cells are unable to properly arrest in G1 in response to nutrient starvation, to initiate sexual differentiation, and to survive under stressed conditions (15, 50). These functions are mediated, at least in part, by Gad8, an AGC family protein kinase that functions downstream of Tor1 (25). Unlike tor1, tor2 is essential for growth. However, it remains unknown how tor2 supports cell growth. We have recently shown that Rhb1 interacts with Tor2 in a GTP-dependent manner and activates it (44). rhb1 encoding Rhb1 is also an essential gene, and its inhibition leads to small, round G0/G1 phase cells (21, 55). To further dissect the function of fission yeast Tor2, we constructed temperature-sensitive tor2 mutants. These mutants arrested in G1 phase and unexpectedly initiated sexual differentiation when shifted to the restrictive temperature. Our results on Tor2 suggest that this TOR protein has functions that are distinct from those of Tor1.
Recent studies in mammalian cells and in budding yeast revealed that TOR proteins exist as multiprotein complexes. In mammalian cells, TOR has been shown to form two types of multiprotein complex called TORC1 and TORC2 (19, 34). TORC1 contains raptor and is sensitive to rapamycin, an inhibitor of TOR kinase. This complex mediates effects on protein synthesis and cell growth. On the other hand, TORC2, which contains rictor, mediates regulation to Akt and also affects actin cytoskeleton (14, 35). Budding yeast has two TOR genes encoding Tor1 and Tor2. Either Tor1 or Tor2 can form TORC1 together with Lst8, Tco89, and the raptor orthologue Kog1, indicating that the two Saccharomyces cerevisiae TOR proteins can perform a redundant function. In addition, Tor2, but not Tor1, constitutes TORC2 with Lst8, Avo1, Avo2, Bit61, and the rictor orthologue Avo3, which regulates a different range of downstream targets from TORC1. Inhibition of budding yeast TORC1, either by mutation or by rapamycin, causes cell cycle arrest at G1 phase, whereas TORC2 appears to carry out an essential function for cellular polarization and cytoskeletal reorganization (54).
In this study we also investigated the composition of fission yeast TOR complexes. We have previously identified the raptor homologue Mip1 (40). In addition, the rictor/Avo3 homologue Ste20 (11), the Lst8 homologue Wat1/Pop3 (16), and the Avo1 homologue Sin1 (52) have been identified in fission yeast. We examined association of these proteins with TOR proteins and found that Tor1 and Tor2 have distinct binding partners.
MATERIALS AND METHODS
General methods and strains.
Table 1 summarizes S. pombe strains used in this study. General genetic procedures for S. pombe were described previously (9, 26). Transformation of S. pombe was done by a lithium acetate method (32). To generate a temperature-sensitive allele of tor2, we introduced random mutations into the tor2 open reading frame carried on a plasmid by a PCR method (56). Cells of JV530 (h90 tor2::kanr harboring pREP42-tor2) were transformed with linear DNA fragments carrying mutagenized tor2 alleles. Integrants of a functional tor2 allele at the correct locus were screened at 26.5°C by monitoring their resistance to fluoroorotic acid (indicating loss of ura4+-based pREP42-tor2) and sensitivity to G418 (indicating loss of kanr). Temperature-sensitive mutants were selected from these integrants by replica plating. To carry out tor2 shutoff experiments, we replaced the authentic tor2 promoter with the nmt81 promoter. When necessary, rapamycin was added to liquid and agar medium to a final concentration of 0.1 μg/ml (51). An equal volume of a drug vehicle solution (1:1 dimethyl sulfoxide-methanol) was added to the controls.
TABLE 1.
S. pombe strains used in this study
| Strain | Descriptiona |
|---|---|
| JT164 | h−ade6-M216 leu1 ste20-3HA ≪ kanr |
| JT176 | h−ade6-M216 leu1 mip1-3HA ≪ kanr |
| JT177 | h90 ade6-M216 leu1 ura4-D18 tor2-ts6 |
| JT178 | h90 ade6-M210 leu1 ura4-D18 tor2-ts10 |
| JT293 | h90 ade6-M216 leu1 ura4-D18 ste20::ura4+ |
| JT294 | h90 ade6-M216 leu1 wat1-13myc ≪ hphr |
| JT295 | h90 ade6-M216 leu1 sin1-13myc ≪ hphr |
| JT296 | h90 ade6 leu1 ura4-D18 tor2-ts6 tor1::ura4+ |
| JT297 | h90 ade6 leu1 ura4-D18 tor2-ts10 tor1::ura4+ |
| JT300 | h90 ade6-M210 leu1 nmt81-3HA-tor2 ≪ kanr |
| JT301 | h90 ade6-M216 leu1 tor2-ts6 mip1-3HA ≪ kanr |
| JT303 | h90 ade6-M210 leu1 tor2-ts10 mip1-3HA ≪ kanr |
| JUp1525 | h−ade6-M216 tsc2::kanMX |
| JUp1527 | h−ade6-M216 tsc2::kanMX tor2-ts6 |
| JUp1529 | h−ade6-M216 |
| JUp1530 | h−ade6-M216 tor2-ts6 |
| JUp1533 | h−ade6-M216 tsc2::kanMX tor2-ts10 |
| JUp1537 | h−ade6-M216 tor2-ts10 |
| JV302 | h90 ade6-M210 leu1 tor2-ts6 |
| JV303 | h90 ade6-M216 leu1 tor2-ts6 |
| JV304 | h−ade6-M216 leu1 tor2-ts6 |
| JV305 | h90 ade6-M210 leu1 tor2-ts10 |
| JV306 | h−ade6-M216 leu1 tor2-ts10 |
| JV530 | h90 ade6 leu1 ura4-D18 tor2::kanrwith pREP42-tor2 |
| JV981 | h−ade6-M216 leu1 nmt81-3HA-tor2 ≪ kanr |
| JW944 | h90 ade6-M216 leu1 ura4-D18 gad8::ura4+ |
| JW950 | h90 ade6-M216 leu1 ura4-D18 tor1::ura4+ |
| JW951 | h90 ade6-M210 leu1 ura4-D18 tor1::ura4+ |
| JX384 | h−ade6-M216 leu1 ura4-D18 pka1::ura4+ |
| JY333 | h−ade6-M216 leu1 |
| JY450 | h90 ade6-M216 leu1 |
| JY476 | h90 ade6-M210 leu1 |
| JY878 | h90 ade6-M216 leu1 ura4-D18 |
≪, insertion of resistance cassette.
Microarray analysis.
JY450 (wild type [wt]) and JV303 (tor2-ts6) were grown to mid-log phase in YES (yeast extract with supplements) medium at 25°C. Cells were collected and resuspended in fresh YES medium prewarmed to 34°C and grown at 34°C. At the indicated times, approximately 2 × 108 cells were collected, and total RNA was extracted using a high-salt and acid phenol method. Briefly, cells were resuspended in 400 μl of high-salt RNA buffer (0.3 M NaCl, 20 mM Tris-HCl [pH 7.5], 10 mM EDTA, 1% sodium dodecyl sulfate). A total of 400 μl of phenol:chloroform (1:1 mix of phenol saturated with 0.1 M citrate buffer, pH 4.3, and chloroform) was added, vortexed for 5 min, and then incubated at 65°C for 5 min. The aqueous layer was separated using heavy phase-lock gel (Eppendorf) and extracted once with chloroform. Total RNA was then ethanol precipitated. Thirty micrograms of total RNA was further purified using RNeasy (QIAGEN) columns and submitted for microarray analysis by the University of California-Los Angeles DNA Microarray Core. Probes were generated and hybridized to the GeneChip Yeast Genome 2.0 Array (Affymetrix). The data were then analyzed using dChip (18).
Immunoprecipitation.
JT176 (mip1-HA), JT164 (ste20-HA), JT294 (wat1-myc), and JT295 (sin1-myc) were transformed with either pREP41-His6Flag2-tor2 or pREP41-His6Flag2-tor1. Cells grown in minimal medium (MM) at 30°C to the concentration of 4 × 106 cells/ml were harvested and disrupted with glass beads in buffer B (50 mM Tris-HCl [pH 7.6], 150 mM KCl, 5 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 0.2% NP-40, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 15 mM para-nitrophenyl phosphate, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Complete Mini EDTA-free; Roche]). Lysates were centrifuged, and 1/10 of each supernatant was saved as “total,” and the rest was subjected to immunoprecipitation. A mixture of 5 μl of anti-FLAG M2 monoclonal antibody (Sigma) and 25 μl of Dynabeads protein G (Dynal) was added to each sample, which was then incubated at 4°C for 30 min. After being washed three times with buffer B without protease inhibitors, precipitates were run in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Mouse anti-hemagglutinin (anti-HA) antibody 12CA5 (Sigma) or mouse anti-myc antibody 9E10 (Santa Cruz) was used to detect Mip1-HA, Ste20-HA, Wat1-myc, and Sin1-myc.
RESULTS
Isolation of tor2-ts mutants.
To investigate the function of Tor2, we randomly introduced mutations into the open reading frame of the chromosomal tor2 gene using PCR-based mutagenesis as described in Materials and Methods. We then screened for strains that could grow at 25°C but not at 37°C. Altogether, 15 clones that satisfied this criterion were isolated. Two of them, named tor2-ts6 and tor2-ts10, showed elevated temperature sensitivity, growing poorly even at 30°C (Fig. 1A). To confirm that the temperature-sensitive growth of these strains was due to mutations in the tor2 gene, we transformed them with a plasmid carrying tor2. The transformants recovered growth at the restrictive temperature (see Fig. 5C), suggesting that they were indeed defective in tor2. Subsequent cloning analysis confirmed that mutations were indeed introduced into the tor2 gene in these temperature-sensitive strains (see below).
FIG. 1.
Loss of tor2 function causes G1 arrest. (A) Temperature-sensitive growth of the tor2-ts strains. Cells of JY878 (wt), JT177 (tor2-ts6), and JT178 (tor2-ts10) were streaked on nutrient medium YE and incubated either at 25°C or 30°C for 3 days. (B) Cells of JY476 (wt), JV302 (tor2-ts6), and JV305 (tor2-ts10) were cultured in liquid YE medium at 25°C to exponential phase and shifted to 30°C at a concentration of about 1 × 106 cells/ml. Aliquots were taken at indicated time points, and the cellular DNA content of each sample was measured by FACS analysis. (C) Cells of JV981, in which tor2 is driven by the nmt81 promoter, were grown in liquid MM medium to exponential phase (2 × 106 cells/ml), and thiamine was added to a half of the culture to a concentration of 2 μM to shut off the transcription from the nmt81 promoter. Aliquots were taken at indicated time points, and the cellular DNA content of each sample with (+) or without (−) thiamine was measured by FACS analysis.
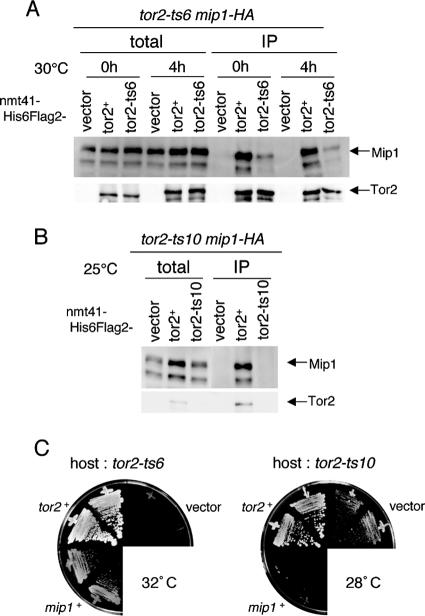
FIG. 5.
Interaction of the temperature-sensitive Tor2 proteins with Mip1. (A) Physical interaction of Tor2-ts6 with Mip1, examined by IP as described in the legend of Fig. 4A. (B) Physical interaction of Tor2-ts10 with Mip1, examined by IP as described in the legend of Fig. 4A. Only samples prepared from cells grown at the permissive temperature 25°C are shown. (C) Genetic interaction: suppression of tor2-ts6 by overexpression of mip1. JV304 (tor2-ts6) and JV306 (tor2-ts10) were transformed with vector pREP41, pREP41-tor2, and pREP41-mip1. Transformants of JV304 were incubated on MM at 32°C for 9 days, whereas transformants of JV306 were incubated on MM at 28°C for 8 days.
Loss of tor2 function results in G1 arrest and ectopic sexual development.
Cells of JV302 (h90 tor2-ts6) and JV305 (h90 tor2-ts10), grown to exponential phase in YE (yeast extract) medium at 25°C, were shifted to 30°C. They were sampled at intervals and subjected to fluorescence-activated cell sorting (FACS) analysis as shown in Fig. 1B. Whereas control wt cells (JY476) continued to grow at 30°C, tor2-ts6 and tor2-ts10 cells gradually arrested in G1 phase after the temperature shift, suggesting that the function of tor2 is necessary to traverse G1 during the cell cycle. With tor2-ts10, the G1 peak was detected at earlier time points than with tor2-ts6. In addition, with tor2-ts10, a peak of cells with 4C DNA content was observed at 24 h after the shift. This may represent cells undergoing meiosis, as described below.
To confirm that the arrest in G1 observed in tor2-ts mutants was due to loss of the Tor2 activity rather than acquisition of an abnormal activity, we constructed a system in which production of Tor2 could be shut off artificially by the use of the thiamine-repressible promoter nmt81. When expression of tor2 from the nmt81 promoter was blocked in heterothallic JV981 cells by the addition of thiamine to the medium, the cells gradually arrested in G1, like tor2-ts cells, indicating that loss of tor2 function causes G1 arrest in the cell cycle (Fig. 1C).
To our surprise, microscopic observation of homothallic tor2-ts cells incubated at the restrictive temperature for 24 h revealed that they contained zygotes and asci (Fig. 2A). This was a unique cell cycle mutant phenotype, which to our knowledge has never been described in fission yeast. Both temperature-sensitive mutants exhibited increased mating efficiency at the restrictive temperature of 30°C (Fig. 2B). The tor2-ts10 mutant showed a higher mating frequency than the tor2-ts6 mutant. However, the former grew slightly more slowly than the latter at the permissive temperature 25°C (Fig. 1A), implying that Tor2-ts10 is more labile than Tor2-ts6 and may already be partially inactive at the permissive temperature (see below). Sporulation was observed in homothallic (Fig. 2A) but not in heterothallic (Fig. 2C) tor2-ts cells subjected to the temperature shift, suggesting that the tor2 deficiency does not provoke haploid meiosis such as that induced by the pat1-ts mutation (13, 30). However, heterothallic tor2-ts cells became smaller at the restrictive temperature, suggesting that they might have entered a state of quiescence (Fig. 2C). We integrated the tor2 shutoff system into a homothallic strain (JT300). This strain grew very poorly even in the absence of thiamine, and microscopic observation indicated that about half of the cells had initiated mating and sporulation in growth medium (Fig. 2D). This is probably because the nmt81 promoter used to drive tor2 in this strain was not strong enough under the derepressed conditions. When thiamine was added, the cells stopped growing completely and generated many asci, confirming that loss of Tor2 function provokes sexual development.
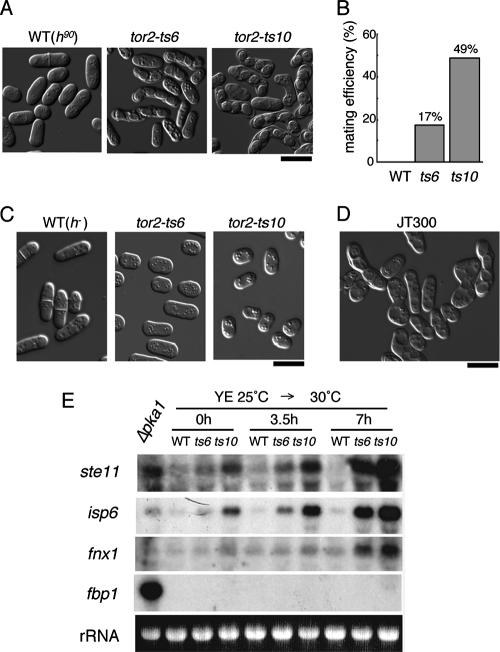
FIG. 2.
Sexual development of tor2-ts cells grown at the restrictive temperature. (A) The three homothallic haploid strains analyzed in the experiment shown in Fig. 1B were examined microscopically after 24 h of incubation at the restrictive temperature. Bar, 10 μm. (B) Calculated mating efficiency of the three strains shown in panel A. (C) Cells of heterothallic haploid strains incubated at the restrictive temperature for 24 h. wt, JY333; tor2-ts6, JV304; and tor2-ts10, JV306. Bar, 10 μm. (D) Cells of a homothallic strain JT300, in which tor2 is driven by the nmt81 promoter, were grown vegetatively on MM plates (− thiamine). They have undergone mating and sporulation even before shutoff of the promoter. Bar, 10 μm. (E) Expression of starvation-responsive genes in tor2-ts cells. Expression of three nitrogen starvation-responsive genes (ste11, isp6, and fnx1), and one glucose starvation-responsive gene (fbp1) was measured in wt (JY333), tor2-ts6 (JV304), and tor2-ts10 (JV306) cells at time zero and 3.5 and 7 h after the shift to the restrictive temperature. Their expression in pka1-defective cells (JX384) was also examined. rRNA stained with ethidium bromide is shown as a loading control.
Loss of tor2 function mimics nitrogen starvation.
The above observations suggested that loss of tor2 function might result in a cellular physiology similar to one induced by nutrient starvation. This condition appears to be sufficient to provoke conjugation and subsequent sporulation, as zygotes and asci are observed at nonpermissive temperature. To further examine this idea, we investigated expression of three typical nitrogen starvation-responsive genes, namely ste11, isp6, and fnx1, in the absence of tor2 function. The ste11 gene encodes an HMG family transcription factor, which regulates a number of genes necessary for sexual development (42). The isp6 gene encodes a vacuolar protease homologous to S. cerevisiae PRB1 involved in autophagy (37). Expression of isp6 is highly enhanced by nitrogen starvation but is independent of ste11 (28, 29). The fnx1 gene encodes a protein with sequence similarity to the proton-driven plasma membrane transporters (8). Expression of ste11 and fnx1 has been reported to be induced by the disruption of rhb1 (21, 43). As shown in Fig. 2E, transcription of these three genes in the tor2-ts mutants was elevated significantly at the restrictive temperature, suggesting that disruption of Tor2 function can mimic nitrogen starvation. Induction of fnx1 expression, however, was slower than that of the other two genes, which may suggest that fnx1 is regulated by tor2 but indirectly. Unlike these nitrogen starvation-responsive genes, fbp1, which encodes fructose-1,6-bisphosphatase, is known to respond sharply to the lack of glucose or a decrease of the cyclic AMP-dependent protein kinase A activity (46, 12) (Fig. 2E). We find that fbp1 does not show any significant elevation in transcription in response to the loss of tor2 (Fig. 2E). Therefore, loss of tor2 function appears to mimic starvation for a nitrogen source but not for a carbon source.
Nitrogen starvation-responsive genes are largely controlled by tor2.
To determine alterations in global gene expression profile due to the inhibition of Tor2, we performed microarray analysis of the tor2-ts6 mutant after shifting the cultures to 34°C for 0, 1, 3, and 8 h. A similar time course was performed with a wt strain to control for gene expression changes that may occur due to the temperature shift. RNA preparation was converted to cDNA and was used to hybridize against an oligonucleotide array of S. pombe genome. The data were analyzed using dChip. Genes showing a variation (standard deviation/mean) of greater than 0.5 were filtered and clustered.
Figure 3 shows hierarchical clustering analysis of the filtered genes to identify gene clusters. We looked for a set of genes whose expression is specifically altered in response to loss of tor2 function. A total of 194 genes are identified to be induced specifically (Fig. 3, cluster induced by loss of tor2). It is interesting that almost all the genes with altered expression show increased expression by the loss of tor2 function. This may suggest that fission yeast Tor2 generally functions to repress expression of genes.
FIG. 3.

Microarray analysis of loss of tor2 activity. Global expression profiles from tor2+ (JY450) or tor2-ts6 (JV303) incubated for the indicated times at 34°C were assessed using the GeneChip Yeast Genome 2.0 Array. The raw data were normalized and modeled using dChip. Genes showing a variation (standard deviation/mean) of >0.5 were clustered. The cluster of genes that are induced by loss of tor2 is indicated. Red indicates induction, and green indicates suppression relative to expression of the gene in tor2+ cells at 0 h.
Table S1 in the supplemental material lists all 194 genes that are found in the cluster induced due to the loss of tor2 function. Among these, we find a number of genes that are induced during mating and sporulation, according to the Sanger Centre Database (Table 2). They include the major regulators of meiosis, ste11 and mei2. Other sterile genes, ste4, ste6, and ste7, as well as another mei gene, mei4, are also found in the cluster. We find genes involved in pheromone response such as gpa1, rgs1, ste6, mfm1, mfm2, mfm3, map1, map2, map3, mam1, mam2, and mat1-Mc. It is interesting to find genes involved in G protein function, ste6 (GDP/GTP exchange factor for the small G protein, Ras1) and gpa1 (α-subunit of a heterotrimeric G protein), to be transcriptionally induced in response to loss of tor2 function. In addition, we find fus1 and ste4, which are involved in conjugation. meu13 is required for meiotic recombination. This analysis also identified crs1, a gene whose transcript is meiotically spliced and encodes a putative cyclin (3). The isp genes including isp6 are induced during sporulation (37). The wtf genes are a family of Tf element-containing sequences which are transcribed during meiosis (5, 53).
TABLE 2.
Mating and meiosis genes induced by loss of tor2
| Gene identifier | Gene name | Description | Relative expression ina:
|
|||
|---|---|---|---|---|---|---|
| JV303
|
JY450
|
|||||
| 0 h | 8 h | 0 h | 8 h | |||
| SPCC1795.06 | map2 | P-factor | 1.26 | 2.34 | 1.00 | 0.46 |
| SPAC13G7.13c | msa1 | Involved in sporulation (predicted) | 1.11 | 2.81 | 1.00 | 0.87 |
| SPBC29B5.02c | isp4 | Involved in sexual differentiation | 1.04 | 9.90 | 1.00 | 0.91 |
| SPAC1039.09 | isp5 | Involved in sexual differentiation | 10.04 | 131.73 | 1.00 | 4.59 |
| SPAC513.03 | mfm2 | M-factor precursor | 0.80 | 3.60 | 1.00 | 0.41 |
| SPAC222.15 | meu13 | Meiosis specific transcript | 0.68 | 24.27 | 1.00 | 10.51 |
| SPAC25B8.13c | isp7 | 2 OG-Fe(II) oxygenase superfamily | 1.36 | 9.11 | 1.00 | 1.20 |
| SPBC1711.02 | matmc | Mating-type m-specific polypeptide mc | 1.14 | 5.35 | 1.00 | 1.50 |
| SPAC11E3.06 | map1 | Transcription factor | 0.54 | 8.66 | 1.00 | 1.87 |
| SPAC4A8.04 | isp6 | Serine protease | 1.03 | 4.76 | 1.00 | 0.89 |
| SPAC1565.04c | ste4 | Involved in conjugation | 0.75 | 3.82 | 1.00 | 0.98 |
| SPBC32C12.02 | ste11 | Transcription factor | 0.80 | 4.10 | 1.00 | 0.97 |
| SPBPJ4664.03 | mfm3 | M-factor precursor | 1.71 | 11.25 | 1.00 | 1.44 |
| SPAC11H11.04 | mam2 | Pheromone p-factor receptor | 1.18 | 6.23 | 1.00 | 0.97 |
| SPAPB8E5.05 | mfm1 | M-factor precursor | 1.29 | 105.95 | 1.00 | 2.25 |
| SPBPJ4664.03 | mfm3 | M-factor precursor | 1.11 | 9.41 | 1.00 | 1.81 |
| SPBC25B2.02c | mam1 | ABC transporter family | 0.96 | 4.49 | 1.00 | 1.30 |
| SPBC24C6.06 | gpa1 | GTP binding (alpha-1 subunit) | 0.94 | 5.40 | 1.00 | 1.24 |
| SPAC23E2.03c | ste7 | Involved in conjugation | 1.06 | 5.37 | 1.00 | 1.28 |
| SPAC3F10.10c | map3 | Pheromone M-factor receptor | 0.86 | 2.57 | 1.00 | 0.85 |
| SPAC27D7.03c | mei2 | RNA-binding protein | 0.60 | 8.71 | 1.00 | 0.52 |
| SPCC1183.10 | wtf10 | wtf element | 0.84 | 3.39 | 1.00 | 1.46 |
| SPBC32H8.11 | mei4 | Transcription factor (meiotic) | 0.82 | 2.24 | 1.00 | 1.02 |
| SPCC970.11c | wtf9 | wtf element | 0.83 | 2.38 | 1.00 | 1.23 |
| SPCC285.07c | wtf18 | wtf element | 0.99 | 2.51 | 1.00 | 1.30 |
| SPAC22F3.12c | rgs1 | Regulator of G-protein signaling | 0.39 | 4.19 | 1.00 | 1.22 |
| SPCC548.03c | wtf4 | wtf element | 1.00 | 2.87 | 1.00 | 1.25 |
| SPCC794.02 | wtf5 | wtf element | 0.90 | 4.80 | 1.00 | 1.46 |
| SPCC1442.01 | ste6 | Guanyl-nucleotide exchange factor activity | 0.82 | 4.42 | 1.00 | 1.49 |
| SPCC1450.08c | wtf16 | wtf element | 1.14 | 7.21 | 1.00 | 4.26 |
| SPCC1450.08c | wtf16 | wtf element | 0.87 | 5.70 | 1.00 | 3.09 |
| SPAC20G4.02c | fus1 | Formin | 0.74 | 7.86 | 1.00 | 3.40 |
| SPCC830.02 | wtf24 | wtf element | 0.83 | 5.93 | 1.00 | 3.00 |
| SPBC2G2.09c | crs1 | Cyclin (predicted) | 0.59 | 2.78 | 1.00 | 1.61 |
| SPCC794.02 | wtf5 | wtf element | 0.58 | 7.50 | 1.00 | 1.89 |
| SPAC2E12.05 | wtf1 | wtf element | 0.89 | 3.53 | 1.00 | 1.66 |
| SPCC576.16c | wtf22 | wtf element | 1.06 | 11.28 | 1.00 | 3.41 |
| SPCC285.06c | wtf17 | wtf element | 0.97 | 9.16 | 1.00 | 3.26 |
| SPCC548.02c | wtf3 | wtf element | 1.45 | 15.94 | 1.00 | 4.68 |
| SPCC1739.15 | wtf21 | wtf element | 0.74 | 8.65 | 1.00 | 3.60 |
| SPCC1906.04 | wtf20 | wtf element | 1.00 | 7.27 | 1.00 | 2.50 |
| SPCC1906.04 | wtf20 | wtf element | 0.71 | 8.53 | 1.00 | 2.68 |
| SPCC1906.04 | wtf20 | wtf element | 0.80 | 7.48 | 1.00 | 2.40 |
Relative expression of the indicated genes was measured by microarray assay in strains JV303 (tor2-ts6) and JY450 (wt) cultured at 34°C for 0 or 8 h. Values for each gene were normalized to its expression in JY450 at 0 h. For original raw data, see Table S1 in the supplemental material.
Many of the above genes are known to be induced by nitrogen starvation. To examine this point further, we compared our results with those from microarray analysis of diploid cells after nitrogen starvation (23). A total of 151 of our 194 genes induced by the loss of tor2 are found in the list of roughly 1,000 genes found to be induced by nitrogen starvation at least fourfold over vegetative cells (23). These genes are separated into four classes: class I genes are involved in response to nutritional changes (215 genes), class II (early) genes are involved in premeiotic S phase and recombination (111 genes), class III (middle) genes are involved in meiotic divisions (561 genes), and class IV (late) genes are involved in spore formation (133 genes). Roughly two-thirds (97) of the 151 genes are in the first class of genes (class I) that are involved in response to nutritional changes. We find 16 early, 22 middle, and 17 late genes. These results point to an important role that Tor2 plays in nitrogen starvation and mating response. Furthermore, these findings are in agreement with the observation that shifting the tor2-ts6 strain to nonpermissive temperatures induces these cells to undergo sexual differentiation.
Induction of permease and transporter genes by the loss of tor2.
Another group of genes we found to be induced by the loss of tor2 function can be classified as permeases and transporters (Table 3). We find known as well as predicted permeases and transporters for amino acids and other nutrients such as purine and thiamine. We have previously shown that Rhb1 regulates uptake of amino acids and that Rhb1 directly interacts with Tor2 (44). Thus, part of the Rhb1 effects may involve alteration of expression of amino acid permeases. Alteration of expression of genes encoding amino acid permeases has also been observed in tsc mutants (45, 27). In fact, we find a number of transporters and permeases (isp5, SPAC869.10c, SPAC11D3.18c, SPAC1039.01, mam1, and SPBPB2B2.01) in our cluster whose expression levels are also altered by the loss of tsc function.
TABLE 3.
Transporters and permeases induced by loss of tor2
| Gene identifier | Gene name | Description | Relative expression ina:
|
|||
|---|---|---|---|---|---|---|
| JV303
|
JY450
|
|||||
| 0 h | 8 h | 0 h | 8 h | |||
| SPBC1683.12 | Membrane transporter | 1.42 | 6.09 | 1.00 | 1.58 | |
| SPAC869.10c | Amino acid transporter (predicted) | 1.38 | 6.38 | 1.00 | 1.43 | |
| SPBC29B5.02c | isp4 | Involved in sexual differentiation | 1.04 | 9.90 | 1.00 | 0.91 |
| SPAC1039.09 | isp5 | Involved in sexual differentiation | 10.04 | 131.73 | 1.00 | 4.59 |
| SPAC1039.01 | Amino acid permease family | 1.19 | 6.82 | 1.00 | 0.54 | |
| SPCC1840.12 | Oligopeptide transporter family | 0.98 | 4.40 | 1.00 | 2.08 | |
| SPAC3H1.06c | Transporter (predicted) | 1.61 | 11.30 | 1.00 | 1.07 | |
| SPCPB1C11.02 | Amino acid permease family (predicted) | 2.19 | 10.97 | 1.00 | 1.89 | |
| SPAC11D3.18c | Membrane transporter | 2.07 | 31.31 | 1.00 | 4.23 | |
| SPAC1399.03 | fur4 | Uracil permease | 1.04 | 6.56 | 1.00 | 1.37 |
| SPBC1271.09 | Membrane transporter | 1.09 | 4.26 | 1.00 | 0.67 | |
| SPBC1683.05 | Thiamine transporter (predicted) | 1.02 | 3.75 | 1.00 | 0.94 | |
| SPCC417.10 | Membrane transporter | 1.05 | 12.07 | 1.00 | 1.46 | |
| SPCC777.04 | Amino acid transporter (predicted) | 1.33 | 93.52 | 1.00 | 2.10 | |
| SPBC25B2.02c | mam1 | ABC transporter family | 0.96 | 4.49 | 1.00 | 1.30 |
| SPAC1399.01c | Purine permease (predicted) | 1.07 | 9.73 | 1.00 | 1.53 | |
| SPAC29B12.14c | Purine transporter (predicted) | 0.87 | 5.20 | 1.00 | 1.29 | |
| SPBPB2B2.01 | Amino acid permease family | 0.95 | 6.96 | 1.00 | 3.07 | |
| SPBC359.01 | Amino acid permease family | 0.84 | 3.75 | 1.00 | 1.67 | |
| SPAC1F8.01 | ght3 | Hexose transporter | 1.37 | 6.24 | 1.00 | 3.62 |
| SPCC965.06 | Potassium channel subunit ??? (predicted) | 1.26 | 7.36 | 1.00 | 1.68 | |
Relative expression of the indicated genes was measured by microarray assay in strains JV303 (tor2-ts6) and JY450 (wt) cultured at 34°C for 0 or 8 h. Values for each gene were normalized to its expression in JY450 at 0 h. For original raw data, see Table S1 in the supplemental material.
TOR complexes in fission yeast.
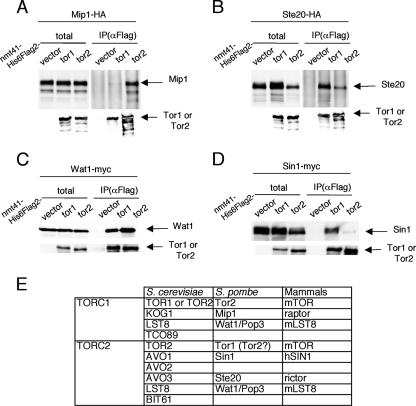
Mammalian raptor, like its budding yeast orthologue Kog1, is a component of TORC1 and is thought to provide a scaffold for the interaction of TOR kinase and its substrate. The structural homologue of raptor/Kog1 in fission yeast, Mip1, is a protein of 1,313 amino acids, and its truncated form missing the N-terminal 173 amino acids (Mip1ΔN) was originally identified in our study as a suppressor of ectopic meiosis induced by an activated form of Mei2 (40). mip1, like tor2, is an essential gene, loss of which results in small round cells arrested in G1. We thus tested physical interaction of Tor2 with Mip1 by immunoprecipitation (IP). Tor1 and other homologues to TORC1 and TORC2 members were also examined in this analysis. Flag-tagged Tor1 or Tor2 was expressed from the nmt41 promoter in cells expressing HA-tagged Mip1 from its authentic promoter. Either Tor1 or Tor2 was precipitated with anti-Flag antibodies, and coprecipitation of Mip1 was examined (Fig. 4A). Tor2 coprecipitated Mip1 far more efficiently than Tor1, suggesting that Tor2 is the major TOR kinase to form a complex with the raptor/Kog1 homologue Mip1 in vivo. In analogous IP analysis, the rictor/Avo3 homologue Ste20 showed substantial affinity for Tor1 and moderate affinity for Tor2 (Fig. 4B), suggesting that it may form a complex with both Tor1 and Tor2 in vivo. Sin1, a homologue of S. cerevisiae Avo1, exhibited a similar binding spectrum to Ste20, whereas Wat1, a homologue of S. cerevisiae Lst8, appeared to bind significantly with both Tor1 and Tor2 (Fig. 4C and D). From these results, we assign the composition of putative fission yeast TORC1 and TORC2 as in Fig. 4E. In addition, we note that the amount of Ste20 and Sin1 appears to be reduced in tor2-overexpressing cells (Fig. 4B and D). It may be that the abundance of Ste20 and Sin1 is regulated negatively by Tor2.
FIG. 4.
Physical interaction of TOR kinases with raptor Mip1, rictor Ste20, and two other TORC members. (A) IP assay to detect interaction of Mip1 with Tor1 or Tor2. Detailed procedures are given in Materials and Methods. (B) IP assay to detect interaction of Ste20 with Tor1 or Tor2. (C) IP assay to detect interaction of Wat1 with Tor1 or Tor2. (D) IP assay to detect interaction of Sin1 with Tor1 or Tor2. (E) A summary of known components of TORC1 and TORC2 in three kinds of organisms. α, anti.
Analysis of ste11 expression supported the above assignment of TORC1 and TORC2. Transcription of ste11 was not induced by nitrogen starvation in tor1Δ and ste20Δ, which were all viable at 30°C. In contrast, tor2Δ, rhb1Δ, and mip1Δ were lethal, and artificial shutoff of each gene resulted in ectopic expression of ste11 (data not shown). Curiously, while Wat1 appeared to constitute both TORC1 and TORC2, deletion of wat1 has been reported to be viable (16, 31) (see Discussion), and wat1Δ failed to express ste11 under nitrogen starvation (data not shown).
Further characterization of the tor2-ts mutants.
We set out to identify the mutated amino acid residue responsible for the temperature sensitivity in the tor2-ts6 and tor2-ts10 alleles. DNA sequencing revealed that each allele carries as many as four substituted residues. Subsequent analysis indicated that two mutations in the HEAT domain, S550P and K711M, either of which confers only weak temperature sensitivity, are together responsible for tor2-ts6. In the case of tor2-ts10, the temperature sensitivity stems from the combination of A1399E in the FAT domain and F2198L in the kinase domain, neither of which alone confers temperature sensitivity. The latter observation appears intriguing, as we have seen that tor2-activating mutations cluster in either the FAT domain or the kinase domain (J. Urano et al., unpublished results).
To examine the nature of the defects in Tor2-ts proteins, we tested their ability to bind to Mip1 by coimmunoprecipitation as before. Compared to wt Tor2, Tor2-ts6 bound Mip1 poorly, even at the permissive temperature, suggesting that the affinity for Mip1 is affected in this mutant protein (Fig. 5A). The affinity was further lowered after a 4-h incubation at the restrictive temperature. Tor2-ts10 also appeared to have little affinity for Mip1, but more striking was its scarcity in the cell, even at the permissive temperature (Fig. 5B). We suspect that Tor2-ts10 may be nonfunctional because it is labile and degrades readily.
Genetic interaction between TOR kinase and its associated proteins.
In addition to physical interaction, we found genetic interaction between mip1 and tor2. The tor2-ts6 mutation was suppressed partially by overexpression of wt mip1 at 32°C (Fig. 5C). The tor2-ts10 mutation was not suppressed by overexpression of mip1. This is consistent with the idea that Tor2-ts6 is stable but lowered in its affinity for Mip1, whereas Tor2-ts10 is unstable and readily destroyed. Interestingly, it seems that mip1 overexpression rather enhances the temperature sensitivity of tor2-ts10 (Fig. 5C) (see Discussion).
The above observations showing that Tor2 and Mip1 cooperate intimately in function reinforce the finding that they constitute the fission yeast TORC1. Also, as Tor1 and Ste20 are both members of the fission yeast TORC2, it is likely that they function together. However, this point has not been shown. Thus, we compared phenotypes of the two mutant strains. Results are summarized in Fig. 6. Both tor1Δ and ste20Δ cells exhibited an elongated morphology (Fig. 6A), and their growth was sensitive to high osmotic pressure (Fig. 6B) and high temperature (data not shown) just like gad8Δ cells. Importantly, ste20Δ could be partially suppressed by an activated form of gad8 (gad8-S527D/S546D) (Fig. 6C), as was the case with tor1Δ (25). Thus, it is highly likely that Tor1 and Ste20 cooperate in the same biological process.
FIG. 6.
Comparison of phenotypes between tor1Δ and ste20Δ. (A) Comparison of cell morphology. Cells of each homothallic haploid strain were grown on YE medium at 30°C for 4 days. (B) Osmo-sensitive growth was examined on YE medium containing 1 M KCl. Incubation was done at 30°C for 4 days. wt, JY450; tor1Δ, JW950; ste20Δ, JT293; and gad8Δ, JW944. (C) Suppression of tor1Δ (JW950) and ste20Δ (JT293) by gad8-S527D/S546D. The wt and mutant gad8 alleles were expressed from plasmid pR3C (25). Growth on MM containing 0.6 M KCl at 30°C was followed for 4 days. (D) Examination of mutual suppression between tor1Δ and tor2-ts. The double mutants and control strains were tested for growth at 32°C. (E) Suppression of the growth defect of ade6-M216 tsc2Δ under low adenine supply by tor2-ts mutations. Indicated strains were grown on Edinburgh minimal medium plates supplemented with either 1 mg/ml or 0.25 mg/ml of adenine.
Because Tor1 and Tor2 apparently function in opposite directions with regard to the control of sexual development, we wondered whether tor1Δ and tor2-ts might mutually suppress each other. If this is the case, we argued that introducing the tor1Δ mutation into the tor2-ts cells would rescue their growth defect at 32°C. However, the results were negative: the double mutants could not grow at 32°C (Fig. 6D).
We also tested whether Rhb1, an activator of Tor2 (44), could be a suppressor of tor2-ts6 and tor2-ts10. However, an activated rhb1 allele, rhb1-N153T (44), did not suppress temperature sensitivity of the two mutants (data not shown). Deletion of tsc2, which codes for a negative regulator of Rhb1, also did not significantly affect the temperature sensitivity of tor2-ts6 and tor2-ts10 (data not shown). In contrast, we found that the poor growth of the tsc2Δ ade6 mutant on medium containing a low concentration of adenine (24) could be suppressed by either tor2-ts6 or tor2-ts10 (Fig. 6E). Whereas the tsc2::kanMX ade6-M216 strains grew poorly on minimal Edinburgh minimal medium plates containing 0.25 mg/ml adenine, the tsc2::kanMX ade6-M216 tor2-ts triple mutants showed healthy growth almost wt levels at 25°C. These results reinforce the idea that Tsc2 and Rhb1 function upstream of Tor2.
In S. cerevisiae, TORC2, which contains Tor2, is involved in actin organization (38). To see whether putative fission yeast TORC1 or TORC2 was to play a similar role, we stained F-actin with BODIPY FL phallacidin in tor1Δ cells at 30°C and in tor2-ts6 and tor2-ts10 cells at the permissive (25°C) and restrictive (30°C) temperatures. Consequently no significant disorganization of actin was observed, except that actin cables in tor1Δ cells might be slightly thicker than ones in the wt (data not shown). We therefore suppose that fission yeast TOR may not be directly relevant to actin organization.
DISCUSSION
Tor2 function in nitrogen signaling.
In this study, we have presented evidence that Tor2 plays critical roles in the nitrogen signaling pathway. Inhibition of Tor2 function leads to the accumulation of G0/G1 phase cells. These accumulated cells are small and round. In addition, expression of the ste11, isp6, and fnx1 genes is induced upon the loss of Tor2 function. Microarray analysis further provided strong evidence for the idea that Tor2 functions in the nitrogen signaling pathway, as genes induced by the loss of Tor2 function overlap significantly with the genes induced by nitrogen starvation. It is interesting to point out that these phenotypes of tor2 loss of function strongly resemble those that are observed by the loss of rhb1 function. Both genes are essential, and their inhibition leads to small, round G0/G1 cells (21, 55). Inhibition of rhb1 also causes induction of ste11, mei2, and fnx1 genes (21, 43). These results support the idea that Rhb1 and Tor2 function in the same manner to stimulate cell growth and cell cycle progression. This is in line with our recent observation that Rhb1 interacts directly with the Tor2 complex (44). We have shown that adenine uptake in the tsc2Δ strain can be rescued by either the tor2-ts6 or the tor2-ts10 mutation; this result further supports the idea that fission yeast possesses the TSC-Rheb-TOR pathway similar to mammalian cells.
Our microarray analysis revealed global negative regulation of the expression of a number of permease and transporter genes by Tor2. These genes include amino acid permeases, purine permeases, and thiamine transporter. Transcriptional regulation of permease genes has also been reported from the analyses of tsc mutants (27, 45). In these cases, expression of these genes is suppressed. Regulation of permeases may be a response to starved conditions. Indeed, a large number of permeases and transporters are shown to be upregulated upon nitrogen starvation (23).
We have compared the cluster of genes upregulated in the tor2-ts6 mutant with genes whose expression is altered in the tsc1 and tsc2 mutants (27, 45). Four genes in our cluster (SPCC1223.09, isp5, isp4, and SPAC869.10c) are found among 14 genes reported to be downregulated in both tsc1Δ and tsc2Δ (45). Six genes (SPAC1039.01, SPAC11D3.03c, mei2, gpa1, SPBC1683.02, and SPBPB2B2.01) are found among 31 genes whose expression is not induced in response to nitrogen starvation in tsc1Δ and tsc2Δ (27). Although there are clear overlaps, they may not be as extensive as one might expect. The reason for this limited overlapping is uncertain. It may reflect differences in experimental parameters such as resolution or setting of the thresholds or indicate that growing tsc1Δ and tsc2Δ cells have undergone certain physiological adaptation. Alternatively, it may indicate that Tsc2 is not the only regulator for the Tor2 pathway, as might be presumed from the observation that tsc2Δ cells are still able to arrest in G1 and mate (24), even though they show a delay in nitrogen starvation responses. It is also possible that there are Tor-independent functions for Tsc2. Further analyses are necessary to fully understand the regulation of TOR by Tsc2 in fission yeast.
The initiation of sexual development in fission yeast depends largely on nutrient conditions, as in many other microorganisms. Our results suggest that Tor2 negatively regulates sexual development, as the inhibition of Tor2 leads to increased meiosis. Previous studies have shown that both nitrogen and glucose are important for sexual development; although depletion of nitrogen is crucial for sexual development, depletion of glucose is not. However, reduction of glucose facilitates sexual development, and a high concentration of glucose suppresses it. Our study has shown clearly that the function of Tor2 is related to the recognition of a nitrogen source but not a carbon source and is likely to be independent of the cyclic AMP-protein kinase A pathway. In S. cerevisiae, it has been shown that Tor1 and Tor2 are involved in nitrogen catabolite repression, a regulatory event in which transcription of certain genes is downregulated by a good nitrogen source such as glutamine but upregulated by a poor nitrogen source such as proline (4, 6, 7, 10, 17). Therefore, the involvement of TOR in nitrogen signaling may be a widely conserved phenomenon among various organisms.
In contrast to tor2, tor1 is not an essential gene. However, tor1 mutants exhibit phenotypes that are distinct from those of the tor2 mutants (48, 15). These mutants exhibit deficiency in properly arresting in G1 in response to nitrogen starvation and in initiating sexual development. This is opposite from our results on Tor2 that show that the inhibition of fission yeast Tor2 promotes G1 arrest and the initiation of sexual development. This sharp contrast between tor1 and tor2 mutant phenotypes suggests that Tor1 and Tor2 have opposing functions. However, loss of either function cannot be complemented by loss of the other, indicating that the two proteins are likely to be involved in distinct biological processes.
Possible TOR kinase complexes.
Our IP analysis showed that the raptor homologue Mip1 is likely to form a complex predominantly with Tor2. This complex, which may correspond to budding yeast TORC1, appears to be necessary to repress nitrogen starvation-responsive genes and to stimulate cell cycle progression at G1. On the other hand, the rictor/Avo3 homologue Ste20 forms a complex with Tor1, and the ste20Δ strain is phenotypically quite similar to tor1Δ. This suggests that Tor1 is likely to be the major partner of Ste20, although our analysis has suggested that Tor2 may also form a complex with Ste20. It is currently unclear whether this complex is physiologically significant or simply an artifact due to overproduction of Tor2 in our analysis. So far, our trials to detect interaction of Tor1 or Tor2 with their associated proteins under physiological conditions (i.e., with no overexpression) have not given IP bands clear enough to deliver unambiguous conclusions. We tentatively suppose that Tor1 and Ste20, together with the Avo1 homologue Sin1, constitute a complex that corresponds to budding yeast TORC2. This putative fission yeast TORC2 is required for G1 arrest and sexual development. Thus, with regard to nitrogen response and sexual development, TORC2 appears to have effects opposite from those of TORC1. In addition, TORC2 may control stress response, as the tor1Δ strain exhibits reduced stress resistance. Our analysis suggests that neither TORC1 nor TORC2 plays a significant role in actin organization in fission yeast. However, as disorganization of actin patches has been reported in the wat1Δ mutant (16), we cannot exclude the possibility that TORC1 and TORC2 control actin in a redundant fashion. Alternatively, Wat1 may have a TOR-unrelated function in actin organization. It is also currently unexplained why wat1Δ is not lethal, although Wat1 is apparently a component of both TORC1 and TORC2. Deletion of S. cerevisiae LST8, the homologue of wat1, is lethal (33). These observations altogether may suggest that, although fission yeast, like the budding yeast, has two TOR kinases and two TOR complexes, the functions of each do not necessarily match those of the budding yeast counterpart.
In mammalian cells and budding yeast, TORC1 is sensitive to rapamycin, while TORC2 is relatively insensitive to this drug (36, 54). In fission yeast, however, rapamycin affects some Tor1-dependent functions but generally does not inhibit TOR-related growth functions (47, 49, 51). Our results agree with the idea that Tor2 is insensitive to rapamycin. First, we find that rapamycin does not significantly block growth of wt cells, as initially reported by Weisman (49). In addition, our study revealed that the inhibition of Tor2 leads to induction of sexual development. This is in contrast to the observation that rapamycin blocks sexual development, due to the inhibition of Fkh1 (50). Finally, we have seen that addition of rapamycin does not activate nitrogen starvation-responsive genes (unpublished results).
A conceivable explanation for the lack of effect by rapamycin on wt cells may be that both TORC1 and TORC2 are inhibited, and because they have opposite effects on nitrogen response and sexual development, inhibition of both may cancel out each other. However, this is unlikely to be the case, as tor1Δ is not able to suppress the temperature sensitivity of the tor2-ts mutants. Taken together, it appears that TORC1 in fission yeast is insensitive to rapamycin.
Two alleles of tor2 temperature-sensitive mutants.
Our characterization of the tor2 temperature-sensitive mutants has indicated that tor2-ts6 and tor2-ts10 represent two different mutant alleles, the former of which may be impaired in the interaction with Mip1, whereas the latter may generate a labile gene product. Interestingly, they behaved quite differently when suppression of the temperature-sensitive growth by mip1ΔN (Mip1 lacking the N-terminal region) was examined. Mip1ΔN is defective in function but can act in a dominant fashion to inhibit meiosis (40). Overexpression of mip1ΔN (mip1-15) suppressed temperature sensitivity of tor2-ts10 but not tor2-ts6 (unpublished results). As described above, overexpression of wt mip1 suppressed temperature sensitivity of tor2-ts6 but enhanced temperature sensitivity of tor2-ts10. Further characterization of these two tor2 temperature-sensitive alleles and the mip1ΔN mutation may provide insight into how TORC1 regulates growth and meiosis in fission yeast.
ADDENDUM
While we were revising this article, two papers containing data that partially overlap with ours were published (1a, 44a).
Supplementary Material
Acknowledgments
We thank Kayoko Tanaka and Akira Yamashita for helpful discussion. Microarray analysis was carried out in the UCLA/JCCC DNA microarray core.
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to Y.M. and by NIH grant CA41996 and NSF grant CCF-0326605 to F.T.
Footnotes
Published ahead of print on 29 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abraham, R. T. 2004. PI 3-kinase related kinases: “big” players in stress-induced signaling pathways. DNA Repair 3:883-887. [DOI] [PubMed] [Google Scholar]
- 1a.Álvarez, B., and S. J. Moreno. 2006. Fission yeast Tor2 promotes cell growth and represses cell differentiation. Cell Sci. 119:4475-4485. [DOI] [PubMed] [Google Scholar]
- 2.Aspuria, P. J., and F. Tamanoi. 2004. The Rheb family of GTP-binding proteins. Cell Signal 16:1105-1112. [DOI] [PubMed] [Google Scholar]
- 3.Averbeck, N., S. Sunder, N. Sample, J. A. Wise, and J. Leatherwood. 2005. Negative control contributes to an extensive program of meiotic splicing in fission yeast. Mol. Cell 18:491-498. [DOI] [PubMed] [Google Scholar]
- 4.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689-692. [DOI] [PubMed] [Google Scholar]
- 5.Bowen, N. J., I. K. Jordan, J. A. Epstein, V. Wood, and H. L. Levin. 2003. Retrotransposons and their recognition of Pol II promoters: a comprehensive survey of the transposable elements from the complete genome sequence of Schizosaccharomyces pombe. Genome Res. 13:1984-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas, M. E., N. S. Cutler, M. C. Lorenz, C. J. Di Como, and J. Heitman. 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13:3271-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crespo, J. L., T. Powers, B. Fowler, and M. N. Hall. 2002. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc. Natl. Acad. Sci. USA 99:6784-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrov, K., and S. Sazer. 1998. The role of fnx1, a fission yeast multidrug resistance protein, in the transition of cells to a quiescent G0 state. Mol. Cell. Biol. 18:5239-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutz, H., H. Heslot, U. Leupold, and N. Loprieno. 1974. Schizosaccharomyces pombe, p. 395-446. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Press, New York, NY. [Google Scholar]
- 10.Hardwick, J. S., F. G. Kuruvilla, J. K. Tong, A. F. Shamji, and S. L. Schreiber. 1999. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl. Acad. Sci. USA 96:14866-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilti, N., D. Baumann, A. M. Schweingruber, P. Bigler, and M. E. Schweingruber. 1999. Gene ste20 controls amiloride sensitivity and fertility in Schizosaccharomyces pombe. Curr. Genet. 35:585-592. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman, C. S., and F. Winston. 1991. Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev. 5:561-571. [DOI] [PubMed] [Google Scholar]
- 13.Iino, Y., and M. Yamamoto. 1985. Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol. Gen. Genet. 198:416-421. [DOI] [PubMed] [Google Scholar]
- 14.Jacinto, E., R. Loewith, A. Schmidt, S. Lin, M. A. Ruegg, A. Hall, and M. N. Hall. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6:1122-1128. [DOI] [PubMed] [Google Scholar]
- 15.Kawai, M., A. Nakashima, M. Ueno, T. Ushimaru, K. Aiba, H. Doi, and M. Uritani. 2001. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr. Genet. 39:166-174. [DOI] [PubMed] [Google Scholar]
- 16.Kemp, J. T., M. K. Balasubramanian, and K. L. Gould. 1997. A wat1 mutant of fission yeast is defective in cell morphology. Mol. Gen. Genet. 254:127-138. [DOI] [PubMed] [Google Scholar]
- 17.Komeili, A., K. P. Wedaman, E. K. O'Shea, and T. Powers. 2000. Mechanism of metabolic control. Target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J. Cell Biol. 151:863-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, C., and W. H. Wong. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loewith, R., E. Jacinto, S. Wullschleger, A. Lorberg, J. L. Crespo, D. Bonenfant, W. Oppliger, P. Jenoe, and M. N. Hall. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10:457-468. [DOI] [PubMed] [Google Scholar]
- 20.Long, X., Y. Lin, S. Ortiz-Vega, K. Yonezawa, and J. Avruch. 2005. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15:702-713. [DOI] [PubMed] [Google Scholar]
- 21.Mach, K. E., K. A. Furge, and C. F. Albright. 2000. Loss of Rhb1, a Rheb-related GTPase in fission yeast, causes growth arrest with a terminal phenotype similar to that caused by nitrogen starvation. Genetics 155:611-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcotte, L., and P. B. Crino. 2006. The neurobiology of the tuberous sclerosis complex. Neuromolecular Med. 8:531-546. [DOI] [PubMed] [Google Scholar]
- 23.Mata, J., R. Lyne, G. Burns, and J. Bahler. 2002. The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32:143-147. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, S., A. Bandyopadhyay, D. J. Kwiatkowski, U. Maitra, and T. Matsumoto. 2002. Role of the Tsc1-Tsc2 complex in signaling and transport across the cell membrane in the fission yeast Schizosaccharomyces pombe. Genetics 161:1053-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo, T., Y. Kubo, Y. Watanabe, and M. Yamamoto. 2003. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 22:3073-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno, S., A. Klar, and P. Nurse. 1990. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-826. [DOI] [PubMed] [Google Scholar]
- 27.Nakase, Y., K. Fukuda, Y. Chikashige, C. Tsutsumi, D. Morita, S. Kawamoto, M. Ohnuki, Y. Hiraoka, and T. Matsumoto. 2006. A defect in protein farnesylation suppresses a loss of Schizosaccharomyces pombe tsc2+, a homolog of the human gene predisposing tuberous sclerosis complex (TSC). Genetics 173:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima, A., T. Hasegawa, S. Mori, M. Ueno, S. Tanaka, T. Ushimaru, S. Sato, and M. Uritani. 2006. A starvation-specific serine protease gene, isp6+, is involved in both autophagy and sexual development in Schizosaccharomyces pombe. Curr. Genet. 49:403-413. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima, A., M. Ueno, T. Ushimaru, and M. Uritani. 2002. Involvement of a CCAAT-binding complex in the expression of a nitrogen-starvation-specific gene, isp6+, in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 66:2224-2227. [DOI] [PubMed] [Google Scholar]
- 30.Nurse, P. 1985. Mutants of the fission yeast Schizosaccharomyces pombe which alter the shift between cell proliferation and sporulation. Mol. Gen. Genet. 198:497-502. [Google Scholar]
- 31.Ochotorena, I. L., D. Hirata, K. Kominami, J. Potashkin, F. Sahin, K. Wentz-Hunter, K. L. Gould, K. Sato, Y. Yoshida, L. Vardy, and T. Toda. 2001. Conserved Wat1/Pop3 WD-repeat protein of fission yeast secures genome stability through microtubule integrity and may be involved in mRNA maturation. J. Cell Sci. 114:2911-2920. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki, K., N. Okazaki, K. Kume, S. Jinno, K. Tanaka, and H. Okayama. 1990. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18:6485-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberg, K. J., S. Bickel, N. Rowley, and C. A. Kaiser. 1997. Control of amino acid permease sorting in the late secretory pathway of Saccharomyces cerevisiae by SEC13, LST4, LST7 and LST8. Genetics 147:1569-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarbassov, D. D., S. M. Ali, D. H. Kim, D. A. Guertin, R. R. Latek, H. Erdjument-Bromage, P. Tempst, and D. M. Sabatini. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296-1302. [DOI] [PubMed] [Google Scholar]
- 35.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov, D. D., S. M. Ali, and D. M. Sabatini. 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17:596-603. [DOI] [PubMed] [Google Scholar]
- 37.Sato, S., H. Suzuki, U. Widyastuti, Y. Hotta, and S. Tabata. 1994. Identification and characterization of genes induced during sexual differentiation in Schizosaccharomyces pombe. Curr. Genet. 26:31-37. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt, A., M. Bickle, T. Beck, and M. N. Hall. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531-542. [DOI] [PubMed] [Google Scholar]
- 39.Shaw, R. J., and L. C. Cantley. 2006. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424-430. [DOI] [PubMed] [Google Scholar]
- 40.Shinozaki-Yabana, S., Y. Watanabe, and M. Yamamoto. 2000. Novel WD-repeat protein Mip1p facilitates function of the meiotic regulator Mei2p in fission yeast. Mol. Cell. Biol. 20:1234-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, E. M., S. G. Finn, A. R. Tee, G. J. Browne, and C. G. Proud. 2005. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J. Biol. Chem. 280:18717-18727. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto, A., Y. Iino, T. Maeda, Y. Watanabe, and M. Yamamoto. 1991. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 5:1990-1999. [DOI] [PubMed] [Google Scholar]
- 43.Tabancay, A. P., Jr., C. L. Gau, I. M. Machado, E. J. Uhlmann, D. H. Gutmann, L. Guo, and F. Tamanoi. 2003. Identification of dominant negative mutants of Rheb GTPase and their use to implicate the involvement of human Rheb in the activation of p70S6K. J. Biol. Chem. 278:39921-39930. [DOI] [PubMed] [Google Scholar]
- 44.Urano, J., M. J. Comiso, L. Guo, P. J. Aspuria, R. Deniskin, A. P. Tabancay, Jr., J. Kato-Stankiewicz, and F. Tamanoi. 2005. Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol. Microbiol. 58:1074-1086. [DOI] [PubMed] [Google Scholar]
- 44a.Uritani, M., H. Hidaka, Y. Hotta, M. Ueno, T. Ushimaru, and T. Toda. 2006. Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells 11:1367-1379. [DOI] [PubMed] [Google Scholar]
- 45.van Slegtenhorst, M., E. Carr, R. Stoyanova, W. D. Kruger, and E. P. Henske. 2004. tsc1+ and tsc2+ regulate arginine uptake and metabolism in Schizosaccharomyces pombe. J. Biol. Chem. 279:12706-12713. [DOI] [PubMed] [Google Scholar]
- 46.Vassarotti, A., and J. D. Friesen. 1985. Isolation of the fructose-1,6-bisphosphatase gene of the yeast Schizosaccharomyces pombe. Evidence for transcriptional regulation. J. Biol. Chem. 260:6348-6353. [PubMed] [Google Scholar]
- 47.Weisman, R. 2004. The fission yeast TOR proteins and the rapamycin response: an unexpected tale. Curr. Top. Microbiol. Immunol. 279:85-95. [DOI] [PubMed] [Google Scholar]
- 48.Weisman, R., and M. Choder. 2001. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 276:7027-7032. [DOI] [PubMed] [Google Scholar]
- 49.Weisman, R., M. Choder, and Y. Koltin. 1997. Rapamycin specifically interferes with the developmental response of fission yeast to starvation. J. Bacteriol. 179:6325-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisman, R., S. Finkelstein, and M. Choder. 2001. Rapamycin blocks sexual development in fission yeast through inhibition of the cellular function of an FKBP12 homolog. J. Biol. Chem. 276:24736-24742. [DOI] [PubMed] [Google Scholar]
- 51.Weisman, R., I. Roitburg, T. Nahari, and M. Kupiec. 2005. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson, M. G., T. S. Pino, S. Tournier, V. Buck, H. Martin, J. Christiansen, D. G. Wilkinson, and J. B. Millar. 1999. Sin1: an evolutionarily conserved component of the eukaryotic SAPK pathway. EMBO J. 18:4210-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne, A. Stewart, J. Sgouros, N. Peat, J. Hayles, S. Baker, D. Basham, S. Bowman, K. Brooks, D. Brown, S. Brown, T. Chillingworth, C. Churcher, M. Collins, R. Connor, A. Cronin, P. Davis, T. Feltwell, A. Fraser, S. Gentles, A. Goble, N. Hamlin, D. Harris, J. Hidalgo, G. Hodgson, S. Holroyd, T. Hornsby, S. Howarth, E. J. Huckle, S. Hunt, K. Jagels, K. James, L. Jones, M. Jones, S. Leather, S. McDonald, J. McLean, P. Mooney, S. Moule, K. Mungall, L. Murphy, D. Niblett, C. Odell, K. Oliver, S. O'Neil, D. Pearson, M. A. Quail, E. Rabbinowitsch, K. Rutherford, S. Rutter, D. Saunders, K. Seeger, S. Sharp, J. Skelton, M. Simmonds, R. Squares, S. Squares, K. Stevens, K. Taylor, R. G. Taylor, A. Tivey, S. Walsh, T. Warren, S. Whitehead, J. Woodward, G. Volckaert, R. Aert, J. Robben, B. Grymonprez, I. Weltjens, E. Vanstreels, M. Rieger, M. Schafer, S. Muller-Auer, C. Gabel, M. Fuchs, A. Dusterhoft, C. Fritzc, E. Holzer, D. Moestl, H. Hilbert, K. Borzym, I. Langer, A. Beck, H. Lehrach, R. Reinhardt, T. M. Pohl, P. Eger, W. Zimmermann, H. Wedler, R. Wambutt, B. Purnelle, A. Goffeau, E. Cadieu, S. Dreano, S. Gloux, et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 54.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 55.Yang, W., A. P. Tabancay, Jr., J. Urano, and F. Tamanoi. 2001. Failure to farnesylate Rheb protein contributes to the enrichment of G0/G1 phase cells in the Schizosaccharomyces pombe farnesyltransferase mutant. Mol. Microbiol. 41:1339-1347. [DOI] [PubMed] [Google Scholar]
- 56.Zhou, Y. H., X. P. Zhang, and R. H. Ebright. 1991. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 19:6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.