Abstract
Neuronal activity greatly influences the formation and stabilization of synapses. Although receptors for sphingosine-1-phosphate (S1P), a lipid mediator regulating diverse cellular processes, are abundant in the central nervous system, neuron-specific functions of S1P remain largely undefined. Here, we report two novel actions of S1P using primary hippocampal neurons as a model system: (i) as a secretagogue where S1P triggers glutamate secretion and (ii) as an enhancer where S1P potentiates depolarization-evoked glutamate secretion. Sphingosine kinase 1 (SK1), a key enzyme for S1P production, was enriched in functional puncta of hippocampal neurons. Silencing SK1 expression by small interfering RNA as well as SK1 inhibition by dimethylsphingosine resulted in a strong inhibition of depolarization-evoked glutamate secretion. Fluorescence recovery after photobleaching analysis showed translocation of SK1 from cytosol to membranes at the puncta during depolarization, which resulted in subsequent accumulation of S1P within cells. Fluorescent resonance energy transfer analysis demonstrated that the S1P1 receptor at the puncta was activated during depolarization and that depolarization-induced S1P1 receptor activation was inhibited in SK1-knock-down cells. Importantly, exogenously added S1P at a nanomolar concentration by itself elicited glutamate secretion from hippocampal cells even when the Na+-channel was blocked by tetrodotoxin, suggesting that S1P acts on presynaptic membranes. Furthermore, exogenous S1P at a picomolar level potentiated depolarization-evoked secretion in the neurons. These findings indicate that S1P, through its autocrine action, facilitates glutamate secretion in hippocampal neurons both by secretagogue and enhancer actions and may be involved in mechanisms underlying regulation of synaptic transmission.
One of the remarkable features of the central nervous system (CNS) is its ability to integrate and store enormous information. Neuronal information is rapidly transferred through the chemical synapse to specialized regions of the postsynaptic plasma membrane, where neurotransmitter receptors are concentrated. Neurotransmitter secretion in the CNS shares many features with constitutive membrane trafficking (4, 19); however, it also exhibits several unique features, including storage of enormous amounts of information and plasticity, that indicate the presence of additional regulators. There is considerable interest in identifying such regulators that modulate the speed and potency of neurotransmitter release. Among various regulators sphingolipid metabolites such as sphingosine-1-phosphate (S1P) have recently attracted attention for their role in the regulation of neuronal function (9).
S1P, a phosphorylated product of sphingosine catalyzed by sphingosine kinase (SK), has been implicated as an important lipid mediator acting both inside and outside the cells (26, 33). Extracellular S1P binds to members of GTP-binding protein (G-protein)-coupled S1P receptor family (S1P1-5), triggering diverse cellular effects including angiogenesis, cardiac development, immunity, cell motility, and neurite extension (34, 36, 44). On the other hand, S1P has been shown to function intracellularly, mediating mobilization of cellular calcium, cell growth, and suppression of apoptosis (16, 40). It has been reported that high KCl-induced depolarization caused accumulation of S1P in PC12 cells (1). More recently, it has been shown that newly synthesized S1P is released from cerebellar granule cells and astrocytes (3). Although evidence is accumulating to suggest the abundance of SK1 (14, 41) and several isotypes of S1P receptors (15) in the CNS, the physiological relevance of this lipid mediator in neuron-specific functions such as neurotransmitter release remains unknown. The present studies were undertaken to identify and characterize the role of S1P in the regulation of transmitter secretion. We present evidence that exogenously added S1P itself causes glutamate secretion in primary hippocampal neurons. We also show that depolarization-evoked glutamate secretion is strongly potentiated by autocrine/paracrine action of S1P produced during depolarization. Implication of S1P in spontaneous secretion is also discussed in this article.
MATERIALS AND METHODS
Plasmid construction.
Hemagglutinin-murine SK1 (HA-mSK1) and HA-rat SK2 (rSK2) plasmids were constructed as described previously (18) and cloned into pCMV5 (Clontech, Palo Alto, CA). Human SK1 (hSK1) (GenBank accession number AF266756) and rSK1 (GenBank accession number NM_133386) cDNAs were amplified from human brain cDNA (Invitrogen) and rat brain cDNA (Clontech), respectively, by PCR (sense primer, 5′-GCCGGTACCCCGCGGGTCGAGGTTATGG-3′, and antisense primer, 5′-GCCGGTACCTCATAAGGGCTCTTCTGGC-3′, for hSK1; sense primer, 5′-CGGGGTACCATGCAACCAGCAGACTGTCCC-3′, and antisense primer, 5′-CGGGGTACCTCATATTGGTTCTTCTGGAGGTGG-3′, for rSK1) to make N-terminally HA-tagged constructs. Site-directed mutagenesis was performed using a Stratagene (La Jolla, CA) QuickChange site-directed mutagenesis kit to prepare a catalytically inactive mutant, hSK1G82D. Constructs were verified by sequencing the entire coding sequence. The construct encoding C-terminally green fluorescent protein (GFP)-fused rat synaptophysin was reconstructed from synaptophysin-cyan fluorescent protein (CFP) in pECFP (a kind gift from S. Okabe, Tokyo Medical and Dental University). Murine S1P1 (mS1P1) (GenBank accession number NM_007901) cDNA was amplified from mouse brain cDNA, which had been reverse transcribed from fetal mouse brain mRNA (Invitrogen) by PCR (sense primer, 5′-CGGAATTCGCCACCATGGTGTCCACTAGCATCC-3′; antisense primer, 5′-CGGAATTCGGGAAGAAGAATTGACGTTTCCAG-3′) to make a C-terminally CFP-fused construct in pECFP-N1. Mouse β-arrestin 2 (GenBank accession number NM_004313) cDNA was amplified from mouse brain cDNA, which had been reverse transcribed from fetal mouse brain mRNA (Invitrogen) by PCR (sense primer, 5′-GCCGGTACCCCGCGGGTCGAGGTTATGG-3′; antisense primer 5′-GCCGGTACCTCATAAGGGCTCTTCTGGC-3′) to make an N-terminally yellow fluorescent protein (YFP)-fused construct in pEYFP-C1.
Cell cultures.
All animals used in this study were handled in compliance with the Kobe University Guidelines for the use of animals. Hippocampal neurons were prepared from embryonic day 18 rats (Wistar). Neurons were cultured on glass-bottomed culture dishes (Matsunami Glass, Osaka, Japan) coated with 300 μg/ml poly-d-lysine and 25 μg/ml laminin in neurobasal medium with added supplement B-27 (Invitrogen), 1 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 atmosphere. Cultured neurons (5 × 104 at a density of 38,000/cm2) were transfected with different cDNAs (0.53 μg) using Lipofectamine 2000 (Invitrogen) 2 to 5 days before experiments. All experiments were performed at 10 to 14 days in vitro.
siRNAs.
Small interfering RNAs (siRNAs) for rSK1 (5′-GGGCAAGGCUCUGAAGCUCdTdT-3′ and 5′-GAGCUUCAGAGCCUUGCCCdTdT-3′; dT is deoxyribosylthymine throughout), for the control siRNA (5′-UUCUCCGAACGUGUCACGUdTdT-3′ and 5′-ACGUGACACGUUCGGAGAAdTdT-3′), for S1P1 (5′-CUGACUUCAGUGGUGUUCAdTdT-3′ and 5′-UGAACACCACUGAAGUCAGdTdT-3′), and for S1P3 (5′-CCCUCUACUCCAAGAAAUACA-3′ and 5′-UAUUUCUUGGAGUAGAGGGGC-3′) were synthesized at Japan Bio Services (Saitama, Japan). Hippocampal neurons were transfected both with siRNAs and either empty vector or various expression vector constructs 2 to 5 days before the assays. Transfection efficiency of siRNAs in neurons was determined by using a commercially available kit (Block-iT Alexa Fluor Red Fluorescent Oligo; Invitrogen).
SK1 antibody.
A rabbit polyclonal anti-mouse SK1 antibody was raised against the synthetic peptide GSRDAPSGRDSRRGPPPEEP (amino acid residues 362 to 381) conjugated to glutathione S-transferase. The antibody was affinity purified by using immunogen-immobilized Sepharose 4B.
Immunoprecipitation.
Cultured hippocampal neurons were harvested using cell lysis buffer (20 mM Tris-HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 150 mM NaCl, 1% [wt/vol] Triton X-100, and protease inhibitors; Roche Applied Science). Lysates were then sonicated and centrifuged at 10,000 × g. The supernatants were incubated with anti-SK1 antibody (diluted 1:100) for 1 h at 4°C and then with protein A-Sepharose for an additional 1 h. Samples were centrifuged for 5 min at 2,000 × g at 4°C, and pellets were washed three times with the lysis buffer without detergent. Finally, the pellets were suspended in cell lysis buffer without detergent and used for either immunoblotting analysis or SK assay.
Immunofluorescence.
Rat hippocampal neurons were immunostained at 10 to 14 days in vitro as previously described (13). Antibodies used were polyclonal anti-SK1 (1:100; see above), polyclonal anti-SK2 (1:1,000), monoclonal anti-Tau (1:100; Sigma Aldrich), monoclonal anti-microtubule-associated protein 2 (anti-MAP2; 1:100) (Sigma Aldrich), and monoclonal antisynaptophysin (1:50; CHEMICON International, Temecula, CA). The fluorescence of Alexa488 and Alexa594 was observed under a confocal laser scanning microscope (LSM 510 META; Carl Zeiss, Jena, Germany), with excitation at 488 nm using a 505- to 530-nm band-pass barrier filter and with excitation at 543 nm using a 560-nm long-pass barrier filter. In some experiments neurons were transfected with a plasmid DNA encoding GFP-SK1, and active puncta were detected after FM4-64 dye loading (see below).
FM4-64 imaging.
Labeling of actively recycling presynaptic vesicles with the fluorescent styryl membrane probe FM4-64 (Molecular Probes, Eugene, OR) was carried out essentially as described previously (37). Briefly, vesicles were loaded for 1 min with 15 μM FM4-64 in 50 mM KCl followed by rinsing in dye-free solution for 10 min. High-potassium (50 mM KCl)- or glutamate-induced exocytosis was measured in buffer solution (135 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose, and 5 mM HEPES, pH 7.3). FM4-64 fluorescence was monitored using a Zeiss LSM 510 META confocal microscope (excitation at 488 nm using a 640-nm long-pass barrier filter). Fluorescence in the puncta (at least 100 different regions) was analyzed using Zeiss LSM 510 software. The fluorescence values from three independent experiments were averaged and plotted to generate a time course of FM4-64 decrease. For dominant negative SK and siRNA experiments, the FM4-64 fluorescence of GFP-positive puncta, where synaptophysin-GFP was expressed, was monitored.
Measurement of glutamate.
Glutamate released from cultured hippocampal neurons was measured using an Amplex Red glutamic acid/glutamate oxidase assay kit (Invitrogen). Primary hippocampal neurons were untreated or pretreated with 10 μM dimethylsphingosine (DMS) (Sigma) or 50 μM 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (HACPT; Calbiochem, La Jolla, CA) for 30 min and then left untreated or treated with 50 mM KCl or 10 nM S1P for 1 min. Then the medium was collected and analyzed for glutamate content according to the manufacturer's instructions. The resulting increase in fluorescence over time was measured at an excitation of 540 nm and emission of 590 nm using a fluorescence spectrophotometer (F-2500; Hitachi, Tokyo).
Mass measurement of S1P in hippocampal neurons.
Mass levels of S1P in hippocampal neurons were determined essentially as described previously (12) with some modifications. In brief, either control or KCl-treated hippocampal neurons (2.5 × 106 cells/sample) were collected with methanol. The extraction procedure was downsized to one-fifth of the original scale. Extracted S1P was dephosphorylated by alkaline phosphatase from bovine intestinal mucosa (Sigma) and rephosphorylated by a recombinant mSK1 with [γ-32P]ATP. Radioactive S1P was quantitated after thin-layer chromatography using authentic S1P as a standard.
Real-time quantitative reverse transcription-PCR.
Total RNA was extracted from rat hippocampal neurons (4 × 105 cells) using Isogen (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. cDNA synthesis and quantitative PCR were as described previously (30). The primer sequences (sense and antisense) were as follows: for rat S1P1, 5′-TTGTTGCAAATGCCCCAACG-3′ and 5′-TTTGCTGCGGCTAAATTCCATG-3′; for rat S1P2, 5′-TCGCCAAGGTCAAGCTCTACG-3′ and 5′-AGACAATTCCAGCCCAGGATGG-3′; for rat S1P3, 5′-CACCTGACCATGATCAAGATGAG-3′ and 5′-ACCCAGCGAGAAGGCAATTAGC-3′; for rat S1P4, 5′-TGTGTATGGCTGCATCGGTCTG-3′ and 5′-GAGCACATAGCCCTTGGAGTAG-3′; for rat S1P5, 5′-GCTCTACGCCAAGGCCTATGTG-3′ and 5′-GCACCTGACAGTAAATCCTTGC-3′; for rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-TGCCCCCATGTTTGTGATG-3′ and 5′-TGTGGTCATGAGCCCTTCC-3′; and for rSK1, 5′-CTGGAGGAGGCTGAGGTATC-3′ and 5′-CCAGTGACCCAGTTCTTCTGC-3′. The expression of each mRNA was normalized to GAPDH mRNA expression.
FRAP.
Fluorescence recovery after photobleaching (FRAP) analysis was performed with a Zeiss LSM 510 META confocal laser scanning microscope as described previously (8). Briefly, the primary hippocampal neurons transfected with GFP-SK1, synaptophysin-GFP, or GFP alone were untreated or were treated with 50 mM KCl or 100 μM glutamate for 1 min. Then each circular region of a punctum of interest was photobleached by scanning for 8 s with an argon laser at the highest power. Recovery of fluorescence in the selected regions was then analyzed by confocal fluorescence microscopy with low laser power at the indicated times (see the figure legends) after photobleaching. For all of the images, the noise levels were reduced by line scan averaging. Then FRAP in fractional units was calculated using the following equation: [F(t) − Fp]/[Fi − Fp] × 100, where Fi is the fluorescence at the selected region before photobleaching, Fp is the fluorescence at the selected region immediately after photobleaching, and F(t) is the fluorescence at the selected region at time t after photobleaching (35).
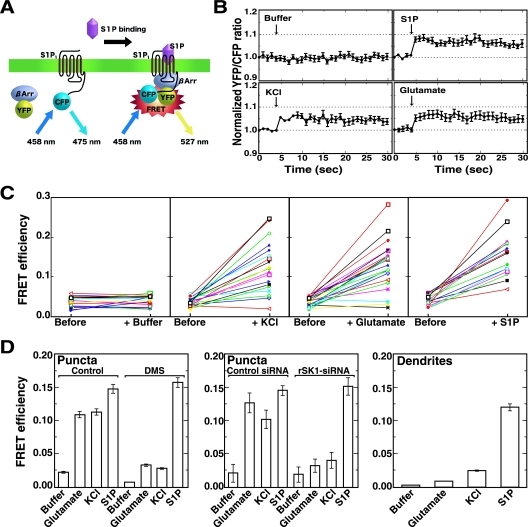
FRET.
Primary hippocampal neurons cultured in glass-bottomed culture dishes were cotransfected with S1P1-CFP (donor) and YFP-β-arrestin (acceptor) at a donor/acceptor ratio of 1:1. Following excitation at 458 nm, CFP and YFP emission spectra were collected (from cells expressing S1P1-CFP or YFP-β-arrestin alone) in eight channels, each with a 20-nm width, from 473 to 633 nm using the lambda mode of the Zeiss LSM 510 META confocal microscope and analytical software. Two days after cotransfection, neurons were treated with various agonists, as indicated in the legend to Fig. 5, and each area at the puncta of interest was subjected to fluorescence resonance energy transfer (FRET) analysis. FRET efficiency was measured after the acceptor photobleaching method as described previously (5). A mixed spectral image of cotransfected cells was collected, and an area of the puncta or dendrites was then selected for photobleaching of YFP. A protocol was then used which recorded pre- and postbleaching images using 458-nm excitation at 10% laser power to limit photobleaching, with a bleaching of the selected area with 100% 514-nm laser power for 2 s (acceptor photobleaching). The images obtained via lambda stacks were separated using the emission fingerprinting method. Two-channel (CFP and YFP) images were generated by applying linear unmixing to the lambda stacks. FRET was resolved as an increase in the CFP (donor) signal after photobleaching of YFP (acceptor). FRET efficiency (E) can be determined from the relative fluorescence intensity of the energy donor (CFP) before (Ipre) and after (Ipost) photobleaching of the energy acceptor (YFP): E = 1 − (Ipre/Ipost) (28).
FIG. 5.
Depolarization-induced S1P receptor activation demonstrated by FRET analysis. (A) The strategy to detect S1P1 interaction with β-arrestin (βArr) after S1P1 activation using FRET is depicted. (B) Hippocampal neurons transfected with expression plasmids encoding S1P1-CFP and YFP-β-arrestin were treated either with depolarization stimuli (50 mM KCl or 100 μM glutamate) or with 10 nM S1P and were analyzed for FRET in living cells. A representative emission ratio of the two fluorophores (excited at 458 nm) from five independent experiments is shown. Arrows indicate the addition of either control (buffer vehicle) or agonists. (C) Hippocampal neurons cotransfected with expression plasmids encoding S1P1-CFP and YFP-β-arrestin were treated without (buffer) or with 50 mM KCl, 100 μM glutamate, or 10 nM S1P and were analyzed for FRET in living cells. Emission detected from an increase in donor fluorescence after acceptor photobleaching of puncta of interest was measured and expressed as FRET efficiency. Note that depolarization induced either by KCl or glutamate as well as S1P treatment caused a significant increase in FRET efficiencies (n = 50; a representative experiment of four independent experiments is shown; P < 0.01, Student's paired t test). (D) Neurons cotransfected with S1P1-CFP and YFP-β-arrestin plasmids were untreated or treated with 10 μM DMS for 30 min before agonist stimulation. In some experiments neurons were transfected with control or rSK1-siRNAs together with plasmids encoding the fluorophore-conjugated proteins. Neurons were stimulated without (buffer) or with 100 μM glutamate, 50 mM KCl, or 10 nM S1P and fixed and measured for FRET efficiency after photobleaching of puncta or dendrite areas of interest. Data are means ± standard errors of the means of three independent experiments carried out in triplicate.
Other procedures.
Immunoblot analysis was carried out as described previously (23). SK1 activity was measured as reported earlier (18) except that 0.5% Triton X-100 was added to the reaction mixture to inhibit SK2 activity (27).
RESULTS
Expression of SK1 in the functional puncta of hippocampal axons.
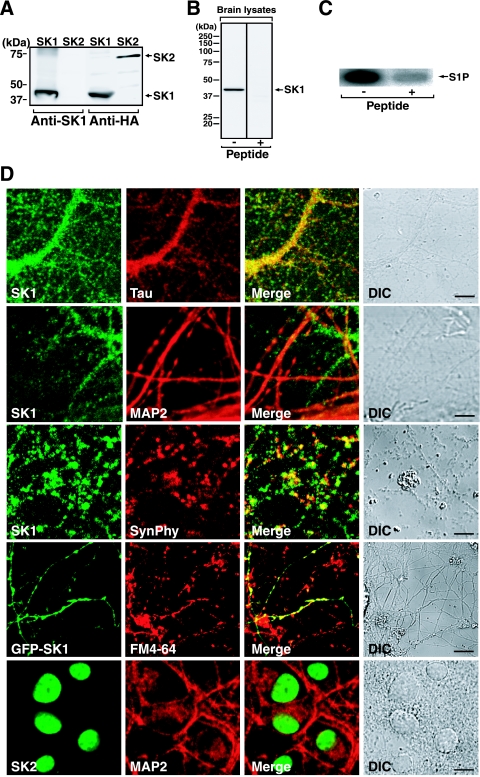
To assess the role of S1P in neuronal function, we first examined the subcellular distribution of SK in hippocampal neurons, a model system to study neuron-specific functions in the present studies. Primary cultures of dissociated rat hippocampal neurons were stained with antibodies against SK1 together with several marker proteins. The anti-SK1 antibody raised against mSK1 specifically recognized rSK1 but not rSK2 (Fig. 1A). Immunoblot analysis of rat brain lysates using this antibody showed mostly a single band at around 40 kDa, which corresponds to recombinant rSK1. This band completely disappeared when the immunogen peptide was included in the primary antibody reaction (Fig. 1B). In addition, this antibody could immunoprecipitate SK activity from rat brain lysates (Fig. 1C), validating the antibody specificity. In immunocytochemical studies of primary hippocampal neurons, SK1 was stained in a punctate pattern along the neurites, and almost all of SK1 was colocalized with Tau, an axonal marker, but not with MAP2, a marker of dendrites (Fig. 1D). The punctate pattern of SK1 was also colabeled with synaptophysin, a marker of presynaptic exocytotic vesicles. In addition, by using the membrane dye FM4-64 to label actively recycling presynaptic vesicles, almost all GFP-SK1-containing puncta were colabeled with FM4-64 (Fig. 1D). These results indicate that SK1 is localized at the functional presynaptic puncta of hippocampal neurons. In contrast, another isozyme, SK2, was localized mainly in the nuclei of hippocampal neurons, which is consistent with our recent observations (18, 30), but not in axonal puncta or dendritic spines. In terms of neuron-specific functions, SK2 was not pursued any further in the present studies.
FIG. 1.
Expression of SK1 in the functional puncta of hippocampal neurons. (A) COS7 cells transiently expressing HA-rSK1 or HA-rSK2 were subjected to immunoblot analysis using anti-SK1 or anti-HA antibody. (B) Rat brain lysates were analyzed for endogenous SK1 expression by immunoblotting experiments in the presence or absence of the immunogen peptide using anti-SK1 antibody. (C) SK activity was immunoprecipitated from rat brain lysates in the absence or presence of the immunogen peptide. Immunoprecipitates were assayed for SK activity. (D) Primary rat hippocampal neurons were double stained with both anti-SK1 or anti-SK2 and anti-Tau, anti-MAP2, or antisynaptophysin (SynPhy) antibodies. In some experiments neurons transiently expressing GFP-SK1 were prelabeled with membrane dye FM4-64 and analyzed for fluorescence localization in living cells. Differential interference contrast (DIC) and merged images are also presented. Scale bar, 10 μm.
Involvement of SK1 in depolarization-induced secretion in hippocampal neurons.
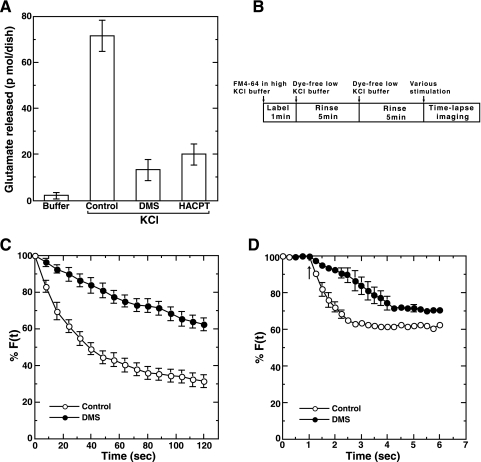
The observation that SK1 was localized at active presynaptic puncta prompted us to ask whether SK1 was involved in neuron-specific functions such as neurotransmitter release. First, the effect of a competitive inhibitor of SK1, DMS, on a neurotransmitter secretion was studied. Glutamate, a neurotransmitter released from hippocampal neurons, was quantitated by an enzymatic fluorometric assay. Glutamate secreted from the neurons during high-potassium-induced depolarization was strongly suppressed by DMS treatment (Fig. 2A). Similarly, a nonsphingoid SK inhibitor, HACPT, caused an inhibition of depolarization-induced glutamate release, suggesting the involvement of SK activity in the phenomena. For more detailed characterization, the involvement of SK in depolarization-induced neurotransmitter secretion was assessed by the FM dye method. Hippocampal neurons were untreated or pretreated with DMS, and then actively recycling presynaptic vesicles of the neurons were labeled with FM4-64 for a short time (1 min) under depolarization conditions using buffer containing 50 mM KCl (Fig. 2B). Secretion was assessed by quantification of selective unloading of FM4-64 after various depolarizing stimuli. DMS caused a marked inhibition of high-potassium-induced depolarization-evoked secretion (Fig. 2C), confirming the involvement of SK activity in the secretion. FM dye emission spectra before and after depolarization were unchanged (data not shown), supporting the notion that fluorescence changes observed here were not the result of changes in the membrane lipid environment after depolarization. DMS itself had no significant effect on fluorescence labeling efficiency (data not shown). An inhibitory effect of DMS was also observed in secretion induced by glutamate, a physiological agonist for hippocampal neurons (Fig. 2D). Glutamate-induced secretion followed a more rapid time course than that caused by high-potassium stimulation.
FIG. 2.
Inhibition of depolarization-evoked glutamate secretion by DMS. (A) Neurons cultured in 6-cm dishes were washed three times with phosphate-buffered saline. Neurons were pretreated without (vehicle) or with 10 μM DMS or 50 μM HACPT for 30 min and analyzed for glutamate secretion 1 min after treatment with either buffer or buffer containing 50 mM KCl. (B) Schematic representation of the protocol for the measurement of neurotransmitter secretion using the FM4-64 dye method. (C) Rat hippocampal neurons were treated without (vehicle; control) or with 10 μM DMS for 30 min. Neurons were washed and labeled with FM dye, which was incorporated into active presynaptic vesicles. The fluorescence of the dye at each punctum of interest was sequentially monitored after depolarization induced by 50 mM KCl. (D) Control or DMS-treated neurons were labeled with FM dye as in panel C, and the fluorescence of the dye was monitored after treatment with 100 μM glutamate. The arrow indicates the addition of glutamate. Data are means ± standard errors of the means of three independent experiments carried out in triplicate. For F(t), fluorescence at the selected region at time t, see text.
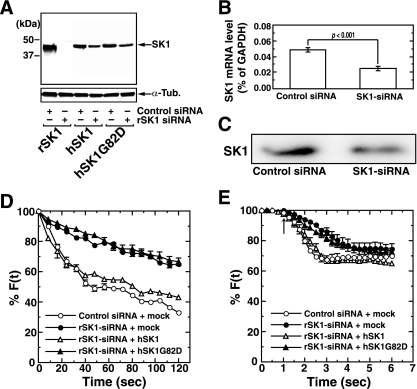
The involvement of endogenous SK1 in neurotransmitter secretion was further demonstrated by SK1-targeted siRNA experiments. rSK1-siRNA treatment of cultured hippocampal neurons strongly inhibited the expression of recombinant rSK1 but not hSK1 or hSK1G82D (Fig. 3A). This rSK1-siRNA treatment of hippocampal neurons suppressed the SK1 mRNA (Fig. 3B) and protein (Fig. 3C) expression by 50% and 60%, respectively. The transfection efficiency of siRNAs was calculated to be 34.6%. Treatment of hippocampal neurons with rSK1-siRNA resulted in a marked inhibition of high-potassium (50 mM)-induced secretion (Fig. 3D). This inhibition of secretion in rSK1-siRNA-treated neurons was almost completely rescued by the coexpression of wild-type hSK1 but not by the kinase-inactive mutant hSK1G82D, suggesting a requirement for the catalytic activity of SK1 in the potentiation of depolarization-evoked secretion. rSK2-siRNA transfection caused no obvious change in KCl-induced secretion (data not shown). When glutamate was used to trigger secretion, secretion was suppressed in rSK1-siRNA-treated neurons, especially during the early phase of secretion (Fig. 3E). Again, this suppression was rescued by the coexpression of wild-type hSK1 but not by catalytically inactive hSK1G82D. Overexpression of wild-type SK1 caused no further potentiation of KCl- or glutamate-induced secretion compared with control siRNA-transfected cells (Fig. 3D and E). Endogenous SK1 may be sufficient for the potentiation. These results indicate that endogenous SK1, presumably through S1P production, plays a role in the up-regulation of depolarization-evoked neurotransmitter secretion in the hippocampal neurons.
FIG. 3.
Involvement of SK1 in depolarization-evoked secretion. (A) Rat hippocampal neurons were transfected either with control or rSK1-siRNA together with expression vectors encoding rSK1, hSK1, or hSK1G82D. Two days after transfection neurons were analyzed for SK1 expression by immunoblot analysis. Endogenous SK1 mRNA and protein were quantitated in neurons transfected with either control or rSK1-siRNA by real-time quantitative PCR (B) or by immunoblot analysis using anti-SK1 antibody (C). Neurons transfected with either control or rSK1-siRNA together with vectors encoding hSK1, hSK1G82D, or an empty vector (mock) were prelabeled with FM4-64 and treated with 50 mM KCl (D) or 100 μM glutamate (E), and the fluorescence of the dye at each punctum of interest was sequentially monitored. The arrow indicates the addition of glutamate. Data are means ± standard errors of the means of three independent experiments carried out in triplicate (D and E). For F(t), fluorescence at the selected region at time t, see text.
Translocation of SK1 from the cytosol to plasma membrane during depolarization.
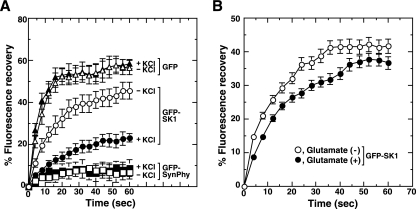
Recent lines of evidence suggest that, upon treatment of cells with various agonists, SK1 undergoes stimulation and subsequent translocation to plasma membrane or membranes of organelles (10, 20, 32, 42). To learn about any depolarization-induced topological changes in SK1 at axonal puncta, where SK1 was enriched (Fig. 1D), we undertook FRAP experiments. Hippocampal neurons were transiently transfected with plasmids encoding various GFP-fused proteins, and the dynamics of the fusion proteins after photobleaching at presynaptic puncta were measured. Under resting conditions cytosolic protein GFP behaved in a freely diffusible manner, and the presynaptic membrane protein synaptophysin was nondiffusible, whereas SK1 behaved in a dual manner: the majority of the SK1 was in a freely diffusible pool but some was attached to membranes and behaved in a nondiffusible manner (Fig. 4A). Upon depolarization induced by KCl, the proportion of SK1 in the nondiffusible pool increased, suggesting that KCl-induced depolarization caused translocation of SK1 from the cytosol to membranes at puncta. The distribution patterns of the other marker proteins such as GFP did not change during depolarization (Fig. 4A and see Fig. S1 in the supplemental material). Similar changes in SK1 behavior were observed upon glutamate-induced depolarization, although the percentage of shift was smaller than that caused by KCl-induced depolarization (Fig. 4B).
FIG. 4.
Translocation of SK1 during depolarization. Hippocampal neurons were transiently transfected with expression vectors encoding GFP-SK1, free GFP, or SynPhy-GFP as indicated. Two days after transfection living cells were subjected to FRAP analysis using confocal laser scanning microscopy. After depolarization induced either by 50 mM KCl (A) or 100 μM glutamate (B), axonal puncta expressing GFP-fused proteins were photobleached. Subsequently, images were collected at the indicated time points. For the graphs the fluorescence recovery immediately after photobleaching (lowest fluorescence intensity, 0 s) at each punctum of interest was measured and is given as percent fluorescence recovery based on the initial value before bleaching. Data are means ± standard errors of the means of three independent experiments carried out in triplicate.
Activation of S1P receptor during depolarization in a manner dependent on SK1 activity.
Our observations that depolarization induced translocation of SK1 from the cytosol to the membranes at puncta (Fig. 4A and B) and that SK1 activity was required for potentiation of transmitter secretion (Fig. 3D and E) prompted us to ask whether S1P newly generated by SK1 was activating S1P receptors. To resolve this issue we carried out FRET analyses to assess S1P receptor activation. Following upon the observation that β-arrestin binds to the G-protein-coupled receptor soon after receptor activation (6, 24), vectors were constructed to express two fusion proteins: S1P1-CFP and YFP-β-arrestin. If β-arrestin binds to S1P1 and the CFP and YFP are in sufficiently close proximity, then excitation of CFP should stimulate emission from the YFP fluorophore in a FRET assay (Fig. 5A). This approach provided the first direct evidence of spatiotemporal activation of S1P receptors in cells. These constructs were transiently transfected in hippocampal neurons, and their emission profiles were examined with an excitation wavelength of 458 nm after various stimuli (Fig. 5B). Upon stimulation with S1P, FRET was observed rapidly (within 1 s), and no detectable FRET was observed under unstimulated conditions, validating proper functioning of this FRET system. When neurons were transfected with control plasmid vectors encoding CFP and YFP proteins, no FRET was observed after KCl-induced depolarization (data not shown). Importantly, during depolarization induced either by KCl or glutamate, FRET occurred on a time scale and with an intensity similar to S1P stimulation, suggesting S1P1 activation during depolarization. The validity of the FRET signals produced by the two fluorophore-conjugated proteins was further confirmed by using FRET efficiency values based on dequenching of the CFP signal after specific photobleaching of the acceptor fluorophore YFP. Again, FRET efficiency significantly increased by KCl- or glutamate-induced depolarization to the same extent as S1P stimulation of the receptor (Fig. 5C and D and see Fig. S2 and S3 in the supplemental material), confirming the S1P1 stimulation during depolarization. Treatment of neurons either with DMS or rSK1-siRNA abrogated the KCl- or glutamate-triggered depolarization-induced increase in FRET efficiency but not that induced by S1P (Fig. 5D), indicating the activation of S1P1 receptor in close proximity to areas where S1P is generated during depolarization. This notion is consistent with the present results that depolarization-evoked secretion was inhibited by treatment with DMS (Fig. 2) or rSK1-siRNA (Fig. 3D and E). The importance of SK1-catalyzed generation of S1P during depolarization was further strengthened by the observation that a depolarization-induced increase in FRET efficiency was detected only in axonal puncta (Fig. 5D), where SK1 was enriched, but not in dendrites, where the amount of SK1 was low (Fig. 1D).
Role of exogenous S1P in neurotransmitter secretion.
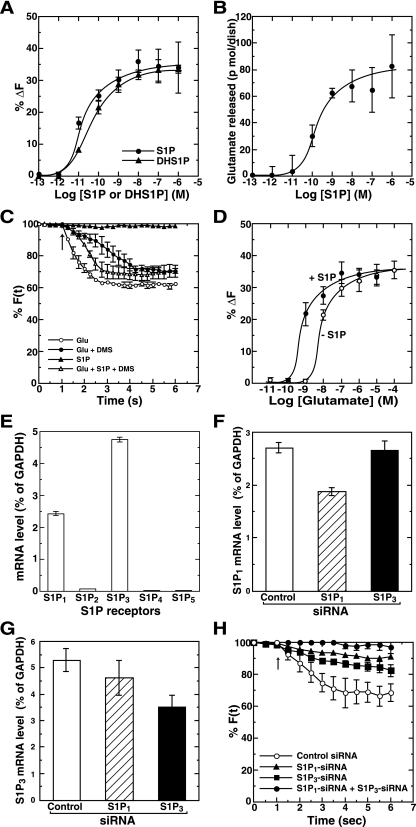
What is the outcome of S1P receptor activation during depolarization? To answer this question we examined the effect of exogenous S1P and dihydroS1P (DHS1P), physiological products of SK1, on secretion in hippocampal neurons. Unexpectedly, S1P by itself elicited secretion in a dose-dependent manner with a 50% effective concentration of ∼20 pM, with the maximal effects being observed at 10 nM as measured by the FM dye method (Fig. 6A). DHS1P also caused secretion with a potency similar to S1P, suggesting a receptor-mediated but not intracellular action. Similar results were also obtained by the direct measurement of glutamate release from hippocampal neurons. Exogenous S1P caused the release of glutamate in the same range of S1P concentrations as determined using FM dye method (Fig. 6B). The effect of S1P was rapid, with maximum exocytosis taking place in 2 s (Fig. 6C). To characterize further the receptor-mediated S1P action during glutamate-induced secretion, the effect of S1P was analyzed in combination with glutamate. Glutamate-induced secretion was potently (75%) inhibited by DMS treatment (Fig. 2D and 6C), confirming the necessity of SK activity for the glutamate action. Importantly, this inhibition by DMS was overcome by the simultaneous addition of a suboptimal concentration (1 pM) of S1P (Fig. 6C), which alone was insufficient to cause exocytosis (Fig. 6A and C, filled triangles), demonstrating potentiation of glutamate-induced transmitter secretion by S1P as well as induction of secretion by this lipid itself. In fact, the dose dependence curve for glutamate-induced exocytosis was shifted to the left by the exogenous addition of 1 pM S1P (Fig. 6D). To demonstrate that this exogenous action of S1P was mediated directly through S1P receptor activation, S1P-mediated secretion was measured in S1P receptor knock-down cells. First, expression of S1P receptors in hippocampal neurons was assessed by real-time quantitative PCR analysis. Of the five S1P receptor subtypes, S1P1 and S1P3 mRNAs were expressed mainly in the primary neurons (Fig. 6E). Next, the effect of S1P receptor siRNAs on their endogenous mRNA expression in the neurons was checked. S1P1 and S1P3 siRNA treatment caused the inhibition of the corresponding endogenous mRNA level by 30% and 35%, respectively (Fig. 6F and G). When either S1P1 or S1P3 receptor siRNA was transfected in the neurons, it caused an obvious inhibition of secretion induced by S1P compared with control siRNA-transfected cells (Fig. 6H). Since S1P1 is known to couple with the heterotrimeric G protein, Gi (36), the involvement of S1P1 receptor in S1P-induced secretion was further ascertained by pertussis toxin sensitivity assays. S1P-induced secretion was partially (50%) inhibited by pertussis toxin treatment (see Fig. S4 in the supplemental material), further supporting the results obtained from S1P1 receptor knock-down cells. Combination of S1P1 and S1P3 receptor siRNA caused an almost complete inhibition of S1P-induced secretion, indicating that S1P-induced secretion is mediated by both S1P1 and S1P3 receptor activation.
FIG. 6.
Functional roles of exogenous S1P as both an inducer and an enhancer of secretion. (A) Primary rat hippocampal neurons were prelabeled with FM4-64. FM dye-labeled cells were stimulated with various concentrations of either S1P or DHS1P for 6 s, and the changes in fluorescence intensity were monitored. At all points vehicle (methanol) concentrations were kept constant. (B) Neurons washed three times with PBS were stimulated with various concentrations of S1P for 6 s. Secreted glutamate was measured by an enzymatic fluorometric assay. (C) Neurons were treated without (vehicle) or with 10 μM DMS for 30 min and labeled with FM dye. Neurons were then stimulated with various combinations of 1 pM S1P and 100 μM glutamate for the indicated time, and the changes in fluorescence intensity were monitored. The arrow indicates the addition of agonists. (D) FM dye-prelabeled neurons were treated with various concentrations of glutamate without or with 1 pM S1P for 6 s, and the changes in fluorescence intensity were monitored. (E) Individual S1P receptor mRNAs were quantitated from primary rat hippocampal neurons by real-time quantitative reverse transcription PCR. Values of mRNA amounts were normalized to GAPDH expression. (F and G) Hippocampal neurons were transfected with control or S1P1 or S1P3 siRNA. Two days after transfection S1P1 mRNA (F) or S1P3 mRNA (G) levels were quantitated by real-time quantitative reverse transcription PCR. (H) Hippocampal neurons transfected with various combinations of control or S1P1 and S1P3 siRNAs were prelabeled with FM4-64. Neurons were stimulated with 10 nM S1P, and the changes in fluorescence intensity were monitored. The arrow indicates the addition of S1P. Data are the means ± standard errors of the means of five independent experiments carried out three (A to D and H) or six (E) times. For F(t), fluorescence at the selected region at time t, see text.
Essential role of S1P in the induction and potentiation of glutamate secretion through its autocrine action.
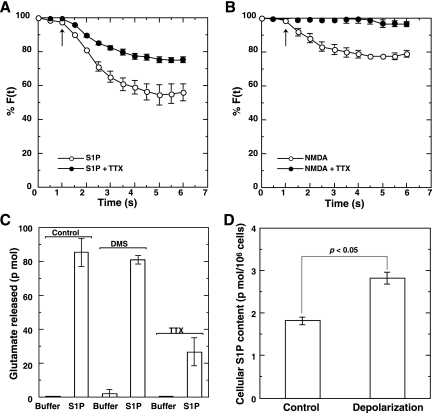
Receptor-mediated secretagogue actions of S1P (Fig. 6A and B) were further characterized using the Na+-channel blocker tetrodotoxin (TTX). When neurons were treated with TTX, S1P-induced secretion was moderately (50%) inhibited by the blocker (Fig. 7A), whereas secretion induced by N-methyl-D-aspartate (NMDA), whose receptors reside mainly at postsynaptic membranes, was almost completely abolished by TTX treatment (Fig. 7B). This indicates that S1P causes secretion by two mechanisms: (i) a direct glutamate secretion from presynaptic puncta that is insensitive to TTX and (ii) a potentiation of depolarization-evoked secretion via glutamate (NMDA) receptor activation that is sensitive to TTX. In fact, S1P-induced glutamate secretion was also partially inhibited by TTX treatment (Fig. 7C), suggesting the involvement of two different mechanisms (i.e., induction and potentiation of secretion) in S1P-induced glutamate secretion. In contrast to a strong inhibition of KCl-induced glutamate secretion by DMS (Fig. 2A), S1P-induced glutamate secretion was insensitive to the SK inhibitor (Fig. 7C). This indicated that S1P produced during depolarization in the localized area, where SK1 was translocated, was finally activating the proximal S1P receptor(s) to elicit biological effects, i.e., induction of glutamate secretion from presynaptic sites and potentiation of glutamate-induced transmitter secretion. In support of this, we observed a colocalization of either S1P1 or S1P3 receptor with FM4-64-stained actively recycling presynaptic vesicles (see Fig. S5 in the supplemental material). To further substantiate this notion, the cellular content of S1P was measured before and after depolarization. Upon high-potassium treatment of neurons, the amount of cellular S1P increased by about 50% compared with untreated neurons (Fig. 7D), suggesting that S1P produced during depolarization plays a pivotal role in both induction and potentiation of glutamate secretion.
FIG. 7.
Essential role of S1P in the induction and potentiation of glutamate secretion. FM dye-prelabeled hippocampal neurons were untreated or treated with 1 μM TTX for 10 min. Neurons were then stimulated with 10 nM S1P (A) or 100 μM NMDA (B), and the changes in fluorescence intensity were monitored. The arrow indicates the addition of agonists. (C) Neurons cultured in 6-cm dishes were washed three times with phosphate-buffered saline. Neurons were pretreated without (vehicle) or with 10 μM DMS for 30 min or with 1 μM TTX for 10 min and analyzed for glutamate secretion 1 min after treatment with either buffer or buffer containing 10 nM S1P. (D) Neurons cultured in 10-cm dishes were washed three times with phosphate-buffered saline. Neurons were treated without (control) or with 50 mM KCl (depolarization) for 1 min. Lipids were extracted from the neurons and analyzed for mass level quantification of S1P (n = 5; P < 0.05, Student's paired t test). For F(t), fluorescence at the selected region at time t, see text.
DISCUSSION
We have demonstrated two novel actions of S1P using dissociated hippocampal neurons as a model, i.e., a secretagogue action whereby S1P triggers glutamate secretion (Fig. 6A and B) and an enhancer action whereby S1P potentiates depolarization-evoked glutamate secretion (Fig. 6C and D). Both of these actions may participate in the up-regulation of S1P-induced glutamate secretion. TTX treatment only partially inhibited S1P-induced glutamate secretion although it abolished NMDA-induced secretion completely (Fig. 7). The TTX-sensitive component may correspond to an enhancer action, and the TTX-insensitive part may reflect a secretagogue action of S1P (Fig. 7A). These two S1P-induced actions may facilitate the formation of a positive activation cycle in excitatory neurons such as glutaminergic neurons. These S1P actions may not be limited to hippocampal neurons but may also apply to other neurons, because immunohistochemical studies revealed that SK1 was enriched in olfactory bulb and cerebellar cortex as well as hippocampus (data not shown). In fact, it has recently been reported that intracellular S1P enhances nerve growth factor-induced excitability in rat sensory neurons (45). S1P functions in these SK1-enriched brain areas needs to be clarified.
S1P has been suggested to exert its actions either intracellularly as a second messenger or extracellularly as a ligand for S1P receptors (26, 33). The present results demonstrate that S1P produced during depolarization exerts its actions by activating S1P1 or S1P3 receptor (Fig. 5 and 6H). High-potassium-induced depolarization causes rapid formation of S1P in hippocampal neurons (Fig. 7D) and in PC12 cells (1). This newly synthesized S1P may be released into extracellular space (3) and may activate S1P receptors. This autocrine/paracrine action of S1P in hippocampal neurons may reflect a wider phenomenon operating as well in other cases such as in immune cells (34). S1P1 activation is known to induce cytoskeletal rearrangements through small G-protein Rac activation via Gi (25, 31). Rac is proposed to facilitate synaptic vesicle fusion to plasma membranes to induce transmitter secretion (11, 17). The precise mechanisms underlying S1P receptor activation leading to neurotransmitter secretion need to be elucidated.
It has been thought that many presynaptic receptors belong to the relatively slow-acting metabotropic G-protein-coupled class, exerting mainly inhibitory effects on the release machinery through inhibition of voltage-gated Ca2+ channels or by increasing K+-channel activity (7, 43). However, our present results clearly indicate that S1P receptor activation causes induction or potentiation of glutamate release from hippocampal neurons. To support our observation, it has recently been reported that pregnenolone sulfate acts through a Gi/o-coupled sigma 1-like receptor to enhance short-term presynaptic facilitation in adult hippocampal CA1 neurons (38). Hippocampal neurons may be unique in the modulation of transmitter release compared with other types of neurons.
Although it has long been known that neurotransmitter(s) is also released spontaneously, independent of action potentials (22), the molecular mechanisms and physiological relevance of spontaneous or quantal secretion remain unknown. Based on the present results showing a functional role for S1P in triggering secretion by itself (Fig. 7A), it is plausible that the SK1/S1P pathway is one, if not the main, determinant to elicit spontaneous secretion in neurons.
It has recently been shown that SK1/SK2 double-knockout mice as well as S1P1 receptor-null mice showed severe defects in neurogenesis, including neural tube closure (29). In addition, there is a line of evidence indicating that neural stem cells in the adult mammalian brain continuously generate new neurons, predominantly in the hippocampus and olfactory bulb (2, 21). We have recently observed that SK1 is enriched in the hippocampus and olfactory bulb in adult mice (data not shown). The ongoing neurogenesis in the adult has recently been implicated in the formation of hippocampus-dependent memories such as trace memories (39). This implies that the new neurons must be integrated into preexisting neuronal networks with functional synaptic transmission through active synaptogenesis and acquisition of synaptic plasticity. The notion of up-regulation of neurotransmitter secretion by the SK1/S1P signaling pathway as demonstrated in the present studies may provide some clues to adult as well as embryonic CNS development.
Supplementary Material
Acknowledgments
We thank T. Terashima, Kobe University Graduate School of Medicine, for valuable comments on the manuscript. We also thank M. Homma for her skillful secretarial assistance.
This work was supported in part by a Grant-in-Aid for COE Research and a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Science, Sports and Culture of Japan and a Grant-in-Aid for JSPS Fellows from Japan Society for the promotion of Science.
Footnotes
Published ahead of print on 26 February 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alemany, R., B. Kleuser, L. Ruwisch, K. Danneberg, H. Lass, R. Hashemi, S. Spiegel, K. H. Jakobs, and D. Meyer zu Heringdorf. 2001. Depolarisation induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett. 509:239-244. [DOI] [PubMed] [Google Scholar]
- 2.Altman, J., and G. D. Das. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124:319-335. [DOI] [PubMed] [Google Scholar]
- 3.Anelli, V., R. Bassi, G. Tettamanti, P. Viani, and L. Riboni. 2005. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J. Neurochem. 92:1204-1215. [DOI] [PubMed] [Google Scholar]
- 4.Bajjalieh, S. M. 1999. Synaptic vesicle docking and fusion. Curr. Opin. Neurobiol. 9:321-328. [DOI] [PubMed] [Google Scholar]
- 5.Bastiaens, P. I., I. V. Majoul, P. J. Verveer, H. D. Söling, and T. M. Jovin. 1996. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 15:4246-4253. [PMC free article] [PubMed] [Google Scholar]
- 6.Benovic, J. L., H. Kuhn, I. Weyand, J. Codina, M. G. Caron, and R. J. Lefkowitz. 1987. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc. Natl. Acad. Sci. USA 84:8879-8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackmer, T., E. C. Larsen, M. Takahashi, T. F. Martin, S. Alford, and H. E. Hamm. 2001. G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292:293-297. [DOI] [PubMed] [Google Scholar]
- 8.Carrero, G., E. Crawford, J. Th'ng, G. de Vries, and M. J. Hendzel. 2004. Quantification of protein-protein and protein-DNA interactions in vivo, using fluorescence recovery after photobleaching. Methods Enzymol. 375:415-442. [DOI] [PubMed] [Google Scholar]
- 9.Colombaioni, L., and M. Garcia-Gil. 2004. Sphingolipid metabolites in neural signalling and function. Brain Res. Rev. 46:328-355. [DOI] [PubMed] [Google Scholar]
- 10.Delon, C., M. Manifava, E. Wood, D. Thompson, S. Krugmann, S. Pyne, and N. T. Ktistakis. 2004. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J. Biol. Chem. 279:44763-44774. [DOI] [PubMed] [Google Scholar]
- 11.Doussau, F., S. Gasman, Y. Humeau, F. Vitiello, M. Popoff, P. Boquet, M. F. Bader, and B. Poulain. 2000. A Rho-related GTPase is involved in Ca2+-dependent neurotransmitter exocytosis. J. Biol. Chem. 275:7764-7770. [DOI] [PubMed] [Google Scholar]
- 12.Edsall, L. C., and S. Spiegel. 1999. Enzymatic measurement of sphingosine-1-phosphate. Anal. Biochem. 272:80-86. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, T., T. Okada, S. Hayashi, S. Jahangeer, N. Miwa, and S. Nakamura. 2004. Delta-catenin/NPRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. Biochem. J. 382:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda, Y., A. Kihara, and Y. Igarashi. 2003. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem. Biophys. Res. Commun. 309:155-160. [DOI] [PubMed] [Google Scholar]
- 15.Harada, J., M. Foley, M. A. Moskowitz, and C. Waeber. 2004. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J. Neurochem. 88:1026-1039. [DOI] [PubMed] [Google Scholar]
- 16.Hla, T., M. J. Lee, N. Ancellin, C. H. Liu, S. Thangada, B. D. Thompson, and M. Kluk. 1999. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem. Pharmacol. 58:201-207. [DOI] [PubMed] [Google Scholar]
- 17.Humeau, Y., M. R. Popoff, H. Kojima, F. Doussau, and B. Poulain. 2002. Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J. Neurosci. 22:7968-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi, N., T. Okada, S. Hayashi, T. Fujita, S. Jahangeer, and S. Nakamura. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278:46832-46839. [DOI] [PubMed] [Google Scholar]
- 19.Jahn, R., and T. C. Sudhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863-911. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, K. R., K. P. Becker, M. M. Facchinetti, Y. A. Hannun, and L. M. Obeid. 2002. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J. Biol. Chem. 277:35257-35262. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M. S., and J. W. Hinds. 1977. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092-1094. [DOI] [PubMed] [Google Scholar]
- 22.Katz, B. 1969. The release of neural transmitter substances. Liverpool University Press, Liverpool, United Kingdom.
- 23.Kosaka, Y., K. Ogita, K. Ase, H. Nomura, U. Kikkawa, and Y. Nishizuka. 1988. The heterogeneity of protein kinase C in various rat tissues. Biochem. Biophys. Res. Commun. 151:973-981. [DOI] [PubMed] [Google Scholar]
- 24.Krasel, C., M. Bunemann, K. Lorenz, and M. J. Lohse. 2005. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J. Biol. Chem. 280:9528-9535. [DOI] [PubMed] [Google Scholar]
- 25.Lee, M. J., S. Thangada, K. P. Claffey, N. Ancellin, C. H. Liu, M. Kluk, M. Volpi, R. I. Sha'afi, and T. Hla. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99:301-312. [DOI] [PubMed] [Google Scholar]
- 26.Le Stunff, H., S. Milstien, and S. Spiegel. 2004. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell. Biochem. 92:882-899. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., M. Sugiura, V. E. Nava, L. C. Edsall, K. Kono, S. Poulton, S. Milstien, T. Kohama, and S. Spiegel. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275:19513-19520. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J., S. A. Ernst, S. E. Gladycheva, Y. Y. Lee, S. I. Lentz, C. S. Ho, Q. Li, and E. L. Stuenkel. 2004. Fluorescence resonance energy transfer reports properties of syntaxin1a interaction with Munc18-1 in vivo. J. Biol. Chem. 279:55924-55936. [DOI] [PubMed] [Google Scholar]
- 29.Mizugishi, K., T. Yamashita, A. Olivera, G. F. Miller, S. Spiegel, and R. L. Proia. 2005. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25:11113-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada, T., G. Ding, H. Sonoda, T. Kajimoto, Y. Haga, A. Khosrowbeygi, S. Gao, N. Miwa, S. Jahangeer, and S. Nakamura. 2005. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 280:36318-36325. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, H., N. Takuwa, T. Yokomizo, N. Sugimoto, S. Sakurada, H. Shigematsu, and Y. Takuwa. 2000. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol. Cell. Biol. 20:9247-9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitson, S. M., P. A. Moretti, J. R. Zebol, H. E. Lynn, P. Xia, M. A. Vadas, and B. W. Wattenberg. 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 22:5491-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyne, S., J. S. Long, N. T. Ktistakis, and N. J. Pyne. 2005. Lipid phosphate phosphatases and lipid phosphate signaling. Biochem. Soc. Trans. 33:1370-1374. [DOI] [PubMed] [Google Scholar]
- 34.Rosen, H., and E. J. Goetzl. 2005. Sphingosine-1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560-570. [DOI] [PubMed] [Google Scholar]
- 35.Samuel, A. D., R. A. Silva, and V. N. Murthy. 2003. Synaptic activity of the AFD neuron in Caenorhabditis elegans correlates with thermotactic memory. J. Neurosci. 23:373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez, T., and T. Hla. 2004. Structural and functional characteristics of S1P receptors. J. Cell Biochem. 92:913-922. [DOI] [PubMed] [Google Scholar]
- 37.Sankaranarayanan, S., P. P. Atluri, and T. A. Ryan. 2003. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 6:127-135. [DOI] [PubMed] [Google Scholar]
- 38.Schiess, A. R., and L. D. Partridge. 2005. Pregnenolone sulfate acts through a G-protein-coupled sigma 1-like receptor to enhance short term facilitation in adult hippocampal neurons. Eur. J. Pharmacol. 518:22-29. [DOI] [PubMed] [Google Scholar]
- 39.Shors, T. J., G. Miesegaes, A. Beylin, M. Zhao, T. Rydel, and E. Gould. 2001. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372-376. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel, S., and S. Milstien. 2003. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem. Soc. Trans. 31:1216-1219. [DOI] [PubMed] [Google Scholar]
- 41.Terada, N., Y. Banno, N. Ohno, Y. Fujii, T. Murate, J. R. Sarna, R. Hawkes, Z. Zea, T. Baba, and S. Ohno. 2004. Compartmentation of the mouse cerebellar cortex by sphingosine kinase. J. Comp. Neurol. 469:119-127. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, C. R., S. S. Iyer, N. Melrose, R. VanOosten, K. Johnson, S. M. Pitson, L. M. Obeid, and D. J. Kusner. 2005. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: inhibition of SK1 translocation by Mycobacterium tuberculosis. J. Immunol. 174:3551-3561. [DOI] [PubMed] [Google Scholar]
- 43.Wu, L. G., and P. Saggau. 1997. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 20:204-212. [DOI] [PubMed] [Google Scholar]
- 44.Yatomi, Y. 2006. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr. Pharm. Des. 12:575-587. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Y. H., M. R. Vasko, and G. D. Nicol. 2006. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J. Physiol. 575:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.