Abstract
The cyclin-dependent kinase inhibitor p21Cip1 regulates multiple cellular functions and protects cells from genotoxic and other cellular stresses. Activation of apoptosis signal-regulating kinase 1 (ASK1) induced by inhibition of mTOR signaling leads to sustained phospho-c-Jun that is suppressed in cells with functional p53 or by forced expression of p21Cip1. Here we show that small deletions of p21Cip1 around S98 abrogate its association with ASK1 but do not affect binding to Cdk1, hence distinguishing between the cell cycle-regulating functions of p21Cip1 and its ability to suppress activation of the ASK1/Jun N-terminal protein kinase (JNK) pathway. p21Cip1 is phosphorylated in vitro by both ASK1 and JNK1 at S98. In vivo phosphorylation of p21Cip1, predominantly carried out by ASK1, is associated with binding to ASK1 and inactivation of ASK1 kinase function. Binding of p21Cip1 to ASK1 requires ASK1 kinase function and may involve phosphorylation of S98.
The cyclin-dependent kinase (CDK) inhibitor p21Cip1 is known to control the cell cycle and DNA replication. In addition, it has been shown to be involved in many biological processes, including differentiation, stress response, apoptosis, development, and tumorigenesis (reviewed in references 7 and 12). Consistent with its numerous cellular functions, p21Cip1 has been found to participate in a number of specific protein-protein interactions. In addition to associating with cyclin CDKs and PCNA (22, 58, 63), p21Cip1 has also been reported to bind to transcription factors (c-Myc [33], E2F [11], C/EBP-α [54], and STAT3 [10]), proteins involved in apoptosis (procaspase 3 [48], stress-associated protein kinase [47], and apoptosis signal-regulating kinase 1 [ASK1] [4]), and other proteins (SET [14], calmodulin [52], HPV-16 E7 [16], GADD45 [30], TOK-1 [41], and protein kinase CK 2 [20]).
p21Cip1 plays a dual role in cell cycle progression, regulating both DNA synthesis and CDK activity. p21Cip1 binds to cyclin E-CDK complexes via an amino-terminal binding site, and this interaction inhibits the activity of these complexes, resulting in a block in cell cycle progression (46). p21Cip1 also blocks cell cycle progression in S phase due to its ability to bind and inhibit PCNA, a processivity factor for replication polymerases (31), through a carboxyl-terminal interaction (21) and leads to inhibition of DNA synthesis (58). However, more recent studies have shown that p21Cip1 also has positive effects on G1 progression (46). p21Cip1 was shown to stabilize interactions between Cdk4 and cyclin D and promote the formation of active complexes in a concentration-dependent manner (60). Several studies (15, 62) have demonstrated that p21Cip1 mediates the assembly and activation of cyclin D1-CDK4/6 complexes (36), which function as sensors of growth factors at G1-S phase.
p21Cip1 has also been shown to play a role in enhancing cell survival. For example, terminally differentiated cells, such as muscle cells (37), hematopoietic stem cells (9), and macrophages (2, 21), were found to contain elevated levels of p21Cip1, and the inhibition of the up-regulation of this protein resulted in apoptotic cell death. Furthermore, p21Cip1 can protect various types of cells from death following exposure to cytotoxic agents and ionizing radiation. The level of p21Cip1 in cells determines the sensitivity to cisplatin and ionizing radiation. p21Cip1 overexpressed in inducible expression systems or adenovirus gene transfer increases cell survival against prostaglandin A2 or p53 overproduction (17, 18). The reduction or absence of p21Cip1 expression, however, sensitizes cells to doxorubicin hydrochloride, camptothecin etoposide, ionizing radiation, or prostaglandin A2 (42, 53, 59, 60). Because the p21Cip1 protein level is elevated in a diversity of tumors, particularly in late-stage, aggressive tumors such as glioblastoma multiforme, the complex functions of p21Cip1 suggest that advanced tumors take advantage of the positive effects of p21Cip1 on cell cycle progression and cell survival (3, 13, 15).
The mechanisms by which p21Cip1 can prevent cells from undergoing apoptosis are not well understood. One mechanism is assumed to involve p21Cip1-dependent cell cycle arrest (often at G2/M) that would permit repair of DNA damage (43). Another distinct mechanism is linked to the ability of p21Cip1 to bind and inactivate cyclin A/Cdk2 complexes. It has been shown that caspase 3-mediated cleavage of p21Cip1 is an important mechanism of cyclin A/Cdk2 activation associated with death in different cell types (1, 29, 39). Apoptosis induced by various stimuli appears to be mediated by caspase 3 cleavage of p21Cip1 and subsequent up-regulation of cyclin A/Cdk2 activity. Caspase-dependent Cdk2 activity is a requisite effector of apoptotic death, and it may be necessary for death-associated chromatin condensation, cell shrinking, and loss of adhesion to substrate (23).
Recent data also point to more direct mechanisms by which p21Cip1 may inhibit cell death. p21Cip1 interacts with procaspase 3 through the amino terminus and suppresses its activation by masking the serine proteinase cleavage site (29, 40, 48, 49). Procaspase 3-p21Cip1 complex formation occurred in mitochondria and required phosphorylation of p21Cip1 by protein kinase A (50). Importantly, phosphorylation of p21Cip1 at T145 by Akt may cause relocalization of p21Cip1 to the cytoplasm (65), where it may form a complex with ASK1 and inhibit the stress-activated mitogen-activated protein kinase (MAPK) cascade and cell death (25).
ASK1, a mammalian MAPK kinase kinase (MAPKKK), activates the Jun N-terminal protein kinase (JNK) and p38 pathways and is activated in response to various cytotoxic stresses, including hydrogen peroxide (H2O2), Fas ligation, tumor necrosis factor (TNF), serum withdrawal, and cancer chemotherapeutic agents (8, 19, 27, 55). Overexpression of ASK1 in epithelial cells in low serum conditions induced apoptosis (27), and ASK1-deficient cells were resistant to H2O2- and TNF-induced apoptosis (56), indicating that ASK1 plays a pivotal role in stress-induced apoptosis. The kinase activity of ASK1 is tightly regulated within cells; under nonstressed conditions ASK1 is inhibited by association with its physiological inhibitor, thioredoxin (Trx). When cells are exposed to H2O2 or TNF, reactive oxygen species-dependent oxidation of Trx occurs, resulting in dissociation of Trx from ASK1 and thereby activation of ASK1 (40). Oligomerization-dependent autophosphorylation appears to be the next step required for full activation of ASK1 after the release from Trx (19). On the other hand, the mechanism(s) of how the activated ASK1 returns to an inactive form has not been elucidated.
Recently we found that the association of p21Cip1 with ASK1 can block rapamycin-induced apoptosis in human and murine cells (25). However, the mechanism by which p21Cip1 associates with ASK1 is not understood. Here we report that p21Cip1 association with ASK1 involves a small region around S98. ASK1 can phosphorylate p21Cip1 at S98 both in vivo and in vitro, and this phosphorylation appears to be important for the association of p21Cip1 with ASK1.
MATERIALS AND METHODS
Chemicals and antibodies.
The JNK inhibitor SP600125, the p38 inhibitor SB203580, and anisomycin (AN), an activator of JNK and p38, were purchased from A. G. Scientific (San Diego, CA). ASK C-terminal antibodies (Sc-5294), p21Cip1 rabbit polyclonal antibody (Sc-756), and mouse antihemagglutinin (anti-HA) monoclonal antibody (Sc-7392) were from Santa Cruz Biotechnology (Santa Cruz, CA). p21Cip1 mouse monoclonal antibody was from BD Pharmingen (Franklin Lakes, NJ). Anti-HA rabbit polyclonal antibody was from BD Clontech (Mountain View, CA). Purified JNK1 and ASK1 kinases were obtained from Upstate Biotechnology (Lake Placid, NY).
Cell lines and growth conditions.
The Rh30 human rhabdomyosarcoma cell line expresses both wild-type (WT) and mutant (R273C) p53 alleles and was grown in antibiotic-free RPMI 1640 under conditions described previously (25). HEK293T cells were maintained in antibiotic-free Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2.
Cell transfection.
293T cells were transfected by using FuGene 6 (Roche). Briefly, HEK293T cells were seeded at 2 × 106 cells/10-cm-diameter dish. Transfection was performed 48 h after seeding the cells. Nine microliters of FuGene 6 was added directly to serum-free DMEM, to a total volume of 100 μl, with gentle mixing. Two micrograms of p21Cip1 plasmid DNA (or plasmids encoding mutants) and 1 μg of ASK1 plasmid DNA were added to prediluted FuGene 6 in 100 μl of DMEM. The mixture was gently tapped to ensure adequate mixing and set at room temperature for 30 to 45 min. The DNA-FuGene 6 transfection mixture was added to the dish dropwise, and at the same time the plate contents were swirled to ensure even dispersal.
Adenoviral infection.
Stable lines expressing Rh30/p21 (WT) and Rh30/ΔNLSp21 (truncated at residue 140 and lacking the nuclear localization signal) under control of the metallothionein promoter have been described previously (25). Both cell lines were infected with replication-deficient adenovirus that expresses WT or N-terminally or C-terminally deleted human ASK1 constructs. First, all infections were performed in 2% FBS-DMEM for 2 h, and then cells were incubated in 10% FBS-DMEM for 22 h, followed by culture in serum-free medium. The expression of p21Cip1 or NLSp21Cip1 was induced with Zn (60 μM) for 24 h, at which time cell lysates were prepared for immunoprecipitation and Western blot analysis.
Immunoprecipitation and Western blot analysis.
Cells were briefly washed with ice-cold phosphate-buffered saline. On ice, cells were lysed in 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) buffer (40 mM HEPES [N-2-hydroxyethylpiperazine-NN-2-ethanesulfonic acid], pH 7.4, 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM glycophosphate, 50 mM NaF, 1.5 mM Na3VO4, 0.3% CHAPS, protease inhibitor mixture [1:1,000; Sigma]). For immunoprecipitation, lysates were sonicated for 10 s and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA). Immune immunoprecipitation and Western blot analyses were performed as previously described (25), with minor modifications.
Protein purification.
p21Cip1 WT and p21Cip1-S98E were expressed in Escherichia coli with a C-terminal His epitope tag using the expression vector pET24d. The harvested E. coli cells were disrupted by ultrasonication and centrifuged. The pellet was dissolved in 6 M urea and purified from the soluble cellular fraction by Ni2+ affinity chromatography in the presence of 6 M urea. p21Cip1-containing fractions were further purified by reverse-phase C4 high-performance liquid chromatography using a 0.1% trifluoroacetic acid/acetonitrile buffer system. The protein sequence was confirmed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS). The purified p21Cip1 proteins were used for in vitro kinase assays.
In vitro kinase assay.
Cells were briefly washed with ice-cold phosphate-buffered saline and disrupted in CHAPS buffer and then immunoprecipitated with rabbit polyclonal antibodies to ASK1 (8, 25) and protein A/G-agarose. The activity of ASK1 was measured according to the phosphorylation of Mal-E-MKK6 in the presence of [γ-P32]ATP, and radiolabeled material was quantitated using a Storm 860 phosphorimager (Amersham Biosciences, Sunnyvale, CA). After detection of phosphorylation of MKK6, polyvinylidene difluoride membranes were immunoblotted with ASK1 antibody. For the phosphorylation of p21Cip1 by JNK or ASK1 in vitro, full-length p21Cip1 and mutant p21-S98E were purified from bacteria. One microgram of purified p21Cip1 or its mutant, p21-S98E, was incubated with purified active ASK1 or JNK1 kinase (Upstate Biotechnology) in the presence of [γ-P32]ATP in kinase buffer (10 mM Tris, pH 7.4, 10 mM MgCl2) (25). Radiolabeling material incorporated into p21Cip1 was quantitated as described above. The same membrane was blotted using anti-p21Cip1 antibody.
Mass spectral analysis of the phosphorylation of p21Cip1 at serine 98.
Kinase reactions using recombinant p21Cip1 and ASK1 proteins were carried out as described above. Reaction mixtures were aliquoted into separate tubes. One aliquot of each reaction mixture was treated with 10 U of calf intestinal alkaline phosphatase and incubated at 37°C for 2 h to dephosphorylate the protein. The control aliquots and alkaline phosphatase-treated aliquots were run on a 10 to 20% sodium dodecyl sulfate gel and stained with SYPRO Ruby protein stain. Bands of interest were excised, reduced, and alkylated with iodoacetamide, and chymotrypsin-trypsin double digests were prepared. The digests were desalted using C18 reversed phase zip tips (Millipore Corporation, Bedford, MA), spotted on MALDI-TOF plates, and subjected to peptide mass fingerprinting-MS.
MS was performed using the model 4700 proteomics analyzer from Applied Biosystems (Foster City, CA). It employs MALDI in conjunction with tandem TOF mass analyzers. The digest was introduced into the instrument in a crystalline matrix of α-cyano-4-hydroxycinnamic acid with diammonium citrate as the additive (final concentration, 2 mM). Database searches were performed with Applied Biosystems GPS explorer software, which uses the Mascot search engine. Protein assignments were made on the basis of MS and MS-MS spectra. NCBInr (November 2004) was used for protein identification.
In vivo kinase assay.
p21Cip1 or its mutants were coexpressed with ASK1 or JNK1 in 293T cells. Twenty-four hours after transfection, the cells were washed in phosphate-free DMEM medium and labeled overnight with [32P]orthophosphate in the phosphate-free DMEM medium. AN (10 μM) was added 3 h before harvest of the cells. The JNK inhibitor SP600125 (10 μM) was added 10 min before harvesting the cells. Cells were lysed in CHAPS buffer and immunoprecipitated with p21Cip1 antibody. Phosphorylated p21Cip1 was quantitated as described above. The same membrane was probed using anti-p21Cip1 antibody.
Plasmid construction.
p21Cip1 expression vector pMT/6CB+/p21 has been described previously (25). pcDNA/ASK1-WT-HA and pcDNA/ASK1-KM-HA express WT and kinase-dead ASK1, respectively. All the new p21Cip1 plasmids were constructed in the Flag-tagged vector pCMV-Tag4A. Full-length p21Cip1 and ΔNLS p21Cip1 were generated by inserting the fragment into pCMV-Tag4A at BamHI and EcoRI sites, yielding pCMV-Tag4A164 and pCMV-Tag4A140, respectively. All the point mutations and small deletions were made using the QuikChange mutagenesis kit (Stratagene). The ASK1 cDNA was cloned into pMH, a mammalian expression vector (SacII/NotI site), and expressed with an HA epitope tag. The point mutations of ASK1 were constructed based on pcDNA/ASK1-WT-HA, and all mutations were confirmed by restriction enzyme digestion and DNA sequencing. The primers used in the constructions are listed in Table S1 in the supplemental material.
RESULTS
The kinase domain of ASK1 is essential for its association with p21Cip1.
Previously, we demonstrated that rapamycin, a specific inhibitor of mTOR, induced apoptosis under conditions of serum-free growth only in cells lacking functional p53. In contrast, rapamycin induced cytostasis in fibroblasts with functional p53, or in p53−/− embryo fibroblasts where p53 had been reexpressed (24). Rapamycin-induced apoptosis was associated with a prolonged stress response resulting from activation of ASK1 and leading to increased phospho-c-Jun (p-c-Jun). In contrast to the apoptosis induced by rapamycin in cells lacking p53, inhibition of mTOR resulted in a transient elevation of p-c-Jun in cells with functional p53 or in which p21Cip1 was overexpressed in the absence of p53. In these cells, p21Cip1 was found to associate with ASK1 and inhibit its kinase activity (25). However, the mechanism by which p21Cip1 interacts with ASK1 and the signaling pathway(s) involved remain to be elucidated.
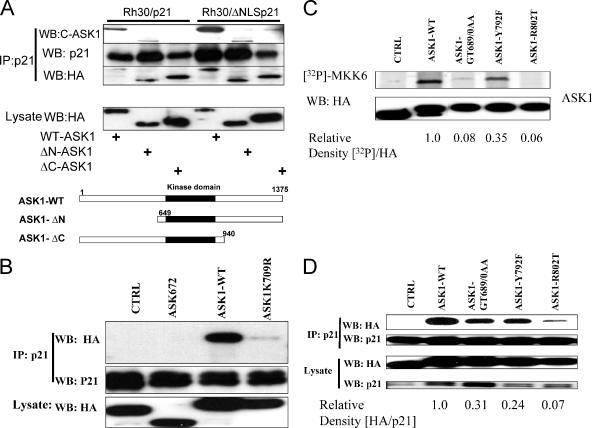
Our first objective was to determine which domain(s) of ASK1 plays a role in association with p21Cip1. Rh30/p21 or Rh30/ΔNLSp21 cells, engineered to stably express full-length p21 or ΔNLSp21 (amino acids [aa] 1 to 140) lacking the nuclear localization sequence under a metallothionein promoter (25) were infected with replication-deficient adenovirus expressing WT, HA-tagged ASK1, or mutants with deletions in the N or C terminus. Adenovirus-infected cells were induced with Zn for 24 h to allow the expression of p21Cip1 and ΔNLS p21Cip1. The results (Fig. 1A) show that both full-length p21Cip1 and ΔNLS p21Cip1 immunoprecipitated with ASK1 and ASK1 peptides lacking either the N or C terminus, as determined by anti-HA or by using a monoclonal antibody against a C-terminal epitope (aa 1076 to 1375) of ASK1. These results indicate that neither the N terminus nor the C terminus was involved in p21Cip1 association but suggest that the overlapping region, the ASK1 kinase domain (aa 678 to 936), may be involved in association of ASK1 with p21Cip1. To further define the region of ASK1 that interacts with p21Cip1, we generated plasmids that would encode small regions of the N- or C-terminal regions of ASK1 as well as point mutants in the kinase domain. Most of these ASK1 deletion constructs failed to be expressed at a detectable level in Rh30 or HEK293T cells; however, as shown in Fig. 1B, the N-terminal fragment of ASK1 (aa 1 to 672), which is readily detected, fails to bind p21Cip1. In addition, the K709R mutation that abrogates ASK1 kinase activity substantially abolished their association (Fig. 1B). This ASK1-K709R mutant lacks kinase activity and has dominant-negative activity (26). We wondered, therefore, whether binding of p21Cip1 to ASK1 was disrupted by this mutation or whether ASK1 kinase activity was required for association.
FIG. 1.
The ASK1 kinase domain is involved in its association with p21Cip1. (A) Rh30/p21 or Rh30/ΔNLSp21 cells were infected by adenoviral vectors that express HA-tagged full-length (WT-ASK1) or epitope-tagged constructs with N- or C-terminal deletions (ΔN-ASK1, ΔC-ASK1). Cell lysates were immunoprecipated (IP) with anti-p21 antibody and immunoblotted (WB) with either a monoclonal antibody recognizing a C-terminal epitope (C-ASK1) or anti-HA or anti-p21 antibody. Expression of ASK1 and deletion mutants in whole-cell lysates is shown in the bottom panel. (B) 293T cells were transfected with the HA-tagged WT (ASK1-WT), the kinase-dead mutant (ASK1K709R), or a plasmid encoding the N-terminal 672 aa (ASK672) and a plasmid encoding ΔNLSp21. Cell lysates were immunoprecipated with anti-p21 antibody and probed with anti-HA antibody. Control cells (CTRL) were tranfected with the ΔNLSp21 plasmid and HA-tagged β-galactosidase. (C) Mutations that inhibit ASK1 catalytic activity also reduce ASK1 association with p21. HA-tagged WT or mutant ASK1 constructs were expressed in 293T cells. Lysates were immunoprecipitated using anti-HA antibody, and ASK1 kinase activity was determined in vitro using the MKK6 peptide as a substrate. (D) 293T cells were cotransfected with the ΔNLSp21 plasmid together with plasmids encoding HA-tagged WT or mutant ASK1. The association of ASK1 with p21Cip1 was determined as described above.
To distinguish between these possibilities we generated several ASK1 kinase domain point or double point mutations and expressed these in 293T cells. In vitro kinase assays for ASK1 activity showed that the GT689/690AA mutant had markedly decreased kinase activity, whereas no activity was detected for the R802T mutant (Fig. 1C). Mutation at the ATP binding site (GT689/690AA) or at the catalytic site (R802T) of ASK1 essentially abolished its association with p21Cip1 (Fig. 1D). In contrast, the Y792F mutant retained some kinase activity (∼35% relative to ASK1-WT), consistent with decreased p21Cip1 binding activity (∼24% binding relative to ASK1-WT). Thus, ASK1 mutants with diminished kinase activity appear to bind less readily to p21Cip1, suggesting that ASK1 activity is important in its association with p21Cip1.
The JNK inhibitor SP600125 disrupts the association of ASK1 and p21Cip1.
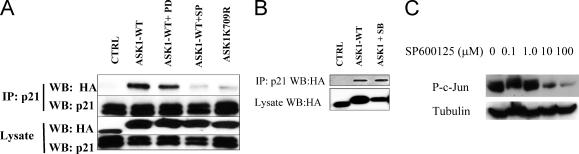
ASK1 is a MAPKKK and phosphorylates and activates a subgroup of MAPKK, such as MKK3/6, and MKK4/7 kinases upstream of p38MAPK and JNK, respectively (8, 19, 27, 55). We hypothesized that either ASK1 or a kinase downstream modified p21Cip1 and that this modification mediated the interaction of p21Cip1 with ASK1. To test whether JNK or p38 plays any role in the association, we used specific kinase inhibitors in the association experiments. HEK293T cells were transfected with expression plasmids encoding HA-ASK1 and ΔNLS p21Cip1. Cells were exposed to SP600125 (JNK inhibitor) or SB203580 (p38 inhibitor) for 10 min prior to cell harvesting. WT ASK1, but not the K709R mutant, associated with ΔNLS p21Cip1. The JNK inhibitor (Fig. 2A) and not the p38 inhibitor (Fig. 2B) disrupted the association of ASK1 with p21Cip1. As a control we used a CDK4/6 inhibitor (PD332991) which did not affect the ASK1/p21Cip1 interaction (Fig. 2A). As shown in Fig. 2C, SP600125 (10 μM, 10 min) suppressed p-c-Jun. Taken at face value, the results indicate that JNK activation is essential for the possible modification of p21Cip1 and subsequent association with ASK1. However, this assumes the specificity of SP600125 for inhibition of JNK.
FIG. 2.
The JNK1 kinase inhibitor SP600125 disrupts the association of ASK1 and p21Cip1. (A) 293T cells were transfected with HA-ASK1 or dominant-negative ASK1 (ASK1K709R) plasmids together with the ΔNLSp21 expression plasmid, ΔNLSp21, or HA-tagged β-galactosidase (CTRL). After 24 h, cells were exposed to SP600125 (10 μM) or a CDK4/6 inhibitor (PD332991 [PD]; 10 μM) for 10 min before preparation of lysates. Cell lysates were immunoprecipitated using anti-p21 antibody, and membranes were probed with anti-HA and anti-p21 antibodies. Whole-cell lysates were also probed to determine the expression of p21 and HA-ASK1 (bottom panels). (B) Cells were prepared as above but exposed to the p38 inhibitor SB203580 (SB; 10 μM) for 10 min prior to lysis. The results show that SB203580 does not block the association of p21 with ASK1. (C) Cells were exposed to increasing concentrations of SP600125 (10 min), and the levels of phosphorylated c-Jun in cell lysates were determined. β-Tubulin was used to normalize protein loading. IP, immunoprecipitation; WB, Western blotting.
Mutations in p21Cip1 around S98 modulate ASK1 association.
To investigate the possible role of JNK in the p21Cip1/ASK1 association, we generated p21Cip1 mutations (T57A and S130A) at reported JNK phosphorylation sites in the ΔNLSp21 construct (32). However, we did not see any effect of these two mutations on association of these proteins (data not shown). In light of this result, we considered two possibilities: that a kinase other than JNK modulates p21/ASK1 association (e.g., ASK1) or that there is an unknown JNK phosphorylation site(s) in p21Cip1, in addition to T57 and S130, that may be important for p21/ASK1 interactions.
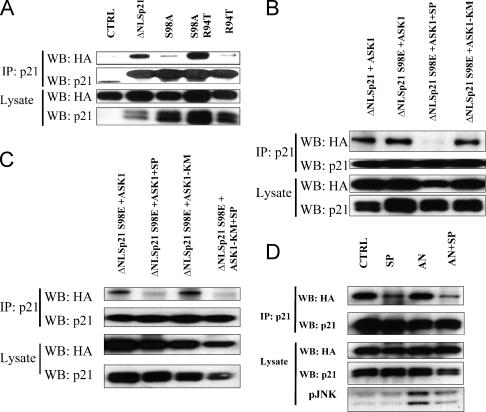
The sequence of p21Cip1 indicates a putative JNK phosphorylation site at S98. Consequently, we generated ΔNLSp21S98A and cotransfected it with HA-ASK1 plasmids into HEK293 cells or cotransfected ΔNLSp21 with HA-β-galactosidase (control). No coimmunoprecipitation between p21 and HA-β-galactosidase was detected, whereas ΔNLSp21 coprecipitated with HA-ASK1 (Fig. 3A, lanes CTRL and ΔNLSp21). The S98A mutation almost completely abolished association with ASK1 (Fig. 3A). Two other p21Cip1 mutants were tested, R94T and a double mutant, S98A/R94T. The R94T mutant also failed to bind ASK1, whereas the double mutant (S98A/R94T) strongly associated with ASK1, suggesting that the domain around S98 may be critical for association of p21Cip1 with ASK1. The S98E mutation, which mimics the phosphorylation of S98, associates with ASK1 and also kinase-dead ASK1. However, somewhat surprisingly, this latter interaction was also abrogated by SP600125 (Fig. 3B). Together these data suggest that phosphorylation of S98 may be important for ASK1 association but also that activation of ASK1 or JNK is necessary, as the interaction between ΔNLS p21S98E and ASK1 was blocked by SP600125. As shown in Fig. 3C, SP600125 also blocked the interaction of p2198E with kinase-dead ASK1. To determine whether JNK activity was required, we used different small interfering RNA constructs in an attempt to down-regulate JNK in 293T cells. However, while JNK2 could be reduced (∼60%), JNK1 was refractory to this approach. Similarly, attempts to use the JNK-interacting peptide (6, 38) did not attenuate phosphorylation of c-Jun. Also, jnk1−/− jnk2−/− double-knockout cells or jnk1−/− cells with conditional regulation of jnk2 (28, 35, 44) were not available. We therefore used a different approach, by asking whether stimulation of JNK increased the binding of p21 with ASK1. 293T cells were transfected with HA-Ask1 together with ΔNLS p21 and after 24 h were stimulated with the JNK activator AN (0.1 μg/ml) with or without SP600125 (10 μM). AN stimulated JNK activity (Fig. 3D), but this did not increase the binding of ASK1 and p21. SP600125 reduced binding both in the absence and presence of AN, even when JNK activation was greater in cells treated with AN and SP600125. Thus, SP600125 inhibited binding of p21 to ASK1 at concentrations that did not completely inhibit AN-induced activation of JNKs.
FIG. 3.
Mutations of p21Cip1 around S98 modulate association with ASK1. (A) 293T cells were cotransfected with HA-ASK1 and p21-empty vector (CTRL) or with HA-ASK1 and plasmids encoding ΔNLSp21, ΔNLSp21S98A (S98A), ΔNLSp21R94T (R94T), or a mutant with both mutations (S98A R94T). After 24 h, cell lysates were prepared and immunoprecipitated with anti-p21 antibody, and membranes were probed with antibodies against HA and p21. Whole-cell lysates were probed with anti-p21 and anti-HA antibodies to determine expression levels following transfection (bottom panels). (B) The phosphomimetic p21S98E mutant associates with kinase-dead ASK1 but is blocked by SP600125. 293T cells were transfected with plasmids encoding ΔNLSp21 or ΔNLSp21S98E or WT HA-ASK1 or a kinase-dead dominant-negative mutant (ASK1-KM). After 24 h, SP600125 (SP; 10 μM) was added, and after an additional 10 min lysates were prepared and processed as above. (C) 293T cells were transfected with HA-ASK1 or dominant-negative ASK1 (K709R) plasmids together with the NLSp21S98E mutant expression plasmid. After 24 h of transfection, cells were exposed to SP600125 (10 μM) for 10 min before preparation of lysates. Cell lysates were immunoprecipitated using anti-p21 antibody, and membranes were probed with anti-HA and anti-p21 antibodies. Whole-cell lysates were also probed to determine the expression of p21 and HA-ASK1 (bottom panels). (D) 293T cells were transfected with HA-ASK1 plasmids together with the NLSp21 expression plasmid. After 24 h of transfection, cells were exposed to SP600125 (10 μM), the JNK activator AN (0.1 μg/ml), or SP600125 (10 μM) in combination with AN (0.1 μg/ml) for 10 min before preparation of lysates. Cell lysates were immunoprecipitated using anti-p21 antibody, and membranes were probed with anti-HA and anti-p21 antibodies. Whole-cell lysates were also probed to determine the expression of p21 and HA-ASK1 (bottom panels). IP, immunoprecipitation; WB, Western blotting.
ASK1 phosphorylates p21Cip1 in vitro.
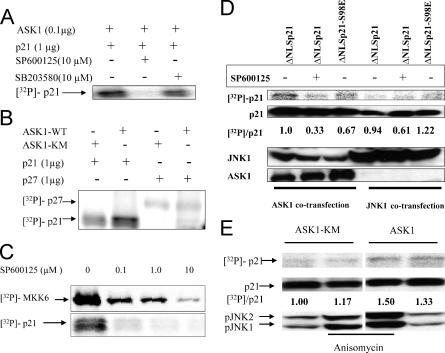
While ASK1 has not been reported to phosphorylate p21Cip1, we found that p21Cip1 can be phosphorylated in vitro by recombinant ASK1 kinase (Fig. 4A). Interestingly, this phosphorylation was inhibited by the JNK-specific inhibitor SP600125 but was not significantly inhibited by the p38 inhibitor SB203580. ASK1 immunoprecipitated from HEK293T cell lysates also phosphorylated recombinant p21Cip1 in vitro, whereas lysates from cells transfected with ASK-KM, a dominant-negative kinase-inactive mutant, had far less activity. As a control we used recombinant p27Kip1, which is not phosphorylated by ASK1 (Fig. 4B). In vitro SP600125 potently inhibited phosphorylation of both MKK6 substrate and p21Cip1 by recombinant ASK1 at concentrations as low as 0.1 μM (Fig. 4C). Thus, the effect of SP600125 inhibiting ASK1/p21Cip1 binding in vivo could be through inhibition of either ASK1 or JNK.
FIG. 4.
Ask1 phosphorylates p21Cip1 in vitro and in vivo. (A) Bacterially expressed purified p21Cip1 was incubated with recombinant ASK1 in the presence of [γ-32P]ATP with or without addition of SP600125 (10 μM) or SB203580 (10 μM). In vitro kinase assays were carried out as described in Materials and Methods. (B) 293T cells were transfected with plasmids encoding HA-ASK1 or the epitope-tagged kinase-dead mutant ASK-KM. After 24 h, cell lysates were prepared and immunoprecipitated using anti-ASK1 antibody. Immunoprecipitates were used in vitro to phosphorylate either purified p21 or purified p27Kip1. (C). The JNK inhibitor SP600125 potently inhibits ASK1-mediated in vitro phosphorylation of MKK6 or p21Cip1. Recombinant ASK1 was incubated with purified Mal-E-MKK6 peptide, a known substrate, or purified p21Cip1 in the presence of [γ-32P]ATP and increasing concentrations of SP600125 as described in Materials and Methods. (D) ASK1 can phosphorylate p21çip1 in vivo. 293T cells were transfected with plasmids encoding ΔNLSp21 or its mutant S98E and cotransfected with ASK1 or JNK1 expression plasmids. The JNK inhibitor SP600125 was added 10 min before harvesting of cells. Cell lysates were immunoprecipated using anti-p21Cip1 antibody, and phosphorylated p21Cip1 was detected using a PhosphorImager. The same membrane was blotted using antibodies against p21Cip1. Levels of ASK1 and JNK1 were determined on whole-cell lysates (bottom panel). Relative activities for phosphorylation of ΔNLSp21 were calculated from the ratio of optical densities of the 32P-p21Cip1/p21Cip1 Western blot signals and normalized to that for ΔNLSp21. (E) ASK1 or ASK1-KM expression plasmids were cotransfected together with the ΔNLSp21 plasmid into 293T cells. After 24 h, cells were either not stimulated or stimulated with AN (10 μg/ml), a known activator of JNK1 and -2, in the presence of [32P]orthophosphate. After 3 h, p21Cip1 was immunoprecipitated, and phosphorylated p21 was detected using a PhosphorImager. The membrane was reprobed using anti-p21 antibody. The lower panels show levels of pJNK1, and the level of pJNK2 in whole-cell lysates was determined using anti-phospho-JNK antibody. Relative activities for phosphorylation of ΔNLSp21 were calculated from the ratio of optical densities of the 32P-p21Cip1/p21Cip1 Western blot signals.
ASK1 phosphorylates ΔNLS p21 in vivo.
To determine whether in vivo S98 of ΔNLSp21 can be phosphorylated, HEK293T cells were transiently transfected with ASK1 or JNK1 plasmids and coexpressed with ΔNLS p21Cip1 WT or the p21S98E mutant. Twenty-four hours after transfection, cells were washed with phosphate-free medium and incubated with [32P]orthophosphate in phosphate-free medium overnight. Cell lysates were immunoprecipitated with p21Cip1 polyclonal antibody, and incorporated radiolabeled material in p21Cip1 was quantified (density of the 32P image/p21 Western blot image). As shown in Fig. 4D, p21Cip1 is phosphorylated by ASK1 in vivo, although the signal is quite low. There is some reduction in phosphorylation of the p21S98E mutant by ASK1, indicating that S98 may be a predominant but not exclusive phosphorylation site for ASK1 in vivo. Very little ΔNLSp21Cip1 was phosphorylated in cells overexpressing JNK1.
To further test whether JNK phosphorylates p21Cip1 in vivo, we expressed the WT ASK1 or dominant-negative ASK1-KM mutant (K709R) together with ΔNLS p21Cip1 in 293T cells. Thus, where the dominant-negative ASK-KM mutant is expressed, only JNK and not ASK1 is activated when cells are treated with AN. As shown in Fig. 4E, AN potently activates JNK isoenzymes. However, there was no increase of phosphorylation on p21Cip1, indicating that JNK may not play a role in phosphorylation of p21Cip1. This is also consistent with other results, where we did not find any detectable association of JNK with p21Cip1 in coprecipitation assays (data not shown). In contrast, p21Cip1 was phosphorylated, albeit at a low level, in cells transfected with ASK1 irrespective of AN treatment.
Rapamycin induces endogenous ASK1-p21Cip1 interaction.
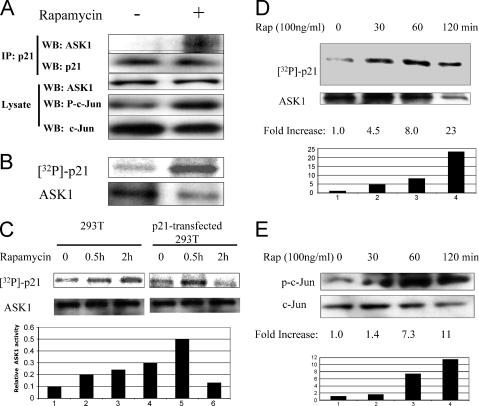
Previously, we demonstrated that rapamycin activated ASK1 in rhabdomyosarcoma cells and that this resulted in association with p21Cip1 when overexpressed. To determine whether rapamycin induced endogenous ASK1 activation and association with endogenous p21Cip1, we treated 293T cells with rapamycin for 1 h and immunoprecipitated endogenous p21Cip1. Although levels of p21Cip1 are very low in these cells, ASK1 coprecipitated only in the presence of rapamycin. Consistent with previous studies, rapamycin induced phosphorylation of c-Jun (Fig. 5A). Rapamycin also activated ASK1, as determined by in vitro kinase assay using recombinant p21Cip1 as a substrate (Fig. 5B). Consistent with previously collected data (24), rapamycin (10 ng/ml) also induced prolonged activation of ASK1 in 293T cells which have low p21Cip1 but induced only transient activation in these cells when transfected with full-length p21Cip1 plasmid (Fig. 5C). The latter result is consistent with our previous observation of suppression of the rapamycin-induced stress response (p-c-Jun) in cells with high p21ip1 expression (24). Further, as shown in Fig. 5D, rapamycin induces prolonged activation of ASK1 in p21−/− mouse embryo fibroblasts (MEFs). p21−/− MEFs were treated with rapamycin for up to 2 h, and ASK1 was immunoprecipitated and used in an in vitro kinase reaction with recombinant p21. These results are consistent with previous studies showing that prolonged activation of ASK1 induced by rapamycin occurred only in cells with mutated p53 or deficient in p21cip1 (24). Consistent with ASK1 activation, phosphorylation of c-Jun was increased by rapamycin treatment (Fig. 5E).
FIG. 5.
Rapamycin treatment induces ASK1 activation and binding of p21Cip1. (A) 293T cells were treated with rapamycin (100 ng/ml) for 1 h, and lysates were prepared, immunoprecipitated (IP) (anti-p21 antibody), and processed as described in Materials and Methods. In the presence of rapamycin, ASK1 coimmunoprecipitated with p21Cip1, and rapamycin also induced phosphorylation of c-Jun. WB, Western blotting. (B) 293T cells were treated with rapamycin (100 ng/ml) for 1 h, and ASK1 was immunoprecipitated and incubated with recombinant p21Cip1 in the presence of [γ-32P]ATP. In vitro kinase assays were carried out as described in Materials and Methods. (C) 293T cells (left) or cells transfected with the p21 plasmid (right) were exposed to rapamycin (10 ng/ml) for up to 2 h. ASK1 was immunoprecipitated, and the activity in phosphorylating recombinant p21Cip1 was assessed in vitro. ASK1 activity relative to the control is quantitated in the histogram. (D) Rapamycin (Rap) induces prolonged ASK1 activation in the absence of p21. p21 null MEFs were treated with rapamycin (100 ng/ml) for various lengths of time, from 0 to 2 h, in 10% fetal calf serum medium. Cell lysates were immunoprecipitated with ASK1 antibody. Bacterially expressed purified p21 as ASK1 kinase substrate was incubated with immunoprecipitated ASK1 in the presence of [32P]ATP. In vitro kinase assays were carried out as described in Materials and Methods. (E) The activation of ASK1 was also confirmed by the increased phosphorylation of c-Jun by rapamycin treatment. The same cell lysate was subjected to Western blotting by p-c-Jun and c-Jun.
Determination of p21Cip1 phosphorylation at S98 by MS analysis.
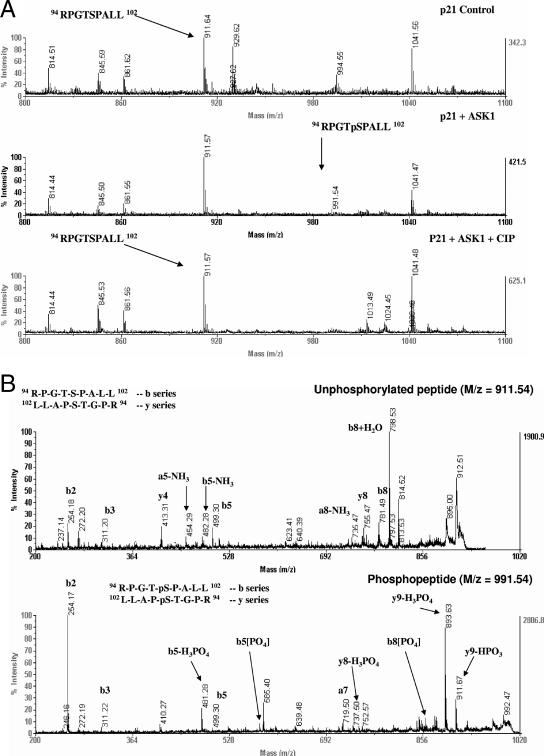
Phosphorylation of p21Cip1 at S98 was investigated by MS characterization of chymotrypsin-trypsin peptides derived from the kinase-treated recombinant p21Cip1 protein. Phosphorylation at S98 was confirmed based on the following: an ion signal of m/z 911.64 was observed (Fig. 6A) which corresponds (within experimental error) to the m/z of 911.53 calculated for the protonated molecular ion of the unphosphorylated peptide of sequence RPGTSPALL (aa 94 to 102). Furthermore, an ion of m/z 991.54 was observed only in the ASK1 kinase-treated p21Cip1 protein sample. This corresponds, within experimental error, to the m/z of 991.49 calculated for the protonated molecular ion of the phosphorylated peptide of sequence RPGTSpPALL. The identity and phosphorylation state of the ion were confirmed by tandem MS (MS-MS). Postsource decay yielded fragments showing the loss of 80 and 98 Da from the intact peptide, corresponding to the loss of HPO3 and H3PO4, respectively, and sequence ions consistent with a peptide of the expected sequence, phosphorylated at the serine residue in the fifth position (S98), were also observed (Fig. 6B). Similar results were obtained in p21Cip1 samples incubated with JNK1 (data not shown).
FIG. 6.
Determination of phosphorylation of p21(S98) by MS. Phosphorylation of S98 in p21Cip1 protein was identified by MS characterization of chymotrypsin-trypsin peptides derived from the ASK1-treated recombinant p21Cip1 protein. MS and MS-MS spectra were obtained on a model 4700 MALDI-tandem TOF spectrometer. MS (A) and MS-MS (B) spectra derived from the singly charged unphosphorylated tryptic peptide RPGTSPALL (panel A, upper spectrum) and the phosphorylated tryptic peptide RPGTpSPALL (panel A, center spectrum) are shown. (B) The amino acid sequence from the peptide could be identified from the b and y ion fragment peaks.
Association of ASK1 with p21Cip1 inactivates ASK1 kinase activity.
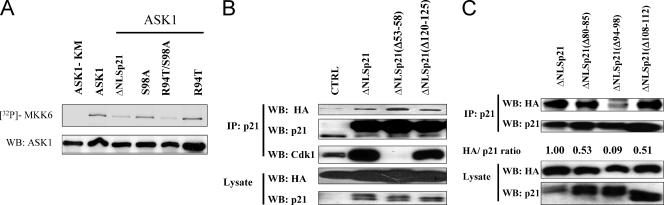
Our results suggest that phosphorylation of p21Cip1 by ASK1, probably at S98, appears to mediate association with ASK1; however, the biological significance of this association is not well understood. To determine whether association of p21Cip1 with ASK1 inhibited its kinase function, HEK293T cells were transfected with ASK1 and ΔNLSp21 WT or mutant plasmids or ASK1 plasmid alone as a control. Twenty-four hours after transfection, cell lysates were prepared and incubated with anti-HA antibody to precipitate ASK1. In vitro kinase assays were conducted using recombinant MKK6 as a substrate in the presence of [γ-32P]ATP. After detection of phosphorylation of MKK6, membranes were probed with ASK1 antibody. Expression of ΔNLSp21 or the R94T/S98A double mutant resulted in decreased ASK1 activity (Fig. 7A). In contrast, expression of ΔNLSp21 S98A and R94T mutants did not alter the ASK1 kinase activity. These data are consistent with the coimmunoprecipitation results, in which S98A and R94T mutants did not associate with ASK1 and did not inhibit ASK1 kinase activity, while the double mutant R94T/S98A, which restores the association with ASK1, also restores the ability to inactivate ASK1 kinase activity.
FIG. 7.
The association of ASK1 with p21Cip1 inactivates ASK1 kinase activity and requires a domain (aa 94 to 98) for association. (A) 293T cells were transfected with epitope-tagged ASK1 or ASK1-KM alone or ASK1 cotransfected with ΔNLSp21, ΔNLSp21S98A, ΔNLSp211R94T, or the double mutant. Cell lysates were immunoprecipated using anti-HA antibody, and precipitated samples were used for in vitro kinase assays with purified MKK6 as the substrate. The phosphorylation of MKK6 was detected using a PhosphorImager. The same membrane was probed using anti-HA antibody. WB, Western blotting. (B) 293T cells were either mock transfected (CTRL) or cotransfected with HA-ASK1 and WT ΔNLSp21 or ΔNLSp21 deletion mutants (Δ53-58, lacking the cdk interaction domain) or a control mutant (Δ120-125). After 24 h, cell lysates were prepared, and p21 was immunoprecipitated (IP). Membranes were probed with antibodies against p21, Cdk1, and HA. The lower panel shows levels of HA-ASK1 and p21 in whole-cell lysates. (C) Identification of the region of p21 involved in association with ASK1. 293T cells were cotransfected with HA-ASK1 and ΔNLSp21 or ΔNLSp21 deletion mutants (Δ80-85, Δ94-98, or Δ108-112). After 24 h, cell lysates were prepared, and p21 was immunoprecipitated. Membranes were probed with anti-p21 and anti-HA antibodies. The lower panel shows levels of HA-ASK1 and p21 in whole-cell lysates. The association of ASK1 with ΔNLSp21 or mutants was derived from the ratio of HA to p21 signal in the Western analyses and normalized to the ratio of ASK1 to ΔNLSp21.
A region of p21Cip1 (aa 94 to 98) modulates its interaction with ASK1.
p21Cip1 binds to several proteins, including CDKs and cyclins as well as PCNA (22, 58, 63), through well-defined domains. We were interested, therefore, in identifying such a domain of p21Cip1 that mediates its association with ASK1. The kinase domain of ASK1 has significant homology to the kinase domains of cdk1 and cdk2. The domain of p21Cip1 that interacts with cdk's maps to amino acid residues 49 to 80 (61). To determine whether this region also plays a role in association with ASK1, we generated a ΔNLSp21 mutant expressing a peptide with a small deletion at positions 53 to 58 (p21Δ53/8). As a control we expressed ΔNLSp21Δ120/5, a deletion mutant lacking residues 120 to 125. The results (Fig. 7B) show that both deletion mutants still interact with ASK1, while p21Δ53/8 does not associate with Cdk1. This indicates that different regions of p21Cip1 are involved in interaction with cdk's and ASK1. We hypothesized that a region around residues 94 to 98 may be involved in association of p21Cip1 with ASK1. To test this idea we generated deletion mutants (Δ80-85, Δ94-98, and Δ108-112) around this region. The results show that the Δ94-98 peptide did not coprecipitate with ASK1, whereas the other two deletions did not effect association with ASK1 (Fig. 7C). Therefore, a region (aa 94 to 98) of p21Cip1 appears to be essential for ASK1 association.
DISCUSSION
The multiple functions of p21Cip1 are determined by protein partners that form specific protein complexes and by its own subcellular localization. Nuclear p21Cip1 functions to inhibit cell cycle progression, while cytoplasmic p21Cip1 appears to prevent apoptosis. However, the mechanisms by which p21Cip1 can prevent cells from undergoing apoptosis and promote cell survival are not well understood. Here, we provide data to demonstrate that p21Cip1 interacts with ASK1 and down-regulates its kinase activity, thereby attenuating the stress response induced by inhibition of mTOR signaling by rapamycin. Our results indicate that the interaction of p21Cip1 with ASK1 is mediated by a small region of p21Cip1 (residues 94 to 98) that includes S98 and that is phosphorylated by both Ask1 and JNK in vitro. Phosphorylation of p21Cip1 at S98, which in vivo appears to be regulated by ASK1, may therefore mediate negative feedback in the ASK1 signaling pathway.
The C terminus of p21Cip1 (residues 141 to 164) has several important functions (57, 58); this region contains the NLS, and hence the ΔNLS p21Cip1 (aa 1 to 140) is predominantly cytoplasmic (25, 45). We found that ΔNLS p21Cip1 has increased association with ASK1 compared to wild-type full-length p21Cip1. Since ASK1 is localized in the cytoplasm (2), it is reasonable that ΔNLS p21Cip1 has greater opportunity to interact with ASK1. Furthermore, the ΔNLS p21Cip1 lacks the site that binds the C8 α-subunit of the 20S proteasome and hence is more stable than the full-length p21Cip1. This may be another reason why ΔNLS p21Cip1 shows increased association with ASK1.
As a cell cycle regulator, p21Cip1 has been shown to associate with CDKs (22) (63) through their kinase domains. The high similarity between CDK1, CDK2, and ASK1 kinase domains (over 40% alignment) suggests that ASK1 may also use its kinase domain to interact with p21Cip1. Our data do demonstrate that the kinase domain of ASK1 is involved in its interaction with p21Cip1. Since p21Cip1 has a very flexible structure and performs a variety of functions (34), it is possible that p21Cip1 interacts with other kinases via their similar kinase domains, affecting their biological function. For example, it has been shown that p21Cip1 associates with JNK and affects its kinase activity (64). p21Cip1 also forms a complex with Rho kinase and inhibits its activity in vivo and in vitro (51).
We observed that not only the kinase domain per se but also the kinase activity of ASK1 is required for its association with p21Cip1. This suggests that ASK1 or a kinase(s) downstream modifies p21Cip1 and that the modification is necessary for p21Cip1-ASK1 association. It is known that ASK1 can activate JNK and p38 (8, 27, 55). To determine which kinase(s) is essential for association of p21Cip1 with ASK1, we examined their association in the presence of JNK and p38 inhibitors. We found that the JNK inhibitor SP600125 almost completely inhibited p21Cip1 binding to ASK1. The immediate suggestion from these data is that JNK modifies p21Cip1 and leads to its association with ASK1. SP600125 is a reversible ATP competitive inhibitor with more than 20-fold greater selectivity for JNK than a range of kinases and other enzymes (4). However, our data show that SP600125 can also potently inhibit recombinant ASK1 activity both in vitro with MKK6 or p21Cip1 as a substrate and also in vivo. Consequently, phosphorylation of p21Cip1 by ASK1 may also mediate the association of these proteins.
ASK1 is a member of the MAPKKK family, and its normal substrate is a subgroup of MAPKKs. The present study represents, to our knowledge, the first time that a MAPKKK/ASK1 has been shown to phosphorylate p21Cip1. In contrast, we did not observe phosphorylation of recombinant p27Kip1 in vitro. Using purified p21Cip1 as a substrate in the in vitro kinase assay, we found several 32P-labeled bands, perhaps indicating that there is more than one ASK1 phosphorylation site besides S98. Furthermore, rapamycin treatment activated ASK1, which phosphorylated p21Cip1 in vitro. Using MS we identified S98 phosphorylation but failed to identify additional sites after in vitro kinase reactions with recombinant ASK1 or JNK1. Several kinases have been reported to phosphorylate p21Cip1 and mediate important biological processes. For example, phosphorylation of T145 by AKT leads to cytoplasmic localization and switches p21Cip1 function from cell cycle inhibition to protection from apoptosis (65).
A mutant that mimics phosphorylation at S98, p21S98E, associates with ASK1 in coimmunoprecipitation assays; however, it was unexpected that the JNK inhibitor SP600125 would also disrupt this association. It is intriguing that a relatively short exposure to SP600125 results in dissociation of p21Cip from ASK1 that may be a consequence of inhibitor-induced conformational change. This may account for the observation that SP600125 blocked the association of the p21R94T/S98A mutant with ASK1 and similarly blocked association of the p21S98E mutant with kinase-dead ASK1.
Even though in vitro JNK1 can phosphorylate p21Cip1 at S98, our data do not support JNKs as the predominant in vivo kinases. Overexpression of JNK1, or activation by AN, failed to increase p21Cip1 phosphorylation in vivo. Furthermore, SP600125 inhibited p21-ASK1 association at concentrations that did not completely block AN-induced activation of JNK. To unambiguously define whether JNK played a role in p21S98 phosphorylation in vivo, we attempted to down-regulate JNK1/2 using small interfering RNA or block JNK activation using a peptide that prevents JNK from binding to JNK-interacting peptide (6, 38), but we were unable to modify JKN activity in either 293T or 3T3 cells. jnk1−/− jnk2−/− double-knockout (35, 44) or jnk1−/− cells with conditional jnk2 (28) were not available for these experiments. Single mutations at other JNK consensus sites (T57, S130) did not decrease p21Cip1-ASK1 interactions (data not shown). However, a mutation that blocks phosphorylation of S98 (S98A) severely attenuates binding of p21Cip1 to ASK1. These data, together with those for the S98E mutant, strongly implicate phosphorylation of p21S98 as being important for binding of WT p21 to ASK1.
Rather surprisingly, the p21R94T mutation restored the ability of the S98A mutant to bind ASK1. This suggested that a region around residues 94 to 98 could be important in this association. To narrow the domain of p21Cip1 involved in the interaction with ASK1, we produced a series of p21Cip1 mutants with small deletions (Fig. 6). As a positive control we introduced deletions into the cdk binding domain (Δ53-58) and showed that it failed to associate with Cdk1 in coprecipitation assays. Deletion of a small sequence around S98 (Δ94-98) significantly (>90%) decreased binding to ASK1, whereas other deletions (Δ80-85, Δ108-112) had no observable effect.
Our results suggest that in vivo ASK1 phosphorylates p21Cip1 at S98 and that this phosphorylation is in part necessary for its association with ASK1. However, the finding that the p21R94T/S98A binds strongly to ASK1 suggests additional complexities, and further experimentation will be required to fully elucidate the molecular detail of association of these proteins. We next questioned the biological significance of p21Cip1-ASK1 association. We found that binding of p21Cip1 inactivates ASK1 kinase activity. In contrast, ASK1 remained active in rapamycin-treated p21Cip1 null MEFs. These data are consistent with our previous finding that suppression of rapamycin-induced activation of ASK1 correlated with p21Cip1 binding (25). The details of the mechanism by which p21Cip1 inactivates ASK1 kinase activity are not completely understood. Possibly, p21Cip1 may mask the kinase domain and prevent its activation of downstream kinases, such as MKK3/6 or MKK4/7. We observed that only a relatively small fraction of ASK1 associated with p21Cip1. We have shown previously (26) that rapamycin treatment leads to a rapid suppression of the activity of protein phosphatase 5 (PP5) that is physically associated with ASK1 and dissociation of the PP2A" regulatory subunit (PR72) from the ASK1 complex. One possibility is that p21Cip1 stabilizes this complex, maintaining PP5 activity, and negatively regulates phosphorylation of T838 and T845 of ASK1 and its kinase activity. Alternatively, p21Cip1 binding to ASK1 may recruit another PP. This possibility is under investigation.
The role of p21Cip1 in attenuating apoptosis induced by various stimuli is well established. Results from many studies suggest that protection of cells is a consequence of cell cycle arrest, potentially allowing for damage repair in the case of DNA-damaging stimuli. Interestingly, rapamycin, a specific inhibitor of mTOR, in some cells blocks the translation of p21Cip1 subsequent to DNA damage induced by agents such as cisplatin, selectively potentiating cytotoxicity in cells with functional p53/p21Cip1 (5, 64). Our previous studies using cell lines with mutations of p53 showed that inhibition of mTOR resulted in a rapid and sustained activation of ASK1 and JNK, increased p-c-Jun, and apoptosis. In cells expressing WT p53 this activation was rapidly suppressed, and suppression was p21Cip1 dependent. Thus, p21Cip1 may protect cells from DNA damage by inducing cell cycle arrest and by suppressing stress responses caused by nutritional deprivation: for example, amino acid starvation that is mimicked by rapamycin treatment.
Supplementary Material
Acknowledgments
Limin Xiao is acknowledged for assistance preparing p21-S98E for these studies.
This work was supported by Public Health Service awards CA77776 (P.J.H.), CA96996 (P.J.H.), and CA82491 (R.W.K.); grant CA21785 (Cancer Center Support Grant) from the National Cancer Institute; and American, Lebanese, Syrian Associated Charities (ALSAC).
Footnotes
Published ahead of print on 26 February 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adachi, S., H. Ito, M. Tamamori-Adachi, Y. Ono, T. Nozato, S. Abe, M. Ikeda, F. Marumo, and M. Hiroe. 2001. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ. Res. 88:408-414. [DOI] [PubMed] [Google Scholar]
- 2.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barboule, N., P. Mazars, V. Baldin, S. Vidal, S. Jozan, P. Martel, and A. Valette. 1995. Expression of p21WAF1/CIP1 is heterogeneous and unrelated to proliferation index in human ovarian carcinoma. Int. J. Cancer 63:611-615. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuvink, I., A. Boulay, S. Fumagalli, F. Zilbermann, S. Ruetz, T. O'Reilly, F. Natt, J. Hall, H. A. Lane, and G. Thomas. 2005. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 120:747-759. [DOI] [PubMed] [Google Scholar]
- 6.Bonny, C., A. Oberson, S. Negri, C. Sauser, and D. F. Schorderet. 2001. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes 50:77-82. [DOI] [PubMed] [Google Scholar]
- 7.Boulaire, J., A. Fotedar, and R. Fotedar. 2000. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol. Biol. (Paris) 48:190-202. [PubMed] [Google Scholar]
- 8.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860-1863. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, T., N. Rodrigues, H. Shen, Y. Yang, D. Dombkowski, M. Sykes, and D. T. Scadden. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287:1804-1808. [DOI] [PubMed] [Google Scholar]
- 10.Coqueret, O., and H. Gascan. 2000. Functional interaction of STAT3 transcription factor with the cell cycle inhibitor p21WAF1/CIP1/SDI1. J. Biol. Chem. 275:18794-18800. [DOI] [PubMed] [Google Scholar]
- 11.Delavaine, L., and N. B. La Thangue. 1999. Control of E2F activity by p21Waf1/Cip1. Oncogene 18:5381-5392. [DOI] [PubMed] [Google Scholar]
- 12.Dotto, G. P. 2000. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim. Biophys. Acta 1471:M43-M56. [DOI] [PubMed] [Google Scholar]
- 13.Erber, R., W. Klein, T. Andl, C. Enders, A. I. Born, C. Conradt, J. Bartek, and F. X. Bosch. 1997. Aberrant p21(CIP1/WAF1) protein accumulation in head-and-neck cancer. Int. J. Cancer 74:383-389. [DOI] [PubMed] [Google Scholar]
- 14.Estanyol, J. M., M. Jaumot, O. Casanovas, A. Rodriguez-Vilarrupla, N. Agell, and O. Bachs. 1999. The protein SET regulates the inhibitory effect of p21(Cip1) on cyclin E-cyclin-dependent kinase 2 activity. J. Biol. Chem. 274:33161-33165. [DOI] [PubMed] [Google Scholar]
- 15.Esteve, V., N. Canela, A. Rodriguez-Vilarrupla, R. Aligue, N. Agell, I. Mingarro, O. Bachs, and E. Perez-Paya. 2003. The structural plasticity of the C terminus of p21Cip1 is a determinant for target protein recognition. Chembiochem 4:863-869. [DOI] [PubMed] [Google Scholar]
- 16.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorospe, M., C. Cirielli, X. Wang, P. Seth, M. C. Capogrossi, and N. J. Holbrook. 1997. p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene 14:929-935. [DOI] [PubMed] [Google Scholar]
- 18.Gorospe, M., X. Wang, K. Z. Guyton, and N. J. Holbrook. 1996. Protective role of p21(Waf1/Cip1) against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol. Cell. Biol. 16:6654-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotoh, Y., and J. A. Cooper. 1998. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 273:17477-17482. [DOI] [PubMed] [Google Scholar]
- 20.Gotz, C., P. Wagner, O. G. Issinger, and M. Montenarh. 1996. p21WAF1/CIP1 interacts with protein kinase CK2. Oncogene 13:391-398. [PubMed] [Google Scholar]
- 21.Gulbis, J. M., Z. Kelman, J. Hurwitz, M. O'Donnell, and J. Kuriyan. 1996. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell 87:297-306. [DOI] [PubMed] [Google Scholar]
- 22.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 23.Harvey, K. J., D. Lukovic, and D. S. Ucker. 2000. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J. Cell Biol. 148:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, S., L. N. Liu, H. Hosoi, M. B. Dilling, T. Shikata, and P. J. Houghton. 2001. p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res. 61:3373-3381. [PubMed] [Google Scholar]
- 25.Huang, S., L. Shu, M. B. Dilling, J. Easton, F. C. Harwood, H. Ichijo, and P. J. Houghton. 2003. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Mol. Cell 11:1491-1501. [DOI] [PubMed] [Google Scholar]
- 26.Huang, S., L. Shu, J. Easton, F. C. Harwood, G. S. Germain, H. Ichijo, and P. J. Houghton. 2004. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J. Biol. Chem. 279:36490-36496. [DOI] [PubMed] [Google Scholar]
- 27.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke, A., M. Karasarides, J. J. Ventura, A. Ehrhardt, C. Zhang, R. A. Flavell, K. M. Shokat, and R. J. Davis. 2006. JNK2 is a positive regulator of the cJun transcription factor. Mol. Cell 23:899-911. [DOI] [PubMed] [Google Scholar]
- 29.Jin, Y. H., K. J. Yoo, Y. H. Lee, and S. K. Lee. 2000. Caspase 3-mediated cleavage of p21WAF1/CIP1 associated with the cyclin A-cyclin-dependent kinase 2 complex is a prerequisite for apoptosis in SK-HEP-1 cells. J. Biol. Chem. 275:30256-30263. [DOI] [PubMed] [Google Scholar]
- 30.Kearsey, J. M., P. J. Coates, A. R. Prescott, E. Warbrick, and P. A. Hall. 1995. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene 11:1675-1683. [PubMed] [Google Scholar]
- 31.Kelman, Z., and M. O'Donnell. 1995. Structural and functional similarities of prokaryotic and eukaryotic DNA polymerase sliding clamps. Nucleic Acids Res. 23:3613-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, G. Y., S. E. Mercer, D. Z. Ewton, Z. Yan, K. Jin, and E. Friedman. 2002. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J. Biol. Chem. 277:29792-29802. [DOI] [PubMed] [Google Scholar]
- 33.Kitaura, H., M. Shinshi, Y. Uchikoshi, T. Ono, S. M. Iguchi-Ariga, and H. Ariga. 2000. Reciprocal regulation via protein-protein interaction between c-Myc and p21(cip1/waf1/sdi1) in DNA replication and transcription. J. Biol. Chem. 275:10477-10483. [DOI] [PubMed] [Google Scholar]
- 34.Kriwacki, R. W., L. Hengst, L. Tennant, S. I. Reed, and P. E. Wright. 1996. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc. Natl. Acad. Sci. USA 93:11504-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuan, C. Y., D. D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667-676. [DOI] [PubMed] [Google Scholar]
- 36.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 37.Lawlor, M. A., and P. Rotwein. 2000. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 20:8983-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, Y. H., J. Giraud, R. J. Davis, and M. F. White. 2003. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278:2898-2902. [DOI] [PubMed] [Google Scholar]
- 39.Levkau, B., H. Koyama, E. W. Raines, B. E. Clurman, B. Herren, K. Orth, J. M. Roberts, and R. Ross. 1998. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell 1:553-563. [DOI] [PubMed] [Google Scholar]
- 40.Liu, H., H. Nishitoh, H. Ichijo, and J. M. Kyriakis. 2000. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 20:2198-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono, T., H. Kitaura, H. Ugai, T. Murata, K. K. Yokoyama, S. M. Iguchi-Ariga, and H. Ariga. 2000. TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase. J. Biol. Chem. 275:31145-31154. [DOI] [PubMed] [Google Scholar]
- 42.Polyak, K., T. Waldman, T. C. He, K. W. Kinzler, and B. Vogelstein. 1996. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 10:1945-1952. [DOI] [PubMed] [Google Scholar]
- 43.Raj, K., P. Ogston, and P. Beard. 2001. Virus-mediated killing of cells that lack p53 activity. Nature 412:914-917. [DOI] [PubMed] [Google Scholar]
- 44.Sabapathy, K., W. Jochum, K. Hochedlinger, L. Chang, M. Karin, and E. F. Wagner. 1999. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev. 89:115-124. [DOI] [PubMed] [Google Scholar]
- 45.Schepers, H., M. Geugien, B. J. Eggen, and E. Vellenga. 2003. Constitutive cytoplasmic localization of p21(Waf1/Cip1) affects the apoptotic process in monocytic leukaemia. Leukemia 17:2113-2121. [DOI] [PubMed] [Google Scholar]
- 46.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 47.Shim, J., H. Lee, J. Park, H. Kim, and E. J. Choi. 1996. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature 381:804-806. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, A., Y. Tsutomi, K. Akahane, T. Araki, and M. Miura. 1998. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 17:931-939. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, A., Y. Tsutomi, M. Miura, and K. Akahane. 1999. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene 18:1239-1244. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki, A., Y. Tsutomi, N. Yamamoto, T. Shibutani, and K. Akahane. 1999. Mitochondrial regulation of cell death: mitochondria are essential for procaspase 3-p21 complex formation to resist Fas-mediated cell death. Mol. Cell. Biol. 19:3842-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka, H., T. Yamashita, M. Asada, S. Mizutani, H. Yoshikawa, and M. Tohyama. 2002. Cytoplasmic p21(Cip1/WAF1) regulates neurite remodeling by inhibiting Rho-kinase activity. J. Cell Biol. 158:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taules, M., A. Rodriguez-Vilarrupla, E. Rius, J. M. Estanyol, O. Casanovas, D. B. Sacks, E. Perez-Paya, O. Bachs, and N. Agell. 1999. Calmodulin binds to p21(Cip1) and is involved in the regulation of its nuclear localization. J. Biol. Chem. 274:24445-24448. [DOI] [PubMed] [Google Scholar]
- 53.Tian, H., E. K. Wittmack, and T. J. Jorgensen. 2000. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 60:679-684. [PubMed] [Google Scholar]
- 54.Timchenko, N. A., T. E. Harris, M. Wilde, T. A. Bilyeu, B. L. Burgess-Beusse, M. J. Finegold, and G. J. Darlington. 1997. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol. Cell. Biol. 17:7353-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobiume, K., T. Inage, K. Takeda, S. Enomoto, K. Miyazono, and H. Ichijo. 1997. Molecular cloning and characterization of the mouse apoptosis signal-regulating kinase 1. Biochem. Biophys. Res. Commun. 239:905-910. [DOI] [PubMed] [Google Scholar]
- 56.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touitou, R., J. Richardson, S. Bose, M. Nakanishi, J. Rivett, and M. J. Allday. 2001. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. EMBO J. 20:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waga, S., G. J. Hannon, D. Beach, and B. Stillman. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574-578. [DOI] [PubMed] [Google Scholar]
- 59.Waldman, T., C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1996. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381:713-716. [DOI] [PubMed] [Google Scholar]
- 60.Waldman, T., Y. Zhang, L. Dillehay, J. Yu, K. Kinzler, B. Vogelstein, and J. Williams. 1997. Cell-cycle arrest versus cell death in cancer therapy. Nat. Med. 3:1034-1036. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Y., I. Filippov, C. Richter, R. Luo, and R. W. Kriwacki. 2005. Solution NMR studies of an intrinsically unstructured protein within a dilute, 75 kDa eukaryotic protein assembly; probing the practical limits for efficiently assigning polypeptide backbone resonances. ChemBioChem 6:2242-2246. [DOI] [PubMed] [Google Scholar]
- 62.Weiss, R. H., A. Joo, and C. Randour. 2000. p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J. Biol. Chem. 275:10285-10290. [DOI] [PubMed] [Google Scholar]
- 63.Xiong, Y., G. J. Hannon, H. Zhang, D. Casso, R. Kobayashi, and D. Beach. 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366:701-704. [DOI] [PubMed] [Google Scholar]
- 64.Xue, Y., N. T. Ramaswamy, X. Hong, and J. C. Pelling. 2003. Association of JNK1 with p21waf1 and p53: modulation of JNK1 activity. Mol. Carcinog. 36:38-44. [DOI] [PubMed] [Google Scholar]
- 65.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.