Abstract
The anaphase-promoting complex/cyclosome (APC/C) is an E3 ubiquitin ligase mediating targeted proteolysis through ubiquitination of protein substrates to control the progression of mitosis. The APC/C recognizes its substrates through two adapter proteins, Cdc20 and Cdh1, which contain similar C-terminal domains composed of seven WD-40 repeats believed to be involved in interacting with their substrates. During the transition from metaphase to anaphase, APC/C-Cdc20 mediates the ubiquitination of securin and cyclin B1, allowing the activation of separase and the onset of anaphase and mitotic exit. APC/C-Cdc20 and APC/C-Cdh1 have overlapping substrates. It is unclear whether they are redundant for mitosis. Using a gene-trapping approach, we have obtained mice which lack Cdc20 function. These mice show failed embryogenesis. The embryos were arrested in metaphase at the two-cell stage with high levels of cyclin B1, indicating an essential role of Cdc20 in mitosis that is not redundant with that of Cdh1. Interestingly, Cdc20 and securin double mutant embryos could not maintain the metaphase arrest, suggesting a role of securin in preventing mitotic exit.
Protein degradation via the ubiquitin and proteasome system is an essential mechanism which ensures the smooth transition between cell cycle phases. In mitosis, the degradation of key regulators is realized through the action of the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase (3, 4, 12, 18, 19). Among the APC/C's substrates are securin and cyclin B1. The degradation of securin allows the activation of separase, a CD clan protease of the caspase family (29). Separase-mediated cleavage of the cohesin-component Scc1 is essential for sister chromatid separation (8, 28). The degradation of cyclin B1 promotes mitotic exit and contributes to the activation of separase in higher eukaryotes as well, where separase is inhibited by Cdk1/cyclin B1 (5, 10, 25). The APC/C recognizes its substrates through two adapter proteins, Cdc20 and Cdh1, which contain similar C-terminal domains composed of seven WD-40 repeats believed to be involved in interacting with their substrates (7, 9, 13, 20, 22). Destruction boxes or KEN boxes are motifs frequently found in APC/C's substrates, but other motifs are also possible for the recognition by APC/C-Cdc20 or APC/C-Cdh1 (7). The association of Cdc20 with the APC/C requires phosphorylation of the latter (14). Several mitotic kinases, including Plk1 and cyclin B1/Cdk1, have been implicated in this phosphorylation. The dependency of Cdc20 binding upon APC/C phosphorylation provides a mechanism to ensure that APC/C-Cdc20 is active only during mitosis. Prior to anaphase, APC/C-Cdc20 activity is restrained by the tumor suppressor RASSF1A (24) and the spindle assembly checkpoint (17, 31).
APC/C-Cdc20 and APC/C-Cdh1 have overlapping as well as distinct substrates. In budding yeast (Saccharomyces cerevisiae), APC/C-Cdc20 is required for the degradation of Pds1 (budding yeast securin) and the B-type cyclin Clb5, whereas APC/C-Cdh1 promotes the degradation of Clb2 (21, 23). Deletion of CDC20 causes cell cycle arrest at metaphase due to the accumulation of Pds1 (15, 23). Deletion of both CDC20 and PDS1 allows the cells to separate their sister chromatids, but the cells cannot exit mitosis due to the presence of Clb5 (15, 23).
It is unclear if CDC20 plays an essential role in mitosis in mammals. Small interfering RNA-mediated knockdown of CDC20 in cultured human cells seems compatible with cellular viability (1), suggesting that it might not be required for the progression of mitosis as in budding yeast or that CDH1 may function to compensate for the reduced expression of CDC20. To address this issue, we analyzed mice derived from a gene trap mouse embryonic stem cell line (26). We report here that the trapped Cdc20 allele is null and homozygous embryos die at the two-cell stage. Further, by breeding into a securin null background, we demonstrated that the loss of securin could not rescue the lethality but causes sister chromatid separation.
MATERIALS AND METHODS
Generation and analysis of Cdc20 gene trap mice.
The gene trap clone, XE368, from BayGenomics, was injected into mouse blastocysts to generate chimeric mice. Breeding of the chimeras with C57BL/6 mice produced animals carrying the trapped allele. We used the following primers for PCR genotyping: primer a (Fig. 1A), 5′-AAGGTGGCTGAGCTCAAAGG-3′; primer b, 5′-GCCTTGGTGGATGAGGCTAC-3′; and primer c, 5′-GTTATCGATCTGCGATCTGC-3′. To visualize the expression of Cdc20, we analyzed the expression of LacZ from the trapped allele. Embryonic day 12.5 (E12.5) embryos were fixed in 0.2% glutaraldehyde-2% formaldehyde-5 mM EDTA-2 mM MgCl2-0.1 M phosphate buffer (pH 7.3) for 1 h, washed three times (30 min each) with washing buffer (0.1% sodium dodecyl sulfate [SDS]-0.2% NP-40-2 mM MgCl2-0.1 M phosphate buffer, pH 7.3), and incubated overnight in the staining solution (1 mg/ml X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]-5 mM potassium ferricyanide-5 mM potassium ferrocyanide in washing buffer). To analyze Cdc20 protein levels, we isolated E10.5 embryos and lysed them in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4-1% NP-40-0.25% sodium deoxycholate-150 mM NaCl-1 mM EDTA-protease inhibitor cocktail [Roche]). Protein concentration was determined by using Bio-Rad protein dye reagent, and 50-μg portions of protein were resolved with SDS-polyacrylamide gel electrophoresis and immunoblotted with anti-Cdc20 antibody (Santa Cruz Biotechnology) followed with enhanced chemiluminescence detection.
FIG. 1.
Generation and analysis of Cdc20 gene trap mice. (A) Diagram of the trapped allele. a, b, and c indicate the positions of the genotyping primers. (B) PCR analysis of two-cell-stage embryos by use of the primers indicated in panel A. (C) Western analysis of E10.5 embryos. Whole-embryo lysates were prepared and separated on SDS-polyacrylamide gel electrophoresis gels. (D) LacZ staining of an E12.5 heterozygous embryo. WT, wild type; Mut, mutant.
Embryo culture and immunostaining.
All embryos were produced through natural mating, and at least three litters of embryos were harvested for each experiment. Two-cell embryos (E1.5) were harvested and cultured in KSOM (CHEMICON) alone or in KSOM supplemented with nocodazole at 100 ng/ml. To genotype preimplantation embryos, we performed two rounds of PCR using the same primer set. For immunostaining, embryos were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.4% Triton X-100 in phosphate-buffered saline (PBS) for 15 min, blocked with 5% goat serum in PBS, incubated with anti-cyclin B1 antibodies (Santa Cruz Biotechnology) or antisecurin antibodies (Novocastra Laboratories) for 2 h at room temperature, and washed with PBS, and the primary antibodies were visualized with Cy3-conjugated goat anti-rabbit secondary antibodies (1:500; Jackson ImmunoResearch Laboratories) for 60 min at room temperature. The embryos were counterstained with DAPI (4′,6′-diamidino-2-phenylindole). The fluorescence image was captured using a Nikon ECLIPSE E800 microscope. The exposure time was set at 100 ms with two-by-two binning for both the mutant and the control embryos.
Time-lapse microscopy.
Two-cell embryos were cultured in KSOM medium until at least one embryo had divided and then transferred into the medium containing 2 ng/ml bis-benzimide H 33342 (Hoechst 33342) (Sigma) at 37°C. The dish was placed on a temperature-controlled stage in a Zeiss Axiovert 200 microscope. The temperature was maintained at 37°C and the CO2 level at 10% with a CTI 3700 controller (Zeiss). The embryos were imaged every 30 min for 24 h by use of a 10× phase-contrast objective. ZEISS IMAGING WS/20A imaging software was used to analyze the progression of mitosis of individual embryos. After the experiment, genotyping of the embryos was performed as described above.
Chromosome spread.
Chromosome spread was performed as previously described (27). Briefly, two-cell embryos (E1.5) were isolated and cultured in KSOM medium for 18 h, transferred into hypotonic solution (1% sodium citrate), and incubated for 10 min at room temperature. The embryos in a microdrop were placed on a grease-free glass slide, and the fixative (methanol:acetic acid [3:1]) was dropped onto the embryos. Fixative dropping was repeated two more times. The slide was air dried and stained with Giemsa solution.
RESULTS AND DISCUSSION
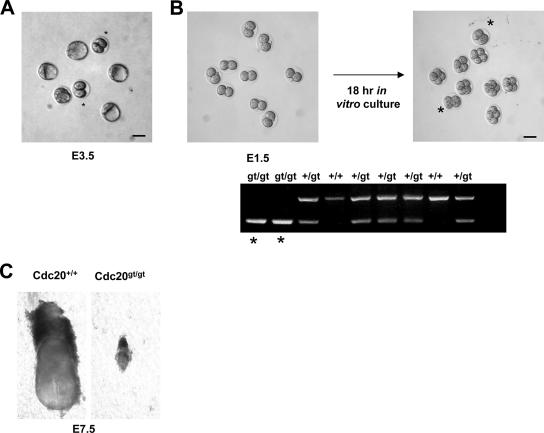
To identify the in vivo function of Cdc20, we derived mice from a gene trap mouse embryonic stem cell clone (XE368) isolated and characterized by BayGenomics (26). The gene trap construct, containing splicing acceptor and β-geo, was inserted in intron 9 of Cdc20 (Fig. 1A). Although the insertion prevents the expression of only the last 59 amino acid residues (out of 499 total residues), it fuses Cdc20 with β-geo in frame, leading to the expression of a much larger fusion protein (Fig. 1C). We named this allele Cdc20gt. It was successfully transmitted through the germ line (Fig. 1B shows PCR genotyping). Western blot analysis of E10.5 whole-embryo lysates with antibodies recognizing epitopes in the N terminus of Cdc20 demonstrated a 50% reduction in the amount of Cdc20 protein in Cdc20+/gt embryos from that in the wild type (Fig. 1C). Due to the fusion between Cdc20 and β-geo, the expression of Cdc20 in Cdc20+/gt embryos could easily be monitored through a colorimetric assay for LacZ. As shown in Fig. 1D, Cdc20 is widely expressed through out the whole embryo, consistent with its role in mitosis.
Given the fusion of a large protein (LacZ-Neo, 1,204 amino acid residues) at the C terminus, we expected that Cdc20gt allele would not be functional, as LacZ-Neo might interfere with Cdc20's interaction with its substrates. Further, we expected that the loss of Cdc20 function would be embryonic lethal, given its role in mitosis. To test these predictions, we intercrossed Cdc20+/gt mice. As shown in Table 1, we did not obtain any neonatal Cdc20gt/gt mice out of 186 offspring produced, while 46 such animals were expected according to the Mendelian law of inheritance. Thus, Cdc20gt/gt mice either cannot survive embryogenesis or die shortly after birth. Further analysis of embryos derived from the same intercrosses indicated that Cdc20gt/gt mice died very early in embryogenesis. No homozygous embryos were recovered beyond E7.5. When embryos were harvested and analyzed at E3.5, we found two classes of them, one at the expected blastocyst stage and the other at two-cell stage (Fig. 2A). Genotyping of the two-cell stage embryos was unsuccessful due to the degeneration of the embryos. The two-cell embryos must have been dead for some time and would have been absorbed soon thereafter, since we never saw two-cell embryos beyond E3.5. To circumvent the degeneration issue, we harvested embryos at E1.5, when they are at two-cell stage, and cultured them for 18 h. During this in vitro incubation, most embryos divided and arrived at the four-cell stage; however, there were a few that stayed in the two-cell stage (Fig. 2B). Genotyping of the two-cell-stage embryos indicated that they were Cdc20gt/gt. Among 75 embryos analyzed, 22% of them were arrested at the two-cell stage and were Cdc20gt/gt. These results demonstrate that Cdc20 is required for embryogenesis beyond the two-cell stage and that the gene trap allele is most likely a null allele.
TABLE 1.
Genotype frequencies for neonates from Cdc20+/gt intercross
| Genotype | No. | Expected (%) | Actual (%) |
|---|---|---|---|
| Cdc20+/+ | 75 | 25 | 40 |
| Cdc20+/gt | 111 | 50 | 60 |
| Cdc20gt/gt | 0 | 25 | 0 |
FIG. 2.
Cdc20 is essential for embryogenesis. (A) Micrograph of a litter from a heterozygote-to-heterozygote intercross at E3.5. Asterisks indicate embryos arrested at the two-cell stage. (B) In vitro culture of two-cell embryos. E1.5 embryos were isolated, cultured, and genotyped. The homozygous mutants are marked by asterisks. (C) A rare survivor of the loss of Cdc20. Scale bars, 20 μm.
The fact that there were fewer than the expected 25% of Cdc20gt/gt embryos arrested at the two-cell stage suggested that there might be some homozygous mutants, which could develop further. Indeed, we found 3 Cdc20gt/gt embryos out of 41 (7.3%) genotyped at E7.5, albeit they were much smaller than their wild-type littermates (Fig. 2C). These embryos were unlikely to develop any further. They probably had an exceptionally high maternal supply of Cdc20.
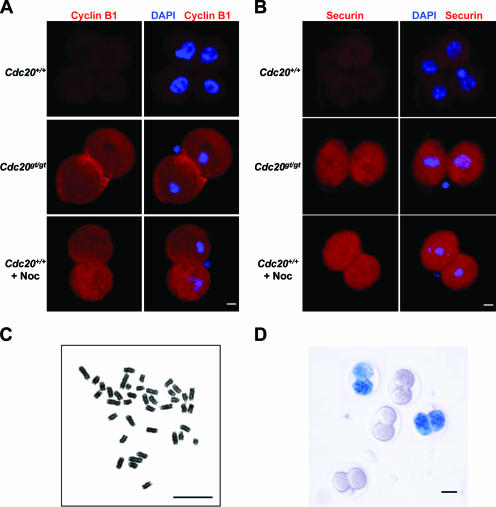
Given Cdc20's role in mediating the ubiquitination of cyclin B1 and securin by the APC, the arrest at the two-cell stage observed for Cdc20gt/gt embryos is likely a result of their inability to pass metaphase. Indeed, immunostaining demonstrated the presence of high levels of cyclin B1 (Fig. 3A) and securin (Fig. 3B) in the arrested Cdc20gt/gt embryos. DNA staining demonstrated metaphase chromosome configuration in these embryos (Fig. 3A and B). To confirm that the detected cyclin B1 and securin levels in the arrested embryos are comparable to the levels seen for normal mitoses, we incubated E1.5 embryos (at the two-cell stage) with and without nocodazole for 16 h to arrest the embryos treated with the microtubule poison at prometaphase. The embryos were then subjected to immunofluorescence analyses of cyclin B1 and securin. As shown in Fig. 3A and B, the levels of cyclin B1 and securin were the same between nocodazole-arrested wild-type embryos and Cdc20 mutant embryos, supporting the notion that the gene trap allele of Cdc20 is null. Furthermore, chromosome spread showed that the arrest caused by the lack of Cdc20 is in metaphase (Fig. 3C). There was no separation of sister chromatids, and the arm cohesion remained intact as well. We do not know how long the metaphase arrest can last in vivo. Given the fact that when the embryos were isolated at the two-cell stage (E1.5) they were still alive and could progress to mitosis in vitro but were dead by E3.5, the duration of the arrest should be less than 48 h.
FIG. 3.
Cdc20 is essential for mitosis. (A) Immunofluorescence staining of cyclin B1. E1.5 embryos were cultured for 18 h in the presence or absence of nocodazole (Noc) and processed for the staining. (B) Immunofluorescence staining of securin. The embryos were processed as for panel A. (C) Chromosome spread of a two-cell embryo after 18 h of in vitro culturing. (D). Expression of Cdh1 in early embryos. E1.5 embryos were fixed and stained for LacZ activities. Scale bars in panels A to C, 5 μm; in panel D, 20 μm.
These data demonstrate that mammalian Cdc20 is essential for mitosis. However, this essentiality could be a result of the absence of Cdh1 expression in the early embryos, which would mask the redundancy between these two APC/C adapter proteins. Therefore, we analyzed the expression of Cdh1 in two-cell mouse embryos. We have generated mice carrying a similarly trapped allele of Cdh1 in the lab (unpublished data), which provided a convenient way to assess Cdh1 expression. We harvested a litter at E1.5 from a cross between Cdh1+/gt and Cdh1+/+ mice and stained for LacZ activities expressed from the trapped Cdh1 locus. Two Cdh1+/gt embryos showed robust LacZ staining (Fig. 3D). This result demonstrates that Cdh1 is expressed in the early embryos. Thus, Cdc20 is not redundant with Cdh1 for the transition from metaphase to anaphase.
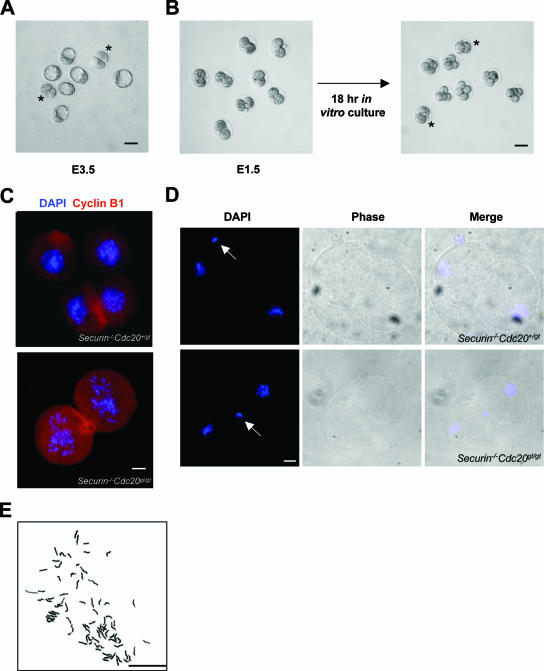
In budding yeast, the deletion of CDC20 causes metaphase arrest (15, 23). The arrest depends on the function of PDS1, the budding yeast securin analog (30). In cdc20 pds1 double mutants, the cells arrest at late anaphase but could not exit mitosis due to the accumulation of Clb5, one of the six B-type cyclins in budding yeast. Since mammalian separase is phosphorylated and inhibited by Cdk1/cyclin B1 (5, 25), we reasoned that unlike what is seen for the budding yeast, in which separase (ESP1) is not known to be inhibited by Cdk/cyclin, deleting securin under a Cdc20 null background in mouse would not lead to separase activation and sister chromatid separation. In other words, the double mutant should arrest at metaphase as the Cdc20 single mutant does. To determine if that is the case, we intercrossed securin−/− Cdc20+/gt mice to obtain securin−/− Cdc20gt/gt animals. No live securin−/− Cdc20gt/gt pups were born from the cross, indicating that the loss of securin could not rescue the lethality caused by Cdc20 deficiency. Analysis of E3.5 embryos derived from the crosses again revealed the presence of embryos arrested at the two-cell stage (Fig. 4A). In vitro culturing of E1.5 embryos for 18 h demonstrated that there were embryos which could not divide any further and were arrested at the two-cell stage (Fig. 4B). Genotyping indicated that the arrested embryos were securin−/− Cdc20gt/gt. With a total of 55 embryos cultured, 12 (21.8%) were securin−/− Cdc20gt/gt and were arrested at two-cell stage, results which were similar to those obtained for Cdc20+/gt intercrosses. As expected, these two-cell embryos also showed high levels of cyclin B1 (Fig. 4C).
FIG. 4.
Loss of sister cohesion in Cdc20 and securin double mutants. (A) Micrograph of a litter from an intercross between securin−/− Cdc20+/gt mice at E3.5. Asterisks indicate embryos arrested at the two-cell stage. (B) In vitro culture of two-cell embryos. E1.5 embryos were isolated, cultured, and genotyped. The double homozygous mutants are marked by asterisks. Immunofluorescence staining of cyclin B1. E1.5 embryos were stained after 18 h of in vitro culturing. Two-cell-stage embryos were cultured for 12 h. Arrows indicate polar bodies. (E) Chromosome spread of a two-cell embryo after 18 h of in vitro culturing. The embryo was obtained from an intercross between securin−/− Cdc20+/gt mice. Scale bars in panels A and B, 20 μm; in panels C to E, 5 μm.
Unexpectedly, however, chromosomes in the double mutants did not display metaphase configuration but were scattered (Fig. 4C). If the embryos were analyzed after a shorter (12-h) in vitro culture, they were at metaphase (Fig. 4D), indicating that the double mutants are not impaired in progressing into metaphase and that the scattering of chromosomes is likely a result of sister separation. To verify that the sister chromatids were separated in the double mutants, we incubated E1.5 embryos derived from securin−/− Cdc20+/gt intercrosses for 18 h and spread the chromosomes of the arrested (hence the double mutant) two-cell embryos. As shown in Fig. 4E, the sister chromatids were completely separated. Furthermore, we subjected the embryos to time-lapse microscopy. Hoechst 33342 was added to the media after 8 to 9 h, when there was at least one embryo in the litter that had divided. As shown in Fig. 5, the two-cell embryos from securin−/− Cdc20+/gt intercrosses stayed in metaphase for about 4 h. After that, chromosomes started wandering away from the metaphase plates. In contrast, the two-cell embryos from Cdc20+/gt intercrosses remained in metaphase. Taken together, these data suggest that the metaphase arrest caused by lack of Cdc20 depends on the function of securin. To eliminate the possibility that the genetic background from securin knockout mice might have influenced the result, we intercrossed securin+/− Cdc20+/gt mice to generate securin+/+ Cdc20gt/gt and securin−/− Cdc20gt/gt embryos in the same litter. Similar results were obtained (data not shown).
FIG. 5.
Time-lapse microscopic analysis of Cdc20 and securin mutant embryos. Cdc20gt/gt (A) and Cdc20gt/gt securin−/− (B) E1.5 embryos were cultured in KOSM for 8 to 9 h before the addition of Hoechst 3344 dye to the medium. Time zero was set at 30 min after the Hoechst dye addition. Scale bars, 10 μm.
Given the fact that securin−/− mouse and human cells could hold sister chromatids together even after prolonged treatments with spindle poisons (10, 11, 16), it is unexpected to find that securin and Cdc20 double mutants could not arrest at metaphase. The question is why the inhibitory phosphorylation of separase can prevent sister separation in securin−/− embryonic stem cells treated with nocodazole (10) but cannot do so in securin and Cdc20 double mutants. One possibility is that the inhibitory phosphorylation of separase does not function in the early embryos. However, when the two-cell embryos were treated with nocodazole, all embryos, including securin−/− embryos and the double mutants, could arrest in prometaphase without the separation of sister chromatids (data not shown), suggesting that the inhibitory phosphorylation of separase is functional at this stage. One difference between the nocodazole-induced arrest and the lack of Cdc20-induced arrest is that the former relies on the spindle assembly checkpoint, whereas the latter does not. In fact, the checkpoint should have been inactivated in Cdc20−/− or securin−/− Cdc20−/− embryos once they established bivalent spindle attachment and arrived at metaphase. Indeed, when Mad2 localization was analyzed, we could not detect the protein in Cdc20 mutant embryos, whereas Mad2 was found associated with the chromosomes in nocodazole-arrested embryos (Fig. 6), indicating no spindle checkpoint activation in the metaphase-arrested mutant embryos. Therefore, we speculate that perhaps there is a separase phosphatase(s) whose activity is controlled by the spindle assembly checkpoint. Thus, in Cdc20−/− or securin−/− Cdc20−/− embryos, as soon as the spindle checkpoint is relieved, the phosphatase(s) starts to dephosphorylate separase and work against the inhibition by Cdk1/cyclin B1, leading to a gradual loss of sister cohesion (without the degradation of cyclin B1, the loss could not be as abrupt as in normal anaphase) in the double mutants but not in Cdc20 single mutants because of the inhibition of separase by securin. The identity of this putative phosphatase will be sought in the future.
FIG. 6.
Spindle assembly checkpoint is not activated in Cdc20-deficient embryos. Two-cell-stage embryos were incubated with (+ Noc) and without (− Noc) nocodazole and processed for Mad2 immunostaining. Scale bar, 5 μm.
Furthermore, our results indicate that the endogenous levels of cyclin B1 are not sufficient to arrest the cells in metaphase in the absence of securin, consistent with the finding by Hagting et al. (6) that low levels of nondegradable cyclin B1 could not arrest the cells in metaphase. About two times the endogenous amount of cyclin B1 is needed for the arrest (6). These results disagree with the notion that even a low level of ectopic expression (∼30% of the endogenous level) of nondegradable cyclin B1 could block the cells in metaphase (2). Our analysis of Cdc20-deficient embryos unequivocally shows that preventing the degradation of cyclin B1 alone cannot block the progression of mitosis at metaphase in mammals, unless the spindle assembly checkpoint is activated at the same time or the level of cyclin B1 is raised unphysiologically high.
In summary, we demonstrated that Cdc20 is essential for metaphase-to-anaphase transition. The loss of Cdc20 causes mouse embryogenesis to stop at the two-cell stage.
Acknowledgments
We thank R. Zhang for microinjection and L. Garza for excellent technical help.
P.Z. is supported by grants from NIH. J.P.Y. is supported by a graduate training grant from NIH.
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 2.Chang, D. C., N. Xu, and K. Q. Luo. 2003. Degradation of cyclin B is required for the onset of anaphase in mammalian cells. J. Biol. Chem. 278:37865-37873. [DOI] [PubMed] [Google Scholar]
- 3.Dej, K. J., and T. L. Orr-Weaver. 2000. Separation anxiety at the centromere. Trends Cell Biol. 10:392-399. [DOI] [PubMed] [Google Scholar]
- 4.Fang, G., H. Yu, and M. W. Kirschner. 1999. Control of mitotic transitions by the anaphase-promoting complex. Philos. Trans. R. Soc. Lond. B 354:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorr, I. H., D. Boos, and O. Stemmann. 2005. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19:135-141. [DOI] [PubMed] [Google Scholar]
- 6.Hagting, A., N. Den Elzen, H. C. Vodermaier, I. C. Waizenegger, J. M. Peters, and J. Pines. 2002. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157:1125-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16:2179-2206. [DOI] [PubMed] [Google Scholar]
- 8.Hauf, S., I. C. Waizenegger, and J. M. Peters. 2001. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293:1320-1323. [DOI] [PubMed] [Google Scholar]
- 9.Hendrickson, C., M. A. Meyn III, L. Morabito, and S. L. Holloway. 2001. The KEN box regulates Clb2 proteolysis in G1 and at the metaphase-to-anaphase transition. Curr. Biol. 11:1781-1787. [DOI] [PubMed] [Google Scholar]
- 10.Huang, X., R. Hatcher, J. P. York, and P. Zhang. 2005. Securin and separase phosphorylation act redundantly to maintain sister chromatid cohesion in mammalian cells. Mol. Biol. Cell 16:4725-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jallepalli, P. V., I. C. Waizenegger, F. Bunz, S. Langer, M. R. Speicher, J. M. Peters, K. W. Kinzler, B. Vogelstein, and C. Lengauer. 2001. Securin is required for chromosomal stability in human cells. Cell 105:445-457. [DOI] [PubMed] [Google Scholar]
- 12.Koshland, D. E., and V. Guacci. 2000. Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr. Opin. Cell Biol. 12:297-301. [DOI] [PubMed] [Google Scholar]
- 13.Kraft, C., H. C. Vodermaier, S. Maurer-Stroh, F. Eisenhaber, and J. M. Peters. 2005. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18:543-553. [DOI] [PubMed] [Google Scholar]
- 14.Kramer, E. R., N. Scheuringer, A. V. Podtelejnikov, M. Mann, and J. M. Peters. 2000. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11:1555-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim, H. H., P. Y. Goh, and U. Surana. 1998. Cdc20 is essential for the cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast. Curr. Biol. 8:231-234. [DOI] [PubMed] [Google Scholar]
- 16.Mei, J., X. Huang, and P. Zhang. 2001. Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 11:1197-1201. [DOI] [PubMed] [Google Scholar]
- 17.Nasmyth, K. 2005. How do so few control so many? Cell 120:739-746. [DOI] [PubMed] [Google Scholar]
- 18.Nasmyth, K., J. M. Peters, and F. Uhlmann. 2000. Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288:1379-1385. [DOI] [PubMed] [Google Scholar]
- 19.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9:931-943. [DOI] [PubMed] [Google Scholar]
- 20.Pfleger, C. M., E. Lee, and M. W. Kirschner. 2001. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15:2396-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab, M., A. S. Lutum, and W. Seufert. 1997. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90:683-693. [DOI] [PubMed] [Google Scholar]
- 22.Schwab, M., M. Neutzner, D. Mocker, and W. Seufert. 2001. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20:5165-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirayama, M., A. Toth, M. Galova, and K. Nasmyth. 1999. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature 402:203-207. [DOI] [PubMed] [Google Scholar]
- 24.Song, M. S., S. J. Song, N. G. Ayad, J. S. Chang, J. H. Lee, H. K. Hong, H. Lee, N. Choi, J. Kim, H. Kim, J. W. Kim, E. J. Choi, M. W. Kirschner, and D. S. Lim. 2004. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat. Cell Biol. 6:129-137. [DOI] [PubMed] [Google Scholar]
- 25.Stemmann, O., H. Zou, S. A. Gerber, S. P. Gygi, and M. W. Kirschner. 2001. Dual inhibition of sister chromatid separation at metaphase. Cell 107:715-726. [DOI] [PubMed] [Google Scholar]
- 26.Stryke, D., M. Kawamoto, C. C. Huang, S. J. Johns, L. A. King, C. A. Harper, E. C. Meng, R. E. Lee, A. Yee, L. L'Italien, P. T. Chuang, S. G. Young, W. C. Skarnes, P. C. Babbitt, and T. E. Ferrin. 2003. BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 31:278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarkowska, A. 1966. An air-drying method for chromosome preparations from mouse eggs. Cytogenetics 5:394-400. [Google Scholar]
- 28.Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400:37-42. [DOI] [PubMed] [Google Scholar]
- 29.Uhlmann, F., D. Wernic, M. A. Poupart, E. V. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103:375-386. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto, A., V. Guacci, and D. Koshland. 1996. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol. 133:99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu, H. 2002. Regulation of APC-Cdc20 by the spindle checkpoint. Curr. Opin. Cell Biol. 14:706-714. [DOI] [PubMed] [Google Scholar]