Abstract
Cadherins are the most crucial membrane proteins for the formation of tight and compact cell-cell contacts. Cadherin-based cell-cell adhesions are dynamically established and/or disrupted during various physiological and pathological processes. However, the molecular mechanisms that regulate cell-cell contacts are not fully understood. In this paper, we report a novel functional role of casein kinase 1 (CK1) in the regulation of cell-cell contacts. Firstly, we observed that IC261, a specific inhibitor of CK1, stabilizes cadherin-based cell-cell contacts, whereas the overexpression of CK1 disrupts them. CK1 colocalizes with E-cadherin and phosphorylates the cytoplasmic domain of E-cadherin in vitro and in a cell culture system. We show that the major CK1 phosphorylation site of E-cadherin is serine 846, a highly conserved residue between classical cadherins. Constitutively phosphorylated E-cadherin (S846D) is unable to localize at cell-cell contacts and has decreased adhesive activity. Furthermore, phosphorylated E-cadherin (S846D) has weaker interactions with β-catenin and is internalized more efficiently than wild-type E-cadherin. These data indicate that CK1 is a novel negative regulator of cadherin-based cell-cell contacts.
In multicellular organisms, individual cells are often connected to each other via cell-cell adhesions to form three-dimensionally-structured tissues or organs. The formation of tight and compact cell-cell adhesions suppresses free cell movement and provides cells with a positional cue for proper polarization. Among many junctional proteins, cadherins, especially classical cadherins, are the most crucial membrane proteins for the establishment of intercellular adhesions (for reviews, see references 15 and 32). Approximately 20 classical cadherins have been identified, and each displays a characteristic tissue distribution. For example, E-cadherin, the prototype and the best-characterized classical cadherin, is expressed primarily in epithelial cells. N-cadherin is expressed in neuronal and fibroblastic cells, while VE-cadherin is expressed in endothelial cells. The extracellular domains of cadherins form Ca2+-dependent homophilic adhesions between neighboring cells. The cytoplasmic region of cadherins includes two domains: a membrane-proximal CH2 domain and a more distal CH3 domain (CH denotes cadherin homology, and these domains are found in most of the classical cadherins) (34). The CH2 and CH3 domains interact with p120 catenin and β-catenin/γ-catenin, respectively. β-Catenin binds to α-catenin, which in turn associates with actin filaments. While the extracellular domain of cadherin induces cell-cell adhesion in the presence of Ca2+, an interaction between the cytoplasmic domain of cadherin and the underlying actin cytoskeleton is also required for the construction of tight and compact cell-cell adhesions (for a review, see reference 32).
Cadherin-based cell-cell contacts are not static but are often dynamically modulated during various physiological and pathological processes including mitosis, oncogenesis, and epithelial-mesenchymal transition during embryonic development. In all these processes, cadherin has been reported to be down-regulated by endocytosis (1, 19, 29, 39). However, the molecular mechanisms for the induction of endocytosis are not clearly understood. In cell culture systems, two experimental stimuli enhance the endocytosis of E-cadherin: the activation of tyrosine kinases and low-Ca2+ treatment. Upon the activation of tyrosine kinases such as Src, hepatocyte growth factor and epidermal growth factor receptors, E-cadherin and its binding proteins become tyrosine phosphorylated, which induces the recruitment of the E3-ubiquitin ligase Hakai and the ubiquitination of the E-cadherin complex. The complex is then internalized and sorted into lysosomes for protein degradation (11, 31). In contrast, low-Ca2+ treatment triggers the internalization of E-cadherin that occurs independently of tyrosine phosphorylation and ubiquitination, and the internalized E-cadherin is recycled back to the plasma membrane (8, 21, 31). The molecular mechanism for this process largely remains to be clarified.
Casein kinase 1 (CK1) is a serine/threonine kinase that is evolutionarily conserved from yeast to mammals (for a review, see reference 20). In mammals, at least seven CK1 isoforms (α, β, γ1, γ2, γ3, δ, and ɛ) and their splice variants have been identified, which are all ubiquitously expressed. All CK1 isoforms are highly homologous within their kinase domains, but they differ in the lengths and primary structures of their noncatalytic domains. CK1 phosphorylates many substrates that are involved in various cellular processes including cell differentiation, proliferation, membrane transport, and oncogenesis. For example, in Saccharomyces cerevisiae, CK1 phosphorylates and enhances the endocytosis of many membrane proteins including the α-factor pheromone receptor and uracil permease (16, 24, 25). In both invertebrates and vertebrates, several CK1 isoforms have been reported to have a regulatory role in the wingless (Wnt) signaling pathway (10, 12, 23, 40, 41). In the present study, we report that the cytoplasmic domain of cadherin is a novel substrate for CK1 and that the subsequent phosphorylation of E-cadherin negatively regulates cadherin-based cell-cell adhesions.
MATERIALS AND METHODS
Antibodies, plasmids, and materials.
The antibody to the cytoplasmic portion of E-cadherin from Transduction Laboratories (San Diego, CA) was used for immunoprecipitation and Western blotting. Antibodies to the extracellular portion of E-cadherin (ECCD2 and HECD1) from Zymed (South San Francisco, CA) were used for immunofluorescence of MDCK cells and MCF-7 cells, respectively. Anti-N-cadherin, -α-catenin, -β-catenin, -CK1ɛ, and -EEA1 antibodies were from Transduction Laboratories. Chicken anti-CK1α antibody from EnCor Biotechnology (Alachua, FL) was used for Western blotting, and goat anti-CK1α antibody from Santa Cruz (Santa Cruz, CA) was used for immunofluorescence. Mouse anti-Myc (4A6) antibody was obtained from Upstate (Charlottesville, VA), and rabbit anti-Myc (A14) antibody was from Santa Cruz. All antibodies were used at dilutions of 1:1,000 for Western blotting and 1:100 for immunofluorescence, except for anti-CK1α antibody, which was used at a dilution of 1:5,000 for Western blotting.
To construct pCAN-Myc-CK1α (full length) and pCAN-Myc-CK1ɛ (full-length), the cDNAs of rat CK1α and Xenopus laevis CK1ɛ were amplified from pCS2-CK1α and pCS2-CK1ɛ by PCR using the primers 5′-CGAATTCGGATCCGCATGGCGAGCAGCAGCGGCTCC-3′ and 5′-GGAATTCGCGGCCGCTTAGAAACCTGTGGGGGTTTGGGCC-3′ and 5′-CATCGATGTCGACACATGGAGCTGAGAGTGGGGAAC-3′ and 5′-CATCGATATCGATACATGGAGCTGAGAGTGGGGAAC-3′, respectively. pCS2-CK1α and pCS2-CK1ɛ were kindly provided by X. He (Harvard Medical School, Boston, MA). The amplified cDNAs of CK1α and CK1ɛ were cloned into BamHI/NotI and ClaI/NotI sites of the pCAN-Myc vector, respectively. To construct pcDNA4/TO/GFP-CK1α, the cDNA of rat CK1α was excised from pCAN-Myc-CK1α (BamHI/NotI) and, after blunting the ends, inserted into an EcoRI site of vector pcDNA/TO/GFP. To obtain pcDNA4/TO/GFP, the cDNA of green fluorescent protein (GFP) was amplified from pEGFP-C2 by PCR and was inserted into the BamHI/ApaI site of vector pcDNA4/TO. Vectors pcDNA6/TR and pcDNA4/TO were obtained from Invitrogen (Paisley, United Kingdom). pcDNA-E-cadherin-Myc and pGEX-E-cadherin were described previously (11, 17). pGEX-TEV-β-catenin was kindly provided by B. Weise (Stanford University School of Medicine, Stanford, CA). Recombinant β-catenin was produced by cleavage of the glutathione S-transferase (GST) tag using AcTEV protease (Invitrogen) at 4°C overnight, followed by affinity chromatography according to the manufacturer's instructions.
IC261 (CK1 inhibitor), H-89 (protein kinase A [PKA] inhibitor), glycogen synthase kinase 3β (GSK-3β) inhibitor II, and cycloheximide were purchased from Calbiochem (Darmstadt, Germany). A His6-tagged constitutively active form of the CK1δ protein was purchased from Sigma, and protein kinase Cζ (PKCζ) and casein kinase 2 (CK2) proteins were obtained from Calbiochem. An active form of GSK-3β protein was obtained from Upstate. Lipofectamine Plus reagent and essential amino acids were obtained from Invitrogen. [γ-32P]ATP and [32P]orthophosphate were purchased from GE Healthcare (Piscataway, NJ). Site-directed mutagenesis was performed using a QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA).
Immunoprecipitation, GST-E-cadherin pull-down assay, and Western blotting.
Immunoprecipitation was performed as described previously by using 1% Triton X-100 lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 1% Triton X-100) containing 5 μg ml−1 leupeptin, 50 mM phenylmethylsulfonyl fluoride, and 7.2 trypsin inhibitor units of aprotinin (17). To exclude β-catenin from the E-cadherin immunoprecipitate, MCF-7 cells were lysed in 1% Triton X-100 lysis buffer containing 1% sodium dodecyl sulfate (SDS). The lysate was then diluted 10-fold with 1% Triton X-100 lysis buffer before immunoprecipitation.
For GST-E-cadherin pull-down assays, 10 μl of glutathione-Sepharose beads (Pharmacia) attached to 6 μg GST or GST-E-cadherin protein was incubated with cell lysates in 1% Triton X-100 lysis buffer, followed by the same procedures as described above. For GST-E-cadherin mutant pull-down assays, radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS containing the same protease inhibitors as described above) was used. Western blotting was performed as described previously (17).
Cell culture, immunofluorescence, microinjection, RNA interference, endocytosis assay, and membrane-targeting experiment.
HEK293, MCF-7, MDCK, and L fibroblast cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin at 37°C and ambient air supplemented with 5% CO2. MDCK epithelial cells transformed with a temperature-sensitive Src mutant (ts-Src MDCK cells) were cultured as described previously (2). To obtain cells expressing E-cadherin mutants, MDCK and L fibroblast cells were transfected with the pcDNA-Myc-E-cadherin wild type or S846A or S846D mutants using Lipofectamine Plus reagent, and stably transfected cells were selected in medium containing 800 μg ml−1 of G418 (Calbiochem). More than five stable clones were obtained from two independent transfections for each construct. It should be noted that the expression of E-cadherin S846D decreases in both MDCK and L fibroblast cells as the passage proceeds. Thus, cells in earlier passages were used for the experiments. Since we could not obtain MDCK cells stably expressing CK1 by a conventional method, a Tet-ON inducible system was used. First, MDCK cells were transfected with pcDNA6/TR, followed by selection in medium containing 5 μg ml−1 of blasticidin (Invitrogen). pcDNA4/TO/GFP-CK1α was then used for the second transfection, and the doubly transfected cells were selected in medium containing 5 μg ml−1 of blasticidin and 400 μg ml−1 of zeocin (Invitrogen). Sixteen hours after the addition of tetracycline, induced expression of GFP-CK1α was examined. For the experiments indicated as being “under low-confluence conditions” or “at low density,” 2 × 105 and 5 × 105 cells were plated in 6-well plates and 6-cm dishes, respectively, and after 16 h, experiments were carried out. Otherwise, 6 × 105, 1.5 × 106, and 5 × 106 cells were plated in 6-well plates and 6- and 9-cm dishes, respectively, and experiments were started after 24 to 48 h. IC261 (10 μM), H-89 (200 nM), and GSK-3β inhibitor II (1 μM) were added for 4 h unless otherwise indicated.
Calcium was depleted from fetal calf serum, and the low-calcium medium was reconstituted as described previously (3). Immunofluorescence was performed as previously described (17). Microinjection was performed as described previously (3). pCAN-Myc-CK1α or pCAN-Myc-CK1ɛ (0.1 μg μl−1 phosphate-buffered saline [PBS]) was microinjected into the nucleus of MCF-7 cells. After microinjection, cells were incubated in normal calcium medium for 6 h, followed by immunostaining with the indicated antibodies. Cell aggregation and cell dissociation assays were performed as described previously (30, 38). In a dissociation assay for cells with IC261, cell clumps were incubated for 2 h after medium was replaced with that containing 10 μM IC261. Validated small interfering RNA oligonucleotides for CK1α or CK1ɛ were obtained from QIAGEN. Oligonucleotides were transfected into MCF-7 cells using Hi-Perfect reagent (QIAGEN) according to the manufacturer's instructions. Maximally, 50% reduction of endogenous CK1 protein was obtained by either small interfering RNA. The endocytosis assay was performed as previously described (11), except that after biotinylation, cells were incubated at 18°C to block the recycling of internalized E-cadherin back to the plasma membrane (21), and bafilomycin was not used. To examine the targeting of newly synthesized E-cadherin into the plasma membrane, we combined pulse-chase and surface biotinylation assays. First, cells were metabolically labeled as described previously (11), except that [35S]methionine and cysteine were used for 30 min. Cells were then incubated with 0.5 mg ml−1 sulfo-N-hydroxysulfosuccinimide-SS-biotin in the presence or absence of 10 μM IC261 in Krebs-Ringer buffer at 37°C for 30 min or 2 h. Surface-biotinylated E-cadherin was pulled down with 20 μl of monomeric avidin beads, followed by elution with 2 mM d-biotin in PBS and immunoprecipitation with anti-E-cadherin antibody. An Immobilized Monomeric Avidin kit (Pierce, Rockford, IL) was used for the purification of biotinylated proteins.
Immunofluorescent images were analyzed by confocal microscopy unless otherwise indicated. To quantify the images in Fig. 9d, images were captured at every 0.5-μm interval, and the image where colocalization of E-cadherin and EEA-1 was maximally observed was chosen for analysis. To obtain epifluorescence and confocal images, we used a Zeiss Axioskop 1 with a Roper Scientific Coolsnap camera and a Bio-Rad MRC 1024 mounted on a Nikon Optiphot 2 microscope, respectively. To obtain phase-contrast images, we used a Leica DMIRB microscope with a Hamamatsu C4742-95 Orca camera. Images were captured and analyzed using Openlab (Improvision) and ImageJ 1.36b (National Institutes of Health).
FIG. 9.
Mutations in the major CK1 phosphorylation residue of E-cadherin affect endocytosis of E-cadherin. (a) Effect of IC261 on endocytosis of endogenous E-cadherin (E-cad) in MCF-7 cells. E-cadherin in MCF-7 cells was surface biotinylated and incubated in normal (N) or low-Ca2+ (L) medium in the presence or absence of 10 μM IC261 for 5 h. Biotinylated proteins on the plasma membrane were then stripped off by glutathione treatment. Biotinylated E-cadherin inside cells was recovered on streptavidin beads and analyzed by Western blotting (WB) with anti-E-cadherin antibody (Ab). Input indicates total biotinylated E-cadherin. (b and c) Effect of E-cadherin mutation on its endocytosis in MDCK cells. (b) Wild-type and nonphosphorylatable (S846A) E-cadherin on the respective stable clones (WT28 and A28) were surface biotinylated and incubated in normal (N) or low-Ca2+ (L) medium for 8 h. After glutathione treatment, biotinylated E-cadherin inside cells was recovered on streptavidin beads and analyzed by Western blotting with anti-Myc antibody. Input indicates total biotinylated Myc-E-cadherin. (c) Wild-type E-cadherin (WT) and pseudophosphorylated E-cadherin (S846D) on the respective stable clones (WT25 and D23) were analyzed as described above, except that cells were incubated in normal (N) or low-Ca2+ (L) medium for 3 h. Input indicates total biotinylated Myc-E-cadherin. The lower panel shows the quantification of endocytosed E-cadherin compared with total biotinylated E-cadherin from the results of three independent experiments. It should be noted that different incubation times were used in b (8 h) and c (3 h) in order to maximize the difference in endocytosis between respective mutants. (d) Effect of E-cadherin mutation on its endocytosis in MDCK cells by immunofluorescence staining. Myc-tagged wild-type or pseudophosphorylated (S846D) E-cadherin was transiently expressed in MDCK cells and incubated with 2.5 μg ml−1 cycloheximide for 8 h. Cycloheximide was used to block the entry of newly synthesized E-cadherin into endosomes. The subcellular localization of exogenously expressed E-cadherin and early endosomes was analyzed using anti-Myc and anti-EEA1 antibodies. Scale bar, 20 μm. Expression of E-cadherin S846D induced flattening of cells as seen in Fig. 7b, and more early endosomes were observed in the same plane as E-cadherin in confocal microscopic analyses. Neither the E-cadherin wild type nor S846D affected the morphology of early endosomes.
In vitro phosphorylation assay.
Prior to phosphorylation assays, 2 μg of GST-E-cadherin wild-type or mutant proteins was coupled to glutathione-Sepharose beads; otherwise, endogenous E-cadherin protein was immunoprecipitated with anti-E-cadherin antibody using MCF-7 cells cultured in a 15-cm dish, followed by intensive washing with phosphorylation buffer (20 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 1 mM EGTA, and 40 μM cold ATP). The E-cadherin beads were then incubated in 30 μl of phosphorylation buffer with 0.36 μCi [γ-32P]ATP and the indicated kinase at 30°C for 8 min while shaking at 1,400 rpm, followed by washing with ice-cold phosphorylation buffer three times, boiling in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, and SDS-PAGE. For CK1, to optimize phosphorylation conditions, the time-dependent and kinase dose-dependent reactions were first studied, and we used an 8-min reaction time and 0.2 μg CK1δ protein for all the experiments except for maximal phosphorylation. To examine maximal phosphorylation of E-cadherin by CK1δ, the phosphorylation reaction was performed with 1.2 μg CK1δ for 30 min. A total of 0.2 μg PKCζ, 0.2 μg GSK-3β, or 250 U of CK2 was also used for a phosphorylation assay.
In vivo phosphorylation assay.
Krebs-Ringer buffer (20 mM HEPES-NaOH [pH 7.4], 118 mM NaCl, 4.75 mM KCl, 1.2 mM MgCl2, 0.26 mM CaCl2, 25 mM NaHCO3, 0.45% glucose, and 1× essential amino acids) was used for the phosphorylation assay. For low-Ca2+ treatment, CaCl2 was excluded from the buffer. MCF-7 cells in a 6-cm dish were first preincubated in Krebs-Ringer buffer for 1 h and further incubated in 1 ml of Krebs-Ringer buffer containing 0.5 mCi [32P]orthophosphate in the presence or absence of IC261 for 2 h, when [32P]orthophosphate was converted into [32P]ATP inside cells followed by the phosphorylation of proteins. IC261 did not grossly affect the radioactivity of total cell lysates, suggesting that it did not block the conversion from orthophosphate to ATP. Cells were then washed twice with PBS and precleared by mouse control immunoglobulin G beads prior to immunoprecipitation with anti-E-cadherin antibody.
Statistical analysis.
Descriptive statistics on the dissociation indices (DI) were calculated with NCSS software. Student's t tests were then generated with a threshold equal to 5% (α = 0.05). The DI data were transformed to arcsin (square DI), and Student's t tests were achieved with NCSS software using these values because this test requires variables with no fixed limits.
RESULTS
CK1 inhibitor stabilizes cadherin-based cell-cell contacts.
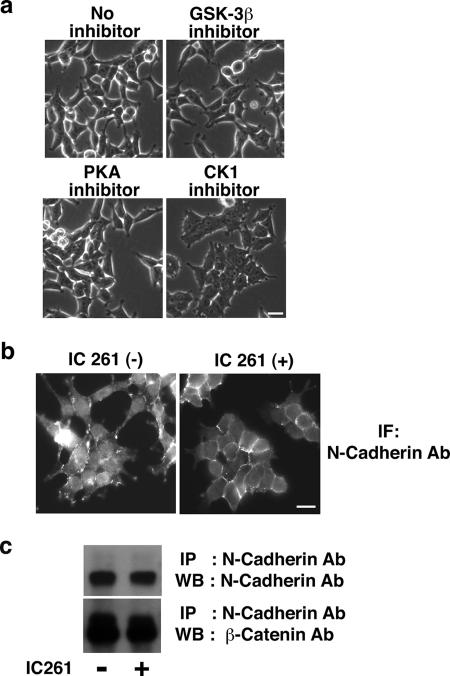
In the process of testing the effect of several serine/threonine kinase inhibitors on HEK293 cells, we realized that an inhibitor of CK1, IC261, induced a prominent morphological change. Although HEK293 cells express moderate levels of N-cadherin, they do not form tight cell-cell contacts and have a fibroblastic morphology (Fig. 1a, upper left panel). Upon the addition of IC261, however, the cells started to adhere together and form cell-cell contacts reminiscent of the mesenchymal-epithelial transition (Fig. 1a, lower right panel). Other kinase inhibitors such as GSK-3β and PKA inhibitor had no obvious effect (Fig. 1a). In the presence of IC261, N-cadherin was recruited to cell-cell contact sites and formed more regular and compact adhesions (Fig. 1b), although the expression levels of N-cadherin and β-catenin were not affected (Fig. 1c). IC261 is the most widely used CK1 inhibitor that blocks CK1 in a highly specific manner (26). CK1 isoforms show different sensitivity to IC261, with a 50% inhibitory concentration of ∼1 μM for CK1δ and CK1ɛ and a 50% inhibitory concentration of ∼10 μM for CK1α (26). The cell-cell contact stabilization effect was seen in the presence of 2.5 μM IC261 (data not shown); however, the maximal effect was observed with 10 μM IC261, suggesting that the inhibition of not only CK1δ and CK1ɛ but also other isoforms including CK1α is attributed to the effect of IC261.
FIG. 1.
CK1 inhibitor IC261 stabilizes N-cadherin-based cell-cell contacts in HEK293 cells. (a) Effect of various kinase inhibitors on HEK293 cells. HEK293 cells were incubated with 1 μM GSK-3β inhibitor II, 200 nM H-89 (PKA inhibitor), or 10 μM IC261 (CK1 inhibitor) for 4 h. The effect was examined by phase-contrast microscopy. Scale bar, 20 μm. (b) Effect of IC261 on the localization of N-cadherin in HEK293 cells. HEK293 cells were incubated in the presence (+) or absence (−) of 10 μM IC261 for 4 h, and the localization of N-cadherin was examined by immunofluorescence with anti-N-cadherin antibody using epifluorescence microscopy. Scale bar, 20 μm. (c) Effect of IC261 on the expression of the N-cadherin complex. HEK293 cells were incubated in the presence or absence of 10 μM IC261 for 4 h, followed by immunoprecipitation (IP) with anti-N-cadherin antibody and Western blotting (WB) with anti-N-cadherin and anti-β-catenin antibodies.
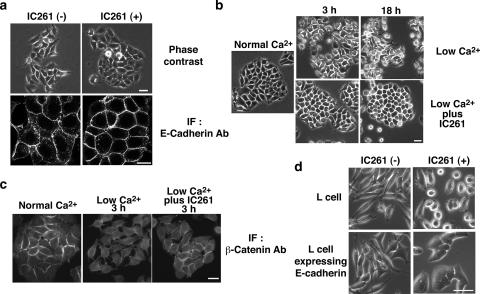
Since HEK293 cells do not express E-cadherin, we examined whether IC261 also affects E-cadherin-based cell-cell contacts in MCF-7 cells. MCF-7 cells are epithelial cells derived from human breast cancer cells that express E-cadherin but not N-cadherin. Under low-confluence conditions, MCF-7 cells adhere to each other weakly and form relatively immature E-cadherin-based cell-cell contacts (Fig. 2a, left panels). However, upon the addition of IC261, they formed more compact cell-cell contacts at which there was a higher accumulation of E-cadherin (Fig. 2a, right panels). The localization of ZO-1, a marker for tight junctions, was not significantly affected by IC261 (data not shown). When cultured at higher densities, MCF-7 cells formed tighter cell-cell contacts, and under these conditions, IC261 had no significant effect (data not shown). The expression of E-cadherin and β-catenin was not affected by IC261 (see Fig. 5a and b).
FIG. 2.
CK1 inhibitor IC261 stabilizes E-cadherin-based cell-cell contacts in MCF-7 and L fibroblast cells. (a) Effect of IC261 on MCF-7 cells under low-confluence conditions. MCF-7 cells cultured at low density were incubated in the presence or absence of 10 μM IC261 for 4 h. The effect of IC261 was examined by phase-contrast and immunofluorescence (IF) microscopy with anti-E-cadherin antibody (Ab). Scale bars, 20 μm. (b and c) Effect of IC261 on low-Ca2+-induced cell separation in MCF-7 cells. MCF-7 cells were incubated in low-Ca2+ medium in the presence or absence of 10 μM IC261 for the indicated times. The effect of the treatment was examined by phase-contrast microscopy (b) and immunofluorescence microscopy with anti-β-catenin antibody (c). Scale bars, 20 μm. (d) Requirement of E-cadherin for the effect of IC261 on cell-cell contacts. L cells or L cells expressing E-cadherin were incubated in the presence or absence of 10 μM IC261 for 4 h. The effect of IC261 was examined by phase-contrast microscopy. Scale bar, 20 μm.
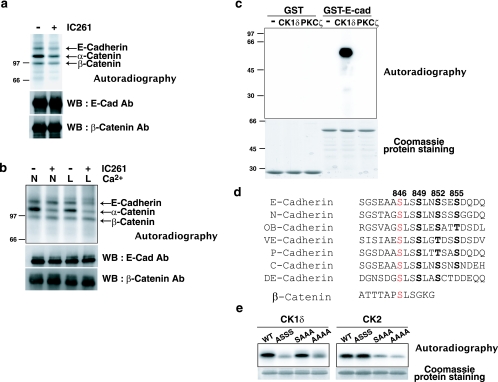
FIG. 5.
CK1 phosphorylates the cytoplasmic domain of E-cadherin. (a and b) Effect of IC261 on phosphorylation of the E-cadherin complex in MCF-7 cells in an in vivo phosphorylation assay. MCF-7 cells were cultured at low (a) or high (b) density and incubated with [32P]orthophosphate in the presence or absence of 10 μM IC261 for 2 h. In b, cells were incubated in either normal (N) or low-Ca2+ (L) medium. Cell lysates were immunoprecipitated with anti-E-cadherin antibody, followed by SDS-PAGE, autoradiography, and Western blotting (WB) with the indicated antibodies (Ab). The proteins in the autoradiography bands were identified by comparing them with the accompanying Western blotting results. (c) Phosphorylation of the cytoplasmic domain of E-cadherin by an in vitro phosphorylation assay. GST or the GST-tagged cytoplasmic domain of E-cadherin was incubated with [γ-32P]ATP in the presence of CK1δ or PKCζ. Phosphorylated proteins were subjected to SDS-PAGE, followed by autoradiography and Coomassie protein staining. (d) Amino acid sequence alignment of the cytoplasmic domains of classical cadherins. The sequence of E-, N-, OB-, VE-, and P-cadherins are from a mouse protein database. C- and DE-cadherins are E-cadherin counterparts from frog and fly, respectively. The numbers indicate the amino acid numbers for mouse E-cadherin. The potential major CK1 phosphorylation site of cadherins is in red. An analogous CK1 phosphorylation site of β-catenin is also shown. (e) Determination of the major CK1 phosphorylation site of E-cadherin by an in vitro phosphorylation assay. The GST-tagged wild type (WT) and nonphosphorylatable E-cadherin mutants were incubated with [γ-32P]ATP in the presence of CK1δ or CK2, followed by autoradiography and Coomassie protein staining. ASSS, S846A; SAAA, S849A, S852A, and S855A; AAAA, S846A, S849A, S852A, and S855A.
When MCF-7 cells are incubated in low-Ca2+ medium (Ca2+ ≅ 100 μM), the extracellular domains of E-cadherin form only weak and transient homophilic interactions, and cells dissociate from one another (Fig. 2b, upper panels). Interestingly, when IC261 was added to the low-Ca2+ medium, the dissociation process was prevented. The cells remained attached to each other (Fig. 2b, lower panels), and β-catenin was still localized at cell-cell contacts (Fig. 2c), suggesting that E-cadherin-based intercellular adhesions are maintained. To quantify the effect of IC261, we incubated cells in suspension in low-Ca2+ medium with or without 10 μM IC261 for 2 h, and cell aggregates were counted after trypsin treatment in the presence of Ca2+ (TC treatment) or EGTA (TE treatment) (30). The cell DI (NTC/NTE, where NTC and NTE are the total particle numbers after the TC and TE treatment) were 0.34 and 0.14 in the cells cultured in the absence and presence of IC261, respectively, and the difference was statistically significant (P < 0.005). To further examine this effect, IC261 was added to cells following dissociation in low-Ca2+ medium. After 2 to 3 h of IC261 incubation, the cells re-formed cell-cell contacts even in low-Ca2+ medium (see Fig. S1a in the supplemental material). Thus, IC261 not only blocks cell separation but also reverts cell-cell contact formation under low-Ca2+ conditions.
To investigate whether the effect of IC261 depends on cadherin, we used L fibroblast cells that do not express endogenous classical cadherins and thus do not form stable cell-cell contacts. The addition of IC261 to L cells did not induce the formation of cell-cell contacts, and some cells rounded up, probably due to effects on the cytoskeleton (Fig. 2d, upper panels). In contrast, L cells stably expressing E-cadherin formed weak and immature cell-cell adhesions when plated at low densities (Fig. 2d, lower left panels). Upon the addition of IC261, they tightly adhered to each other and formed compact cell-cell contacts (Fig. 2d, lower right panel), indicating that IC261 requires E-cadherin to promote cell-cell contact formation.
To examine whether IC261 affects the disruption of E-cadherin-based cell-cell contacts induced by Src, we used ts-Src MDCK cells. At 40.5°C (the nonpermissive temperature for ts-Src activity), cells formed tight intercellular adhesions. In contrast, when shifted to 35°C (the permissive temperature for ts-Src activity), cells dissociated from one another and acquired a spindle-like morphology (see Fig. S1b, upper panels, in the supplemental material) (2, 11). This shape change was accompanied by a redistribution of E-cadherin from the plasma membrane to intracellular compartments (see Fig. S1b, lower panels, in the supplemental material). The addition of IC261 did not significantly affect either this morphological change or the E-cadherin relocalization at 35°C (see Fig. S1b in the supplemental material). Thus, IC261 does not affect tyrosine kinase- and ubiquitin-dependent disruption of cell-cell contacts.
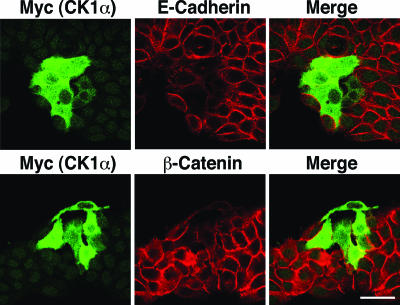
We also investigated the effect of the overexpression of CK1 on E-cadherin-based cell-cell contacts by microinjecting the cDNA encoding CK1α into the nucleus of MCF-7 epithelial cells. Between microinjected cells, the level of E-cadherin and β-catenin at cell-cell contacts was significantly reduced, compared with that between nonmicroinjected cells (Fig. 3). The microinjection of the cDNA encoding CK1ɛ also induced a similar effect but to a lesser extent (data not shown), which may be due to its autoinhibitory domain. Microinjection of the empty vector had no effect (data not shown). These data suggest that CK1 has an inhibitory role in the formation of E-cadherin-based cell-cell contacts, consistent with the observation that IC261 stabilizes them.
FIG. 3.
Overexpression of CK1 disrupts E-cadherin-based cell-cell contacts. The cDNA of Myc-tagged CK1α was microinjected into the nucleus of MCF-7 cells. The effect of expression of CK1α on E-cadherin-based cell-cell contacts was analyzed by immunofluorescence microscopy with the indicated antibodies. Scale bar, 40 μm.
CK1 colocalizes and interacts with E-cadherin.
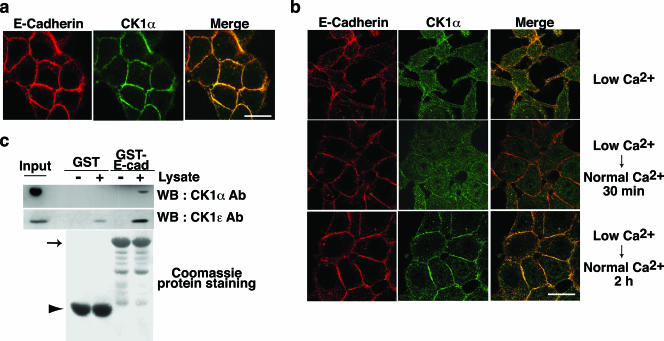
We analyzed the subcellular localization of CK1α in MDCK cells by immunostaining with anti-CK1α antibody. At steady state, CK1α accumulated at cell-cell contact sites, where it colocalized with E-cadherin (Fig. 4a). We further examined whether CK1 localization is dynamically regulated under Ca2+ switch conditions. In low-Ca2+ medium, MDCK cells lost their tight E-cadherin-based cell-cell contacts, and CK1α was diffusely distributed into the cytosol and was no longer concentrated at the plasma membrane (Fig. 4b, upper panels). Upon reversion to normal Ca2+ medium, E-cadherin accumulated at cell-cell adhesion sites. CK1α was recruited to these newly forming cell-cell contact sites, although the time course of this recruitment was slightly slower than that of E-cadherin (Fig. 4b, middle and lower panels). By using MDCK cells stably expressing GFP-tagged CK1α, we observed a similar subcellular localization of CK1α (see Fig. S2 in the supplemental material). Thus, CK1α colocalizes with E-cadherin at cell-cell contact sites, and this localization is dynamically regulated during the formation of cell-cell adhesions.
FIG. 4.
CK1 colocalizes and interacts with E-cadherin. (a and b) Colocalization between CK1α and E-cadherin in MDCK cells at steady state (a) or during Ca2+ switch (b). The subcellular localization of E-cadherin and CK1α was examined in MDCK cells using anti-E-cadherin and anti-CK1α antibodies. Scale bars, 10 μm. (c) Interaction between CK1 and E-cadherin (E-cad) by GST pull-down assays. Beads coupled to GST or the GST-tagged E-cadherin cytoplasmic domain were incubated with MCF-7 cell lysates. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting (WB) with anti-CK1α and anti-CK1ɛ antibodies (Ab). The arrow and arrowhead indicate the positions of GST-E-cadherin and GST, respectively.
We next examined the interaction between CK1 and E-cadherin using a GST pull-down assay. Endogenous CK1α from MCF-7 cells bound to GST-E-cadherin-coupled beads but not to GST-coupled beads (Fig. 4c, upper panel). Endogenous CK1ɛ also showed a strong preference for GST-E-cadherin beads (Fig. 4c, middle panel). The interaction was further examined by immunoprecipitation using MCF-7 or MDCK cells. However, we could not consistently detect coimmunoprecipitation between E-cadherin and CK1 (data not shown), suggesting that the interaction occurs transiently or catalytically in cells.
CK1 phosphorylates the cytoplasmic domain of E-cadherin.
We used an in vivo phosphorylation assay to examine whether CK1 phosphorylates the components of the E-cadherin complex. MCF-7 cells were incubated with 32P-labeled orthophosphate in the presence or absence of IC261, followed by immunoprecipitation with anti-E-cadherin antibody, SDS-PAGE, and autoradiography. In the E-cadherin complex, phosphorylation of E-cadherin, α-catenin, and β-catenin was detected (Fig. 5a and b). Under low-confluence conditions, the addition of IC261 reduced the phosphorylation of E-cadherin and α-catenin by approximately 50% (Fig. 5a). Both proteins remained phosphorylated even in the presence of IC261, suggesting that kinases other than CK1 also phosphorylate E-cadherin and α-catenin. When cells were cultured at higher densities, the phosphorylation of E-cadherin was not affected by IC261 (Fig. 5b); thus, the confluence of cells affects the phosphorylation of E-cadherin. However, when cells were incubated in low-Ca2+ medium, IC261 suppressed the phosphorylation of E-cadherin (Fig. 5b). IC261 reduced the phosphorylation of α-catenin under both normal and low-Ca2+ conditions but had no effect on the phosphorylation of β-catenin. The amounts of β-catenin and α-catenin bound to E-cadherin were not affected under these experimental conditions (Fig. 5a and b and data not shown). These data indicate that CK1 phosphorylates both E-cadherin and α-catenin in MCF-7 cells.
It has been reported that CK1 phosphorylates serine 45 of β-catenin, which primes the subsequent phosphorylation by GSK-3β and enhances the proteasomal degradation of β-catenin (23, 40). We produced nonphosphorylatable (S45A) and pseudophosphorylated (S45D) β-catenin mutants and studied whether such mutations affect the adhesive properties of β-catenin in epithelial cells. However, both mutants localized at cell-cell contact sites in MDCK cells and bound equally to E-cadherin in a GST-E-cadherin pull-down assay (data not shown). Thus, we could not obtain any data indicating a significant role of CK1-mediated phosphorylation of β-catenin in the formation of cell-cell contacts. Therefore, we focused on the CK1-mediated phosphorylation of E-cadherin. The significance of CK1-mediated phosphorylation of α-catenin is currently being investigated.
Next, we studied whether CK1 can directly phosphorylate E-cadherin. Using an in vitro phosphorylation assay, we found that purified CK1δ protein efficiently phosphorylates GST-E-cadherin but not GST (Fig. 5c). By contrast, another kinase, PKCζ, did not phosphorylate GST-E-cadherin, suggesting that CK1 specifically phosphorylates E-cadherin. About 0.8 mol of phosphate was maximally incorporated into 1 mol of E-cadherin (data not shown), indicating that E-cadherin is indeed a prominent substrate of CK1.
There are three known substrate consensus sequences for CK1 phosphorylation: D/EXXS/T, PO4-S/TXXS/T, and SLS (where X is any amino acid and S/T in boldface indicates the phosphorylation site). In the case of β-catenin, S45 matches with the third consensus sequence, SLS (Fig. 5d). To determine the CK1 phosphorylation sites of E-cadherin, we first aligned the amino acid sequences of the cytoplasmic domains of several classical cadherins from different species. Interestingly, in the β-catenin-binding site, there is a highly conserved region that includes SLS at amino acid position 846 in murine E-cadherin (Fig. 5d). There are other conserved serine/threonine residues at amino acid positions 849, 852, and 855 in murine E-cadherin. They satisfy the second consensus sequence, PO4-S/TXXS/T, that could be sequentially phosphorylated following the primed S846 phosphorylation. To identify the CK1 phosphorylation sites on E-cadherin, we tested a series of nonphosphorylatable mutants of E-cadherin (at amino acids 846, 849, 852, and/or 855) for phosphorylation in vitro. The single-amino-acid substitution S846A reduced CK1-catalyzed phosphorylation by 70 to 80%, while mutations at positions 849, 852, and 855 did not affect the level of phosphorylation (Fig. 5e, left panels). This indicates that S846 is the major phosphorylation site for CK1. We also tested mutations of other residues (T750 and S848), but phosphorylation was not affected by these mutations (data not shown). Thus, other minor CK1 phosphorylation sites on E-cadherin remain to be clarified. CK2 is another serine/threonine kinase that has been reported to phosphorylate E-cadherin and enhance E-cadherin-β-catenin interactions (22). For CK2, the mutation at position 846 did not affect phosphorylation; instead, the mutations at positions 849, 852, and 855 reduced phosphorylation (Fig. 5e, right panels). These data indicate that CK1 and CK2 phosphorylate distinct serine residues.
It was shown previously that CK1-catalyzed phosphorylation primes the subsequent phosphorylation of β-catenin by GSK-3β (23). E-cadherin also contains a serine residue at S842, although not conserved in fly, which matches with a substrate consensus sequence for GSK-3β phosphorylation (S/TXXXS-PO4) following the CK1-catalyzed phosphorylation at S846 (Fig. 5d). However, CK1-catalyzed phosphorylation of E-cadherin did not prime the phosphorylation by GSK-3β in in vitro phosphorylation assays (see Fig. S3 in the supplemental material), and GSK-3β inhibitor did not affect the effect of IC261 on cell dissociation (data not shown).
CK1-induced phosphorylation of E-cadherin attenuates its adhesive function at cell-cell contact sites.
To explore the functional significance of the CK1-induced phosphorylation of E-cadherin, we mutated the major phosphorylation residue S846 into alanine (S846A) and aspartic acid (S846D) to produce nonphosphorylatable and pseudophosphorylated mutant forms of E-cadherin, respectively. The Myc-tagged wild-type and mutant forms of E-cadherin were stably expressed in L fibroblast cells, and more than five independent clones were analyzed for each of the different forms of E-cadherin. Upon expression of wild-type or nonphosphorylatable E-cadherin, L cells formed stable cell-cell contacts under high-confluence conditions and became epithelium-like (Fig. 6b, left and middle panels). In contrast, L cells expressing the pseudophosphorylated E-cadherin mutant did not form tight cell-cell contacts and remained fibroblastic (Fig. 6b, right panel), despite a similar level of E-cadherin expression (Fig. 6a). Consistently, when cultured in suspension, L cells expressing wild-type or nonphosphorylatable E-cadherin formed large cell aggregates, while those expressing the pseudophosphorylated E-cadherin did not (Fig. 6c). Differences in the strengths of cell-cell adhesions were quantified using TC treatment or TE treatment (30). Cell aggregates were counted, and the cell DI (NTC/NTE, where NTC and NTE are the total particle numbers after the TC and TE treatment) was calculated (Table 1). This index was 0.64, 0.46, and 0.88 in the cells expressing wild type, S846A, and S846D E-cadherin, respectively. The differences between the wild type and S846A and between the wild type and S846D are statistically significant (P < 0.01 and P < 0.005, respectively). Furthermore, we examined the effect of the mutations on the localization of E-cadherin by immunofluorescence. Both wild-type and nonphosphorylatable E-cadherin accumulated at cell-cell contact sites, while pseudophosphorylated E-cadherin was diffusely distributed in the cytosol and on the plasma membrane (Fig. 6d). Taken together, these data indicate that the single-amino-acid substitution in the major CK1 phosphorylation site of E-cadherin influences the adhesive activity of E-cadherin and that upon phosphorylation on S846, E-cadherin is unable to localize at cell-cell contact sites or to mediate stable intercellular adhesions.
FIG. 6.
Mutations in the major CK1 phosphorylation residue of E-cadherin affect adhesiveness of cell-cell contacts in L fibroblast cells stably expressing E-cadherin mutants. L fibroblast clones stably expressing the Myc-tagged wild type and nonphosphorylatable (S846A) and pseudophosphorylated (S846D) mutants of E-cadherin were obtained. More than five independent clones were analyzed for each of the different types of E-cadherin, and analogous data were obtained between clones expressing the same type of E-cadherin. The data using representative clones (WT2, A5, and D13 for wild-type, S846A, and S846D E-cadherin, respectively) are shown. (a) Expression level of E-cadherin mutants in the L fibroblast clones. Cell lysates (20 μg of proteins) from the indicated clones were analyzed by Western blotting (WB) with anti-Myc antibody (Ab). (b) Effect of mutations of E-cadherin on cell-cell contact formation. Nontransfected cells or the indicated clones were cultured at high density and analyzed by phase-contrast microscopy. Scale bar, 40 μm. (c) Effect of mutations of E-cadherin on formation of cell aggregates. Nontransfected cells or the indicated clones were cultured in suspension and examined by phase-contrast microscopy. Scale bar, 40 μm. (d) Effect of mutations on subcellular localization of E-cadherin. The indicated clones were cultured at low density and analyzed by immunostaining with anti-Myc antibody. Scale bar, 20 μm. IF, immunofluorescence. (e) Effect of mutations of E-cadherin on IC261-induced stabilization of cell-cell contacts. The indicated clones were cultured at a low density in the presence or absence of 10 μM IC261 for 4 h and analyzed by phase-contrast microscopy. Scale bar, 20 μm.
TABLE 1.
Cell DI of L cells expressing E-cadherin mutants in the presence or absence of IC261a
| Treatment | DI (NTC/NTE)
|
|||
|---|---|---|---|---|
| Parental L cell | L cell expressing E-cad (WT) WT2 | L cell expressing E-cad (S846A) A5 | L cell expressing E-cad (S846D) D13 | |
| − IC261 | 0.83 ± 0.09 | 0.64 ± 0.09a | 0.46 ± 0.09**b | 0.88 ± 0.04***c |
| + IC261 | 0.91 ± 0.05 | 0.52 ± 0.05*d | 0.41 ± 0.09*e | 0.72 ± 0.06 |
*, P < 0.05 (a and d, b and e); **, P < 0.01 (a and b); ***, P < 0.005 (a and c). The data were obtained from more than four independent experiments. E-cad, E-cadherin; WT, wild type.
We also tested the effect of IC261 on these transfected cells. At low cell densities, L cells expressing wild-type E-cadherin formed more stable cell-cell contacts in the presence of IC261 (Fig. 2d and 6e, upper panels). Interestingly, IC261 also affected cells expressing nonphosphorylatable E-cadherin in a similar manner (Fig. 6e, middle panels). In contrast, IC261 did not clearly promote cell-cell adhesions between cells expressing pseudophosphorylated E-cadherin (Fig. 6e, lower panels), although the cell DI showed a minor effect (Table 1). The effect of IC261 on these cells was also confirmed by quantifying the cell DI (Table 1). Thus, the stabilizing effect of IC261 on cell-cell contacts is partly attributed to the modulation of S846 of E-cadherin. However, the phosphorylation of other proteins and/or other minor phosphorylation sites on E-cadherin are also required for the IC261 effect.
To investigate the significance of CK1-mediated phosphorylation of E-cadherin further, we established MDCK epithelial cells stably expressing the E-cadherin mutants (Fig. 7A). The expression of wild-type or S846A E-cadherin did not induce obvious morphological changes, and both exogenous and endogenous E-cadherin accumulated at cell-cell contact sites (Fig. 7b, left and middle panels). In contrast, cells expressing S846D E-cadherin were more flattened, with some fibroblastic characteristics also observed, and both exogenous S846D and endogenous E-cadherin failed to accumulate at cell-cell adhesions (Fig. 7b, right panel). As the cell density increased, however, both endogenous and exogenous S846D E-cadherin accumulated at cell-cell contact sites (data not shown). Thus, in epithelial cells, the expression of pseudophosphorylated E-cadherin induced a dominant-negative effect, inhibiting the formation of cell-cell contacts under conditions of low confluence.
FIG. 7.
Mutations in the major CK1 phosphorylation site of E-cadherin affect localization of E-cadherin in MDCK cells stably expressing E-cadherin mutants. MDCK cell clones stably expressing the Myc-tagged wild type and nonphosphorylatable (S846A) and pseudophosphorylated (S846D) mutants of E-cadherin were obtained. More than five independent clones were analyzed for each of the different types of E-cadherin, and analogous data were obtained between clones expressing the same types of E-cadherin. Data using representative clones (WT25 and WT28, A28, and D23 for wild-type, S846A, and S846D E-cadherin, respectively) are shown. (a) Expression level of E-cadherin mutants in MDCK cell clones. Cell lysates (20 μg of proteins) from the indicated clones were analyzed by Western blotting (WB) with anti-Myc and anti-E-cadherin antibodies (Ab). The arrowhead and arrow indicate the positions of exogenous Myc-tagged E-cadherin and endogenous E-cadherin, respectively. (b) Effect of mutations in the major CK1 phosphorylation site of E-cadherin on its localization in MDCK cells. The indicated clones were examined by phase-contrast microscopy and immunofluorescence (IF) microscopy with anti-Myc and anti-E-cadherin antibodies. It should be noted that anti-E-cadherin antibody detects both exogenous and endogenous E-cadherin. Scale bars, 20 μm.
CK1 phosphorylation of E-cadherin decreases the interaction between E-cadherin and β-catenin and enhances endocytosis of the E-cadherin complex.
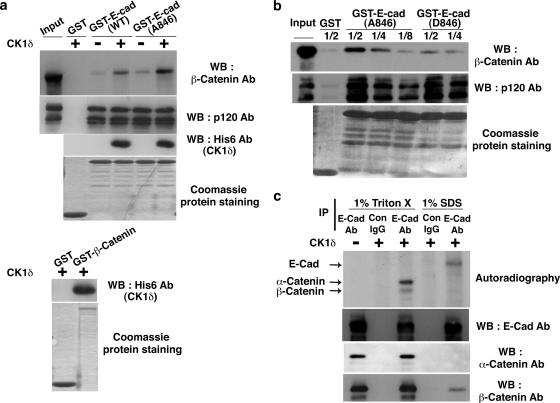
Finally, we explored the molecular mechanism by which CK1 regulates cell-cell contacts. First, we examined the effect of CK1 on the interaction of E-cadherin with the binding proteins. The GST-E-cadherin wild type or S846A was first incubated with ATP and CK1δ at 30°C and then mixed with HEK293 cell lysates, followed by GST pull-down assays. The amounts of E-cadherin-bound β-catenin or p120 catenin from the lysates were examined by Western blotting. CK1δ bound both the E-cadherin wild type and S846A and enhanced the interaction of E-cadherin with β-catenin but not with p120 (Fig. 8a, upper panels). This effect of CK1δ was observed even when CK1δ and E-cadherin were incubated with ATPγS (see Fig. S4a in the supplemental material). Taken together with the data showing that CK1δ can directly interact with β-catenin (Fig. 8a, lower panels), the effect of CK1δ on the increased binding between E-cadherin and β-catenin is not through phosphorylation but is through the formation of a ternary complex between three proteins. Interestingly, in the presence of CK1δ, a higher amount of β-catenin bound to E-cadherin S846A than to the E-cadherin wild type (Fig. 8a, upper panel). Next, we tested whether CK1 phosphorylation of E-cadherin itself affects the affinity of E-cadherin for its binding proteins. Beads coupled to GST-E-cadherin S846A or S846D were incubated with HEK293 cell lysates. When titration was performed with decreasing amounts of cell lysates, it was revealed that the interaction of β-catenin with pseudophosphorylated E-cadherin was weaker than that with nonphosphorylatable E-cadherin (Fig. 8b, upper panel). Interactions with p120 were not suppressed by the mutations (Fig. 8b, middle panel). These results indicate that E-cadherin, β-catenin, and CK1δ can form a ternary protein complex but that once E-cadherin is phosphorylated, direct interactions between E-cadherin and β-catenin are reduced.
FIG. 8.
CK1-catalyzed phosphorylation of E-cadherin affects the interaction between E-cadherin and β-catenin and vice versa. (a) Effect of CK1 on the interaction between E-cadherin and the binding proteins. (Upper panel) GST or the GST-tagged cytoplasmic domain of wild-type (WT) or nonphosphorylatable (S846A) E-cadherin was coupled to glutathione-Sepharose beads and incubated with ATP in the presence or absence of His6-tagged CK1δ at 30°C for 30 min. The beads were then incubated with HEK293 cell lysate at 4°C, followed by GST pull-down assays. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting (WB) with anti-His6 antibody (Ab). (Lower panel) GST or GST-β-catenin was coupled to glutathione-Sepharose beads and incubated with His6-CK1δ, followed by GST pull-down assays. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting with the indicated antibodies. (b) Effect of mutations of E-cadherin on its interaction with binding proteins. GST or the GST-tagged cytoplasmic domain of nonphosphorylatable (S846A) or pseudophosphorylated (S846D) E-cadherin was coupled to glutathione-Sepharose beads. The beads were then incubated with the titrated amount of HEK293 cell lysate (one-half, one-quarter, or one-eighth of cell lysate from an 80% confluent culture in a 9-cm plate). The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting with the indicated antibodies. (c) Interaction between E-cadherin and β-catenin prevents phosphorylation of E-cadherin by CK1. To uncouple β-catenin from E-cadherin, MCF-7 cells were lysed in 1% SDS lysis buffer. The lysate was then diluted 10-fold prior to immunoprecipitation (IP). Otherwise, immunoprecipitation was performed in 1% Triton X-100 lysis buffer. After immunoprecipitation, beads were intensively washed, and in vitro phosphorylation assays were performed with [γ-32P]ATP and CK1δ, followed by autoradiography and Western blotting with anti-E-cadherin, -α-catenin, and -β-catenin antibodies. Arrows indicate the positions of E-cadherin, α-catenin, and β-catenin.
We also studied whether the interaction between E-cadherin and β-catenin blocks CK1-induced phosphorylation of E-cadherin. E-cadherin was immunoprecipitated from MCF-7 cell lysates under mild- or harsh-detergent conditions to precipitate the E-cadherin-β-catenin complex or E-cadherin free from β-catenin, respectively (11), followed by an in vitro phosphorylation assay. E-cadherin that did not bind to β-catenin was more efficiently phosphorylated by CK1 than E-cadherin that was bound to β-catenin (Fig. 8c). When recombinant β-catenin was added to E-cadherin, the phosphorylation of E-cadherin was reduced (see Fig. S4b in the supplemental material). Both α-catenin and β-catenin that bound to E-cadherin were phosphorylated by CK1 (Fig. 8c). Taken together, these data suggest that dissociation from β-catenin promotes the phosphorylation of E-cadherin by CK1.
We then examined whether CK1 is involved in the endocytosis of E-cadherin by using surface biotinylation assays. Under low-Ca2+ conditions, the endocytosis of E-cadherin is enhanced, leading to a disruption of E-cadherin-based cell-cell contacts (21). In the presence of IC261, this low-Ca2+-induced separation of cell-cell adhesions is strongly suppressed in MCF-7 cells (Fig. 2b and c). Indeed, the biotinylation assay revealed that the internalization of E-cadherin induced by low-Ca2+ treatment was suppressed by IC261 (Fig. 9a). We also examined whether CK1 phosphorylation of E-cadherin affects the endocytosis of E-cadherin in MDCK cells. Upon low-Ca2+ treatment, nonphosphorylatable E-cadherin (S846A) was not efficiently endocytosed compared with wild-type E-cadherin (Fig. 9b). In contrast, pseudophosphorylated E-cadherin (S846D) was more efficiently internalized from the plasma membrane than wild-type E-cadherin (Fig. 9c). The endocytosis of E-cadherin S846D was not significantly suppressed by IC261 (see Fig. S4c in the supplemental material). The internalization of the E-cadherin wild type and that of S846D were further compared by using immunofluorescent analysis. As shown in Fig. 9d, higher amounts of E-cadherin S846D were localized in early endosomes than those of the E-cadherin wild type. The quantification of the immunostaining intensity showed that the percentages of endosomal E-cadherin relative to overall E-cadherin were 5.7% and 13.3% for the E-cadherin wild type and S846D, respectively (statistically different; P < 0.01 [n = 10]). These data indicate that CK1 plays a positive role in the endocytosis of E-cadherin and that E-cadherin is an important substrate of CK1 in this process.
Since the association of E-cadherin with β-catenin has been reported to affect cadherin targeting to the plasma membrane (7), we also examined the involvement of CK1-catalyzed phosphorylation in this process by combining pulse-chase and surface biotinylation assays. Newly synthesized E-cadherin was metabolically labeled with 35S, and its transport to the plasma membrane was monitored by biotinylation with NHS-SS-biotin in the presence or absence of IC261 for 45 min (data not shown) or 2 h (see Fig. S4d in the supplemental material). We found that the addition of IC261 did not significantly influence the transport of newly synthesized E-cadherin to the plasma membrane, suggesting that CK1 is not involved in cadherin membrane targeting.
DISCUSSION
In this study, we provide evidence for a novel mechanism that regulates E-cadherin-based cell-cell adhesions: CK1 phosphorylation of the cytoplasmic domain of E-cadherin. Upon phosphorylation, the endocytosis of E-cadherin is enhanced, and E-cadherin loses its stable localization at cell-cell contact sites. In yeast, CK1 has been reported to be involved in the endocytosis of many membrane proteins, including the α-factor pheromone receptor and uracil permease (16, 24, 25). CK1-induced phosphorylation of these membrane proteins primes the subsequent ubiquitination, leading to their internalization and/or sorting to the vacuole for protein degradation. In mammals, however, the mode of regulation of endocytosis by CK1 may be different. Our findings did not suggest the involvement of ubiquitination in the CK1-induced endocytosis of E-cadherin. Firstly, IC261 did not affect E-cadherin endocytosis or disruption of E-cadherin-based cell-cell contacts induced by Src, a process that is dependent on the ubiquitination of the E-cadherin complex (11). In addition, we did not detect the ubiquitination of the pseudophosphorylated (S846D) E-cadherin (data not shown).
The function of CK1 is regulated by both its subcellular localization and catalytic activity (for a review, see reference 20). In epithelial cells, we found that CK1α is localized at cell-cell contact sites, and its localization is dynamically regulated during the modulation of cell-cell contacts. When cells separate from each other, CK1α does not accumulate on the plasma membrane. However, as cells reform intercellular adhesions, CK1α is recruited at cell-cell contact sites. The molecular mechanism for this recruitment remains to be resolved. In addition, several molecular mechanisms regulate the catalytic activity of CK1. First, the C-terminal domain of CK1 contains inhibitory autophosphorylation sites. The truncation of the C terminus or the dephosphorylation of autophosphorylation sites by phosphatases increases CK1 activity (5, 6, 13). Second, in some cell types, an increase of the plasma membrane concentration of phosphatidylinositol 4,5-biphosphate reduces CK1α activity (4, 14). It is not clearly understood what physiological stimuli regulate CK1 activity in epithelial cells. One possible stimulus is the Wnt signaling pathway. Activation by Wnt-3a has been reported to activate CK1ɛ in HEK293 cells (37). Moreover, CK1 phosphorylates various components of the Wnt signaling pathway, regulating this pathway either positively or negatively (10, 12, 23, 40, 41). Taken together with our finding that CK1-mediated phosphorylation of E-cadherin attenuates its interaction with β-catenin, CK1 may regulate both Wnt signaling and cell-cell contacts simultaneously in epithelial cells.
There are seven isoforms (α, β, γ1, γ2, γ3, δ, and ɛ) of CK1 that have distinct substrate specificities and subcellular localizations (for a review, see reference 20). Whether these isoforms have distinct functions or compensate for each other seems signaling pathway and cell context dependent. For example, in a canonical Wnt signaling pathway, CK1α is a negative regulator (23), whereas CK1γ3, CK1δ, and CK1ɛ positively regulate the pathway (10, 12, 27, 33, 35). In contrast, in a noncanonical Wnt pathway, CK1α cooperates with CK1ɛ positively in planar cell polarity signaling (36). We show here that the overexpression of either CK1α or CK1ɛ destabilizes E-cadherin-based cell-cell contacts. RNA interference of CK1α and/or CK1ɛ in MCF-7 cells, inducing maximally a 50% reduction of endogenous CK1 proteins, did not induce any significant effects on cell morphology (data not shown). Thus, it is possible that multiple CK1 isoforms may be involved in the regulation of cell-cell contacts, depending on the cell type, which needs to be studied in the future. To further characterize the functional role of CK1 in vivo, it may be advantageous to study lower organisms that contain fewer CK1 isoforms.
Within the CH3 domain of cadherins, there are serine/threonine clusters that overlap largely with the β-catenin-binding site. The finding that IC261 did not completely abolish E-cadherin phosphorylation suggests that another kinase(s) also phosphorylates E-cadherin. Indeed, CK2 has been shown to phosphorylate serine/threonine residues in the CH3 domain, which enhances the interaction of E-cadherin with β-catenin (18, 22). However, the suggested CK2 phosphorylation sites (S840, S853, and S855) are not well conserved among classical cadherins of different species. In contrast, we found that S846 shows striking conservation within classical cadherins and that it is the major CK1 phosphorylation site of E-cadherin. In addition, the phosphorylation at S846 decreases the interaction with β-catenin. Thus, the balance of CK1- and CK2-catalyzed phosphorylation may determine the binding affinity between E-cadherin and β-catenin. Furthermore, the effect of IC261 on the stabilization of cell-cell contacts cannot be attributed solely to the S846 phosphorylation of E-cadherin (Fig. 6e), suggesting the existence of other substrate proteins for CK1. Indeed, CK1 has been reported to phosphorylate other cell-cell adhesion proteins such as occludin and connexin, although the functional significance of these phosphorylations remains to be clarified (9, 28). It is plausible that CK1 phosphorylates multiple junctional proteins in order to dynamically regulate the different types of cell-cell junctions in a coordinated manner.
Cadherin-based cell-cell adhesions are dynamically regulated during cancer metastasis, mitosis, and epithelial-mesenchymal transitions in embryonic development. It is now important to determine whether CK1-mediated phosphorylation of cadherin is involved in these processes. To resolve this, efficient experimental assays to monitor the activity of CK1 and specific antibodies against phosphorylated cadherin need to be established. Other important questions are what stimuli activate CK1 in vivo and how CK1-mediated phosphorylation of E-cadherin enhances the endocytosis of E-cadherin. Future investigations into these questions will lead us to further understand this vital molecular mechanism that dynamically regulates cell-cell adhesions.
Supplementary Material
Acknowledgments
We thank Martin Raff and Mark Marsh for critical reading of the manuscript. Norberto Serpente is acknowledged for technical help. We also thank Xi He (Boston) and Bill Weis (Stanford) for providing CK1 constructs and pGEX-TEV-β-catenin, respectively.
S.D.-C. was supported by an FEBS Long Term Fellowship. A.F. was supported by a postdoctoral fellowship from Ministerio de Educacion, Cultura y Deporte of Spain. This work is supported by MRC funding to the Cell Biology Unit.
Footnotes
Published ahead of print on 12 March 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bauer, A., H. Lickert, R. Kemler, and J. Stappert. 1998. Modification of the E-cadherin-catenin complex in mitotic Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 273:28314-28321. [DOI] [PubMed] [Google Scholar]
- 2.Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga, V. 2002. Cell-cell interactions: a practical approach. Oxford University Press, Oxford, England.
- 4.Brockman, J. L., and R. A. Anderson. 1991. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J. Biol. Chem. 266:2508-2512. [PubMed] [Google Scholar]
- 5.Carmel, G., B. Leichus, X. Cheng, S. D. Patterson, U. Mirza, B. T. Chait, and J. Kuret. 1994. Expression, purification, crystallization, and preliminary X-ray analysis of casein kinase-1 from Schizosaccharomyces pombe. J. Biol. Chem. 269:7304-7309. [PubMed] [Google Scholar]
- 6.Cegielska, A., K. F. Gietzen, A. Rivers, and D. M. Virshup. 1998. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J. Biol. Chem. 273:1357-1364. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y. T., D. B. Stewart, and W. J. Nelson. 1999. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 144:687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citi, S. 1992. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J. Cell Biol. 117:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, C. D., and P. D. Lampe. 2002. Casein kinase 1 regulates connexin-43 gap junction assembly. J. Biol. Chem. 277:44962-44968. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, G., W. Wu, J. Shen, J. Bilic, U. Fenger, P. Stannek, A. Glinka, and C. Niehrs. 2005. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438:867-872. [DOI] [PubMed] [Google Scholar]
- 11.Fujita, Y., G. Krause, M. Scheffner, D. Zechner, H. E. Leddy, J. Behrens, T. Sommer, and W. Birchmeier. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4:222-231. [DOI] [PubMed] [Google Scholar]
- 12.Gao, Z. H., J. M. Seeling, V. Hill, A. Yochum, and D. M. Virshup. 2002. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. USA 99:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gietzen, K. F., and D. M. Virshup. 1999. Identification of inhibitory autophosphorylation sites in casein kinase I epsilon. J. Biol. Chem. 274:32063-32070. [DOI] [PubMed] [Google Scholar]
- 14.Gross, S. D., D. P. Hoffman, P. L. Fisette, P. Baas, and R. A. Anderson. 1995. A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J. Cell Biol. 130:711-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumbiner, B. M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6:622-634. [DOI] [PubMed] [Google Scholar]
- 16.Hicke, L., B. Zanolari, and H. Riezman. 1998. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J. Cell Biol. 141:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan, C., N. Serpente, P. Cogram, C. R. Hosking, C. U. Bialucha, S. M. Feller, V. M. Braga, W. Birchmeier, and Y. Fujita. 2004. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 24:6690-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, A. H., and W. I. Weis. 2001. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 105:391-402. [DOI] [PubMed] [Google Scholar]
- 19.Jarrett, O., J. L. Stow, A. S. Yap, and B. Key. 2002. Dynamin-dependent endocytosis is necessary for convergent-extension movements in Xenopus animal cap explants. Int. J. Dev. Biol. 46:467-473. [PubMed] [Google Scholar]
- 20.Knippschild, U., A. Gocht, S. Wolff, N. Huber, J. Lohler, and M. Stoter. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell. Signal. 17:675-689. [DOI] [PubMed] [Google Scholar]
- 21.Le, T. L., A. S. Yap, and J. L. Stow. 1999. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146:219-232. [PMC free article] [PubMed] [Google Scholar]
- 22.Lickert, H., A. Bauer, R. Kemler, and J. Stappert. 2000. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J. Biol. Chem. 275:5090-5095. [DOI] [PubMed] [Google Scholar]
- 23.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 24.Marchal, C., S. Dupre, and D. Urban-Grimal. 2002. Casein kinase I controls a late step in the endocytic trafficking of yeast uracil permease. J. Cell Sci. 115:217-226. [DOI] [PubMed] [Google Scholar]
- 25.Marchal, C., R. Haguenauer-Tsapis, and D. Urban-Grimal. 2000. Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines signal endocytosis of yeast uracil permease. J. Biol. Chem. 275:23608-23614. [DOI] [PubMed] [Google Scholar]
- 26.Mashhoon, N., A. J. DeMaggio, V. Tereshko, S. C. Bergmeier, M. Egli, M. F. Hoekstra, and J. Kuret. 2000. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J. Biol. Chem. 275:20052-20060. [DOI] [PubMed] [Google Scholar]
- 27.McKay, R. M., J. M. Peters, and J. M. Graff. 2001. The casein kinase I family in Wnt signaling. Dev. Biol. 235:388-396. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie, J. A., K. Riento, and A. J. Ridley. 2006. Casein kinase I epsilon associates with and phosphorylates the tight junction protein occludin. FEBS Lett. 580:2388-2394. [DOI] [PubMed] [Google Scholar]
- 29.Miller, J. R., and D. R. McClay. 1997. Characterization of the role of cadherin in regulating cell adhesion during sea urchin development. Dev. Biol. 192:323-339. [DOI] [PubMed] [Google Scholar]
- 30.Nagafuchi, A., S. Ishihara, and S. Tsukita. 1994. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 127:235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palacios, F., J. S. Tushir, Y. Fujita, and C. D'Souza-Schorey. 2005. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 25:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Moreno, M., C. Jamora, and E. Fuchs. 2003. Sticky business: orchestrating cellular signals at adherens junctions. Cell 112:535-548. [DOI] [PubMed] [Google Scholar]
- 33.Peters, J. M., R. M. McKay, J. P. McKay, and J. M. Graff. 1999. Casein kinase I transduces Wnt signals. Nature 401:345-350. [DOI] [PubMed] [Google Scholar]
- 34.Rimm, D. L., and J. S. Morrow. 1994. Molecular cloning of human E-cadherin suggests a novel subdivision of the cadherin superfamily. Biochem. Biophys. Res. Commun. 200:1754-1761. [DOI] [PubMed] [Google Scholar]
- 35.Sakanaka, C., P. Leong, L. Xu, S. D. Harrison, and L. T. Williams. 1999. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc. Natl. Acad. Sci. USA 96:12548-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strutt, H., M. A. Price, and D. Strutt. 2006. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr. Biol. 16:1329-1336. [DOI] [PubMed] [Google Scholar]
- 37.Swiatek, W., I. C. Tsai, L. Klimowski, A. Pepler, J. Barnette, H. J. Yost, and D. M. Virshup. 2004. Regulation of casein kinase I epsilon activity by Wnt signaling. J. Biol. Chem. 279:13011-13017. [DOI] [PubMed] [Google Scholar]
- 38.Thoreson, M. A., P. Z. Anastasiadis, J. M. Daniel, R. C. Ireton, M. J. Wheelock, K. R. Johnson, D. K. Hummingbird, and A. B. Reynolds. 2000. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148:189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulrich, F., M. Krieg, E. M. Schotz, V. Link, I. Castanon, V. Schnabel, A. Taubenberger, D. Mueller, P. H. Puech, and C. P. Heisenberg. 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9:555-564. [DOI] [PubMed] [Google Scholar]
- 40.Yanagawa, S., Y. Matsuda, J. S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng, X., K. Tamai, B. Doble, S. Li, H. Huang, R. Habas, H. Okamura, J. Woodgett, and X. He. 2005. A dual-kinase mechanism for Wnt coreceptor phosphorylation and activation. Nature 438:873-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.