Abstract
The insulin-like growth factors (insulin-like growth factor I [IGF-I] and IGF-II) exert important effects on growth, development, and differentiation through the IGF-I receptor (IGF-IR) transmembrane tyrosine kinase. The insulin receptor (IR) is structurally related to the IGF-IR, and at high concentrations, the IGFs can also activate the IR, in spite of their generally low affinity for the latter. Two mechanisms that facilitate cross talk between the IGF ligands and the IR at physiological concentrations have been described. The first of these is the existence of an alternatively spliced IR variant that exhibits high affinity for IGF-II as well as for insulin. A second phenomenon is the ability of hybrid receptors comprised of IGF-IR and IR hemireceptors to bind IGFs, but not insulin. To date, however, direct activation of an IR holoreceptor by IGF-I at physiological levels has not been demonstrated. We have now found that IGF-I can function through both splice variants of the IR, in spite of low affinity, to specifically activate IRS-2 to levels similar to those seen with equivalent concentrations of insulin or IGF-II. The specific activation of IRS-2 by IGF-I through the IR does not result in activation of the extracellular signal-regulated kinase pathway but does induce delayed low-level activation of the phosphatidylinositol 3-kinase pathway and biological effects such as enhanced cell viability and protection from apoptosis. These findings suggest that IGF-I can function directly through the IR and that the observed effects of IGF-I on insulin sensitivity may be the result of direct facilitation of insulin action by IGF-I costimulation of the IR in insulin target tissues.
The insulin-like growth factors (insulin-like growth factor I [IGF-I] and IGF-II) are members of a complex signaling network that regulates growth, development, and differentiation at the tissue level and proliferation and survival at the cellular level (13, 38, 39). In addition to the ligands, the IGF signaling system also includes the IGF-I receptor (IGF-IR), the IGF-II/cation-independent mannose-6-phosphate receptor (IGF-IIR), and the insulin receptor (IR). The IGF-IIR specifically binds IGF-II but is devoid of signal transduction capability, and its primary function with respect to IGF action is as a clearance receptor that can modulate the bioavailability of extracellular IGF-II (20, 45). The IGF-IR, on the other hand, is generally considered to mediate the majority of the biological effects of both IGF-I and IGF-II (25). The IGF-IR is a transmembrane heterotetrameric (α2-β2) tyrosine kinase that is comprised of two extracellular, ligand-binding α subunits that are linked by disulfide bonds to each other and to the transmembrane β subunits that contain intrinsic tyrosine kinase activity. The IR, which is primarily activated by insulin, is structurally related to the IGF-IR (10). Both the IGF-IR and the IR undergo autophosphorylation after ligand activation; tyrosine-phosphorylated residues in the juxtamembrane domain of the β subunits then recruit insulin receptor substrate 1 (IRS-1) and IRS-2, which serve as scaffolding/adaptor proteins that couple the activated IGF-IR or IR to upstream components of the phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase (Erk) signal transduction cascades (23, 52). A final component of the IGF signaling system is a family of six high-affinity IGF-binding proteins (IGFBPs) that occur in cell surface-associated forms and in the circulation and in extracellular fluids. The IGFBPs can modulate IGF actions both positively and negatively through effects on IGF half-life and receptor interaction (2, 14, 17).
While, under normal conditions, the IGFs function primarily through the IGF-IR, and insulin functions exclusively through the IR (26), there is ample experimental evidence for cross talk between the IGF and insulin ligands and their respective receptors. At high, nonphysiological concentrations, IGFs exhibit activation of the IR and insulin can activate the IGF-IR. These effects are typically seen at high nanomolar concentrations and are not felt to reflect a general biological phenomenon. In the case of insulin activation of the IGF-IR, the concentrations required are orders of magnitude greater than the maximal levels seen in vivo, even in cases of extreme hyperinsulinemia (1, 43).
In contrast to the largely artificial nature of insulin activation of the IGF-IR, there are two distinct molecular mechanisms that allow IGF cross talk with the IR. The first of these is the existence of hybrid receptors consisting of covalently linked IGF-IR and IR α-β hemireceptors that are thought to represent a fraction of the levels of IGF-IR and IR holoreceptors in cells expressing significant levels of both (15, 29). A number of studies have shown that these hybrid receptors retain the ability to bind IGF-I and IGF-II but do not appreciably bind insulin (26, 47). This differential binding may reflect the ability of a single IGF-IR α-subunit ligand-binding domain to associate with an IGF molecule and the current model of the insulin-IR interaction, which posits that a single insulin molecule bridges two distinct binding sites on two apposed α subunits in the high-affinity conformation (9). In other words, hybrid receptors have the minimal IGF-IR α subunit sufficient for IGF binding, but not the two IR α subunits necessary to constitute the insulin-binding site. Thus, hybrid receptors allow IGF ligands to activate IGF-IR and IR β subunits simultaneously. The biological effects of the trans-activated IR β subunit versus an activated IR holoreceptor are potentially distinct (30). The overall contribution of hybrid receptors to IGF/insulin action is unclear; however, the relative proportion of hybrid receptors is significant in certain tissues and is also high in breast cancer cells (3, 31).
A second pathway for IGF activation of the IR is the result of alternative splicing of exon 11 of the IR transcript, which encodes a 12-amino-acid sequence at the carboxyl terminus of the α subunit (28). The IR mRNA that lacks the exon 11 sequence encodes the IR-A protein isoform, which exhibits the expected high affinity for insulin, an intermediate affinity for IGF-II, and a low affinity for IGF-I (11, 18). The IR mRNA that contains the exon 11 sequence encodes the IR-B isoform, which displays the classical behavior of the IR, i.e., high-affinity binding to insulin alone. The IR-A version tends to be expressed in fetal and tumor tissues and exhibits more proliferative than metabolic downstream effects. The IR-B variant, on the other hand, is highly expressed in differentiated tissues, including insulin target tissues, and is more coupled to insulin-stimulated metabolic effects.
In this work, we describe a third, novel pathway for IGF action through the IR. Specifically, we demonstrate that IGF-I, at physiological levels, acts through both versions of the IR to preferentially activate IRS-2 and downstream biological actions. These effects are not associated with significant activation of the IR itself or of IRS-1 or the Erk pathway. The existence of these effects in IGF-IR-deficient cells and with IGF analogs that do not interact with IGFBPs strongly supports the concept of direct IGF-I activation of the IR that does not require robust IR autophosphorylation but that results in specific coupling to IRS-2 and downstream signaling pathways, potentially including the PI3K/Akt cascade. These actions may have particular relevance to the reported effects of IGF-I on insulin sensitivity in vivo, as well as the action of the IR in the central nervous system (CNS).
MATERIALS AND METHODS
Cell lines and culture.
IGF-IR-deficient (R−) mouse embryo fibroblast lines engineered to express similar levels of human IR-A (R− IR-A) and IR-B (R− IR-B) and vector-transfected controls have been previously described (11, 12). Cells were serum starved overnight and treated with 10 nM IGF-I, IGF-II, Long R3 IGF-I, or insulin for the indicated periods of time before cell lysates were prepared for direct sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses (for IR Y960 and Y1158/1162/1163 phosphorylation, Akt, and phospho-Akt S473) or immunoprecipitation (for IR, IRS-1, and IRS-2 activation). NIH 3T3 cells overexpressing human IR-A have been previously described (16) and were treated and analyzed similarly to the R−, R− IR-A, and R− IR-B cells.
Antibodies and other reagents.
Antibodies against the following proteins were obtained from the indicated sources: IRS-1, IRS-2, IR, and phosphotyrosine (PY20) from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-IR (Y960), phospho-IR (Y1158/1162/1163), and phospho-Akt S473 from Biosource International (Hopkinton, MA); and Akt from Cell Signaling Technology, Inc. (Danvers, MA). IGF-I, IGF-II, and Long R3 IGF-I were obtained from GroPep Pty., Ltd. (Adelaide, Australia). Human insulin was from Sigma-Aldrich (St. Louis, MO).
Immunoprecipitation and Western immunoblotting.
Lysate preparation, immunoprecipitation, Western immunoblotting, enhanced chemiluminescence detection, and scanning densitometry were performed as described previously (12).
siRNA transfection.
ON-TARGETplus Smartpool small interfering RNA (siRNA) (containing a mixture of four siRNAs) for human IRS-2, ON-TARGETplus siCONTROL nontargeting pool (negative control), and DharmaFECT 3 transfection reagent were purchased from Dharmacon, Inc. (Lafayette, CO).
Apoptosis and proliferation assays.
The ability of IGF-I, IGF-II, and insulin to protect against butyrate-induced apoptosis was assessed as previously described (12). For determination of overall growth/survival, cells were plated in triplicate in 96-well plates (0.5 × 103 cells/well), transfected with both negative-control and IRS-2 siRNAs for 36 h according to the manufacturer's recommendations, incubated in serum-free Dulbecco modified Eagle medium for 16 h, and treated with 10 nM insulin, IGF-II, IGF-I, or an equivalent volume of vehicle (10 mM HCl). At the indicated time points, cell monolayers were incubated for 30 min at 37°C with 10 μl/well of WST-1 reagent (Roche Applied Science; Indianapolis, IN). Absorbance readings were obtained at 450 and 600 nm on a microplate reader.
Statistical analysis.
INSTAT from Graphpad Software, Inc. (San Diego, CA) was used to carry out all statistical analysis. Unless otherwise indicated, one-way analysis of variance with Tukey's posttest was performed. All data points are means and standard errors of the means (SEM) from at least three separate experiments and representative blots shown as needed.
RESULTS
Relative activation of IR-A and -B by IGFs and insulin.
As a foundation for the studies that follow, Fig. 1 illustrates the relative activation of IR-A and IR-B by insulin, IGF-I, and IGF-II. At equivalent 10 nM concentrations, insulin induced strong activation of both isoforms, although the increase in IR tyrosine phosphorylation was greater with IR-B, consistent with the intrinsically greater in vitro kinase activity of this receptor isoform reported previously (24). These data demonstrate the greater activation of IR-B versus IR-A in a cellular context. This difference was seen with phosphorylation of the triple tyrosine cluster in the catalytic domain (Fig. 1A), Y960 (Fig. 1B), and total tyrosine phosphorylation (Fig. 1C and D). In contrast, IGF-I activation of both isoforms was negligible or absent in each case. IR activation by IGF-II was greater than with IGF-I for IR-A and IR-B. While the relative level of activation of IR-A by IGF-II versus insulin was higher than that for IR-B, as expected based upon previous studies demonstrating the higher affinity of this isoform for IGF-II (11, 18), it is important to note that the absolute level of activation, as measured by the relative increase over the basal level (Fig. 1A, B and C) by IGF-II was significantly higher for IR-B than for IR-A.
FIG. 1.
Activation of IR-A and IR-B by insulin, IGF-II, and IGF-I. R− IR-A and IR-B cells were serum starved overnight and treated with 10 nM ligand for 5 min. (A and B) Lysates were blotted for IR pY960 (A) or IR pY1158/1162/1163 (B), and phospho-IR levels were normalized for IR β-subunit levels in either R− IR-A or R− IR-B cells by stripping and reprobing with an IR β-subunit antibody. (C and D) IR immunoprecipitates were blotted with anti-phosphotyrosine (PY20) antibody. Total IR phosphorylation was also normalized for IR β-subunit levels by stripping and reprobing with an IR β-subunit antibody. Panel D shows a representative blot of the experiment shown in panel C and also illustrates that the R− IR-B cells express ∼50% of the level of IR in the R− IR-A cells. The data in panel C are represented as percentages of the maximal (max) level of phosphorylation seen with insulin, since the lack of background signal in the basal PY20 immunoprecipitation samples precluded calculation of the increase over basal values. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to basal levels of phosphorylation or, where indicated, between the bracketed samples. P values are represented as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. IP, immunoprecipitation; WIB, Western immunoblotting.
Time course of IRS-1 and IRS-2 activation.
In order to examine downstream IR signaling, we next determined the relative activation of IRS-1 and IRS-2. Figure 2A depicts the time course of IRS-1 activation by insulin, IGF-I, and IGF-II. For IR-A, insulin induced a rapid and sustained activation of IRS-1, while activation by IGF-II followed a similar, but lower, pattern of activation. As expected, activation by IGF-I was minimal. As shown in Fig. 2B, IRS-1 activation through IR-B exhibited a distinct transient pattern, but with a rank order of potency similar to that seen with IR-A. The relative activation of IRS-1 by IGF-II versus insulin through IR-B was greater than their relative activation through IR-A. Thus, while IGF-II activated IR-A to a greater extent than IR-B as a proportion of the activation elicited by insulin itself (Fig. 1), the relative activation of IRS-1 by IGF-II versus insulin was greater through IR-B. Therefore, IGF-II-stimulated IR and IRS-1 phosphorylation per se was greater through IR-B than IR-A, in spite of the higher affinity of IGF-II for IR-A.
FIG. 2.
Time course of IRS-1 and IRS-2 activation by insulin, IGF-II, and IGF-I. Cells were treated as described in the legend to Fig. 1, and the lysates were immunoprecipitated (IP) with anti-IRS-1 and IRS-2 antibodies, followed by Western immunoblotting with anti-IRS and PY20 antibodies. (A) IRS-1 activation in R− IR-A cells; (B) IRS-1 activation in R− IR-B cells; (C) IRS-2 activation in R− IR-A cells; (D) IRS-2 activation in R− IR-B cells. Representative blots are shown beneath each graph. In each case, the PY20 signal was normalized for IRS-1 or IRS-2 levels as determined by blotting with the respective antibody separately for each sample. Error bars represent SEM of three independent experiments. The bottom portion of the gel image in each case corresponds to a single representative IRS-1 or IRS-2 control blot. max, maximum; pIRS-1, phosphorylated IRS-1; pIRS-2, phosphorylated IRS-2.
Surprisingly, the activation of IRS-2, as shown in Fig. 2C and D, was rapid, transient, and similar for each ligand. Specifically, the activation of IRS-2 by IGF-II and, in particular, IGF-I, was greater than predicted based upon their relative affinities for the IR and their stimulation of IR tyrosine phosphorylation. Thus, IGF-I stimulated robust IRS-2 activation, in spite of its negligible activation of IR-A or IR-B. A second interesting aspect of these findings was the specific activation of IRS-2 by IGF-I in contrast to activation of both IRS-1 and IRS-2 by IGF-II and insulin. Figure 3 summarizes these findings by compiling the maximal 5-min activation data for IRS-1 and IRS-2 activation in R− IR-A (Fig. 3A and C) and R− IR-B (Fig. 3B and D) cells. This figure shows that the activation of IRS-1 by all ligands through both IR-A and IR-B was essentially proportional to IR activation itself, while IRS-2 activation was not. Specifically, insulin activated both IRS-1 and IRS-2, IGF-II activated IRS-1 less than insulin but activated IRS-2 as well as insulin, whereas IGF-I activated only IRS-2, but as well as it activated insulin and IGF-II. These data also show that there were no significant differences in the abilities of insulin, IGF-II, and IGF-I to activate IRS-2. Figure 4 shows that this same pattern was seen in NIH 3T3 cells overexpressing IR-A. Thus, this activation pattern was not a unique feature of the R− cell system.
FIG. 3.
Graphical summary of the 5-min activation of IRS-1 and IRS-2 from Fig. 2. (A) IRS-1 activation in R− IR-A cells; (B) IRS-1 activation in R− IR-B cells; (C) IRS-2 activation in R− IR-A cells; (D) IRS-2 activation in R− IR-B cells. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to basal levels of phosphorylation or, where indicated, between the bracketed samples. P values are represented as follows: **, P < 0.01; ***, P < 0.001. NS, not significant; max, maximum.
FIG. 4.
IRS-1 and IRS-2 activation by insulin, IGF-II, and IGF-I in NIH 3T3/IR-A cells. Cells were treated and analyzed as described in the legend to Fig. 2. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to basal levels of phosphorylation or, where indicated, between the bracketed samples. P values are represented as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant; IP, immunoprecipitation; WB, Western blotting; max, maximum.
IRS-2 activation by IGF-I is IGFBP independent.
The unexpected ability of IGF-I to specifically activate IRS-2 could conceivably have been due to effects through one or more IGFBPs. Although such a relatively indirect effect is not clearly consistent with the rapid time course of IRS-2 activation, we addressed this question directly by evaluating the ability of an IGF-I analog, Long R3 IGF-I, which does not exhibit high affinity for IGFBPs, to activate IRS-2. As shown in Fig. 5, Long R3 IGF-I was as active as IGF-I (and insulin and IGF-II) in activating IRS-2 in both R− IR-A cells (Fig. 5A) and NIH 3T3/IR-A cells (Fig. 5B), suggesting that the observed activation was not mediated through an IGFBP-dependent mechanism. Similar results were obtained with R− IR-B cells (data not shown).
FIG. 5.
Effect of Long R3 IGF-I on IRS-2 activation in (A) R− IR-A cells and (B) NIH 3T3/IR-A cells. Cells were treated and analyzed as described in the legend to Fig. 2. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to basal levels of phosphorylation or, where indicated, between the bracketed samples. P values are represented as follows: **, P < 0.01; ***, P < 0.001. NS, not significant; max, maximum; IP, immunoprecipitation; WB, Western blotting.
Effect of IR transfection on IRS-2 activation.
To ensure that the distinct effects of IGF-I on IRS activation could be ascribed to IR-A and IR-B activation, rather than to some undefined IR-independent mechanism, IRS-2 activation by IGF-I and Long R3 IGF-I was compared in R− IR-A and IR-B cells and parental, control R− cells that express only low levels of endogenous mouse IR (Fig. 6). It is clear that, while some IRS-2 activation was seen in R− cells (presumably through the endogenous mouse IR), IRS-2 activation was significantly increased in cells expressing human IR-A or IR-B. In other experiments, IRS-2 activation by insulin and IGF-II was also minimal in R− cells compared to R− IR-A and IR-B cells (data not shown).
FIG. 6.
Effect of IR transfection on IGF-I and Long R3 IGF-I activation of IRS-2. Control R− cells and RI− R-A and R− IRB cells were treated with 10 nM IGF-I (open bars) or Long R3 IGF-I (closed bars) and analyzed as described in the legend to Fig. 2. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to levels of phosphorylation in IGF-I-treated R− cells or, where indicated, between the bracketed samples. P values are represented as follows: **, P < 0.01; ***, P < 0.001. NS, not significant; max, maximum; IP, immunoprecipitation; WB, Western blotting.
Activation of downstream signaling pathways.
We have previously shown that IGF-I did not activate the PI3K or Erk pathway in either R− IR-A or R− IR-B cells at 5 min (12). To ascertain whether IGF-I activated the canonical PI3K or Erk pathways at other time points, the time course experiment shown in Fig. 7 was performed. As shown, IGF-I can activate the PI3K/Akt pathway, albeit to a lesser degree than insulin, and with a significant increase in Akt phosphorylation only evident at 60 min. Of note, the pattern of Akt activation by insulin was biphasic, with a rapid peak at 1 min, followed by a decline at 5 min, and a subsequent gradual increase up to 60 min. IGF-I did not induce Erk phosphorylation over a 60-min time course (data not shown).
FIG. 7.
Time course of Akt activation by insulin and IGF-I in R− IR-B cells. Cells were treated with 10 nM ligand over 60 min. (A) Graphical representation of three experiments. Results are expressed as a percentage of the maximal (max) Akt phosphorylation seen with insulin. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to basal levels of Akt phosphorylation. P values are represented as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Representative Western immunoblot. pAKT, phosphorylated Akt.
IGF effects on survival and viability.
While the experiments described above establish the ability of IGF-I to directly activate IRS-2 through IR-A and IR-B and, to some extent, the PI3K/Akt signal transduction cascade, an important question is whether this IGF-I/IR/IRS-2 pathway produces a biological effect. Toward this end, we evaluated the effects of IGF-I, IGF-II, and insulin on survival and viability of R− IR-A and R− IR-B cells. As shown in Fig. 8, IGF-I protected cells from butyrate-induced apoptosis in R− IR-B cells (Fig. 8B), and, to a lesser extent, in R− IR-A cells (Fig. 8A), and the former effect was equivalent to that seen with IGF-II. In these assays, insulin and IGF-II were equipotent through IR-A, whereas IGF-II and IGF-I were equipotent through IR-B. The effect of IGF-I on cell growth/viability through IR-B was also seen with a WST assay (Fig. 9B and C). In these experiments, all three ligands stimulated significant growth/survival over a 72-h time course, with the final levels of stimulation being similar (Fig. 9B). It is particularly noteworthy that these effects were seen in cells expressing the IR-B isoform that has been considered to be specific for insulin action. Similar data were obtained with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (data not shown).
FIG. 8.
Effect of 10 nM insulin, IGF-II, and IGF-I on R− IR-A (A) and R− IR-B (B) cell survival from butyrate-induced apoptosis relative to serum-free and 5 mM butyrate controls. Results are expressed as a percentage of the maximal survival response stimulated by 200 nM insulin. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to butyrate-treated control. P values are represented as follows: *, P < 0.05; ***, P < 0.001.
FIG. 9.
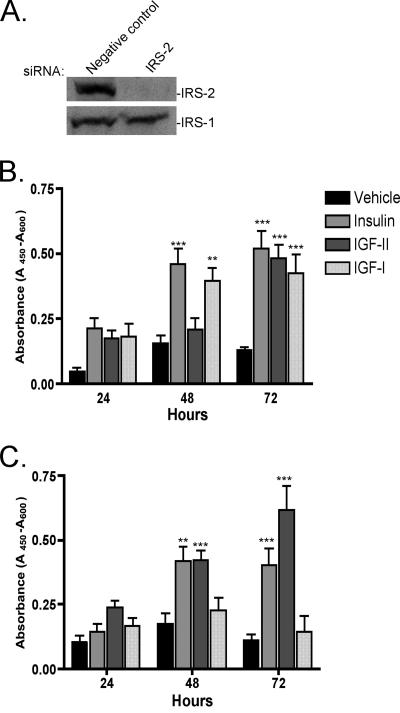
Proliferation/viability of R− IR-B cells after IRS-2 knockdown with siRNA transfection. (A) Western immunoblot of IRS-2 and IRS-1 after 48-h transfection of negative-control and IRS-2 siRNAs. (B) WST assay with negative-control siRNA Smartpool. (C) WST assay with IRS-2 siRNA Smartpool. Error bars represent SEM of three independent experiments. Statistical significance shown is in relation to vehicle control at each time point. P values are represented as follows: **, P < 0.01; ***, P < 0.001.
Role of IRS-2 in IGF-I-mediated effects through the IR.
In order to ascertain the requirement for IRS-2 activation in the biological effects elicited by IGF-I, we employed an siRNA-mediated knockdown strategy. As shown in Fig. 9A, treatment of R− IR-B cells with an IRS-2 siRNA pool, but not a negative-control siRNA, eliminated IRS-2 expression but had no effect on IRS-1 expression. IRS-2 knockdown effectively inhibited IGF-I-stimulated growth/survival in the WTS assay (Fig. 9C) but had no effect on insulin or IGF-II-mediated growth/survival (compare Fig. 9B and C).
DISCUSSION
The data presented in this paper show that IGF-I can function through both splice variants of the IR to specifically activate IRS-2, resulting in biological effects on cellular survival and migration. The inhibition of IGF-I-stimulated cell growth/survival by siRNA-mediated IRS-2 knockdown supports the conclusion that IRS-2 activation is necessary for these biological effects of IGF-I through the IR. These results are in line with previous reports showing that IRS-2 activation is important for cell migration (8, 22) and cell survival (27, 50, 51). The level of activation of IRS-2 by IGF-I was similar to that elicited by insulin and IGF-II, in spite of the significantly lower affinity of IGF-I for the IR and the lack of significant IGF-I-stimulated autophosphorylation of either IR isoform. Similarly, the relative activation of both IRS-1 and IRS-2 by IGF-II versus insulin through IR-B was greater than would have been predicted based upon their relative affinities for, and activation of, the IR. These findings are reminiscent of earlier studies that described IR action in the absence of detectable IR phosphorylation (35, 40, 42). While these reports were subsequently explained as reflecting insufficient detection of receptor phosphorylation and as being inconsistent with the inactivity of kinase-deficient mutants, the current data suggest that some of these previous findings may have been prematurely discounted. Of potential relevance to the data reported here, we have previously reported IGF-I action through a Y1175 IGF-IR mutant lacking detectable IGF-I-induced autophosphorylation (4, 48), and Yau et al. (53) have described IGF-I activation of the Erk pathway in H4IIE hepatoma cells in the absence of IGF-IR or IRS-1 activation.
A second interesting aspect of the IGF-I activation of IRS-2 reported in this work was the differential activation of IRS-2 versus IRS-1. While insulin and IGF-II activated both IRS-1 and IRS-2, IGF-I activation was specific for IRS-2. In fact, an examination of the data of Fig. 2 and 3 shows that insulin maximally activated IRS-1 and IRS-2, IGF-II partially activated IRS-1 and robustly activated IRS-2 (to levels similar to insulin), while IGF-I activated IRS-2, but not IRS-1. Thus, insulin and the IGFs exerted a spectrum of effects on IRS-1 and -2 that did not reflect their relative activation of either IR-A or IR-B. One clue to the differential activation of IRS-1 versus IRS-2 by IGF-I is that the interaction of IRS-2 with the IR is thought to differ from the interaction of IRS-1 with the IR. While both IRS-1 and IRS-2 bind to the IR via an interaction between their N-terminal PTB domains and phosphorylated Y960 in an NPXY motif in the juxtamembrane region of the IR (49), IRS-2 contains a unique IR-binding domain within residues 591 to 786 (21, 41). Mutation of Y960 to alanine in the IR did not affect ligand-mediated phosphorylation of IRS-2 but severely decreased IRS-1 phosphorylation (6). These data suggest that IRS-1 and IRS-2 phosphorylation can be regulated via distinct mechanisms, independent of IR phosphorylation, that may be selectively induced by IGF ligand binding to the IR as reported here. Although the divergent activation of IRS proteins has not been previously described for IGFs, an insulin analog has been described that, like IGF-I, specifically activates IRS-2 (36). It may be instructive to compare in more detail the structural similarities between that analog and IGF-I to identify possible residues that may contribute to IRS-specific activation through the IR.
A third novel aspect of IR-mediated IRS-2 activation by IGF-I is the existence of resulting biological effects (i.e., protection from butyrate-induced apoptosis and cell viability) observed in the absence of stimulation of the Erk pathway that is usually implicated in IGF action. The small and delayed activation of the PI3K pathway seen with IGF-I may underlie these effects. Alternatively, it is conceivable that IRS-2 activation downstream of the IGF-I-activated IR engages other signaling cascades, such as phosphatidylinositol-dependent kinase 1 targets other than Akt/protein kinase B, various protein kinase C isoforms (32), or signaling pathways not previously linked to IGF or insulin action.
Another intriguing observation made in the course of these studies was the difference in the time course and ligand sensitivity of IRS-1 activation in IR-A- and IR-B-expressing cells (Fig. 2). Specifically, IRS-1 activation through IR-A (greatest with insulin) was rapid and sustained over the 1-h period examined, while IRS-1 activation through IR-B was transient. Additionally, IRS-1 activation through IR-A by IGF-II was less than the relative activation of IR itself by IGF-II, whereas IRS-1 activation in IR-B-expressing cells by IGF-II was greater than would have been predicted based upon the relative activation of IR-B by IGF-II versus insulin. Thus, the differential splicing of exon 11 modulates both the time course of IRS-1 activation and the relationship between IGF-II activation of IRS-1 versus the IR.
It is tempting to speculate on the potential ramifications of these findings for IGF and insulin action in vivo. While essentially all studies of insulin action at the molecular level have involved treatment of serum-starved cells with insulin alone, it is important to recall that insulin-sensitive target tissues, such as the pancreas, liver, muscle, and fat, are exposed to elevated insulin levels postprandially in a setting of continuous exposure to circulating and locally produced IGF-I and IGF-II. Thus, treatment of cells with insulin alone constitutes an artificial situation. We propose that IGF-I, in particular, may function in vivo as a tonic facilitator of insulin action by virtue of its ability to generate a basal level of activated IRS-2 and, potentially, enhanced downstream signaling. Such an effect may explain the ability of IGF-I to mimic insulin action and to increase insulin sensitivity in numerous experimental and clinical studies. Specifically, IGF-I can induce hypoglycemia in animal models and human subjects and can also reduce insulin requirements of type 1 diabetic patients (37, 46). It will, therefore, be of interest to assess the molecular effects of simultaneous treatment with IGF-I and/or IGF-II and insulin on insulin-mediated signal transduction and biological activity, as well as the necessity of IGF-IR action in IGF-mediated effects on insulin-like action in cells expressing both IR and IGF-IR.
It is also reasonable to consider the implications of the data reported here for the proposed role of IR action in the CNS (7, 19, 33, 34). IR expression is widespread in the CNS, and intracerebroventricular administration of insulin elicits important biological effects. The evidence for physiological levels of CNS insulin, with the possible exception of the hypothalamus, remains less than compelling, however. We propose that the natural ligand for the majority of the IR present in the CNS may be IGF-I (5), either acting alone or by facilitating the effects of the low levels of insulin derived from the circulation. The phenotype of neuronal IR knockout mice is consistent with this hypothesis (44).
Acknowledgments
This work was supported, in part, by a grant from the McCoy Foundation to C.T.R. and a bursary from the Novartis Foundation to A.D. and C.T.R.
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Andersen, A. S., T. Kjeldsen, F. C. Wiberg, H. Vissing, L. Schaffer, J. S. Rasmussen, P. De Meyts, and N. P. Moller. 1992. Identification of determinants that confer ligand specificity on the insulin receptor. J. Biol. Chem. 267:13681-13686. [PubMed] [Google Scholar]
- 2.Bach, L. A., S. J. Headey, and R. S. Norton. 2005. IGF-binding proteins-the pieces are falling into place. Trends Endocrinol. Metab. 16:228-234. [DOI] [PubMed] [Google Scholar]
- 3.Bailyes, E. M., B. T. Nave, M. A. Soos, S. R. Orr, A. C. Hayward, and K. Siddle. 1997. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 327:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blakesley, V. A., H. Kato, C. T. Roberts, Jr., and D. LeRoith. 1995. Mutation of a conserved amino acid residue (tryptophan 1173) in the tyrosine kinase domain of the IGF-I receptor abolishes autophosphorylation but does not eliminate biologic function. J. Biol. Chem. 270:2764-2769. [DOI] [PubMed] [Google Scholar]
- 5.Bondy, C. A., and C. M. Cheng. 2004. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 490:25-31. [DOI] [PubMed] [Google Scholar]
- 6.Chaika, O. V., N. Chaika, D. J. Volle, H. Hayashi, Y. Ebina, L. M. Wang, J. H. Pierce, and R. E. Lewis. 1999. Mutation of tyrosine 960 within the insulin receptor juxtamembrane domain impairs glucose transport but does not inhibit ligand-mediated phosphorylation of insulin receptor substrate-2 in 3T3-L1 adipocytes. J. Biol. Chem. 274:12075-12080. [DOI] [PubMed] [Google Scholar]
- 7.Craft, S., and G. S. Watson. 2004. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 3:169-178. [DOI] [PubMed] [Google Scholar]
- 8.Cui, X., H. J. Kim, I. Kuiatse, H. Kim, P. H. Brown, and A. V. Lee. 2006. Epidermal growth factor induces insulin receptor substrate-2 in breast cancer cells via c-Jun NH2-terminal kinase/activator protein-1 signaling to regulate cell migration. Cancer Res. 66:5304-5313. [DOI] [PubMed] [Google Scholar]
- 9.De Meyts, P. 2004. Insulin and its receptor: structure, function and evolution. Bioessays 26:1351-1362. [DOI] [PubMed] [Google Scholar]
- 10.De Meyts, P., and J. Whittaker. 2002. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat. Rev. Drug Discov. 1:769-783. [DOI] [PubMed] [Google Scholar]
- 11.Denley, A., E. R. Bonython, G. W. Booker, L. J. Cosgrove, B. E. Forbes, C. W. Ward, and J. C. Wallace. 2004. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol. Endocrinol. 18:2502-2512. [DOI] [PubMed] [Google Scholar]
- 12.Denley, A., G. V. Brierley, J. M. Carroll, A. Lindenberg, G. W. Booker, L. J. Cosgrove, J. C. Wallace, B. E. Forbes, and C. T. Roberts, Jr. 2006. Differential activation of insulin receptor isoforms by insulin-like growth factors is determined by the C domain. Endocrinology 147:1029-1036. [DOI] [PubMed] [Google Scholar]
- 13.Denley, A., L. J. Cosgrove, G. W. Booker, J. C. Wallace, and B. E. Forbes. 2005. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 16:421-439. [DOI] [PubMed] [Google Scholar]
- 14.Duan, C. 2002. Specifying the cellular responses to IGF signals: roles of IGF binding proteins. J. Endocrinol. 175:41-54. [DOI] [PubMed] [Google Scholar]
- 15.Entingh-Pearsall, A., and C. R. Kahn. 2004. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J. Biol. Chem. 279:38016-38024. [DOI] [PubMed] [Google Scholar]
- 16.Faria, T. N., V. A. Blakesley, H. Kato, B. Stannard, D. LeRoith, and C. T. Roberts, Jr. 1994. Role of the carboxyl-terminal domains of the insulin and insulin-like growth factor I receptors in receptor function. J. Biol. Chem. 269:13922-13928. [PubMed] [Google Scholar]
- 17.Firth, S. M., and R. C. Baxter. 2002. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23:824-854. [DOI] [PubMed] [Google Scholar]
- 18.Frasca, F., G. Pandini, P. Scalia, L. Sciacca, R. Mineo, A. Costantino, I. D. Goldfine, A. Belfiore, and R. Vigneri. 1999. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 19:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerozissis, K. 2003. Brain insulin: regulation, mechanisms of action and functions. Cell. Mol. Neurobiol. 23:1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkes, C., and S. Kar. 2004. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res. Rev. 44:117-140. [DOI] [PubMed] [Google Scholar]
- 21.He, W., A. Craparo, Y. Zhu, T. J. O'Neill, L. M. Wang, J. H. Pierce, and T. A. Gustafson. 1996. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J. Biol. Chem. 271:11641-11645. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, J. G., X. Zhang, T. Yoneda, and D. Yee. 2001. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene 20:7318-7325. [DOI] [PubMed] [Google Scholar]
- 23.Johnston, A. M., L. Pirola, and E. Van Obberghen. 2003. Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signalling. FEBS Lett. 546:32-36. [DOI] [PubMed] [Google Scholar]
- 24.Kosaki, A., T. S. Pillay, L. Xu, and N. J. Webster. 1995. The B isoform of the insulin receptor signals more efficiently than the A isoform in HepG2 cells. J. Biol. Chem. 270:20816-20823. [DOI] [PubMed] [Google Scholar]
- 25.LeRoith, D., H. Werner, D. Beitner-Johnson, and C. T. Roberts, Jr. 1995. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 16:143-163. [DOI] [PubMed] [Google Scholar]
- 26.Li, G., E. J. Barrett, H. Wang, W. Chai, and Z. Liu. 2005. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146:4690-4696. [DOI] [PubMed] [Google Scholar]
- 27.Lingohr, M. K., L. M. Dickson, C. E. Wrede, I. Briaud, J. F. McCuaig, M. G. Myers, Jr., and C. J. Rhodes. 2003. Decreasing IRS-2 expression in pancreatic beta-cells (INS-1) promotes apoptosis, which can be compensated for by introduction of IRS-4 expression. Mol. Cell. Endocrinol. 209:17-31. [DOI] [PubMed] [Google Scholar]
- 28.Mosthaf, L., K. Grako, T. J. Dull, L. Coussens, A. Ullrich, and D. A. McClain. 1990. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 9:2409-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moxham, C. P., V. Duronio, and S. Jacobs. 1989. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulinlike growth factor I and insulin receptor heterodimers. J. Biol. Chem. 264:13238-13244. [PubMed] [Google Scholar]
- 30.Pandini, G., F. Frasca, R. Mineo, L. Sciacca, R. Vigneri, and A. Belfiore. 2002. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 277:39684-39695. [DOI] [PubMed] [Google Scholar]
- 31.Pandini, G., R. Vigneri, A. Costantino, F. Frasca, A. Ippolito, Y. Fujita-Yamaguchi, K. Siddle, I. D. Goldfine, and A. Belfiore. 1999. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin. Cancer Res. 5:1935-1944. [PubMed] [Google Scholar]
- 32.Pillay, T. S., S. Xiao, L. Keranen, and J. M. Olefsky. 2004. Regulation of the insulin receptor by protein kinase C isoenzymes: preferential interaction with beta isoenzymes and interaction with the catalytic domain of betaII. Cell Signal. 16:97-104. [DOI] [PubMed] [Google Scholar]
- 33.Plum, L., M. Schubert, and J. C. Bruning. 2005. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 16:59-65. [DOI] [PubMed] [Google Scholar]
- 34.Porte, D., Jr., D. G. Baskin, and M. W. Schwartz. 2005. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54:1264-1276. [DOI] [PubMed] [Google Scholar]
- 35.Rafaeloff, R., B. A. Maddux, A. Brunetti, P. Sbraccia, C. K. Sung, R. Patel, D. M. Hawley, and I. D. Goldfine. 1991. Transmembrane signalling by insulin via an insulin receptor mutated at tyrosines 1158, 1162, and 1163. Biochem. Biophys. Res. Commun. 179:912-918. [DOI] [PubMed] [Google Scholar]
- 36.Rakatzi, I., S. Ramrath, D. Ledwig, O. Dransfeld, T. Bartels, G. Seipke, and J. Eckel. 2003. A novel insulin analog with unique properties: LysB3, GluB29 insulin induces prominent activation of insulin receptor substrate 2, but marginal phosphorylation of insulin receptor substrate 1. Diabetes 52:2227-2238. [DOI] [PubMed] [Google Scholar]
- 37.Ranke, M. B. 2005. Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol. Metab. 16:190-197. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, C. T., Jr. 2003. Insulin-like growth factor signaling, p. 354-359. In H. L. Henry and A. W. Norman (ed.), Encyclopedia of hormones. Academic Press, Boston, MA.
- 39.Roberts, C. T., Jr. 2004. The role of the insulin-like growth factor system in pre- and post-natal growth, development, and tumorigenesis, p. 121-132. In S. M. Houston, J. M. P. Holly, and E. A. Feldman (ed.), IGF, nutrition, and health. Humana Press, Totowa, NJ.
- 40.Rolband, G. C., J. F. Williams, N. J. Webster, D. Hsu, and J. M. Olefsky. 1993. Deletion of exon 21 of the insulin receptor eliminates tyrosine kinase activity but preserves mitogenic signaling. Biochemistry 32:13545-13550. [DOI] [PubMed] [Google Scholar]
- 41.Sawka-Verhelle, D., S. Tartare-Deckert, M. F. White, and E. Van Obberghen. 1996. Insulin receptor substrate-2 binds to the insulin receptor through its phosphotyrosine-binding domain and through a newly identified domain comprising amino acids 591-786. J. Biol. Chem. 271:5980-5983. [DOI] [PubMed] [Google Scholar]
- 42.Sbraccia, P., K. Y. Wong, A. Brunetti, R. Rafaeloff, V. Trischitta, D. M. Hawley, and I. D. Goldfine. 1990. Insulin down-regulates insulin receptor number and up regulates insulin receptor affinity in cells expressing a tyrosine kinase-defective insulin receptor. J. Biol. Chem. 265:4902-4907. [PubMed] [Google Scholar]
- 43.Schaffer, L. 1994. A model for insulin binding to the insulin receptor. Eur. J. Biochem. 221:1127-1132. [DOI] [PubMed] [Google Scholar]
- 44.Schubert, M., D. Gautam, D. Surjo, K. Ueki, S. Baudler, D. Schubert, T. Kondo, J. Alber, N. Galldiks, E. Kustermann, S. Arndt, A. H. Jacobs, W. Krone, C. R. Kahn, and J. C. Bruning. 2004. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 101:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott, C. D., and S. M. Firth. 2004. The role of the M6P/IGF-II receptor in cancer: tumor suppression or garbage disposal? Horm. Metab. Res. 36:261-271. [DOI] [PubMed] [Google Scholar]
- 46.Simpson, H. L., N. C. Jackson, F. Shojaee-Moradie, R. H. Jones, D. L. Russell-Jones, P. H. Sonksen, D. B. Dunger, and A. M. Umpleby. 2004. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J. Clin. Endocrinol. Metab. 89:425-432. [DOI] [PubMed] [Google Scholar]
- 47.Slaaby, R., L. Schaffer, I. Lautrup-Larsen, A. S. Andersen, A. C. Shaw, I. S. Mathiasen, and J. Brandt. 2006. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 281:25869-25874. [DOI] [PubMed] [Google Scholar]
- 48.Stannard, B., V. Blakesley, H. Kato, C. T. Roberts, Jr., and D. LeRoith. 1995. Single tyrosine substitution in the insulin-like growth factor I receptor inhibits ligand-induced receptor autophosphorylation and internalization, but not mitogenesis. Endocrinology 136:4918-4924. [DOI] [PubMed] [Google Scholar]
- 49.Sun, X. J., L. M. Wang, Y. Zhang, L. Yenush, M. G. Myers, Jr., E. Glasheen, W. S. Lane, J. H. Pierce, and M. F. White. 1995. Role of IRS-2 in insulin and cytokine signalling. Nature 377:173-177. [DOI] [PubMed] [Google Scholar]
- 50.Tseng, Y. H., K. Ueki, K. M. Kriauciunas, and C. R. Kahn. 2002. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J. Biol. Chem. 277:31601-31611. [DOI] [PubMed] [Google Scholar]
- 51.Valverde, A. M., I. Fabregat, D. J. Burks, M. F. White, and M. Benito. 2004. IRS-2 mediates the antiapoptotic effect of insulin in neonatal hepatocytes. Hepatology 40:1285-1294. [DOI] [PubMed] [Google Scholar]
- 52.White, M. F. 2002. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 283:E413-E422. [DOI] [PubMed] [Google Scholar]
- 53.Yau, L., H. Lukes, H. McDiarmid, J. Werner, and P. Zahradka. 1999. Insulin-like growth factor-I (IGF-I)-dependent activation of pp42/44 mitogen-activated protein kinase occurs independently of IGF-I receptor kinase activation and IRS-1 tyrosine phosphorylation. Eur. J. Biochem. 266:1147-1157. [DOI] [PubMed] [Google Scholar]