Abstract
Friend erythroleukemia virus has long served as a paradigm for the study of the multistage progression of leukemia. Friend virus infects erythroid progenitor cells, followed by an initial polyclonal expansion of infected cells, which is driven by the activation of a naturally occurring truncated form of the Stk receptor tyrosine kinase (Sf-Stk). Subsequently, the accumulation of additional mutations in p53 and the activation of PU.1 result in full leukemic transformation. The early stages of transformation induced by Friend virus are characterized in vitro by the Epo-independent growth of infected erythroblasts. We have shown previously that this transforming event requires the kinase activity and Grb2 binding site of Sf-Stk and the recruitment of a Grb2/Gab2 complex to Sf-Stk. Here, we demonstrate that Stat3 is required for the Epo-independent growth of Friend virus-infected cells and that the activation of Stat3 by Sf-Stk is mediated by a novel Stat3 binding site in Gab2. These results underscore a central role for Stat3 in hematopoietic transformation and describe a previously unidentified role for Gab2 in the recruitment and activation of Stat3 in response to transforming signals generated by tyrosine kinases.
Friend virus induces in mice acute erythroleukemia characterized by two distinct stages, an initial polyclonal expansion of infected erythroid progenitor cells mediated by the viral glycoprotein gp55, followed by the acquisition of additional genetic alterations, including the mutation of p53 and retroviral insertion into the PU.1 locus, resulting in the expansion of leukemic clones (21). Genetic analysis has led to the identification of several signaling pathways required for the early stage of progenitor cell expansion in response to viral infection. The W, Sl, Fv2, and f loci have been demonstrated to regulate the expansion of infected progenitor cells in vivo. The W locus, encoding the Kit receptor tyrosine kinase, is required for the normal development of target cells for the virus in the spleen (32), whereas an analysis of the f locus, encoding Madh5 (17), has suggested a role for the BMP4/Smad5 signaling pathway in the acquisition of additional target cells in the spleen following infection (A. Subramanian, submitted for publication).
The Friend virus susceptibility locus 2, Fv2, encodes a naturally occurring, N-terminally truncated form of the Stk receptor tyrosine kinase, the murine homologue of the human Ron receptor, called Sf-Stk (25). The expression of Sf-Stk in erythroid progenitor cells is driven by an internal promoter, and the translation of this transcript generates a protein that lacks the extracellular ligand binding domain of full-length Stk, while retaining the transmembrane and kinase domains. C57BL/6 mice harbor mutations in the internal promoter in the gene encoding Stk, resulting in reduced Sf-Stk expression and Friend virus resistance. Sf-Stk is required for Epo-independent colony formation induced by Friend virus infection. Exogenous expression of Sf-Stk rescues the Epo-independent growth of Friend virus-infected erythroid progenitor cells in vitro (8) and renders C57BL/6 mice susceptible to Friend erythroleukemia in vivo (25).
The viral glycoprotein gp55, from the spleen focus forming virus, interacts with the erythropoietin receptor (EpoR) (18) and Sf-Stk (23), and this signaling complex drives the Epo-independent expansion of Friend virus-infected cells. We have shown previously that the kinase domain and Grb2 binding site of Sf-Stk are required for this response to Friend virus infection (8) and that a Grb2/Gab2 complex downstream of Sf-Stk mediates the growth of primary erythroid progenitor cells infected in vitro with Friend virus (33). Recent studies have demonstrated that mice harboring a human EpoR knocked in to the murine locus (which fails to interact with gp55) develop leukemia, but not the characteristic polycythemia, in response to Friend virus infection (40). Taken together, these data suggest that signaling through Sf-Stk plays a central role in the polyclonal expansion of infected progenitor cells, whereas EpoR regulates the differentiation of these cells.
The activation of Stat5 by EpoR has been extensively studied and plays a key role in the differentiation of erythroid progenitor cells in response to Epo. However, while Epo and gp55 both lead to the activation of Stat5 in erythroid cells, nuclear translocation and DNA binding of Stat5 are not observed in response to gp55, suggesting that Stat5 may not play an essential role in the transmission of the cell growth signals in gp55-induced erythroleukemia cells (37). Furthermore, mice with a targeted deletion in Stat5 develop erythroleukemia in response to the polycythemia-inducing strain of Friend virus (FVP), but without the characteristic development of polycythemia (40), indicating that like EpoR, Stat5 is required for the differentiation, but not the expansion, of Friend virus target cells (40).
Stat1 and Stat3 are also activated by EpoR and have been suggested to play distinct roles in erythropoiesis (15, 16), although the mechanism of tyrosine phosphorylation of Stat1 and Stat3 by EpoR is distinct from that of Stat5 (12, 14). Mice harboring a targeted deletion in Stat1 exhibit overall reductions in erythroid progenitor cells (11); however, the role of Stat3 in erythropoiesis in vivo has not been elucidated. Constitutive activation of Stat1 and Stat3 has been demonstrated in primary erythroleukemic cells (13) and cell lines infected with spleen focus forming virus (24). Taken together, these data suggest potential roles for Stat1 and Stat3 in regulating the aberrant growth of erythroid progenitor cells in Friend disease.
Here, we set out to determine the role of Stat3 in the progression of Friend erythroleukemia. We demonstrate that Stat3 is required for the Epo-independent growth of Friend virus-infected erythroid progenitor cells. Furthermore, we identified a novel Stat3 binding site in Gab2 which is required for this process, suggesting that the activation of Stat3 in Friend virus-infected cells is mediated by an Sf-Stk/Grb2/Gab2 signaling pathway. These studies highlight a critical role for Stat3 activation in the progression of Friend erythroleukemia and identify a novel mechanism for the activation of Stat3 downstream of tyrosine kinases. Interestingly, Gab2 is also required for the transformation of hematopoietic cells by BCR/Abl (29), which suggests that the observations described here could have broader implications for the function of Gab2 in various hematopoietic malignancies.
MATERIALS AND METHODS
Mice.
Gab2−/− mice, on an FV2-sensitive BALB/c background, were genotyped as previously described (34). Floxed stat3 mice (28) on a C57BL/6 background were obtained from Charles Drake (John Hopkins University) and were crossed for two generations onto the BALB/c background. Genotypes of Fv2 loci were determined by PCR with appropriate primers. For preparing genomic DNA, about 5 mm of mouse tails was clipped and lysed in lysis buffer (100 mM Tris-Cl, pH 8.0, 5 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 100 μl proteinase K) at 55°C overnight. After centrifugation, 2 volumes of ethanol were added to cleared lysates to precipitate the genomic DNA. The pellet was washed with 75% ethanol, air dried, and resuspended in 100 μl of 10 mM Tris-Cl, pH 8.0. For the genotype of the floxed Stat3 allele, the primer pair 5′-CCTGAAGACCAAGTTCATCTGTGTGAC-3′ (forward) and 5′-CACACAAGCCATCAAACTCTGGTCTCC-3′ (reverse) was used. The PCR parameters were 95°C for 1 min, followed by 30 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 1 min and 30 s. The PCR products were loaded onto a 1.5% agarose gel for electrophoresis and visualized with ethidium bromide staining. The wild-type allele amplifies a 200-bp fragment, and the floxed allele amplifies a 250-bp fragment. For the Fv2 loci, the primers were 5′-GGTGGGTTTAACGGTTAGGG-3′ (forward) and 5′-TCTGGGCTCTGCCTCCTTAT-3′ (reverse). The PCR parameters were 95°C for 1 min, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The PCR products were then loaded on a 12.5% acrylamide gel for electrophoresis to visualize the DNA fragments by ethidium bromide staining. The sensitive allele amplifies a 53-bp fragment, and the resistant allele amplifies a 50-bp fragment. All research involving the use of mice was performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee of Pennsylvania State University.
DNA constructs.
Gab1/2 chimeric molecules were obtained by three-way ligation. The primers used were 1S1, 5′-CGGGGTACCATGTATGATGTTCCTGATTATGCTAGCCTCAGCGGTGGTGAAGTGGTCTG-3′; 1S2, 5′-CCAACAGAAGAAGATCCTGTG-3′; 1S3, 5′-CATCCAGCTCATGACCGATC-3′; 1S4, 5′-AACCCAAACCTGTCCAGTGAAG-3′; 1AS1, 5′-CCGGAATTCTCATTTCACACTCTTCGCTGGCG-3′; 1AS2, 5′-ATTAAACCCACAGATGTCACAAATAC-3′; 1AS3, 5′-TGGTTTCGGTGGCCGAGGTG-3′; 1AS4, 5′-CATGGGAACATAATTCTCTTCAC-3′; 2S1, 5′-CGGGGTACCATGTATGATGTTCCTGATTATGCTAGCCTCAGCGGCGGCGGCGACGTGG-3′; 2S2, 5′-CAGGCTGAGGAGAGCACAG-3′; 2S3, 5′-AGTCAGGCAGAAACACCTCG-3′; 2S4, 5′-CAAAACCCAGTGTCTGCATCTC-3′; 2AS1, 5′-GCGGATCCTCTCATCACAGCTTG-3′; 2AS2, 5′-ATTGAAGCCACAGATCTGGCAG-3′; 2AS3, 5′-TGGCTTGGGGGGGCGGG-3′; and 2AS4, 5′-CATAGGGACATAGTTCTCTTCG-3′. Partial cDNA fragments of Gab1 and Gab2 were synthesized independently by PCRs with the appropriate primer pairs, digested with restriction enzymes, and inserted into the pcDNA3 vector. For each fragment, primer pairs were as follows: for CM1, Gab12-592 (1S1 and 1AS4) and Gab2588-676 (2S4 and 2AS1); for CM2, Gab12-348 (1S1 and 1AS3) and Gab2359-676 (2S3 and 2AS1); for CM3, Gab22-118 (1S1 and 1AS2) and Gab2120-676 (2S2 and 2AS1); for CM4, Gab22-119 (2S1 and 2AS2) and Gab1119-694 (1S2 and 1AS1); for CM5, Gab22-358 (2S1 and 2AS3) and Gab1349-694 (1S3 and 1AS1); and for CM6, Gab22-587 (2S1 and 2AS4) and Gab1593-694 (1S4 and 1AS1). For the purpose of ligation reactions, phosphate groups were added to the 5′ end of the primers prior to PCRs. These oligonucleotides (250 pmol) were incubated with 10 U T4 polynucleotide kinase (Biolabs) and 1 mM ATP in a 25-μl reaction volume for 30 min at 37°C. The enzyme was deactivated by 10 min of incubation at 70°C.
MSCV-neo-myc/sf-stk, MSCV-neo-myc/sf-stk(Y429F), and MSCV-neo-myc/sf-stk(Y436F) were described previously (8). MSCV-neo-myc/sf-stk(KD) was produced by site-directed mutagenesis using the Stratagene QuikChange kit (Stratagene), following the manufacturer's protocol. Using MSCV-neo-myc/sf-stk as a template, the mutation was introduced with the oligonucleotide (sense) 5′-GCGTCCTAGACAAGGAATTCTTCAGTGTTCGCCAGCATC-3′.
The murine MSCV-neo-Gab2 and MSCV-neo-myc/sf-stk/Gab2 cDNAs were described previously (34). The QuikChange mutagenesis kit was used to mutate MSCV-neo-Gab2 and MSCV-neo-myc/sf-stk/Gab2 to MSCV-neo-Gab2(Y/F) and MSCV-neo-myc/sf-stk/Gab2(Y/F), respectively, by using the oligonucleotide (sense) 5′-CACCTCAGGAGTATCTCTTCTTGCACCAGTGCATAAGC-3′. The MSCV-neo-myc/sf-stk(KD)/Gab2 cDNA was introduced by site-directed mutagenesis (Stratagene QuikChange kit) following the manufacturer's protocol. The template was MSCV-neo-myc/sf-stk/Gab2, and the primer was (sense) 5′-CCACTGTGCCATCATGTCTCTGAGTCGG-3′.
The dominant negatives pRc CMV-Stat3(Y705F) and pRc CMV-Stat3(ZZ/VVV) (kind gifts of C. Horvath, Northwestern University) were excised from the pRc cytomegalovirus vector with NotI and ApaI and subcloned into MSCV-neo at the HpaI site to produce MSCV-neo-Stat3(Y705F) and MSCV-neo-Stat3(ZZ/VVV). Wild-type MSCV-neo-Stat3 was generated by site-directed mutagenesis (Stratagene QuikChange kit) following the manufacturer's protocol. The template was MSCV-neo-Stat3(Y705F) and the primer was (sense) 5′-ACCTTCCTACTGCGCTTCAG-3′.
Transient transfection.
The 293T cell line was grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, penicillin-streptomycin, and l-glutamine. Cells were transiently transfected by the TransIT-293 transfection reagent (Mirus Corporation) by using 1 to 2 μg pEco and the appropriate murine stem cell virus (MSCV)-neo constructs (1 to 10 μg) at 37°C for 48 to 72 h prior to harvest of the viral supernatant. Protein expression from the various plasmids was verified by Western blot analysis.
In vitro infection and colony assay.
For in vitro infection of bone marrow, cells were harvested from femurs and tibias of >6-week-old BALB/c, C57BL/6, or Gab2−/− mice and washed in phosphate-buffered saline. Bone marrow was infected with viral supernatant from the transient transfections for 20 h as previously described (8). For Epo-independent colony analysis, total bone marrow cells from different strains of mice were harvested and incubated with supernatant from cells expressing FVP (derived from FP63 cells [Alan Bernstein, Mount Sinai Hospital, Toronto, Ontario, Canada]) or Dulbecco's modified Eagle's medium (10% fetal bovine serum, penicillin-streptomycin, and l-glutamine) on ice for 1 h, as previously described (34). Cells were added to MethoCult medium M3234 (StemCell Technologies), along with 2.5 ng interleukin-3 (IL-3) (PeproTech), in triplicate with or without 1 U/ml Epo (R&D Systems). Cultures were incubated for 2 to 8 days in 5% CO2 at 37°C. Erythroid colonies (erythroid burst-forming unit [BFU-E] and CFU-E) were visualized by acid-benzidine staining as previously described (8).
Antibodies, immunoprecipitation, and Western blotting.
Mouse anti-Myc (1:1,000), rabbit anti-stat3 (1:1,000), and rabbit anti-phospho-stat3(Y705) (1:1,000) antibodies were purchased from Cell Signaling. Mouse anti-Gab1 was purchased from Santa Cruz. Rabbit anti-Gab2 and rabbit anti-phospho-stat3 (S727) antibodies were purchased from Upstate. Rabbit anti-β-actin antibody was purchased from Sigma. 293T cells were transiently transfected with plasmids expressing the desired protein. Twenty-four hours after transfection, cells were lysed in lysis buffer (1% NP-40, 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholate, 2 mM Na3VO4, 10 mM NaF, and 2 mM phenylmethylsulfonyl fluoride). After 15 min of centrifugation, the cleared cell lysates were incubated with the indicated antibodies and protein A (or G)-Sepharose at 4°C overnight. The immunoprecipitates were analyzed by reduced SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene difluoride membrane. The blots were incubated with the appropriate antibodies, and bands were detected with ECL (Amersham).
Flow cytometry analysis.
Bone marrow cells were collected after in vitro infection, fixed with 2% paraformaldehyde for 10 min on ice, and analyzed by flow cytometry for green fluorescent protein (GFP) expression.
RESULTS
The region between Q120 and P358 of Gab2 is required for hematopoietic transformation and activation of Stat3 downstream of the Stk receptor.
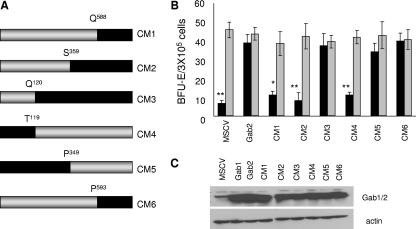
Previous studies from our laboratory demonstrated a requirement for Gab2 in the transformation of primary hematopoietic cells by Friend erythroleukemia virus both in vitro and in vivo. Furthermore, we found that exogenous expression of Gab2, but not the closely related Gab1, rescued the ability of Gab2−/− hematopoietic progenitor cells to form cytokine-independent colonies in response to Friend virus infection, suggesting a unique role played by Gab2, but not Gab1, in hematopoietic transformation. Here, we set out to map the domains of Gab2 required for this response. A series of six Gab1/Gab2 chimeric proteins (Fig. 1A) were generated and tested for their abilities to rescue the Epo-independent colony formation of Gab2−/− hematopoietic cells in response to Friend virus infection. As shown in Fig. 1B, while Gab2−/− hematopoietic cells infected with empty vector (MSCV) failed to form Epo-independent colonies following Friend virus infection, the transduction of these cells with MSCV harboring wild-type Gab2 completely rescued this defect. Exogenous expression of the Gab1/Gab2 fusion proteins demonstrated that the region between amino acids Q120 and P358 in Gab2 is essential to support the transformation of Friend virus-infected erythroblasts.
FIG. 1.
The region between Q120 and P358 of Gab2 is essential to support the transformation of Friend virus-infected erythroblasts. (A) Schematic diagram of the chimeric Gab1/2 constructs used in this study. Gray bars, sequence from Gab1; black bars, sequence from Gab2. (B) Total bone marrow from Gab2−/− mice was transduced with empty vector (MSCV), Gab2, and the indicated Gab1/2 chimeras (CM1 to CM6). Transduced cells were plated in methylcellulose containing IL-3 (2.5 ng/ml) in the presence of FV or Epo (1 U/ml). BFU-E cells were stained with acid-benzidine and scored on day 5. Black columns, Friend virus; gray columns, Epo. Bars denote the means ± SD of one of three independent experiments performed in replicates of three and reflect normalized values. *, P < 0.05; **, P < 0.01. (C) Expression of Gab1, Gab2, and Gab1/2 chimeric proteins in 293T cells, determined by Western blot analysis using a mixture of anti-Gab1 and anti-Gab2 antibodies.
The transformation of primary erythroblasts by Friend virus requires the activation of a truncated form of the Stk receptor tyrosine kinase (Sf-Stk). Previously, we demonstrated that Y436 in the C-terminal tail of Sf-Stk is required for its ability to mediate the transformation of infected cells. Further, we found that a fusion protein in which the C-terminal tail of Sf-Stk, including Y436, was replaced with Gab2 (Sf-Stk/Gab2) was fully able to support Epo-independent growth induced by Friend virus, suggesting that the primary role of Y436 was to recruit Gab2 to the signaling complex. However, a similar fusion with Gab1 (Sf-Stk/Gab1) failed to support this response, even though both fusion proteins induced strong activation of Erk and Akt in 293 cells. Because Stat3 is often activated downstream of receptor tyrosine kinases, it is phosphorylated in Friend virus-infected cells and has been implicated in the progression of a number of malignancies; we set out to test whether the differences in the abilities of Sf-Stk/Gab1 and Sf-Stk/Gab2 fusions to support the Epo-independent growth of Friend virus-infected cells could be due to differences in activation of Stat3.
The Y194LHQ motif in Gab2 interacts with Stat3 and mediates tyrosine phosphorylation of Stat3 downstream of Stk.
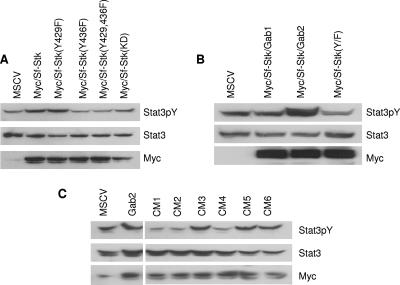
To determine whether Stat3 is downstream of Sf-Stk, we coexpressed Sf-Stk and Stat3 in 293 cells. Here, we demonstrate that Sf-Stk induces tyrosine phosphorylation of Stat3 in 293 cells and that this phosphorylation is dependent on Y436 (Fig. 2A). In addition, the expression of the Sf-Stk/Gab2 fusion, but not Sf-Stk/Gab1, enhanced tyrosine phosphorylation of Stat3 in these cells (Fig. 2B). Therefore, we tested the abilities of the Gab1/Gab2 fusion proteins to mediate Stat3 activation when coexpressed with Sf-Stk. Interestingly, the ability of Gab2 to enhance tyrosine phosphorylation of Stat3 in the presence of Sf-Stk mapped to the same region of Stat3 that was required for hematopoietic transformation in response to Friend virus infection (Fig. 2C).
FIG. 2.
The region between Q120 and P358 of Gab2 is required to mediate Stat3 activation when coexpressed with Sf-Stk. 293T cells were transiently cotransfected with Stat3 and plasmids expressing (A) wild-type or mutant forms of Sf-Stk or (B) wild-type or mutant forms of Sf-Stk/Gab fusions as indicated. Cell lysates were probed with anti-phospho-Stat3, anti-Stat3, and anti-Myc. (C) 293T cells were transiently cotransfected with Myc-Sf-Stk, Stat3, and plasmids expressing the various Gab1/2 fusions as indicated. Cell lysates were probed with anti-phospho-Stat3, anti-Stat3, and anti-Gab1/2.
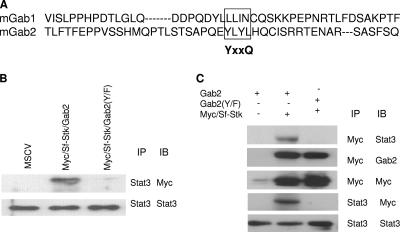
An analysis of the amino acid sequence in this region of Gab2 revealed a YxxQ motif (a potential Stat3 binding site) at position 194 (Fig. 3A), which is conserved in the human sequence. Therefore, we mutated this sequence to FxxQ in the context of both the Sf-Stk/Gab2 [Sf-Stk/Gab2(Y/F)] fusion protein and wild-type Gab2 [Gab2(Y/F)]. Figure 3B demonstrates that Sf-Stk/Gab2, but not Sf-Stk/Gab2(Y/F), immunoprecipitates with Stat3 in 293 cells. Further, the expression of wild-type Sf-Stk with Gab2 in 293 cells resulted in the coimmunoprecipitation of Sf-Stk with Stat3. However, while Sf-Stk retained the ability to recruit Gab2(Y/F) when coexpressed in 293 cells, Stat3 did not coimmunoprecipitate with Sf-Stk under these conditions (Fig. 3C). The expression of Sf-Stk/Gab2(Y/F) in 293 cells also failed to enhance tyrosine phosphorylation of Stat3 in these cells (Fig. 2B), indicating that the recruitment of Stat3 to this motif is required for the tyrosine phosphorylation of Stat3 downstream of Sf-Stk. We failed to detect the interaction of Sf-Stk with Stat1 or Stat5 in the presence of Gab2 in 293 cells, suggesting that this interaction is specific to Stat3 (data not shown).
FIG. 3.
The ability of Gab2 to recruit and activate Stat3 is dependent on the Y194LHQ site in Gab2. (A) Sequence alignment of mGab1 and mGab2 proteins. The potential Stat3 binding site is shown in the box. (B) 293T cells were transiently cotransfected with Stat3 and plasmids expressing wild-type (MSCV) or mutant Sf-Stk/Gab2 fusions as indicated. Cell lysates were immunoprecipitated (IP) with anti-Stat3 antibodies and subsequently analyzed by SDS-PAGE and Western immnunoblotting (IB) with anti-Myc and anti-Stat3 antibodies. (C) 293T cells were transiently cotransfected with Stat3, Sf-Stk, and wild-type or mutant forms of Gab2. Cell lysates were immunoprecipitated with anti-Stat3 and blotted with anti-Gab2, anti-Myc, and anti-Stat3 antibodies. −, absence of; +, presence of.
Stat3 binding to Y194 is required for Friend virus-induced Epo-independent colony formation.
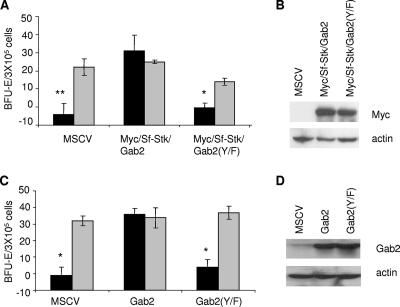
To determine whether the interaction of Stat3 with Gab2 downstream of Sf-Stk is required for the hematopoietic transformation induced by Friend virus, Sf-Stk/Gab2 and Sf-Stk/Gab2(Y/F) were exogenously expressed in primary hematopoietic progenitor cells from resistant C57BL/6 mice lacking endogenous Sf-Stk. Figure 4A shows that while Sf-Stk/Gab2 supported the Epo-independent growth of Friend virus-infected erythroblasts, Sf-Stk/Gab2(Y/F) failed to support this response. Similarly, exogenous expression of Gab2 in hematopoietic cells from Gab2−/− mice rescued the Epo-independent growth of these cells in response to Friend virus infection, as shown previously, while the expression of Gab2(Y/F) did not rescue the defect in the Gab2−/− cells (Fig. 4C). An equivalent expression of the fusion proteins in 293 packaging cells is demonstrated by Western blot analysis (Fig. 4B and D), and equal infection efficiencies of primary bone marrow cells by these vectors were verified by flow cytometry (data not shown). Taken together, these data indicate that the YxxQ motif in Gab2, which is not present in Gab1, mediates the interaction of Gab2 with Stat3 downstream of Sf-Stk and that this interaction is critical in the ability of Gab2 to support hematopoietic transformation in response to Friend erythroleukemia virus.
FIG. 4.
The Y194LHQ site in Gab2 is critical to support Epo-independent colony formation of primary erythroblasts in response to Friend virus infection. (A) Total bone marrow from C57BL/6 mice was transduced with empty vector (MSCV), Myc-Sf-Stk/Gab2, or Myc-Sf-Stk/Gab2 harboring the Y194-to-F mutations. Transduced cells were plated in methylcellulose containing IL-3 (2.5 ng/ml) in the presence or absence of FV or Epo (1 U/ml). BFU-E cells were stained with acid-benzidine and scored on day 5. Black columns, Friend virus; gray columns, Epo. Bars denote the means ± SD of one of three independent experiments performed in replicates of three and reflect normalized values. (B) Expression of Myc-sf-stk/Gab2 and Myc-sf-stk/Gab2(Y/F) in 293T packaging cells. (C) Total bone marrow from Gab2−/− mice was transduced with empty vector, Gab2 or Gab2(Y194/F). Colony assays were performed as described in the legend for panel A. (D) Expression of Gab2 and Gab2(Y/F) in 293T packaging cells. *, P < 0.05; **, P < 0.01.
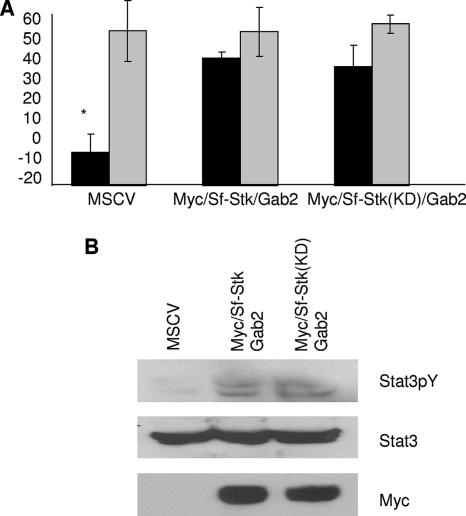
To determine whether the Stat3 binding site in Gab2 is phosphorylated directly by Sf-Stk or indirectly by another kinase, we generated a kinase-dead form of the Sf-Stk/Gab2 fusion protein. We have shown previously that kinase-dead Sf-Stk does not support the Epo-independent growth of Friend virus-infected cells. However, we found that the kinase-dead Sf-Stk/Gab2 fusion at least partially supports the cytokine-independent growth of primary hematopoietic cells following infection with Friend virus (Fig. 5A). Furthermore, the kinase-dead Sf-Stk/Gab2 fusion protein induces Stat3 tyrosine phosphorylation in 293 cells (Fig. 5B). Taken together, these data suggest that the kinase activity of Sf-Stk is required for receptor autophosphorylation but may not be required for the phosphorylation of Gab2 or Stat3 following the recruitment of Gab2 to the receptor complex.
FIG. 5.
The kinase activity of Sf-Stk is not required for the phosphorylation of Stat3 or Epo-independent colony formation of primary erythroblasts in response to Friend virus infection. (A) Total bone marrow from Gab2−/− C57BL/6 mice was transduced with empty vector (MSCV), Myc-Sf-Stk/Gab2, or Myc-Sf-Stk(KD)/Gab2. Transduced cells were plated in methylcellulose containing IL-3 (2.5 ng/ml) in the presence or absence of FV or Epo (1 U/ml). BFU-E cells were stained with acid-benzidine and scored on day 5. Black columns, Friend virus; gray columns, Epo. Bars denote the means ± SD of one of three independent experiments performed in replicates of three and reflect normalized values. *, P < 0.05. (B) 293T cells were transiently cotransfected with Stat3 and plasmids indicated above. Cell lysates were probed with anti-phospho-Stat3, anti-Stat3, and anti-Myc.
Stat3 is tyrosine phosphorylated and interacts with Gab2 in spleens from Friend virus-infected mice.
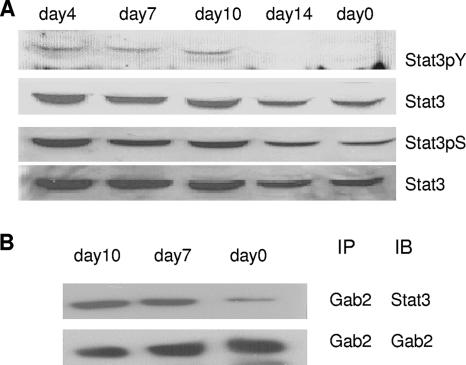
The spleen is the primary site of polyclonal expansion of infected erythroid progenitor cells in vivo. We have shown previously that Gab2 is tyrosine phosphorylated in the spleens of infected mice. Here, we set out to investigate whether the Gab2/Stat3 signaling pathway is active in these cells. Unfractionated spleen cells from mice infected with Friend virus for various amounts of time were collected and Stat3 tyrosine phosphorylation was analyzed. We observed that Stat3 is tyrosine phosphorylated at days 4, 7, and 10 following infection, the time which correlates with the most rapid expansion of erythroblasts in the spleen (Fig. 6A). The immunoprecipitation of these lysates with Gab2 and immunoblotting with Stat3 demonstrated that there is enhanced interaction of Gab2 and Stat3 at days 7 and 10 following infection (Fig. 6B). Taken together, these data indicate that the interaction of Gab2 with Stat3 and the tyrosine phosphorylation of Stat3 are induced in spleens of Friend virus-infected mice during the expansion phase of infected erythroblasts.
FIG. 6.
Interaction of Gab2 with Stat3 and tyrosine phosphorylation of Stat3 in spleens of Friend virus-infected mice. (A) Ten-week-old BALB/cJ mice were injected in their tail veins with FVP at different time points (day 14, day 10, day 7, and day 4). Spleen protein was harvested and subsequently analyzed by SDS-PAGE and Western blotting with anti-phospho-Stat3(Y705), anti-Stat3, and anti-phospho-Stat3(S727). (B) Ten-week-old BALB/cJ mice were injected in their tail veins with FVP at different time points (day 10 and day 7). Spleen protein was immunoprecipitated with anti-Gab2 antibodies and subsequently analyzed by SDS-PAGE and Western blotting with anti-Stat3 and anti-Gab2.
Stat3 is required downstream of Stk for the transformation of primary hematopoietic cells by Friend erythroleukemia virus.
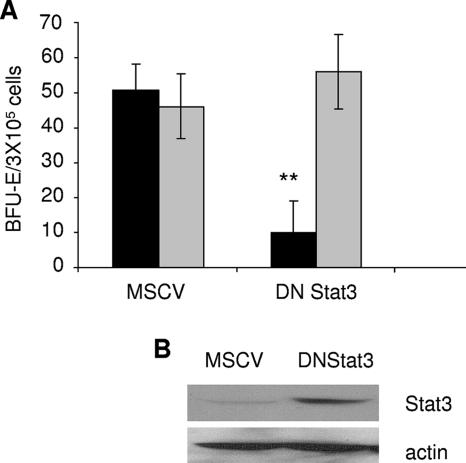
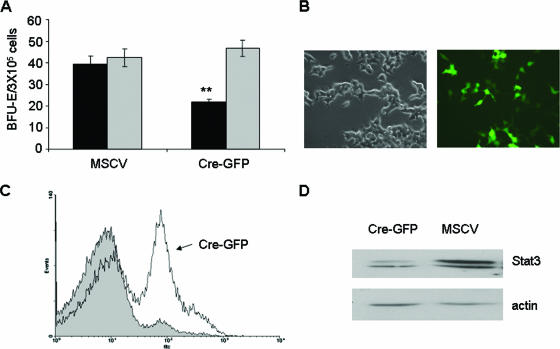
Recent studies have shown that Stat5 is required for Friend virus-induced polycythemia but not for the development of Friend leukemia. However, the potential role of Stat3 in this process has not been addressed. Therefore, we infected primary bone marrow cells from Friend virus-sensitive BALB/c mice with retroviral vectors harboring a dominant negative form of Stat3, followed by infection with Friend leukemia virus and assessment of Epo-independent colony growth. As shown in Fig. 7, the expression of the dimerization mutant Stat3(Y705F) (which acts as a dominant negative Stat3) inhibited Epo-independent colony formation in response to Friend virus infection but had little effect on the Epo-dependent growth of these cells. This experiment was also performed with the DNA binding mutant Stat3(ZZ/VVV), and similar results were obtained (data not shown). To confirm a role for Stat3 in the transformation of primary erythroblasts in response to Friend erythroleukemia virus, we harvested bone marrow from floxed Stat3 mice and infected these cells with a retroviral vector expressing Cre recombinase fused to GFP. Figure 8A shows that the expression of Cre resulted in a reduction in Epo-independent colony formation in response to Friend virus infection, as observed with the dominant negative Stat3. The expression of Cre in 293 packaging cells was verified by fluorescence (Fig. 8B), and that in primary bone marrow cells was verified by flow cytometry (Fig. 8C). Reduced levels of Stat3 in bone marrow cells expressing Cre were verified by Western blot analysis (Fig. 8D). These data support the conclusion that the activation of Stat3 is required for the early stages of transformation of primary erythroblasts by Friend virus.
FIG. 7.
Expression of dominant negative Stat3 inhibits colony formation in response to Friend virus in vitro. (A) Total bone marrow from BALB/c mice was transduced with empty vector (MSCV) or a dominant negative Stat3 [DNStat3(Y705F)]. Transduced cells were plated in methylcellulose containing IL-3 (2.5 ng/ml) in the presence of FV or Epo (1 U/ml). BFU-E cells were stained with acid-benzidine and scored on day 5. Black columns, Friend virus; gray columns, Epo. Bars denote the means ± SD of one of three independent experiments performed in replicates of three and reflect normalized values. **, P < 0.01. (B) Expression of dominant negative Stat3 in 293T packaging cells.
FIG. 8.
Expression of Cre recombinase inhibits colony formation by bone marrow cells from floxed Stat3 mice in response to Friend virus infection. (A) Total bone marrow from floxed Stat3 BALB/c mice was transduced with empty vector or a Cre-GFP fusion plasmid. Transduced cells were plated in methylcellulose containing IL-3 (2.5 ng/ml) in the presence of FV or Epo (1 U/ml). BFU-E cells were stained with acid-benzidine and scored on day 5. Black columns, Friend virus; gray columns, Epo. Bars denote the means ± SD of one of three independent experiments performed in replicates of three and reflect normalized values. **, P < 0.01. (B) Expression of Cre-GFP in 293T packaging cells by immunofluorescence. (C) Total bone marrow from floxed Stat3 mice was infected with vector (shaded area) or Cre-GFP (solid line). Infection efficiency was determined by flow cytometry for GFP. (D) Expression of Stat3 in total bone marrow from floxed Stat3 mice infected with empty vector or Cre-GFP.
DISCUSSION
Following the initial discoveries that Stat3 is required for cellular transformation by v-src (3) and that a constitutive active Stat3 molecule itself can lead to cellular transformation (4), evidence for a critical role for Stat3 in transformation has steadily accumulated. Stat3 is persistently activated in several human cancers, including several hematological malignancies, such as multiple myelomas, leukemias, and lymphomas, in which activated kinases appear to promote its constitutive activity. Recently, Chiarle et al. verified a requirement for Stat3 in the development of B-cell lymphomas by the anaplastic lymphoma kinase in vivo following targeted disruption of Stat3 in the B- and T-cell lineages (6). Here, we have identified an essential role for Stat3 in the early stages of transformation of primary erythroblasts in response to Friend erythroleukemia virus. Therefore, our data further support Stat3 as a potential molecular target in cancer therapy. However, while the critical nature of Stat3 in transformation is becoming increasing clear, the mechanism by which Stat3 is activated in transformed cells has, in many cases, remained elusive.
In v-Eyk, the mutation of the receptor tyrosine kinase c-Eyk results in the presence of a YXXQ motif, a canonical Stat3 binding site (36), leading to the enhanced activation of Stat3 and cellular transformation (2). In addition, enhanced tyrosine phosphorylation of Stat3 has been associated with the mutation of the aspartic acid in the kinase domain of the Kit receptor tyrosine kinase; the activation of this pathway is required for its transforming ability (22), and the juxtamembrane mutations in the Kit receptor found in gastrointestinal stromal tumors have propensities similar to those of activate Stat proteins (5). However, while most tyrosine kinases activate the Stat3 signaling pathway, few contain a canonical Stat3 binding motif. Here we demonstrate, for the first time, a functional Stat3 binding motif in Gab2. The ability of receptor tyrosine kinases to recruit Gab2 through a Grb2-dependent mechanism suggests the possibility that a wide range of receptor tyrosine kinases could activate the Stat3 signaling pathway via a Grb2-/Gab2-dependent mechanism.
There is increasing evidence that the Grb2-/Gab2-dependent signaling pathway may also play a central role in transformation. Gab2 maps to 11q13-14, a region that is commonly amplified in breast cancer, that is found to be overexpressed in human breast cancer, and that potentiates breast carcinogenesis in mice induced by ErbB2 (1, 7). In addition, a Grb2/Gab2 signaling pathway is critical for leukemic transformation by BCR/Abl (30) and the inhibition of Gab2 with RNA interference inhibits colony formation by primary chronic myeloid leukemia cells (30). A Grb2 binding site also contributes to leukemogenesis induced by the Tel/Abl tyrosine kinase (20), and Gab2 mediates mast cell proliferation in response to the Kit receptor tyrosine kinase (39). Taken together, these observations indicate that Gab2 could be activated downstream of tyrosine kinases in hematopoietic cells and propagate signals required for the transforming activity of those kinases.
Gab2 is a large adaptor protein (related to the insulin receptor substrate family of adaptors) which transmits signals from a number of receptor tyrosine kinases and is recruited to active signaling complexes through two mechanisms: a polyproline-rich region that binds the N-terminal SH3 domain of Grb2 and a pleckstrin homology domain that is recruited to the membrane by phospholipids generated by PI3 kinase (10, 19). Two Shp-2 binding motifs and three p85 binding sites conserved in all Gab family members (Gab1, Gab2, and Gab3) have been shown to be critical for the activation of the Erk and PI3 kinase pathways, respectively. Accordingly, we found that the mutation of the Shp2 or p85 binding sites in Gab2 in the context of an Sf-Stk/Gab2 fusion protein reduced cellular transformation of primary erythroblasts in response to Friend virus (34). However, these signaling pathways were retained in an Sf-Stk/Gab1 fusion protein that failed to support the transformation of Friend virus-infected bone marrow cells, indicating that Gab2 must harbor unique functions which are not associated with the activation of Gab1 that are also required for transformation. Our data indicate that the recruitment and activation of Stat3 by Gab2 cooperate with signals generated by Shp2 and p85 and are required for the ability of Gab2 to mediate the transformation of primary hematopoietic cells downstream of the Stk receptor tyrosine kinase.
While the mechanism by which most receptor tyrosine kinases lead to the activation of the Stat3 signaling pathway is unclear, many studies have implicated Src family kinases in this process (9, 27, 35). Recent studies have demonstrated that (i) the hematopoietic cell kinase mediates the phosphorylation of Gab1 and Gab2 in multiple myeloma cells (26), (ii) Lyn and Syk are required for Gab2 tyrosine phosphorylation downstream of FcɛR1 (38), and (iii) granulocyte colony-stimulating factor-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent (41). We have shown here that the kinase activity is not required for the activation of Stat3 and the Epo-independent growth of Friend virus-infected cells in the context of an Sf-Stk/Gab2 fusion protein. This raises the intriguing possibility that the activation of Stat3 downstream of receptor tyrosine kinases, including Stk, could be mediated by the Src-dependent phosphorylation of Gab2, resulting in the recruitment and activation of Stat3. Indeed, our unpublished observations demonstrate a requirement for Src family kinases in the transformation of primary erythroblasts by Friend leukemia virus in vitro. However, our studies with the Lyn knockout mice did not reveal a critical role for the Lyn tyrosine kinase in the early stages of transformation in vivo (31).
In this study, we have identified a novel receptor tyrosine kinase/Grb2/Gab2/Stat3 signaling pathway required for the transformation of primary hematopoietic cells by using Friend erythroleukemia virus as a model system. Although the targeting of tyrosine kinases has become the first line of therapy in treating leukemia and other malignancies, the occurrence of drug resistance has highlighted the necessity for identifying multiple complementary targets for the successful treatment of these diseases. The identification of specific downstream signaling events that mediate transformation will aid in the elucidation of a new generation of targets for drug therapy. Our data indicate that blocking the ability of Gab2 to recruit and activate Stat3 may provide a novel target in the treatment of leukemia and possibly a wider range of human cancers.
Acknowledgments
This work was funded by grant no. R01 HL066571 from the National Institutes of Health and by a scholar award from the Leukemia and Lymphoma Society to P. H. Correll.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Bentires-Alj, M., S. G. Gil, R. Chan, Z. C. Wang, Y. Wang, N. Imanaka, L. N. Harris, A. Richardson, B. G. Neel, and H. Gu. 2006. A role for the scaffolding adapter GAB2 in breast cancer. Nat. Med. 12:114. [DOI] [PubMed] [Google Scholar]
- 2.Besser, D., J. Bromberg, J. Darnell, and H. Hanafusa. 1999. A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol. Cell. Biol. 19:1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromberg, J., C. Horvath, D. Besser, W. Latham, and J. Darnell. 1998. Stat3 activation is required for cellular transformation by v-src. Mol. Cell. Biol. 18:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberg, J., M. Wrzeszczynska, G. Devgan, Y. Zhao, R. Pestell, C. Albanese, and J. Darnell. 1999. Stat3 as an oncogene. Cell 98:295-303. [DOI] [PubMed] [Google Scholar]
- 5.Casteran, N., P. De Sepulveda, N. Beslu, M. Aoubala, S. Letard, E. Lecocq, R. Rottapel, and P. Dubreuil. 2003. Signal transduction by several KIT juxtamembrane domain mutations. Oncogene 22:4710-4722. [DOI] [PubMed] [Google Scholar]
- 6.Chiarle, R., W. J. Simmons, H. Cai, G. Dhall, A. Zamo, R. Raz, J. G. Karras, D. E. Levy, and G. Inghirami. 2005. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 11:623-629. [DOI] [PubMed] [Google Scholar]
- 7.Daly, R., H. Gu, J. Parmar, S. Malaney, R. Lyons, R. Kairouz, D. Head, S. Henshall, B. Neel, and R. Sutherland. 2002. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene 21:5175-5181. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein, L. D., P. A. Ney, Q. P. Liu, R. F. Paulson, and P. H. Correll. 2002. Sf-Stk kinase activity and the Grb2 binding site are required for Epo-independent growth of primary erythroblasts infected with Friend virus. Oncogene 21:3562-3570. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, R., T. Bowman, G. Niu, H. Yu, S. Minton, C. Muro-Cacho, C. Cox, R. Felcome, R. Fairclough, S. Parsons, A. Laudano, A. Gazit, A. Levitzki, A. Raker, and R. Jove. 2001. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499-2513. [DOI] [PubMed] [Google Scholar]
- 10.Gu, H., and B. G. Neel. 2003. The ‘Gab’ in signal transduction. Trends Cell Biol. 13:122. [DOI] [PubMed] [Google Scholar]
- 11.Halupa, A., M. Bailey, K. Huang, N. Iscove, D. Levy, and D. Barber. 2005. A novel role for Stat1 in regulating murine erythropoiesis: deletion of Stat1 results in overall reduction of erythroid progenitor cells and alters their distribution. Blood 105:552-561. [DOI] [PubMed] [Google Scholar]
- 12.Haq, R., A. Halupa, B. Beattie, J. Mason, B. Zanke, and D. Barber. 2002. Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J. Biol. Chem. 277:17359-17366. [DOI] [PubMed] [Google Scholar]
- 13.Kirito, K., T. Nagashima, K. Ozawa, and N. Komatsu. 2002. Constitutive activation of Stat1 and Stat3 in primary erythroleukemia cells. Int. J. Hematol. 75:51-54. [DOI] [PubMed] [Google Scholar]
- 14.Kirito, K., K. Nakajima, T. Watanabe, M. Uchida, M. Tanaka, K. Ozawa, and N. Komatsu. 2002. Identification of the human erythropoietin receptor region required for Stat1 and Stat3 activation. Blood 99:102-110. [DOI] [PubMed] [Google Scholar]
- 15.Kirito, K., M. Uchida, M. Takatoku, K. Nakajima, T. Hirano, Y. Miura, and N. Komatsu. 1998. A novel function of Stat1 and Stat3 proteins in erythropoietin-induced erythroid differentiation of human leukemia cell line. Blood 92:462-471. [PubMed] [Google Scholar]
- 16.Kirito, K., M. Uchida, M. Yamada, Y. Miura, and N. Komatsu. 1997. A distinct function of Stat proteins in erythropoietin signal transduction. J. Biol. Chem. 272:16507-16513. [DOI] [PubMed] [Google Scholar]
- 17.Lenox, L. E., J. M. Perry, and R. F. Paulson. 2005. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 105:2741-2748. [DOI] [PubMed] [Google Scholar]
- 18.Li, J., A. D'Andrea, H. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature 343:762-764. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1. [DOI] [PubMed] [Google Scholar]
- 20.Million, R. P., N. Harakawa, S. Roumiantsev, L. Varticovski, and R. A. Van Etten. 2004. A direct binding site for Grb2 contributes to transformation and leukemogenesis by the Tel-Abl (ETV6-Abl) tyrosine kinase. Mol. Cell. Biol. 24:4685-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ney, P., and A. D'Andrea. 2000. Friend erythroleukemia revisited. Blood 96:3675-3680. [PubMed] [Google Scholar]
- 22.Ning, Z., J. Li, M. McGuinness, and R. Arceci. 2001. STAT3 activation is required for Asp816 mutant c-Kit induced tumorigenicity. Oncogene 20:4528-4536. [DOI] [PubMed] [Google Scholar]
- 23.Nishigaki, K., D. Thompson, C. Hanson, T. Yugawa, and S. Ruscetti. 2001. The envelope glycoprotein of Friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk. J. Virol. 75:7893-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohashi, T., M. Masuda, and S. Ruscetti. 1995. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood 85:1454-1462. [PubMed] [Google Scholar]
- 25.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23:159. [DOI] [PubMed] [Google Scholar]
- 26.Podar, K., G. Mostoslavsky, M. Sattler, Y.-T. Tai, T. Hayashi, L. P. Catley, T. Hideshima, R. C. Mulligan, D. Chauhan, and K. C. Anderson. 2004. Critical role for hematopoietic cell kinase (Hck)-mediated phosphorylation of Gab1 and Gab2 docking proteins in interleukin 6-induced proliferation and survival of multiple myeloma cells. J. Biol. Chem. 279:21658-21665. [DOI] [PubMed] [Google Scholar]
- 27.Ren, Z., and T. S. Schaefer. 2002. ErbB-2 activates Stat3α in a Src- and JAK2-dependent manner. J. Biol. Chem. 277:38486-38493. [DOI] [PubMed] [Google Scholar]
- 28.Sano, S., S. Itami, K. Takeda, M. Tarutani, Y. Yamaguchi, H. Miura, K. Yoshikawa, S. Akira, and J. Takeda. 1999. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 18:4657-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattler, M., M. G. Mohi, Y. B. Pride, L. R. Quinnan, N. A. Malouf, K. Podar, F. Gesbert, H. Iwasaki, S. Li, and R. A. Van Etten. 2002. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1:479. [DOI] [PubMed] [Google Scholar]
- 30.Scherr, M., A. Chaturvedi, K. Battmer, I. Dallmann, B. Schultheis, A. Ganser, and M. Eder. 2006. Enhanced sensitivity to inhibition of SHP2, STAT5, and Gab2 expression in chronic myeloid leukemia (CML). Blood 107:3279-3287. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian, A., S. Hegde, P. H. Correll, and R. Paulson. 2006. Mutation of the Lyn tyrosine kinase delays the progression of Friend virus induced erythroleukemia without affecting susceptibility. Leuk. Res. 30:1141. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian, A., H. E. Teal, P. H. Correll, and R. F. Paulson. 2005. Resistance to Friend virus-induced erythroleukemia in W/Wv mice is caused by a spleen-specific defect which results in a severe reduction in target cells and a lack of Sf-Stk expression. J. Virol. 79:14586-14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teal, H., S. Ni, J. Xu, L. Finkelstein, A. Cheng, R. Paulson, G. Feng, and P. Correll. 2006. Grb2-mediated recruitment of Gab2, but not Gab1, to Sf-Stk supports the expansion of Friend virus-infected erythroid progenitor cells. Oncogene 25:2433-2443. [DOI] [PubMed] [Google Scholar]
- 34.Teal, H. E., S. Ni, J. Xu, L. D. Finkelstein, A. M. Cheng, R. F. Paulson, G. S. Feng, and P. H. Correll. 2006. GRB2-mediated recruitment of GAB2, but not GAB1, to SF-STK supports the expansion of Friend virus-infected erythroid progenitor cells. Oncogene 25:2433. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Y., W. Wharton, R. Garcia, A. Kraker, R. Jove, and W. Pledger. 2000. Activation of Stat3 preassembled with platelet-derived growth factor beta receptors requires Src kinase activity. Oncogene 19:2075-2085. [DOI] [PubMed] [Google Scholar]
- 36.Wiederkehr-Adam, M., P. Ernst, K. Muller, E. Bieck, F. Gombert, J. Ottl, P. Graff, F. Grossmuller, and M. Heim. 2003. Characterization of phosphopeptide motifs specific for the Src homology 2 domains of signal transducer and activator of transcription 1 (Stat1) and Stat3. J. Biol. Chem. 278:16117-16128. [DOI] [PubMed] [Google Scholar]
- 37.Yamamura, Y., H. Senda, Y. Kageyama, T. Matsuzaki, M. Noda, and Y. Ikawa. 1998. Erythropoietin and Friend virus gp55 activate different JAK/STAT pathways through the erythropoietin receptor in erythroid cells. Mol. Cell. Biol. 18:1172-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu, M., C. A. Lowell, B. G. Neel, and H. Gu. 2006. Scaffolding adapter Grb2-associated binder 2 requires Syk to transmit signals from FcɛRI. J. Immunol. 176:2421-2429. [DOI] [PubMed] [Google Scholar]
- 39.Yu, M., J. Luo, W. Yang, Y. Wang, M. Mizuki, Y. Kanakura, P. Besmer, B. G. Neel, and H. Gu. 2006. The scaffolding adapter Gab2, via SHP-2, regulates Kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J. Biol. Chem. 281:28615-28626. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., M. S. Randall, M. R. Loyd, W. Li, R. L. Schweers, D. A. Persons, J. E. Rehg, C. T. Noguchi, J. N. Ihle, and P. A. Ney. 2006. Role of erythropoietin receptor signaling in Friend virus-induced erythroblastosis and polycythemia. Blood 107:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, Q.-S., L. J. Robinson, V. Roginskaya, and S. J. Corey. 2004. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood 103:3305-3312. [DOI] [PubMed] [Google Scholar]