Abstract
P bodies are cytoplasmic domains that contain proteins involved in diverse posttranscriptional processes, such as mRNA degradation, nonsense-mediated mRNA decay (NMD), translational repression, and RNA-mediated gene silencing. The localization of these proteins and their targets in P bodies raises the question of whether their spatial concentration in discrete cytoplasmic domains is required for posttranscriptional gene regulation. We show that processes such as mRNA decay, NMD, and RNA-mediated gene silencing are functional in cells lacking detectable microscopic P bodies. Although P bodies are not required for silencing, blocking small interfering RNA or microRNA silencing pathways at any step prevents P-body formation, indicating that P bodies arise as a consequence of silencing. Consistently, we show that releasing mRNAs from polysomes is insufficient to trigger P-body assembly: polysome-free mRNAs must enter silencing and/or decapping pathways to nucleate P bodies. Thus, even though P-body components play crucial roles in mRNA silencing and decay, aggregation into P bodies is not required for function but is instead a consequence of their activity.
In eukaryotic cells, posttranscriptional processes (e.g., mRNA surveillance, silencing, translational repression, and decay) play a central role in the regulation of gene expression and ultimately determine the expression levels of a significant fraction of the transcriptome (57). These processes are interlinked by the use of common enzymes and by sharing mRNAs and their associated proteins (ribonucleoprotein complexes [mRNPs]) as common substrates. Recently, it has become apparent that posttranscriptional processes acting on cytoplasmic mRNPs are physically tied and can occur in discrete cytoplasmic domains known as mRNA processing bodies or P bodies (3, 17).
The first proteins found in P bodies are those functioning in the degradation of bulk mRNA (3, 17). In eukaryotes, this process is initiated by removal of the poly(A) tail by deadenylases (38, 57). There are several deadenylase complexes in eukaryotes: the PARN2-PARN3 complex is thought to initiate deadenylation, which is then continued by the CAF1-CCR4-NOT complex (38, 57). Following deadenylation, mRNAs are exonucleolytically digested from their 3′ end by the exosome, a multimeric complex with 3′-to-5′ exonuclease activity (24). Alternatively, the cap structure is removed by the decapping enzyme DCP2 after deadenylation, rendering the mRNA susceptible to 5′-to-3′ degradation by the major cytoplasmic exonuclease XRN1 (38, 57).
Decapping requires the activity of several proteins generically termed decapping coactivators, though they may stimulate decapping by different mechanisms (38, 57). In the yeast Saccharomyces cerevisiae, these include DCP1, which forms a complex with DCP2 and is required for decapping in vivo, the enhancer of decapping-3 (EDC3 or LSm16), the heptameric LSm1-7 complex, the DExH/D-box RNA helicase 1 (Dhh1, also known as RCK/p54 in mammals), and Pat1, a protein of unknown function that interacts with the LSm1-7 complex, Dhh1, and XRN1 (28, 38, 57). In human cells, DCP1 and DCP2 are part of a multimeric protein complex that includes RCK/p54, EDC3, and Ge-1 (also known as RCD-8 or Hedls), a protein that is absent in S. cerevisiae (20, 60).
The decapping enzymes, decapping coactivators, and XRN1 colocalize in P bodies (3, 17). Additional P-body components in multicellular organisms include the protein RAP55 (also known as LSm14), which has a putative role in translation regulation (1, 2, 47, 58), and GW182, which plays a role in the microRNA (miRNA) pathway (6, 18, 19, 26, 30, 31, 34, 41).
Many other proteins functioning in diverse posttranscriptional processes have been shown to localize to P bodies. These include (i) proteins involved in nonsense-mediated mRNA decay (NMD), a pathway that degrades mRNAs harboring premature translation termination codons (or nonsense codons) (22, 45, 52); (ii) proteins with roles in AU-rich element (ARE)-mediated mRNA decay, a pathway that degrades mRNAs containing AU-rich elements in their 3′ untranslated regions (UTRs) (27, 32, 46); (iii) the Argonaute proteins, involved in RNA interference (RNAi) and miRNA-mediated gene silencing (6, 26, 30, 31, 34, 40, 43); and (iv) translational repressors, such as eIF4E-transporter (eIF4E-T) and yeast Dhh1 and its vertebrate ortholog RCK/p54 (4, 13, 14, 16, 21).
The presence of mRNA decay enzymes and of effectors of the NMD, ARE-mediated mRNA decay, RNAi, and miRNA pathways in P bodies raises the question of whether the environment of microscopic P bodies is required for these processes to occur or whether these processes take place as efficiently in the diffuse cytoplasm. Recent studies with mammalian cells have shown that P-body integrity is not required for ARE-mediated mRNA decay or silencing mediated by fully or partially complementary exogenous, transfected small interfering RNAs (siRNAs) (13, 46). In this study, we investigated the requirement for P-body integrity in NMD, mRNA decay, and silencing mediated by miRNAs and long double-stranded RNAs (dsRNAs). We show that these pathways are unaffected in cells lacking detectable, wild-type P bodies. However, although P bodies are not required for silencing, active silencing pathways are required for P-body formation in Drosophila melanogaster cells, indicating that P bodies are not the cause but a consequence of silencing.
MATERIALS AND METHODS
Western blots and fluorescence microscopy.
Antibodies to Drosophila Trailer hitch (Tral; full length) and Ge-1 (amino acids 1428 to 1641) were raised in rats immunized with glutathione S-transferase fusions of the proteins expressed in Escherichia coli. For Western blotting, the polyclonal antibodies were diluted 1:1,000. Mouse monoclonal antitubulin antibodies (Sigma) were diluted 1:10,000. Bound primary antibodies were detected with alkaline phosphatase-coupled anti-rat or anti-mouse (1:25,000) antibodies (Western-Star kit from Tropix).
For immunofluorescence, Schneider cells (S2 cells) were allowed 15 min to adhere to poly-d-lysine-coated coverslips, fixed with 4% paraformaldehyde for 15 min, followed by 5 min incubation in methanol at −20°C. Cells were then permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (5 min) and stained with affinity-purified anti-Tral or anti-Ge-1 antibodies diluted 1:250 in phosphate-buffered saline containing 1% bovine serum albumin (1 h). Tetramethyl rhodamine isothiocyanate-coupled goat secondary antibody (Molecular Probes) was used in a dilution of 1:250. Cells were mounted using Fluoromount-G (Southern Biotechnology Associates, Inc.). Images were acquired using a Leica TCS SP2 confocal microscope. For a given experiment, all images were taken with the same settings.
RNAi.
Depletions described in this study were carried out by treating cells with the corresponding dsRNAs on day 0 and day 4 as described previously (6). Thirty micrograms of dsRNA were used per six-well dish containing ca. 2 × 106 cells. In the restoration experiments, luciferase siRNA (5′-CGUACGCGGAAUACUUCGAdTdT) and SMG6 siRNA (5′-GGAAUCUCAUACUGAUAAAUU) were used. The siRNAs were transfected at a final concentration of 26 nM.
Functional assays.
All transfections were performed in 6-well or 24-well dishes by using Effectene transfection reagent (QIAGEN). The NMD and RNAi reporters have been described previously (23, 37). Plasmids allowing the expression of miRNA primary transcripts and miRNA reporters were described previously (6). The transfection mixtures contained 100 ng of firefly luciferase (F-Luc) reporter plasmid (F-Luc-CG10011 or F-Luc-Nerfin), 0.4 μg of the Renilla luciferase (R-Luc) transfection control plasmid (pAc5.1-R-Luc), and 1 μg of plasmids expressing miRNA primary transcripts.
The F-Luc-5BoxB plasmid and a plasmid allowing the expression of a λN-hemagglutinin (HA)-GW182 fusion have been described before (6). The following plasmids were cotransfected: 0.15 μg reporter plasmid (F-Luc-5BoxB), 0.4 μg pAc5.1-R-Luc (as a transfection control), and 0.5 μg pAc5.1λN-HA construct (for the expression of λN-HA fusions). Three days after transfection, F-Luc and R-Luc activities were measured using a dual-luciferase reporter assay system (Promega), and total RNA was isolated using TriFast (peqLab Biotechnologies). RNA samples were analyzed as described previously (6).
RESULTS
P-body components are evolutionarily conserved.
We reported earlier that the P-body marker GW182 localizes to cytoplasmic foci in D. melanogaster S2 cells together with the decapping enzyme DCP2 and the decapping coactivator DCP1 (6), suggesting that these foci represent P bodies. To characterize D. melanogaster P bodies further, we raised antibodies to the D. melanogaster orthologs of two proteins found in human-cell P bodies. These correspond to Ge-1 (20, 60) and Tral (LSm15), which is closely related to human RAP55 (or LSm14; see references 1, 2, 47, 56, and 58). Both antibodies stained the cytoplasm diffusely and also stained discrete cytoplasmic foci with a diameter ranging from 100 nm to 300 nm (Fig. 1A). The antibody signals are specific, as they are lost in cells in which the cognate proteins were depleted (Fig. 1A and B; see the large fields in Fig. S1A in the supplemental material). The foci are present in about 95% of the cell population and are readily detectable because the concentration of Tral or Ge-1 in these foci is significantly higher than that in the surrounding cytoplasm.
FIG. 1.
Conservation of P-body components. (A and B) S2 cells were stained with affinity-purified anti-Tral or anti-Ge-1 antibodies. The antibody staining is specific, as it was strongly reduced in cells in which the corresponding proteins were depleted (knockdown). (B) The efficiency of Tral or Ge-1 depletions was assessed by Western blotting. (C) Confocal fluorescent micrographs of S2 cells expressing GFP fusions of the proteins indicated on the left (green channel). Cells were stained with affinity-purified anti-Tral antibodies (red channel). Scale bar, 5 μm.
We next examined the distribution of green fluorescent protein (GFP)-tagged versions of proteins found in P bodies in yeast and/or human cells. These include DCP1, DCP2, GW182, Me31B (the D. melanogaster ortholog of S. cerevisiae Dhh1 and vertebrate RCK/p54), CG5208 (the D. melanogaster homolog of S. cerevisiae Pat1, referred to as HPat hereafter), and EDC3 (also known as LSm16; see references 3 and 17). All of these proteins formed cytoplasmic foci that costained with the anti-Tral or anti-Ge-1 antibodies (Fig. 1C; for Ge-1, see Fig. S1B in the supplemental material). Importantly, the expression of the GFP-tagged proteins did not significantly alter the number and size of endogenous P bodies (Fig. 1, compare panels C and A). Together, these results indicate that the localization of decapping enzymes and decapping coactivators into P bodies is evolutionarily conserved. The localization of GW182 in Drosophila P bodies is in agreement with the proposal that GW-bodies and P bodies overlap, as reported for mammalian cells by several groups (5, 6, 18, 19, 26, 27, 31, 34, 39, 46, 53, 55, 58-60).
We also examined the localization of proteins implicated in translational regulation in Drosophila oocytes whose corresponding transcripts are detectable in S2 cells, in particular, Smaug and the dsRNA binding protein Staufen. Smaug is a translational repressor that also promotes deadenylation of bound mRNAs by recruiting the CAF1-CCR4-NOT1 complex (61). Both proteins localized to P bodies with endogenous Tral (Fig. 2A). Strikingly, P bodies increased in size in cells expressing Staufen at high levels but not in cells overexpressing GFP fusions of Smaug or of the proteins examined in Fig. 1C, suggesting that Staufen promotes P-body formation. Drosophila Staufen, Tral, DCP1, DCP2, XRN1, and Me31B have also been detected in RNP granules in neuronal cells and/or in oocytes (5, 61), indicating that P bodies and other RNP granules observed in neuronal cells or during development share common components (3, 17).
FIG. 2.
Nontranslating mRNAs and overexpression of translational repressors enhance P-body formation. (A) Confocal fluorescent micrographs of S2 cells expressing GFP fusions of Staufen (Stau) or Smaug (green channel). Cells were stained with affinity-purified anti-Tral antibodies (red channel). Scale bar, 5 μm. (B) S2 cells were treated with RNase A (15 min, 100 μg/ml), cycloheximide (1 h, 10 μg/ml), or puromycin (1 h, 100 μg/ml) and stained with affinity-purified anti-Tral or anti-Ge-1 antibodies. (C) S2 cells were transfected with plasmids expressing F-Luc and R-Luc. Eight hours after transfection, cells were split into three pools. One pool was treated with puromycin, and one was treated with cycloheximide. Luciferase activity was measured 1 h after the addition of the drugs.
P-body formation requires nontranslating mRNPs and/or mRNPs undergoing decapping.
A conserved feature of P bodies in human and yeast cells is that their formation depends on RNA and is enhanced in cells in which the concentration of nontranslating mRNAs or of mRNAs undergoing decapping increases (4, 12, 16, 27, 44, 48, 55). These observations indicate that mRNAs must exit the translation cycle to localize to P bodies (14). In agreement with this, we observed that Drosophila P bodies decline when cells are treated with RNase A or with cycloheximide (which inhibits translation elongation and stabilizes mRNAs into polysomes) (Fig. 2B). In contrast, P-body sizes increase in cells treated with puromycin (Fig. 2B), which causes premature polypeptide chain termination and polysome disassembly (9). Both puromycin and cycloheximide inhibit protein synthesis in S2 cells, as judged by the reduction of F-Luc and R-Luc activities after the treatment of cells transiently expressing these proteins with these drugs (Fig. 2C).
The size of Drosophila P bodies also depends on the fraction of mRNAs undergoing decapping, in agreement with the results reported for yeast and human cells (4, 14, 16, 25, 44, 48, 53). Indeed, blocking mRNA decay at an early stage, for instance, by preventing deadenylation in cells in which NOT1 (a component of the CAF1-CCR4-NOT deadenylase complex) is depleted, leads to the dispersion of P bodies, whereas P bodies are on average more prominent in cells from which DCP2 or XRN1 is depleted (in which decapping and subsequent 5′-to-3′ mRNA decay are inhibited) (Fig. 3).
FIG. 3.
P-body formation requires mRNAs undergoing decapping. S2 cells from which the indicated proteins were depleted were stained with affinity-purified anti-Tral or anti-Ge-1 antibodies. Depletion of NOT1, LSm1, Me31B, HPat, or Ge-1 results in P-body loss, whereas depletion of DCP2 or XRN1 leads to an increase in P-body size. EDC3 or Tral depletions have no detectable effect on P-body integrity. Numbers indicate the fraction of cells exhibiting a staining identical to that shown in the representative panel in three independent knockdowns performed per protein (at least 100 cells were counted per knockdown). Scale bar, 5 μm.
In this study, we considered P bodies dispersed when Ge-1 or Tral were no longer detected in discrete, bright cytoplasmic foci of 100 to 300 nm as observed for control cells. Whether P bodies are simply fragmented into small submicroscopic aggregates or their components are released into soluble protein complexes cannot be discriminated. Nevertheless, the important distinction between wild-type cells and cells lacking P bodies is that in control cells, the concentration of Ge-1 or Tral (and other components) in P bodies is significantly higher than that in the surrounding cytoplasm, whereas in cells in which P bodies are dispersed, the proteins are more homogenously distributed in the cytoplasm.
We then examined P bodies in cells from which various proteins known to stimulate decapping were depleted. We observed that Tral spreads more uniformly within the cytoplasm in cells from which LSm1, LSm3, Me31B, HPat, or Ge-1 was depleted (Fig. 3; see Fig. 6C for LSm3), indicating that these proteins are required for P-body formation. Similarly, Ge-1 staining was more diffuse in cells from which LSm1 (or LSm3), Me31B, or HPat was depleted (Fig. 3). These observations are consistent with the putative role of the LSm1-7 complex, Me31B, and HPat upstream of DCP2 in the 5′-to-3′ mRNA decay pathway (38). Depletion of LSm proteins in human cells also leads to P-body loss (4, 13), but yeast cells lacking LSm1 have larger P bodies than wild-type cells (44).
FIG. 6.
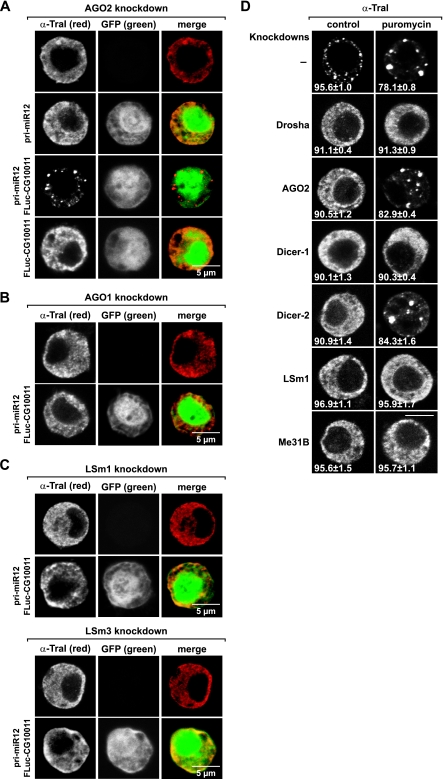
Releasing mRNAs from polysomes is not sufficient for P-body formation. (A) AGO2-depleted cells were transfected with plasmids encoding pri-miR-12, pri-miR-12 together with F-Luc-CG10011 reporter, or the F-Luc-CG10011 reporter alone. A plasmid expressing GFP was cotransfected in order to visualize transfected cells. Cells were stained with affinity-purified anti-Tral antibodies. (B and C) AGO1-, LSm1-, or LSm3-depleted cells were transfected with plasmids encoding pri-miR-12 together with F-Luc-CG10011 reporter. A plasmid expressing GFP was cotransfected in order to visualize transfected cells. Cells were stained with affinity-purified anti-Tral antibodies. (D) S2 cells from which the proteins indicated on the left were depleted were treated with puromycin and stained with affinity-purified anti-Tral antibodies. Numbers indicate the fraction of cells exhibiting a staining identical to that shown in the representative panel as described in the legends to Fig. 3 and 4. Scale bar, 5 μm.
Depletion of Tral or EDC3 (LSm16) does not affect P-body integrity (Fig. 3), although these proteins are depleted efficiently (Fig. 1B and data not shown). In contrast to Tral, human RAP55 (LSm14) is required for P-body integrity (58), suggesting that these proteins may not represent functional analogs, despite their similarities.
P-body formation depends on functional RNA silencing pathways.
P-body formation is linked to miRNA biogenesis, because P-bodies decline in human cells from which Drosha or its partner DGCR8 is depleted (39). Consistently, we observed a reduction in P-body sizes and numbers in cells from which Drosha or Pasha (the Drosophila ortholog of DGCR8) is depleted (Fig. 4).
FIG. 4.
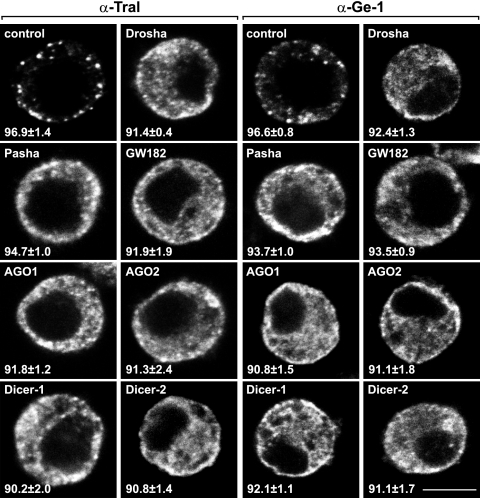
P-body integrity depends on functional silencing pathways. S2 cells from which proteins involved in different steps of RNAi or the miRNA pathway were depleted were stained with affinity-purified anti-Tral or ant-Ge-1 antibodies. The fraction of cells exhibiting a staining identical to that shown in the representative panel was determined by scoring at least 100 cells in each of the three independent knockdowns performed per protein. Scale bar, 5 μm.
We also observed a decline of wild-type P bodies in cells from which Dicer-1, AGO1, and GW182 (Fig. 4), proteins required for miRNA biogenesis and function, have been depleted. Surprisingly, Dicer-2 or AGO2 depletion also led to similar diffuse distributions of Tral and Ge-1 (Fig. 4), even though these depletions do not affect miRNA biogenesis and function in D. melanogaster (36, 41). Thus, blocking RNA silencing pathways at any step (i.e., not only at the biogenesis step), leads to a strong reduction of P-body sizes and numbers and, consequently, to a more diffuse cytoplasmic distribution of Tral or Ge-1. This loss of wild-type P bodies is observed under conditions in which the depletions inhibit the corresponding silencing pathways, the miRNA pathway for Drosha, Pasha, Dicer-1, GW182, or AGO1 knockdowns or the siRNA pathway for Dicer-2 or AGO2 knockdowns (see Fig. 7; data not shown). All together, these results indicate that silencing pathways are likely to generate a significant fraction of nontranslating mRNPs and/or mRNPs committed to decapping required for P-body formation.
FIG. 7.
Silencing by miRNAs or dsRNAs does not require microscopic P bodies. (A) The indicated proteins were depleted from S2 cells (kd, knockdown). Depleted cells were cotransfected with plasmids expressing miRNA reporters (Nerfin or CG10011), plasmids expressing miR-9b or miR-12 or the corresponding empty vector. R-Luc served as a transfection control. F-Luc activity and the corresponding mRNA levels were measured and normalized to those of the Renilla control. Normalized F-Luc activities and mRNA levels in cells transfected with the empty vector were set to 100% for each knockdown (not shown). Error bars represent standard deviations from three independent experiments. (B) S2 cells expressing adh mRNA were treated with a dsRNA targeting a central region of the adh open reading frame or with GFP dsRNA as a control. The levels of adh mRNA were quantitated and normalized to those of a CAT mRNA transfection control. These values were set to 100 in cells treated with GFP dsRNA. The left panel shows results for S2 cells expressing adh mRNA that were treated with adh or GFP dsRNAs. These cells were then treated with dsRNAs targeting the proteins indicated. The levels of adh mRNA were quantitated and normalized to those of a transfection control (CAT mRNA). For each knockdown, the normalized values obtained for the cells in the presence of the adh dsRNA were divided by those obtained for cells treated with GFP dsRNA to compensate for unspecific effects of the depletions. Error bars represent standard deviations from three independent experiments. (C) Depletions shown in panel B resulted in P-body dispersion as shown by fluorescent staining of cells with anti-Tral antibodies. Numbers indicate the fraction of cells exhibiting a staining identical to that shown in the representative panel in the three independent knockdowns performed per protein (at least 100 cells were counted per knockdown). Scale bar, 5 μm.
mRNAs that enter silencing pathways trigger P-body formation.
To assess the possibility that mRNPs that enter silencing pathways elicit P-body formation, we tried to restore P bodies in cells from which Drosha (which is not required for the siRNA pathway) was depleted by transfecting an experimentally validated siRNA that targets an endogenous mRNA effectively (i.e., an siRNA that targets SMG6 and inhibits NMD) (data not shown) or, as a control, an siRNA that does not target an endogenous transcript (we used one complementary to F-Luc [F-Luc siRNA]). The siRNAs were cotransfected with a plasmid expressing GFP to identify transfected cells. We saw no restoration of P bodies in cells transfected with F-Luc siRNA, whereas SMG6 siRNA restored P bodies in about 30% of depleted cells expressing GFP (Fig. 5A). Importantly, SMG6 siRNA did not restore P bodies in cells from which AGO2 was depleted (Fig. 5B), indicating that restoration requires an active siRNA pathway. Furthermore, the siRNAs did not affect P-body sizes or numbers in wild-type cells (data not shown; see also below regarding NMD factors).
FIG. 5.
mRNAs that enter silencing pathways elicit P-body formation. (A) Drosha-depleted cells were transfected with SMG6 siRNA, F-Luc siRNA, or F-Luc siRNA together with a plasmid expressing F-Luc mRNA. A plasmid expressing GFP was cotransfected in order to visualize transfected cells. (B) AGO2-depleted cells were transfected with SMG6 siRNA together with a plasmid expressing GFP in order to visualize transfected cells. (C) S2 cells from which the proteins indicated on the left were depleted were treated with long dsRNAs targeting GFP or the Drosophila CG4415 mRNA. In all panels, cells were stained with affinity-purified anti-Tral antibodies. Numbers indicate the fraction of cells exhibiting a staining identical to that shown in the representative panel as described in the legends to Fig. 3 and 4. Scale bar, 5 μm.
The observation that only SMG6 siRNA restores P bodies suggests that, in addition to the siRNA, an mRNP target must be present for P bodies to assemble. To confirm this, we cotransfected cells lacking P bodies with the F-Luc siRNA and a plasmid expressing F-Luc mRNA. In the presence of F-Luc mRNA, the F-Luc siRNA restored P-body formation in about 30% of transfected cells (Fig. 5A). The SMG6 siRNA or the F-Luc siRNA, in the presence of F-Luc mRNA, also restored P bodies in cells from which additional proteins required for the miRNA pathway (e.g., Pasha, Dicer-1, or AGO1) (data not shown) were depleted.
We then examined whether long dsRNAs could also restore P-body formation in cells from which Drosha, Pasha, Dicer-1, or AGO1, none of which is required for dsRNA processing into siRNAs, was depleted. We found that dsRNAs targeting an endogenous transcript of unknown function (CG4415) restored P-body formation efficiently, whereas a dsRNA targeting GFP did not restore P bodies (Fig. 5C). We obtained similar results with different dsRNAs targeting endogenous mRNAs (data not shown). The dsRNAs did not alter P bodies in control cells (Fig. 5C). More importantly, dsRNAs did not restore P bodies in cells from which AGO2 was depleted (Fig. 5C), again indicating that the RNAi pathway must be functional. These observations support the conclusion that mRNPs that enter the RNAi pathway elicit P-body formation.
Finally, we also investigated whether overexpression of an miR-12 primary transcript (pri-miR-12) could restore P bodies in cells from which AGO2 or Dicer-2 (which are not required for the miRNA pathway) was depleted. P-body formation was restored only when the plasmid encoding pri-miR-12 was cotransfected with a plasmid expressing an F-Luc reporter fused to the 3′ UTR of the Drosophila gene CG10011, which contains miR-12 binding sites (Fig. 6A). Similar results were obtained with pri-miR-9b or pri-bantam (data not shown). The miRNA/target pairs did not restore P bodies in cells from which AGO1 was depleted (Fig. 6B), so in this case, the miRNA pathway must be functioning to restore P bodies. In summary, P-body formation requires silencing of mRNAs by siRNAs, dsRNAs, or miRNAs.
In contrast to the results described above, siRNAs, dsRNAs, or miRNAs do not restore P bodies in cells from which Me31B, HPat, Ge-1, LSm1, or LSm3 is depleted (Fig. 5C, 6C, and 7C and data not shown) even though depletion of these proteins has no effect on siRNA-mediated gene silencing and LSm1 (or LSm3) depletion does not affect miRNA function (see below). Thus, these proteins could be required for the recruitment and/or expression of some essential P-body components or play a direct role in P-body assembly by mediating RNP-RNP interactions required for aggregation of silenced mRNPs into P bodies.
Releasing mRNAs from polysomes is not sufficient to induce P-body formation.
The observations described above indicate that P-body formation requires nontranslating mRNPs or mRNPs undergoing decapping and that silencing pathways are a major source of these mRNPs. Whether P bodies could be nucleated independently of silencing pathways, for instance, by puromycin treatment of cells in which P bodies have been dispersed (as a way to generate nontranslating mRNPs independently of the miRNA pathway), was therefore of interest. Puromycin treatment induces the formation of large P bodies in cells from which proteins required for siRNA-mediated gene silencing (e.g., AGO2 or Dicer-2) are depleted (Fig. 6D). Puromycin treatment does not, however, restore P bodies in cells from which Drosha, Pasha, Dicer-1, AGO1, GW182, Me31B, HPat, LSm1, or Ge-1 was depleted (Fig. 6D and data not shown), suggesting that stripping ribosomes off mRNAs is not sufficient for P-body formation and that polysome-free mRNPs must undergo remodeling steps, undergo changes in RNP composition, and/or be channeled through the miRNA pathway to enter P bodies.
That the RNAi pathway contributes less than the miRNA pathway to P-body formation is in agreement with the observation that depletion of AGO2 from S2 cells affects only about 6% of the transcriptome, whereas transcripts changing levels upon AGO1 depletion accounts for up to 17% of the transcriptome (42). Thus, in the absence of Dicer-2 or AGO2, P bodies can be assembled, given a functional miRNA pathway and an increase of polysome-free mRNPs in the cytoplasm due to the puromycin treatment. In contrast, when the miRNA pathway is impaired (in cells from which Drosha, Pasha, Dicer-1, or AGO1 is depleted), the puromycin treatment is not sufficient to elicit P-body formation.
miRNAs silence gene expression in the absence of discrete, microscopic P bodies.
Given the contribution of the miRNA pathway to P-body formation, we asked whether P bodies are required for miRNA function. In human and D. melanogaster cells, the depletion of GW182 affects both miRNA function and P-body integrity (6, 13, 26, 31, 34, 39, 41, 59), leaving open the question of whether GW182 acts directly or whether localization of the silencing machinery into P bodies is required for miRNA regulation. We therefore investigated the effect of depleting LSm1 or LSm3 on miRNA function.
To monitor miRNA activity, we made use of the two miRNA sensor reporters described before. In these reporters, the coding region of F-Luc is flanked by the 3′ UTRs of the Drosophila genes Nerfin and CG10011. The 3′ UTR of Nerfin has binding sites for miR-9b, whereas the 3′ UTR of CG10011 mRNA contains binding sites for miR-12 (6, 41, 42). Expression of the F-Luc construct fused to the 3′ UTR of Nerfin is silenced in the presence of miR-9b, and this silencing occurs mainly at the translational level. Silencing of CG10011 by miR-12 is due to accelerated mRNA decay, as both luciferase expression and mRNA levels are reduced to similar extents (6).
Depletion of LSm1 or LSm3 had no effect on silencing by miRNAs (Fig. 7A), even though wild-type P bodies were no longer detected in these cells (Fig. 6C and data not shown). As a positive control, depletion of AGO1 impaired miRNA-mediated silencing, as reported before (Fig. 7A) (6, 41). Depletion of Me31B, HPat, or Ge-1 affected both P-body integrity and miRNA function (data not shown), so this was not informative. Thus, although P-body components play roles in gene silencing, miRNA regulation can occur in the absence of detectable microscopic P bodies. Taken together with the observation that silencing pathways are required for P-body formation, these results indicate that the accumulation of Argonaute proteins and miRNAs and their mRNA targets in P bodies (6, 8, 26, 29, 30, 31, 34, 40, 43) is a consequence, rather than the cause, of silencing.
Macroscopic P bodies are not required for RNAi or NMD.
We showed in previous studies that NMD and silencing by dsRNAs are not affected by depletion of GW182 (41), which normally disrupts P bodies (26, 31, 34, 59). However, P-body integrity was not examined in that study. We therefore reexamined these pathways in cells from which LSm1, LSm3, Me31B, HPat, or Ge-1 was depleted. We observed that alcohol dehydrogenase (adh) mRNA levels were reduced 20-fold by a complementary dsRNA targeting a central region of the open reading frame. In cells from which LSm1, LSm3, Me31B, HPat, or Ge-1 was depleted, degradation of adh mRNA by the complementary dsRNA was not affected, whereas adh mRNA levels were partially restored in cells from which AGO2 was depleted (Fig. 7B). Similar results were obtained when an siRNA was used (data not shown), as reported by Chu and Rana (13), in mammalian cells from which LSm1 was depleted. That these depletions indeed result in P-body dispersion was confirmed by immunofluorescent staining of cells using anti-Tral antibodies (Fig. 7C).
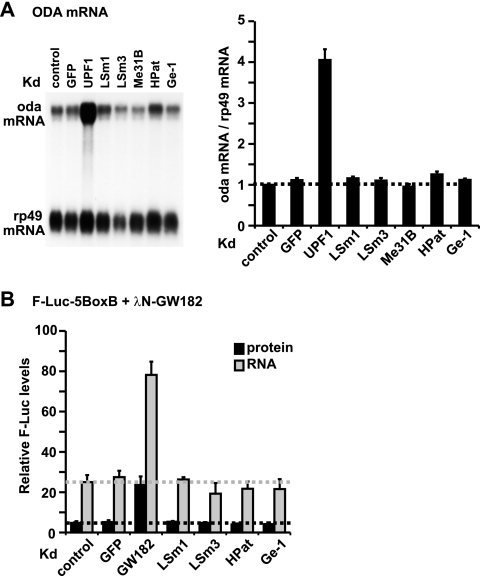
Similarly, depletion of LSm1, LSm3, Me31B, HPat, or Ge-1 did not affect the expression levels of an endogenous target of the NMD pathway, the mRNA encoding ornithine decarboxylase antizyme (ODA mRNA) (Fig. 8A) (23), although these depletions did disrupt P bodies, as judged by the staining of cells with anti-Tral antibodies (as shown in Fig. 3). As a positive control, depletion of UPF1, a key effector of the NMD pathway, led to a fourfold increase of ODA mRNA level relative to that of the rp49 mRNA (encoding ribosomal protein RpL32) (Fig. 8A). We confirmed these results by using NMD reporters based on the alcohol dehydrogenase gene (adh) as described by Rehwinkel et al. (41) (see Fig. S2 in the supplemental material). We conclude that P-body integrity (as judged by detection of these structures by light microscopy) is clearly necessary neither for silencing mediated by dsRNAs or siRNAs nor for NMD.
FIG. 8.
NMD and mRNA decay do not require macroscopic P bodies. (A) The levels of endogenous ODA mRNA were analyzed by Northern blotting of cells from which the indicated proteins were depleted (knockdown [kd]). For each knockdown, ODA mRNA levels were normalized to the levels of rp49 mRNA. These ratios were set to 1 in control cells. Error bars represent standard deviations from three independent experiments. (B) S2 cells from which the indicated proteins were depleted (Kd) were transfected with the F-Luc-5BoxB reporter, a plasmid expressing R-Luc, and vectors expressing the λN peptide or λN-GW182. F-Luc activity and the corresponding mRNA levels were measured and normalized to those of the Renilla control. Normalized F-Luc activities and mRNA levels in cells transfected with the λN peptide alone were set to 100% for each knockdown (not shown). Error bars represent standard deviations from three independent experiments. We confirmed that the depletions shown in panels A and B resulted in P-body dispersion by fluorescent staining of cells with anti-Tral antibodies (see Fig. 3; data not shown).
NMD is mediated by a number of effectors that assemble on mRNAs that terminate translation prematurely and recruit general decay enzymes. These include UPF1, UPF2, UPF3, SMG1, and SMG5-7 (15). P-body integrity is not affected under conditions in which depletion of NMD effectors inhibits NMD, (data not shown), suggesting that P-body formation does not require an active NMD pathway in Drosophila. This is in contrast to the results reported for yeast, in which deletion of upf2 or upf3 genes enhanced P-body formation (45; see Discussion).
GW182-mediated decay occurs independently of detectable P bodies.
GW182 accelerates the decay of bound mRNAs by a deadenylation and decapping mechanism (6). GW182 is essential for P-body integrity (Fig. 4) (17, 26, 31, 34, 59), and proteins involved in GW182-mediated decay (i.e., DCP1, DCP2, CCR4, and CAF1) localize to P bodies (4, 16, 17, 44, 49). If the local concentration of effectors of posttranscriptional processes into P bodies were to play an active role, this specific pathway would be expected to rely on P bodies.
To examine GW182-mediated decay, the protein was tethered to the 3′ UTR of a F-Luc reporter via the interaction between a peptide derived from the N protein of bacteriophage λ (λN peptide) and five RNA binding elements (five BoxB elements) inserted in the 3′ UTR of the reporter (F-Luc-5BoxB). Tethering of GW182 strongly reduces F-Luc expression (Fig. 8B), mainly due to a decrease of F-Luc mRNA levels (Fig. 8B) (6). The ability of GW182 to reduce F-Luc-5BoxB expression is unaffected in cells from which LSm1, LSm3, HPat, or Ge-1 is depleted (Fig. 8B), which lack detectable wild-type P bodies (Fig. 3 and data not shown). These results indicate that even when the effectors of a decay pathway concentrate in P bodies and are required for P-body integrity, this local concentration is not required for function.
DISCUSSION
Several lines of evidence show that P bodies do not serve as storage sites for the effectors of posttranscriptional process but are sites where mRNA degradation and silencing can take place. For instance, P-body formation is RNA dependent, and decay intermediates, siRNAs, and miRNAs and their targets are detected in P bodies (3, 6, 8, 17, 26, 29, 30, 31, 34, 40, 43, 44, 45). Moreover, the size and number of P bodies depends on the fraction of mRNAs undergoing decapping (3, 16, 17, 19, 44). However, the question of whether mRNA decay and silencing require the environment of microscopic, wild-type P bodies to occur or whether these processes can also occur outside of P bodies in soluble protein complexes remains open. In this study, we showed that formation of large P bodies visible in the light microscope as observed in wild-type cells is not required for several processes associated with P-body components, including NMD, mRNA decay, and RNA-mediated gene silencing.
Microscopic versus submicroscopic P bodies.
The question we addressed in this study is whether the environment of macroscopic P bodies is required for posttranscriptional regulation. P bodies are defined as the large cytoplasmic foci visible by light microscopy in wild-type cells. These foci are on average 100 to 300 nm in diameter and are readily detected as bright cytoplasmic dots because the concentration of proteins in these foci is significantly higher than in the surrounding cytoplasm. Nevertheless, most P-body components are also detected diffusely throughout the cytoplasm (4-6, 8, 12-14, 16, 18-20, 25, 29, 43-48, 52, 53, 58-60). For a limited number of examples that have been analyzed, it has been shown that P-body components are not confined to these structures but dynamically exchange with the cytoplasmic pool (27, 29, 55). Quantitative information regarding the fractionation of P-body components between P bodies and the cytoplasm is still lacking, but given the volume of P bodies relative to that of the cytoplasm, it is likely that the diffuse cytoplasmic fraction is significantly larger. This suggests that posttranscriptional processes are likely to occur and may even be initiated in the diffuse cytoplasm or in soluble protein complexes that aggregate to form P bodies (see below). Whether these processes take place in submicroscopic aggregates or soluble protein complexes in the absence of detectable microscopic P bodies remains to be solved. However, we consider that aggregates or large multiprotein assemblies that are not detectable by light microscopy cannot be defined as bodies.
P-body assembly.
Translation factors or ribosomes are generally not present in P bodies (with the exception of cap binding protein eIF4E), indicating that mRNAs leave the translation cycle prior to entering P bodies (12, 27, 48). Consistently, releasing mRNAs from polysomes leads to increases in P-body sizes and numbers, whereas the stabilization of mRNAs into polysomes disrupts P bodies (3, 27, 44). These observations suggest that a critical step in P-body formation is the release of mRNPs from a translationally active state associated with polysomes to a translationally inactive state (14). In this paper, we show that releasing mRNAs from polysomes by puromycin treatment is not sufficient to elicit P-body formation and that functional silencing pathways or proteins generically termed decapping coactivators are required for P-body assembly. These proteins include Me31B (Dhh1 in yeast), HPat (Pat1 in yeast), Ge-1, and the LSm1-7 complex.
What could be the role of these proteins in P-body formation? Me31B is an RNA helicase which could facilitate rearrangements in mRNP composition upon release from polysomes (14). The role of HPat is unclear, but the yeast ortholog interacts with Dhh1, XRN1, and the heptameric LSm1-7 complex (10, 11, 50, 51). Coimmunoprecipitation assays indicate that the interaction between Dhh1 and Pat1 orthologs (i.e., Me31B and HPat) is conserved in Drosophila (A. Eulalio and E. Izaurralde, unpublished observations). Finally, the LSm1-7 complex associates with deadenylated mRNAs and stimulates decapping (10, 11, 50, 51). Clearly, many details regarding the precise molecular function of these proteins remain to be discovered, but their requirement for P-body assembly indicates that mRNAs that are not actively translated do not enter into P bodies by default: the activity of a defined set of proteins is required. Alternatively, nontranslating mRNAs may enter silencing pathways, and this would also lead to changes in mRNP composition due to the recruitment of Argonaute proteins and binding partners, which include P-body components such as GW182, decapping enzymes, and RCK/p54 (6, 13, 26, 30, 31, 34, 43).
Once P-body components are bound to an RNP, P-body formation may then be triggered by protein-protein interactions. Indeed, proteins required for P-body assembly are known to interact to form multimeric protein complexes. Consistently, in addition to the interactions mentioned above, DCP1, DCP2, Ge-1, RCK/p54, and EDC3 form a multimeric protein complex in human cells (20). The absolute requirement of RNA for P-body formation could be explained if affinities between these proteins increased upon RNA binding. Additionally, proteins like GW182 and Ge-1 are multidomain proteins that could bind more than one RNP simultaneously, bringing into close proximity several components and thus nucleating the formation of P bodies.
RNAs targeted by silencing pathways nucleate P bodies.
In this study, we showed that both the RNAi and miRNA pathways contribute to the generation of a pool of nontranslating mRNPs and/or of mRNPs committed to decay which are required for P-body formation. Nevertheless, silencing can occur in the absence of microscopic P bodies. Our results provide support to previous models proposing that silencing is initiated in the cytoplasm and that the localization of the silencing machinery into P bodies is a consequence, rather than the cause, of silencing (40).
An unexpected observation from our studies is that AGO2 and Dicer-2, which function in siRNA-mediated gene silencing in Drosophila (36, 41), are required for P-body integrity. The role of these proteins in P-body assembly is unlikely to be structural, because P bodies are restored upon puromycin treatment in cells from which AGO2 or Dicer-2 is depleted. The most likely explanation for the requirement of these proteins is, therefore, that silencing by siRNAs also generates RNPs that elicit P-body formation. The requirement for AGO2 could be at least partially explained by the observation that the expression levels of a small subset of endogenous miRNA targets are affected in AGO2-depleted cells, suggesting that some miRNAs may be loaded into AGO2-containing RNA-induced silencing complexes (42). Furthermore, the AGO1 and AGO2 genes interact (35), although it is unclear how this interaction affects the activities of these proteins.
The requirement for Dicer-2 in P-body assembly, however, suggests that endogenous siRNA targets also contribute to P-body formation. Because the levels of dsRNA synthesis from endogenous loci that could provide precursors for the production of endogenous siRNAs are currently unknown, the fraction and origin of transcripts regulated by endogenous siRNAs cannot be estimated. Nonetheless, a possible source of endogenous dsRNAs is the bidirectional transcription of pseudogenes and transposable elements, in agreement with the role of the RNAi pathway as a defense mechanism against RNA viruses and mobile genetic elements (33).
The essential role of silencing pathways in P-body formation in Drosophila, and presumably in human cells (39), raises the question of how P bodies are assembled in S. cerevisiae, which lacks silencing pathways. One possibility is that other posttranscriptional processes generate nontranslating mRNPs required to nucleate P bodies. For instance, the NMD pathway contributes to P-body assembly in yeast cells, because depletion of Upf2 or Upf3 leads to increases in P-body size and number in a Upf1-dependent manner (45), whereas similar experiments with Drosophila cells do not affect P bodies (data not shown).
P-body function.
With the exception of the proteins involved in silencing, the composition of P bodies and the effects of drugs such as cycloheximide and puromycin on P-body size and number are strikingly similar in yeast, Drosophila, and human cells, raising the question of what the role of these structures accounting for their conservation in eukaryotic cells could be. Our results show that the environment of microscopic P bodies is not essential for mRNA decay or silencing but do not exclude that the formation of P bodies confers a kinetic advantage. Moreover, our results do not rule out a role for large P bodies in sequestering a specific set of nontranslating mRNPs and reinforcing their repression by shielding them from the translation machinery. In agreement with this possibility, Bhattacharyya et al. (8) have shown that the mRNA encoding the cationic amino acid transporter CAT-1 is repressed by miR-122 in human hepatocarcinoma cells. Repression correlates with the accumulation of this mRNA in P bodies. Under conditions of cellular stress, the RNA binding protein HuR binds to AU-rich elements present in the 3′ UTR of CAT-1 mRNA and reverses silencing. Concomitantly, CAT-1 mRNA exits P bodies and associates with polysomes.
Aggregation into P bodies could also irreversibly commit specific mRNAs to degradation. In addition, one could envisage that the aggregation of decay intermediates into P bodies prevents these fragments from accumulating in the cytoplasm and entering futile cycles of translation and/or titrating limiting (and hence regulatory) RNA binding proteins. This would be particularly important for structured RNA fragments resistant to the action of nucleases.
Finally, the conservation of P bodies may reflect a role for these structures in other cellular processes that we do not yet fully appreciate. A role in some steps of retroviral or retrotransposon life cycles is suggested by the localizations of the antiretroviral proteins APOBEC3G and APOBEC3F in human cell P bodies and of the protein and RNA components of the retrovirus-like element Ty3 in yeast P bodies (7, 54). A link between P bodies and the regulation of retrotransposition would be consistent with the role of RNAi pathways in silencing the expression of transposable elements (33). Because all known essential P-body components play roles in decapping and/or silencing and proteins playing an exclusively structural role in P-body assembly have not yet been identified, it is currently not possible to evaluate the role of P bodies for cell, tissue, or organism survival.
Supplementary Material
Acknowledgments
We are grateful to D. J. Thomas for comments on the manuscript.
This study was supported by the Max Planck Society and the Human Frontier Science Program Organization. A.E. and I.B.-A. are recipients of fellowships from the Portuguese Foundation for Science and Technology and the European Molecular Biology Organization, respectively.
Footnotes
Published ahead of print on 2 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Albrecht, M., and T. Lengauer. 2004. Novel Sm-like proteins with long C-terminal tails and associated methyltransferases. FEBS Lett. 569:18-26. [DOI] [PubMed] [Google Scholar]
- 2.Anantharaman, V., and L. Aravind. 2004. Novel conserved domains in proteins with predicted roles in eukaryotic cell-cycle regulation, decapping and RNA stability. BMC Genomics 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, P., and N. Kedersha. 2006. RNA granules. J. Cell Biol. 172:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrei, M. A., D. Ingelfinger, R. Heintzmann, T. Achsel, R. Rivera-Pomar, and R. Luhrmann. 2005. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11:717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbee, S. A., P. S. Estes, A. M. Cziko, J. Hillebrand, R. A. Luedeman, J. M. Coller, N. Johnson, I. C. Howlett, C. Geng, R. Ueda, A. H. Brand, S. F. Newbury, J. E. Wilhelm, R. B. Levine, A. Nakamura, R. Parker, and M. Ramaswami. 2006. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52:997-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behm-Ansmant, I., J. Rehwinkel, T. Doerks, A. Stark, P. Bork, and E. Izaurralde. 2006. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beliakova-Bethell, N., C. Beckham, T. H. Giddings, Jr., M. Winey, R. Parker, and S. Sandmeyer. 2006. Virus-like particles of the Ty3 retrotransposon assemble in association with P-body components. RNA 12:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya, S. N., R. Habermacher, U. Martine, E. I. Closs, and W. Filipowicz. 2006. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125:1111-1124. [DOI] [PubMed] [Google Scholar]
- 9.Blobel, G., and D. Sabatini. 1971. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc. Natl. Acad. Sci. USA 68:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnerot, C., R. Boeck, and B. Lapeyre. 2000. The two proteins Pat1p (Mrt1p) and Spb8p interact in vivo, are required for mRNA decay, and are functionally linked to Pab1p. Mol. Cell. Biol. 20:5939-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouveret, E., G. Rigaut, A. Shevchenko, M. Wilm, and B. Seraphin. 2000. A Sm-like protein complex that participates in mRNA degradation. EMBO J. 19:1661-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brengues, M., D. Teixeira, and R. Parker. 2005. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu, C. Y., and T. M. Rana. 2006. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4:1122-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coller, J., and R. Parker. 2005. General translational repression by activators of mRNA decapping. Cell 122:875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti, E., and E. Izaurralde. 2005. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 17:316-325. [DOI] [PubMed] [Google Scholar]
- 16.Cougot, N., S. Babajko, and B. Seraphin. 2004. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 165:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eulalio, A., I. Behm-Ansmant, and E. Izaurralde. 2007. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 18.Eystathioy, T., E. K. Chan, S. A. Tenenbaum, J. D. Keene, K. Griffith, and M. J. Fritzler. 2002. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13:1338-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eystathioy, T., A. Jakymiw, E. K. Chan, B. Seraphin, N. Cougot, and M. J. Fritzler. 2003. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA 9:1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenger-Gron, M., C. Fillman, B. Norrild, and J. Lykke-Andersen. 2005. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20:905-915. [DOI] [PubMed] [Google Scholar]
- 21.Ferraiuolo, M. A., S. Basak, J. Dostie, E. L. Murray, D. R. Schoenberg, and N. Sonenberg. 2005. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 170:913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuhara, N., J. Ebert, L. Unterholzner, D. Lindner, E. Izaurralde, and E. Conti. 2005. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol. Cell 17:537-547. [DOI] [PubMed] [Google Scholar]
- 23.Gatfield, D., and E. Izaurralde. 2004. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature 429:575-578. [DOI] [PubMed] [Google Scholar]
- 24.Houseley, J., J. LaCava, and D. Tollervey. 2006. RNA-quality control by the exosome. Nat. Rev. Mol. Cell Biol. 7:529-539. [DOI] [PubMed] [Google Scholar]
- 25.Ingelfinger, D., D. J. Arndt-Jovin, R. Luhrmann, and T. Achsel. 2002. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8:1489-1501. [PMC free article] [PubMed] [Google Scholar]
- 26.Jakymiw, A., S. Lian, T. Eystathioy, S. Li, M. Satoh, J. C. Hamel, M. J. Fritzler, and E. K. Chan. 2005. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 7:1267-1274. [DOI] [PubMed] [Google Scholar]
- 27.Kedersha, N., G. Stoecklin, M. Ayodele, P. Yacono, J. Lykke-Andersen, M. J. Fritzler, D. Scheuner, R. J. Kaufman, D. E. Golan, and P. Anderson. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kshirsagar, M., and R. Parker. 2004. Identification of Edc3p as an enhancer of mRNA decapping in Saccharomyces cerevisiae. Genetics 166:729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung, A. K., J. M. Calabrese, and P. A. Sharp. 2006. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. USA 103:18125-18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., M. A. Valencia-Sanchez, G. J. Hannon, and R. Parker. 2005. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7:719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., F. V. Rivas, J. Wohlschlegel, J. R. Yates III, R. Parker, and G. J. Hannon. 2005. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7:1261-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lykke-Andersen, J., and E. Wagner. 2005. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 19:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meister, G., and T. Tuschl. 2004. Mechanisms of gene silencing by double-stranded RNA. Nature 431:343-349. [DOI] [PubMed] [Google Scholar]
- 34.Meister, G., M. Landthaler, L. Peters, P. Y. Chen, H. Urlaub, R. Luhrmann, and T. Tuschl. 2005. Identification of novel argonaute-associated proteins. Curr. Biol. 15:2149-2155. [DOI] [PubMed] [Google Scholar]
- 35.Meyer, W. J., S. Schreiber, Y. Guo, T. Volkmann, M. A. Welte, and H. A. Muller. 2006. Overlapping functions of argonaute proteins in patterning and morphogenesis of Drosophila embryos. PLoS Genet. 2:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamura, K., A. Ishizuka, H. Siomi, and M. C. Siomi. 2004. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 18:1655-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orban, T. I., and E. Izaurralde. 2005. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA 11:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker, R., and H. Song. 2004. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 11:121-127. [DOI] [PubMed] [Google Scholar]
- 39.Pauley, K. M., T. Eystathioy, A. Jakymiw, J. C. Hamel, M. J. Fritzler, and E. K. Chan. 2006. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 7:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillai, R. S., S. N. Bhattacharyya, C. G. Artus, T. Zoller, N. Cougot, E. Basyuk, E. Bertrand, and W. Filipowicz. 2005. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 309:1573-1576. [DOI] [PubMed] [Google Scholar]
- 41.Rehwinkel, J., I. Behm-Ansmant, D. Gatfield, and E. Izaurralde. 2005. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11:1640-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehwinkel, J., P. Natalin, A. Stark, J. Brennecke, S. M. Cohen, and E. Izaurralde. 2006. Genome-wide analysis of mRNAs regulated by Drosha and Argonaute proteins in Drosophila melanogaster. Mol. Cell. Biol. 26:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen, G. L., and H. M. Blau. 2005. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7:633-636. [DOI] [PubMed] [Google Scholar]
- 44.Sheth, U., and R. Parker. 2003. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300:805-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth, U., and R. Parker. 2006. Targeting of aberrant mRNAs to cytoplasmic processing bodies. Cell 125:1095-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoecklin, G., T. Mayo, and P. Anderson. 2006. ARE-mRNA degradation requires the 5′-3′ decay pathway. EMBO Rep. 7:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka, K. J., K. Ogawa, M. Takagi, N. Imamoto, K. Matsumoto, and M. Tsujimoto. 2006. RAP55, a cytoplasmic mRNP component, represses translation in Xenopus oocytes. J. Biol. Chem. 281:40096-40106. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira, D., U. Sheth, M. A. Valencia-Sanchez, M. Brengues, and R. Parker. 2005. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11:371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Temme, C., S. Zaessinger, S. Meyer, M. Simonelig, and E. Wahle. 2004. A complex containing the CCR4 and CAF1 proteins is involved in mRNA deadenylation in Drosophila. EMBO J. 23:2862-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tharun, S., W. He, A. E. Mayes, P. Lennertz, J. D. Beggs, and R. Parker. 2000. Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404:515-518. [DOI] [PubMed] [Google Scholar]
- 51.Tharun, S., and R. Parker. 2001. Targeting an mRNA for decapping: displacement of translation factors and association of the Lsm1p-7p complex on deadenylated yeast mRNAs. Mol. Cell 8:1075-1083. [DOI] [PubMed] [Google Scholar]
- 52.Unterholzner, L., and E. Izaurralde. 2004. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell 16:587-596. [DOI] [PubMed] [Google Scholar]
- 53.van Dijk, E., N. Cougot, S. Meyer, S. Babajko, E. Wahle, and B. Seraphin. 2002. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 21:6915-6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wichroski, M. J., G. B. Robb, and T. M. Rana. 2006. Human retroviral host restriction factors APOBEC3G and APOBEC3F localize to mRNA processing bodies. PLoS Pathog. 2:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilczynska, A., C. Aigueperse, M. Kress, F. Dautry, and D. Weil. 2005. The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118:981-992. [DOI] [PubMed] [Google Scholar]
- 56.Wilhelm, J. E., M. Buszczak, and S. Sayles. 2005. Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev. Cell 9:675-685. [DOI] [PubMed] [Google Scholar]
- 57.Wilusz, C. J., and J. Wilusz. 2004. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 20:491-497. [DOI] [PubMed] [Google Scholar]
- 58.Yang, W. H., J. H. Yu, T. Gulick, K. D. Bloch, and D. B. Bloch. 2006. RNA-associated protein 55 (RAP55) localizes to mRNA processing bodies and stress granules. RNA 12:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, Z., A. Jakymiw, M. R. Wood, T. Eystathioy, R. L. Rubin, M. J. Fritzler, and E. K. Chan. 2004. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 117:5567-5578. [DOI] [PubMed] [Google Scholar]
- 60.Yu, J. H., W. H. Yang, T. Gulick, K. D. Bloch, and D. B. Bloch. 2005. Ge-1 is a central component of the mammalian cytoplasmic mRNA processing body. RNA 11:1795-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaessinger, S., I. Busseau, and M. Simonelig. 2006. Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 133:4573-4583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.