Abstract
Apoptosis is critical for embryonic development, tissue homeostasis, and tumorigenesis and is determined largely by the Bcl-2 family of antiapoptotic and prosurvival regulators. Here, we report that glycogen synthase kinase 3 (GSK-3) was required for Mcl-1 degradation, and we identified a novel mechanism for proteasome-mediated Mcl-1 turnover in which GSK-3β associates with and phosphorylates Mcl-1 at one consensus motif (155STDG159SLPS163T; phosphorylation sites are in italics), which will lead to the association of Mcl-1 with the E3 ligase β-TrCP, and β-TrCP then facilitates the ubiquitination and degradation of phosphorylated Mcl-1. A variant of Mcl-1 (Mcl-1-3A), which abolishes the phosphorylations by GSK-3β and then cannot be ubiquitinated by β-TrCP, is much more stable than wild-type Mcl-1 and able to block the proapoptotic function of GSK-3β and enhance chemoresistance. Our results indicate that the turnover of Mcl-1 by β-TrCP is an essential mechanism for GSK-3β-induced apoptosis and contributes to GSK-3β-mediated tumor suppression and chemosensitization.
Glycogen synthase kinase 3β (GSK-3β), a key component of the Wnt signaling pathway, plays important roles in embryonic development and tumorigenesis (14, 16). It phosphorylates β-catenin and then creates a recognition motif for β-catenin binding with E3-ubiquitin ligase complex SCFβ-TrCP, which increases β-catenin degradation (1, 46). A number of growth factors, including insulin growth factor 1, epidermal growth factor, platelet-derived growth factor, fibroblast growth factor 2, hepatocyte growth factor, transforming growth factor β, tumor necrosis alpha (TNF-α) (4, 12, 20, 38), and oncogenic proteins, such as hepatitis B virus X protein (13) and the latent membrane protein 2A of Epstein-Barr virus (33), can inactivate GSK-3β through the phosphorylation of GSK-3β at the Ser9 residue (by phosphatidylinositol 3-kinase [PI3-K]/Akt, mitogen-activated protein kinase [MAPK]/p90RSK, or mTOR/S6K pathways), resulting in the stabilization of β-catenin.
In addition to being implicated in tumorigenesis, GSK-3β has been implicated in multiple physiologic processes including protein synthesis, cell proliferation, cell differentiation, microtubule dynamics, and cell motility. It exerts its actions by directly phosphorylating a broad range of substrates, including the translation factor eukaryotic initiation factor 2B, cyclin D1, c-Jun, c-Myc, NFAT, CREB, Tau, and Snail (14, 16, 50). Recent studies suggest that GSK-3β is an upstream regulator of apoptosis and possesses proapoptotic characteristics. The overexpression of GSK-3β induces apoptosis in several cell types, and the catalytically inactive mutant of GSK-3β or treatment with specific GSK-3β inhibitors such as Li+, SB216763, and SB415286 protects these cells against several apoptotic stimuli (2, 8, 19, 37). Moreover, the inactivation of GSK-3β through the phosphorylation of the Ser9 residue can reduce apoptosis (27). It has been proposed that this type of inactivation of GSK-3β by the PI3-K/Akt pathway may mediate the antiapoptotic effects of Akt (37, 43). However, the molecular mechanism by which GSK-3β induces apoptosis has not yet been fully elucidated.
Apoptosis is critical for normal embryonic development, tissue homeostasis, and cell functions, and the deregulation of apoptosis contributes to diverse human diseases such as neurodegenerative disorders and cancers (22, 48). Two alternative pathways can activate initiator caspases to induce apoptosis. The extrinsic pathway is mediated by death receptors on the cell surface (3, 40, 44), including Fas/APO-1/CD5, TNF receptor 1, and TNF-related apoptosis-inducing ligand receptors, which activate caspase 8 and 10. The intrinsic pathway is determined largely by the Bcl-2 family of antiapoptotic and prosurvival regulators, which control cytochrome c release from mitochondria and then caspase 9 activation (10, 17, 26). Mcl-1 (myeloid cell leukemia 1), a Bcl-2-like antiapoptotic protein, was originally characterized in differentiating myeloid cells and contains three BH domains (BH1 to BH3) (25) and a transmembrane domain at its C terminus by which Mcl-1 localizes to various intracellular membranes, especially the outer mitochondrial membrane (47). Mcl-1 is a rapidly turned over protein, which is quickly degraded upon a variety of apoptosis-inducing signals and, in contrast, quickly induced by multiple survival cytokines including epidermal growth factor, VEGF, granulocyte-macrophage colony-stimulating factor, and interleukin-3 through the PI3-K/Akt, MEK/MAPK, and JAK/STAT signaling cascades (7, 32). The rapid induction and degradation of Mcl-1 suggest that it can sense acute environmental changes and maintain the balance between cell survival and cell death (36, 39). The overexpression of Mcl-1, Bcl-XL, and Bcl-2 is often found in various human tumors, and due to rare mutations in these genes, the upstream regulators of these molecules may contribute to tumorigenesis (5, 6). However, the immediate upstream regulator of Mcl-1 is poorly understood.
In an attempt to understand the molecular mechanism of GSK-3β-induced apoptosis, we found that GSK-3β physically associated with and phosphorylated Mcl-1 and that the phosphorylated Mcl-1 was then ubiquitinated and degraded by the E3 ligase β-TrCP, which contributes to GSK-3β-induced apoptosis. An Mcl-1 mutant (Mcl-1-3A), which cannot be degraded by β-TrCP, inhibited GSK-3β-possessed tumor suppression and chemosensitization.
MATERIALS AND METHODS
Constructs and reagents.
pCGN-GSK-3β (wild type [WT]), pGEX-GSK-3β, and GSK-3β-CA (S9A GSK-3β), and pMT2-GSK-3α were kindly provided by A. Kikuchi, M. J. Birnhaum, and J. R. Woodgett. β-TrCP1, β-TrCP2, and the F-box-domain-deleted mutant β-TrCP1/ΔF box were kindly provided by K. Tanaka. pHA-hMcl-1 was kindly provided by H.-Y. Yan-Yen. The full-length hMcl-1 cDNA was then subcloned into the pCMV5-HA, pCMV5-MYC, and pGEX-6P-1 vectors. Using the QuikChange multisite-directed mutagenesis kit (Stratagene, La Jolla, CA), all Mcl-1 mutants and GSK-3β-KD (pCGN-GSK-3β KD), which was based on the above-described constructs, were generated according to the manufacturer's protocol, and all mutations were verified by automated sequencing. The GSK-3β inhibitor TDZD8 and cell-permeable GSK-3β peptide inhibitor L803-mts were purchased from Calbiochem (San Diego, CA). The proteasome inhibitor MG132, cycloheximide, and staurosporine were purchased from Sigma (St. Louis, MO).
Cell culture, UV treatment, and proliferation and apoptosis assays.
Cells were grown in Dulbecco's modified Eagle's medium-F12 medium supplemented with 10% fetal bovine serum. Transient or stable transfections of cells with DNA were performed with an optimal ratio of DNA and liposome. GSK-3β knockout mouse embryonic fibroblast (MEF) cells were kindly provided by J. R. Woodgett. The Mcl-1-expressing MCF-7 stable cell line was isolated with blasticidin selection. HeLa and inducible Mule short hairpin RNA (shRNA) U2OS cells were plated at a density of 3 × 106 cells in a 6-cm dish with Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum for 1 day and then treated with 1.9 J/m2/s of UV irradiation for 2 min or with 0.1 μM staurosporine at the indicated times. The percentage of survival of cells upon chemotherapy drug treatment was assessed by MTT (3-4.5-dimethylthiazol-2,5-diphenyltetrazolium bromide thiazol blue) assay. The apoptotic cells were assessed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) assay or flow cytometry assay (fluorescence-activated cell sorter [FACS]) as previously described (11); briefly, trypsinized cells were washed with phosphate-buffered saline (PBS) and then fixed with 70% ethanol overnight at 4°C. Before FACS analysis, cells were washed with PBS, and fluorochrome solution (50 μg/ml propidium iodide plus 25 μg/ml RNase in PBS) was then added. Each experiment was performed in triplicate, and error bars represent means ± standard errors.
Immunoblotting and immunoprecipitation.
Immunoblotting and immunoprecipitation were done essentially as previously described (13), with the following antibodies: mouse and rabbit anti-Mcl-1, mouse anti-GSK-3β (BD Transduction Labs, San Diego, CA), rabbit anti-GSK-3β (Stressgen Biotechnologies, Victoria, Canada), phospho-(Ser9)-GSK-3β (Calbiochem, San Diego, CA, and Cell Signaling Technology, Beverly, MA), phospho-β-catenin (Ser33/37/Thr41) (Cell Signaling Technology), GSK-3α/β and GSK-3α (Santa Cruz Biotechnology, Santa Cruz, CA), procyclic acidic repetitive protein (PARP) and caspase 9 (Biolegend, San Diego, CA), β-TrCP (Zymed, San Francisco, CA), and hemagglutinin (HA) tag, Myc tag, actin, and tubulin (Sigma, St. Louis, MO). Three specific antibodies against the three phosphorylation sites of Mcl-1 (155Ser, 159Thr, and 163Thr) were generated by Bethyl Laboratories, Inc. (Montgomery, TX).
In vitro kinase assay and phosphorylation analysis.
Purified glutathione S-transferase (GST)-Mcl-1 proteins as indicated were incubated with purified GST-GSK-3β protein or active Erk2 (Upstate Biotechnology, Charlottesville, VA) in the presence of 50 mM ATP in a kinase buffer with or without 5 μCi [γ-32P]ATP for 30 min at 30°C. Reaction products were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then blotted with phospho-Mcl-1 antibody, or 32P-labeled proteins were visualized by autoradiography.
Peptide-binding assay and dot blotting.
The Mcl-1 peptides (ESGNNTSTDGSLPSTPPPAE; phosphorylation sites are in italics) were synthesized (SynPep Corp., Dublin, CA) and coupled to agarose beads using an AminoLink kit (Pierce Biotechnology, Rockford, IL). Mcl-1 peptide and purified GST-Mcl-1 protein (control) were incubated with GST -WT GSK-3β or GST-kinase-dead GSK-3β and then spotted onto a nitrocellulose membrane. The nitrocellulose membrane was reacted with the three phospho-Mcl-1 antibodies to confirm phosphorylation by GSK-3β. The coupled Mcl-1 peptide with or without phosphorylation by GSK-3β was then incubated with in vitro transcription and translation lysates of [35S]methionine-labeled β-TrCP1 or β-TrCP2 produced by the TNT Quick Coupled Transcription/Translation systems (Promega, Madison, WI) in the presence of [35S]methionine (MP Biomedicals, Solon, OH). The agarose beads were extensively washed and analyzed by SDS-PAGE followed by autoradiography.
Ubiquitination assay.
For the in vitro ubiquitination assay, purified Mcl-1 protein was phosphorylated by GSK-3β and then incubated with in vitro-translated β-TrCP1 or deleted-F-box β-TrCP1 in the presence of ubiquitin ligation buffer containing 100 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 2 mM NaF, 10 nM okadaic acid, 2 mM ATP, 0.6 mM dithiothreitol, 60 ng of E1, 300 ng of E2, and 12 mg of His-ubiquitin (Sigma). Reaction mixtures were incubated at 37°C for 60 min and terminated by boiling for 5 min with SDS sample buffer containing 0.1 M dithiothreitol and were then resolved by SDS-PAGE and blotted with an anti-His-ubiquitin antibody. For in vivo ubiquitination assays, 293T cells were transfected with HA-ubiquitin, Mcl-1-WT, or Mcl-1-3A and β-TrCP1 or F-box-deleted β-TrCP1 and then treated with MG132 (10 μM) for 10 h. Mcl-1 was immunoprecipitated and then blotted with anti-HA-ubiquitin.
siRNA transfection.
Cells were transfected with β-TrCP-small interfering RNA (siRNA) (5′-GAGCUCUUGGUGGAUCAUCTT-3′), control siRNA duplex (Dharmacon, Lafayette, CO), and GSK-3α and GSK-3β siRNA expression plasmids pKD-GSK-3α-v3 and pKD-GSK-3β-v1 (Upstate Biotechnology) by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or electroporation using Nucleofector 1 (Amaxa Biosystems, Gaithersburg, MD), and lysates were prepared 48 h after transfection.
Animal studies.
We performed tumorigenicity assays in an orthotopic mammary tumor mouse model as previously described (28). Briefly, 6-week-old female nude mice received subcutaneous interscapular implants of one 17β-estradiol pellet (0.72 mg/pellet). One day later, MCF-7 cells (2 × 106 cells) were injected into the mammary fat pads. Two weeks later, when most tumors exceeded 4 by 4 mm, the tumor-bearing mice were randomly divided into four groups of five mice each. The mice in all treatment groups received an intratumor injection of the liposome complex with GSK-3β twice a week for 2 weeks. Then tumor volumes were measured twice a week, and the data (means ± standard errors) were tested using the t test.
RESULTS
GSK-3 downregulates Mcl-1.
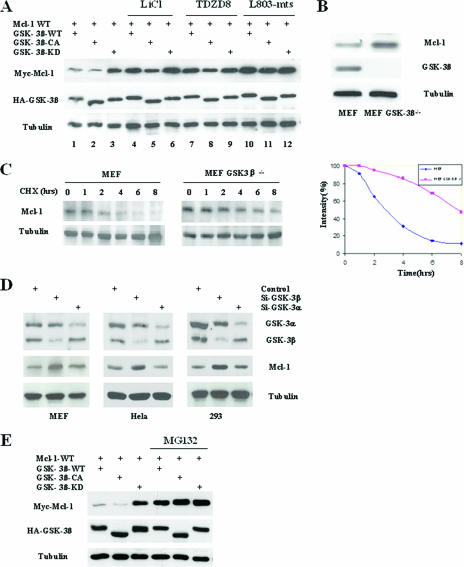
To investigate the mechanism of GSK-3β-induced apoptosis, we searched for correlations between GSK-3β activity and expression of the apoptosis-associated molecule Mcl-l. The overexpression of GSK-3β and Mcl-1 in 293T cells demonstrated that Mcl-1 expression levels were much lower in the presence of constitutively active GSK-3β (GSK-3β-CA) and GSK-3β-WT than in the presence of kinase-dead GSK-3β (GSK-3β-KD) (Fig. 1A, lanes 1 and 2 versus lane 3). Furthermore, when the kinase activity of GSK-3β-WT or GSK-3β-CA was blocked with the GSK-3β inhibitors lithium and TDZD8 and the specific peptide inhibitor L803-mts, expression levels of Mcl-1 were upregulated (Fig. 1A, lanes 4, 5, 7, 8, 10, and 11 versus lanes 1 and 2), indicating that GSK-3β may downregulate Mcl-1. In addition, in the GSK-3β knockout MEF cells, the Mcl-1 level was significantly higher than that in WT MEF cells, which possessed normal GSK-3β kinase activity (Fig. 1B; see Fig. S1 in the supplemental material), and the half-life of endogenous Mcl-1 in GSK-3β-null MEF cells increased from around 3 h to almost 6 to 7 h compared with MEF cell (Fig. 1C). Furthermore, the knockdown of GSK-3β by siRNA upregulated the Mcl-1 level in several cell lines, but the knockdown of GSK-3α did not achieve a similar result (Fig. 1D), and the overexpression of GSK-3α did not downregulate Mcl-1 significantly compared with GSK-3β in 293T cells (see Fig. S2A in the supplemental material), indicating that GSK-3β is the major form of GSK-3 to downregulate Mcl-1. In addition, the proteasome inhibitor MG132 blocked Mcl-1 downregulation by GSK-3β-WT and GSK-3β-CA (Fig. 1F, lanes 4 and 5 versus lanes 1 and 2), suggesting that proteasome-mediated degradation is involved in GSK-3β-induced Mcl-1 downregulation.
FIG. 1.
GSK-3β downregulates Mcl-1. (A) 293T cells transfected with Mcl-1-WT, GSK-3β-WT, GSK-3β-CA, or GSK-3β-KD were treated with the GSK-3β inhibitors LiCl (20 mM), TDZD8 (4 μM), and L803-mts (40 mM), as indicated, for 10 h. Expression of Myc-Mcl-1 and HA-GSK-3β was then analyzed by Western blotting. (B) Lysates of MEF cells and GSK-3β knockout MEF cells were subjected to Western blotting to detect endogenous Mcl-1 and GSK-3β. (C) MEF cells and GSK-3β knockout MEF cells (MEF GSK-3β−/−) were treated with cycloheximide (20 μM) for the indicated times, and endogenous Mcl-1 was then detected by specific mouse anti-Mcl-1 antibody. Equal amounts of protein were subjected to Western blot analyses, as determined by comparing the amount of tubulin (left). Densitometry results for endogenous Mcl-1 after treatment with cycloheximide were normalized by the intensity of tubulin and then plotted (right), and the half-lives of Mcl-1 were determined. (D) Different kinds of human cancer cell lines were transfected with siRNA against GSK-3β and GSK-3α, and cell lysates were then subjected to Western blotting to detect endogenous Mcl-1. (E) 293T cells transfected with Mcl-1-WT, GSK-3β-WT, GSK-3β-CA, or GSK-3β-KD were treated with or without the proteasome inhibitor MG132 (10 μM) for 10 h and analyzed by Western blotting against Myc-Mcl-1 and HA-GSK-3β.
GSK-3 phosphorylates Mcl-1.
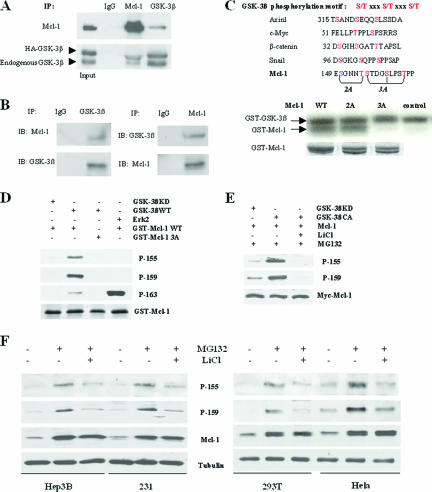
To investigate how GSK-3β degrades Mcl-1, we investigated whether GSK-3β interacts with Mcl-1. To this end, GSK-3β (HA tag) or GSK-3α was transfected into 293T cells, and GSK-3β and Mcl-1 or GSK-3α and Mcl-1 were then immunoprecipitated from cotransfected 293T cell lysates. An association between GSK-3β and Mcl-1 was clearly detected by Western blotting (Fig. 2A); however, the association between GSK-3α and Mcl-1 was not detectable under the same condition (see Fig. S2B in the supplemental material). Furthermore, the in vivo association of endogenous GSK-3β and Mcl-1 was also detected in the breast cancer cell line MDA-MB-453 (Fig. 2B), whereas we could not detect any association between GSK-3β and two other Bcl-2 family proteins, Bcl-2 and Bcl-XL (data not shown). Given the physical association between GSK-3β and Mcl-1, we investigated whether GSK-3 could directly phosphorylate Mcl-1. The kinase assay showed that both GSK-3β and GSK-3α could phosphorylate Mcl-1 in vitro (Fig. 2C; see Fig. S3A in the supplemental material). Interestingly, we noticed that human Mcl-1 contains two putative GSK-3β phosphorylation motifs (150SGNN154T and 155STDG159SLPS163T). To determine the GSK-3β phosphorylation site of Mcl-1, the two potential Mcl-1 phosphorylation motifs were mutated to produce Mcl-1-2A and Mcl-1-3A. Mcl-1-3A completely abolished Mcl-1 phosphorylation by GSK-3β, but Mcl-1-2A did not affect Mcl-1 phosphorylation by GSK-3β (Fig. 2C), suggesting that the motif 155STDG159SLPS163T is the substrate site for GSK-3β kinase. The results of the direct phosphorylation of Mcl-1 by GSK-3β suggest that unlike other substrate of GSK-3β, such as β-catenin, the priming phosphorylation is not required for Mcl-1 phosphorylation by GSK-3β. However, there is a stretch of glutamic acid residues followed by the last threonine of the phosphorylation motif, which may mimic phosphoserine/thereonine and function as priming phosphorylation. This issue requires further investigation in the future. We then generated three specific rabbit antibodies against the three potentially phosphorylated sites of Mcl-1 (p-155S, p-159S, and p-163T) by GSK-3β. Results of an in vitro kinase assay using the three phosphoantibodies showed that GSK-3β phosphorylated Mcl-1 at all three sites (Fig. 2D), whereas Erk phosphorylated Mcl-1 strongly only at the 163T residue, consistent with data from a previous report (15) and suggesting that phosphosphorylation at the 163T residue may be mediated mostly by Erk. Since 155S and 159T are more specific as GSK-3β substrate sites, and there is no cross-reactivity of the two antibodies (data not shown), we used the corresponding specific phosphoantibodies (p-155S and p-159T) to test the phosphorylation of Mcl-1 in transfected 293T cells. The two sites of Mcl-1 were truly phosphorylated by GSK-3β (Fig. 2E) and almost totally blocked by treatment with LiCl. Similar results were found in several other several cell lines (Fig. 2F) when we analyzed the two specific phosphorylation sites of Mcl-1 in vivo after treatment with MG132 or/and LiCl. Taken together, these results indicated that GSK-3β physically associates with Mcl-1 and phosphorylates it at the motif (155 to 163 residues).
FIG. 2.
GSK-3β phosphorylates Mcl-1. (A) GSK-3β (HA tagged) was transfected into 293T cells, and Mcl-1 or GSK-3β was then immunoprecipitated (IP) from transfected 293T cell lysates (1,000 μg/lane) and subjected to Western blotting. IgG, immunoglobulin G. (B) GSK-3β associates with Mcl-1 in vivo. Endogenous GSK-3β and Mcl-1 were immunoprecipitated from the breast cancer cell line MDA-MB-453 and subjected to Western blotting. (C) Comparison of the amino acid sequences of the GSK-3β phosphorylation motif in Mcl-1 and other known GSK-3β substrates. Mutant GST-Mcl-1 protein was incubated with WT GST-GSK-3β, and the kinase assay was performed as described in Materials and Methods. (D) WT or 3A mutant GST-Mcl-1 protein was incubated with WT or kinase-dead GST-GSK-3β protein or active Erk2 as indicated, the kinase assay was performed as described in Materials and Methods, and reaction mixture samples were then subjected to SDS-PAGE and blotted with specific phospho-Mcl-1 antibodies. (E) 293T cells were cotransfected with Mcl-1 and GSK-3β-KD or GSK-3β-CA and, 36 h later, were treated with MG132 alone or with LiCl for 10 h. Cell lysates were then blotted with specific phospho-Mcl-1 antibodies. (F) Cells were treated with MG132 alone (10 μM) or with LiCl (20 mM) for 10 h as indicated (cells are still viable under this condition), and cell lysates were then blotted with specific phospho-Mcl-1 antibodies.
Phosphorylation of Mcl-1 by GSK-3β is required for Mcl-1 degradation.
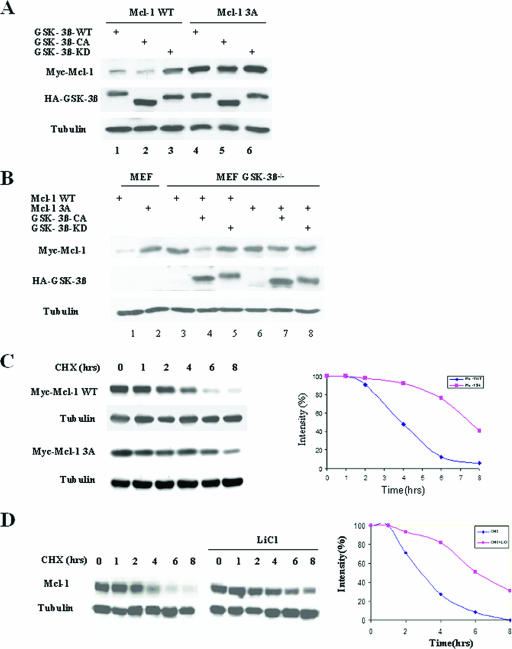
Next, we investigated the effect of Mcl-1 phosphorylation by GSK-3β on the stability of Mcl-1. Mcl-1-WT and the phosphorylation motif mutant Mcl-1-3A were cotransfected into 293T cells with GSK-3β-WT, GSK-3β-CA, or GSK-3β-KD. Mcl-1-3A was resistant to GSK-3β-WT- and GSK-3β-CA-induced degradation compared to Mcl-1-WT (Fig. 3A, lanes 4 and 5 versus lanes 1 and 2). Furthermore, Mcl-1-3A was more stable than Mcl-1-WT in MEF cells (Fig. 3B, lane 2 versus lane 1). However, in GSK-3β knockout MEF cells, Mcl-1-WT was as stable as Mcl-1-3A (Fig. 3B, lane 3 versus lane 6), and the reexpression of GSK-3β-CA downregulated Mcl-1-WT but not Mcl-1-3A (Fig. 3B, lane 4 versus lane 7). In addition, the half-life of Mcl-1-3A was nearly doubled (about 7 h) compared to that of Mcl-1-WT (Fig. 3C). Similar results were observed when the half-life of endogenous Mcl-1 in 293T cells was compared with that in 293T cells treated with the GSK-3β inhibitor lithium (Fig. 3D). These results indicate that the phosphorylation of Mcl-1 by GSK-3β is critical for Mcl-1 degradation.
FIG. 3.
Phosphorylation of Mcl-1 is required for Mcl-1 degradation. (A) Mcl-1-WT or Mcl-1-3A was cotransfected with GSK-3β-WT, GSK-3β-CA, or GSK-3β-KD into 293T cells as indicated. Cell lysates were then analyzed by Western blotting against Myc-Mcl-1 and HA-GSK-3β. (B) Mcl-1-WT or Mcl-1-3A was cotransfected with GSK-3β-CA or GSK-3β-KD in MEF or GSK-3β knockout MEF cells (MEF GSK-3β−/−) as indicated. Cell lysates were analyzed by Western blotting against Myc-Mcl-1 and HA-GSK-3β. (C) Mcl-1-WT or Mcl-1-3A was transfected into 293T cells, and cells were then treated with cycloheximide (CHX) (20 mM) for the indicated times. Cell lysates were analyzed by Western blotting against Myc-Mcl-1. Equal amounts of protein were subjected to Western blot analyses, as determined by comparing amounts of tubulin (left). Densitometry results for Mcl-1-WT and Mcl-1-3A after cycloheximide treatment were plotted, and the half-lives of Mcl-1-WT and Mcl-1-3A were determined (right). (D) 293T cells were treated with cycloheximide (20 μM) or cycloheximide with LiCl (20 mM) for the indicated times. Endogenous Mcl-1 expression was examined by Western blotting, and the half-lives of Mcl-1 were determined as described above (C).
β-TrCP directly mediates degradation of GSK-3β-phosphorylated Mcl-1.
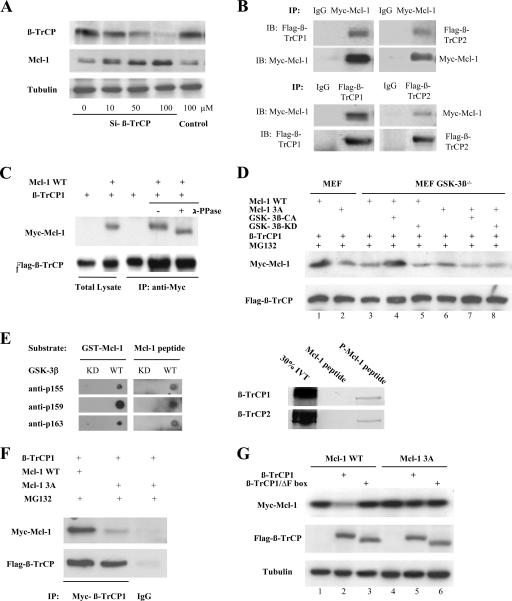
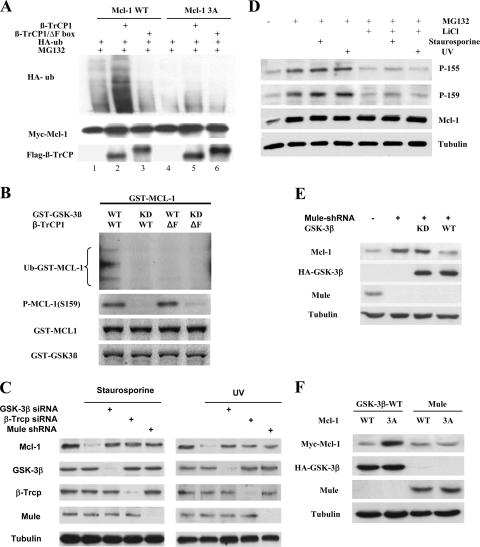
Next, we further investigated how GSK-3β-phosphorylated Mcl-1 is degraded in cells. We recognized that the phosphorylated motif of Mcl-1 phosphorylated by GSK-3β contains a sequence, DGSXXXT, that is similar to the DSGXXS/T destruction motif present in known substrates of the E3 ligase β-TrCP, which has two isoforms, β-TrCP1 and β-TrCP2 (46). Therefore, we tested whether β-TrCP could downregulate Mcl-1. The knockdown of both β-TrCP1 and β-TrCP2 expression in 293T cells by the siRNA method led to the accumulation of Mcl-1 in a dosage-dependent manner (Fig. 4A), and a time course experiment also showed an association between decreasing β-TrCP and increasing Mcl-1 levels (see Fig. S4 in the supplemental material). Furthermore, the results of the coimmunoprecipitation (Fig. 4B) showed that Mcl-1 associated with both β-TrCP1 and β-TrCP2, indicating that both β-TrCP1 and β-TrCP2 may be involved in Mcl-1 degradation. As the two isoforms of β-TrCP are known to be structurally and functionally related to each other, we then used β-TrCP1 to further address more detailed mechanisms. A faster-migrating band of Mcl-1 was generated once the immunocomplexes of Mcl-1 and β-TrCP1 were treated with λ-phosphatase (Fig. 4C), indicating that the Mcl-1 associated with β-TrCP was hyperphosphorylated. In addition, the associations between β-TrCP1 and Mcl-1-WT or Mcl-1-3A were very weak in the GSK-3β−/− MEF cells when β-TrCP1 was cotransfected with Mcl-1-WT or the Mcl-1-3A mutant (Fig. 4D, lanes 3 and 6 versus lane 1). However, in MEF cells and in GSK-3β−/− MEF cells in which GSK-3β activity was recovered by transfection of GSK-3β-CA, the association between β-TrCP1 and Mcl-1-WT was significantly increased and was much stronger than the association between β-TrCP1 and Mcl-1-3A (Fig. 4D, lanes 1 and 4 versus lanes 3, 6, and 7). Also, in these cells, GSK-3β-KD did not increase the association between β-TrCP1 and Mcl-1 (Fig. 4D, lanes 5 and 8). The results indicated that the phosphorylation of Mcl-1 by GSK-3β is required for its interaction with β-TrCP, which has been further verified by a peptide-binding assay, as in vitro-translated β-TrCP associated only with GSK-3β-phosphorylated Mcl-1 peptide (Fig. 4E). Similar results were obtained when β-TrCP1 and Mcl-1-WT or Mcl-1-3A were cotransfected into 293T cells (Fig. 4F). Consistently, β-TrCP1 degraded only Mcl-1-WT but not Mcl-1-3A (Fig. 4G, lane 2 versus lanes 1 and 5), whereas β-TrCP1/ΔF, which lacks an F-box function domain, did not affect the stability of Mcl-1 (Fig. 4G, lane 3 versus lane 1).
FIG. 4.
β-TrCP mediates Mcl-1 degradation. (A) 293T cells were transfected with the indicated doses of siRNA-β-TrCP or nonspecific siRNA (negative control), and cell lysates were then analyzed for endogenous β-TrCP and Mcl-1. (B) β-TrCP1 or β-TrCP2 (Flag tagged) and Mcl-1 (Myc tagged) were cotransfected into 293T cells, and Mcl-1 and β-TrCP1 or Mcl-1 and β-TrCP2 were then immunoprecipitated (IP) from cell lysates and subjected to Western blotting (IB). (C) Immunocomplexes obtained by β-TrCP1 immunoprecipitation were treated with λ-phosphatase (PPase) and analyzed for Mcl-1. (D) β-TrCP1 (Flag tagged) was cotransfected with Mcl-1-WT or Mcl-1-3A (Myc tagged) into MEF with or without GSK-3β knockout cells, and cells were then treated with MG132 (10 μM) for 10 h. Flag-β-TrCP1 was immunoprecipitated from cell lysates and then analyzed for Mcl-1. (E) Mcl-1 peptide and purified GST-Mcl-1 protein were phosphorylated by GST-GSK-3β and then analyzed with three phospho-Mcl-1 antibodies by dot blotting (left). Immobilized Mcl-1-derived peptides, with or without phosphorylation by GSK-3β, were incubated with [35S]methionine-labeled in vitro-translated (IVT) β-TrCP1 or β-TrCP2 protein and analyzed by autoradiography (right). (F) β-TrCP1 (Flag tagged) was cotransfected with Mcl-1-WT or Mcl-1-3A (Myc tagged) into 293T cells, and cells were then treated with MG132 (10 μM) for 10 h. Flag-β-TrCP1 was immunoprecipitated from cotransfected 293T cell lysates and then analyzed for Mcl-1. IgG, immunoglobulin G. (G) 293T cells were transfected with Mcl-1-WT or Mcl-1-3A and β-TrCP1 or F-box-deleted β-TrCP1 as indicated, and cell lysates were then analyzed for Mcl-1.
To investigate whether β-TrCP could directly ubiquitinate Mcl-1, in vivo ubiquitination of Mcl-1 by β-TrCP1 was performed in 293T cells. A much stronger ubiquitination was induced by β-TrCP1 in Mcl-1-WT than in Mcl-1-3A (Fig. 5A, lane 2 versus lane 5), while β-TrCP1 lacking the F box lost the ability to ubiquitinate Mcl-1 (Fig. 5A, lane 3). We then carried out an in vitro ubiquitination assay to further address whether β-TrCP directly ubiquitinated Mcl-1. The addition of purified β-TrCP1, but not dominant-negative β-TrCP1 (F-box-deleted form), stimulated Mcl-1 ubiquitination, and the ubiquitination of Mcl-1 also required phosphorylation by GSK-3β (Fig. 5B). Recently, a new E3 ligase, Mule/ARF-BP1, has been shown to degrade Mcl-1 (49); we then investigated whether GSK-3β/β-TrCP and Mule are a dependent or an independent pathway for Mcl-1 degradation. We found that UV irradiation and the anticancer drug staurosporine could lead to Mcl-1 degradation, and siRNA results showed that both GSK-3β/β-TrCP and the Mule pathway contribute to the UV- or staurosporine-induced Mcl-1 degradation (Fig. 5C). As UV irradiation and staurosporine can both activate GSK-3β (see Fig. S5 in the supplemental material) and phosphorylate Mcl-1 at two identified sites of Mcl-1 (Fig. 5D), we therefore investigated whether Mule may play a role in GSK-3β/β-TrCP-mediated Mcl-1 degradation. Our results indicated that GSK-3β-mediated Mcl-1 degradation is independent of Mule. In the Mule knockout cell, GSK-3β could still downregulate Mcl-1 (Fig. 5E). Mutating the GSK-3β phosphorylation motif of Mcl-1 rendered Mcl-1 able to resist the degradation induced by GSK-3β but not by Mule (Fig. 5F). Taken together, these results indicated that β-TrCP mediates ubiquitination and degradation of phosphorylated Mcl-1 by GSK-3β.
FIG. 5.
β-TrCP directly ubiquitinated GSK-3β-phosphorylated Mcl-1. (A) 293T cells were transfected with HA-ubiquitin, Mcl-1-WT or Mcl-1-3A and β-TrCP1 or F-box-deleted β-TrCP1 as indicated, and cells were then treated with MG132 (10 μM). Mcl-1 was immunoprecipitated and then analyzed with anti-HA-ubiquitin (HA-ub). (B) Purified Mcl-1 protein was phosphorylated by GSK-3β and then incubated with in vitro-translated β-TrCP1 or F-box-deleted β-TrCP1 in the presence of E1, E2, or His-ubiquitin (Ub) as described previously (21). Mcl-1 ubiquitination was examined by anti-His-ubiquitin immunoblotting. (C) Inducible Mule shRNA U2OS cells were transfected with siRNA against GSK-3β or β-TrCP for 48 h or treated with 2 μg/ml tetracycline to induce Mule shRNA for 48 h and then stimulated with UV irradiation and staurosporine for 6 h. The phosphorylation status of GSK-3β, β-TrCP, and Mule and the Mcl-1 level were determined in cell lysates by Western blotting. (D) Cells were treated with MG132, LiCl, staurosporine, or UV irradiation as indicated, and the cell lysates were then blotted with specific phospho-Mcl-1 antibodies. (E) Inducible Mule shRNA U2OS cells treated with 2 μg/ml tetracycline to induce Mule shRNA for 48 h were transfected with GSK-3β-WT or GSK-3β-KD, and the Mcl-1 level in cell lysates was then determined by Western blotting. (F) In 293T cells, the Myc-Mcl-1 WT or 3A mutant was cotransfected with HA-GSK-3β-WT or Flag-Mule for 48 h, and the Mcl-1 level in cell lysates was then determined by Western blotting.
GSK-3β induces apoptosis, tumor suppression, and chemosensitization, which can be overridden by mutated Mcl-1.
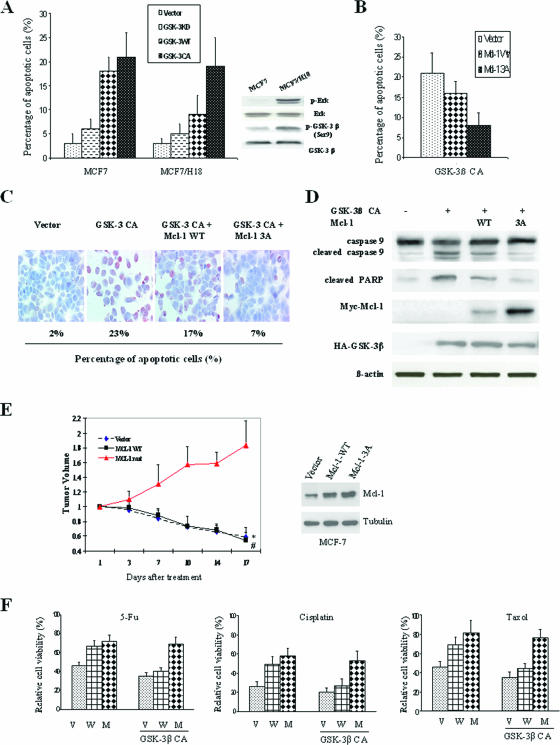
To investigate whether Mcl-1 can inhibit GSK-3β-induced apoptosis, we first examined the apoptotic effects of GSK-3β on breast cancer cells. We found that GSK-3β-induced apoptosis is dependent on its kinase activity. Transfection of GSK-3β-CA caused much more apoptosis than did transfection of GSK-3β-KD in MCF-7 and Her2-transfected MCF-7/H18 cells, whereas in MCF-7/H18 cells, GSK-3β-WT induced only 50% as much apoptosis as that in MCF-7 cells (Fig. 6A, left). This phenomenon may be caused by the Her2-activated Erk/MAPK pathway in MCF-7/H18 cells, in which wild-type GSK-3β can be easily inactivated by the Erk/MAPK pathway as measured by enhanced Ser9 phosphorylation (Fig. 6A, right) (13). When Mcl-1 was cotransfected with GSK-3β-CA into MCF-7 cells, we found that mutated Mcl-1-3A could inhibit GSK-3β-CA-induced apoptosis, as measured by FACS and TUNEL staining assay, while Mcl-1-WT only partially inhibited GSK-3β-CA-induced apoptosis (Fig. 6B and C). In addition, we found that GSK-3β-induced apoptosis was mediated by the activation of an intrinsic apoptosis pathway, as evidenced by the cleavage of caspase 9 and PARP (an alternative marker of the apoptotic pathway), which can be almost totally inhibited by Mcl-1-3A but only partially inhibited by Mcl-1-WT, perhaps because of the rapid degradation of Mcl-1-WT by GSK-3β-CA (Fig. 6D). It provided a plausible mechanism by which Mcl-1 blocked GSK-3β-induced apoptosis.
FIG. 6.
GSK-3β-mediated tumor suppression and chemosensitization can be overridden by expression of mutated Mcl-1. (A) GSK-3β-WT, GSK-3β-CA, or GSK-3β-KD was transfected by electroporation into MCF-7 or Her-2-expressing MCF-7 cells, and the percentages of apoptotic cells were then determined by FACS as described in Materials and Methods. (B) GSK-3β-CA was cotransfected with Mcl-1-WT or Mcl-1-CA into MCF-7 cells, and the percentages of apoptotic cells were then determined by FACS. (C) GSK-3β-CA was cotransfected with Mcl-1-WT or Mcl-1-CA into MCF-7 cells, and the percentages of apoptotic cells were then detected by TUNEL assay. (D) GSK-3β-CA was cotransfected with Mcl-1-WT or Mcl-1-CA into MCF-7 cells, and the cell lysates were then analyzed for caspase 9 and PARP. (E) Tumor-bearing mice were treated with intratumor injection of the liposome complex with GSK-3β, and the tumor volumes were then measured twice a week (* and # indicate that the differences between the group of Mcl-1-WT and the vector control with the group of Mcl-1-3A mutant are statistically significant [P < 0.05]). (F) Vector-transfected (V), Mcl-1-WT-expressing (W) and Mcl-1-3A-expressing (M) MCF-7 cells with or without GSK-3β transfection were treated with the apoptotic drugs 5-fluorouracil (5-Fu) (10 μg/ml), cisplatin (10 μg/ml), and taxol (2 nM) for 48 h. Relative cell viability was measured by an MTT assay, and cells without chemotherapy drug treatment were defined as the 100% control.
As we found that GSK-3β induced apoptosis and that this could be blocked by Mcl-1-3A, we next investigated whether GSK-3β plays a role in tumor suppression and whether Mcl-1 can resist GSK-3β's function. We generated Mcl-1-WT-expressing and Mcl-1-3A-expressing MCF-7 cells and used these cells to create an orthotopic breast cancer model in nude mice (28). The tumor-bearing mice were treated with a liposome complex with GSK-3β. The results showed that GSK-3β significantly inhibited tumor growth in both the vector control MCF-7 and Mcl-1-WT-expressing MCF-7 mouse models (Fig. 6E; see Fig. S6 in the supplemental material), indicating that GSK-3β is associated with tumor-suppressive activity, as expected, and Mcl-1-3A maintained tumor growth owing to its resistance to GSK-3β's function (Fig. 6E; see Fig. S6 in the supplemental material). In addition, we found that Mcl-1-WT-expressing and Mcl-1-3A-expressing MCF-7 cells were resistant to cell death induced by 5-fluorouracil, cisplatin, or taxol compared with the vector control-expressing MCF-7 cells (Fig. 6F). These results were consistent with previous reports that Mcl-1 expression is associated with resistance to chemotherapy (24, 42). However, when GSK-3β-CA was transfected in these stable cells prior to treatment with the three drugs, only Mcl-1-3A-expressing MCF-7 cells were chemoresistant (Fig. 6F); Mcl-1-WT-expressing MCF-7 cells lost their chemoresistance because of rapid degradation by GSK-3β. This result may have important clinical implications: it suggests that activating GSK-3β may be used to sensitize cells to chemotherapy, especially since mutation of Mcl-1 is a rare event in human tumors.
DISCUSSION
One of the hallmarks of tumorigenesis is escape from apoptosis (18). The overexpression of the antiapoptotic Bcl-2 or Bcl-XL gene in transgenic mice can promote the transformation of T lymphocytes, myeloid cells, and mammary epithelial cells and pancreatic cell tumors (23, 29, 34, 41). Mcl-1 promotes cell viability, as do Bcl-2 and Bcl-XL; however, a notable difference in the pattern of expression between Mcl-1 and other members of the Bcl-2 family was observed following a wide range of environmental stimuli (7). It is not yet clear what causes the differential expression between Mcl-1 and other Bcl-2 family members. In the current study, we found that GSK-3β was associated with Mcl-1 but not with Bcl-2 and Bcl-XL and then led to Mcl-1 degradation, which may provide a rationale for the differential expression between Mcl-1 and other members of the Bcl-2 family.
As Mcl-1 is an immediate-response gene and expected to be a short-term survival regulator, the Mcl-1 protein is tightly regulated and subjected to rapid degradation when cells undergo apoptosis in response to various stimuli such as growth factor withdrawal, ionizing radiation, and DNA-damaging reagents (7, 9, 35). In the current study, we noticed that one conservative GSK-3β phosphorylation motif in Mcl-1 is important in regulating Mcl-1, so we screened mutations in the Mcl-1 gene, especially in exon 1, which contains the phosphorylation motifs. We reasoned that mutations in this region might disrupt the phosphorylation of Mcl-1 and stabilize the protein, thus protecting cells from apoptosis. No mutation in exon 1 was found in any of the 16 cell lines screened (data not shown), indicating that a mutation in Mcl-1 that causes aberrant expression and function may be a rare event. This finding is consistent with previous reports (5, 6). Thus, the upstream signaling regulating Mcl-1 expression and function is more likely to play a role in tumorigenicity. Here, we identified a novel mechanism by which Mcl-1 stability is regulated, namely, GSK-3β/β-TrCP-mediated degradation of Mcl-1, in which GSK-3β phosphorylates Mcl-1 at three residues and results in the subsequent ubiquitination and degradation of phosphorylated Mcl-1 by the E3 ligase β-TrCP. Many apoptosis stimulators such as growth factor withdrawal, UV irradiation, and the anticancer drug staurosporine can activate GSK-3β and then downregulate MCl-1, indicating that this mechanism is a general phenomenon.
It is well documented that GSK-3β, a component of the WNT signaling pathway, plays an important role in apoptosis, and GSK-3β activity can be inhibited by the PI3-K/AKT pathway, resulting in resistance to apoptosis (8, 37). However, it is still unclear by which pathway GSK-3β facilitates apoptosis. Two important death receptors in the extrinsic pathway of apoptosis, CD95 (Fas/APO-1) and TNF-related apoptosis-inducing ligand (Apo2), seem to be related to GSK-3β-induced apoptosis (2, 43). However, several lines of evidence indicate that GSK-3β-induced apoptosis is related to the mitochondrion-dependent intrinsic caspase pathway. GSK-3β was found to be an important mediator of hypoxia-induced apoptosis, and GSK-3β-mediated apoptotic effects occur via the activation of the mitochondrial death pathway (30). In addition, GSK-3β exerts some of its proapoptotic effects in neurons by regulating the mitochondrial localization of Bax directly or indirectly (30, 45), which leads to mitochondrial dysfunction followed by caspase activation and apoptosis. Our current study and another recently published study by Maurer et al. (31) indicated that Mcl-1 is a direct substrate of GSK-3β. β-TrCP-associated Mcl-1 turnover mediated GSK-3β-activated intrinsic apoptosis, as indicated by cleaved caspase 9, by which GSK-3β exerted its tumor suppression activity. Although it is not surprising that Mcl-1 gives cells the ability to resist chemotherapy-induced cell death, GSK-3β was able to override Mcl-1-induced chemoresistance (Fig. 6F), suggesting that GSK-3β may be used in the clinic for enhancing chemosensitization, especially in patients with high levels of Mcl-l.
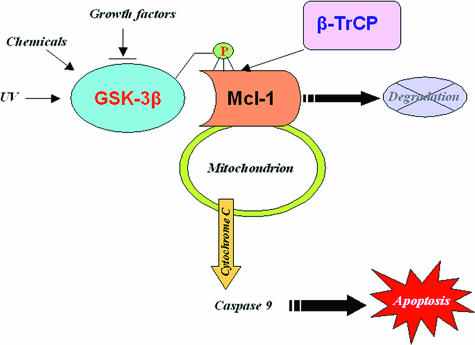
The proapoptotic features of GSK-3β may reflect the notion that insulin and insulin growth factor 1 and its downstream targets PI3-K and protein kinase B as well as Wnt ligand are acknowledged survival factors. Generally, our results, together with previous findings, provide a plausible mechanism (Fig. 7) for how the inactivation of GSK-3β stabilizes Mcl-1 to inhibit cell apoptosis, which provides a direct linkage between GSK-3β and the intrinsic apoptotic cascades through the regulation of Mcl-1 expression. Growth factors/receptors activate the PI3-K/Akt and Erk/MAPK pathways, and activated Akt and Erk will then inactivate GSK-3β through phosphorylation at Ser9, which will lead to Mcl-1 stabilization, because the loss of phosphorylation of Mcl-1 by GSK-3β will render Mcl-1 resistant to the degradation by the E3 ligase β-TrCP. Stabilized Mcl-1 will exert its antiapoptotic effects through the inactivation of the intrinsic apoptotic pathway, which may contribute to the immortalization and tumorigenesis of cells.
FIG. 7.
β-TrCP-associated Mcl-1 turnover mediates GSK-3β-induced apoptosis.
Supplementary Material
Acknowledgments
We thank J. R. Woodgett, A. Kikuchi, M. J. Birnhaum, K. Tanaka, and H.-Y. Yan-Yen for critical reagents and Jeng C. Cheng and Stephanie A. Miller for editing the manuscript.
This work was supported by NIH CA099031, NIH CA109311, the National Breast Cancer Foundation, Inc., the Kadoorie Charitable Foundation (M.-C.H.), a predoctoral fellowship from the U.S. Army Breast Cancer Research Program (grant W81XWH-05-1-0252 to D.-F.L.), and NIH Cancer Center support grant CA16672.
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 26 March 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beurel, E., M. Kornprobst, M. J. Blivet-Van Eggelpoel, C. Ruiz-Ruiz, A. Cadoret, J. Capeau, and C. Desbois-Mouthon. 2004. GSK-3beta inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp. Cell Res. 300:354-364. [DOI] [PubMed] [Google Scholar]
- 3.Budd, R. C. 2002. Death receptors couple to both cell proliferation and apoptosis. J. Clin. Investig. 109:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheon, S. S., P. Nadesan, R. Poon, and B. A. Alman. 2004. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp. Cell Res. 293:267-274. [DOI] [PubMed] [Google Scholar]
- 5.Cory, S., D. C. Huang, and J. M. Adams. 2003. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590-8607. [DOI] [PubMed] [Google Scholar]
- 6.Coultas, L., and A. Strasser. 2003. The role of the Bcl-2 protein family in cancer. Semin. Cancer Biol. 13:115-123. [DOI] [PubMed] [Google Scholar]
- 7.Craig, R. W. 2002. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia 16:444-454. [DOI] [PubMed] [Google Scholar]
- 8.Cross, D. A., A. A. Culbert, K. A. Chalmers, L. Facci, S. D. Skaper, and A. D. Reith. 2001. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 77:94-102. [DOI] [PubMed] [Google Scholar]
- 9.Cuconati, A., C. Mukherjee, D. Perez, and E. White. 2003. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17:2922-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degli Esposti, M. 2004. Mitochondria in apoptosis: past, present and future. Biochem. Soc. Trans. 32:493-495. [DOI] [PubMed] [Google Scholar]
- 11.Deng, J., W. Xia, and M. C. Hung. 1998. Adenovirus 5 E1A-mediated tumor suppression associated with E1A-mediated apoptosis in vivo. Oncogene 17:2167-2175. [DOI] [PubMed] [Google Scholar]
- 12.Desbois-Mouthon, C., A. Cadoret, M. J. Blivet-Van Eggelpoel, F. Bertrand, G. Cherqui, C. Perret, and J. Capeau. 2001. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20:252-259. [DOI] [PubMed] [Google Scholar]
- 13.Ding, Q., W. Xia, J. C. Liu, J. Y. Yang, D. F. Lee, J. Xia, G. Bartholomeusz, Y. Li, Y. Pan, Z. Li, R. C. Bargou, J. Qin, C. C. Lai, F. J. Tsai, C. H. Tsai, and M. C. Hung. 2005. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol. Cell 19:159-170. [DOI] [PubMed] [Google Scholar]
- 14.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domina, A. M., J. A. Vrana, M. A. Gregory, S. R. Hann, and R. W. Craig. 2004. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 23:5301-5315. [DOI] [PubMed] [Google Scholar]
- 16.Frame, S., and P. Cohen. 2001. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulbins, E., S. Dreschers, and J. Bock. 2003. Role of mitochondria in apoptosis. Exp. Physiol. 88:85-90. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 19.Hetman, M., J. E. Cavanaugh, D. Kimelman, and Z. Xia. 2000. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holnthoner, W., M. Pillinger, M. Groger, K. Wolff, A. W. Ashton, C. Albanese, P. Neumeister, R. G. Pestell, and P. Petzelbauer. 2002. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J. Biol. Chem. 277:45847-45853. [DOI] [PubMed] [Google Scholar]
- 21.Hu, J., C. M. McCall, T. Ohta, and Y. Xiong. 2004. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat. Cell Biol. 6:1003-1009. [DOI] [PubMed] [Google Scholar]
- 22.Igney, F. H., and P. H. Krammer. 2002. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 23.Jager, R., U. Herzer, J. Schenkel, and H. Weiher. 1997. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene 15:1787-1795. [DOI] [PubMed] [Google Scholar]
- 24.Kitada, S., J. Andersen, S. Akar, J. M. Zapata, S. Takayama, S. Krajewski, H. G. Wang, X. Zhang, F. Bullrich, C. M. Croce, K. Rai, J. Hines, and J. C. Reed. 1998. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood 91:3379-3389. [PubMed] [Google Scholar]
- 25.Kozopas, K. M., T. Yang, H. L. Buchan, P. Zhou, and R. W. Craig. 1993. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 90:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwana, T., and D. D. Newmeyer. 2003. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 15:691-699. [DOI] [PubMed] [Google Scholar]
- 27.Li, M., X. Wang, M. K. Meintzer, T. Laessig, M. J. Birnbaum, and K. A. Heidenreich. 2000. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol. Cell. Biol. 20:9356-9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y. M., Y. Wen, B. P. Zhou, H. P. Kuo, Q. Ding, and M. C. Hung. 2003. Enhancement of Bik antitumor effect by Bik mutants. Cancer Res. 63:7630-7633. [PubMed] [Google Scholar]
- 29.Linette, G. P., J. L. Hess, C. L. Sentman, and S. J. Korsmeyer. 1995. Peripheral T-cell lymphoma in lckpr-bcl-2 transgenic mice. Blood 86:1255-1260. [PubMed] [Google Scholar]
- 30.Loberg, R. D., E. Vesely, and F. C. Brosius III. 2002. Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J. Biol. Chem. 277:41667-41673. [DOI] [PubMed] [Google Scholar]
- 31.Maurer, U., C. Charvet, A. S. Wagman, E. Dejardin, and D. R. Green. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 21:749-760. [DOI] [PubMed] [Google Scholar]
- 32.Michels, J., P. W. Johnson, and G. Packham. 2005. Mcl-1. Int. J. Biochem. Cell Biol. 37:267-271. [DOI] [PubMed] [Google Scholar]
- 33.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates β-catenin signaling in epithelial cells. J. Virol. 77:12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik, P., J. Karrim, and D. Hanahan. 1996. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 10:2105-2116. [DOI] [PubMed] [Google Scholar]
- 35.Nijhawan, D., M. Fang, E. Traer, Q. Zhong, W. Gao, F. Du, and X. Wang. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opferman, J. T., A. Letai, C. Beard, M. D. Sorcinelli, C. C. Ong, and S. J. Korsmeyer. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671-676. [DOI] [PubMed] [Google Scholar]
- 37.Pap, M., and G. M. Cooper. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929-19932. [DOI] [PubMed] [Google Scholar]
- 38.Papkoff, J., and M. Aikawa. 1998. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem. Biophys. Res. Commun. 247:851-858. [DOI] [PubMed] [Google Scholar]
- 39.Rinkenberger, J. L., S. Horning, B. Klocke, K. Roth, and S. J. Korsmeyer. 2000. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14:23-27. [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider, P., and J. Tschopp. 2000. Apoptosis induced by death receptors. Pharm. Acta Helv. 74:281-286. [DOI] [PubMed] [Google Scholar]
- 41.Strasser, A., A. W. Harris, M. L. Bath, and S. Cory. 1990. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348:331-333. [DOI] [PubMed] [Google Scholar]
- 42.Thallinger, C., M. F. Wolschek, H. Maierhofer, H. Skvara, H. Pehamberger, B. P. Monia, B. Jansen, V. Wacheck, and E. Selzer. 2004. Mcl-1 is a novel therapeutic target for human sarcoma: synergistic inhibition of human sarcoma xenotransplants by a combination of mcl-1 antisense oligonucleotides with low-dose cyclophosphamide. Clin. Cancer Res. 10:4185-4191. [DOI] [PubMed] [Google Scholar]
- 43.Wang, Q., X. Wang, A. Hernandez, M. R. Hellmich, Z. Gatalica, and B. M. Evers. 2002. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J. Biol. Chem. 277:36602-36610. [DOI] [PubMed] [Google Scholar]
- 44.Wang, S., and W. S. El-Deiry. 2003. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22:8628-8633. [DOI] [PubMed] [Google Scholar]
- 45.Watcharasit, P., G. N. Bijur, L. Song, J. Zhu, X. Chen, and R. S. Jope. 2003. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 278:48872-48879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 13:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, T., K. M. Kozopas, and R. W. Craig. 1995. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J. Cell Biol. 128:1173-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhivotovsky, B., and G. Kroemer. 2004. Apoptosis and genomic instability. Nat. Rev. Mol. Cell Biol. 5:752-762. [DOI] [PubMed] [Google Scholar]
- 49.Zhong, Q., W. Gao, F. Du, and X. Wang. 2005. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121:1085-1095. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, B. P., J. Deng, W. Xia, J. Xu, Y. M. Li, M. Gunduz, and M. C. Hung. 2004. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6:931-940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.