Abstract
Dorsal vessel morphogenesis in Drosophila melanogaster serves as a superb system with which to study the cellular and genetic bases of heart tube formation. We used a cardioblast-expressed Toll-GFP transgene to screen for additional genes involved in heart development and identified tailup as a locus essential for normal dorsal vessel formation. tailup, related to vertebrate islet1, encodes a LIM homeodomain transcription factor expressed in all cardioblasts and pericardial cells of the heart tube as well as in associated lymph gland hematopoietic organs and alary muscles that attach the dorsal vessel to the epidermis. A transcriptional enhancer regulating expression in these four cell types was identified and used as a tailup-GFP transgene with additional markers to characterize dorsal vessel defects resulting from gene mutations. Two reproducible phenotypes were observed in mutant embryos: hypoplastic heart tubes with misaligned cardioblasts and the absence of most lymph gland and pericardial cells. Conversely, a significant expansion of the lymph glands and abnormal morphology of the heart were observed when tailup was overexpressed in the mesoderm. Tailup was shown to bind to two DNA recognition sequences in the dorsal vessel enhancer of the Hand basic helix-loop-helix transcription factor gene, with one site proven to be essential for the lymph gland, pericardial cell, and Svp/Doc cardioblast expression of Hand. Together, these results establish Tailup as being a critical new transcription factor in dorsal vessel morphogenesis and lymph gland formation and place this regulator directly upstream of Hand in these developmental processes.
The study of cardiogenesis in Drosophila melanogaster has significantly enhanced our understanding of the genetic and molecular basis of heart formation. The embryonic/larval fly heart, also called the dorsal vessel, is a linear organ for hemolymph circulation that closely resembles the vertebrate heart at its primitive tube stage (4, 30). A gene network that precisely controls cardiac cell specification, dorsal vessel morphogenesis, and cellular diversification therein has been defined (25). Such a regulatory network involves Decapentaplegic- and Wingless-initiated intercellular signaling events, which activate downstream transcriptional effectors such as Tinman (Tin), Pannier (Pnr), Dorsocross (Doc), and Seven-up (Svp). The combinatorial and at times opposing functions of these regulators result in the activation of defined gene expression programs, which lead to cardiac tube formation and the differentiation of functionally distinct cell types (10, 22, 23, 31). That is, of the 104 cardioblasts comprising the dorsal vessel, Tin-positive cells of the heart region will adopt a fate of working contractile myocardium, while Svp/Doc-positive cells will develop into inflow tracts within the heart domain. Of evolutionary relevance, all members of this cardiogenic regulatory network have functional homologues used in the more complex processes needed for heart development in vertebrates (20).
Drosophila has also emerged as an important model for the study of the genetic and cellular bases of hematopoiesis. Mature hemocytes arise during embryogenesis from the cephalic mesoderm and during larval development from the lymph glands, tissues associated with the anterior region of the dorsal vessel (7, 13, 17). A close connection exists between certain genes controlling cardiogenesis and hematopoiesis, as tin and pnr functions are required for the specification of the cardiogenic mesoderm, and lymph gland primordia are derived from a subset of these cells. Additionally, hemanogioblast-like progenitors that divide asymmetrically to generate daughter cells that are cardioblasts contributing to the anterior dorsal vessel and prohemocytes contributing to the lymph glands have been identified (17). Thereafter, hematopoietic factors of the GATA, Friend of GATA, Runx, and Glial Cells Missing classes control the differentiation of hemocyte precursors into defined hematopoietic cell lineages (7, 8). As with the cardiogenic regulators, functional homologues of these genes control various aspects of hematopoiesis in vertebrates.
While important insights into genes controlling heart and blood cell development in Drosophila have been gained, the story remains complex, with the need to discover additional regulatory components of these processes. With this goal in mind, we generated transgenic fly strains that express cardiac enhancer-green fluorescent protein (GFP) constructs that mark cardioblasts of the dorsal vessel. One characterized enhancer includes a cardioblast regulatory module of the Toll gene, which is positively activated by the functions of Tin and Doc (27). Such a reagent has been used in screens of the Drosophila genome for additional genes required for dorsal vessel morphogenesis.
In this report, we identify tailup (tup) as being a gene that is essential for heart formation in Drosophila. Tup is a LIM homeodomain transcription factor (26) expressed in several cell types of the dorsal vessel, and its mutation causes severe cardioblast, pericardial cell, and lymph gland deficiencies. Our studies also establish Tup as being a direct transcriptional activator of the Hand gene, which encodes a heart-expressed basic helix-loop-helix transcription factor (11). The importance of these findings is twofold. First, they provide new mechanistic insights into the transcriptional network controlling heart and blood cell development in Drosophila. Second, as Tup- and Hand-related proteins function in vertebrate heart development, these results further substantiate the evolutionary conservation of genes controlling cardiogenesis and hematopoiesis and implicate a regulatory relationship for the vertebrate genes.
MATERIALS AND METHODS
Drosophila strains.
The Df(2L)D15, tup1, and tupisl-1 strains were obtained from the Bloomington Stock Center. The Toll-nGFP (27), H15-lacZ (19), and Hand-GFP (11) transgenic lines have been previously characterized. The latter two strains were provided by W. Brook and Z. Han. The Handko strain was also described previously (12) and was provided by Z. Han.
Mutant phenotype analyses.
Wild-type and mutant embryos were collected and stained with various antibodies as previously described (27). The following primary antibodies were used in these studies: mouse anti-β-galactosidase at a 1:300 dilution (Promega, Madison, WI), rabbit anti-D-MEF2 at a 1:1,000 dilution (H. Nguyen), mouse anti-Tup at a 1:100 dilution (Developmental Studies Hybridoma Bank), mouse anti-GFP at a 1:200 dilution (BD Biosciences, Palo Alto, CA), mouse anti-Col at a 1:100 dilution (M. Crozatier), rabbit anti-Srp at a 1:500 dilution (D. Hoshizaki), and rabbit anti-Odd at a 1:500 dilution (J. Skeath). GFP expression in living embryos was detected as previously described (27).
Tup and Hand enhancer analyses.
Four partially overlapping DNAs spanning 13 kb of sequence upstream of the tup gene were generated by PCR amplification of the bacterial artificial chromosome (BAC) clone RP98-3E19 obtained from the BACPAC Resource Center (Children's Hospital, Oakland Research Institute). PCR products were initially cloned into the pCRII-TOPO vector using the TA cloning kit from Invitrogen (San Diego, CA) and subsequently moved into the P-element vector pH-Stinger (1). A similar strategy was used to generate Hand-GFP transgenes. DNAs were obtained by PCR amplification of the BAC clone RP98-30M19 with primers designed based on the previously published Hand cardiac and hematopoietic enhancer (HCH) sequence (11). The compositions of primers used to generate wild-type tup and wild-type or mutant Hand DNAs are available upon request. Transgenic lines carrying the tup-GFP or Hand-GFP DNAs were established after the injection of yw67c23 embryos using standard transformation techniques (10). At least five lines were established and analyzed for each DNA tested.
Additional molecular analyses.
To determine the molecular mutations present in the tup1 and tupisl-1 alleles, genomic DNA was isolated from wild-type and heterozygous adults by using a protocol described previously by McGinnis and Beckendorf (18). Primer pairs were designed to generate six overlapping tup DNAs that spanned the complete exon/intron organization of the gene. Specific sequences of the primers used for PCR amplification are available upon request. PCR products were generated using Taq DNA polymerase, amplification reagents, and protocols obtained from Roche Applied Science (Indianapolis, IN). Wild-type and mutated tup DNA sequences were determined by the Macrogen Corporation (Rockville, MD).
For electromobility shift assays with Tup and Hand, the Tup protein was generated using the TnT Quick Coupled Transcription/Translation system (Promega). These assays were performed as described previously by Ranganayakulu et al. (21). Complementary oligonucleotides containing the consensus Tup-1 and Tup-2 binding sites of the Hand HCH enhancer were used. The oligonucleotide sequences for wild-type and competitor probes for the Tup-1 site were Tup-1A (5′-AATCAAACCCCTAATGGATTAAAATG-3′) and Tup-1B (5′-CATTTTAATCCATTAGGGGTTTGATT-3′) (the Tup binding site is underlined). Oligonucleotides with an altered sequence, previously shown to disrupt Isl1 DNA binding (6), served as the mutant competitor DNA. These included Tup-1A mutant (5′-AATCAAACCCCTAggtGATTAAAATG-3′) and Tup-1B mutant (5′-CATTTTAATCaccTAGGGGTTTGATT-3′). The oligonucleotide sequences for wild-type and competitor probes for the Tup-2 site were Tup-2A (5′-CCGCACTTCCATTAGGAATATATCT-3′) and Tup-2B (5′-AGATATATTCCTAATGGAAGTGCGGT-3′) (the Tup binding site is underlined). Oligonucleotides with altered sequences served as the mutant competitor DNA. These included Tup-2A mutant (5′-ACCGCACTTCaccTAGGAATATATCT-3′) and Tup-2B mutant (5′-AGATATATTCCTAggtGAAGTGCGGT-3′) (lowercase type indicates the base changes within the Tup binding sites).
RESULTS
Discovery of tup as being a gene required for dorsal vessel morphogenesis.
The Toll transmembrane protein is expressed on lateral surfaces of all cardioblasts as they align and migrate to the midline during dorsal vessel formation (27). The transcriptional enhancer controlling Toll expression in these cells has been characterized and used to direct GFP expression in transgenic flies (Fig. 1A). This combination resulted in a high-resolution reagent that has been used in a screen for genes of the second chromosome that are required for dorsal vessel formation. Specifically, the Toll-nGFP transgene was crossed into deficiency backgrounds that span most of chromosome 2. Multiple intervals that deleted a gene(s) needed for normal cardiogenesis were identified, and certain deficiency regions were further analyzed by surveying ethyl methylsulfonate (EMS)- or P-element-induced mutations known to map within them. Several genes were identified as being essential for dorsal vessel morphogenesis by using this approach (Y. Tao and R. A. Schulz, unpublished data). This report focuses on the expression and function of tup in the cardiogenic process.
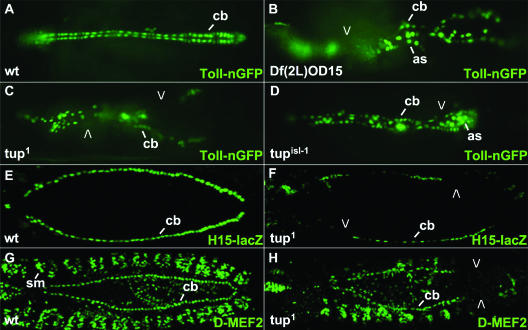
FIG. 1.
Dorsal vessel phenotypes observed in tup mutant embryos. (A to D) Toll-nGFP transgene expression in (A) wild-type (wt), (B) homozygous Df(2L)OD15, (C) homozygous tup1, and (D) homozygous tupisl-1 embryos. Embryos are at stage 16 of development. (E and F) H15-lacZ transgene expression in (E) wild-type and (F) homozygous tup1 embryos. Embryos are at stage 14 of development. (G and H) D-MEF2 protein expression in (G) wild-type and (H) homozygous tup1 embryos. Embryos are at stage 15 of development. Open arrowheads point to missing cardioblasts and pronounced gaps in dorsal vessels of mutant embryos. All embryos are oriented with the anterior to the left. Abbreviations: as, amnioserosa cells; cb, cardioblasts; sm, somatic body wall muscles.
Df(2L)OD15 deletes sequences within the 36F-37B interval of chromosome 2, and homozygous embryos contain a severely distorted dorsal vessel based on Toll-nGFP expression (Fig. 1B). By reviewing information present in the FlyBase database, we assessed the loci present within this chromosome interval and noted the presence of tup, whose homologue, islet1 (isl1), has a proven function in mouse heart development (3). We obtained two EMS-induced alleles of tup (24, 26) and were able to show that mutations specific to this gene resulted in dorsal vessel phenotypes comparable to those observed with the homozygous deficiency condition. That is, homozygous tup1 and tupisl-1 embryos possessed decreased numbers and abnormal organization of cardioblasts based on the altered expression of the dorsal vessel markers Toll-nGFP (Fig. 1C and D), H15-lacZ (19) (Fig. 1F), and D-MEF2 (15) (Fig. 1H). Missing cardioblasts included both Tin- and Svp/Doc-positive cells, with gaps observed at random locations within the cardiac tube. Thus, tup is a locus that is newly identified as being required for correct dorsal vessel formation in Drosophila.
tup expression in multiple cell types of the dorsal vessel.
tup has been studied mostly in the context of its role in patterning the central nervous system. Thus, limited information was available on its expression in the heart tube other than the detection of tup mRNA and protein in undefined cells of the dorsal vessel (FlyBase report on tup) (26). We used an anti-Tup monoclonal antibody to determine the Tup-positive cells within the dorsal vessel. The Tup protein is initially observed in the dorsal mesoderm around stage 10, where it could function in the specification of various dorsal vessel cell types (data not shown). By stage 16, four specific cell types were identified as expressing Tup in the mature linear organ: prohemocytes of the lymph glands, pericardial cells, all cardioblasts, and alary muscles that attach the heart tube to the overlying epidermis (Fig. 2A and B). Such observations were consistent with the requirement of tup for normal dorsal vessel morphogenesis.
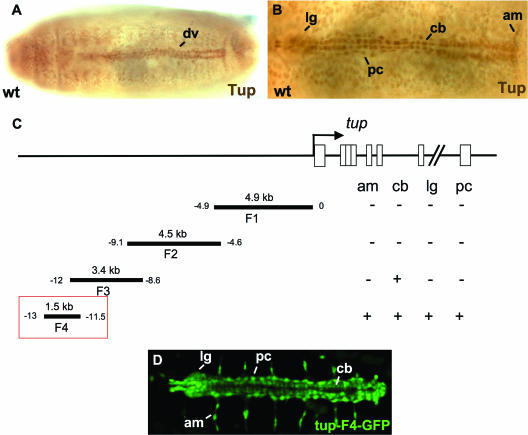
FIG. 2.
tup expression in cells within and associated with the dorsal vessel. (A) Low-magnification (×20) image of a wild-type (wt) embryo immunostained for the Tup protein. Tup expression in the dorsal vessel (dv) is indicated. (B) High-magnification (×40) image of a wild-type embryo immunostained for Tup protein. Tup expression is observed (from anterior to posterior) in lymph glands (lg), pericardial cells (pc), cardioblasts (cb), and alary muscles (am). (C) Mapping the location of regulatory modules controlling tup dorsal vessel expression. Boxes within the tup gene correspond to exon sequences. (D) Expression of the tup-F4-GFP transgene in all four types of Tup-positive cells of the dorsal vessel. All embryos are at stage 16 of development and oriented with the anterior to the left.
In an attempt to identify transcriptional control sequences regulating tup expression in these cells, we tested upstream DNAs for enhancer function in the heart tube. A 1.5-kb DNA located between −13 and −11.5 kb 5′ of the gene was able to direct GFP expression in all four Tup-positive cell types (Fig. 2C and D). The tup-F4-GFP transgene thus served as a sensitive marker for multiple components of dorsal vessel assembly, including the lymph glands, alary muscles, pericardial cells, and cardioblasts.
The tup gene produces two mRNA transcripts due to alternative splicing, which result in two versions of Tup that differ solely in their C-terminal sequences (Fig. 3A). We sought to determine the molecular mutations present in the tup1 and tupisl-1 alleles so as to define potential alterations in the Tup proteins. For the tup1 allele, a missense mutation that changes Cys-57 to Tyr-57 was found. This Cys residue is present in the first zinc finger of the LIM domain and is fully conserved among LIM domain proteins (14). Mutation of this critical amino acid resulted in highly unstable Tup proteins, as tup1 mutant embryos accumulated very low levels of Tup (Fig. 3C). Such an observation was consistent with the strong dorsal vessel phenotype observed in homozygous tup1 embryos. We failed to find a mutation in tup exon or intron sequences in DNA obtained from tupisl-1 mutants, suggesting that this alteration may reside in transcriptional regulatory sequences of the gene.
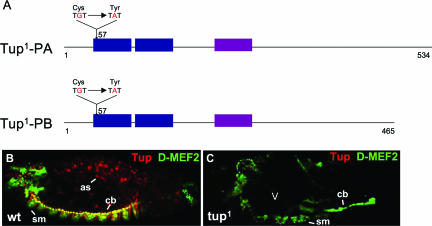
FIG. 3.
Determination of the molecular mutation present in the tup1 allele. (A) Two very similar forms of Tup are generated by the alternative splicing of pre-mRNA. The LIM and homeobox domains of the proteins are indicated. A single nucleotide mutation present in the tup1 allele results in the change of an amino acid crucial for zinc finger formation within the first LIM domain; specifically, the codon for Cys-57 is mutated to one encoding Tyr-57. (B and C) Double staining of (B) wild-type (wt) and (C) homozygous tup1 embryos for Tup and D-MEF2 reveals an absence (open arrowhead) of the Tup protein in mutant animals. The embryos are at stage 13 of development and oriented with the anterior to the left. Abbreviations: as, aminoserosa cells; cb, cardioblasts; sm, somatic body wall muscles.
Detailed analyses of tup dorsal vessel phenotypes.
Given the severity of the tup1 mutation and its strong effect on protein levels in mutant embryos, we used this genetic background to undertake an in-depth analysis of tup dorsal vessel phenotypes. As noted, tup-F4-GFP is expressed in lymph glands, pericardial cells, and cardioblasts. In homozygous tup1 embryos, cardiac hypoplasia was observed, since there was a decrease in the number of cells in the malformed dorsal vessel, including an apparent absence of the lymph glands (Fig. 4B). The Collier (Col) protein serves as a discriminating marker for lymph gland anlagen during embryogenesis and the posterior signaling centers of the lymph glands after dorsal vessel morphogenesis has occurred (5). Double labeling of mutant embryos for Col and GFP expressed under the control of the tup dorsal vessel enhancer showed the presence of rudimentary lymph glands in tup mutants (Fig. 4D). Thus, the comparable finding of Col-positive cells within lymph glands of wild-type and tup1 embryos indicated that at least part of the hematopoietic organs are present in the absence of Tup function.
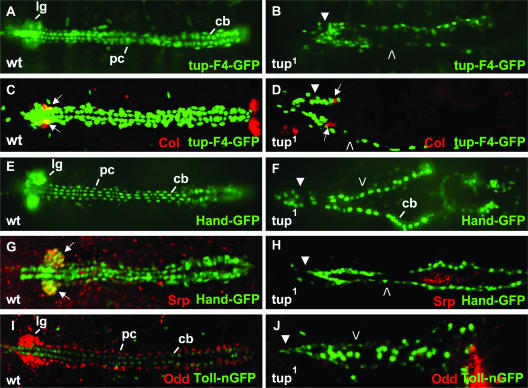
FIG. 4.
High-resolution analyses of tup dorsal vessel and lymph gland phenotypes. (A and B) tup-F4-GFP transgene expression in (A) wild-type (wt) and (B) homozygous tup1 embryos. Lymph glands (lg), pericardial cells (pc), and cardioblasts (cb) of the normal dorsal vessel are indicated in the wild-type embryo. (C and D) Col protein and tup-F4-GFP transgene expression in (C) wild-type and (D) homozygous tup1 embryos. Arrows point to Col expression in the posterior signaling centers of the lymph glands associated with the normal dorsal vessel, and comparable Col staining is observed along the defective heart tube of the mutant embryo. Col expression is also observed in bilateral posterior structures in the wild-type embryo, which are out of focus in the tup1 embryo due to a defect in dorsal closure. (E and F) Hand-GFP transgene expression in (E) wild-type and (F) homozygous tup1 embryos. Prominent GFP expression in the lymph glands, pericardial cells, and cardioblasts in the normal dorsal vessel is highlighted. (G and H) Srp protein and Hand-GFP transgene expression in (G) wild-type and (H) homozygous tup1 embryos. Arrows point to the coincident expression of Srp and Hand in the lymph glands of the wild-type embryo. (I and J) Odd protein and Toll-nGFP transgene expression in (I) wt and (J) tup1 embryos. Prominent Odd expression in the lymph glands and pericardial cells is highlighted, while Toll expression in cardioblasts is also indicated in the wild-type embryo. (B, D, F, H, and J) All five of the markers, or marker combinations, reveal the absence of most lymph gland (closed arrowheads) and pericardial (open arrowheads) cells in defective dorsal vessels found in tup1 mutant embryos. Mutant embryos also exhibit defects in dorsal closure, with prolonged GFP expression in cells of the amnioserosa using the Toll-nuclear GFP (nGFP) marker (J). All embryos are at stage 16 of development and oriented with the anterior to the left.
The observation of depleted numbers of cells in the dorsal vessel and defects in lymph gland development was further supported using the Hand-GFP transgene, which is expressed in lymph glands, pericardial cells, and cardioblasts (11) (Fig. 4E). This probe allowed the identification of decreased and misaligned cardioblasts in mutant embryos and also highlighted the apparent absence of lymph gland and pericardial cells due to the Tup protein mutation (Fig. 4F). The severe lymph gland phenotype was documented by the absence of Serpent (Srp)-expressing prohemocyte cells in the majority of mutant embryos double stained for Srp and Hand-GFP (Fig. 4H). Odd-skipped (Odd) is another high-resolution marker for lymph gland and pericardial cells (28), and the inability to detect the Odd protein in most homozygous tup1 embryos further suggested the severe reduction of both cell types. Thus, it can be concluded that tup function is critical for proper dorsal vessel formation, including the production and/or survival of a correct number of cardioblast, pericardial, and lymph gland cells.
Direct regulation of the Hand dorsal vessel enhancer by Tup.
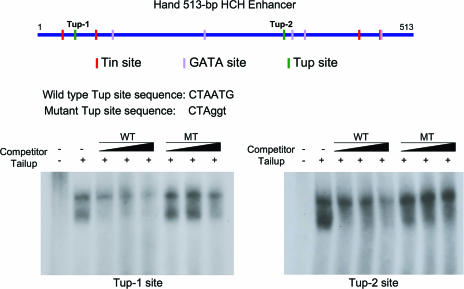
Since tup is expressed and functions in three cell types that also express the Hand gene, we considered the possibility that Tup may be a transcriptional regulator of Hand through its cardiac and hematopoietic enhancer. Previous studies defined the presence of required Tin and GATA recognition elements in the Hand 513-bp HCH enhancer (11), and a scan of this sequence also identified two regions with consensus Isl1 binding sites (6). Electromobility shift assays were undertaken with the Tup protein and the putative binding elements, with the finding that Tup can selectively interact with both of the wild-type recognition sequences (Fig. 5). These molecular data were consistent with the possibility of Tup functioning as a transcriptional activator of Hand dorsal vessel expression through this defined regulatory module.
FIG. 5.
Two Tup DNA recognition sites are present in the Hand 513-bp HCH enhancer. The locations of Tup CTAATG binding elements, relative to previously defined Tin and GATA protein binding sites (15), are indicated in the Hand DNA. At the bottom, electromobility gel shift assays reveal that Tup can selectively bind to double-stranded oligonucleotides containing the Tup-1 or Tup-2 sequence. Abbreviations: MT, Tup-site-mutated oligonucleotide; WT, wild-type oligonucleotide.
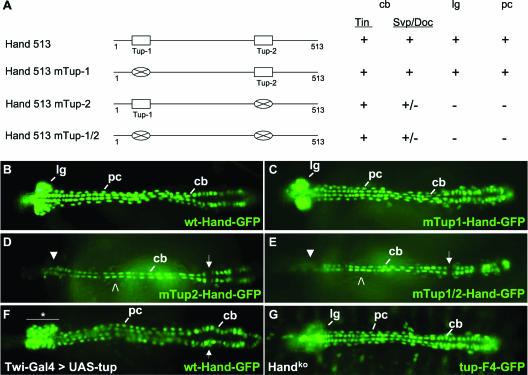
To test this hypothesis, we generated Hand HCH-GFP fusion genes with or without mutations in one or both of the Tup sites within the enhancer and tested DNA activities in transgenic embryos (Fig. 6A). Mutation of the Tup-1 element failed to abrogate enhancer activity, as the GFP reporter was detected in all cardioblast, lymph gland, and pericardial cells (Fig. 6A and C). However, when the Tup-2 site alone or both the Tup-1 and Tup-2 elements were mutated within the HCH sequence, GFP expression was completely lost from lymph gland and pericardial cells (Fig. 6A, D, and E). Additionally, Hand enhancer activity was significantly diminished in Svp/Doc-expressing cardioblasts due to the Tup-2 site mutation, while enhancer activity was maintained in Tin-expressing cardioblasts. Such findings demonstrated the importance of a wild-type Tup-2 element for the normal function of the HCH enhancer and strongly implicated Tup as being a direct transcriptional activator of Hand dorsal vessel expression.
FIG. 6.
Tup is a direct transcriptional regulator of the Hand dorsal vessel enhancer. (A, left) Schematic of the Hand 513-bp HCH enhancer and three Tup site mutant versions thereof. (A, right) Function of wild-type and mutated Hand-GFP DNAs in transgenic embryos. Activities of the various enhancers in Tin or Svp/Doc cardioblasts (cb), lymph glands (lg), or pericardial cells (pc) are indicated as positive (+), negative (−), or greatly reduced (+/−). (B and C) Normal cellular expression profile of GFP in embryos harboring the wt-Hand-GFP or mTup1-Hand-GFP transgenes. (D and E) Representative embryos expressing the mTup2-Hand-GFP or mTup1/2-Hand-GFP construct. With both mutated DNAs, GFP is observed in Tin cardioblasts but absent from the lymph glands (closed arrowheads) and pericardial cells (open arrowheads). Reporter expression is greatly diminished in Svp/Doc cardioblasts (arrows) with both mutations. (F) Twi-Gal4>UAS-tup embryo expressing the wt-Hand-GFP marker. This forced expression condition results in an expanded population of prohemocytes in the lymph glands (horizontal bar and asterisk) and a disorganized heart region (arrow). (G) Expression of the tup-F4-GFP transgene in a homozygous Handko mutant embryo. tup expression appears to be normal in cardioblasts and pericardial cells but reduced in the lymph glands. All embryos are at stage 16 of development and oriented with the anterior to the left.
To further investigate the Tup regulation of Hand, we used the Gal4-upstream activation sequence (UAS) binary transcription system (2) to force the expression of tup throughout the mesoderm. In Twi-Gal4>UAS-tup embryos, we observed a greatly expanded population of Hand-GFP-positive cells in the lymph glands and an abnormal organization of the posterior heart region (Fig. 6F). Thus, increasing the amount of the Tup protein is sufficient to generate supernumerary prohemocytes within the hematopoietic organs. We also assessed the activity of the tup heart enhancer in Hand mutants. In homozygous Handko embryos (12), we observed a normal dorsal vessel structure and proper expression of tup-F4-GFP in cardioblasts and pericardial cells (Fig. 6G). However, transgene expression was diminished in the lymph glands, possibly because of cell death observed in these tissues due to a Hand gene mutation (12). These findings further support the belief that tup resides genetically upstream of Hand in the regulatory network controlling gene expression in cardiac and hematopoietic cells of the dorsal vessel.
DISCUSSION
Our findings identified the Tup LIM homeodomain transcription factor as being a newly discovered player in the regulatory network controlling dorsal vessel morphogenesis and hematopoietic organ formation. Tup is expressed in all cardioblast and pericardial cells of the heart tube, prohemocytes of the lymph glands, and alary muscles needed to secure the dorsal vessel to the epidermis. Our phenotypic studies demonstrated a requirement for tup function in three of these cells types. tup mutant embryos present with a hypoplastic dorsal vessel, with a variable number of cardioblasts that fail to organize into a heart tube structure. It appears that correct numbers of cardioblasts are not specified in mutant embryos, as gaps were observed in the bilateral cardioblast rows early in the process of dorsal vessel formation. Missing cardioblasts included cells of both the Tin- and Svp/Doc-positive subclasses. The late cardioblast misalignment phenotype is likely due to the dorsal closure and germ band retraction defects known to occur in tup embryos.
While the degree of cardioblast hypoplasia is variable in mutant embryos, the severe reduction in prohemocytes of the lymph glands and pericardial cells surrounding the contractile tube is fully penetrant. The Col protein serves as an excellent marker for lymph gland primordia and the posterior signaling centers of lymph glands associated with the mature dorsal vessel (5). Since Col expression is normal in tup mutants, Tup function is not required for the early specification of lymph gland primordia within the dorsal mesoderm. However, the severe reduction of several mature lymph gland markers such as tup-GFP, Hand-GFP, Srp, and Odd suggests that either prohemocytes are present within lymph glands with Tup activity essential for expression of all four of these indicator genes or the cells are absent due to defects in prohemocyte proliferation and/or programmed cell death. The latter is an attractive possibility since Hand knockout embryos show ectopic apoptosis among lymph gland progenitor cells (12).
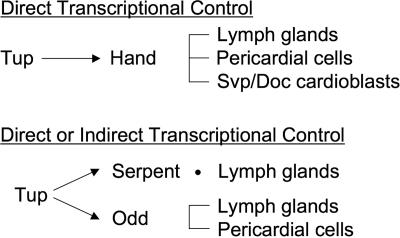
A function for the Hand basic helix-loop-helix transcription factor has been reported for cardioblast, pericardial, and lymph gland cells. This is the same set of dorsal vessel and hematopoietic cells that require Tup function. Through our analysis of the Hand cardiac and hematopoietic enhancer, we demonstrated that Tup is a direct transcriptional regulator of Hand in these cell types. Specifically, mutation of the single Tup-2 element in the HCH regulatory module resulted in a dramatic loss or reduction of Hand enhancer activity in prohemocytes, pericardial cells, and the Svp/Doc cardioblast subtype (9, 16, 22, 31). These findings invoke two possibilities. First, tup phenotypes may be due to the lack of Hand expression and function in cardioblasts, pericardial cells, and lymph gland progenitors. However, Tup function is likely to be even more critical for cardiogenic and hematopoietic events, as the forced expression of tup results in the production of excess prohemocytes, while the ectopic expression of Hand does not. Thus, Tup can be considered to be a seminal upstream regulator of genetic and cellular events controlling lymph gland formation. Second, Tin and GATA factors have been shown to regulate the HCH enhancer. Thus, it is possible the Hand cardiac and hematopoietic transcription occurs due to combinatorial control, specifically via Tup and Doc cofunction in Svp/Doc-expressing cardioblasts and Tup and Srp coactivity in lymph gland progenitors. Ample evidence exists for the function of multiple interacting transcription factors in the regulation of heart and blood cell gene expression in Drosophila (23, 25). To summarize regulatory aspects of its function, the data showing that Tup is a direct transcriptional activator of Hand expression in lymph glands, pericardial cells, and Svp/Doc-positive cardioblasts through the HCH enhancer module are compelling (Fig. 7). Likewise, Tup serves as either a direct or indirect regulator of srp expression in lymph gland cells and odd expression in lymph gland and pericardial cells.
FIG. 7.
Proposed functions for Tup in regulating lymph gland, pericardial cell, and Svp/Doc cardioblast gene expression.
The finding of Tup as a newly identified component of the gene regulatory network controlling heart and hematopoietic formation further documents the evolutionary conservation of transcriptional regulators in these developmental processes. Tup's vertebrate relative, Isl1, has been shown to be required for the development of heart regions contributed by the secondary heart field, with Isl1+-positive cells populating the outflow tract, right ventricle, part of the left ventricle, and most of the atria (3, 29). Our demonstration of a function for Tup in hematopoietic organ formation suggests that Isl1 may likewise function in some aspect of hematopoiesis in vertebrate systems. Hand genes are also conserved between Drosophila and vertebrates, with Hand1 and Hand2 required for specific aspects of ventricular development in the mouse heart (20). The direct regulation of Hand cardiac and hematopoietic expression by Tup suggests that a comparable transcriptional control may be utilized in vertebrate heart development through Isl1 regulation of the Hand1 and/or Hand2 gene.
In summary, Drosophila has served as a powerful system to discover signaling pathways and transcriptional regulators governing heart development. Through the generation of a sensitive cardiac enhancer-GFP transgene and its use in a screen for additional genes required for normal dorsal vessel morphogenesis, we identified an additional evolutionarily conserved component of the gene regulatory network controlling cardiac development. The further detailed study of Tup in terms of its functions in cardiogenesis and hematopoiesis and its molecular interactions with other proven heart and blood cell regulators, should provide needed insights into mechanistic functions of LIM homeodomain class proteins in these vital developmental processes.
Acknowledgments
We are grateful to W. Brook, Z. Han, and the Bloomington Stock Center for providing Drosophila strains and M. Crozatier, D. Hoshizaki, H. Nguyen, J. Skeath, and the Developmental Studies Hybridoma Bank for sending antibody reagents.
This research was supported by grants to R.A.S. from the National Institutes of Health (HL059151 and HL071540).
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Barolo, S., L. A. Carver, and J. W. Posakony. 2000. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques 29:726-732. [DOI] [PubMed] [Google Scholar]
- 2.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 3.Cai, C. L., X. Liang, Y. Shi, P. H. Chu, S. L. Pfaff, J. Chen, and S. Evans. 2003. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5:877-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cripps, R. M., and E. N. Olson. 2002. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev. Biol. 246:14-28. [DOI] [PubMed] [Google Scholar]
- 5.Crozatier, M., J. M. Ubeda, A. Vincent, and M. Meister. 2004. Cellular immune response to parasitization in Drosophila requires EBF orthologue collier. PLoS Biol. 2:1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodou, E., M. P. Verzi, J. P. Anderson, S. M. Xu, and B. L. Black. 2004. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131:3931-3942. [DOI] [PubMed] [Google Scholar]
- 7.Evans, C. J., V. Hartenstein, and U. Banerjee. 2003. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5:673-690. [DOI] [PubMed] [Google Scholar]
- 8.Fossett, N., and R. A. Schulz. 2001. Functional conservation of hematopoietic factors in Drosophila and vertebrates. Differentiation 69:83-90. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski, K., C. Y. Choi, Y. Kim, and R. A. Schulz. 2000. Genetically distinct cardial cells within the Drosophila heart. Genesis 28:36-43. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski, K., N. Fossett, J. D. Molkentin, and R. A. Schulz. 1999. The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development 126:5679-5688. [DOI] [PubMed] [Google Scholar]
- 11.Han, Z., and E. N. Olson. 2005. Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132:3525-3536. [DOI] [PubMed] [Google Scholar]
- 12.Han, Z., P. Yi, X. Li, and E. N. Olson. 2006. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 133:1175-1182. [DOI] [PubMed] [Google Scholar]
- 13.Jung, S. H., C. J. Evans, C. Uemura, and U. Banerjee. 2005. The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132:2521-2533. [DOI] [PubMed] [Google Scholar]
- 14.Kadrmas, J. L., and M. C. Beckerle. 2004. The LIM domain: from the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 5:920-931. [DOI] [PubMed] [Google Scholar]
- 15.Lilly, B., B. Zhao, G. Ranganayakulu, B. M. Paterson, R. A. Schulz, and E. N. Olson. 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267:688-693. [DOI] [PubMed] [Google Scholar]
- 16.Lo, P. C. H., and M. Frasch. 2001. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech. Dev. 104:49-60. [DOI] [PubMed] [Google Scholar]
- 17.Mandal, L., U. Banerjee, and V. Hartenstein. 2004. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 36:1019-1023. [DOI] [PubMed] [Google Scholar]
- 18.McGinnis, W., and S. K. Beckendorf. 1983. Association of a Drosophila transposable element of the roo family with chromosomal deletion breakpoints. Nucleic Acids Res. 11:737-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miskolczi-McCallum, C. M., R. J. Scavetta, P. C. Svendsen, K. H. Soanes, and W. J. Brook. 2005. The Drosophila melanogaster T-box genes midline and H15 are conserved regulators of heart development. Dev. Biol. 278:459-472. [DOI] [PubMed] [Google Scholar]
- 20.Olson, E. N. 2006. Gene regulatory networks in the evolution and development of the heart. Science 313:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranganayakulu, G., B. Zhao, A. Dokidis, J. D. Molkentin, E. N. Olson, and R. A. Schulz. 1995. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 171:169-181. [DOI] [PubMed] [Google Scholar]
- 22.Reim, I., and M. Frasch. 2005. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 132:4911-4925. [DOI] [PubMed] [Google Scholar]
- 23.Sorrentino, R. P., K. M. Gajewski, and R. A. Schulz. 2005. GATA factors in Drosophila heart and blood cell development. Semin. Cell Dev. Biol. 16:107-116. [DOI] [PubMed] [Google Scholar]
- 24.Strecker, T. R., P. Li, S. A. Meghee, D. Ham, S. K. Smith, J. A. Schreck, S. J. Youn, and P. S. H. Kon. 1995. The effects of the glucocorticoid, dexamethasone on the development of the Drosophila embryo. Roux Arch. Dev. Biol. 204:359-368. [DOI] [PubMed] [Google Scholar]
- 25.Tao, Y., and R. A. Schulz. 2007. Heart development in Drosophila. Semin. Cell Dev. Biol. 18:3-15. [DOI] [PubMed] [Google Scholar]
- 26.Thor, S., and J. B. Thomas. 1997. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron 18:397-409. [DOI] [PubMed] [Google Scholar]
- 27.Wang, J., Y. Tao, I. Reim, K. Gajewski, M. Frasch, and R. A. Schulz. 2005. Expression, regulation, and requirement of the Toll transmembrane protein during dorsal vessel formation in Drosophila melanogaster. Mol. Cell. Biol. 25:4200-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward, E. J., and J. B. Skeath. 2000. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 127:4959-4969. [DOI] [PubMed] [Google Scholar]
- 29.Xu, H., and A. Baldini. 2007. Genetic pathways to mammalian heart development: recent progress from manipulation of the mouse genome. Semin. Cell Dev. Biol. 18:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaffran, S., and M. Frasch. 2002. Early signals in cardiac development. Circ. Res. 91:457-469. [DOI] [PubMed] [Google Scholar]
- 31.Zaffran, S., I. Reim, L. Qian, P. C. Lo, R. Bodmer, and M. Frasch. 2006. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development 133:4073-4083. [DOI] [PubMed] [Google Scholar]