Abstract
alpha-4 is an essential gene and is a dominant antiapoptotic factor in various tissues that is a regulatory subunit for type 2A protein phosphatases. A multiplexed phosphorylation site screen revealed that knockdown of alpha-4 by small interfering RNA (siRNA) increased p38 mitogen-activated protein kinase (MAPK) and c-Jun phosphorylation without changes in JNK or ERK. FLAG-alpha-4 coprecipitated hemagglutinin-MEK3 plus endogenous protein phosphatase 2A (PP2A) and selectively enhanced dephosphorylation of Thr193, but not Ser189, in the activation loop of MEK3. Overexpression of alpha-4 suppressed p38 MAPK activation in response to tumor necrosis factor alpha (TNF-α). The alpha-4 dominant-negative domain (DND) (residues 220 to 340) associated with MEK3, but not PP2A, and its overexpression sensitized cells to activation of p38 MAPK by TNF-α and interleukin-1β, but not by ansiomycin or sorbitol. The response was diminished by nocodazole or by siRNA knockdown of the Opitz syndrome protein Mid1 that binds alpha-4 to microtubules. Interference by alpha-4 DND or alpha-4 siRNA increased caspase 3/7 activation in response to TNF-α. Growth of transformed cells in soft agar was enhanced by alpha-4 and suppressed by alpha-4 DND. The results show that alpha-4 targets PP2A activity to MEK3 to suppress p38 MAPK activation by cytokines, thereby inhibiting apoptosis and anoikis.
alpha-4 was originally cloned from a λgt11 library using a monoclonal antibody made against a phosphoprotein that associated with the immunoglobulin alpha (Igα) protein in the BCR complex of immunoglobulin M cross-linked and phorbol myristate acetate-stimulated B cells (23, 28). alpha-4 is the homolog of the yeast protein called Tap42, which in yeast binds the type 2A protein phosphatases Pph21/22 (PP2A), Pph3 (PP4), and Sit4 (PP6) (12). Tap42 functions in the TOR pathway in yeast to suppress phosphatase activity toward transcription factors Gln3 and Msn2 (5, 25, 44). alpha-4 has also been shown to bind different type 2A protein phosphatases (PP2A, PP4, and PP6) in mammals (7, 10, 24, 36-38, 42). We recently reported that alpha-4 exerts opposing kinetic effects on PP6 and PP2A (41). Kong et al. demonstrated that conditional knockout of alpha-4 in thymocytes, as well as in other cell types, results in apoptosis (27). Their conclusion was that alpha-4 acts as a nonredundant and dominant antiapoptotic factor in various tissues.
The p38 MAPK stress pathway has been implicated as a key regulator of apoptosis in cells stimulated with inflammatory cytokines (tumor necrosis factor alpha [TNF-α] or interleukin 1 [IL-1]) (1, 20, 51, 55). Programmed cell death, or apoptosis, can be engaged by either extrinsic or intrinsic pathways. The extrinsic pathway relies on activation of aspartyl proteases in response to inflammatory cytokines (TNF-α or IL-1) or other well-studied ligands, such as FasL. An initiator caspase in the inactive proform (e.g., procaspase 8) is bound via a death effector domain in an adapter protein associated with the C terminus of the receptor and is cleaved upon receptor activation, resulting in its activation (4, 45). This leads to activation of the downstream effector caspases 3 and 7 by proteolysis. The intrinsic pathway involves activation or inactivation of apoptogenic or proapoptogenic proteins involved in mitochondrially meditated apoptosis. These molecules consist of the Bcl-2 family members, functionally divided into two groups: the antiapoptotic Bcl-2 family members (Bcl-2 and Bcl-xL) and the proapoptotic Bcl-2 family members (Bad, Bim, and Bax). Regulation of Bcl-2 or Bcl-xL by p38 mitogen-activated protein kinase (MAPK) has been reported to cause their inactivation and to promote apoptosis (11, 17). Activation of Bad and BimEL by p38 MAPK has also been shown (6, 18). Upon activation of proapoptotic proteins and inactivation of antiapoptotic proteins, cytochrome c, apoptosis-inducing factor, and Smac/DIABLO are released from the mitochondria, resulting in activation of the Apaf complex (apoptosome) (4, 45). This promotes the activation of the initiator caspase 9, resulting in activation of the downstream effector caspases 3 and 7 and apoptosis (4, 45). Thus, assay of activated caspase 3/7 serves as a convenient readout of initiation of apoptosis by either pathway.
The MAPK family of proteins contains three kinases called ERK, p38 MAPK, and JNK. MAPKs are activated by upstream dual-specific serine/threonine and tyrosine kinases, referred to as MAPK/ERK kinases (MEKs), which are activated by MAPK kinase kinases (MKKKs) (8, 40, 43). The ERK pathway is activated by growth factors that promote cellular growth and proliferation (8, 40, 43). The p38 MAPK and JNK pathways are activated by cellular stress, such as inflammatory cytokines, hyperosmolality (sorbitol or NaCl), chemotherapeutics, chemicals (e.g., anisomycin), or UV irradiation (8, 40, 43). These MAPK pathways are negatively regulated by dephosphorylation by multiple phosphatases (14, 26, 34, 35, 50). Primarily, type 2A phosphatases catalyze the initial deactivation, whereas at later times following stimulation, dual-specificity MAPK phosphatases are induced and contribute to down-regulation of MAPK signals (14, 26, 35, 50). Evidence suggests that B regulatory subunits have a dominant role in targeting PP2A to ERK, especially B′ or B56 family members (29, 49). Regulatory-subunit specificity for JNK and p38 MAPK is not well understood.
To better understand the basis for alpha-4 action in apoptosis signaling, we sought to identify substrates of alpha-4-targeted phosphatases by depleting cells of alpha-4 using small interfering RNA (siRNA) and analyzing extracts with a multiplexed phosphorylation site screen offered by Kinexus, Inc. Knockdown of alpha-4 using siRNA selectively increased phosphorylation of p38 MAPK and c-Jun without changing ERK or JNK. We demonstrate that alpha-4 targets the p38 MAPK pathway by binding to the upstream kinase MEK3 and directing site-specific dephosphorylation of Thr193 in the activation loop. Overexpression of alpha-4 reduces cytokine activation of p38 MAPK and apoptosis and enhances cell growth in soft agar. The alpha-4 dominant-negative domain (DND) acts as an interfering protein to compete with endogenous alpha-4 to enhance cytokine activation of p38 MAPK and diminish cell growth in soft agar. The effects of alpha-4 depend on intact microtubules, as well as the alpha-4 binding partner Mid1, a protein mutated in the human disease Opitz syndrome. Thus, alpha-4 functions as a targeting and regulatory subunit for PP2A to oppose inflammatory-cytokine signaling to p38 MAPK by dephosphorylating and inactivating MEK3, thereby inhibiting apoptosis and anoikis.
MATERIALS AND METHODS
Reagents and antibodies.
TNF-α and IL-1β (R&D Systems) were dissolved in phosphate-buffered saline (PBS) at 20 μg/ml and used at the final concentrations noted. Anisomycin (Sigma) was diluted in dimethyl sulfoxide at 20 μg/ml, and sorbitol was dissolved in distilled H2O at 2 M. alpha-4 antibodies were made in rabbits against C-terminal peptide (WKDTHPRGYGNRQNMG) or against full-length His-tagged alpha-4 in chickens (Aves Labs, Inc.). Full-length recombinant PHI-1 was used to produce antibodies in rabbits (13). Antibodies against MEK3/6 (sc-13069) and p-MEK3/6 (sc-8407) (Santa Cruz Biotechnology), JNK (9252) and p-JNK (9251) (Cell Signaling), c-Jun (06-225) and p-c-Jun (06-659) (Upstate Biotechnology Inc.), p38 MAPK (AF8691) and p-p38 MAPK (AF869) (R&D Systems), Erk1/2 (M5670) and p-Erk1/2 (M8159) (Sigma), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (MAB374) (Chemicon) were used at the recommended dilutions for immunoblot analyses. The Mid1 antibodies used in this study were a kind gift from Timothy Cox, University of Washington. Blots were analyzed either by enhanced chemiluminescence (Pierce) or by the LI-COR Odyssey infrared scanner (LI-COR Biosciences) using secondary antibodies labeled with IRdye (680-nm or 800-nm VWR [Rockland]).
Cell culture.
HeLa, HEK293T, and COS7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 1× antibiotic/antimycotic. HEK293 cells were grown in modified Eagle's medium (MEM) supplemented with 10% FBS, 1× antibiotic/antimycotic, and 2 mM l-glutamine. Stable HEK293 cells were grown in MEM supplemented with 10% FBS, 2 mM l-glutamine, and 500 μg/ml Geneticin, and stable MDA-MB-231-luc cells were grown in MEM supplemented with 10% FBS, 2 mM l-glutamine, and 1,000 μg/ml Geneticin. Cell culture supplies were purchased from Invitrogen (Gibco-BRL). Cells were grown at 37°C in a humidified incubator with 5% CO2. Vectors were digested (∼30 μg of plasmid DNA) with NotI for 2 to 3 h at 37°C and were reacted with calf intestine alkaline phosphatase for 1 h at 37°C prior to phenol-chloroform extraction and precipitation with 1/10 volume of 3 M sodium acetate, pH 5.2, and 2 volumes 100% ethanol overnight. Precipitated DNA was dissolved and used to transfect HEK293 or MDA-MB-231-luc cells using the calcium phosphate method (46). The cells were incubated for 3 to 4 days prior to selection with Geneticin for 10 to 14 days, changing medium every 2 to 3 days. Individual clones were removed from the plates by using a cloning disc (Fisher), and 30 to 40 clones were selected for each construct transfected. Clones were selected based on immunoblotting for cells expressing equivalent or slightly greater amounts of FLAG alpha-4 proteins than endogenous alpha-4.
siRNA knockdown.
Either single-region siRNA 21-mers or SMART-POOLs were from Dharmacon. The sequences for control siRNAs used in this study are AAACCCACAGAGGCCUUCA and GUGGGAUGAAAUGAACAUC. The human alpha-4 (P78318) siRNA (678) targeting sequence is GGCAUCAACUUCUAACUCA. The Mid1 SMART-POOL contains four individual siRNA sense sequences specific for human Mid1 (NM_000318): CAGCAAAGACGACAGAUUAUU, GCUGAUAGCUGGAUGAUAGUU, GAACAAGUGUCUGACGAUUUU, and AGAAGAAACUGCUAGAAUGUU. For HeLa cells, six-well plates were seeded with 60,000 to 75,000 cells/well on day 1. On day 2, 160 pmol of siRNA was transfected into cells using Oligofectamine (Invitrogen) as described by the manufacturer's protocol. After 2 days, the cells were transfected with another round of siRNA (160 pmol) in fresh medium. Cells were harvested on day 5 by lysis in 1% sodium dodecyl sulfate (SDS) buffer (1% SDS, 20 mM MOPS [morpholinepropanesulfonic acid], pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1 mM Pefabloc, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 mM dithiothreitol [DTT]) or 2× SDS sample buffer directly. Lysates were boiled at 100°C for 5 min and then centrifuged at top speed for 10 min in a microcentrifuge. Extracts were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting. For HEK293 cells, six-well plates were seeded at 50,000 cells/well on day 1. On day 2, 160 pmol of siRNA was transfected using siIMPORTER (Upstate Biotechnology, Inc.) following the manufacturer's protocol. After 2 days, the cells were transfected with 160 pmol of siRNA in fresh medium. Cells were harvested by direct lysis in 2× SDS sample buffer, and whole-cell lysates were boiled prior to SDS-PAGE and immunoblot analysis.
Immunoprecipitation assays.
To test for binding between alpha-4 and MEK3, 100-mm dishes of 60 to 70% confluent COS7 cells were transfected with 5 to 10 μg DNA with Fugene6 reagent as described in the manufacturer's instructions. FLAG-alpha-4 constructs were made as previously described (30). Hemagglutinin (HA)-MEK3 was a kind gift from Thomas Sturgill (University of Virginia). Cells were transfected for 48 h prior to being harvested with 1% NP-40 buffer (20 mM MOPS, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM Pefabloc, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 μM microcystin-LR [Alexis Biochemicals], 1 mM sodium orthovanadate, and 1 mM DTT). After centrifugation at 13,000 rpm in a microcentrifuge for 20 min, ∼500 to 1,000 μg of extract protein was used for immunoprecipitation with 20 μl of a 50% suspension of α-FLAG (M2) beads (Sigma) for 2 h at 4°C. The immunoprecipitates were washed three times with 1% NP-40 buffer, followed by addition of 40 μl 2× SDS sample buffer. Samples were allowed to incubate at room temperature for 30 to 60 min, and the eluants were subjected to SDS-PAGE and immunoblot analysis.
To test for recruitment of endogenous PP2A to HA-MEK3 by alpha-4, HEK293 stable cell lines expressing FLAG-alpha-4 or stably transfected with empty vector were seeded at 40% confluence and later transfected with 3 μg of HA-MEK3 using the calcium phosphate method (46). The cells were incubated for 48 h, followed by washing in 1× PBS buffer two times and lysis using 1% NP-40 buffer. Extracts were prepared by centrifugation at 14,000 rpm for 15 min at 4°C and immunoprecipitated using 15 μl of a 50% slurry of anti-HA beads (Sigma-Aldrich). The immunoprecipitates were washed three times with NP-40 buffer, eluted with 40 μl of 2× SDS sample buffer, and subsequently boiled for 5 min. Samples were analyzed by immunoblotting with anti-PP2A and anti-HA antibodies.
Coimmunoprecipitation of FLAG-alpha-4 (DND) and endogenous Mid1 was tested using stable cell lines lysed in RIPA buffer (50 mM MOPS, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 20 mM β-glycerophosphate, 1 mM Pefabloc, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 μM microcystin-LR, 1 mM sodium orthovanadate, and 1 mM DTT). Extracts were immunoprecipitated using 5 μl α-FLAG (M2) agarose beads for 2 h at 4°C, followed by washing two or three times with RIPA buffer, and eluted using 40 μl of 2× SDS sample buffer at 100°C for 5 min. Samples were analyzed by SDS-PAGE and immunoblotting.
AP-1 luciferase assay.
HEK293 cells were seeded in 12-well dishes at a density of 25,000 cells/well the night before the first siRNA transfection. Transfection of HEK293 cells was done using siIMPORTER (Upstate-CHEMICON) following the manufacturer's protocol using 160 pmol of control or alpha-4 siRNA in the first transfection for 48 h at 37°C. The medium was changed 6 to 10 h prior to a second transfection using 160 pmol of siRNA plus 200 ng Luc or AP-1-Luc reporter vector and 20 ng pCMV-Renilla to normalize for transfection. The cells were incubated for 16 to 18 h prior to TNF-α stimulation using 20 ng/ml for 6 h. The cells were subsequently washed twice with PBS and frozen at −70°C for 1 h. The cells were lysed using 100 μl of lysis buffer from a Promega Luciferase assay kit (E1500) and allowed to incubate for 20 to 30 min before being assayed for luciferase. Firefly and Renilla luciferase substrates were made as directed by the manufacturer's protocol or as described previously (32). Samples were analyzed on a Berthold LB 953 luminometer. Luciferase activity was normalized as units of firefly luciferase activity relative to units of Renilla luciferase activity.
HA-MEK3 phosphorylation.
HEK293 cell lines expressing alpha-4 were transfected with HA-MEK3 using the calcium phosphate method. Cells were plated at ∼50% confluence in 100-mm dishes, transfected 18 h later with 5 to 10 μg of HA-MEK3, and allowed to incubate for 48 h before being harvested. The plates were washed twice with 1× PBS and then lysed in 1% NP-40 buffer. The lysates were clarified by centrifugation at 14,000 rpm for 15 min, further diluted 1:1 with 2× SDS sample buffer, and boiled for 5 min. Approximately 20 μg of protein was analyzed by immunoblotting with anti-(P-Ser189/P-Ser193) MEK3/(P-Ser207/P-Ser211) MEK6 (M5193; Sigma), anti-(P-Ser189) MEK3/(P-Ser207) MEK6, anti-HA (12CA5), anti-(P-Thr180/P-Tyr182) p38 MAPK, and anti-GAPDH.
HEK293T cells were cotransfected with HA-MEK3 and FLAG-PP2A, FLAG-PP2A (E42A), or FLAG empty vector using Arrest-In (Open BioSystems) following the manufacturer's protocol. The cells were plated at 40 to 50% confluence, transfected 16 h later using 5 μg of HA-MEK3 and 5 μg FLAG-PP2A (wild type [wt] or E42A) or empty vector, and allowed to incubate overnight at 37°C. The cells were harvested and lysed in 1% NP-40 buffer, and extracts were made by centrifugation at 14,000 rpm for 15 min at 4°C. Approximately 600 to 800 μg of extract was precipitated using anti-HA beads (Sigma) for 2 h at 4°C. The immunoprecipitates were washed three times with NP-40 buffer, eluted with 40 μl of 2× SDS sample buffer, and subsequently boiled for 5 min. Samples were analyzed by immunoblotting them with anti-(P-Ser189/P-Ser193) MEK3/(P-Ser207/P-Ser211) MEK6, anti-HA (12CA5), and anti-FLAG (Sigma).
Analysis of p38 MAPK activation to various stress stimuli.
Cells were treated with TNF-α, IL-1β, anisomycin, or sorbitol in a dose-dependent manner. For each stimulus, cell lines with empty vector or FLAG-alpha-4 (full length), (1-249), or (220-340) were seeded in 12-well plates at ∼30,000 cells/well 2 days prior to stimulus. The cells were incubated with a range of doses of TNF-α (0 to 40 ng/ml), IL-1β (0 to 50 ng/ml), anisomycin (0 to 40 ng/ml), or sorbitol (0 to 0.4 M) for 20 to 30 min prior to lysis with 2× SDS sample buffer. Samples were boiled for 5 to 10 min and analyzed by immunoblotting using either enhanced chemiluminescence or the LI-COR Odyssey system for final detection. UV treatment of stable cells was done with one dose (80 kJ/cm2), followed by 20 to 30 min of incubation prior to lysis. Chemical inhibition of p38 MAPK and JNK assays was done using 20 μM SB203580 or 20 μM SB600125, respectively, for 30 min prior to treatment with 20 ng/ml TNF-α. The lysates were analyzed by immunoblotting using anti-(P-S73) c-Jun antibody (Upstate) and anti-GAPDH. Immunoblots were quantitated using the LI-COR Odyssey system and software or by densitometry of X-ray film and analyzed with Image-Quant software.
Active caspase 3/7 detection.
Caspase 3/7 detection was done using Caspase-Glo 3/7 detection assay reagent (G8091; Promega). HeLa cells or stable cell lines were seeded into white-wall clear-bottom 96-well plates (Costar) at a density of 5,000 cells/well in triplicate wells. The HeLa cells were transfected with PHI-1, alpha-4, or Mid1 siRNA as described above using Oligofectamine on day 1 and day 3. On day 4, 20 ng/ml of TNF-α or anisomycin was added for 24 h prior to active caspase 3/7 detection by adding 100 μl of reagent to each well and incubating it at room temperature for 30 min to 3 h. Stable cell lines were grown for 48 h, after which the growth medium was replaced with serum-free (0.5% FBS-MEM-l-glutamine-Geneticin) with either TNF-α (20 ng/ml), anisomycin (20 ng/ml), or vehicle present and were allowed to incubate for either 24 h or 48 h before the caspase 3/7 assay was run as described above. The plate was sealed with white polyester sealing tape (Fisher) and read on a Veritas Luminometer Plate Reader (Turner Biosystems) using a standard protocol. Data were analyzed in Microsoft Excel and plotted after the background was corrected.
Soft-agar assays.
HEK293 cells stably transfected with empty vector or expressing FLAG-alpha-4 or FLAG-alpha-4 (220-340) (DND) were seeded in soft agar using six-well plates prepared as described in the manufacturer's protocol (ECM570; Chemicon). The medium was changed every 2 to 3 days for 28 to 30 days prior to staining for analysis by microscopy. Images were acquired using a Zeiss Axiovert 135 microscope and processed using OpenLab software (Improvision). For these and other assays, data were imported into Microsoft Excel for statistical analysis and graphing by SigmaPlot 9.0 (Systat Software) using Students t test (one tailed), assuming unequal variances, with a P value of <0.05 considered significant.
RESULTS
Protein phosphorylation in response to alpha-4 knockdown by siRNA.
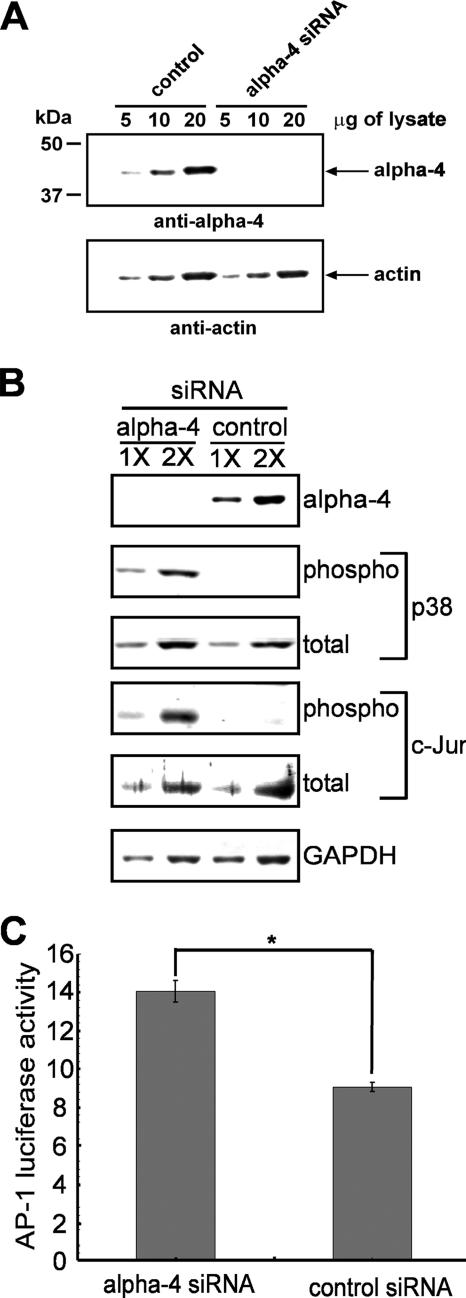
We compared the phosphorylation of proteins from cells with alpha-4 knocked down by siRNA versus controls, using a multiplexed analysis of 27 different phosphorylation sites in signal transduction proteins (Kinetworks PhosphoSite Screen 1.3) (see Table S1 in the supplemental material). Equivalent amounts of total protein from HeLa cells treated with control siRNA or alpha-4 siRNA were immunoblotted to demonstrate that alpha-4 protein was depleted >80% compared to the control, based on twofold dilutions of the samples (Fig. 1A). Actin was immunoblotted as a loading control for equivalent amounts of protein in the analyses. Knockdown of alpha-4 increased the phosphorylation of MEK3/6 (lane 7), c-Jun (lane 11), Akt/protein kinase B (lanes 13 and 14), GSK3α (lanes 15 and 17), p38 MAPKα (lane 18), CREB (lane 20), and Rb (lane 20) (see Fig. S1A and B in the supplemental material). Phosphorylation sites increased by depletion of alpha-4 are potential substrates of alpha-4-bound phosphatases (see Fig. S2A to D in the supplemental material). On the other hand, knockdown of alpha-4 decreased phosphorylation of STAT3 (lane 10), PKR (lane 16), and MEK1/2 (lane 19) (see Fig. S1A and B in the supplemental material). Decreased phosphorylation of proteins implies that alpha-4 restricts phosphatase activity toward these phosphorylation sites, showing that alpha-4 can either increase or decrease phosphatase reactivity with different substrates. As a further demonstration of specificity, no apparent changes in the phosphorylation of PKCα (lanes 5 and 7), JNK (lane 6), PKCβ (lane 7), PKCɛ (lane 9), Raf1 (lane 12), and PKCδ (lane 13) were observed in this screen. Phosphorylation sites unaffected by depletion of alpha-4 presumably are not substrates and are not in pathways affected by alpha-4. We interpreted these results as evidence that alpha-4 regulates phosphatase activity toward specific substrates, consistent with its acting as a targeting subunit for type 2A phosphatases.
FIG. 1.
Enhanced phosphorylation of p38 MAPK and c-Jun by siRNA knockdown of alpha-4. HeLa cells were transfected with control siRNA or alpha-4 siRNA, and extracts were analyzed by immunoblotting. (A) Knockdown of alpha-4 by siRNA. Either 5, 10, or 20 mg of extract protein from cells treated with control siRNA or alpha-4 siRNA were immunoblotted for alpha-4 (top) and actin (bottom) as a loading control. Knockdown of alpha-4 resulted in a reduced yield of total protein in cell extracts compared to the control (0.75 mg/ml versus 1.1 mg/ml, respectively), consistent with the loss of cells due to depletion of alpha-4. (B) Extracts with either 1× or 2× volume were immunoblotted for levels of alpha-4 and for phosphorylation of p38 MAPK and c-Jun versus the total amounts of these proteins, with GAPDH as an additional loading control. (C) Luciferase activity expressed from an AP-1 promoter in HeLa cells treated with control siRNA or alpha-4 siRNA (n = 3); *, P < 0.05 by Student's t test. The error bars indicate standard deviation.
Effects of alpha-4 knockdown on p38 MAPK, JNK, and ERK.
We analyzed in parallel both the phosphorylation sites and the levels of the individual proteins to validate the results of the Kinexus screen for different MAPK pathways. Cells depleted of alpha-4 showed a threefold increase in dual phosphorylated p38 MAPK (T180/Y182) relative to controls, while the levels of p38 MAPK protein were the same (Fig. 1B). Furthermore, phosphorylation of S73 in c-Jun was increased in the same amount of c-Jun protein in cells depleted of alpha-4 as in the control (Fig. 1B). Transfection with a control siRNA effectively depleted its cognate target, PHI-1 (not shown), without increasing the phosphorylation of p38 MAPK or c-Jun, demonstrating that the siRNA effects were specific for alpha-4. In contrast to a threefold increase in phosphorylation of p38 MAPK and c-Jun, siRNA knockdown of alpha-4 did not increase the ratio of phospho- to total MEK3/6, JNK1/2, or ERK1/2 (see Table S2 in the supplemental material), demonstrating the considerable specificity of the effects of alpha-4 among different MAPKs. Our conclusion was that depletion of alpha-4 by siRNA did not affect the phosphorylation of sites in MEK3/6, JNK1/2, or ERK1/2; therefore, alpha-4 selectively targets regulation of p38 MAPK.
Depletion of alpha-4 by siRNA resulted in increased c-Jun phosphorylation and enhanced its activity as a transcription factor. We used an AP-1-Luc/Renilla dual reporter assay to quantitate c-Jun activation in transfected cells. Knockdown of alpha-4 with siRNA resulted in a 1.5-fold increase in AP-1 activation compared to cells treated with control siRNA (Fig. 1C). This significant increase in AP-1 activity reflects the increase in c-Jun S73 phosphorylation. We concluded that knockdown of alpha-4 by siRNA resulted in c-Jun phosphorylation and AP-1 activation.
Selective dephosphorylation of MEK3 by alpha-4::PP2A.
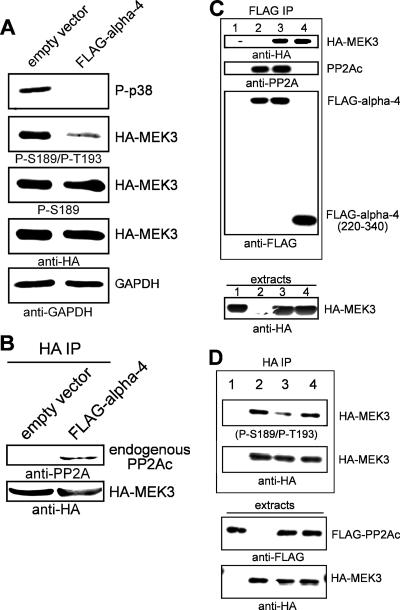
We tested whether alpha-4 targets type 2A phosphatase to inactivate either p38 MAPK directly or its upstream activator, MEK3/6. Phosphorylation site screening showed more than a fourfold increase in p38 MAPK phosphorylation and a twofold increase in MEK3/6 phosphorylation relative to the control, normalized to levels of actin. Our further analyses showed a threefold increase in the ratio of phosphorylated p38 MAPK to total p38 MAPK protein but no increase in the ratio of phosphorylated MEK3/6 (S189/S207) to total MEK3/6 protein in a comparison of cells depleted of alpha-4 by siRNA to cells treated with control siRNA. The differences between the phosphorylation site screening and our own analyses could be due to at least two factors: (i) use of actin as a loading control compared to calculating the phospho-/total protein ratio for p38 MAPK and MEK3/6 and (ii) different methods of detection and quantitation. The commercial phosphorylation site screen uses chemiluminescence for detection, whereas our own immunoblotting uses fluorescence-conjugated secondary antibodies and an infrared scanner (Odyssey; LICOR Industries), which provides a better range of linear response and two-color simultaneous scanning. Furthermore, we realized that the phosphorylation site antibodies were directed toward only a single phosphorylation site, i.e., Ser189 in MEK3 (Ser207 in MEK6), and did not include a second nearby phosphorylation site in both MEK proteins (Thr193 in MEK3 and Thr211 in MEK6). Dual phosphorylation at these neighboring sites is required for MEK3/6 activation (52). Cell lines stably overexpressing FLAG-alpha-4 or empty vector (as a control) were transiently transfected with HA-MEK3, and extracts were analyzed by immunoblotting them with (i) anti-(P-Thr180/P-Tyr182) p38 MAPK, (ii) dual-phosphorylation site anti-(P-Ser189/P-Thr193) MEK3, (iii) single-phosphorylation site anti-(P-Ser189) MEK3, (iv) anti-HA, and (v) anti-GAPDH as a loading control (Fig. 2A). There was little difference in the phosphorylation of Ser189 in HA-MEK3 expressed in control versus alpha-4 cells but a >2-fold decrease in MEK3 dual phosphorylation (P-Ser189/P-Thr193) (Fig. 2A). The relative dephosphorylation of HA-MEK3 in cells overexpressing alpha-4 was reflected in the diminished activation of endogenous p38 MAPK. The enhanced dephosphorylation of MEK3 was due to recruitment of endogenous PP2Ac by alpha-4. HA-MEK3 coprecipitated endogenous PP2Ac in cells stably overexpressing FLAG-alpha-4, but not in control cells stably expressing empty vector (Fig. 2B). This result shows that alpha-4 promotes association between MEK3 and PP2A.
FIG. 2.
Site-specific dephosphorylation of MEK3 by alpha-4::PP2A. (A) HEK293 cells stably expressing empty vector or FLAG-alpha-4 were transiently transfected with HA-MEK3. Immunoblotting of extracts (top to bottom) used dual-site anti-(P-Thr180/P-Tyr182) p38 MAPK, dual-site anti-(P-Ser189/P-Thr193) MEK3 antibody, single-site anti-(P-Ser189) MEK3 antibody, anti-HA to show MEK3 levels, and GAPDH as an extra loading control. (B) HEK293 cells stably expressing empty vector or FLAG-alpha-4 were transiently transfected with HA-MEK3, and anti-HA immunoprecipitates (IP) were analyzed by immunoblotting. HA-MEK3 coprecipitates endogenous PP2Ac in cells expressing FLAG-alpha-4 (right lane), but not in cells with empty vector (left lane). (C) FLAG-alpha-4 and HA-MEK3 were expressed in COS7 cells, and anti-FLAG precipitates were analyzed by immunoblotting (top). FLAG-alpha-4 coprecipitates endogenous PP2A in cells not expressing HA-MEK3 (lane 2). FLAG-alpha-4 coprecipitates HA-MEK3, as well as endogenous PP2A (lane 3). FLAG-alpha-4 (220-340) coprecipitated HA-MEK3, but not PP2A (lane 4). (D) FLAG-PP2A (wt or E42A) and HA-MEK3 were coexpressed in HEK293T cells, and anti-HA precipitates were analyzed by immunoblotting (top). Dual phosphorylation (S189/T193) was reduced when HA-MEK3 was coexpressed with wt FLAG-PP2A (lane 3), but not with FLAG-PP2A (E42A is a point mutant that does not bind alpha-4) (lane 4) (42).
We tested for stable association of alpha-4 with MEK3. Both FLAG-alpha-4 and FLAG-alpha-4 C-terminal domain (220 to 340) coprecipitated HA-MEK3 (Fig. 2C, lanes 3 and 4). On the other hand, FLAG-alpha-4, but not FLAG-alpha-4 C-terminal domain, coprecipitated endogenous PP2A, as previously shown (10, 30, 42) (Fig. 2C, lanes 3 and 4). The results demonstrate stable association between the C-terminal domain of alpha-4 and MEK3 and are consistent with our previous report that the C-terminal domain (220 to 340) of alpha-4 binds substrates, but not PP2A (30). One would expect that residues 220 to 340 of alpha-4 would act as a DND protecting MEK3 from inactivation by PP2A.
In separate experiments, HA-MEK3 was coexpressed with either FLAG-PP2A or FLAG-PP2A (E42A), a point mutant of PP2A that does not bind alpha-4, or FLAG empty vector as a control. HA-MEK3 was recovered by immunoprecipitation, and phosphorylation was assayed by phosphorylation site immunoblotting. Expression of wt FLAG-PP2A reduced dual phosphorylation of HA-MEK3, whereas the E42A mutant of PP2A and control did not (Fig. 2D, lanes 3 and 4). Our conclusion is that the association of PP2A with alpha-4 is required for dephosphorylation of HA-MEK3. The FLAG-tagged PP2A-Aα subunit showed binding to endogenous PP2A, but not to HA-MEK3 (data not shown). Anti-FLAG complexes were reprobed for endogenous p38 MAPK, but no coprecipitation was observed (data not shown). We concluded that PP2A and MEK3 bind to separate domains of alpha-4 to promote PP2A dephosphorylation of Thr193 in MEK3.
Cytokine signaling to p38 MAPK regulated by alpha-4.
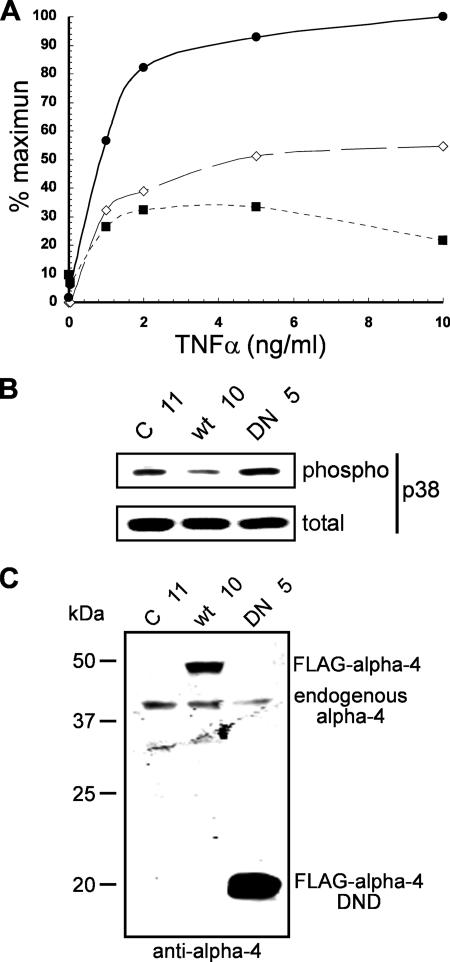
Inflammatory cytokines, such as TNF-α and IL-1β, signal through MEK3/6 kinases to p38 MAPK, whereas other forms of cellular stress activate p38 MAPK even in cells from MEK3−/− knockout animals (21, 22, 47, 53). We made cell lines stably expressing (i) full-length FLAG-tagged murine alpha-4; (ii) FLAG-alpha-4 (220-340), the DND; or (iii) empty vector. These cells were tested for responses to TNF-α (0 to 10 ng/ml) by immunoblotting extracts for P-p38 MAPK (Thr180/Tyr182). Cells stably expressing FLAG-alpha-4 DND (clone DN 5) exhibited enhanced phosphorylation of p38 MAPK at all concentrations of TNF-α (Fig. 3A). There was no change in TNF-α dose dependence compared to control cells (clone C 11) (Fig. 3A). Cells expressing FLAG-alpha-4 (clone wt 10) suppressed activation of p38 MAPK in response to TNF-α (Fig. 3A). Extracts were immunoblotted with anti-phospho-(Thr180/Tyr182) p38 MAPK and anti-p38 MAPK protein to show the relative differences between stable cells lines C 11, wt 10, and DN 5 treated with 10 ng/ml TNF-α (Fig. 3B). Expression of FLAG-alpha-4 and the FLAG-alpha-4 DND relative to endogenous levels of alpha-4 were analyzed using anti-alpha-4 antibodies that reacted with both ectopic and endogenous proteins (Fig. 3C). These results support the hypothesis that alpha-4 regulates TNF-α stimulation of p38 MAPK by acting as a targeting subunit for PP2A and that the alpha-4 DND interferes with targeting by endogenous alpha-4.
FIG. 3.
Effects of alpha-4 on TNF-α activation of p38 MAPK. (A) HEK293 cells stably expressing FLAG-alpha-4, FLAG-alpha-4 DND, or empty vector as a control were treated with various doses of TNF-α for 20 min, and extracts were subjected to immunoblotting. The dose response for TNF-α (ng/ml) versus percent activation of p38 MAPK was measured by the ratio of anti-phospho-(Thr180/Tyr182) p38 MAPK and anti-p38 MAPK proteins. Cells with FLAG-alpha-4 DND (DN 5) are represented by solid circles, cells with FLAG-alpha-4 (wt 10) by solid squares, and cells with empty vector (C 11) by open diamonds. (B) The immunostaining of phospho-p38 MAPK and p38 MAPK proteins in extracts from C 11, wt 10, and DN 5 cells at the maximum dose of TNF-α (10 ng/ml). (C) Immunoblot of extracts from stably transfected cells using anti-alpha-4 to compare the levels of ectopic FLAG-tagged alpha-4 proteins to the endogenous alpha-4.
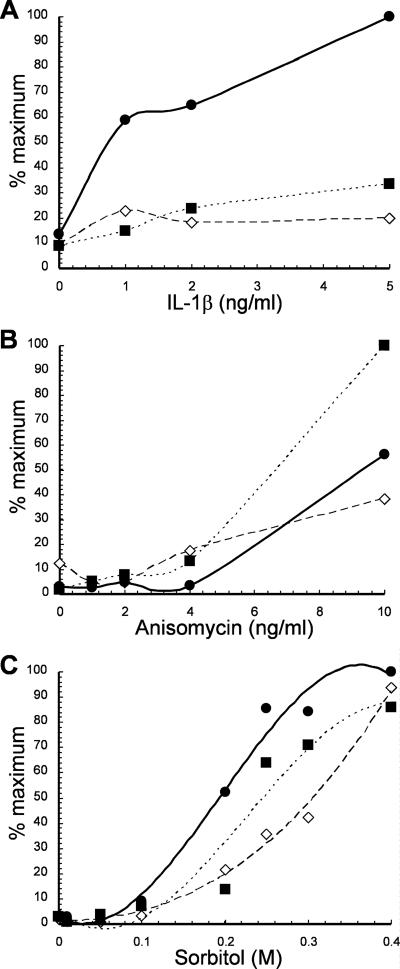
We used these stable cell lines to examine p38 MAPK activation in response to various doses of IL-1β (0 to 5 ng/ml), anisomycin (0 to 10 ng/ml), sorbitol (0 to 0.4 M), or UV (80 kJ/cm2). Cells expressing either FLAG-alpha-4 (Fig. 4A to C) or cells transfected with empty vector (Fig. 4A) responded to IL-1β and sorbitol about the same. At the highest dose of anisomycin, there was a difference between the control and full-length FLAG-alpha-4. Cells expressing FLAG-alpha-4 DND (DN 5) showed enhanced activation of p38 MAPK in response to IL-1β over the entire range of concentrations tested (Fig. 4A), a response similar to that with TNF-α (Fig. 3A). In contrast, the response of cells expressing FLAG-alpha-4 DND (DN 5) to sorbitol, anisomycin, or UV irradiation was not significantly different from that of the control cells (Fig. 4B and C and data not shown). The enhancement of p38 MAPK activation in response to inflammatory cytokines was replicated in multiple independent cell lines expressing FLAG-alpha-4 DND (see Fig. S3A in the supplemental material). There was on average a twofold increase in phospho-p38 MAPK in response to TNF-α in cell lines expressing the FLAG-alpha-4 DND protein compared to control cells. Cell lines expressing the FLAG-alpha-4 DND protein showed enhanced phosphorylation of S73 in c-Jun in response to TNF-α (see Fig. S3B, lane 2, in the supplemental material). c-Jun S73 phosphorylation was reduced by the addition of SB203580, a p38 MAPK inhibitor, and SB600125, a chemical inhibitor of JNK, linking both kinases to c-Jun activation (see Fig. S3B, lanes 3 and 4, in the supplemental material). In cell lines expressing the FLAG-alpha-4 DND protein, there was on average a >4-fold increase in phospho-p38 MAPK in response to IL-1β compared to control cells. The difference in responses to TNF-α versus IL-1β may depend on the level of the receptors or other signaling components. The results demonstrate that overexpression of the alpha-4 DND enhances activation of p38 MAPK by inflammatory cytokines, presumably by competitively interfering with the dephosphorylation and inactivation of MEK3.
FIG. 4.
Activation of p38 MAPK by various stress stimuli. HEK293 cells expressing empty vector or wt or dominant-negative alpha-4 protein were subjected to different stress stimuli (IL-1β, anisomycin, or sorbitol). Dose-response curves depict activation of p38 MAPK as measured by the ratio of immunoblotting for anti-phospho-(Thr180/Tyr182) p38 MAPK versus anti-p38 MAPK protein. (A) Cells treated with 0 to 5 ng/ml IL-1β for 20 min. (B) Cells treated with 0 to 10 ng/ml anisomycin for 20 min. (C) Cells treated with 0 to 0.4 M sorbitol for 20 min. Cells with FLAG-alpha-4 DND are represented by solid circles, cells with FLAG-alpha-4 wt by solid squares, and cells with empty vector by open diamonds.
Microtubule requirements of p38 MAPK activation.
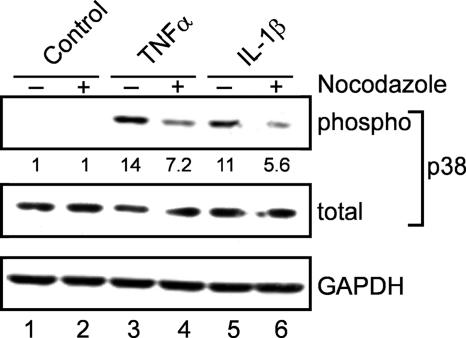
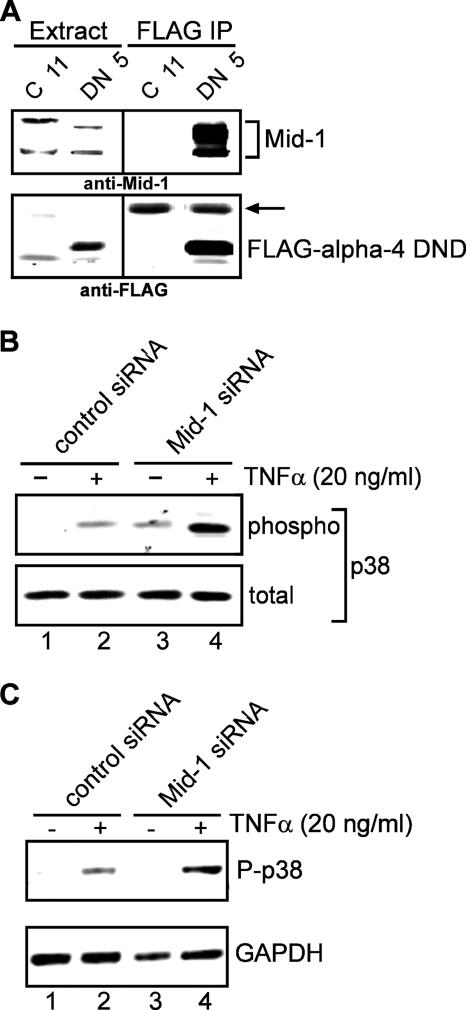
Microtubule integrity is required for cytokine signaling to p38 MAPK via the MEK3/6-dependent pathway, and p150glued binds MEK3/6 to promote its activation (9). The alpha-4 DND-enhancement of p38 MAPK activation in response to inflammatory cytokines (Fig. 3A and 4A) was decreased by about one-half when cells were transiently exposed to nocodazole to disrupt microtubules (Fig. 5) prior to TNF-α or IL-1β stimulation. This result confirms that intact microtubules are required for cytokine activation pf p38 MAPK. We previously found that alpha-4 associates with a microtubule-associated protein called Midline1 (Mid1) (30), and indeed, endogenous Mid1 was concentrated in a coprecipitation assay with FLAG-alpha-4 DND (Fig. 6A, DN 5). Knockdown of Mid1 in HeLa cells by siRNA increased basal phospho-p38 MAPK levels compared to controls (Fig. 6B). Furthermore, siRNA depletion of Mid1 in HeLa (Fig. 6B) and HEK293 (Fig. 6C) cells stimulated with TNF-α produced a two- to threefold increase in p38 MAPK phosphorylation compared to controls. The results demonstrate that Mid1 contributes to the negative control of basal and cytokine-induced p38 MAPK activation. Thus, TNF-α signaling to p38 MAPK depends on intact microtubules and is reduced by Mid1-dependent mechanisms. Our hypothesis is that Mid1 binds and localizes alpha-4 to microtubules and that this contributes to targeting of PP2A to inactivate MEK3.
FIG. 5.
Microtubules are required for enhanced cytokine stimulation of p38 MAPK. HEK293 cells expressing FLAG-alpha-4 DND (clone DN 5) were treated with 1 μg/ml nocodazole for 4 h prior to stimulation with 20 ng/ml TNF-α, 20 ng/ml IL-1β, or vehicle as a control. Extracts were immunoblotted with anti-phospho-(Thr180/Tyr182) p38 MAPK, anti-p38 MAPK protein, and anti-GAPDH as an additional loading control. The ratio of phospho-p38 MAPK to total p38 MAPK was quantitated by fluorescent scanning, and the values were normalized to controls, shown as numbers below the top blot.
FIG. 6.
Mid1 protein in activation of p38 MAPK by TNF-α. HEK293 cells stably expressing FLAG-alpha-4 DND (DN 5) or empty vector (C 11) were used to demonstrate association of Mid1. (A) FLAG-alpha-4 DND (DN 5) coprecipitates endogenous Mid1 and concentrates it from the extract of DN 5 cells. The arrow indicates the light chain of anti-FLAG (M2) serving as another loading control. Samples labeled extract contain 2% of the extract volume used for immunoprecipitation. Extracts and anti-FLAG precipitates were immunoblotted with anti-Mid1 and anti-FLAG antibodies. Either HeLa (B) or HEK293 (C) cells were transfected with control siRNA or siRNA against Mid1 and stimulated with 20 ng/ml TNF-α. Extracts were immunoblotted using anti-phospho-(Thr180/Tyr182) p38 MAPK, with either anti-p38 MAPK protein or GAPDH as a control.
Apoptosis enhanced by interference with alpha-4.
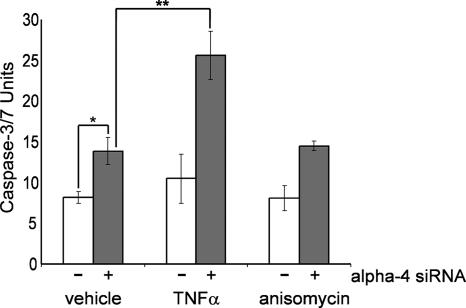
We observed that knockdown of alpha-4 by siRNA yielded less total cell mass (see the legend to Fig. 1), probably due to apoptosis. HeLa cells were seeded in 96-well plates in medium containing 0.5% FBS, transfected with control or alpha-4 siRNA for 72 h, and treated with vehicle or TNF-α for 48 h before being assayed for caspase 3/7 as a reporter of apoptosis. Control knockdown cells (Fig. 7) showed little increase in caspase 3/7 activity in response to TNF-α or anisomycin. However, knockdown of alpha-4 by siRNA significantly increased the activation of caspase 3/7 in cells treated with vehicle or anisomcin (Fig. 7). These cells were especially sensitive to TNF-α, which nearly doubled caspase 3/7 activation compared to controls. Thus, depleting alpha-4 by siRNA knockdown enhanced the activation of caspase 3/7 in response to TNF-α.
FIG. 7.
Knockdown of alpha-4 enhances caspase 3/7 activation in response to TNF-α. HeLa cells were transfected with control siRNA or alpha-4 siRNA and treated with 40 ng/ml TNF-α, 40 ng/ml anisomycin, or vehicle for 48 h, and the cells were stained for activated caspase 3/7 using Promega Caspase-Glo 3/7. The open bars represent control siRNA, and the gray bars represent alpha-4 siRNA; *, P < 0.01, and **, P < 0.005 by Student's t test (n = 3). The error bars represent standard deviations.
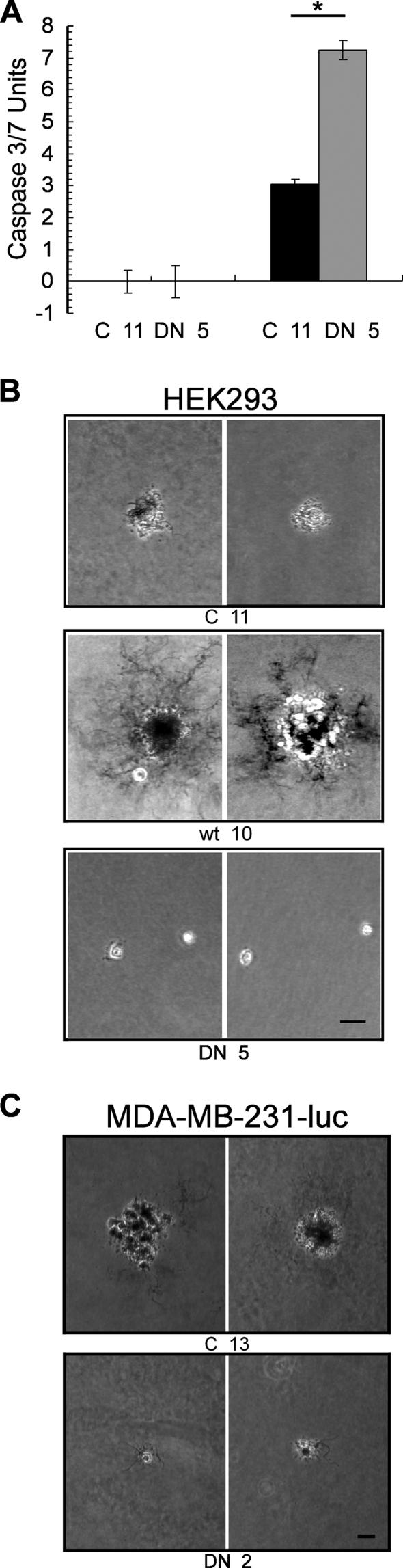
The effects of alpha-4 knockdown were mimicked by the expression of the FLAG-alpha-4 DND. A stable cell line, FLAG-alpha-4 DND (DN 5), was compared to a control HEK293 cell line stably transfected with empty vector (C 11). The cells were treated with vehicle or TNF-α for 48 h before being assayed for caspase 3/7. Whereas vehicle did not cause activation of caspase 3/7, addition of TNF-α to DN 5 cells caused a significant increase (P < 0.01) in caspase 3/7 activity compared to control C 11 cells treated with TNF-α (Fig. 8A). We concluded that interference with alpha-4 function by overexpression of alpha-4 DND or by siRNA knockdown enhanced TNF-α induction of apoptosis. The results demonstrate that alpha-4 DND acts as a proapoptotic factor, increasing apoptosis in response to TNF-α.
FIG. 8.
Dominant-negative alpha-4 enhances caspase 3/7 activation in response to TNF-α and suppresses growth in soft agar. (A) HEK293 cells expressing FLAG-alpha-4 DND (DN 5; gray bar) or empty vector (C 11; black bar) were treated with 40 ng/ml TNF-α or vehicle and assayed for activation of caspase 3/7 (*, P < 0.01 by Student's t test; n = 3). The error bars represent standard deviation. (B) HEK293 cells expressing FLAG-alpha-4, FLAG-alpha-4 DND, or empty vector were grown in soft agar, stained, and photographed under phase illumination (the bar indicates a length of 70 μm). (C) MDA-MB-231-luc cells expressing FLAG-alpha-4 DND (DN 2) or empty vector (C 13) were growth in soft agar and stained as previously described.
alpha-4 and growth in soft agar.
We assayed for anchorage-independent growth of HEK293 cells stably expressing either FLAG-alpha-4, FLAG-alpha-4 DND, or empty vector. Cells were seeded in soft agar at the same density of 5,000 cells per well in a six-well plate. After 30 days, the cultures were stained and photographed by phase-contrast microscopy at 10× magnification (Fig. 8B). Cells transfected with empty vector grew into colonies with an average diameter of 104 μm. Cells expressing FLAG-alpha-4 grew into significantly larger colonies (mean, 137 μm; P < 0.002) that were surrounded by dendritic structures that extended in all directions to a distance that was approximately the same as the diameter of the colony itself. These cultures of FLAG-alpha-4 cells exhibited threefold-higher quantitative staining (Chemicon Stain no. 90318) than controls. In contrast, few cells expressing FLAG-alpha-4 DND survived, and only small colonies (mean, 56 μm; P < 0.001) were observed. Furthermore, in separate experiments, stable overexpression of FLAG-alpha-4 DND in the breast cancer cell line MDA-MB-231-luc greatly decreased colony size (mean, 68 μm; P < 0.001) in a soft-agar assay compared to the control (mean, 150 μm) (Fig. 8C). Thus, expression of alpha-4 yielded more, larger, and qualitatively different colonies than control cells, whereas expression of FLAG-alpha-4 DND caused nearly complete loss of cell growth in soft agar.
DISCUSSION
MEK3 and p38 MAPK regulation by alpha-4.
Our results demonstrate that the protein alpha-4 is a phosphatase regulatory subunit responsible for negative regulation of inflammatory cytokine signaling to p38 MAPK. Genetic knockout of alpha-4 is embryonic lethal in animals, and apoptosis occurs upon expression of Cre in cells derived from various tissues of animals with a Floxed alpha-4 gene (27). Kong et al. concluded that alpha-4 plays a nonredundant and essential role in opposing apoptosis in all cell types (27). By screening for changes in phosphorylation due to knockdown of alpha-4, we identified MEK3-p38 MAPK as a target for phosphatases with an alpha-4 subunit. alpha-4 controls this pathway by physical association with MEK3 to promote specific PP2A dephosphorylation of one of two sites in the activation loop of MEK3. The alpha-4 protein uses a C-terminal region (residues 220 to 340), called here the DND, to associate with MEK3. The alpha-4 DND is separate from the region of alpha-4 (residues 1 to 220) that binds type 2A protein phosphatases (PP2A, PP4, and PP6) (24, 30). This allowed us to overexpress the alpha-4 DND to interfere with the action of endogenous alpha-4 phosphatase complexes. Either overexpression of alpha-4 DND or siRNA knockdown of endogenous alpha-4 enhanced p38 MAPK phosphorylation in response to stimulation with the inflammatory cytokine TNF-α or IL-1β, but not in response to anisomycin, sorbitol, or UV. Signaling to p38 MAPK from other cell stresses is known to involve separate MEK and MEKK, and these apparently are not under the control of alpha-4-mediated dephosphorylation. The assembly of complexes with kinases and phosphatases is now a well-accepted theme for achieving biological specificity in responses to diverse stimuli. We predict that separate MEKs and MEKKs will be under the control of different phosphatase complexes. This organization adds specificity of signaling and affords individual routes for feedback and cross talk regulation.
Microtubules, Mid1, MEK3, and p38 MAPK.
Activation of MEK3 by cytokines requires association with the p150glued subunit of the dyenin complex and depends on intact microtubules (9). Cheung et al. found that depletion of p150glued from cells by siRNA decreased MEK3/6 and p38 MAPK activation in response to stress stimulation. Overexpression of the p50 dynamitin protein, known to bind to dyenin and to disrupt dyenin complexes, decreased stress-induced MEK3/6 activation and p38 MAPK phosphorylation (9). Furthermore, these authors showed that microtubules are required for MEK3/6 activation of p38 MAPK by pretreating cells with nocodazole, taxol, or colchicin prior to cytokine stimulation. We reported previously that the alpha-4 DND associates with microtubules by binding to Mid1 (30). We showed here concentration of Mid1 from extracts by alpha-4 DND, and we suggest this interaction may be the basis for the dominant-negative effect. Mid1 association with microtubules requires phosphorylation by MAPK and is sensitive to inhibition of MEK1/2 activity (30). This provides one pathway for MEK/ERK to reduce activation of p38 MAPK by recruitment of alpha-4 with MEK3 onto microtubules. alpha-4 also targets PP2A to dephosphorylate Mid1 and promotes its release from microtubules (30). This release would limit the negative control of MEK3.
We demonstrated that depletion of Mid1 from cells by siRNA increased basal p38 MAPK phosphorylation and enhanced activation by TNF-α, consistent with Mid1-dependent targeting of alpha-4 phosphatase. Pretreatment of cells expressing alpha-4 DND with nocodazole decreased inflammatory-cytokine activation of p38 MAPK. We propose that alpha-4 binds Mid1 for association with microtubules to position PP2A in proximity to the MEK3-p38 MAPK that is tethered to microtubules by p150glued. Furthermore, Mid1 is a BB-RING finger protein with E3 ubiquitin ligase activity (31, 48). The catalytic subunit of PP2A has been reported as a substrate (31, 48), but other proteins in this signaling complex, such as p150glued, dyenin, MEK3, or MEKKs, may be substrates for Mid1-mediated ubiquitination. The alpha-4 protein is a nexus for multiple signaling pathways.
Apoptosis and alpha-4.
Activation of p38 MAPK by inflammatory cytokines induces apoptosis in certain cell types (3, 17, 18, 55). The p38 MAPK induces cell death by activation of caspases 8, 9, and 3 and release of cytochrome c from mitochondria, and chemical inhibition of p38 MAPK with SB203580 blocks caspase activation, resulting in accumulation of the procaspases, and prevents release of cytochrome c (11, 17, 18, 39). Furthermore, p38 MAPK phosphorylates several anti-apoptotic (Bcl-2 and Bcl-xL) and proapoptotic (Bad and BimEL) proteins, thereby regulating their functions (6, 11, 17, 18). For example, the anti-apoptotic factor Bcl-xL is associated with mitochondria, and phosphorylation by p38 MAPK promotes its degradation, resulting in enhanced apoptosis (17). Apoptosis in response to genetic knockout of alpha-4 probably involves mitochondria, because apoptosis was prevented by overexpression of Bcl-xL (27). We observed enhanced caspase 3/7 activation in response to TNF-α stimulation in cells expressing alpha-4 DND or knocked down for alpha-4 by siRNA. This involved the release of cytochrome c from mitochondria (data not shown). However, p38 MAPK activation alone was not sufficient to trigger apoptosis, because we observed that anisomycin activated p38 MAPK without caspase 3/7 activation. We imagine that cytokine receptors produce multiple signals upstream of MEK3 that are required, together with activation of p38 MAPK, to produce apoptosis.
Anoikis and alpha-4.
Growth in soft agar is an in vitro assay for tumorigenesis that tests for the ability of transformed cells to avoid anoikis, which is induction of apoptosis in the absence of attachment to extracellular matrix (ECM) (15, 33). The lack of ECM engagement induces stress kinase pathways (p38 MAPK or JNK) in certain cell types, leading to anoikis (15). Anoikis can be suppressed either by integrin binding ECM or by overexpression of Bcl-2 antiapoptotic family members (15). Overexpression of alpha-4 in HEK293 cells greatly enhanced colony size in soft agar, and we propose that this was due to suppression of anoikis. The antiapoptotic function of alpha-4 was proposed to be p53 dependent, based on transcriptional responses in genetic knockouts versus controls (27). However, alpha-4 function appears to be p53 independent in HEK293 cells that are transformed by adenovirus and that express the E1A protein that binds p53 and inhibits its function (16). We also tested the human breast cancer cell line MDA-MB-231-luc, which has mutated p53 and increased expression of survivin, an inhibitor of apoptosis (2, 19, 54). Because alpha-4 acted in cell lines without functional p53, we propose that not all alpha-4 actions are p53 dependent. In addition, cells overexpressing alpha-4 grew into colonies that had unusual branched extensions, suggesting that alpha-4 modified cell morphology, perhaps through changes in the cytoskeleton. In contrast, expression of alpha-4 DND in HEK293 cells or in MDA-MB-231-luc cells gave fewer, smaller colonies relative to the control, consistent with enhanced anoikis. Thus, either alpha-4 DND protein or siRNA interference with the action of endogenous alpha-4 abrogates its antiapoptotic function by blocking the dephosphorylation of targeted substrates. Interfering with alpha-4 function offers a new way to induce apoptosis of transformed cells and may be a worthwhile approach to enhance cancer chemotherapy.
Supplementary Material
Acknowledgments
We thank Thomas Sturgill and Carol Chrestensen (Pharmacology Department, University of Virginia) for MEK plasmids and Timothy Cox (University of Washington) for Mid1 antibody. We also thank Jason Kirkbride, Allison Kruszewski, and Abbi Daugherty for technical assistance.
This work was supported by a USPHS National Cancer Institute grant awarded to D.L.B. (CA77584).
Footnotes
Published ahead of print on 16 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aggarwal, B. B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. [DOI] [PubMed] [Google Scholar]
- 2.Altieri, D. C. 2006. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol. 18:609-615. [DOI] [PubMed] [Google Scholar]
- 3.Avdi, N. J., K. C. Malcolm, J. A. Nick, and G. S. Worthen. 2002. A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J. Biol. Chem. 277:40687-40696. [DOI] [PubMed] [Google Scholar]
- 4.Bao, Q., and Y. Shi. 2006. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 14:56-65. [DOI] [PubMed] [Google Scholar]
- 5.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689-692. [DOI] [PubMed] [Google Scholar]
- 6.Cai, B., S. H. Chang, E. B. Becker, A. Bonni, and Z. Xia. 2006. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J. Biol. Chem. 281:25215-25222. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J., R. T. Peterson, and S. L. Schreiber. 1998. α4 associates with protein phosphatases 2A, 4 and 6. Biochem. Biophys. Res. Commun. 247:827-832. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., T. B. Gibson, F. Robinson, L. Silvestro, G. Pearson, B. Xu, A. Wright, C. Vanderbilt, and M. H. Cobb. 2001. MAP kinases. Chem. Rev. 101:2449-2476. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, P. Y., Y. Zhang, J. Long, S. Lin, M. Zhang, Y. Jiang, and Z. Wu. 2004. p150(Glued), Dynein, and microtubules are specifically required for activation of MKK3/6 and p38 MAPKs. J. Biol. Chem. 279:45308-45311. [DOI] [PubMed] [Google Scholar]
- 10.Chung, H., A. C. Nairn, K. Murata, and D. L. Brautigan. 1999. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the alpha 4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry 38:10371-10376. [DOI] [PubMed] [Google Scholar]
- 11.De Chiara, G., M. E. Marcocci, M. Torcia, M. Lucibello, P. Rosini, P. Bonini, Y. Higashimoto, G. Damonte, A. Armirotti, S. Amodei, A. T. Palamara, T. Russo, E. Garaci, and F. Cozzolino. 2006. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J. Biol. Chem. 281:21353-21361. [DOI] [PubMed] [Google Scholar]
- 12.Di Como, C. J., and K. T. Arndt. 1996. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 10:1904-1916. [DOI] [PubMed] [Google Scholar]
- 13.Eto, M., A. Karginov, and D. L. Brautigan. 1999. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry 38:16952-16957. [DOI] [PubMed] [Google Scholar]
- 14.Farooq, A., and M. M. Zhou. 2004. Structure and regulation of MAPK phosphatases. Cell Signal. 16:769-779. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore, A. P. 2005. Anoikis. Cell Death Differ. 12(Suppl. 2):1473-1477. [DOI] [PubMed] [Google Scholar]
- 16.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 17.Grethe, S., M. P. Ares, T. Andersson, and M. I. Porn-Ares. 2004. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp. Cell Res. 298:632-642. [DOI] [PubMed] [Google Scholar]
- 18.Grethe, S., and M. I. Porn-Ares. 2006. p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial apoptosis. Cell Signal. 18:531-540. [DOI] [PubMed] [Google Scholar]
- 19.Hui, L., Y. Zheng, Y. Yan, J. Bargonetti, and D. A. Foster. 2006. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene 25:7305-7310. [DOI] [PubMed] [Google Scholar]
- 20.Ichijo, H. 1999. From receptors to stress-activated MAP kinases. Oncogene 18:6087-6093. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, T., D. L. Boyle, M. Corr, D. Hammaker, R. J. Davis, R. A. Flavell, and G. S. Firestein. 2006. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 103:5484-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue, T., D. Hammaker, D. L. Boyle, and G. S. Firestein. 2005. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J. Immunol. 174:4301-4306. [DOI] [PubMed] [Google Scholar]
- 23.Inui, S., K. Kuwahara, J. Mizutani, K. Maeda, T. Kawai, H. Nakayasu, and N. Sakaguchi. 1995. Molecular cloning of a cDNA clone encoding a phosphoprotein component related to the Ig receptor-mediated signal transduction. J. Immunol. 154:2714-2723. [PubMed] [Google Scholar]
- 24.Inui, S., H. Sanjo, K. Maeda, H. Yamamoto, E. Miyamoto, and N. Sakaguchi. 1998. Ig receptor binding protein 1 (α4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood 92:539-546. [PubMed] [Google Scholar]
- 25.Jacinto, E., B. Guo, K. T. Arndt, T. Schmelzle, and M. N. Hall. 2001. TIP41 interacts with TAP42 and negatively regulates the TOR signaling pathway. Mol. Cell. 8:1017-1026. [DOI] [PubMed] [Google Scholar]
- 26.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439.11171037 [Google Scholar]
- 27.Kong, M., C. J. Fox, J. Mu, L. Solt, A. Xu, R. M. Cinalli, M. J. Birnbaum, T. Lindsten, and C. B. Thompson. 2004. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science 306:695-698. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara, K., T. Matsuo, J. Nomura, H. Igarashi, M. Kimoto, S. Inui, and N. Sakaguchi. 1994. Identification of a 52-kDa molecule (p52) coprecipitated with the Ig receptor-related MB-1 protein that is inducibly phosphorylated by the stimulation with phorbol myristate acetate. J. Immunol. 152:2742-2752. [PubMed] [Google Scholar]
- 29.Letourneux, C., G. Rocher, and F. Porteu. 2006. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 25:727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., T. D. Prickett, E. Elliott, G. Meroni, and D. L. Brautigan. 2001. Phosphorylation and microtubule association of the Opitz syndrome protein Mid-1 is regulated by protein phosphatase 2A via binding to the regulatory subunit α4. Proc. Natl. Acad. Sci. USA 98:6650-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massiah, M. A., B. N. Simmons, K. M. Short, and T. C. Cox. 2006. Solution structure of the RBCC/TRIM B-box1 domain of human MID1: B-box with a RING. J. Mol. Biol. 358:532-545. [DOI] [PubMed] [Google Scholar]
- 32.Melhuish, T. A., and D. Wotton. 2006. The Tgif2 gene contains a retained intron within the coding sequence. BMC Mol. Biol. 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meredith, J. E., Jr., B. Fazeli, and M. A. Schwartz. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell. 4:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millward, T. A., S. Zolnierowcz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 35.Mumby, M. C., and G. Walter. 1993. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol. Rev. 73:673-699. [DOI] [PubMed] [Google Scholar]
- 36.Murata, K., J. Wu, and D. L. Brautigan. 1997. B cell receptor-associated protein α4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 94:10624-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanahoshi, M., T. Nishiuma, Y. Tsujishita, K. Hara, S. Inui, N. Sakaguchi, and K. Yonezawa. 1998. Regulation of protein phosphatase 2A catalytic activity by α4 protein and its yeast homolog Tap42. Biochem. Biophys. Res. Commun. 251:520-526. [DOI] [PubMed] [Google Scholar]
- 38.Nanahoshi, M., Y. Tsujishita, C. Tokunaga, S. Inui, N. Sakaguchi, K. Hara, and K. Yonezawa. 1999. Alpha4 protein as a common regulator of type 2A-related serine/threonine protein phosphatases. FEBS Lett. 446:108-112. [DOI] [PubMed] [Google Scholar]
- 39.Park, M. T., J. A. Choi, M. J. Kim, H. D. Um, S. Bae, C. M. Kang, C. K. Cho, S. Kang, H. Y. Chung, Y. S. Lee, and S. J. Lee. 2003. Suppression of extracellular signal-related kinase and activation of p38 MAPK are two critical events leading to caspase-8- and mitochondria-mediated cell death in phytosphingosine-treated human cancer cells. J. Biol. Chem. 278:50624-50634. [DOI] [PubMed] [Google Scholar]
- 40.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 41.Prickett, T. D., and D. L. Brautigan. 2006. The alpha-4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J. Biol. Chem. 281:30503-30511. [DOI] [PubMed] [Google Scholar]
- 42.Prickett, T. D., and D. L. Brautigan. 2004. Overlapping binding sites in protein phosphatase 2A for association with regulatory A and alpha-4 (mTap42) subunits. J. Biol. Chem. 279:38912-38920. [DOI] [PubMed] [Google Scholar]
- 43.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68:320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santhanam, A., A. Hartley, K. Duvel, J. R. Broach, and S. Garrett. 2004. PP2A phosphatase activity is required for stress and Tor kinase regulation of yeast stress response factor Msn2p. Eukaryot. Cell 3:1261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz, D. R., and W. J. Harrington, Jr. 2003. Apoptosis: programmed cell death at a molecular level. Semin. Arthritis Rheum. 32:345-369. [DOI] [PubMed] [Google Scholar]
- 46.Stefansson, B., and D. L. Brautigan. 2006. Protein phosphatase 6 subunit with conserved Sit4-associated protein domain targets IκBɛ. J. Biol. Chem. 281:22624-22634. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka, N., M. Kamanaka, H. Enslen, C. Dong, M. Wysk, R. J. Davis, and R. A. Flavell. 2002. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 3:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trockenbacher, A., V. Suckow, J. Foerster, J. Winter, S. Krauss, H. H. Ropers, R. Schneider, and S. Schweiger. 2001. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat. Genet. 29:287-294. [DOI] [PubMed] [Google Scholar]
- 49.Van Kanegan, M. J., D. G. Adams, B. E. Wadzinski, and S. Strack. 2005. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J. Biol. Chem. 280:36029-36036. [DOI] [PubMed] [Google Scholar]
- 50.Wadgaonkar, R., J. W. Pierce, K. Somnay, R. L. Damico, M. T. Crow, T. Collins, and J. G. Garcia. 2004. Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells. Am. J. Respir. Cell Mol. Biol. 31:423-431. [DOI] [PubMed] [Google Scholar]
- 51.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 52.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 53.Wysk, M., D. D. Yang, H. T. Lu, R. A. Flavell, and R. J. Davis. 1999. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl. Acad. Sci. USA 96:3763-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, L., Z. Cao, H. Yan, and W. C. Wood. 2003. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 63:6815-6824. [PubMed] [Google Scholar]
- 55.Zarubin, T., and J. Han. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11-18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.