Abstract
The induction of Bcl-xL is critical for the survival of late proerythroblasts. The erythroid-specific transcriptional network that regulates Bcl-xL expression in erythropoiesis remains unclear. The activation of the central erythropoietic transcriptional factor, GATA-1, leads to the early, transient induction of a transcription repressor, Gfi-1B, followed by the late induction of Bcl-xL during erythroid maturation in G1ER cells. Chromatin immunoprecipitation assays demonstrated that a constant level of GATA-1 binds to the Bcl-x promoter throughout the entire induction period, while Gfi-1B is transiently associated with the promoter in the early phase. The sustained expression of Gfi-1B abolished GATA-1-induced Bcl-xL expression. Here, we present evidence that GATA-1 binds to the noncanonical GATT motif of the Bcl-x promoter for trans-activation. Gfi-1B expressed at increased levels is recruited to the Bcl-x promoter through its association with GATA-1, suppressing Bcl-xL transcription. Therefore, the down-regulation of Gfi-1B in the late phase of erythroid maturation is necessary for Bcl-xL induction. Furthermore, we show that the inhibition of Bcr-Abl kinase by treatment with imatinib caused the up-regulation of Gfi-1B in K562 cells, where Gfi-1B also cooperated with GATA-1 to repress Bcl-xL transcription. Gfi-1B knockdown by RNA interference diminished imatinib-induced apoptosis, while the overexpression of Gfi-1B sensitized K562 cells to arsenic-induced death. These findings illuminate the role of Gfi-1B in GATA-1-mediated transcription in the survival aspect of erythroid cells.
During erythrocyte development, it is essential that committed precursors be protected from cell death. It has been well documented that the increased expression of Bcl-xL during terminal erythroid maturation provides the antiapoptotic function for maintaining the viability of mature, definitive erythroid cells (1, 9, 32). GATA-1, an erythroid-specific transcription activator, has been shown to be an upstream activator of Bcl-xL expression, because the restoration of GATA-1 function in immature G1E erythroblasts derived from GATA-1-deficient embryonic stem cells (38) triggers erythroid maturation through Bcl-xL induction (9, 39). Despite the presence of a typical GATA sequence in the Bcl-x promoter, it was previously reported that mutations in the proximal GATA-1 binding motif of the Bcl-x promoter have little effect on the promoter activity (9). Since the expression of Bcl-xL mRNA displayed delayed kinetics after GATA-1 induction, it has been proposed that GATA-1 acts as an indirect activator of Bcl-xL transcription (9). The details of the Bcl-xL transcriptional control by the GATA-1-regulated transcriptional network remain undefined.
Gfi-1B (growth factor independence 1B) is a Gfi family transcriptional repressor that contains a SNAG domain to mediate transcriptional repression and a zinc finger domain at its carboxyl terminus for DNA binding to the TAAATCAC(A/T)GCA recognition sequence (the binding site motif is highlighted in bold) (11, 35, 41). Only a small number of Gfi-1B-regulated genes are known, including p21, Socs1, Socs3, and the Gfi-1B gene's own promoter (14, 16, 35). Gene targeting experiments have shown that Gfi-1B gene disruption results in embryonic lethality due to a loss of red blood cell formation, indicating the necessary role of Gfi-1B in erythropoiesis (25). In a previous study, the enforced expression of Gfi-1B in early erythroid progenitor cells induced a drastic expansion of erythroblast colonies, independent of erythropoietin. These Gfi-1B-transduced cells exhibited massive apoptosis after 7 to 10 days of culture, with a significant reduction of Bcl-xL expression (22). Because the canonical consensus sequence recognized by Gfi-1B was not found in the Bcl-x promoter region, it was proposed that Gfi-1B does not exert its transcriptional repression function directly on the Bcl-x promoter (7, 22).
By searching the microarray database (39) for profiles of gene expression after early and late GATA-1 activation in G1ER cells, we found that Gfi-1B is an early gene induced by GATA-1 activation and that its expression level declines before the rise of the Bcl-xL transcript level in the late phase. Our recent study has demonstrated that Gfi-1B interacts with GATA-1 via the zinc finger domain of Gfi-1B. When Gfi-1B expression is elevated, Gfi-1B forms a complex with GATA-1 which binds to the AATC motif of the Gfi-1B promoter to repress transcription, conferring a negative feedback loop (14). Interestingly, the Bcl-x promoter also contains several conserved AATC motifs. These clues compelled us to investigate whether both GATA-1 and Gfi-1B participate directly in the regulation of Bcl-xL expression. Here, our data demonstrate that GATA-1 binds to the Bcl-x promoter at the GATT (AATC for the reverse strand) site for trans-activation and that the enforced expression of Gfi-1B represses the Bcl-x promoter in a GATA-1-dependent manner. Thus, Gfi-1B converts GATA-1-mediated activation to repression. These results suggest that the up-regulation of Gfi-1B is involved in suppressing Bcl-xL transcription in the early phase of GATA-1 activation in G1ER cells, while the decrease of Gfi-1B expression in the late phase allows GATA-1-mediated Bcl-xL transcription.
In this study, we used K562 cells to investigate the regulation of Gfi-1B expression in apoptotic induction. K562 is an erythroleukemic cell line established by erythroblasts isolated from a patient with chronic myelogenous leukemia (CML), which is a myeloproliferative disorder associated with t(9;22) translocation, which produces a BCR-ABL oncogene hybrid that encodes a chimeric protein, p210, with a hyperactive tyrosine kinase (3, 8). The p210Bcr-Abl protein activates several pathways, including the Ras/MEK/extracellular signal-regulated kinase 1 and 2/phosphatidylinositol 3-kinase/Akt (26, 33), NF-κB (19, 23), and signal transducer and activator of transcription (STAT) protein pathways (2, 13, 30, 31). Imatinib mesylate, an inhibitor of Bcr-Abl tyrosine kinase, is now widely used in CML treatment (4, 5, 17, 27, 34). The action of imatinib in killing cells expressing Bcr-Abl has been attributed to the inhibition of Bcl-xL transcription by blocking Bcr-Abl/STAT5 signaling (6, 13, 18, 21, 36). Here, we found that the treatment of K562 cells with imatinib increased Gfi-1B expression, resulting in Gfi-1B occupancy of the Bcl-x promoter through the formation of a complex of Gfi-1B and GATA-1. As a result, Bcl-xL transcription was suppressed. Finally, we demonstrated that the depletion of Gfi-1B expression in K562 cells diminished imatinib-induced apoptosis and that the overexpression of Gfi-1B increased the susceptibility to arsenic-induced apoptosis. These results indicate the role of Gfi-1B in apoptosis and emphasize the importance of GATA-1-Gfi-1B interaction in transcriptional control for erythroid development.
MATERIALS AND METHODS
Materials.
Imatinib mesylate (STI571; Gleevec) was provided by Novartis Pharmaceuticals (Basel, Switzerland). Bcl-xL (54H6), STAT5, and phospho-STAT5 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Gfi-1B (B7) and GATA-1 (C-20 and N6) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). As2O3, anti-β-tubulin, and anti-Flag were from Sigma (St. Louis, MO).
Plasmid construction.
The Gfi-1B cDNA was obtained by reverse transcription-PCR (RT-PCR) from K562 mRNA and cloned in-frame into the BamHI site of pCS2-MT. The gene expressing a nonfunctional mutant form of Gfi-1B, expressed by pCS2-P2A-Gfi-1B, was derived from pCS2-Gfi-1B by using the QuikChange XL site-directed mutagenesis kit (Stratagene). The pGL2-3.2 vector containing 3.2 kb of the murine Bcl-x promoter region fused to the promoterless pGL2 firefly luciferase reporter (10) was a generous gift of Gabriel Núnez. The pGL2-3.2 plasmid was digested with HindIII to generate a 0.6-kb fragment of the Bcl-x promoter region, which was subcloned into the pGL2 vector, producing pGL2-0.6. Mutant versions of pGL2-0.6 (pGL2-0.6mut1 and pGL2-0.6mut2) were generated using the QuikChange site-directed mutagenesis kit with a specific mutated primer that converts GATT (positions −404 to −401 relative to the translation start site) to GATC or AATT. pCMV2-GATA-1 and the Gfi-1B promoter constructs pGL2-hG (−145 to +19) and pCMV2-Gfi-1B were described previously (14, 15). The MIGR1 and MIGR-CFlag-Gfi-1B plasmids were from Dominique Duménil. The pRK5-STAT5A and pRK5-STAT5AΔ713 plasmids were from James Ihle. The pEQ-PAM3(-E) and pSV-A-MLV-env plasmids were from Stephen Nimer.
Cell culture.
G1ER cells were grown in IMDM (Invitrogen Life Technologies) with 15% heat-inactivated fetal bovine serum (HyClone), erythropoietin (2 U/ml), and rat stem cell factor (50 ng/ml). The addition of β-estradiol (Sigma, St. Louis, MO) at 10−7 mol/liter was used to activate the conditional GATA-1. K562 and 293T cells were maintained in RPMI 1640 medium and Dulbecco's modified Eagle's medium (Invitrogen Life Technologies), respectively, supplemented with 10% heat-inactivated fetal bovine serum. NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum.
RNA isolation and RT-PCR.
Total RNA was isolated from cells at the indicated times by using RNAzol B reagent (Tel-Test). cDNA was synthesized using ImProm-II reverse transcriptase (Promega). Semiquantitative PCRs were performed with cycling parameters of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s (27 cycles for the glyceraldehyde-3-phosphate dehydrogenase [GAPDH] gene and 32 cycles for Bcl-xL and Gfi-1B). The primer sequences were as follows: human Gfi-1B sense primer, 5′-AACTTCGAGTGCCGCATGTGC-3′, and antisense primer, 5′-TGATGAGGTTGGAGCTCTGGC-3′; human Bcl-xL sense primer, 5′-TTCAGTGACCTGACATCCCAG-3′, and antisense primer, 5′-TGCATTGTTCCCATAGAGTTCC-3′; human GAPDH gene sense primer, 5′-CATGGCACCGTCAAGG-3′, and antisense primer, 5′-CACCATGGGGGCATCAGC-3′; mouse Bcl-xL sense primer, 5′-CTCTCCTACAAGCTTTCCCAG-3′, and antisense primer, 5′-CCAGCGGTTGAAGCGCTCC-3′; mouse Gfi-1B sense primer, 5′-TTGGAGCAGCATACTCACGTC-3′, and antisense primer, 5′-AGATTGTGTTGACTCTCACG-3′; and mouse β-actin sense primer, 5′-ATGACCCAGATCATGTTTGAG-3′, and antisense primer, 5′-GTACGACCAGAGGCATACAG-3′.
Retroviral production and cell infection.
293T cells were transiently transfected with MIGR, pEQ-PAM3(-E), and pSV-A-MLV-env (20). Viral supernatants were collected at 48 h. The supernatant was centrifuged and filtered through a 0.45-μm-pore-size filter. Cells were incubated with the retroviral supernatant for 48 h in the presence of hexadimethrine bromide (8 μg/ml; Sigma). Two consecutive transduction rounds were performed before starting the assays.
Transfection and luciferase assays.
NIH 3T3 cells were transfected using a DNA mixture containing Lipofectamine (Invitrogen Life Technologies). After transfection for 24 h, cells were analyzed for reporter activity.
K562 cells (5 × 106) were suspended in 0.4 ml of RPMI 1640 medium in the presence of DNA mixtures for electroporation by Gene Pulser (Bio-Rad) at 300 V and 960 μF. Transfected cells were assayed for both firefly and Renilla luciferase activities by using the dual luciferase assay system (Promega).
Preparation of nuclear extracts and gel shift analysis.
The preparation of nuclear extracts from 293T cells and gel shift reactions were as described previously (15). A competitive DNA oligomer or GATA-1 (C-20; Santa Cruz, CA) antibody was added to the nuclear extracts for 30 min on ice prior to the DNA binding reaction. After the DNA binding reaction proceeded at room temperature for 25 min, samples were analyzed by electrophoresis at 150 V for 1.5 h through nondenaturing 4% polyacrylamide gels. Gels were then dried for autoradiography.
ChIP and re-ChIP assays.
Chromatin immunoprecipitation (ChIP) was performed as described previously (14). In brief, cells (108) were treated with formaldehyde at a final concentration of 1% for 10 min at room temperature. Glycine was added at a final concentration of 125 mM to quench cross-linking. Cell lysates were sonicated to generate DNA fragments averaging <1 kb. After preclearance, cell extracts were incubated with 2 μg of antibody at 4°C overnight, followed by precipitation with protein A-Sepharose beads. After washing, elution, deproteination, and heating, immunoprecipitated DNA was extracted and ethanol precipitated. DNA was resuspended in 50 μl of Tris-EDTA buffer. For chromatin reimmunoprecipitation (re-ChIP), chromatin cross-linked to the immunocomplex was eluted by incubation with 50 μl of dithiothreitol (10 mM) at 37°C for 30 min. After centrifugation, the supernatant was diluted with a 20× volume of buffer (20 mM Tris-HCl [pH 8.1], 150 mM NaCl, 2 mM EDTA, 1% Triton X-100) and subjected to the ChIP procedure as described above. PCR was applied to the immunoprecipitated DNA with the following thermal cycling program: 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C (30 cycles for the first ChIP and 35 cycles for the re-ChIP), followed by a 5-min extension step at 72 °C. PCR products were analyzed on 1.5% agarose gel and visualized by ethidium bromide staining.
Immunoprecipitation.
Cells lysed in 50 mM Tris-HCl buffer (pH 7.5) containing 1% Triton X-100, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 5 mM NaF, and proteinase inhibitors (Sigma) were centrifuged at 10,000 × g for 5 min and precleared by incubation with normal mouse immunoglobulin G (IgG) and protein A beads for 30 min at 4°C. The supernatants were immunoprecipitated using GATA-1 antibody and protein A beads. The beads were then washed four times with the lysis buffer, and the proteins on the beads were separated by SDS-10% polyacrylamide gel electrophoresis.
Biotinylated-oligonucleotide pulldown assay.
Nuclear extracts from K562 cells (5 × 107) were prepared as described previously (15). Biotinylated double-stranded oligonucleotides (wild type, 5′-AGGCGGATTTGAATG-3′, and mutant, 5′-AGGCGAATTTGAATG-3′ [the mutated and corresponding wild-type sequences are underlined]) coupled to streptavidin-agarose beads were incubated with 50 μg of the extracts at 30°C for 10 min, followed by six washings with the binding buffer containing 150 mM NaCl prior to separation on SDS-polyacrylamide gel for immunoblot analysis. For competition experiments, the extracts were incubated with the wild-type-oligonucleotide-coupled beads in the presence of a 50-fold molar excess of either the wild-type (5′-CACTTGATACAGAAAGTGATAACTCT-3′) or mutant (5′-CACTTCTTACAGAAAGTCTTAACTCT-3′) oligonucleotide.
RNA interference experiments.
Small interfering RNAs (siRNAs) targeting human Gfi-1B and GATA-1 were obtained from Dharmacon siGenome SMART pools, and control siRNAs targeting the firefly luciferase gene were purchased from Dharmacon. Cells (105) were electroporated with 0.4 μg of siRNA by using a Bio-Rad apparatus (voltage, 280 V; capacitance, 250 μF) (7).
Apoptosis assay.
An annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Oncogene) was used for measuring apoptosis. Cells were harvested after treatment and stained with 1.25 μl of FITC-conjugated annexin V reagent. After 15 min of incubation at room temperature, cells were added to 1 μl of propidium iodide and then analyzed by flow cytometry. Flow cytometry was performed with a FACScan II analyzer (Becton Dickinson). Annexin V staining was detected in the FL1 channel, whereas propidium iodide staining was monitored in the FL2 channel.
RESULTS
Enforced expression of Gfi-1B in G1ER cells repressed GATA-1-induced Bcl-xL expression.
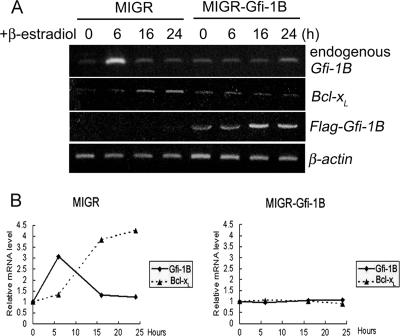
As an initial attempt to know whether Gfi-1B is involved in the regulation of Bcl-xL expression in erythroid cells, we tested the effect of the enforced expression of Gfi-1B in G1ER cells on Bcl-xL expression. G1ER cells that stably express GATA-1 fused to the ligand binding domain of the human estrogen receptor (9, 38, 40) were exposed to β-estradiol to activate GATA-1, by which synchronous erythroid maturation was triggered. We infected G1ER cells with retrovirus generated from full-length Flag-Gfi-1B or the empty MIGR retroviral vector and measured Bcl-xL transcript levels in response to GATA-1 activation by the semi-RT-PCR method. At 6 h after the β-estradiol treatment of cells infected with the control virus, the Gfi-1B RNA level was markedly elevated, and it declined to the basal level at the time point of 16 h, while the Bcl-xL RNA level did not increase until 16 h after GATA-1 activation and remained elevated at 24 h (Fig. 1A and B, left panel). In general, patterns of Gfi-1B and Bcl-xL RNA expression in G1ER cells infected with the control retrovirus were similar to the data deposited in the G1EDb microarray database (data not shown) (39). The early induction of Gfi-1B after GATA-1 activation is consistent with our previous finding that GATA-1 is a direct activator of the Gfi-1B promoter (15). Interestingly, the infection of G1ER cells with Flag-Gfi-1B-carrying retrovirus abolished the GATA-1-induced expression of Bcl-xL, indicating that the sustained expression of Gfi-1B negatively modulates Bcl-xL transcription during erythroid maturation. Infection with Flag-Gfi-1B-carrying virus also caused low levels of endogenous Gfi-1B mRNA throughout the 24-h period of GATA-1 activation (Fig. 1A and B, right panel), which was consistent with our previous observation showing negative autoregulation of the Gfi-1B gene.
FIG. 1.
Induction of Bcl-xL mRNA by GATA-1 in G1ER cells was repressed by the enforced expression of Gfi-1B. (A) G1ER cells infected with Flag-Gfi-1B-encoding (MIGR-Gfi-1B) or control (MIGR) retroviruses were treated with β-estradiol (10−7 M) to activate GATA-1, after which cells were harvested at the times indicated for RNA preparation followed by RT-PCR analysis using primers specific for mouse Gfi-1B, human Gfi-1B (for MIGR-CFlag-Gfi-1B), mouse Bcl-xL, and mouse β-actin. (B) Densitometric evaluation of relative levels of mouse endogenous Gfi-1B and Bcl-xL RNA normalized by the β-actin mRNA level in each sample of MIGR1 (left panel)- or MIGR-CFlag-Gfi-1B (right panel)-transfected G1ER cells.
Gfi-1B and GATA-1 occupancy of the Bcl-x promoter in the early phase of erythroid maturation.
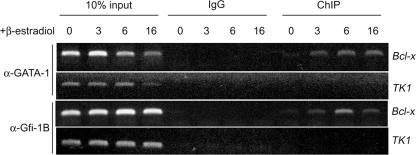
Next, we performed chromatin cross-linking and ChIP to know whether Gfi-1B is directly involved in regulating the Bcl-x promoter during GATA-1 activation in G1ER cells. Primers specific to the proximal murine Bcl-x promoter were used for the PCR. The result showed that Gfi-1B was associated with the Bcl-x promoter and that the degree of occupancy was inversely correlated with the level of Bcl-xL expression while the extent of the binding of GATA-1 to the Bcl-x promoter remained constant throughout the period of GATA-1 induction (Fig. 2). PCR using mouse TK1 promoter primers was performed as a negative control. These results suggest that both GATA-1 and Gfi-1B interact with the Bcl-x promoter during erythroid maturation and that Gfi-1B acts as a transcriptional repressor of the Bcl-x promoter.
FIG. 2.
Occupancy of the Bcl-x promoter by GATA-1 and Gfi-1B in G1ER cells. In vivo cross-linked protein-DNA complexes were isolated from G1ER cells treated with β-estradiol for the indicated periods and were subjected to immunoprecipitation with IgG (control), Gfi-1B (α-Gfi-1B), or GATA-1 (α-GATA-1) antibody. The coimmunoprecipitated DNA was amplified by PCR using the Bcl-x proximal-promoter primers or TK1 primers as a negative control.
Repression of the Bcl-x promoter by the enforced expression of Gfi-1B in K562 cells.
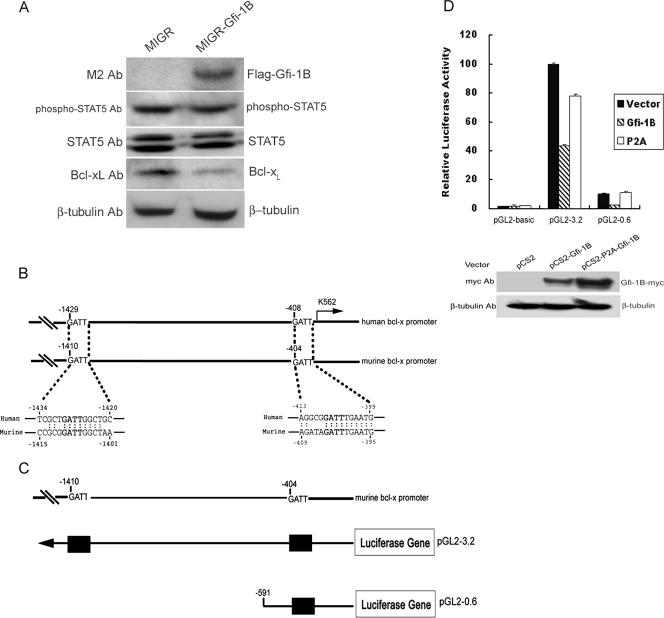
Similar to that of G1ER cells, the infection of K562 cells by transduction with retroviral Flag-Gfi-1B decreased Bcl-xL expression. Because STAT5 is a direct activator of the Bcl-x gene in hematopoietic cells (31), we then examined the effect of the overexpression of Gfi-1B on STAT5 activation by measuring the phosphorylation status of STAT5. It appeared that the elevation of Gfi-1B expression had no effect on the intensity of STAT5 phosphorylation (Fig. 3A).
FIG. 3.
Gfi-1B represses the Bcl-x promoter activity. (A) K562 cells were infected with Flag-Gfi-1B-encoding (MIGR-Gfi-1B) or control (MIGR) retroviruses and analyzed by Western blotting with anti-Bcl-xL, M2, STAT5, phospho-STAT5, or β-tubulin antibody (Ab). (B) Sequences of putative Gfi-1B binding sites on human and murine Bcl-x promoters are indicated in bold letters. Each sequence is numbered relative to the first nucleotide of the translation start site, which was set at +1. The arrow indicates the major transcriptional start site. (C) Schematic representation of the murine Bcl-x promoter reporter constructs. The upper diagram shows the putative Gfi-1B binding sites in the murine Bcl-x promoter, and the lower one shows the constructed murine Bcl-x promoter reporter plasmids. Putative Gfi-1B binding sites, with the sequence AATC, are shown as boxes. (D) K562 cells were cotransfected with the murine Bcl-x promoter reporter constructs and pRL-TK in the presence of pCS2, pCS2-Gfi-1B, or the pCS2-P2A-Gfi-1B mutant plasmid expressing a nonfunctional form of Gfi-1B (P2A) as indicated. The luciferase activities normalized by Renilla luciferase activities were calculated relative to that in the cells transfected with full-length pGL2-3.2 and the control vector, which was set arbitrarily at 100. Values are the averages of three independent determinations. Total lysates from the cells transfected with the pGL2-3.2 plasmid together with pCS2, pCS2-Gfi-1B, or pCS2-P2A-Gfi-1B as indicated were subjected to Western blot analysis using anti-myc (9E10) and β-tubulin antibodies.
To study the functional effect of Gfi-1B on the Bcl-x promoter, we analyzed the sequence within the proximal promoter region of the human Bcl-x gene (10), which revealed several occurrences of the Gfi-1B binding core sequence, the AATC motif, located at −1429, −1024, −924, and −408 upstream of the translation start site. As shown here, the GATT (AATC for the reverse strand) motif at −1429 and −408 is conserved in the murine promoter (10) (Fig. 3B). It was noted that the sequences surrounding these two sites do not match the consensus Gfi-1B binding sequence. To understand whether the enforced expression of Gfi-1B can directly repress the Bcl-x promoter activity, we constructed two reporter plasmids in which 3.2- and 0.6-kb fragments of the murine Bcl-x promoter were linked to the luciferase gene (Fig. 3C). K562 cells were transfected with these two constructed Bcl-x reporter plasmids, along with the Gfi-1B expression vector (pCS2-Gfi-1B), the plasmid expressing a nonfunctional mutant Gfi-1B (pCS2-P2A-Gfi-1B), or an empty vector as a control. The mutant form of Gfi-1B expressed by pCS2-P2A-Gfi-1B carries a mutation in the SNAG domain that disrupts the Gfi-1B transcriptional repression function (11, 16, 37). The coexpression of the wild-type Gfi-1B in K562 cells repressed the activities of pGL2-3.2 and pGL2-0.6, while the overexpression of the pCS2-P2A-Gfi-1B-encoded mutant protein had little effect on the Bcl-x promoter activity (Fig. 3D). These data clearly show Gfi-1B as a repressor of the minimal Bcl-x promoter.
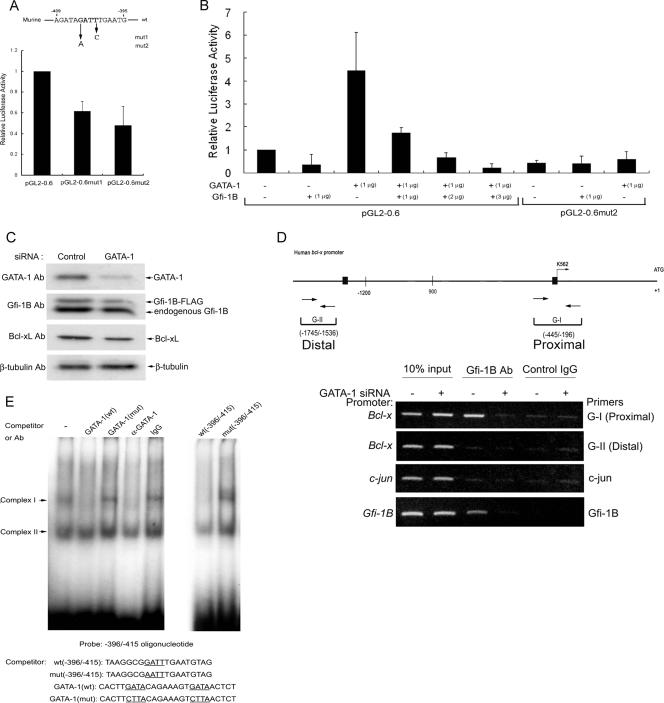
GATA-1 mediates the Gfi-1B repression of the Bcl-x promoter through binding at the AATC site.
We then introduced a mutation at the −404 GATT (the AATC reverse strand) site of the murine minimal Bcl-x promoter, which corresponds to the −408 GATT site of the human Bcl-x promoter. However, the mutation converting GATT to GATC or AATT decreased the Bcl-x promoter activity, an indication that this site is a positive cis element for trans-activation (Fig. 4A). To test whether GATA-1 may also mediate the trans-activation of the Bcl-x promoter through this site, we expressed GATA-1 in NIH 3T3 cells, a nonerythroid line, to measure its effect on the Bcl-x promoter activity. It turned out that the ectopic expression of GATA-1 significantly stimulated luciferase activity derived from the pGL2-0.6 construct and that the mutation of the GATT site abolished GATA-1-mediated trans-activation. The coexpression of Gfi-1B inhibited the GATA-1-dependent stimulation of wild-type promoter activity in a dosage-dependent manner (Fig. 4B). These results led us to propose that GATA-1 activates the Bcl-x promoter in K562 cells by binding to the GATT motif of the Bcl-x promoter and that the elevation of Gfi-1B expression converts GATA-1-mediated activation to repression.
FIG. 4.
GATA-1 activates the Bcl-x promoter and mediates Gfi-1B repression. (A) K562 cells were transfected with the Bcl-x reporter constructs pGL2-0.6 and pGL2-0.6mut1 (mut1; carrying a mutation at −401 in the GATT sequence) and pGL2-0.6mut2 (mut2; carrying a mutation at −404 in the GATT sequence), and luciferase activities were determined and expressed as described in the legend to Fig. 3D, with the activity in cells transfected with full-length pGL2-3.2 and the control vector set at 1. The error bars represent standard deviations. wt, wild type. (B) NIH 3T3 cells were transfected with the minimal Bcl-x reporter construct pGL2-0.6 or pGL2-0.6mut2 along with pCMV2-GATA-1 in combination with different amounts of pCS2-Gfi-1B as indicated. The luciferase activities were normalized and expressed relative to that in cells transfected with pGL2-0.6 and control vectors, which was set arbitrarily at 1. Values are the averages of three independent determinations. The error bars represent standard deviations. −, absent. (C) K562 cells with pS2-Gfi-1B-Flag retroviral integration were electroporated with GATA-1 siRNA or control siRNA for 48 h. Whole-cell lysates were subjected to Western blot analysis using anti-GATA-1, Gfi-1B, Bcl-xL, and β-tubulin antibodies (Ab). (D) Cells were treated with formaldehyde for ChIP assays using anti-Gfi-1B antibody or normal mouse IgG. DNA isolated from immunoprecipitates was amplified by PCR with primers specific for the Bcl-x (G-I [−445 to −196] and G-II [−1745 to −1536]), Gfi-1B, or c-jun promoter. +, present. (E) Results from gel shift assays of the human Bcl-x promoter region from −396 to −415. The 32P-labeled double-stranded oligonucleotide containing the sequence from −396 to −415 of the Bcl-x promoter was used for the assays in the absence or presence of an unlabeled competitor (at a 10-fold excess) with 2.5 μg of nuclear extracts from 293T cells overexpressing Flag-GATA-1, as indicated. The sequence of each competitor is shown below. Mutated and corresponding wild-type sequences are underlined. Control IgG and GATA-1(C-20) antibodies were added to the reaction mixtures where indicated. mut, mutant.
GATA-1-dependent Gfi-1B occupancy of the Bcl-x promoter.
To assess the role of GATA-1 in Gfi-1B recruitment to the Bcl-x promoter, K562 cells that stably express Flag-Gfi-1B (14) were transfected with GATA-1 siRNA to decrease GATA-1 expression (Fig. 4C). In the Western analysis, we noticed that the level of Flag-Gfi-1B in this cell line had become lower than that of endogenous Gfi-1B, which was different from our previous results showing that the enforced expression of Flag-Gfi-1B inhibits endogenous Gfi-1B expression (14). We reasoned that the exogenous gene established in Flag-Gfi-1B-expressing K562 cells might have been inactivated after long-term culture, allowing more endogenous Gfi-1B to be expressed. Since this cell line expresses a higher level of Gfi-1B, we used this cell line to knock down GATA-1 to test whether GATA-1 is required for Gfi-1B occupancy of the Bcl-x promoter. Cross-linked chromatins of the control and GATA-1-depleted cells were immunoprecipitated by Gfi-1B antibody for PCRs using primers specific for the promoter regions indicated below. ChIP assays showed that Gfi-1B was associated with the proximal, but not the distal, region of the Bcl-x promoter in K562 cells. This indicates that the distal −1429 GATT sequence is probably not a Gfi-1B-interacting site. The depletion of GATA-1 abolished Gfi-1B binding to the Bcl-x promoter, demonstrating the requirement of GATA-1 for Gfi-1B occupancy of the Bcl-x proximal promoter. Similar results were observed when ChIP assays were performed using primers specific for the Gfi-1B promoter in the PCR, consistent with our previous finding that Gfi-1B binds to its own promoter through GATA-1 (14). As a negative control, primers specific for the c-jun promoter were applied in a PCR using the same immunoprecipitated chromatin samples (Fig. 4D). Based on these data, we conclude that GATA-1 is a direct activator of the Bcl-x promoter and mediates the recruitment of Gfi-1B to the promoter for transcriptional repression.
To examine whether GATA-1 can bind to the −408 GATT site of the human Bcl-x promoter, we performed a gel shift assay using a radiolabeled DNA fragment covering the region from −396 to −415 of the Bcl-x promoter sequence. The incubation of this probe with nuclear extracts from 293T cells ectopically expressing GATA-1 gave rise to DNA-protein complexes I and II (Fig. 4E). Complex I was abolished by an excess amount of the unlabeled oligomer but not by the oligomer carrying a mutation in the GATT site. An excess amount of the unlabeled GATA consensus oligonucleotide or the specific GATA-1 antibody also abolished the formation of complex I. No amount of the competitor had an effect on complex II formation, indicating that complex II is a nonspecific DNA-protein complex. Accordingly, we conclude that GATA-1 binds to the −408 GATT site of the human Bcl-x promoter.
Up-regulation of Gfi-1B participates in the imatinib-induced repression of Bcl-xL expression.
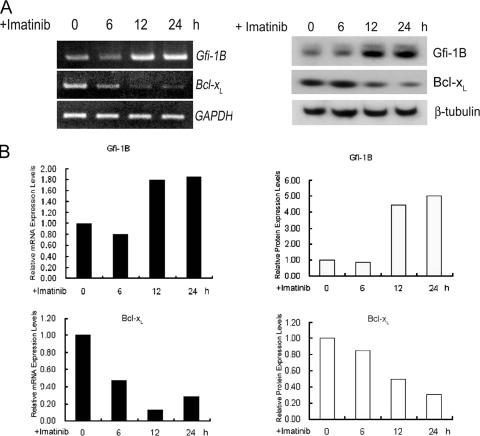
Since it is well established that the inhibition of Bcl-xL expression is responsible for imatinib-induced apoptosis in CML (6, 13, 18, 21, 36), we then used this system to consider the question of whether Gfi-1B is involved in the imatinib-induced down-regulation of Bcl-xL expression. To address this question, we first examined the effect of imatinib treatment on levels of Gfi-1B and Bcl-xL mRNA expression in K562 cells by semiquantitative RT-PCR. Following the imatinib treatment, the level of Gfi-1B mRNA was markedly increased, with a concurrent decrease in Bcl-xL mRNA expression (Fig. 5, left panels). A similar relationship was reflected in the levels of Gfi-1B and Bcl-xL protein expression as determined by Western blot analysis (Fig. 5, right panels).
FIG. 5.
Inverse correlation between Gfi-1B and Bcl-xL expression in response to imatinib treatment. (A) K562 cells treated with imatinib for the indicated periods were processed for RT-PCR analysis (left panel) using primers specific for Gfi-1B, Bcl-xL, and the GAPDH gene and for Western blotting (right panel) with anti-Gfi-1B, anti-Bcl-xL, and β-tubulin antibody. (B) Densitometric analysis of Gfi-1B and Bcl-xL at the RNA (left panel) and protein (right panel) levels, with results normalized by the GAPDH mRNA level and the β-tubulin level, respectively, in each sample.
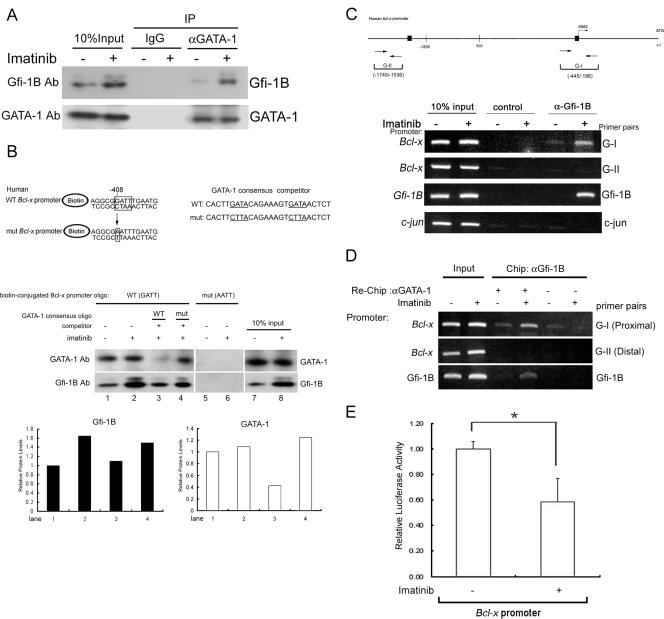
Given that the imatinib treatment did not change the GATA-1 expression level, we then investigated the extent of GATA-1-Gfi-1B complex formation by immunoprecipitation from imatinib-treated cells. As shown in Fig. 6A, endogenous Gfi-1B was coimmunoprecipitated with endogenous GATA-1 by anti-GATA-1 antibody, and imatinib treatment increased the amount of Gfi-B in the GATA-1 complex. To verify that GATA-1 binds to the GATT motif of the human Bcl-x promoter, we performed the streptavidin pulldown assay with biotin-labeled oligonucleotides covering the region from −399 to −413 of the wild-type human Bcl-x promoter sequence and the corresponding sequence mutated at the −408 GATT site. The biotin-labeled oligonucleotides were incubated with nuclear extracts from K562 cells treated with or without imatinib. Western blot analysis of the pulldown proteins showed the association of both GATA-1 and Gfi-1B with the wild-type oligonucleotide but not the one mutated at the −408 GATT site (Fig. 6B). Consistently, the imatinib treatment enhanced the binding of Gfi-1B to the beads containing the wild-type Bcl-x promoter sequence without affecting the amount of GATA-1 binding to DNA. An excess amount of an oligonucleotide containing the typical GATA-1 binding sequence, but not the oligonucleotide carrying the GATA-to-CTTA conversion, was capable of abolishing the binding of GATA-1 to the wild-type sequence and diminishing the imatinib-induced increased association with Gfi-1B. These results support the notion that GATA-1 binds to the GATT motif of the Bcl-x promoter and that elevated amounts of Gfi-1B in imatinib-treated cells are brought to the promoter through GATA-1.
FIG. 6.
Imatinib-induced Gfi-1B formed a complex with GATA-1 on the Bcl-x promoter. (A) K562 cells were treated with or without imatinib (1 μM). After 24 h, whole-cell lysates were prepared and immunoprecipitated with anti-GATA-1 antibody (αGATA-1) or control IgG. The immunoprecipitates (IP) were subjected to immunoblot analysis with anti-Gfi-1B antibody (Ab), followed by reprobing with anti-GATA-1 antibody to confirm that GATA-1 was successfully immunoprecipitated. −, absent; +, present. (B) Pulldown assay using biotin-labeled oligonucleotides containing the Gfi-1B binding sequence (−408 to −405) on the human Bcl-x promoter. Nuclear extracts prepared from untreated or imatinib-treated K562 cells were incubated with biotin-labeled double-stranded oligonucleotides containing the wild-type (WT) or mutated (mut) Gfi-1B binding site. The bound proteins were pulled down with streptavidin-agarose and were analyzed by immunoblotting using antibody against Gfi-1B or GATA-1. Competitor oligonucleotides (oligo) specific for typical and mutated GATA-1 canonical consensus sequences were each included in the reaction mixture as indicated (lanes 3 and 4). Relative amounts of GATA-1 and Gfi-1B protein in lanes 1 to 4 were evaluated by densitometric determination and are shown in the lower panel. (C) In vivo cross-linked protein-DNA complexes isolated from untreated and imatinib-treated K562 cells were subjected to immunoprecipitation with IgG (control) or Gfi-1B antibody (α-Gfi-1B) as indicated. The coimmunoprecipitated DNA was amplified by PCR using the Bcl-x primers G-I (−445 to −196) and G-II (−1745 to −1536), the primer pair for c-jun, or the primer pair specific for the Gfi-1B promoter. Putative Gfi-1 and Gfi-1B binding sites are shown by boxes. The PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. (D) Following ChIP with Gfi-1B antibody, the complexes were subjected to a second immunoprecipitation (re-ChIP) with either anti-GATA-1 antibody (αGATA-1) or control IgG. The coimmunoprecipitated DNA was amplified by PCR using the indicated primers: G-I, G-II, the primer pair specific for the Gfi-1B promoter, or the c-jun primer pair as a control. (E) K562 cells were transfected with the Bcl-x promoter construct, pGL2-3.2, together with pRL-TK. After 24 h, cells were treated with or without 1 μM imatinib for an additional 24 h. Cells were then harvested for the luciferase assay. Values are the averages of three independent determinations. The error bars represent standard deviations. *, P < 0.05.
Although Gfi-1B in K562 nuclear extracts could still bind to this GATT site in the presence of the GATA-1 binding competitor, an in vivo ChIP analysis showed a lack of Gfi-1B occupancy of the Bcl-x promoter in GATA-1-depleted cells (Fig. 4D), indicating that the in vivo recruitment of Gfi-1B to the promoter is highly dependent on GATA-1. The discrepancy may be that under the in vitro binding conditions, Gfi-1B is capable of binding to the oligonucleotide covering the GATT site of the Bcl-x promoter but that this binding capability is too weak to be detected within the in vivo chromatin environment.
Imatinib treatment increases the cooccupancy of the Bcl-x promoter by Gfi-1B and GATA-1.
Using ChIP analysis, we also tested the effect of imatinib treatment on the in vivo occupancy of the Bcl-x promoter by Gfi-1B and GATA-1. The results showed an imatinib-responsive increase in the amount of Gfi-1B bound to the proximal Bcl-x promoter region in vivo. The chromatins covering the distal region of the Bcl-x promoter or the c-jun promoter were not precipitated in this ChIP assay (Fig. 6C). To know whether Gfi-1B and GATA-1 simultaneously cooccupy the Bcl-x promoter in vivo, we performed a re-ChIP analysis. First, ChIP was performed using Gfi-1B antibody. The precipitated chromatin fragments were eluted and subjected to reprecipitation using GATA-1 antibody. The same DNA fragment with Gfi-1B and GATA-1 simultaneously bound would be amplified with the subsequent PCR. As shown in Fig. 6D, re-ChIP assays showed that the imatinib treatment increased the amounts of GATA-1 and Gfi-1B bound on the Bcl-x and Gfi-1B promoter regions in K562 cells. In parallel, we observed that the imatinib treatment caused a 41% reduction in luciferase activity expressed from the full-length Bcl-x reporter plasmid (pGL2-3.2) in K562 cells (Fig. 6E). Collectively, these results indicate that the in vivo association of Gfi-1B with the Bcl-x promoter correlates with the increase of Gfi-1B complexed with GATA-1 and the reduction of Bcl-xL transcription in imatinib-treated cells.
Role of Gfi-1B in apoptosis.
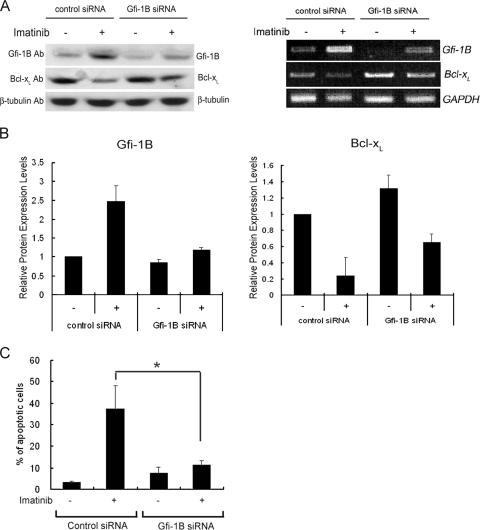
Next, we questioned whether the up-regulation of Gfi-1B is necessary for the imatinib-induced repression of Bcl-xL, since there is a possibility that the lack of phopho-STAT5-dependent transcription in imatinib-treated cells is by itself sufficient to cause a reduction of Bcl-xL transcription. To address this question, we tested the effect of Gfi-1B knockdown on the imatinib-induced repression of Bcl-xL and apoptosis. Without imatinib treatment, K562 cells transfected with Gfi-1B siRNA showed reduced Gfi-1B expression at both the mRNA and protein levels (Fig. 7A). The induction of Gfi-1B by imatinib treatment was diminished in cells transfected with Gfi-1B siRNA, with a concurrent increase in Bcl-xL mRNA and protein levels (Fig. 7B). In parallel, cells were harvested and analyzed by flow cytometry with annexin V staining as an apoptotic indicator. As expected, cells transfected with control siRNA displayed a marked increase in the apoptosis-positive population induced by imatinib treatment. In contrast, transfection with Gfi-1B siRNA effectively abolished imatinib-induced apoptosis (Fig. 7C). Thus, an elevation of Gfi-1B expression is required for imatinib-induced apoptosis.
FIG. 7.
Gfi-1B is required for imatinib-induced apoptosis in K562 cells. K562 cells were transfected with Gfi-1B siRNA or control siRNA. After 24 h, cells were treated with or without 1 μM imatinib for an additional 48 h. (A) Western blot analysis with anti-Gfi-1B, anti-Bcl-xL, or β-tubulin antibody (Ab; left panel) and semiquantitative RT-PCR assay using primers specific for Gfi-1B, Bcl-xL, and the GAPDH gene (right panel). −, absent; +, present. (B) The effect of Gfi-1B siRNA transfection on the imatinib-induced up-regulation of Gfi-1B (left panel) and down-regulation of Bcl-xL (right panel) was evaluated by densitometric determination of the protein expression levels, which were normalized by the β-tubulin level in the corresponding sample. Values are the averages of results from three independent experiments. The error bars represent standard deviations. (C) Cells were also harvested and analyzed by annexin V-FITC staining. Values are the averages of three independent determinations. The error bars represent standard deviations. *, P < 0.05.
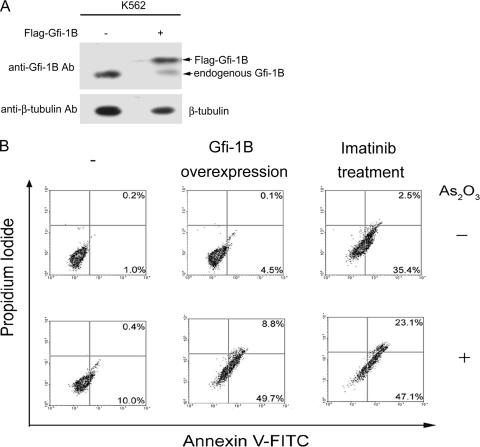
Finally, we addressed whether the overexpression of Gfi-1B can sensitize K562 cells to death induced by other drugs. It has been shown previously that As2O3 treatment induces K562 cell apoptosis without decreasing Bcl-xL expression, and the 50% inhibitory concentration was determined to be 10 μM (29). Herein, we tested whether the overexpression of Gfi-1B can make K562 cells susceptible to As2O3-induced apoptosis. K562 cells were transfected with pCMV2-Flag-Gfi-1B or an empty vector and were treated with or without As2O3 at 0.5 μM for apoptotic analysis. For comparison, parallel sets of control cells were treated with imatinib alone or in combination with As2O3. As shown in Fig. 8B, treatment with As2O3 at a low dosage slightly increased the apoptosis-positive population among cells transfected with the control vector. In contrast, the population of cells overexpressing Gfi-1B showed a significant increase in apoptotic cells in response to As2O3 treatment, as seen among the control cells cotreated with imatinib and As2O3. Thus, the overexpression of Gfi-1B in K562 cells, like cotreatment with imatinib, enhances susceptibility to As2O3-induced apoptosis.
FIG. 8.
Overexpression of Gfi-1B renders K562 cells susceptible to As2O3-induced apoptosis. K562 cells were transfected with pCMV2-Flag-Gfi-1B or an empty vector. (A) After 24 h, total lysates were subjected to Western blot analysis using anti-Gfi-1B and β-tubulin antibodies (Ab). −, absent; +, present. (B) Transfected cells were also treated with or without As2O3 (0.5 μM) as indicated for an additional 48 h. For comparison, a parallel set of cells transfected with an empty vector was treated with imatinib (1 μM) in the presence or absence of As2O3 (0.5 μM). Cells were then harvested for annexin V-FITC and propidium iodide staining prior to flow cytometric analysis.
DISCUSSION
In this study, we demonstrated that GATA-1 is a direct activator of Bcl-xL transcription through binding to the GATT motif. The elevation of Gfi-1B expression in the early stage of erythroid maturation and in imatinib-treated K562 cells suppresses Bcl-xL transcription via the association of Gfi-1B with GATA-1 on the Bcl-x promoter. Thus, Bcl-x is a gene target of the GATA-1-Gfi-1B repression complex. Our results suggest that the down-regulation of Gfi-1B relieves repression, allowing GATA-1 to activate Bcl-xL transcription in the late phase of GATA-1-activated erythroid cells. Thus, GATA-1 and Gfi-1B interplay to control the timing of Bcl-xL expression during erythroid development. Moreover, in this report we present the first evidence that Gfi-1B contributes to apoptotic induction in imatinib-treated K562 cells via its repressor role in Bcl-xL transcription.
Induction of Gfi-1B by inhibiting Bcr-Abl/STAT5 signaling in K562 cells.
The overexpression of Gfi-1B in G1ER cells did not lead to apoptosis because G1ER cells have been transformed by Bcl-2 for the maintenance of a culture of these stem cells as an immortalized cell line (38). In this study, we used K562 cells to induce apoptosis by blocking Bcr-Abl signaling with imatinib treatment. Very interestingly, the inhibition of Bcr-Abl/STAT5 signaling in K562 cells resulted in the up-regulation of Gfi-1B (Fig. 5 and 7A). Given that the depletion of GATA-1 abolished the in vivo binding of Gfi-1B to the Bcl-x promoter while the depletion of Gfi-1B expression diminished imatinib-induced apoptosis (Fig. 4D and 7C), we conclude that the induction of Gfi-1B in imatinib-treated K562 cells contributes to the inhibition of Bcl-xL transcription in a GATA-1-dependent manner.
We also transfected K562 cells with the Gfi-1B promoter reporter plasmid pGL2-hG (−145 to +19) to test whether the trans-activation of Gfi-1B is stimulated by imatinib treatment. We found that imatinib treatment increased the Gfi-1B promoter activity (data not shown). Since the transfection of cells with increasing amounts of a STAT5 (dominant negative) expression vector decreased the Gfi-1B promoter activity stimulated by the imatinib treatment (data not shown), it is conceivable that Gfi-1B expression is negatively modulated by STAT5 signaling in K562 cells. As STAT5 is also a transcriptional activator of Bcl-x, it is likely that blocking STAT5 signaling not only eliminates the activation machinery but also adds a repressor to suppress Bcl-xL expression.
Relationship between GATA-1 and Gfi-1B in binding to the Bcl-x promoter.
It is noted that the Bcl-x promoter contains characteristic motifs for the binding of several transcription factors, including Ets-1, activator protein 4, nuclear factor-erythroid 2, Evi, GATA-1, and activator protein 1 (10), suggesting that Bcl-xL may be induced by different transcription mechanisms dependent on the cellular context and stimulated signals. As mentioned earlier, the role of GATA-1 in Bcl-xL expression has been obscure because mutations of the typical GATA binding site did not affect GATA-1-activated Bcl-xL transcription (9). Although the conserved GATT motif (AATC for the reverse strand), the core of a Gfi-1B binding consensus sequence, is present in the minimal Bcl-x promoter, three different experimental findings obtained from this study indicated that GATA-1 binds to the GATT site directly to regulate transcription. First, the ectopic expression of GATA-1 in NIH 3T3 cells activated the wild-type Bcl-x promoter but not the promoter carrying a mutation in this GATT motif (Fig. 4B). Second, an oligonucleotide covering the GATT site of the Bcl-x promoter could pull down GATA-1 from nuclear extracts from K562 cells, while the mutation of this site disrupted the binding (Fig. 4E and 6B). Third, an oligonucleotide with the consensus GATA-1 binding sequence, but not a mutated GATA oligonucleotide, could compete with GATA-1 for binding to the wild-type sequence of the Bcl-x promoter (Fig. 6B). Since GATA-1 is also a direct activator of Gfi-1B transcription (15), our data suggest that the early induction of Gfi-1B works as a transient repressor of the Bcl-x promoter. Apparently, the GATA-1-mediated activation of the Bcl-x promoter is not switched on until Gfi-1B expression is down-regulated in terminal differentiated erythrocytes. Presumably, the feedback inhibition of Gfi-1B transcription and the instability of Gfi-1B RNA in erythroid cells (half-life, approximately 30 min) (data not shown) are the important mechanisms contributing to the down-regulation of Gfi-1B and allowing GATA-1 to mediate Bcl-xL transcription in the later phase of erythroid maturation.
The GATA-1-Gfi-1B repression complex resides on the Bcl-x promoter.
Very recently, it has been shown that Gfi-1B forms a complex with GATA-1 in murine erythroleukemia cells (12, 24, 28) and that both Gfi-1B and GATA-1 are bound to the myc and myb promoters in vivo (12, 24). Accordingly, it has been proposed that GATA-1 forms a complex with Gfi-1B to repress the expression of myc and myb. Our previous results demonstrate that the Gfi-1B promoter is indeed the target of this repression complex (14). In addition to the results of the oligonucleotide pulldown analysis using imatinib-treated K562 extracts (Fig. 6B), the re-ChIP experimental data proved the simultaneous association of GATA-1 and Gfi-1B on the Bcl-x promoter in vivo (Fig. 6D). Thus, the Bcl-x promoter is another target of the GATA-1-Gfi-1B repression complex.
Adding the apoptotic regulatory role for Gfi-1B.
It has been reported previously that erythroblasts constitutively expressing Gfi-1B failed to differentiate beyond the proerythroblast stage and showed massive apoptosis due to a lack of Bcl-xL expression (22). Data presented in this study offer an explanation of why the enforced expression of Gfi-1B in CD34-positive progenitor cells promotes apoptosis (22). In support of the role of Gfi-1B in apoptotic regulation, we showed that the Gfi-1B-mediated repression of Bcl-xL effectively sensitizes K562 cells to undergo arsenic-induced apoptosis. The mode of action of Gfi-1B on the Bcl-x promoter is similar to what we previously proposed for the feedback inhibition of Gfi-1B transcription (14), which ensures that the level of Gfi-1B expression is controlled within a proper range in erythroid cells. The results shown here exemplify a specific case in which the limitation of Gfi-1B expression is important in minimizing its function of repression of the survival gene Bcl-x.
Experimental results from two independent studies, by Osawa et al. and Garcon et al., suggested that Gfi-1B may have distinct effects at the various stages during erythroid differentiation (7, 22). In immature erythroid progenitors, through the zinc finger domains, Gfi-1B would trans-activate target genes implicated in cell proliferation for erythroblast expansion. At the onset of differentiation, Gfi-1B would regulate genes that have to be repressed for differentiation induction. In this study, we add new information that GATA-1 and Gfi-1B interplay to regulate gene expression in the survival aspect of terminal erythroid differentiation.
Acknowledgments
We are grateful to Dave Turner (University of Michigan, Ann Arbor, MI), Gabriel Núnez (University of Michigan, Ann Arbor, MI), and Dominique Duménil (Institut Gustave Roussy, Paris, France) for kindly providing pCS2-MT, pGL2-3.2, and MIGR-CFlag-Gfi-1B plasmids. We thank James Ihle (St. Jude Children's Research Hospital, Memphis, TN) and Stephen Nimer (Memorial Sloan-Kettering Cancer Center, New York, NY) for providing pRK5-STAT5A, pRK5-STAT5AΔ713, pEQ-PAM3(-E), and pSV-A-MLV-env plasmids. We also thank Stuart H. Orkin (Children's Hospital and Dana Farber Cancer Institute, Howard Hughes Medical Institute, Boston, MA) and Novartis Pharmaceuticals (Basel, Switzerland) for the generous gift of the G1ER cell line and imatinib.
This study was supported by grants NSC95-3112-B-002-006, NSC95-2752-B-002-006-PAE, and NSC94-2320-B-002-018 from the National Science Council, Taiwan, Republic of China.
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Benito, A., M. Silva, D. Grillot, G. Nunez, and J. L. Fernandez-Luna. 1996. Apoptosis induced by erythroid differentiation of human leukemia cell lines is inhibited by Bcl-XL. Blood 87:3837-3843. [PubMed] [Google Scholar]
- 2.de Groot, R. P., J. A. Raaijmakers, J. W. Lammers, and L. Koenderman. 2000. STAT5-dependent cyclin D1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol. Cell Biol. Res. Commun. 3:299-305. [DOI] [PubMed] [Google Scholar]
- 3.Deininger, M. W., J. M. Goldman, and J. V. Melo. 2000. The molecular biology of chronic myeloid leukemia. Blood 96:3343-3356. [PubMed] [Google Scholar]
- 4.Druker, B. J., C. L. Sawyers, H. Kantarjian, D. J. Resta, S. F. Reese, J. M. Ford, R. Capdeville, and M. Talpaz. 2001. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344:1038-1042. [DOI] [PubMed] [Google Scholar]
- 5.Druker, B. J., M. Talpaz, D. J. Resta, B. Peng, E. Buchdunger, J. M. Ford, N. B. Lydon, H. Kantarjian, R. Capdeville, S. Ohno-Jones, and C. L. Sawyers. 2001. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344:1031-1037. [DOI] [PubMed] [Google Scholar]
- 6.Ferrao, P. T., M. J. Frost, S. P. Siah, and L. K. Ashman. 2003. Overexpression of P-glycoprotein in K562 cells does not confer resistance to the growth inhibitory effects of imatinib (STI571) in vitro. Blood 102:4499-4503. [DOI] [PubMed] [Google Scholar]
- 7.Garcon, L., C. Lacout, F. Svinartchouk, J. P. Le Couedic, J. L. Villeval, W. Vainchenker, and D. Dumenil. 2005. Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood 105:1448-1455. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh, A., and H. E. Broxmeyer. 1997. The function of BCR/ABL and related proto-oncogenes. Curr. Opin. Hematol. 4:3-11. [DOI] [PubMed] [Google Scholar]
- 9.Gregory, T., C. Yu, A. Ma, S. H. Orkin, G. A. Blobel, and M. J. Weiss. 1999. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood 94:87-96. [PubMed] [Google Scholar]
- 10.Grillot, D. A., M. Gonzalez-Garcia, D. Ekhterae, L. Duan, N. Inohara, S. Ohta, M. F. Seldin, and G. Nunez. 1997. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J. Immunol. 158:4750-4757. [PubMed] [Google Scholar]
- 11.Grimes, H. L., T. O. Chan, P. A. Zweidler-McKay, B. Tong, and P. N. Tsichlis. 1996. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol. Cell. Biol. 16:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosveld, F., P. Rodriguez, N. Meier, S. Krpic, F. Pourfarzad, P. Papadopoulos, K. Kolodziej, G. P. Patrinos, A. Hostert, and J. Strouboulis. 2005. Isolation and characterization of hematopoietic transcription factor complexes by in vivo biotinylation tagging and mass spectrometry. Ann. N. Y. Acad. Sci. 1054:55-67. [DOI] [PubMed] [Google Scholar]
- 13.Horita, M., E. J. Andreu, A. Benito, C. Arbona, C. Sanz, I. Benet, F. Prosper, and J. L. Fernandez-Luna. 2000. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J. Exp. Med. 191:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, D. Y., Y. Y. Kuo, and Z. F. Chang. 2005. GATA-1 mediates auto-regulation of Gfi-1B transcription in K562 cells. Nucleic Acids Res. 33:5331-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, D. Y., Y. Y. Kuo, J. S. Lai, Y. Suzuki, S. Sugano, and Z. F. Chang. 2004. GATA-1 and NF-Y cooperate to mediate erythroid-specific transcription of Gfi-1B gene. Nucleic Acids Res. 32:3935-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jegalian, A. G., and H. Wu. 2002. Regulation of Socs gene expression by the proto-oncoprotein GFI-1B: two routes for STAT5 target gene induction by erythropoietin. J. Biol. Chem. 277:2345-2352. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian, H., C. Sawyers, A. Hochhaus, F. Guilhot, C. Schiffer, C. Gambacorti-Passerini, D. Niederwieser, D. Resta, R. Capdeville, U. Zoellner, M. Talpaz, B. Druker, J. Goldman, S. G. O'Brien, N. Russell, T. Fischer, O. Ottmann, P. Cony-Makhoul, T. Facon, R. Stone, C. Miller, M. Tallman, R. Brown, M. Schuster, T. Loughran, A. Gratwohl, F. Mandelli, G. Saglio, M. Lazzarino, D. Russo, M. Baccarani, and E. Morra. 2002. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346:645-652. [DOI] [PubMed] [Google Scholar]
- 18.Kindler, T., F. Breitenbuecher, S. Kasper, T. Stevens, B. Carius, H. Gschaidmeier, C. Huber, and T. Fischer. 2003. In BCR-ABL-positive cells, STAT-5 tyrosine-phosphorylation integrates signals induced by imatinib mesylate and Ara-C. Leukemia 17:999-1009. [DOI] [PubMed] [Google Scholar]
- 19.Kirchner, D., J. Duyster, O. Ottmann, R. M. Schmid, L. Bergmann, and G. Munzert. 2003. Mechanisms of Bcr-Abl-mediated NF-kappaB/Rel activation. Exp. Hematol. 31:504-511. [DOI] [PubMed] [Google Scholar]
- 20.Mulloy, J. C., J. Cammenga, K. L. MacKenzie, F. J. Berguido, M. A. Moore, and S. D. Nimer. 2002. The AML1-ETO fusion protein promotes the expansion of human hematopoietic stem cells. Blood 99:15-23. [DOI] [PubMed] [Google Scholar]
- 21.Oetzel, C., T. Jonuleit, A. Gotz, H. van der Kuip, H. Michels, J. Duyster, M. Hallek, and W. E. Aulitzky. 2000. The tyrosine kinase inhibitor CGP 57148 (ST1 571) induces apoptosis in BCR-ABL-positive cells by down-regulating BCL-X. Clin. Cancer Res. 6:1958-1968. [PubMed] [Google Scholar]
- 22.Osawa, M., T. Yamaguchi, Y. Nakamura, S. Kaneko, M. Onodera, K. Sawada, A. Jegalian, H. Wu, H. Nakauchi, and A. Iwama. 2002. Erythroid expansion mediated by the Gfi-1B zinc finger protein: role in normal hematopoiesis. Blood 100:2769-2777. [DOI] [PubMed] [Google Scholar]
- 23.Reuther, J. Y., G. W. Reuther, D. Cortez, A. M. Pendergast, and A. S. Baldwin, Jr. 1998. A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 12:968-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, P., E. Bonte, J. Krijgsveld, K. E. Kolodziej, B. Guyot, A. J. Heck, P. Vyas, E. de Boer, F. Grosveld, and J. Strouboulis. 2005. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 24:2354-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleque, S., S. Cameron, and S. H. Orkin. 2002. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 16:301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattler, M., and J. D. Griffin. 2003. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin. Hematol. 40(Suppl. 2):4-10. [DOI] [PubMed] [Google Scholar]
- 27.Sawyers, C. L., A. Hochhaus, E. Feldman, J. M. Goldman, C. B. Miller, O. G. Ottmann, C. A. Schiffer, M. Talpaz, F. Guilhot, M. W. Deininger, T. Fischer, S. G. O'Brien, R. M. Stone, C. B. Gambacorti-Passerini, N. H. Russell, J. J. Reiffers, T. C. Shea, B. Chapuis, S. Coutre, S. Tura, E. Morra, R. A. Larson, A. Saven, C. Peschel, A. Gratwohl, F. Mandelli, M. Ben-Am, I. Gathmann, R. Capdeville, R. L. Paquette, and B. J. Druker. 2002. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99:3530-3539. [DOI] [PubMed] [Google Scholar]
- 28.Schuh, A. H., A. J. Tipping, A. J. Clark, I. Hamlett, B. Guyot, F. J. Iborra, P. Rodriguez, J. Strouboulis, T. Enver, P. Vyas, and C. Porcher. 2005. ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol. Cell. Biol. 25:10235-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shim, M. J., H. J. Kim, S. J. Yang, I. S. Lee, H. I. Choi, and T. Kim. 2002. Arsenic trioxide induces apoptosis in chronic myelogenous leukemia K562 cells: possible involvement of p38 MAP kinase. J. Biochem. Mol. Biol. 35:377-383. [DOI] [PubMed] [Google Scholar]
- 30.Shuai, K., J. Halpern, J. ten Hoeve, X. Rao, and C. L. Sawyers. 1996. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene 13:247-254. [PubMed] [Google Scholar]
- 31.Sillaber, C., F. Gesbert, D. A. Frank, M. Sattler, and J. D. Griffin. 2000. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood 95:2118-2125. [PubMed] [Google Scholar]
- 32.Silva, M., D. Grillot, A. Benito, C. Richard, G. Nunez, and J. L. Fernandez-Luna. 1996. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood 88:1576-1582. [PubMed] [Google Scholar]
- 33.Steelman, L. S., S. C. Pohnert, J. G. Shelton, R. A. Franklin, F. E. Bertrand, and J. A. McCubrey. 2004. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 18:189-218. [DOI] [PubMed] [Google Scholar]
- 34.Talpaz, M., R. T. Silver, B. J. Druker, J. M. Goldman, C. Gambacorti-Passerini, F. Guilhot, C. A. Schiffer, T. Fischer, M. W. Deininger, A. L. Lennard, A. Hochhaus, O. G. Ottmann, A. Gratwohl, M. Baccarani, R. Stone, S. Tura, F. X. Mahon, S. Fernandes-Reese, I. Gathmann, R. Capdeville, H. M. Kantarjian, and C. L. Sawyers. 2002. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99:1928-1937. [DOI] [PubMed] [Google Scholar]
- 35.Tong, B., H. L. Grimes, T. Y. Yang, S. E. Bear, Z. Qin, K. Du, W. S. El-Deiry, and P. N. Tsichlis. 1998. The Gfi-1B proto-oncoprotein represses p21WAF1 and inhibits myeloid cell differentiation. Mol. Cell. Biol. 18:2462-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida, M., T. Watanabe, M. Kunitama, M. Mori, S. Kikuchi, K. Yoshida, K. Kirito, T. Nagai, K. Ozawa, and N. Komatsu. 2004. Erythropoietin overcomes imatinib-induced apoptosis and induces erythroid differentiation in TF-1/bcr-abl cells. Stem Cells 22:609-616. [DOI] [PubMed] [Google Scholar]
- 37.Vassen, L., K. Fiolka, S. Mahlmann, and T. Moroy. 2005. Direct transcriptional repression of the genes encoding the zinc-finger proteins Gfi1b and Gfi1 by Gfi1b. Nucleic Acids Res. 33:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss, M. J., C. Yu, and S. H. Orkin. 1997. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17:1642-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch, J. J., J. A. Watts, C. R. Vakoc, Y. Yao, H. Wang, R. C. Hardison, G. A. Blobel, L. A. Chodosh, and M. J. Weiss. 2004. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood 104:3136-3147. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, P., X. Zhang, A. Iwama, C. Yu, K. A. Smith, B. U. Mueller, S. Narravula, B. E. Torbett, S. H. Orkin, and D. G. Tenen. 2000. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96:2641-2648. [PubMed] [Google Scholar]
- 41.Zweidler-Mckay, P. A., H. L. Grimes, M. M. Flubacher, and P. N. Tsichlis. 1996. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol. Cell. Biol. 16:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]