Abstract
Gradients of Wnt/β-catenin signaling coordinate development and physiological homeostasis in metazoan animals. Proper embryonic development of the fruit fly Drosophila melanogaster requires the Naked cuticle (Nkd) protein to attenuate a gradient of Wnt/β-catenin signaling across each segmental anlage. Nkd inhibits Wnt signaling by binding the intracellular protein Dishevelled (Dsh). Mice and humans have two nkd homologs, nkd1 and nkd2, whose encoded proteins can bind Dsh homologs (the Dvl proteins) and inhibit Wnt signaling. To determine whether nkd genes are necessary for murine development, we replaced nkd exons that encode Dvl-binding sequences with IRES-lacZ/neomycin cassettes. Mutants homozygous for each nkdlacZ allele are viable with slightly reduced mean litter sizes. Surprisingly, double-knockout mice are viable, with subtle alterations in cranial bone morphology that are reminiscent of mutation in another Wnt/β-catenin antagonist, axin2. Our data show that nkd function in the mouse is dispensable for embryonic development.
Our expanding knowledge of molecular mechanisms that govern vertebrate development stems from a legacy of discovery using model genetic organisms—in particular, the fruit fly Drosophila melanogaster. Just over a quarter century ago, Nüsslein-Volhard, Wieschaus, and their colleagues in Tübingen saturated the fly genome with embryonic lethal mutations that produce altered body patterns, the cloning of which ultimately revealed an evolutionarily conserved “toolkit” of genes—composed predominantly of transcription factors and signal transducers—that executes the development of all of the animals studied to date (8, 55). Because teleost fish—from which modern mammals derive—underwent two rounds of genome duplication 300 to 400 million years ago (27), genes that are present in single copy in protostomes such as Drosophila are often present in multiple copies and act with partial or complete redundancy in mammals. For example, mammals have three hedgehog (hh) and four Notch (N) genes, each of which is present in single copy in flies (2, 29, 48). Duplicate toolkit genes may evolve mutable tissue- or stage-specific functions or may exhibit functional redundancy that is not evident unless all paralogs are simultaneously mutated.
Wnt proteins are a family of intercellular signaling proteins with widespread roles in animal development, tissue homeostasis, and human disease (11, 47). Flies have 7 wnt genes, while mice and humans have 19 (http://www.stanford.edu/∼rnusse/wntwindow.html). Diversification of Wnt proteins occurred early in animal evolution, as the basal cnidarian Nematostella vectensis has 12 wnt genes whose expression in discrete domains along the anterior-posterior axis is reminiscent of fly and mammalian homeotic gene expression (41). Fundamental insights into the mechanism of Wnt signaling emerged from the study of wingless (wg), a Drosophila wnt gene with numerous sequential roles in nearly all of the tissues and life stages of the fly (37). The earliest requirement for wg is during embryo segmentation, where its expression in ectodermal stripes prefigures the segmented body plan (3, 52). When the secreted Wg protein encounters adjacent cells, it elicits a complex signaling cascade, termed the canonical Wnt/β-catenin pathway, that culminates in accumulation of the transcriptional cofactor β-catenin and transactivation of tissue-specific target genes (54, 56, 61). Proper embryonic development requires the graded action of Wg and other signals across each segmental anlage (4, 24, 58). In the absence of wg or key downstream signal transducers, the transcription of target genes such as hh and engrailed (en) ceases, with dramatic consequences for pattern formation; conversely, increased Wg signaling leads to expanded domains of hh and en expression, with opposite but equally dramatic phenotypic consequences (4, 5, 43, 52, 53, 60, 62, 75). Subsequent investigations of Wg and other Wnt proteins have revealed several “noncanonical” signaling pathways, some of which, like the canonical pathway, act through Frizzled (Fz) and Arrow/LRP receptors, some of which may act through Fz and cadherin-family molecules, and others of which act through N or Ryk/Derailed (Drl) receptors (12, 16, 25, 30, 39, 40, 49, 63, 65).
Among the original Tübingen mutant collection is the naked cuticle (nkd) gene (34). Shortly after the onset of Wg action, nkd mutants develop markedly elevated levels of β-catenin and expanded domains of Wg target gene expression despite an apparently normal quantity and distribution of Wg, suggesting that nkd mutant cells are hypersensitive to Wg (5, 52, 67, 75). Molecular characterization of nkd revealed a novel gene whose transcript is Wg inducible, thereby forming a negative feedback loop (75). Nkd can bind and inactivate Dishevelled (Dsh) or its mammalian homologs, the Dvl proteins, a family of intracellular “scaffold” proteins that transduces several types of Wnt signal but whose mechanisms of action remain mysterious and controversial (7, 51, 57, 68-71). Although Dsh is thought to be a hub of cytoplasmic signaling, a recent report suggests that Dsh may also carry Wnt signals into the nucleus (32). Likewise, the mechanism of Nkd action on Dsh in Drosophila remains puzzling but also involves nuclear transport (67).
Mice and humans have two nkd genes, nkd1 and nkd2, that share sequence similarity with fly nkd in two regions: an EF hand-containing domain—termed the EFX domain—that binds Dsh and a C-terminal histidine-rich region (35, 70-72). Alignments of insect and mammalian Nkd proteins reveal four conserved sequence motifs interspersed by mostly unrelated sequence, suggesting a common arrangement of functional motifs in the ancestral Nkd protein (67, 70). Our studies of Drosophila Nkd showed that protein truncations N terminal of Dsh-binding regions produced embryonic lethality with the strongest phenotypic consequences (67, 75). Similarly, mutation or deletion of the mouse Nkd1 EF hand impaired the mutant protein's ability to inhibit Wnt/β-catenin signaling in cultured cells (71). We hypothesized that truncating mutations similar to those that cause strong nkd phenotypes and lethality in Drosophila, when introduced into the mammalian nkd genes, would produce null genetic lesions, possibly resulting in embryonic lethality and/or phenotypes indicative of increased Wnt signaling.
Here we report the generation of mice in which an internal ribosome entry site-β-galactosidase (IRES-lacZ) fusion gene replaces exons encoding nkd1 or nkd2 EFX domains and the results of our breeding experiments. Each nkdlacZ mouse expresses β-galactosidase in patterns that mimic endogenous nkd expression (70). Mice homozygous for each of our nkdlacZ alleles are viable, with slight reductions in mean litter size. Our nkd1 mutant mice do not exhibit the reduced testis mass that was observed in mice homozygous for a distinct nkd1 allele reported by another group during the course of our studies (45). By mating nkd1 to nkd2 mutant mice in a mixed genetic background, we have obtained a small number of adult homozygous double-mutant mice that are viable and fertile. Unlike the strict genetic requirement for nkd in the fly embryo, mouse nkd activity is apparently dispensable for embryonic development.
MATERIALS AND METHODS
Animals.
All of the experiments in this study were performed according to guidelines approved by the Institutional Animal Care and Use Committee (IACUC).
Cloning of nkd1 and nkd2 genomic DNAs.
Bacterial artificial chromosome clones encompassing the mouse nkd1 and nkd2 coding regions were obtained from mouse 129Sv libraries by Southern blotting and PCR screens performed by Research Genetics (now owned by Invitrogen). Each bacterial artificial chromosome was digested with restriction enzymes, and DNA fragments hybridizing to nkd1 or nkd2 cDNA probes by Southern blotting were subcloned into Bluescript-IIKS+ (Stratagene). Subclones encompassing exons 5 to 10 (nkd1) or 4 to 10 (nkd2) were restriction mapped with multiple six-cutter enzymes, and genomic maps consistent with the Celera mouse genomic sequence were assembled as shown in Fig. 1A and 2A.
FIG. 1.
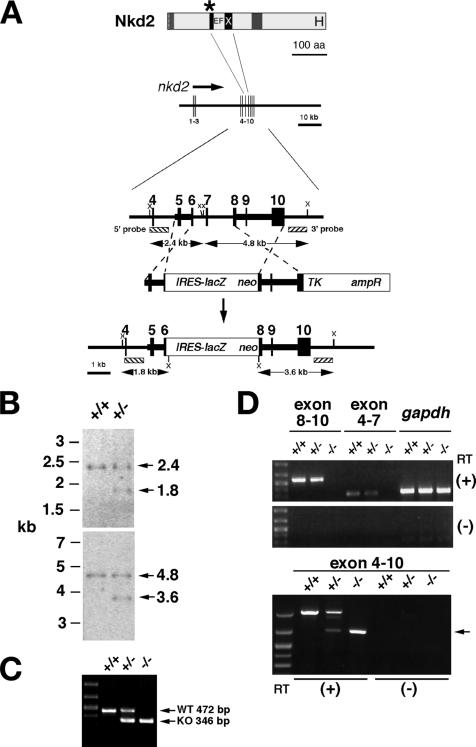
Targeting of mouse nkd1. (A) Schematic of the Nkd1 protein above the genomic region, with exons numbered 1 to 10 and the direction of transcription designated by the arrow. The asterisk designates the position of the stop codon introduced into exon 5. Homologous recombination (dashed lines) replaced exons 5 to 10 with the IRES-lacZ/neo cassette. Enzyme sites: V, EcoRV; R, EcoRI. aa, amino acids. (B) Southern blotting of mouse tail DNA cut with EcoRV (top) or EcoRI (bottom) from the indicated genotypes and probed with the 5′ (top) or 3′ (bottom) external probes as designated in panel A. (C) PCR genotyping with tail DNAs from mice of the indicated genotypes amplified with primers specific for the wild-type (WT) and nkd1lacZ (KO) chromosomes. (D) Northern blotting of adult lung mRNAs from mice of the indicated genotypes probed with nkd1 exon 10 probe (top) and then stripped and reprobed with gapdh as a loading control (bottom). Note that +/− has approximately half the signal from the major 1.8-kb band of +/+ mice and that −/− lung tissue has no specific hybridization signal. (E) Western blotting of extracts from 15.5-dpc nkd1 +/+, +/−, and −/− mouse embryos. Note the reduction of the ∼60-kDa band in the +/− lane and its absence from the −/− lane (arrow). The arrowhead designates a cross-reactive band that confirms equal loading in each lane.
FIG. 2.
Targeting of mouse nkd2. (A) Schematic of Nkd2 protein above the nkd2 genomic region as described for Fig. 1. Homologous recombination replaced exons 6 to 8 with the IRES-lacZ/neo cassette. Enzyme site: X, XbaI. aa, amino acids. (B) Southern blotting of tail DNAs from mice of the indicated genotypes cut with XbaI (top and bottom) and probed with 5′ (top) or 3′ (bottom) external probes as designated in panel A. (C) PCR genotyping with tail DNA from mice of the indicated genotypes amplified by primers specific for the wild-type (WT) and nkd2lacZ (KO) chromosomes. (D) RT-PCR analysis of brain RNA from adult mice of the indicated nkd2 genotypes with primers spanning the designated nkd2 exons or the control gene gapdh, with (+) or without (−) reverse transcriptase (RT). Markers are loaded in the leftmost lanes. (Top) −/− reactions lack specific bands spanning exons 8 to 10 (579 bp) or 4 to 7 (323 bp) that were deleted by the targeting event. gapdh (350 bp) is a positive control that is amplified from all three samples. (Bottom) +/+ mice have a 1,025-bp band corresponding to the full-length exon 4 to 10 product. +/− mice have 1,025- and 699-bp bands, while −/− mice only have the 699-bp band (arrow). Sequencing of the 699-bp band revealed exon 5-9-10 splicing, predicting the synthesis of an out-of-frame, truncated protein (data not shown).
Targeting constructs.
We used the targeting vector pGKneoTK3.IRESlacZ made by Andreas Kispert (cited in reference 76). For the 5′ arm of nkd1, a 1.2-kb SalI/XhoI PCR product that introduced a stop codon at amino acid 91 in exon 5 was cloned into the SalI site; for the 3′ arm, a 3-kb SspI/HindIII fragment within the 3′ untranslated region was cloned into the NotI/BamHI site to make the construct shown in Fig. 1A. For the 5′ arm of nkd2, a 0.8-kb SalI/XhoI PCR product that introduced a stop codon into exon 6 at amino acid 121 was cloned into the SalI site; for the 3′ arm, a 2.2-kb SacI/NcoI fragment encompassing exons 8 to 10 was cloned into the NotI/BamHI site to make the construct shown in Fig. 2A.
ES cell culture.
SalI-linearized targeting constructs for each gene were electroporated into 129SvEv embryonic stem (ES) cells by standard methods, the cells were subjected to positive and negative selection, and surviving clones were screened by PCR. The targeting frequencies were as follows: nkd1, 2/900 clones; nkd2, 4/1,460 clones. Each was verified by PCR and Southern blotting with 5′ and 3′ probes external to each targeting arm as shown in Fig. 1A and 2A.
Chimeric-mouse generation.
Chimeric mice were generated from two independent ES cell clones for each gene (nkd1, 1G3 and 3H7; nkd2, 5C12 and 9H3), and similar genotyping, expression, and breeding results were obtained for each clone. The nkd1lacZ and nkd2lacZ mice generated in this study have been deposited and are available through Jackson Laboratories (stock no. 6958 and 6960).
Southern blotting.
Tail DNA was digested with EcoRV or EcoRI (for nkd1) or XbaI (for nkd2) at 37°C overnight, separated on a 0.8% agarose gel, transferred to Hybond-N+ (Amersham Pharmacia Biotech) membrane by alkaline transfer, probed with the indicated 32P-labeled DNA fragments schematized in Fig. 1A and 2A, and exposed for autoradiography.
PCR genotyping.
The wild-type nkd1 allele was detected with primers from intron 4 (N1-IN4-5p, CCTGCTGAAGTGGTTGGTAG) and exon 5 (N1-EX5-3p, GAGAACTCCTCCCATTTAGATG) to generate a 487-bp product, while the recombined nkd1lacZ allele was detected with primer N1-IN4-5p and a primer from the IRES-lacZ vector (ILZ-3p, TAGAGCGGCCTACGTTTAAACATCG) to generate a 346-bp product. The wild-type nkd2 allele was detected with primers in intron 5 (N2-IN5-5p, TGGTCAGGGAACGACAGAG) and exon 6 (N2-IN6-3p, GTTCCAGATCTCAAGAGGTC) to generate a 472-bp product, while the recombined nkd2lacZ allele was detected with primers N2-IN5-5p and ILZ-3p to generate a 346-bp product. PCR amplification of genomic DNA was performed with 35 cycles of denaturation at 94°C, annealing at 58°C, and extension at 72°C, and DNA was visualized on a 1.2% agarose gel with ethidium bromide as shown in Fig. 1C and 2C.
Northern blotting.
Mouse tissues were flash frozen in liquid N2 and Dounce homogenized, and RNA was isolated by the protocol provided with the TRIzol reagent (Invitrogen). Oligo(dT)-selected mRNA was separated on a 1% agarose gel in morpholinepropanesulfonic acid (MOPS) buffer, transferred to Hybond-N+ membrane, and hybridized to 32P-labeled DNA probes as shown in Fig. 1D.
Reverse transcription (RT)-PCR.
RNA was isolated from mouse brain of the genotypes indicated in Fig. 2D as described above, reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen), and subject to PCR as follows. nkd2 exons 4 to 7 were detected with exon 4 (N2-EX4-5p, AGGAGCTGCTCAATGGAGA) and exon 7 (N2-EX7-3p, ACCTCGTAGATGGTGTGCAT) primers to synthesize a 323-bp product; exons 8 to 10 were detected with exon 8 (N2-EX8-5p, CGTGGCAGAACAGAGATTG) and exon 10 (N2-EX10-3p, TACCGCTTGTGACCATAGG) primers to synthesize a 579-bp product; and exons 4 to 10 were detected with primers N2-EX4-5p and N2-EX10-3p to synthesize a 1,025-bp product from the wild-type chromosome or a 699-bp product from the nkd2lacZ chromosome. Sequencing of the 699-bp band from nkd2lacZ/lacZ animals confirmed splicing between exons 5 and 9 that predicts the synthesis of an out-of-frame, prematurely truncated protein (data not shown).
Western blotting.
Embryos of each genotype at 15.5 days postcoitus (dpc) were pulverized in liquid N2 and then homogenized with a Teflon-glass homogenizer in triple-detergent buffer (50 mM Tris, 50 mM NaCl [pH 7.4], 1% NP-40, 0.1% sodium dodecyl sulfate, 0.5% Na-deoxycholate, 5 mM EDTA, 1 mM EGTA, 1 mM Na-orthovanadate, 50 mM NaF, 1× complete protease inhibitor [Roche]), and extracts were spun at 1,000 × g for 10 min. A 200-μg sample of soluble extract, quantitated by Bradford assay (Bio-Rad), was resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked in 5% dry milk for 1 h at room temperature and then incubated with a rabbit polyclonal antibody against Nkd1 (1:1,000; Cell Signaling Technology) or four different Nkd2 antibodies (one made by the University of Texas [UT] Southwestern Antibody Production Core and three made by Cell Signaling Technology; data not shown) at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:1,500; Pierce) at room temperature for 1 h. Signals were visualized with the SuperSignal West Chemiluminescent Substrate kit (Pierce).
X-Gal staining.
Embryos and adult tissues were dissected in phosphate-buffered saline, fixed in 4% paraformaldehyde-phosphate-buffered saline for 1 h at 4°C, rinsed three times for 30 min each time in rinse buffer (100 mM sodium phosphate, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40), and then incubated in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining solution (100 mM sodium phosphate, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml X-Gal) overnight at 37°C to detect β-galactosidase activity. Sectioned X-Gal-stained tissues were counterstained in nuclear fast red.
Immunohistochemistry.
β-Catenin immunostaining was performed in duplicate on testis tissue from three animals of each genotype at room temperature with the Animal Research Kit (Dako) and antigen retrieval reagent BORG (BioCare Medical). Anti-β-catenin monoclonal antibody E-5 (Santa Cruz Biotechnology) was used at 1:40, and slides were counterstained with hematoxylin.
Skeletal preparations.
Adult mice of the ages indicated were euthanized by CO2 inhalation, skinned, eviscerated, fixed in 95% ethanol overnight, and then rocked in acetone overnight to clear adipose tissues. The specimens were stained with alcian blue staining solution (4 volumes 95% ethanol, 1 volume acetic acid, and 0.15 mg alcian blue/ml) for 48 h, rinsed in 95% ethanol for >5 h, treated with 2% KOH overnight to remove soft tissues, and then stained with alizarin red staining solution (1% KOH plus 0.05 mg alizarin red/ml) for 30 to 90 min. The specimens were cleared in 1% KOH plus 20% glycerol for >2 days and then photographed in 50% ethanol-50% glycerol.
Dual-energy X-ray absorptiometry scans.
Percent body fat and bone mineral density were determined with a GE-Lunar PIXImus equipped with software version 2.1.
Statistical analysis.
The significance of mouse body and testis weights in Fig. 4 and 5B was calculated with a Student unpaired t test.
FIG. 4.
Growth curves of wild-type and nkd1 and nkd2 mutant mice. Shown are mean weights ± standard deviations for the indicated numbers of male (top) and female (bottom) mice at the indicated postnatal weeks of age. At 12 weeks, nkd1lacZ/lacZ but not nkd2lacZ/lacZ mice are significantly smaller than wild-type mice, with P values indicated.
FIG. 5.
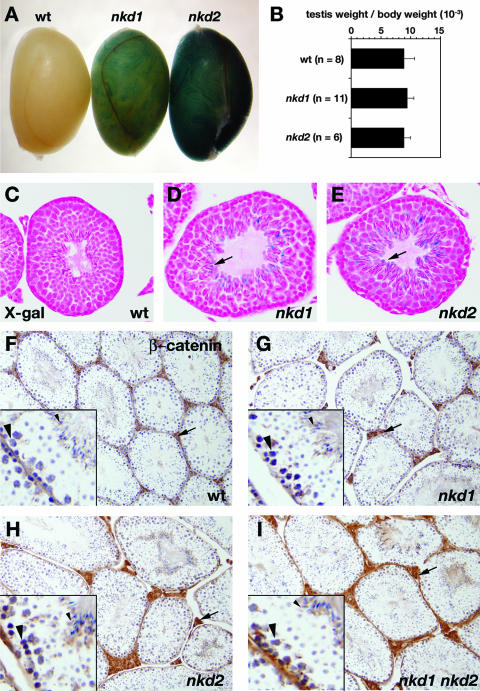
nkd mutant testes resemble wild-type testes. (A) X-Gal-stained wild-type (wt, left), nkd1lacZ/lacZ (middle), and nkd2lacZ/lacZ (right) testis tissues from 3-month-old males. (B) Ratios of combined testis weight to body weight for the indicated numbers of 3-month-old wild-type (top bar) and homozygous nkd1 (middle bar) or nkd2 (bottom bar) mutant male mice. (C to E) High-power magnifications of X-Gal-stained testis tubules from wild-type (C), nkd1lacZ/lacZ (D), and nkd2lacZ/lacZ (E) mice. Note the X-Gal-positive elongating sperm near the tubular lumen (arrows) in panels D and E. (F to I) β-Catenin immunohistochemistry of wild-type (F), nkd1lacZ/lacZ (G), nkd2lacZ/lacZ (H), and nkd1lacZ/lacZ nkd2lacZ/lacZ (I) testes revealed by DAB staining (brown) shows similar distribution and intensity of intertubular (Leydig cell, arrow) staining, as well as basal spermatocyte nuclei (large arrowhead in lower left of each inset) and elongating spermatids (small arrowhead in upper right of each inset). Note the similar staining of all four genotypes.
RESULTS
Targeting of the nkd1 and nkd2 genes.
nkd1 and nkd2 are single-copy genes on mouse chromosomes 8C1 and 13C4, respectively; humans have single orthologs of each gene on chromosomes 16q12.1 and 5p15.3, with no additional closely related sequences or pseudogenes identified by genomic BLAST searches of the mouse and human genomes. In order to generate null mutations in nkd1 and nkd2, we made mice harboring stop codons 5′ of each encoded EFX domain by replacing exons 5 to 10 (nkd1) or 6 to 8 (nkd2) with IRES-lacZ (Fig. 1A and 2A; see Materials and Methods). Southern blot assays, PCR, and genomic sequencing confirmed each targeting event, both in ES cells and following germ line transmission in mice (Fig. 1B and C and 2B and C and data not shown). Northern blotting and/or RT-PCR of tissues from homozygous mutant animals confirmed the predicted molecular nature of each targeting event (Fig. 1D and 2D and data not shown). Western analysis of 15.5-dpc embryo extracts of each nkd1 genotype with affinity-purified anti-Nkd1 antisera confirmed the absence of Nkd1 protein in homozygous mutants (Fig. 1E). Unfortunately, four independent antisera against Nkd2 were unable to detect endogenous Nkd2 from either lysates or immunoprecipitates of 15.5-dpc embryos, despite the facts that nkd2 mRNA and nkd2lacZ are abundant at that time and that each antiserum was able to detect overproduced Nkd2 by Western blotting of Nkd2-transfected cultured cells (70) (Fig. 3D and data not shown). We verified the homozygous viable nature of nkd2 mutation by introducing a genetic lesion of comparable severity to nkd2lacZ by excising exon 5 via Cre-lox technology (T.C. and K.W., unpublished data).
FIG. 3.
Expression patterns of nkd1lacZ and nkd2lacZ. (A and B) X-Gal staining of 12.5-dpc embryos harvested from nkd1lacZ/+ (A) or nkd2lacZ/+ (B) heterozygous intercrosses reveals an intensity of blue staining that increases with the nkdlacZ copy number. (C) Ten and one-half day postcoitus nkd1lacZ/+ (left) or nkd2lacZ/+ (right) embryo. Note dorsal CNS staining in nkd1lacZ that is absent from nkd2lacZ (arrows) but similar somite (arrowheads) and tail bud (asterisks) expression. (D) Fifteen and one-half day postcoitus nkd1lacZ/+ (left) and nkd2lacZ/+ (right) embryos. Note the similar expression of each gene in the snout, ear, and hair follicles (panels D′ to D″) but distinct staining patterns in the distal limb buds. (E and F) Liver tissue from adult nkd1lacZ/+ (E) or nkd2lacZ/+ (F) mice showing pericentral hepatocyte staining in the former but not the latter. (G) Snout tissue of a 15.5-dpc nkd1lacZ/+ mouse showing expression in whisker mesenchyme (arrow) and dermal papillae (arrowhead). (H) nkd2lacZ/+ mouse intestine tissue showing staining of the wall musculature (arrowhead).
nkd1lacZ and nkd2lacZ expression resembles endogenous nkd mRNA.
As expected, the intensity of X-Gal staining in nkdlacZ embryos or tissues strictly correlated with the gene dosage, with homozygous mutants consistently staining more intensely than heterozygotes (Fig. 3A and B). We observed patterns of X-Gal staining in each nkdlacZ mouse that closely mimic the dynamic patterns of endogenous nkd1 and nkd2 mRNAs (e.g., cf. reference 70 and Fig. 3C), making each nkdlacZ strain potentially useful as a reporter for Wnt-dependent transcriptional activity. For example, while nkd1lacZ and nkd2lacZ are each expressed in the somites and tail bud through mid-embryogenesis, nkd1lacZ but not nkd2lacZ is expressed in the dorsal embryonic CNS and in adult pericentral hepatocytes (Fig. 3C, E, and F), and both reporters are expressed in the intestinal musculature (Fig. 3H and data not shown). The expression of nkd1lacZ in some pericentral hepatocytes may reflect regulation by Wnt/β-catenin signals (6). By 15.5 dpc, nkd1lacZ and nkd2lacZ are expressed in similar patterns within the mesenchyme and dermal papillae of developing vibrissae and pelage hair follicles (Fig. 3D, D″, and G), where the roles for Wnt signaling in stem cell maintenance and follicle morphogenesis are well recognized (1).
nkd1 or nkd2 knockout mice are viable and fertile.
In a mixed 129-Bl6 genetic background, heterozygous intercrosses of each nkdlacZ allele gave rise to viable progeny in roughly Mendelian ratios (Table 1) and animals homozygous mutant for each nkd allele were viable and fertile (Table 1). Growth curves for 4- to 12-week-old pups fed ad libitum showed a slight yet statistically significant retardation of growth (males, 15%; females, 4.6%) for nkd1lacZ/lacZ by 12 weeks, similar to a previously reported nkd1 allele (45), but no growth abnormality in nkd2lacZ/lacZ (Fig. 4). Histology and weights of major organs from 3-month-old mice were comparable between the wild type and each mutant (n = 6 to 11 animals of each genotype; data not shown). We then crossed our original allele-transmitting chimeras into the 129SvEv background and found that heterozygous intercrosses of mice carrying each nkd allele also yielded roughly Mendelian progeny ratios but reduced mean litter sizes relative to the mixed background (Table 1).
TABLE 1.
Breeding results from nkd1lacZ and nkd2lacZ crossesa
| Background and cross | No. of pairs | No. of litters | No. of pups/litter | No. of mice of each genotype
|
||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | ||||
| Bl6-129 hybrid | ||||||
| nkd1lacZ/+ × nkd1lacZ/+ | 9 | 15 | 7.3 | 32 | 55 | 23 |
| nkd2lacZ/+ × nkd2lacZ/+ | 5 | 14 | 7.4 | 25 | 52 | 26 |
| 129 | ||||||
| nkd1lacZ/+ × nkd1lacZ/+ | 10 | 23 | 4.9 | 29 | 50 | 27 |
| nkd2lacZ/+ × nkd2lacZ/+ | 3 | 4 | 5.8 | 9 | 7 | 7 |
| Total (both) | ||||||
| nkd1lacZ/+ × nkd1lacZ/+ | 61 | 105 | 50 | |||
| nkd2lacZ/+ × nkd2lacZ/+ | 34 | 59 | 33 | |||
| Bl6-129 hybrid | ||||||
| nkd1lacZ/lacZ × nkd1lacZ/lacZ | 6 | 19 | 5.3 | |||
| nkd2lacZ/lacZ × nkd2lacZ/lacZ | 9 | 33 | 6.4 | |||
| 129 | ||||||
| nkd1lacZ/lacZ × nkd1lacZ/lacZb | 6 | 1 | 1 | |||
| nkd2lacZ/lacZ × nkd2lacZ/lacZ | 6 | 20 | 4.5 | |||
For each cross, the number of breeding pairs, the number of litters, and the mean number of pups per litter are indicated.
bAmong six breeding pairs, a single litter consisting of a single pup was produced during a 3-month period. No histologic abnormalities were noted in the testes or ovaries of nkd1lacZ/lacZ animals in the 129 background, although one of the males had unilateral testicular agenesis.
nkd1lacZ/lacZ and nkd2lacZ/lacZ mice in a mixed background were fertile but with slightly reduced mean litter sizes compared to those of heterozygotes (Table 1). In view of the important role for Wnt/β-catenin signaling in the maintenance of bone mass (21, 38), examination of alizarin red- and alcian blue-stained 3-month-old female skeletons (two wild type, four nkd1lacZ/lacZ, and two nkd2lacZ/lacZ), as well as dual-energy X-ray absorptiometry scans and subsequent histologic sections of knee joints (four wild type, five nkd1lacZ/lacZ, and five nkd2lacZ/lacZ), did not yield any consistent differences between wild-type and nkd1 or nkd2 mutant animals (data not shown).
In the course of our studies, another group reported that homozygosity for a distinct targeted mutation in nkd1 caused a slight reduction in testis size and reduced numbers of elongated sperm (45). In contrast to their report, our 3-month-old nkd1lacZ/lacZ (and nkd2lacZ/lacZ) mice had testis-to-body weight ratios similar to those of the wild type (Fig. 5B). X-Gal staining of mutant testis tissue revealed β-galactosidase activity in elongating spermatids within testicular tubules (Fig. 5C to E), consistent with previous observations (45). However, in contrast to the previously reported nkd1 mutant mice, we did not observe any obvious differences in β-catenin abundance or distribution by diaminobenzidine (DAB) immunohistochemistry between wild-type and nkd1 or nkd2 mutant testes (three testes per genotype) (Fig. 5F to H). Unlike our knockout alleles, which are each predicted to encode proteins truncated near their N termini, the previously reported nkd1 allele encodes an internally deleted ∼45-kDa Nkd1 protein that lacks the Dvl-binding EFX domain, and its persistence in the previously reported knockouts (45) may interfere with Wnt/β-catenin signaling and/or Dvl homeostasis in a manner distinct from that of our mutations.
nkd1 nkd2 double-knockout mice are viable.
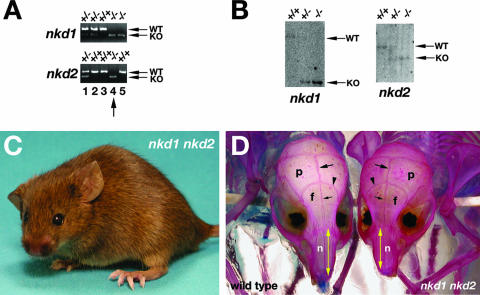
nkd1 and nkd2 are expressed in partially redundant patterns throughout gestation (e.g., Fig. 3D), so the absence of visible embryonic phenotypes could be due to genetic redundancy. To test this possibility, we mated nkd1lacZ and nkd2lacZ mice in the mixed genetic background. nkd1lacZ/+ nkd2lacZ/+ mice were viable and fertile, as were their progeny with the genotypes nkd1lacZ/lacZ nkd2lacZ/+, nkd1lacZ/+ nkd2lacZ/lacZ, and nkd1lacZ/lacZ nkd2lacZ/lacZ. However, females possessing no or one wild-type copy of nkd1 or nkd2 did not tend to their young as well as nkd1 or nkd2 homozygous mutants, as 35% of 46 litters from mothers with no or one wild-type copy of nkd1 or nkd2 were cannibalized by their mothers prior to weaning, compared to 14% of 51 litters from nkd1lacZ/lacZ or nkd2lacZ/lacZ single-mutant mothers. Nevertheless, by breeding male and female compound heterozygous mice, we raised two male and three female nkd1lacZ/lacZ nkd2lacZ/lacZ mice from 12 litters, confirming their genotype by PCR and Southern blotting (e.g., Fig. 6A to C). No histologic abnormalities were observed in major organs of the five double-knockout mice obtained (data not shown), and the abundance and distribution of β-catenin by DAB immunohistochemistry in double-knockout testes resembled the wild type and the single mutants (Fig. 5F to I). However, skeletal preparations of male and female 2-month-old double-knockout mice revealed a slightly shortened skull and nasal bones (Fig. 6D and data not shown) that are similar to but less severe than those of animals homozygous mutant for another antagonist of Wnt/β-catenin signaling, axin2 (46, 73). While axin2 mutant cranial bone sutures underwent premature fusion (73) in a manner that is perhaps similar to human craniosynostosis, such fusions were not evident in the two nkd double-knockout animals examined (Fig. 6D and data not shown), suggesting that the nkd mutant phenotype is not as severe as that of axin2.
FIG. 6.
nkd1 nkd2 double-mutant mice are viable. (A) PCR genotyping of a subset of progeny of an nkd1lacZ/+ nkd2lacZ/+ intercross for nkd1 and nkd2 gene status. Note that lane 4 DNA is from a double knockout. WT, wild type; KO, knockout. (B) Southern blotting of wild-type (left lanes), nkd1lacZ/+ nkd2lacZ/+ (middle lanes), and nkd1lacZ/lacZ nkd2lacZ/lacZ (right lanes) DNAs probed for nkd1 (left blot) and nkd2 (right blot) status. (C) Three-month-old nkd1lacZ/lacZ nkd2lacZ/lacZ adult female with a slightly short nose. (D) Rostral view of wild-type (left) and nkd1lacZ/lacZ nkd2lacZ/lacZ (right) 2-month-old female skeleton preparations. Cranial bones: parietal (p), frontal (f), and nasal (n). Sutures: sagittal (large arrows), coronal (arrowheads), and posterior frontal (small arrows). The double line of alizarin red stain at sutures suggests an absence of craniosynostosis. Note that the length of the nasal bone, from the frontonasal suture to its anterior tip, is decreased in an nkd1lacZ/lacZ nkd2lacZ/lacZ mouse (double yellow arrows).
DISCUSSION
The discovery of a discrete “toolkit” of conserved genes that govern the development of all animals was one of the great epiphanies of 20th century biology. Wnt proteins are among the most ancient of the toolkit proteins and remain a subject of intense biomedical interest not only for their roles in a diverse array of physiological processes but also due to frequent misregulation of Wnt signaling in human disease. The widespread activation of Wnt, Hedgehog, EGF, and Notch signaling in human cancer underscores each cell's critical task of controlling these signaling systems in their normal contexts, where they enforce stem cell and tissue homeostasis (9, 28, 64). Multiple control mechanisms have evolved to ensure that each of these influential signals is active only at the right place and for a limited time. In addition to restricting the synthesis of pathway-stimulating ligands, feedback regulation is a key mechanism that limits the action of these signals (17).
Our previous work showed that nkd is a negative feedback regulator for Wg signaling in Drosophila and that mice and humans have two nkd-related genes, nkd1 and nkd2 (70, 75). Guided by our studies of Drosophila nkd function, we generated early truncating lesions in each mouse nkd gene to investigate whether they are essential for development. To our surprise, nkd genes are dispensable for mouse development, as we only observed subtly reduced fertility, increased cannibalization of litters, and altered cranial bone morphology in mutant animals. We surmise that each of these phenotypes is modifiable by genetic background, as litter sizes were consistently reduced in the pure 129 background compared to the mixed Bl6-129 background. We also observed shortened nasal bones in nkd1lacZ/lacZ single-mutant animals in the 129 background—similar to those seen in the double-knockout animals in the mixed background—which was not evident for nkd1lacZ/lacZ in the mixed background (data not shown), indicating that the cranial abnormalities in our nkd mutants are also strain dependent.
The similarity between nkd- and axin2-encoded mutant cranial phenotypes is likely not a coincidence because both genes are inducible inhibitors of Wnt/β-catenin signaling (50). Mice express another axin gene, axin1, that is not regulated by Wnt signaling but which is essential for early development (74). Any differences in axin1 and axin2 function are the result of distinct expression patterns, because the endogenous mouse axin1 coding region can be replaced with the axin2 cDNA without phenotypic consequence (10). It is intriguing that just as our nkd mutant mouse phenotype is less severe than the axin2 phenotype, the cuticle phenotype of Drosophila nkd mutants is typically not as severe as that of axin mutants (23, 34, 67). Genetic epistasis has also revealed an important difference between the nkd and axin genes in Drosophila, with the nkd phenotype being signal dependent and axin signal independent (5, 23, 57). If this relationship is also true in mammals, then one may predict that the nkd mutant but not the axin2 mutant phenotype should be suppressed by reducing the wnt or dvl gene dosage. In spite of this conjecture, the axin2 mutant cranial phenotype is partially suppressed by heterozygosity for β-catenin (46), consistent with axin1-dependent regulation of β-catenin levels in axin2 mutant adults.
If the mouse nkd genes are dispensable for development, then why are they conserved in evolution? Let us first reconsider the function of nkd in Drosophila. Early in fly development, just prior to the time when Wg is active, nkd is broadly transcribed in the embryo (75). nkd accumulation in proportion to the Wg signal then limits β-catenin accumulation and Wg target gene expression (14, 42, 43, 52, 62, 75). Despite the complex, Wg-dependent striped pattern of nkd expression evident during segmentation, ubiquitous Nkd can rescue an nkd mutant to adulthood, indicating that nkd regulation by Wg signaling is not strictly necessary for Nkd activity (67). In an otherwise wild-type background, Nkd overproduction had little effect on the fly embryo pattern (33, 75). Similarly, we were unable to observe any obvious consequences for development or physiology when mouse Nkd1 was expressed in transgenic mice under the control of the ubiquitous elongation factor 1-α promoter or the inducible metallothionein promoter (M. Zhang, M. Amanai, and K. A. Wharton, Jr., unpublished observations). However, in Drosophila, severe loss-of-Wg signaling phenotypes can be induced in the embryo when Nkd is overproduced in a genetic background compromised for Wg pathway activity (33, 75) or when Nkd is overproduced through several days of larval and pupal development, resulting in a spectrum of adult phenotypes indicative of reduced Wg signaling or dsh activity (57, 75). Thus, it is possible that our nkd1 transgenes were unable to drive expression to levels high enough to produce visible phenotypes and/or that mammalian Nkd1 is an intrinsically weaker antagonist of Wnt signaling than Drosophila Nkd, as we have observed when either is misexpressed in Drosophila or Xenopus (70, 75; P. S. Klein, unpublished observation).
In a fashion reminiscent of the Wg-dependent expression of nkd in the fly, each mouse nkd gene is expressed in dynamic, gradient-like patterns throughout embryonic development, presumably as a consequence of regulation by Wnt and other signaling pathways (13, 31, 59, 70). In addition to a common organization of sequence motifs in all known Nkd proteins (67), the exquisite conservation of several putative ion chelation residues in the “loop” of the EFX domain in all of the Nkd proteins sequenced to date (70, 75; K.W., unpublished data) strongly suggests conserved functions involving Dsh/Dvl and possibly other proteins that associate with this domain. However, a mutant Nkd protein—albeit overproduced—that lacks the EFX domain can rescue a fly nkd mutant (67), indicating that the EFX domain, at least in Drosophila, is not strictly required for Nkd activity. The present study also shows that Nkd EFX domains are not required for mouse development; however, in contrast to the situation in the fly (67), we show that the mouse nkd genes are themselves dispensable for embryonic development.
At least three hypotheses to account for the present results can be entertained. First, an absolute requirement for the nkd genes may not be revealed when breeding mice in a controlled environment under artificial selection. Consistent with this idea is our observation that mothers with no or one residual copy of wild-type nkd had an increased propensity to cannibalize their young after birth, suggesting behavioral or stress-related reproductive phenotypes that will be the subject of future investigation.
A second possibility is that any alterations in Wnt/β-catenin signaling that arise in animals lacking nkd activity during embryonic development may be compensated for by altered expression of other Wnt pathway antagonists, such as those of the secreted Frizzled-related protein (sFRP), Cerberus, WIF, and Dickkopf (Dkk) families, which act extracellularly (36), or Axin2 and Lef1, which act intracellularly (26, 44, 50, 72). Indeed, the requirement for nkd in Drosophila may be absolute because flies do not possess homologs of the extracellular antagonist sFRP, Cerberus, or Dkk families, and the lone Drosophila WIF protein is dedicated to regulating Hh and not Wnt signaling (20, 22). In contrast to the mammalian genes, there have been no reports of transcriptional regulation of the Drosophila axin or tcf gene, and other genes such as wingful and notum appear to play more important roles in regulating postembryonic Wg signaling than nkd (18, 19, 75).
Finally, the function of nkd genes and their likely retention throughout much of the animal kingdom may be rooted in the phenomenon of canalization, a term that describes the capacity of evolved life to minimize phenotypic variation in the face of perturbations in the environment or genetic background (66; reviewed in reference 15). The mammalian nkd genes may fine-tune the levels and/or activities of the Dvl proteins so that downstream signaling events occur with high fidelity. Thus, a strict requirement for nkd genes may be revealed by experiments that measure the extent to which nkd mutant animals are susceptible to genetic or pharmacologic perturbation in Wnt or Dvl signaling. Future behavioral experiments, or experiments in which mutations in the murine nkd genes are combined with mutations in other Wnt signaling regulators, may reveal additional roles for these evolutionarily well-conserved genes.
Acknowledgments
Special thanks go to Matthew Scott, in whose laboratory the genomic characterization of murine nkd genes was initiated. Thanks go to Jon Graff for the targeting vector; Robert Hammer, Robin Nguyen, and John Ritter for expert ES cell culture and chimeric-mouse production; Makoto Kuro-O and Ljiljana Milenkovic for transgenic mouse vectors; Jim Richardson and John Shelton for histology; Wayne Lai and the UT Southwestern Antibody Core for antibodies; Xiaowu Zhang and Cell Signaling Technology for generously providing anti-Nkd1 and anti-Nkd2 antibodies; Mike Arnold, Yuri Kim, and Eric Olson for advice regarding skeletal preparations; Kevin White for introducing K.A.W. to the concept of canalization; and Chih-Chiang Chan, Jin Jiang, and Robert Hammer for comments on the manuscript.
K.A.W. acknowledges Matthew Scott for support of J.K. by the Howard Hughes Medical Institute. K.A.W. is a W. W. Caruth, Jr., Scholar in Biomedical Research at UT Southwestern and has received support from the Texas affiliate of the American Heart Association, an Institutional Junior Faculty Research Grant from the American Cancer Society, UT Southwestern Dean's funds, the Department of Pathology, NIH K08-HD01164, and R01-GM65404.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Alonso, L., and E. Fuchs. 2003. Stem cells in the skin: waste not, Wnt not. Genes Dev. 17:1189-1200. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas, S., K. Matsuno, and M. E. Fortini. 1995. Notch signaling. Science 268:225-232. [DOI] [PubMed] [Google Scholar]
- 3.Baker, N. E. 1988. Localization of transcripts from the wingless gene in whole Drosophila embryos. Development 103:289-298. [DOI] [PubMed] [Google Scholar]
- 4.Bejsovec, A., and A. Martinez Arias. 1991. Roles of wingless in patterning the larval epidermis of Drosophila. Development 113:471-485. [DOI] [PubMed] [Google Scholar]
- 5.Bejsovec, A., and E. Wieschaus. 1993. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 119:501-517. [DOI] [PubMed] [Google Scholar]
- 6.Benhamouche, S., T. Decaens, C. Godard, R. Chambrey, D. S. Rickman, C. Moinard, M. Vasseur-Cognet, C. J. Kuo, A. Kahn, C. Perret, and S. Colnot. 2006. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell 10:759-770. [DOI] [PubMed] [Google Scholar]
- 7.Boutros, M., and M. Mlodzik. 1999. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83:27-37. [DOI] [PubMed] [Google Scholar]
- 8.Carroll, S. B., J. K. Grenier, and S. D. Weatherbee. 2001. From DNA to diversity: molecular genetics and the evolution of animal design. Blackwell Science, Inc., Malden, MA.
- 9.Casci, T., and M. Freeman. 1999. Control of EGF receptor signalling: lessons from fruitflies. Cancer Metastasis Rev. 18:181-201. [DOI] [PubMed] [Google Scholar]
- 10.Chia, I. V., and F. Costantini. 2005. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol. Cell. Biol. 25:4371-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevers, H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469-480. [DOI] [PubMed] [Google Scholar]
- 12.Couso, J. P., and A. Martinez Arias. 1994. Notch is required for wingless signaling in the epidermis of Drosophila. Cell 79:259-272. [DOI] [PubMed] [Google Scholar]
- 13.Dequeant, M. L., E. Glynn, K. Gaudenz, M. Wahl, J. Chen, A. Mushegian, and O. Pourquie. 2006. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 314:1595-1598. [DOI] [PubMed] [Google Scholar]
- 14.DiNardo, S., J. M. Kuner, J. Theis, and P. H. O'Farrell. 1985. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43:59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flatt, T. 2005. The evolutionary genetics of canalization. Q. Rev. Biol. 80:287-316. [DOI] [PubMed] [Google Scholar]
- 16.Fradkin, L. G., M. van Schie, R. R. Wouda, A. de Jong, J. T. Kamphorst, M. Radjkoemar-Bansraj, and J. N. Noordermeer. 2004. The Drosophila Wnt5 protein mediates selective axon fasciculation in the embryonic central nervous system. Dev. Biol. 272:362-375. [DOI] [PubMed] [Google Scholar]
- 17.Freeman, M. 2000. Feedback control of intercellular signalling in development. Nature 408:313-319. [DOI] [PubMed] [Google Scholar]
- 18.Gerlitz, O., and K. Basler. 2002. Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 16:1055-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldez, A. J., R. R. Copley, and S. M. Cohen. 2002. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell 2:667-676. [DOI] [PubMed] [Google Scholar]
- 20.Glise, B., C. A. Miller, M. Crozatier, M. A. Halbisen, S. Wise, D. J. Olson, A. Vincent, and S. S. Blair. 2005. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev. Cell 8:255-266. [DOI] [PubMed] [Google Scholar]
- 21.Gong, Y., R. B. Slee, N. Fukai, G. Rawadi, S. Roman-Roman, A. M. Reginato, H. Wang, T. Cundy, F. H. Glorieux, D. Lev, M. Zacharin, K. Oexle, J. Marcelino, W. Suwairi, S. Heeger, G. Sabatakos, S. Apte, W. N. Adkins, J. Allgrove, M. Arslan-Kirchner, J. A. Batch, P. Beighton, G. C. Black, R. G. Boles, L. M. Boon, C. Borrone, H. G. Brunner, G. F. Carle, B. Dallapiccola, A. De Paepe, B. Floege, M. L. Halfhide, B. Hall, R. C. Hennekam, T. Hirose, A. Jans, H. Juppner, C. A. Kim, K. Keppler-Noreuil, A. Kohlschuetter, D. LaCombe, M. Lambert, E. Lemyre, T. Letteboer, L. Peltonen, R. S. Ramesar, M. Romanengo, H. Somer, E. Steichen-Gersdorf, B. Steinmann, B. Sullivan, A. Superti-Furga, W. Swoboda, M. J. van den Boogaard, W. Van Hul, M. Vikkula, M. Votruba, B. Zabel, T. Garcia, R. Baron, B. R. Olsen, and M. L. Warman. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513-523. [DOI] [PubMed] [Google Scholar]
- 22.Gorfinkiel, N., J. Sierra, A. Callejo, C. Ibanez, and I. Guerrero. 2005. The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev. Cell 8:241-253. [DOI] [PubMed] [Google Scholar]
- 23.Hamada, F., Y. Tomoyasu, Y. Takatsu, M. Nakamura, S. Nagai, A. Suzuki, F. Fujita, H. Shibuya, K. Toyoshima, N. Ueno, and T. Akiyama. 1999. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283:1739-1742. [DOI] [PubMed] [Google Scholar]
- 24.Hatini, V., and S. DiNardo. 2001. Divide and conquer: pattern formation in Drosophila embryonic epidermis. Trends Genet. 17:574-579. [DOI] [PubMed] [Google Scholar]
- 25.Hing, H. K., X. Sun, and S. Artavanis-Tsakonas. 1994. Modulation of wingless signaling by Notch in Drosophila. Mech. Dev. 47:261-268. [DOI] [PubMed] [Google Scholar]
- 26.Hovanes, K., T. W. Li, J. E. Munguia, T. Truong, T. Milovanovic, J. Lawrence Marsh, R. F. Holcombe, and M. L. Waterman. 2001. β-Catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 28:53-57. [DOI] [PubMed] [Google Scholar]
- 27.Hurley, I., M. E. Hale, and V. E. Prince. 2005. Duplication events and the evolution of segmental identity. Evol. Dev. 7:556-567. [DOI] [PubMed] [Google Scholar]
- 28.Hynes, N. E., and H. A. Lane. 2005. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5:341-354. [DOI] [PubMed] [Google Scholar]
- 29.Ingham, P. W., and A. P. McMahon. 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15:3059-3087. [DOI] [PubMed] [Google Scholar]
- 30.Inoue, T., H. S. Oz, D. Wiland, S. Gharib, R. Deshpande, R. J. Hill, W. S. Katz, and P. W. Sternberg. 2004. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 118:795-806. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa, A., S. Kitajima, Y. Takahashi, H. Kokubo, J. Kanno, T. Inoue, and Y. Saga. 2004. Mouse Nkd1, a Wnt antagonist, exhibits oscillatory gene expression in the PSM under the control of Notch signaling. Mech. Dev. 121:1443-1453. [DOI] [PubMed] [Google Scholar]
- 32.Itoh, K., B. K. Brott, G. U. Bae, M. J. Ratcliffe, and S. Y. Sokol. 2005. Nuclear localization is required for Dishevelled function in Wnt/β-catenin signaling. J. Biol. 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, W. M., and A. Bejsovec. 2005. RacGap50C negatively regulates wingless pathway activity during Drosophila embryonic development. Genetics 169:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jürgens, G., E. Wieschaus, C. Nüsslein-Volhard, and H. Kluding. 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Wilhelm Roux Arch. Dev. Biol. 193:283-295. [DOI] [PubMed] [Google Scholar]
- 35.Katoh, M. 2001. Molecular cloning, gene structure, and expression analyses of NKD1 and NKD2. Int. J. Oncol. 19:963-969. [PubMed] [Google Scholar]
- 36.Kawano, Y., and R. Kypta. 2003. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116:2627-2634. [DOI] [PubMed] [Google Scholar]
- 37.Klingensmith, J., and R. Nusse. 1994. Signaling by wingless in Drosophila. Dev. Biol. 166:396-414. [DOI] [PubMed] [Google Scholar]
- 38.Koay, M. A., and M. A. Brown. 2005. Genetic disorders of the LRP5-Wnt signalling pathway affecting the skeleton. Trends Mol. Med. 11:129-137. [DOI] [PubMed] [Google Scholar]
- 39.Korswagen, H. C. 2002. Canonical and non-canonical Wnt signaling pathways in Caenorhabditis elegans: variations on a common signaling theme. Bioessays 24:801-810. [DOI] [PubMed] [Google Scholar]
- 40.Kühl, M. 2004. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front. Biosci. 9:967-974. [DOI] [PubMed] [Google Scholar]
- 41.Kusserow, A., K. Pang, C. Sturm, M. Hrouda, J. Lentfer, H. A. Schmidt, U. Technau, A. von Haeseler, B. Hobmayer, M. Q. Martindale, and T. W. Holstein. 2005. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433:156-160. [DOI] [PubMed] [Google Scholar]
- 42.Larsen, C. W., E. Hirst, C. Alexandre, and J. P. Vincent. 2003. Segment boundary formation in Drosophila embryos. Development 130:5625-5635. [DOI] [PubMed] [Google Scholar]
- 43.Lee, J. J., D. P. von Kessler, S. Parks, and P. A. Beachy. 1992. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71:33-50. [DOI] [PubMed] [Google Scholar]
- 44.Leung, J. Y., F. T. Kolligs, R. Wu, Y. Zhai, R. Kuick, S. Hanash, K. R. Cho, and E. R. Fearon. 2002. Activation of AXIN2 expression by β-catenin/TCF: a feedback repressor pathway regulating Wnt signaling. J. Biol. Chem. 277:21657-21665. [DOI] [PubMed] [Google Scholar]
- 45.Li, Q., T. O. Ishikawa, H. Miyoshi, M. Oshima, and M. M. Taketo. 2005. A targeted mutation of Nkd1 impairs mouse spermatogenesis. J. Biol. Chem. 280:2831-2839. [DOI] [PubMed] [Google Scholar]
- 46.Liu, B., H. M. Yu, and W. Hsu. 2007. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of β-catenin in proliferation and differentiation. Dev. Biol. 301:298-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logan, C. Y., and R. Nusse. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781-810. [DOI] [PubMed] [Google Scholar]
- 48.Louvi, A., and S. Artavanis-Tsakonas. 2006. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7:93-102. [DOI] [PubMed] [Google Scholar]
- 49.Lu, W., V. Yamamoto, B. Ortega, and D. Baltimore. 2004. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119:97-108. [DOI] [PubMed] [Google Scholar]
- 50.Lustig, B., B. Jerchow, M. Sachs, S. Weiler, T. Pietsch, U. Karsten, M. van de Wetering, H. Clevers, P. M. Schlag, W. Birchmeier, and J. Behrens. 2002. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22:1184-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malbon, C. C., and H. Y. Wang. 2006. Dishevelled: a mobile scaffold catalyzing development. Curr. Top. Dev. Biol. 72:153-166. [DOI] [PubMed] [Google Scholar]
- 52.Martinez Arias, A., N. E. Baker, and P. W. Ingham. 1988. Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development 103:157-170. [DOI] [PubMed] [Google Scholar]
- 53.Noordermeer, J., P. Johnston, F. Rijsewijk, R. Nusse, and P. A. Lawrence. 1992. The consequences of ubiquitous expression of the wingless gene in the Drosophila embryo. Development 116:711-719. [DOI] [PubMed] [Google Scholar]
- 54.Noordermeer, J., J. Klingensmith, N. Perrimon, and R. Nusse. 1994. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367:80-83. [DOI] [PubMed] [Google Scholar]
- 55.Nüsslein-Volhard, C., and E. Wieschaus. 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287:795-801. [DOI] [PubMed] [Google Scholar]
- 56.Peifer, M., D. Sweeton, M. Casey, and E. Wieschaus. 1994. Wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120:369-380. [DOI] [PubMed] [Google Scholar]
- 57.Rousset, R., J. A. Mack, K. A. Wharton, Jr., J. D. Axelrod, K. M. Cadigan, M. P. Fish, R. Nusse, and M. P. Scott. 2001. naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 15:658-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanson, B. 2001. Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep. 2:1083-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt, C., A. Otto, G. Luke, P. Valasek, W. R. Otto, and K. Patel. 2006. Expression and regulation of Nkd-1, an intracellular component of Wnt signalling pathway in the chick embryo. Anat. Embryol. 211:525-534. [DOI] [PubMed] [Google Scholar]
- 60.Siegfried, E., T. B. Chou, and N. Perrimon. 1992. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71:1167-1179. [DOI] [PubMed] [Google Scholar]
- 61.Siegfried, E., E. L. Wilder, and N. Perrimon. 1994. Components of wingless signalling in Drosophila. Nature 367:76-80. [DOI] [PubMed] [Google Scholar]
- 62.Tabata, T., S. Eaton, and T. B. Kornberg. 1992. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 6:2635-2645. [DOI] [PubMed] [Google Scholar]
- 63.Tada, M., M. L. Concha, and C. P. Heisenberg. 2002. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin. Cell Dev. Biol. 13:251-260. [DOI] [PubMed] [Google Scholar]
- 64.Taipale, J., and P. A. Beachy. 2001. The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349-354. [DOI] [PubMed] [Google Scholar]
- 65.Veeman, M. T., J. D. Axelrod, and R. T. Moon. 2003. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5:367-377. [DOI] [PubMed] [Google Scholar]
- 66.Waddington, C. H. 1942. Canalization of development and the inheritance of acquired characters. Nature 150:563-565. [Google Scholar]
- 67.Waldrop, S., C. C. Chan, T. Cagatay, S. Zhang, R. Rousset, J. Mack, W. Zeng, M. Fish, M. Zhang, M. Amanai, and K. A. Wharton, Jr. 2006. An unconventional nuclear localization motif is crucial for function of the Drosophila Wnt/wingless antagonist naked cuticle. Genetics 174:331-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wallingford, J. B., and R. Habas. 2005. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132:4421-4436. [DOI] [PubMed] [Google Scholar]
- 69.Wharton, K. A., Jr. 2003. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253:1-17. [DOI] [PubMed] [Google Scholar]
- 70.Wharton, K. A., Jr., G. Zimmermann, R. Rousset, and M. P. Scott. 2001. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev. Biol. 234:93-106. [DOI] [PubMed] [Google Scholar]
- 71.Yan, D., J. B. Wallingford, T. Q. Sun, A. M. Nelson, C. Sakanaka, C. Reinhard, R. M. Harland, W. J. Fantl, and L. T. Williams. 2001. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. USA 98:3802-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan, D., M. Wiesmann, M. Rohan, V. Chan, A. B. Jefferson, L. Guo, D. Sakamoto, R. H. Caothien, J. H. Fuller, C. Reinhard, P. D. Garcia, F. M. Randazzo, J. Escobedo, W. J. Fantl, and L. T. Williams. 2001. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/β-catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. USA 98:14973-14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu, H. M., B. Jerchow, T. J. Sheu, B. Liu, F. Costantini, J. E. Puzas, W. Birchmeier, and W. Hsu. 2005. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132:1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng, L., F. Fagotto, T. Zhang, W. Hsu, T. J. Vasicek, W. L. Perry III, J. J. Lee, S. M. Tilghman, B. M. Gumbiner, and F. Costantini. 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181-192. [DOI] [PubMed] [Google Scholar]
- 75.Zeng, W., K. A. Wharton, Jr., J. A. Mack, K. Wang, M. Gadbaw, K. Suyama, P. S. Klein, and M. P. Scott. 2000. naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403:789-795. [DOI] [PubMed] [Google Scholar]
- 76.Zhu, Y., J. A. Richardson, L. F. Parada, and J. M. Graff. 1998. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94:703-714. [DOI] [PubMed] [Google Scholar]