Abstract
Replicative senescence of human diploid fibroblasts (HDFs) is largely implemented by the cyclin-dependent kinase (CDK) inhibitors p16INK4a and p21CIP1. Their accumulation results in a loss of CDK2 activity, and cells arrest with the retinoblastoma protein (pRb) in its hypophosphorylated state. It has become standard practice to bypass the effects of p16INK4a by overexpressing CDK4 or a variant form that is unable to bind to INK4 proteins. Although CDK4 and CDK6 and their INK4-insensitive variants can extend the life span of HDFs, they also cause a substantial increase in the levels of endogenous p16INK4a. Here we show that CDK4 and CDK6 can extend the life span of HDFs that have inactivating mutations in both alleles of INK4a or in which INK4a levels are repressed, indicating that overexpression of CDK4/6 is not equivalent to ablation of p16INK4a. However, catalytically inactive versions of these kinases are unable to extend the replicative life span, suggesting that the impact of ectopic CDK4/6 depends on their ability to phosphorylate as yet unidentified substrates rather than to sequester CDK inhibitors. Since p16INK4a deficiency, CDK4 expression, and p53 or p21CIP1 ablation have additive effects on replicative life span, our results underscore the idea that senescence is an integrated response to diverse signals.
Cellular senescence is now recognized as a general response to a variety of oncogenic and genotoxic stresses but was originally observed in cultures of primary human diploid fibroblasts (HDFs) as they reached the end of their proliferative life span (21). After what appears to be a predetermined number of population doublings (PDs), HDFs enter a permanent state of growth arrest, termed M1, and develop a characteristic phenotype (49, 59). In HDFs, a critical determinant of M1 is the erosion of the telomeres that occurs with each division (20), but it is clear that there are additional telomere-independent mechanisms that limit proliferative life span, collectively referred to as culture stress (11, 51, 62). In the classical HDF system, it was found that senescence could be delayed by interfering with the retinoblastoma (pRb) and p53 tumor suppressor pathways, for example, by using DNA tumor virus oncoproteins that bind to either or both pRb and p53 (49). This results in a significant increase in the maximum number of PDs, but the continued erosion of telomeres during this period eventually leads to chromosome fusion and breakage and the cultures reach a state referred to as M2 or crisis, where cell division is still occurring but is offset by extensive cell death (49, 59).
A distinctive feature of senescent HDFs is that they express elevated levels of the p16INK4a and p21CIP1 cyclin-dependent kinase (CDK) inhibitors (1, 19, 35, 54, 61). The expression of p21CIP1 peaks as cells approach M1, presumably reflecting a p53-mediated signal from the damaged telomeres (9, 22), whereas the accumulation of p16INK4a is more pronounced after cell proliferation has ceased (1, 6, 54). It is tacitly assumed that these CDK inhibitors are responsible for implementing the senescence arrest by preventing the CDK-mediated phosphorylation of pRb and its relatives. Whereas p16INK4a interacts specifically with CDK4 and CDK6 and blocks their association with D-type cyclins (40, 47), p21CIP1 interacts with multiple cyclin-CDK complexes (reviewed in reference 50). When bound to cyclin E-CDK2 and cyclin A-CDK2, the CIP/KIP proteins act as potent inhibitors of catalytic activity (42), but their impact on the cyclin D-dependent kinases is more enigmatic.
Various pieces of evidence suggest that the CIP/KIP proteins promote the assembly of cyclin D-CDK complexes, and indeed, most of the D-type cyclins in the cell are present in these stable ternary complexes (8, 27, 31, 36, 41). However, it remains a matter of debate whether such complexes have catalytic activity or simply provide a buffering system that controls the availability of CIP/KIP proteins to inhibit CDK2 (37, 52). In either case, p16INK4a has the capacity to inhibit CDK2 as well as CDK4 and CDK6 by causing the redistribution of CIP/KIP proteins onto CDK2-containing complexes, where they function as inhibitors, and by promoting the formation of inactive cyclin D-CDK2 complexes (26, 31-33, 54).
The prospect that the D-type cyclins have both kinase-dependent and kinase-independent functions has important implications (for example, see references 28 and 63). In the context of senescence, it has not been rigorously established whether the catalytic activity of cyclin D-CDK complexes is extinguished at M1, when the levels of p21CIP1 and p16INK4a are changing dramatically. In early senescence, the peak of p21CIP1 might be expected to promote the formation of ternary cyclin D-CDK-p21CIP1 complexes while simultaneously inhibiting cyclin E-CDK2. As the total levels of p21CIP1 start to decline in late senescence, the accumulating p16INK4a would potentially ensure that enough CIP/KIP proteins are redistributed onto cyclin E-CDK2 by displacing them from the CDK4 and CDK6 complexes. However, the situation is further complicated by the fact that the levels of cyclin D1, CDK4, and CDK6 are also modestly elevated in senescent cells (13, 54; unpublished observations of the authors).
To try to gain further insight into the role of the cyclin D-CDK complexes at senescence, we have explored the ability of exogenous CDK4 and CDK6 to extend the replicative life span of HDFs. As previously reported (34), both wild-type CDK4 and CDK6 and mutated versions of these kinases (R24C and R31C, respectively) that are unable to interact with INK4 proteins elicit a modest increase in the maximum number of populations doublings. The cells arrest with an M1 phenotype but at a stage that is intermediate between M1 and M2. HDFs respond to excess CDK4/6 by expressing substantially elevated levels of p16INK4a and p21CIP1, perhaps as a stress response or because of homeostatic mechanisms that maintain an appropriate balance between cyclins, CDKs, and CDK inhibitors. Importantly, CDK4/6 can also delay senescence in INK4a-deficient HDFs, implying that the effect must be independent of p16INK4a. Since p16INK4a-deficient HDFs have a partially extended life span that can be further extended by CDK4/6 and by p53 ablation, our results reveal previously unexpected facets in the implementation of senescence. Moreover, we find that catalytically inactive variants of CDK4/6 are unable to extend cellular life span, suggesting that the effects must be dependent on phosphorylation of an as yet unknown substrate(s) rather than sequestration of CDK inhibitors.
MATERIALS AND METHODS
Cell culture and retroviral infection.
Cell stocks were maintained at 37°C and 5% CO2 in Dulbecco modified Eagle's medium supplemented with 10% fetal calf serum. The fibroblast cultures were routinely passaged at a 1:4 or 1:8 split ratio as soon as they reached confluence and were therefore assumed to have undergone two or three PDs, respectively, at each passage. To permit uptake of ecotropic retroviruses, the fibroblasts were infected with an amphotropic retrovirus encoding the mouse basic amino acid transporter (pWXL-Neo-Eco) and selected in medium containing G418 (150 μg/ml). Ecotropic retroviral stocks were prepared by transient transfection of BOSC-23 cells. For retroviral infections, pools of G418-resistant cells were plated at 25 to 50% confluence and incubated overnight. The culture medium was replaced with 5 ml of filtered viral supernatant together with 3 ml of fresh medium and the equivalent of 4 μg/ml polybrene. After 24 h the medium was replaced, and selection in medium containing either 1.25 μg/ml puromycin (Calbiochem) or 50 to 100 μg/ml hygromycin (Sigma) was initiated on day 2 postinfection.
The wild-type and mutant versions of CDK4 and CDK6 cDNAs were transferred into the pBABE retroviral vector as BamHI-EcoRI fragments and have been described previously (34). In some cases, one or two copies of the hemagglutinin (HA) epitope tag were added at the carboxy terminus. Retroviral vectors encoding mouse Bmi1 and Cbx7 have been described elsewhere (6, 15). A short-hairpin RNA (shRNA) targeting human p21CIP1 was generated using the 19-nucleotide sequence 5′-CTTCGACTTTGTCACCGAG-3′. This sequence was used as the basis for complementary 59-mer oligonucleotides capable of forming a hairpin and flanked by sites for the BglII and HindIII restriction enzymes. The annealed oligonucleotides were cloned into pRetroSuper vectors (7) that confer either puromycin or hygromycin resistance.
Immunoprecipitation and immunoblotting.
For direct immunoblotting, cells were lysed in 62.5 mM Tris-HCl (pH 6.8) containing 2% (wt/vol) sodium dodecyl sulfate (SDS) and the protein concentration was estimated using the bicinchoninic acid (BCA) assay (Pierce). Mercaptoethanol and bromophenol blue were added to make the final composition equivalent to that of Laemmli sample buffer. Samples were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) in either 12 or 15% gels and blotted onto Immobilon-P membranes (Millipore). Membranes were probed with primary antibodies diluted in phosphate-buffered saline, 0.2% Tween 20, and 5% milk powder and processed as previously described. Antibody binding was visualized using G.E. Healthcare ECL reagents.
For immunoprecipitation, cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 1% [vol/vol] NP-40) containing freshly added protease inhibitors (a 1:1,000 dilution from a stock solution of 20 mM aminoethylbenzenesulfonyl fluoride, 10 mM EDTA, 1.3 mM bestatin, 140 μM E-64, 10 μM leupeptin, and 3 μM aprotinin). The protein concentration was estimated using the BCA assay (Pierce). Samples (500 μg) of protein in a total volume of 500 μl were immunoprecipitated with 2 μl of antibody and 20 μl of protein A beads (for rabbit polyclonal antibodies) or protein G beads (for mouse monoclonal antibodies). The samples were rotated at 4°C overnight. The beads were washed four times with 1 ml of cold NP-40 lysis buffer containing protease inhibitors. The beads were then boiled for 10 min in the presence of 25 μl 2× sample buffer and the released proteins fractionated by SDS-PAGE in 12 or 15% gels. Proteins were detected by immunoblotting as described above.
Antisera.
Rabbit polyclonal antibodies against CDK2 (sc-163), CDK4 (sc-601), CDK6 (sc-177), and p21CIP1 (sc-397) were obtained from Santa Cruz. Rabbit polyclonal antiserum against MEK1/2 (no. 9122) was from Cell Signaling. Monoclonal antibodies against CDK4 (DCS31), CDK6 (K6.83), and cyclin D1 (DCS6) were obtained from Neomarkers. The monoclonal antibodies against p16INK4a (DCS50 and JC8) and polyclonal antibodies against cyclin D1 (287.3), CDK6 (LBO-1), and p16INK4a (DPAR12) have been described previously (3, 40).
Gel filtration chromatography.
Cells were washed once with phosphate-buffered saline and then lysed in Tween lysis buffer (50 mM HEPES, pH 8.0, 0.1% Tween 20, 1 mM EDTA, 2.5 mM EGTA, 150 mM NaCl, 1 mM dithiothreitol, 1 mM sodium fluoride, 0.1 mM sodium orthovanadate, 2 μg of aprotinin per ml, and 100 μg of phenylmethylsulfonyl fluoride per ml) and frozen rapidly on dry ice. Lysates were subjected to freeze-thawing three times, followed by clarification by centrifugation at 14,000 × g for 10 min, and the protein concentrations were determined using the Pierce BCA protein assay reagents according to the manufacturer's instructions.
Gel filtration chromatography was carried out using a Superdex 200 HR 10/30 column (Pharmacia) with a fast-performance liquid chromatography system (BioLogic System; Bio-Rad). Samples of 2.5 mg of cell lysate in 250 μl of Tween lysis buffer were loaded onto the column and separated in Tween lysis buffer at a flow rate of 0.4 ml per min. The molecular mass standards (Sigma) used to calibrate the column were as follows: alcohol dehydrogenase, 150 kDa; bovine serum albumin, 66 kDa; carbonic anhydrase, 29 kDa; and cytochrome C, 12.4 kDa. Fifty microliters of each fraction was taken for immunoblot analysis, and 350 μl from each fraction was used for immunoprecipitation.
RESULTS
Persistence of cyclin D1-CDK4-p21CIP1 complexes in senescent fibroblasts.
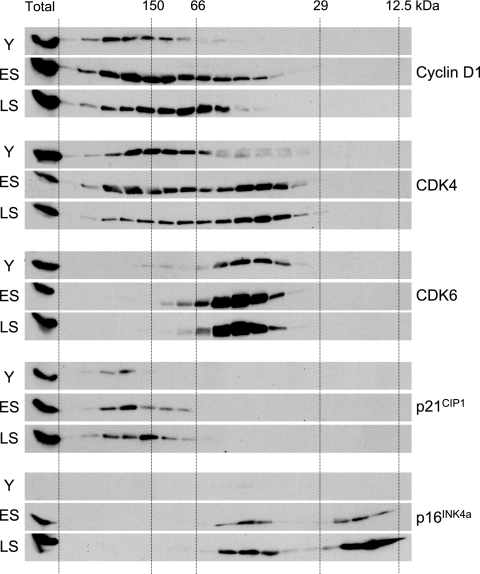
Although it is well established that the levels of p21CIP1 and p16INK4a increase dramatically at M1, with the peak of p21CIP1 preceding that of p16INK4a, there have been relatively few studies on the impact this has on the composition of cyclin D-CDK complexes (1, 33, 54). Given the dynamic nature and multiplicity of the complexes involved, we tried to facilitate such analyses by incorporating a gel filtration step to separate complexes of different sizes. Lysates from proliferating, early senescent, and late senescent fibroblasts (TIG3 cells) were fractionated on a Superdex 200 column, and samples of each fraction were analyzed by direct immunoblotting with antibodies against cyclin D1, CDK4, CDK6, p21CIP1, and p16INK4a (Fig. 1). To confirm suspected associations, residual samples were also subjected to immunoprecipitation followed by immunoblotting (see Fig. S1 in the supplemental material). Given the volume of data generated by this approach, only illustrative examples are shown here, and we have excluded the high-molecular-weight complexes (≥400 kDa) that contain heat shock and chaperone proteins (10, 30, 31).
FIG. 1.
Comparison of cyclin D1-CDK-CKI complexes in young and senescent HDFs. Equal amounts of cell lysate (2.5 mg) from young (Y), early senescence (ES), or late senescence (LS) TIG3 cells were subjected to gel filtration chromatography on a Superdex 200 column. Equivalent amounts (1/20th) of the individual fractions were separated by SDS-PAGE in a 12% acrylamide gel and immunoblotted with antisera against cyclin D1, CDK4, CDK6, p21CIP1, and p16INK4a. The lane on the left of each panel corresponds to a sample (12.5 μg) of total protein analyzed directly (i.e., without gel filtration).
In young HDFs, most of the cyclin D1 and p21CIP1 was present in complexes of around 150 to 200 kDa (Fig. 1), as reported for other cell types (10, 30, 31, 36, 41, 43). Immunoprecipitation and immunoblotting confirmed that cyclin D1 and p21CIP1 were associated with CDK4 and CDK6 in complexes of this size (see Fig. S1 in the supplemental material; also data not shown). However, whereas a substantial proportion of the CDK4 eluted in the 150- to 200-kDa size range, only a minority of the CDK6 was found in these fractions, with the remainder concentrated in a complex of approximately 50 kDa. This would be consistent with a 1:1 association between CDK6 and one of the INK4 proteins, and although the levels of p16INK4a were very low in early-passage HDFs, immunoprecipitation with a CDK6 antibody confirmed that this complex contained both p16INK4a and p18INK4c (see Fig. S1 in the supplemental material; also data not shown). An equivalent CDK4-p16INK4a complex was also present in these cells, and the proportion of CDK4 that eluted in the 50-kDa size range increased significantly in early and late senescent HDFs following the marked accumulation of p16INK4a (Fig. 1).
Surprisingly, a substantial proportion of p16INK4a in senescent HDFs eluted with the predicted molecular mass of the monomeric protein. In principle, this free p16INK4a should have been capable of sequestering CDK4 and CDK6. Indeed, virtually all of the CDK6 in senescent cells was found in the 50-kDa complex. Although there was a change in the size distribution of CDK4 between proliferating and senescent cells with a shift into the 50-kDa complexes, a considerable amount of the CDK4 remained associated with cyclin D1 and p21CIP1 in the 150- to 200-kDa size range (Fig. 1; also see Fig. S1 in the supplemental material). There was also a slight reduction in the mean size of p21CIP1 complexes in senescent HDFs but no evidence for any free p21CIP1. Taken together, these data imply that cyclin D1-CDK4-p21CIP1 complexes persist at M1 despite an excess of free p16INK4a.
Life span extension by overexpression of CDK4 or CDK6.
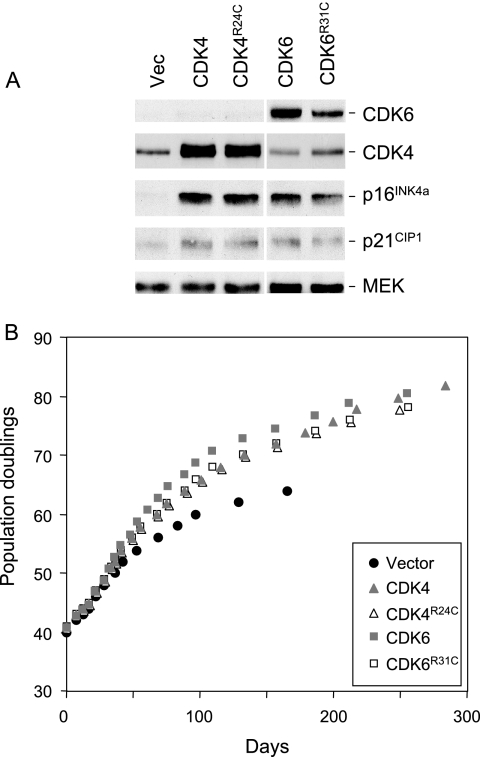
The substantial amount of free p16INK4a in senescent cells has interesting implications for the accepted practice of using ectopic CDK4 or CDK6 to override the effects of p16INK4a. It is generally assumed that overexpression of wild-type CDK4 will sequester p16INK4a, as well as other INK4 proteins, and promote the formation of cyclin D-CDK complexes. Whether these have catalytic activity or act as a sink for p21CIP1 is a moot point. An alternative strategy is to use the R24C variant of CDK4 or the analogous R31C variant of CDK6, both of which are unable to interact with any INK4 proteins (16, 34, 60). These should in principle allow the formation of active cyclin D-CDK complexes that are insensitive to p16INK4a, as well as sequestering p21CIP1 in ternary complexes with the D cyclins. We compared these strategies by using recombinant retroviruses to express the relevant cDNAs in normal HDFs. Expression of the ectopic proteins was confirmed by immunoblotting with antibodies against CDK4 and CDK6, respectively (Fig. 2A). The levels of CDK4 were considerably increased relative to those of the endogenous protein in the vector-only control cells, but quantification was complicated by the appearance of a doublet in SDS-PAGE. This is consistently observed following overexpression of catalytically active CDK4, but the molecular explanation remains unclear.
FIG. 2.
Extension of HDF life span by wild-type and mutant versions of CDK4 and CDK6. (A) Hs68 cells were infected at PD40 with retroviruses encoding wild-type CDK4, the R24C mutant of CDK4, wild-type CDK6, the R31C mutant of CDK6, or the empty vector as indicated. Following selection in puromycin, the cell pools were analyzed by immunoblotting with antibodies against CDK4, CDK6, p16INK4a, and p21CIP1. MEK was used as a control for loading. Note that the samples were analyzed on the same gel, but the scanned images were subsequently edited for continuity of presentation. (B) Infected cell pools were passaged under standard conditions of tissue culture until they reached M1 senescence, as judged by failure to double in 4 weeks. The curves show cumulative PDs at each time point.
Compared to the vector-only controls, Hs68 cells transduced with either wild-type or mutant versions of CDK4 and CDK6 achieved an additional 10 to 12 PDs (Fig. 2B). Similar life span extensions were obtained with TIG3 cells (see Fig. S2 in the supplemental material) and have been reported previously for the BJ (HCA2) strain of HDFs (34). In each case, the cells arrested with an M1-like phenotype. Importantly, there was no appreciable difference between the effects of the wild-type and INK4-insensitive versions of CDK4 and CDK6 in these assays.
Induction of p16INK4a by ectopic CDK4 and its effect on cyclin D-CDK complexes.
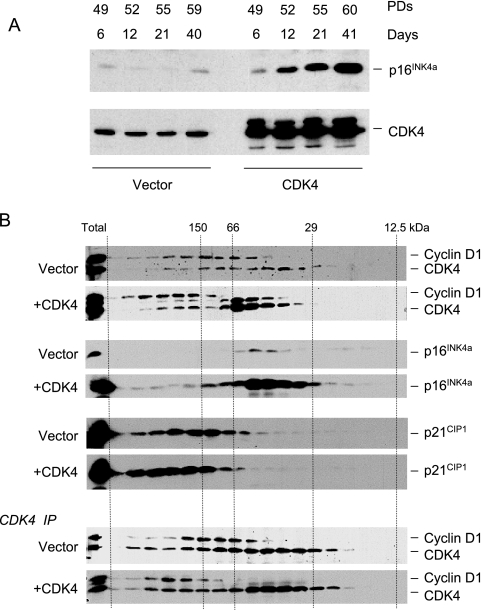
A striking and consistent consequence of introducing ectopic CDK4 or CDK6 was that cells produced significantly elevated levels of p16INK4a, as well as moderately elevated levels of p21CIP1 (Fig. 2A). The increased abundance of p16INK4a occurred within days of retroviral infection and was maintained over numerous PDs. In the example shown in Fig. 3A, TIG3 cells expressing exogenous CDK4 accumulated up to 30-fold more p16INK4a compared to age-matched cells infected with the empty vector control. The effects therefore appear to be an acute reaction to the exogenous CDKs rather than an indirect consequence of life span extension.
FIG. 3.
Induction of p16INK4a by ectopic CDK4 and its effect on cyclin D-CDK complexes. (A) TIG3 cells were infected with a retrovirus encoding wild-type CDK4 or the empty-vector control. Following drug selection (6 days), the cells were passaged under standard conditions and cell lysates prepared at various time points. Cumulative PDs at each time point are as indicated. Samples (30 μg) of total protein were fractionated by SDS-PAGE and immunoblotted for p16INK4a and CDK4. (B) Extracts from TIG3 cells infected with a retrovirus encoding wild-type CDK4 or the empty-vector control were subjected to gel filtration and analyzed as in Fig. 1. Cyclin D1 and CDK4 were visualized on the same immunoblot, with the upper band corresponding to cyclin D1. Ectopic expression of CDK4 resulted in two immunoreactive bands, as discussed in the text. In the lower panels, samples of each fraction were immunoprecipitated with an antibody against CDK4 prior to SDS-PAGE and immunoblotting.
If the cells respond to the additional CDK4 by producing more p16INK4a, then the impact on cyclin D-CDK complexes is likely to be self-limiting. To address this possibility, we used gel filtration to compare the size distribution of cyclin D1, CDK4, and p16INK4a in TIG3 cells transduced with the empty vector or with the CDK4 retrovirus (Fig. 3B). The most obvious difference in the CDK4-transduced cells was that a greater proportion of the CDK4 was present in 50-kDa complexes, because of its association with p16INK4a, and there was also an increase in the average size of the cyclin D1-CDK4 complexes. There was a concomitant shift in the distribution of total p21CIP1, reversing the trend noted in Fig. 1, which would be consistent with the assembly of ternary cyclin D1-CDK4-p21CIP1 complexes. Importantly, the additional p16INK4a in the CDK4-transduced cells was predominantly in the 50-kDa complexes. Because of the magnitude of the p16INK4a peak and the spillover of the signal, it was not possible to judge what proportion of the p16INK4a remained monomeric in the cells expressing exogenous CDK4.
Life span extension by CDK4 is independent of its association with p16INK4a.
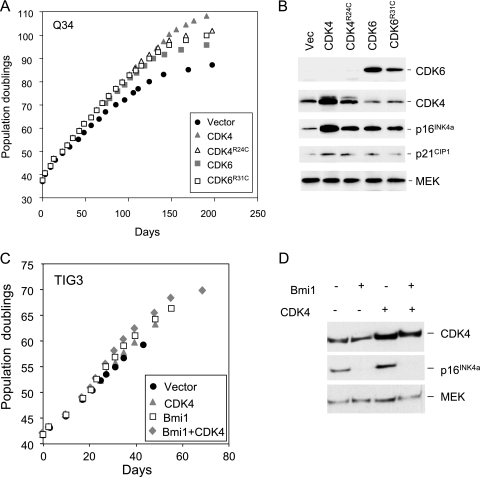
The above findings implied that overexpression of wild-type CDK4 has a relatively minor impact on its availability to bind cyclin D1, because much of it becomes bound to the extra p16INK4a that is produced. The variant forms of CDK4 and CDK6 that are unable to interact with p16INK4a should potentially avoid this problem, but the situation is complicated because the endogenous CDK4 and CDK6 in the transduced cells would remain able to associate with the extra p16INK4a. To try to clarify matters, we asked whether ectopic CDK4 and CDK6 would extend the life span of p16INK4a-deficient HDFs. One such strain, designated Q34 HDFs, has different missense mutations in each copy of the gene and produces two full-length but functionally compromised variants of p16INK4a (24). Surprisingly, ectopic expression of wild-type CDK4 or CDK6 or the respective R24C and R31C mutants caused significant life span extension relative to the Q34 cells infected with the empty vector (Fig. 4A). The magnitude of the effect was comparable to that observed with normal HDFs and was confirmed with two independent strains of p16INK4a-deficient HDFs that we have studied in detail (see Fig. S3 in the supplemental material). Immunoblotting of the cell lysates showed that the exogenous CDKs were expressed at equivalent levels and, importantly, that they caused up-regulation of the nonfunctional p16INK4a (Fig. 4B). These results imply that exogenous CDK4 and CDK6 extend HDF life span by mechanisms that are independent of p16INK4a.
FIG. 4.
CDK4 and CDK6 can extend the life span of p16INK4a-deficient HDFs. (A) The Q34 strain of p16INK4a-deficient HDFs was infected at PD37 with retroviruses encoding wild-type CDK4, the R24C mutant of CDK4, wild-type CDK6, the R31C mutant of CDK6, or the empty vector, as indicated. Following selection, the infected cell pools were passaged under standard conditions of tissue culture until they reached M1 senescence. Curves show cumulative PDs at each time point. (B) Lysates prepared from infected cell pools were analyzed by immunoblotting with antibodies against CDK4, CDK6, p16INK4a, and p21CIP1. MEK was used as a control for loading. (C and D) The TIG3 strain of HDFs was infected at PD42 with retroviruses encoding CDK4 or Bmi1 or both, along with appropriate vector controls. Following selection, the infected cell pools were passaged under standard conditions of tissue culture until they reached M1 senescence. The curves show cumulative PDs at each time point. Lysates prepared from the infected cell pools were analyzed by immunoblotting with antibodies against CDK4, p16INK4a, and MEK.
As further verification, we also asked whether CDK4 could extend the life span of control fibroblasts in which expression of the wild-type INK4a gene was repressed. To this end, TIG3 cells were infected with a retrovirus encoding the Polycomb group protein Bmi1, which is known to repress transcription from the INK4a locus (25) (Fig. 4C). The cells were then superinfected with a retrovirus encoding CDK4, along with appropriate empty vector controls. As shown in Fig. 4D, Bmi1 and CDK4 were both capable of extending the replicative life span and had additive effects when coexpressed. Although Bmi1 is likely to have other target genes, its ability to delay senescence is largely attributable to repression of p16INK4a. Thus, Bmi1 is unable to extend the life span of p16INK4a-deficient HDFs (6; R. Jones and G. Peters, unpublished results). Furthermore, we obtained similar results (see Fig. S4 in the supplemental material) using a different Polycomb group protein, Cbx7, which can repress INK4a independently of Bmi1 (15).
Life span extension by CDK4 requires kinase activity.
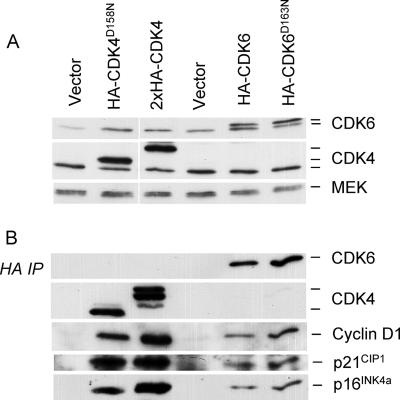
The most straightforward explanation for the effects of exogenous CDK4 and CDK6 would be that they promote the sequestration of CIP/KIP proteins in ternary complexes with the D cyclins, thereby delaying the point at which p21CIP1 is able to inhibit CDK2. The change in the size distribution of the cyclin D-CDK4 complexes in Fig. 3B could be viewed as consistent with this interpretation. However, without knowing the composition and stoichiometry of the 150- to 200-kDa complexes, we reasoned that evidence for or against this mechanism would be difficult to obtain by standard immunoprecipitation and immunoblotting. As an alternative strategy, we asked whether catalytically inactive versions of CDK4 and CDK6 are able to extend the replicative life span of HDFs. These mutants were generated by altering a single Asp residue in the ATP binding site of the kinase (56), and the proteins therefore retain the capacity to bind to cyclin D1 and p21CIP1. To confirm this, we used CDK4D158N and CDK6D163N constructs that carried an HA epitope tag at the carboxy terminus. Both variants were expressed in TIG3 cells, along with wild-type and empty-vector controls, and the levels of endogenous and exogenous proteins were monitored by immunoblotting for CDK4 and CDK6 (Fig. 5A). Equivalent samples of the cell lysates were then immunoprecipitated with an HA-specific antibody, followed by immunoblotting for cyclin D1, p21CIP1, and p16INK4a (Fig. 5B). From these and additional experiments (not shown), we concluded that the kinase-dead variants were as capable of interacting with endogenous proteins as the wild-type CDKs.
FIG. 5.
Catalytically inactive versions of CDK4 and CDK6 are able to associate with cyclin D1, p21CIP1, and p16INK4a. TIG3 cells at PD42 were infected with retroviruses encoding HA-tagged versions of wild-type CDK4, CDK4D158N, wild-type CDK6, or CDK6D163N or empty-vector controls. (A) Following drug selection, cell lysates were prepared and samples (25 μg) of total protein were analyzed by SDS-PAGE in a 12% gel and immunoblotted with antibodies against CDK4 and CDK6. MEK served as a control for loading. As discussed in the text, ectopic expression of CDK4 resulted in multiple bands, the exact provenance of which remains unclear. Note also that the wild-type CDK4 construct contained two copies of the HA epitope. (B) Samples (500 μg) of protein were immunoprecipitated with a monoclonal antibody against the HA epitope, fractionated by SDS-PAGE, and immunoblotted for CDK4, CDK6, cyclin D1, p21CIP1, or p16INK4a as indicated.
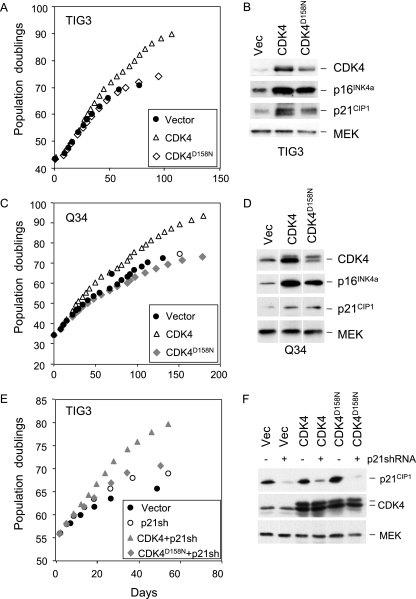
However, in long-term cell proliferation assays, the D158N version of CDK4 had little if any effect on the life span of TIG3 or p16INK4a-deficient Q34 HDFs compared to the vector-only controls (Fig. 6A and C). In the same experiment, wild-type CDK4 had the previously documented effects. CDK6D163N was also unable to extend the life span of TIG3 cells (not shown). Importantly, overexpression of CDK4D158N did cause up-regulation of endogenous p16INK4a and p21CIP1 to a degree similar to that for wild-type CDK4 (Fig. 6B and D). This suggests that the homeostatic or stress response mechanism that balances the levels of CDK4 and p16INK4a is registering the amount of protein rather than its catalytic activity.
FIG. 6.
Catalytically inactive CDK4 does not extend the life span of HDFs. TIG3 cells (A and B) or Q34 cells (C and D) were infected with retroviruses encoding wild-type CDK4, the kinase-dead version, CDK4D158N, or empty vector. The infected cell pools were passaged under standard conditions of tissue culture until they reached M1 senescence. The curves (A and C) show cumulative PDs at each time point. Samples of cell lysate were immunoblotted with antibodies against CDK4, p16INK4a, and p21CIP1 (B and D). MEK was used as a control for loading. (E and F) TIG3 cells transduced with either CDK4 or CDK4D158N or the empty vector were infected with a retrovirus containing shRNA against p21CIP1. The curves show cumulative PDs at each time point (E). Samples of cell lysate were immunoblotted with antibodies against p21CIP1, CDK4, and MEK (F).
To further exclude the possibility that life span extension involves the sequestration of p21CIP1, we used shRNA to knock down the expression of p21CIP1 in TIG3 cells. As shown in Fig. 6F, the levels of p21CIP1 were substantially reduced, irrespective of the coexpression of CDK4 or CDK4D158N. Importantly, knock-down of p21CIP1 provided a significant life span extension, which was further extended by overexpression of the catalytically active version but not the catalytically inert version of CDK4 (Fig. 6E).
Multiple intermediate stages between M1 and M2.
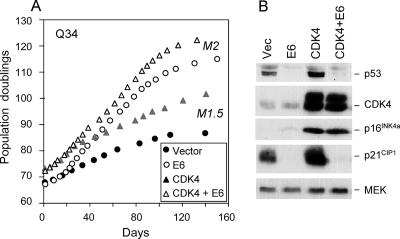
It was previously shown that overexpression of CDK4 or CDK6 enables normal HDFs to senesce at an intermediate stage between M1 and M2, operationally defined as MINT (34). This was based on the fact that subsequent ablation of p53 by human papillomavirus (HPV) E6 provided an additional life span extension that culminated in crisis. We used a similar argument to infer that p16INK4a-deficient cells or cells expressing Bmi1 also senesce at an MINT or M1.5 stage, rather than at M1 (6). The obvious question, therefore, is how to rationalize the ability of CDK4 and CDK6 to extend the life span of p16INK4a-deficient cells that are already destined to senesce at M1.5. To approach this issue, we introduced HPV E6 into Q34 cells that had been previously transduced with exogenous CDK4 and CDK6 or with the empty vector. As illustrated in Fig. 7A, HPV E6 provides a proliferative benefit to HDFs, as others have noted (12), presumably due to the loss of p53 and reduced expression of p21CIP1 (Fig. 7B). In both cases, introduction of E6 also resulted in a significantly extended life span (Fig. 7A), which culminated with an M2 phenotype (6; data not shown). Importantly, cells expressing both E6 and CDK4 reached a higher number of PDs than with either agent alone (Fig. 7A), and a similar though less-dramatic trend was noted with coexpression of E6 and CDK6 (not shown). The maximum life span of approximately 124 PDs is the highest that we have recorded for human fibroblasts without the introduction of telomerase. Taken together, the data suggest there are at least three intermediate stages between M1 and M2, one that is dependent on p16INK4a, another that is dependent on p53, and a third stage that can be bypassed by the kinase activities of CDK4 and CDK6.
FIG. 7.
Effects of p53 ablation and CDK4 overexpression are additive. (A) Q34 cells that had been previously infected with a retrovirus encoding wild-type CDK4 or vector-only control cells were superinfected with a virus encoding the E6 protein from HPV16. No drug selection was required because of the proliferative advantage gained by cells that express E6. The infected cell pools were passaged under standard conditions until cultures failed to double in 4 weeks. The curves show cumulative PDs at each time point. Note that cultures expressing E6 ended with an M2 phenotype, as demonstrated by BrdU incorporation and extensive cell death. The phenotype of the control cultures was indistinguishable from M1. (B) Samples of cell lysate were analyzed by immunoblotting for p53, CDK4, p16INK4a, or p21CIP1, as indicated. MEK was used as a control for loading.
DISCUSSION
The ability of exogenous CDK4 and CDK6 to overcome aspects of replicative senescence, whether induced by telomere dysfunction or proliferative stress, has been well documented (18, 34, 44, 46, 48, 53, 58). The data we describe here are consistent with these findings but question assumptions about the underlying mechanisms. Our study was initially prompted by the fact that the dramatic increase in the level of p16INK4a in senescent human fibroblasts does not prevent the assembly of cyclin D-CDK4 complexes (Fig. 1) (33, 54). Although the corresponding cyclin D-CDK6 complexes are disrupted, in line with indications that the INK4 family may bind preferentially to CDK6 (17, 39, 41), it is not clear what effect this would have in the face of sustained cyclin D-CDK4 function. Measurement of CDK4- and CDK6-associated kinase activity in human cells remains unreliable, and it is therefore difficult to tell whether the cyclin D-CDK4 complexes, which also contain p21CIP1 or p27KIP1, are catalytically active in senescent fibroblasts. Data on the inhibition of cyclin E-CDK2 are more robust (33, 54), and despite recent doubts about its importance in cell proliferation (4, 38, 55), loss of CDK2 activity remains the most likely explanation for the arrest of senescent cells.
Against this background, we were interested in exploring how overexpression of CDK4 and CDK6 can delay senescence. Based on our findings, the idea that they simply titrate p16INK4a is untenable. Firstly, cells respond to ectopic CDK4 and CDK6 by expressing substantially more p16INK4a. Gel filtration analyses suggest that much of the extra p16INK4a becomes bound to CDKs (Fig. 3B) and that the excess CDK4 drives the assembly of cyclin D-CDK4-p21CIP1 ternary complexes. Secondly, the CDK4R24C and CDK6R31C mutants, which are unable to bind to any of the endogenous INK4 proteins, cause life span extension and induce p16INK4a as effectively as the respective wild-type proteins (Fig. 2) (34). Thirdly, and most importantly, the exogenous CDKs can also extend the life span of fibroblasts that lack functional p16INK4a (Fig. 4). This cannot be explained by sequestration of p15INK4b, p18INK4c, and p19INK4d, since the INK4-insensitive variants, CDK4R24C and CDK6R31C, produce the same effect.
As an alternative explanation, we considered whether the expression of additional CDK4 and CDK6 could promote the formation of cyclin D-CDK complexes, thereby sequestering a greater proportion of the intracellular p21CIP1 and p27KIP1. While it is difficult to exclude this possibility, shRNA-mediated knockdown of p21CIP1 did not prevent life span extension by ectopic CDKs, and the results obtained with catalytically inactive versions of CDK4 and CDK6 suggest a different mechanism. Since these variants are evidently capable of binding to cyclin D1, p21CIP1, and p16INK4a, they should in principle be able to cause the same subunit rearrangements as wild-type CDKs. Interestingly, they are also capable of causing increased expression of p16INK4a and p21CIP1. However, in several independent experiments, CDK4D158N had virtually no effect on fibroblast life span, irrespective of p16INK4a status, and similar results were obtained with catalytically inactive CDK6.
The inevitable conclusion from these findings is that life span extension by CDK4 and CDK6 is not caused by subunit rearrangement but by their ability to phosphorylate a critical substrate or substrates. What is less clear is whether these are known proteins, such as pRb and its relatives, or previously unrecognized substrates. In this context, there are conflicting views about the need for cyclin D-dependent kinase activity to bypass pRb-mediated growth arrest (2, 29). One way to address this issue would be to ask whether CDK4 can delay senescence in cells lacking pRb. However, this experiment is problematic, because the selective inactivation of pRb, for example, with HPV E7, causes E2F-mediated expression of p14ARF and up-regulation of p53. E7 also has direct effects on p21CIP1 (14), and although senescence is delayed, the cells enter a precocious M2-like state (5; our unpublished observations). Similarly, HDFs in which pRb has been inactivated by homologous recombination have an extended life span that apparently ends in crisis (57). It is partly because of these problems that strategies for overriding the effects of p16INK4a rather than pRb have so much appeal. The remarkable feature of our findings was that two such strategies, CDK4 overexpression and p16INK4a deficiency, produced additive effects on life span yet still allowed the HDFs to adopt an M1-like state. Subsequent ablation of p53 elicited a further extension of life span, at which point the cells acquired an M2 phenotype. This implies that the effects of CDK4-associated kinase activity are independent of p53. In support of this, expression of HPV E6 caused a similar increase in the number of PDs in both CDK4-transduced and control cells. The inference is that CDK4 kinase activity might be targeting an additional pathway that contributes to the implementation of senescence.
Although some studies have drawn attention to differences between CDK4 overexpression and p16INK4a deficiency (23), most have assumed that they are mechanistically equivalent (18, 34, 46, 48, 58). A common approach has been to use the CDK4R24C mutant or a fusion protein in which this variant is linked to cyclin D1 (45) because of the additional benefit that this protein should be immune to all members of the INK4 family. Mice in which the endogenous Cdk4 locus has been replaced by the Cdk4R24C variant are tumor prone, and the resultant mouse embryonic fibroblasts are immortal and highly susceptible to transformation (44, 53). Our findings suggest that the underlying mechanisms warrant further investigation and point to the existence of an additional pathway that regulates proliferative life span via a mechanism that is sensitive to the catalytical activity of cyclin D-dependent kinases. Given the prevalence of cyclin D1-CDK4 dysregulation in human cancers, elucidation of this pathway is a high priority.
Supplementary Material
Acknowledgments
We thank Marc Rodriguez-Niedenführ and Goedele Maertens for comments on the manuscript and both past and present members of the lab for patience and support.
Footnotes
Published ahead of print on 9 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alcorta, D. A., Y. Xiong, D. Phelps, G. Hannon, D. Beach, and J. C. Barrett. 1996. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 93:13742-13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, G. L., M. W. Landis, and P. W. Hinds. 2005. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle 4:330-338. [PubMed] [Google Scholar]
- 3.Bates, S., L. Bonetta, D. MacAllan, D. Parry, A. Holder, C. Dickson, and G. Peters. 1994. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 9:71-79. [PubMed] [Google Scholar]
- 4.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13:1775-1785. [DOI] [PubMed] [Google Scholar]
- 5.Bond, J. A., M. F. Haughton, J. M. Rowson, P. J. Smith, V. Gire, D. Wynford-Thomas, and F. S. Wyllie. 1999. Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol. Cell. Biol. 19:3103-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookes, S., J. Rowe, A. Gutierrez Del Arroyo, J. Bond, and G. Peters. 2004. Contribution of p16(INK4a) to replicative senescence of human fibroblasts. Exp. Cell Res. 298:549-559. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d'Adda di Fagagna, F., P. M. Reaper, L. Clay-Farrace, H. Fiegler, P. Carr, T. Von Zglinicki, G. Saretzki, N. P. Carter, and S. P. Jackson. 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194-198. [DOI] [PubMed] [Google Scholar]
- 10.Diehl, J. A., W. Yang, R. A. Rimerman, H. Xiao, and A. Emili. 2003. Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol. Cell. Biol. 23:1764-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drayton, S., and G. Peters. 2002. Immortalisation and transformation revisited. Curr. Opin. Genet. Dev. 12:98-104. [DOI] [PubMed] [Google Scholar]
- 12.Dulic, V., G. E. Beney, G. Frebourg, L. F. Drullinger, and G. H. Stein. 2000. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol. Cell. Biol. 20:6741-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulic, V., L. F. Drullinger, E. Lees, S. I. Reed, and G. H. Stein. 1993. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc. Natl. Acad. Sci. USA 90:11034-11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil, J., D. Bernard, D. Martinez, and D. Beach. 2004. Polycomb CBX7 has a unifying role in cellular lifespan. Nat. Cell Biol. 6:67-72. [DOI] [PubMed] [Google Scholar]
- 16.Grossel, M. J., G. L. Baker, and P. W. Hinds. 1999. cdk6 can shorten G(1) phase dependent upon the N-terminal INK4 interaction domain. J. Biol. Chem. 274:29960-29967. [DOI] [PubMed] [Google Scholar]
- 17.Guan, K. L., C. W. Jenkins, Y. Li, M. A. Nichols, X. Wu, C. L. O'Keefe, A. G. Matera, and Y. Xiong. 1994. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 8:2939-2952. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara, E., R. Smith, D. Parry, H. Tahara, S. Stone, and G. Peters. 1996. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 16:859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harley, C. B. 1991. Telomere loss: mitotic clock or genetic time bomb? Mutat. Res. 256:271-282. [DOI] [PubMed] [Google Scholar]
- 21.Hayflick, L., and P. S. Moorhead. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585-621. [DOI] [PubMed] [Google Scholar]
- 22.Herbig, U., W. A. Jobling, B. P. Chen, D. J. Chen, and J. M. Sedivy. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14:501-513. [DOI] [PubMed] [Google Scholar]
- 23.Holland, E. C., W. P. Hively, V. Gallo, and H. E. Varmus. 1998. Modeling mutations in the G1 arrest pathway in human gliomas: overexpression of CDK4 but not loss of INK4a-ARF induces hyperploidy in cultured mouse astrocytes. Genes Dev. 12:3644-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huot, T. J., J. Rowe, M. Harland, S. Drayton, S. Brookes, C. Gooptu, P. Purkis, M. Fried, V. Bataille, E. Hara, J. Newton-Bishop, and G. Peters. 2002. Biallelic mutations in p16(INK4a) confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol. Cell. Biol. 22:8135-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164-168. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, H., H. S. Chou, and L. Zhu. 1998. Requirement of cyclin E-Cdk2 inhibition in p16(INK4a)-mediated growth suppression. Mol. Cell. Biol. 18:5284-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 28.Landis, M. W., B. S. Pawlyk, T. Li, P. Sicinski, and P. W. Hinds. 2006. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9:13-22. [DOI] [PubMed] [Google Scholar]
- 29.Leng, X., L. Connell-Crowley, D. Goodrich, and J. W. Harper. 1997. S-phase entry upon ectopic expression of G1 cyclin-dependent kinases in the absence of retinoblastoma protein phosphorylation. Curr. Biol. 7:709-712. [DOI] [PubMed] [Google Scholar]
- 30.Mahony, D., D. A. Parry, and E. Lees. 1998. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene 16:603-611. [DOI] [PubMed] [Google Scholar]
- 31.McConnell, B. B., F. J. Gregory, F. J. Stott, E. Hara, and G. Peters. 1999. Induced expression of p16(INK4a) inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol. Cell. Biol. 19:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra, J., C. Y. Dai, K. Somasundaram, W. S. El-Deiry, K. Satyamoorthy, M. Herlyn, and G. H. Enders. 1999. Induction of p21(WAF1/CIP1) and inhibition of Cdk2 mediated by the tumor suppressor p16(INK4a). Mol. Cell. Biol. 19:3916-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisaki, H., A. Ando, Y. Nagata, O. Pereira-Smith, J. R. Smith, K. Ikeda, and M. Nakanishi. 1999. Complex mechanisms underlying impaired activation of Cdk4 and Cdk2 in replicative senescence: roles of p16, p21, and cyclin D1. Exp. Cell Res. 253:503-510. [DOI] [PubMed] [Google Scholar]
- 34.Morris, M., P. Hepburn, and D. Wynford-Thomas. 2002. Sequential extension of proliferative lifespan in human fibroblasts induced by over-expression of CDK4 or 6 and loss of p53 function. Oncogene 21:4277-4288. [DOI] [PubMed] [Google Scholar]
- 35.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 36.Obaya, A. J., I. Kotenko, M. D. Cole, and J. M. Sedivy. 2002. The proto-oncogene c-myc acts through the cyclin-dependent kinase (Cdk) inhibitor p27(Kip1) to facilitate the activation of Cdk4/6 and early G(1) phase progression. J. Biol. Chem. 277:31263-31269. [DOI] [PubMed] [Google Scholar]
- 37.Olashaw, N., T. K. Bagui, and W. J. Pledger. 2004. Cell cycle control: a complex issue. Cell Cycle 3:263-264. [DOI] [PubMed] [Google Scholar]
- 38.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25-31. [DOI] [PubMed] [Google Scholar]
- 39.Palmero, I., B. McConnell, D. Parry, S. Brookes, E. Hara, S. Bates, P. Jat, and G. Peters. 1997. Accumulation of p16INK4a in mouse fibroblasts as a function of replicative senescence and not of retinoblastoma gene status. Oncogene 15:495-503. [DOI] [PubMed] [Google Scholar]
- 40.Parry, D., S. Bates, D. J. Mann, and G. Peters. 1995. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 14:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parry, D., D. Mahony, K. Wills, and E. Lees. 1999. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol. Cell. Biol. 19:1775-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavletich, N. P. 1999. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287:821-828. [DOI] [PubMed] [Google Scholar]
- 43.Ragione, F. D., G. L. Russo, A. Oliva, C. Mercurio, S. Mastropietro, V. D. Pietra, and V. Zappia. 1996. Biochemical characterization of p16INK4- and p18-containing complexes in human cell lines. J. Biol. Chem. 271:15942-15949. [DOI] [PubMed] [Google Scholar]
- 44.Rane, S. G., S. C. Cosenza, R. V. Mettus, and E. P. Reddy. 2002. Germ line transmission of the Cdk4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell. Biol. 22:644-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao, R. N., N. B. Stamm, K. Otto, S. Kovacevic, S. A. Watkins, P. Rutherford, S. Lemke, K. Cocke, R. P. Beckmann, K. Houck, D. Johnson, and B. J. Skidmore. 1999. Conditional transformation of rat embryo fibroblast cells by a cyclin D1-cdk4 fusion gene. Oncogene 18:6343-6356. [DOI] [PubMed] [Google Scholar]
- 46.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano, M., G. J. Hannon, and D. Beach. 1993. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704-707. [DOI] [PubMed] [Google Scholar]
- 48.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 49.Shay, J. W., W. E. Wright, and H. Werbin. 1991. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1072:1-7. [DOI] [PubMed] [Google Scholar]
- 50.Sherr, C. J. 2000. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 60:3689-3695. [PubMed] [Google Scholar]
- 51.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 52.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 53.Sotillo, R., P. Dubus, J. Martin, E. de la Cueva, S. Ortega, M. Malumbres, and M. Barbacid. 2001. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20:6637-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein, G. H., L. F. Drullinger, A. Soulard, and V. Dulic. 1999. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 19:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tetsu, O., and F. McCormick. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3:233-245. [DOI] [PubMed] [Google Scholar]
- 56.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 57.Wei, W., U. Herbig, S. Wei, A. Dutriaux, and J. M. Sedivy. 2003. Loss of retinoblastoma but not p16 function allows bypass of replicative senescence in human fibroblasts. EMBO Rep. 4:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei, W., W. A. Jobling, W. Chen, W. C. Hahn, and J. M. Sedivy. 2003. Abolition of cyclin-dependent kinase inhibitor p16Ink4a and p21Cip1/Waf1 functions permits Ras-induced anchorage-independent growth in telomerase-immortalized human fibroblasts. Mol. Cell. Biol. 23:2859-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei, W., and J. M. Sedivy. 1999. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp. Cell Res. 253:519-522. [DOI] [PubMed] [Google Scholar]
- 60.Wolfel, T., M. Hauer, J. Schneider, M. Serrano, C. Wolfel, E. Klehmann-Hieb, E. De Plaen, T. Hankeln, K. H. Meyer zum Buschenfelde, and D. Beach. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269:1281-1284. [DOI] [PubMed] [Google Scholar]
- 61.Wong, H., and K. Riabowol. 1996. Differential CDK-inhibitor gene expression in aging human diploid fibroblasts. Exp. Gerontol. 31:311-325. [DOI] [PubMed] [Google Scholar]
- 62.Wright, W. E., and J. W. Shay. 2002. Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 20:682-688. [DOI] [PubMed] [Google Scholar]
- 63.Yu, Q., E. Sicinska, Y. Geng, M. Ahnstrom, A. Zagozdzon, Y. Kong, H. Gardner, H. Kiyokawa, L. N. Harris, O. Stal, and P. Sicinski. 2006. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9:23-32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.