Abstract
Leptin stimulates fatty acid oxidation in skeletal muscle through the activation of AMP-activated protein kinase (AMPK) and the induction of gene expression, such as that for peroxisome proliferator-activated receptor α (PPARα). We now show that leptin stimulates fatty acid oxidation and PPARα gene expression in the C2C12 muscle cell line through the activation of AMPK containing the α2 subunit (α2AMPK) and through changes in the subcellular localization of this enzyme. Activated α2AMPK containing the β1 subunit was shown to be retained in the cytoplasm, where it phosphorylated acetyl coenzyme A carboxylase and thereby stimulated fatty acid oxidation. In contrast, α2AMPK containing the β2 subunit transiently increased fatty acid oxidation but underwent rapid translocation to the nucleus, where it induced PPARα gene transcription. A nuclear localization signal and Thr172 phosphorylation of α2 were found to be essential for nuclear translocation of α2AMPK, whereas the myristoylation of β1 anchors α2AMPK in the cytoplasm. The prevention of α2AMPK activation and the change in its subcellular localization inhibited the metabolic effects of leptin. Our data thus suggest that the activation of and changes in the subcellular localization of α2AMPK are required for leptin-induced stimulation of fatty acid oxidation and PPARα gene expression in muscle cells.
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is thought to function in mammalian cells to yeast as a cellular “fuel gauge,” signaling when energy stores are full or depleted (9, 16). The activation of AMPK results in the phosphorylation of several target molecules and consequent stimulation of fatty acid oxidation, glucose transport in muscle, and cardiac glycolysis as well as the inhibition of anabolic processes and ion channel activities (9, 16). AMPK is a heterotrimer consisting of a catalytic α subunit (α1 or α2) and regulatory β (β1 or β2) and γ (γ1, γ2, or γ3) subunits (9, 16). Phosphorylation of the α subunit (on Thr172) by the upstream kinase AMPKK or allosteric modulation by AMP binding is essential for the activation of AMPK (8, 38). Four kinases, LKB1 (1), Ca2+/calmodulin-dependent protein kinase kinase (10, 13, 39), ATM (32), and TAK1 (24), have been proposed to function as AMPKKs.
The activity of AMPK is regulated by hormones (21, 22, 41) and growth factors (32, 33) as well as by changes in cellular energy status. Leptin is a hormone produced by adipocytes that regulates food intake and fuel metabolism (3). Leptin exerts metabolic effects in peripheral tissues both directly and through the central nervous system (22, 25). We have previously shown that leptin stimulates fatty acid oxidation in skeletal muscle by activating the α2 subunit-containing AMPK (α2AMPK) both directly at the muscle level and indirectly through the hypothalamus and the sympathetic nervous system (22). Activated α2AMPK phosphorylates acetyl coenzyme A (CoA) carboxylase (ACC), resulting in the inhibition of its activity and reduced formation of malonyl-CoA. The latter effect in turn results in the activation of carnitine palmitoyltransferase 1, a step required for the stimulation of fatty acid oxidation in mitochondria (27). In addition, leptin induces the expression of genes whose products contribute to fatty acid oxidation, at least in part in a manner dependent on the transactivation activity of peroxisome proliferator-activated receptor α (PPARα) (18, 36). The activation of AMPK increases PPARα gene expression in muscle cells (19). However, the molecular mechanisms by which leptin activates fatty acid oxidation through α2AMPK and induces PPARα gene expression have remained unknown.
We have now examined how leptin increases fatty acid oxidation and PPARα gene expression via AMPK activation in the mouse muscle cell line C2C12. We found that leptin achieves these effects through the activation of α2AMPK, but not through that of α1AMPK. Activated AMPK consisting of α2, β1, and γ1 subunits was shown to phosphorylate ACC, thereby stimulating fatty acid oxidation, whereas activated AMPK consisting of α2, β2, and γ1 subunits translocates to the nucleus and induces PPARα gene expression. These results indicate that leptin stimulates fatty acid oxidation and gene expression in muscle cells by activating α2AMPK and changing its subcellular localization, with the regulatory β subunits of AMPK playing an important role in this process.
MATERIALS AND METHODS
Cell lines, culture, and transfection.
The mouse myoblastoma C2C12 cell line as well as 3T3-L1 and L6 cells were obtained from the American Type Culture Collection. Cells were cultured in six-well plates and maintained in Dulbecco's modified Eagle's medium (DMEM) containing glucose at 4.5 mg/ml (Sigma) and were supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and nonessential amino acids (Sigma). Transient transfection (5 μg of DNA per well) of C2C12 cells was performed with the TransFast transfection reagent (Promega) in serum-free DMEM for 4 h; cells were subjected to experiments after 48 h. The transfection efficiency was assessed with a vector encoding green fluorescent protein and was found to be >90%. To generate C2C12 cells that stably express Flag epitope-tagged α1 or α2 subunits of human AMPK, we seeded cells at a density of 2.5 × 105 per well and subjected them to transfection as described above; after 48 h, the cells were harvested by exposure to trypsin, seeded in 100-cm2 culture dishes, and cultured in the presence of G418 (1 mg/ml). G418-resistant cells expressing Flag-α1 or Flag-α2 were maintained in the G418-containing medium. For glucose deprivation, C2C12 cells were incubated for 1 or 6 h in glucose-free DMEM (Invitrogen) supplemented with 10% dialyzed fetal bovine serum and nonessential amino acids. Cells were stimulated with leptin, adiponectin, or 5-amino-4-imidazolecarboxamide ribose (AICAR) at a concentration of 10 ng/ml, 100 ng/ml, or 500 μM, respectively.
Antibodies, recombinant proteins, and plasmids.
Antibodies to the α subunits of AMPK (total α or Thr172 phosphorylated), to β-actin, or to cytochrome c were obtained from Cell Signaling Technology; those to ACC (total ACC or Ser79 phosphorylated) were from Upstate Biotechnology; those to Flag or to the hemagglutinin epitope (HA) were from Sigma; those to the β1 or β2 subunits of AMPK or to lamin B were from Santa Cruz Biotechnology; and those to Bcl-2 were from Transduction Laboratories. We also prepared affinity-purified antibodies that recognize either α1 or α2 subunits of AMPK by injecting rabbits with subunit-specific peptides (H2N-Cys-TSPPDSFLDDHHLTR-COOH for α1; H2N-Cys-MDDSAMHIPPGLKPH-COOH for α2) (40). Human recombinant leptin and human recombinant adiponectin were obtained from PeproTech and Alexis, respectively. AICAR was obtained from Sigma. Expression vectors [pcDNA3.1(+)] containing cDNAs for Flag-tagged (NH2-terminal) human α1 or α2 subunits of AMPK and for the nontagged mouse β1 subunit were kindly provided by H. Esumi (National Cancer Center, Kashiwa, Japan) and T. Ogura (Hokuriku University, Japan). The pCR2.1-Topo TA cloning vector and pLITMUS-28i vector were obtained from Invitrogen and New England Biolabs, respectively.
Immunofluorescence staining.
Cells were fixed with 4% paraformaldehyde for 20 min at room temperature. Nonspecific sites were blocked with fetal bovine serum in phosphate-buffered saline (PBS), and the cells were then incubated for 16 h at 4°C with rabbit polyclonal antibodies to Flag, to HA, or to β1 or β2 subunits of AMPK, washed with PBS, and incubated for 1 h at 37°C with fluorescein isothiocyanate-conjugated goat antibodies to rabbit immunoglobulin G (Santa-Cruz). The cells were then washed twice with PBS and observed with a fluorescence microscope (IX70 and DP Controller; Olympus).
RNA extraction and RT-PCR.
Total RNA was extracted from cells with Isogen (Wako), and portions of the RNA (100 ng) were subjected to reverse transcription (RT) with avian myeloblastosis virus reverse transcriptase and an oligo(dT)16 primer (Takara Biochemicals). The resulting first-strand cDNA was subjected to PCR with an LA PCR kit, version 2.1 (Takara), and gene-specific primers (see Table S1 in the supplemental material).
Preparation of siRNAs.
Small interfering RNAs (siRNAs) specific for each subunit of AMPK were prepared with a HiScribe RNA interference (RNAi) transcription kit (New England Biolabs). PCR was performed with first-strand DNA from C2C12 cells as a template and specific primer pairs (see Table S1 in the supplemental material), and the PCR products were subcloned into the pCR2.1-Topo vector. After sequencing, the vectors were digested with BamHI and XhoI, and the insert fragments were ligated into the pLITMUS-28i vector for RNAi. Double-stranded RNA was prepared from the resulting vectors (or from the empty vector as a control) by in vitro transcription with T7 RNA polymerase and was digested with RNase III (New England Biolabs) before application to cells. The efficacy of RNAi was assessed on the basis of the depletion of the target mRNA and protein as monitored by RT-PCR and immunoblot analyses.
Mutagenesis.
Mutagenesis was performed by PCR. PCR products were obtained with a reverse mutagenesis primer (see Table S2 in the supplemental material) and a T7 promoter primer, with the corresponding pcDNA3.1(+) vector as a template, as well as with a forward mutagenesis primer (see Table S2 in the supplemental material) and a reverse primer specific for the polyadenylation signal of bovine growth hormone cDNA, again with the corresponding pcDNA3.1(+) vector as a template. Both PCR products were purified and then mixed, annealed, and amplified by PCR with the T7 promoter and bovine growth hormone reverse primers. The resulting PCR product was purified, digested with restriction enzymes, and ligated into pcDNA3.1(+).
Subcellular fractionation.
Cells were suspended in a solution containing 2 mM EDTA and 10 mM Tris-HCl (pH 7.5) and incubated on ice for 10 min, after which an equal volume of a solution containing 0.5 M sucrose, 0.1 M KCl, 10 mM MgCl2, 2 mM CaCl2, 2 mM EDTA, and 10 mM Tris-HCl (pH 7.5) was added. The cell lysate was centrifuged at 2,700 rpm (700 × g) for 10 min at 4°C, and the resulting pellet and supernatant were collected as the nuclear and cytoplasmic fractions, respectively. The cytoplasmic fraction was then further centrifuged at 15,000 rpm (20,000 × g) for 3 h at 4°C, and the resulting supernatant and pellet were saved as the soluble and insoluble fractions, respectively.
Immunoprecipitation.
Cell lysates prepared by incubation of cells for 30 min at 4°C with 0.1% NP-40 in PBS were centrifuged at 15,000 × g for 15 min at 4°C, and the resulting supernatants (1 mg of protein) were subjected to immunoprecipitation with specific antibodies and protein G-Sepharose (Amersham). The beads were separated by centrifugation, washed six times with 0.1% NP-40 in PBS, and subjected to immunoblot analysis or an assay of kinase activity.
Immunoblot analysis.
Cells were lysed as for immunoprecipitation with the exception that the NP-40 concentration was 1%, and the supernatants were subjected to immunoblot analysis with specific primary antibodies. Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) and ECL reagents (Amersham).
Assay of AMPK activity.
The AMPK activities of cell extracts and immunoprecipitates were measured with the SAMS peptide and [γ-32P]ATP as described previously (17), with a small modification. A recombinant protein comprising maltose binding protein (MBP) fused with three copies of the SAMS peptide (MBP-SAMS) was used as the substrate. After incubation with [γ-32P]ATP, the fusion protein was purified with amylose resin (Amersham) and the associated radioactivity was measured with a scintillation counter (Beckman Coulter). We confirmed that the efficiency of phosphorylation of the MBP-SAMS protein by AMPK was similar to that of phosphorylation of the SAMS peptide fused to glutathione S-transferase (17).
Measurement of fatty acid oxidation.
Cells were exposed to [14C]palmitic acid (American Radiolabeled Chemicals, Inc.) for 30 min after incubation with or without leptin. The culture supernatant was then transferred to a 50-ml tube (Falcon) and mixed with a 1/10 volume of 1 M HCl. The 14CO2 produced during incubation of the mixture for 50 min at 30°C was trapped with a paper filter soaked with NaOH. The paper filter was then transferred to 1 ml of H2O and agitated for 5 min, after which the radioactivity in the H2O was measured with a scintillation counter.
Statistics.
All experiments were repeated at least three times, and each experiment was performed with duplicate or triplicate samples. Data are presented as means ± standard errors of the means (SEM). Statistical analysis was performed by analysis of variance, followed by Dunnett's posthoc test. Data (such as cell number) expressed as a percentage were analyzed after the arcsine transformation (30). A P value of <0.05 was considered statistically significant.
RESULTS
Leptin stimulates fatty acid oxidation by activating α2AMPK.
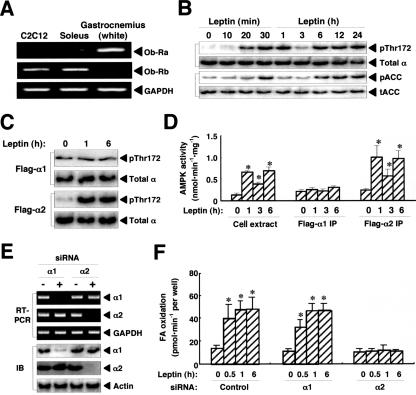
We previously showed that leptin activates α2AMPK and thereby stimulates fatty acid oxidation in red muscle types, including the soleus (22). Similar to the soleus muscle, the mouse myoblastoma C2C12 cell line was found to express the leptin receptor Ob-Rb (Fig. 1A). Leptin (10 ng/ml) induced the phosphorylation of the catalytic α subunit of AMPK on Thr172 in C2C12 cells; this effect was biphasic, with the early phase peaking at 30 min to 1 h and the second phase manifesting between 6 and 24 h (Fig. 1B). The phosphorylation of ACC (on Ser79) also increased in a biphasic manner in parallel with that of the α subunit of AMPK (Fig. 1B). A Flag-tagged version of the human α2 subunit of AMPK (Flag-α2), but not Flag-α1, was also phosphorylated on Thr172 (Fig. 1C) and activated (Fig. 1D) in C2C12 cells at both 1 and 6 h after leptin stimulation. To examine the relative roles of α2 and α1 in leptin-induced fatty acid oxidation in C2C12 cells, we depleted cells of each subunit individually by RNAi (Fig. 1E). The siRNA specific for α2, but not that for α1, abolished leptin-induced fatty acid oxidation in C2C12 cells (Fig. 1F). These data thus indicated that leptin stimulates fatty acid oxidation in C2C12 cells directly by activating α2AMPK. They further showed that long-term stimulation with leptin activates α2AMPK in a biphasic manner.
FIG. 1.
Leptin stimulates fatty acid oxidation through specific activation of α2AMPK in C2C12 cells. (A) Total RNA extracted from C2C12 cells or mouse skeletal muscle (soleus or white gastrocnemius) was subjected to RT-PCR analysis of Ob-Ra, Ob-Rb, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal control) mRNAs. The soleus and white gastrocnemius were used as exemplars of red and white types of skeletal muscle, respectively. (B) Cells were exposed to leptin (10 ng/ml) for the indicated times, after which cell extracts were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated (pThr172) AMPK or total α subunits of AMPK or to Ser79-phosphorylated (pACC) or total ACC (tACC). (C) Cells stably expressing Flag-α1 or Flag-α2 were stimulated with leptin for the indicated times, after which cell extracts were subjected to immunoprecipitation with antibodies to Flag. The resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated AMPK or total α subunits of AMPK. (D) Parental cells and cells stably expressing Flag-α1 or -α2 were treated with leptin for the indicated times, after which AMPK activity in extracts of the parental cells and in immunoprecipitates (IP) prepared with anti-Flag from the transfected cells was measured. Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero. (E) Cells were transfected with siRNAs specific for α1 or α2 subunits of AMPK (+) or with a control siRNA (−). After 48 h, total RNA was isolated from the cells and subjected to RT-PCR analysis of α1, α2, and GAPDH mRNAs (upper panels) and cell extracts were subjected to immunoblot analysis (IB) of α1, α2, and β-actin (internal control). (F) Cells transfected with control, α1, or α2 siRNAs were stimulated with leptin for the indicated times, and the rate of fatty acid (FA) oxidation was measured. Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero.
α2AMPK translocates to the nucleus concomitant with its activation.
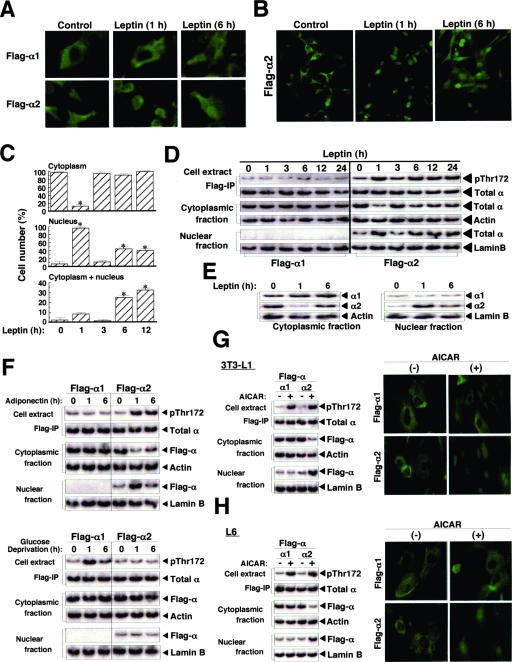
In a variety of cell types, including cell lines (29) and cells of the central nervous system (6, 34), immunofluorescence studies have revealed that α2 localizes to the nucleus or cytoplasm (or both), whereas α1 is restricted to the cytoplasm. With immunofluorescence analysis, we found that both Flag-α2 and -α1 were present in the cytoplasm of C2C12 cells under basal conditions (Fig. 2A and B). In cells exposed to leptin, however, Flag-α2 was detected in the nucleus at 1 h, had returned to the cytoplasm by 3 h, and was present in both the cytoplasm and the nucleus at 6 h after stimulation (Fig. 2A and B). Flag-α1, which was not phosphorylated on Thr172 in response to leptin stimulation (Fig. 1C), remained in the cytoplasm in leptin-treated cells (Fig. 2A). To quantify the changes in the subcellular localization of Flag-α2, we counted the number of cells in which Flag-α2 was detected in the cytoplasm, nucleus, or both compartments (Fig. 2C). The translocation of Flag-α2 to the nucleus at 1 h and its return to the cytoplasm by 3 h after leptin stimulation were apparent in >90% of cells. At 6 or 12 h after stimulation, Flag-α2 was detected in the nucleus of ∼40% of cells and in both the cytoplasm and the nucleus of ∼25 to 30% of cells. Similarly, immunoblot analysis revealed that Flag-α2, but not Flag-α1, translocated to the nucleus in a biphasic manner in parallel with Thr172 phosphorylation of the α subunit (Fig. 2D). Furthermore, endogenous α2, but not α1, had translocated to the nucleus at 1 h and appeared in both the cytoplasm and nucleus by 6 h after leptin stimulation (Fig. 2E). These observations thus showed that leptin changes the subcellular localization of α2 concomitant with its induction of Thr172 phosphorylation of α2 and its activation of α2AMPK.
FIG. 2.
Leptin induces subcellular redistribution of α2AMPK. (A and B) C2C12 cells stably expressing Flag-α1 or -α2 were treated with leptin for 0 (control), 1, or 6 h, fixed, immunostained with antibodies to Flag, and examined with a fluorescence microscope at a magnification of ×400 (A). Immunostained cells expressing Flag-α2 were also examined at a magnification of ×200 (B). (C) C2C12 cells stably expressing Flag-α1 or -α2 were exposed to leptin for the indicated times, fixed, immunostained with anti-Flag, and examined by fluorescence microscopy. The numbers of cells in which Flag-α2 was detected in the cytoplasm, the nucleus, or both the cytoplasm and the nucleus were counted (total of 500 cells in each well). Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero. (D) C2C12 cells stably expressing Flag-α1 or -α2 were stimulated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. The cytoplasmic and nuclear fractions as well as anti-Flag immunoprecipitates (IP) of cell extracts were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated AMPK or total α subunits of AMPK, to β-actin (cytoplasmic marker), or to lamin B (nuclear marker), as indicated. (E) C2C12 cells were treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was subjected to immunoblot analysis with antibodies to α1, to α2, to β-actin, or to lamin B. (F) C2C12 cells stably expressing Flag-α1 or -α2 were exposed to adiponectin (100 ng/ml) or to glucose-free medium for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. The cytoplasmic and nuclear fractions were subjected to immunoblot analysis with antibodies to Flag, to β-actin, or to lamin B, as indicated. Cell extracts were also subjected to immunoprecipitation with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated AMPK or total α subunits of AMPK. (G and H) Mouse fibroblast 3T3-L1 (G) or rat myoblastoma L6 (H) cells transiently expressing Flag-α1 or -α2 were incubated in the absence (−) or presence (+) of 0.5 mM AICAR for 1 h. Cell lysates were then separated into cytoplasmic and nuclear fractions, and these fractions as well as anti-Flag immunoprecipitates of cell extracts were subjected to immunoblot analysis (left panels) as described for panel F. Cells treated with AICAR were also fixed and immunostained with anti-Flag (right panels).
To examine whether the induction of the subcellular redistribution of α2 is specific to leptin, we examined the effects of adiponectin and glucose deprivation on the subcellular localization of Flag-α1 and -α2 in C2C12 cells (Fig. 2F). Adiponectin-induced Thr172 phosphorylation of Flag-α2, but not that of Flag-α1, was apparent at 1 and 6 h after stimulation, whereas glucose deprivation induced the phosphorylation of Flag-α1 but not that of Flag-α2. Similar to the effect of leptin, adiponectin induced translocation of Flag-α2, but not that of Flag-α1, to the nucleus in cells stimulated for 1 h and Flag-α2 was localized to both the nucleus and cytoplasm at 6 h after stimulation (Fig. 2F). In contrast, glucose deprivation did not affect the subcellular localization of either Flag-α1 or -α2. AICAR, an activator of α1AMPK and α2AMPK (8), also induced the translocation of Flag-α2, but not that of Flag-α1, to the nucleus of 3T3-L1 (Fig. 2G), L6 (Fig. 2H), and C2C12 (data not shown) cells stimulated for 1 h. It also induced Thr172 phosphorylation of both Flag-α1 and -α2. These results thus showed that α2 translocates to the nucleus in a variety of cell types in parallel with its phosphorylation on Thr172, whereas α1 does not translocate to the nucleus even when activated.
Nuclear translocation of α2AMPK is dependent on a nuclear localization signal (NLS) in the α2 subunit.
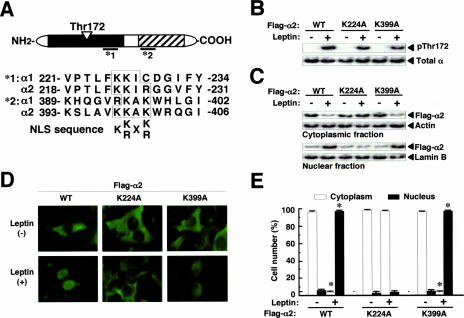
To explore the mechanism by which α2 is preferentially translocated to the nucleus, we searched for an NLS in α1 and α2. The minimum requirement for an NLS is Lys-(Lys/Arg)-X-(Lys/Arg) (12), and two amino acid sequences in human α2, but none in α1, met this criterion. One of these two sequences is present in the catalytic domain (Lys223 to Arg226) of α2, and the other is in the regulatory domain (Lys398 to Lys401) of α2 (Fig. 3A). These sequences are also conserved in mouse and rat α2 (data not shown). We constructed NLS mutants (K224A and K399A) of human α2 to examine the role of these sequences in the leptin-induced nuclear translocation of α2AMPK. Leptin induced Thr172 phosphorylation of both mutant proteins to a level similar to that observed with the wild-type protein [α2(WT)] (Fig. 3B). However, whereas leptin stimulation for 1 h induced the nuclear translocation of Flag-α2(K399A), it did not trigger that of Flag-α2(K224A) (Fig. 3C to E). These results thus indicated that the NLS at Lys223 to Arg226 of α2 is essential for nuclear translocation in response to leptin.
FIG. 3.
Nuclear translocation of α2AMPK is dependent on an NLS in the α2 subunit. (A) Amino acid sequences of putative NLSs in human α2. The filled and striped boxes in the schematic depiction of the α subunit represent the catalytic and regulatory domains, respectively. *1 and *2, amino acid sequences of putative NLSs in human α2. (B) C2C12 cells transiently expressing Flag-α2 (WT, K224A, or K399A) were treated (+) or not treated (−) with leptin for 1 h, after which cell extracts were subjected to immunoprecipitation with anti-Flag and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated or total α subunits. (C) C2C12 cells transiently expressing Flag-α2 (WT, K224A, or K399A) were exposed (+) or not exposed (−) to leptin for 1 h, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was then subjected to immunoblot analysis with antibodies to Flag, to β-actin, or to lamin B, as indicated. (D) C2C12 cells transiently expressing Flag-α2 (WT, K224A, or K399A) were incubated in the absence (−) or presence (+) of leptin for 1 h and then examined by immunofluorescence analysis with anti-Flag. (E) The numbers of cells in which Flag-α2 was detected in the cytoplasm or nucleus after treatment and analysis, as described for panel D, were counted (total of 500 cells per well). Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for cells not exposed to leptin.
Phosphorylation of α2 on Thr172 and the presence of β and γ subunits are essential for nuclear translocation of α2AMPK.
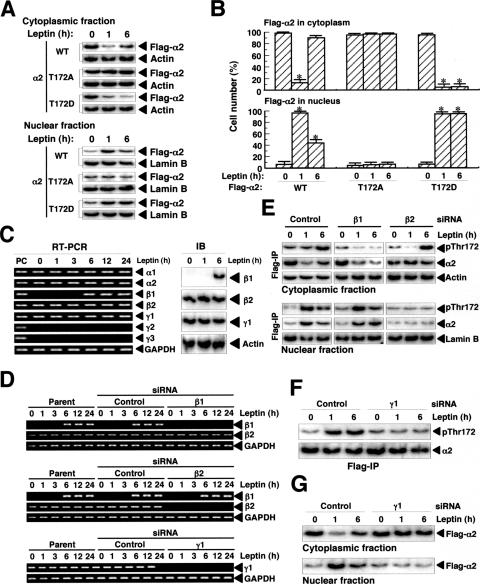
We next examined whether Thr172 phosphorylation is required for the nuclear translocation of α2 in response to leptin stimulation. We constructed two Thr172 mutants of human α2, α2(T172D) and α2(T172A), which mimic the phosphorylated and nonphosphorylated forms of the protein, respectively. In cells stimulated with leptin, Flag-α2(WT) had translocated to the nucleus at 1 h and was apparent in both the cytoplasm and nucleus at 6 h, whereas Flag-α2(T172A) did not translocate to the nucleus in response to leptin (Fig. 4A and B). In contrast, Flag-α2(T172D), which was present in the cytoplasm before leptin stimulation, had translocated to the nucleus at 1 h but had not relocated to the cytoplasm at 6 h after exposure of cells to leptin. These results thus indicated that Thr172 phosphorylation is essential for the nuclear translocation of α2 in response to leptin and that the subsequent dephosphorylation of this residue is necessary for the relocation of α2 to the cytoplasm apparent at 6 h. Furthermore, the cytoplasmic localization of Flag-α2(T172D) apparent before leptin stimulation indicated that some other factor in addition to Thr172 phosphorylation is required for leptin-induced nuclear translocation of α2.
FIG. 4.
Phosphorylation of α2 on Thr172 and the presence of β and γ regulatory subunits are necessary for the leptin-induced changes in the subcellular localization of α2AMPK in C2C12 cells. (A) Cells transiently expressing Flag-α2 (WT, T172D, or T172A) were treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was then subjected to immunoblot analysis with antibodies to Flag, to β-actin, or to lamin B, as indicated. (B) Cells transiently expressing Flag-α2 (WT, T172A, or T172D) were treated with leptin for the indicated times, fixed, and immunostained with anti-Flag. The numbers of cells in which Flag-α2 was detected in the cytoplasm or in the nucleus were counted (total of 500 cells per well). Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero. (C) Cells were treated with leptin for the indicated times, after which total RNA was isolated and subjected to RT-PCR analysis of AMPK-subunit and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs (left panel); total RNA from mouse skeletal muscle (mixture of soleus and red and white types of gastrocnemius) was used as a positive control (PC). Alternatively, extracts of the leptin-treated cells were subjected to immunoblot (IB) analysis with antibodies to β1, β2, or γ1 subunits of AMPK and to β-actin. (D) Cells transfected with control, β1, β2, or γ1 siRNAs or parental cells were treated with leptin for the indicated times, after which total RNA was isolated and subjected to RT-PCR analysis of β1, β2, γ1, or GAPDH mRNAs. (E) Cells stably expressing Flag-α2 were transfected with control, β1, or β2 siRNAs and then treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. These fractions were subjected to immunoblot analysis with antibodies to β-actin or to lamin B, respectively. The fractions were also subjected to immunoprecipitation (IP) with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated α subunits of AMPK and to α2. (F) Cells stably expressing Flag-α2 were transfected with control or γ1 siRNAs and treated with leptin for the indicated times, after which cell extracts were subjected to immunoprecipitation with anti-Flag and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated α subunits of AMPK and to α2. (G) Cells stably expressing Flag-α2 were transfected with control or γ1 siRNAs and treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was subjected to immunoblot analysis with anti-Flag.
We next examined the potential role of regulatory subunits of AMPK in the changes in the subcellular localization of α2AMPK in response to leptin. C2C12 cells express endogenous α1, α2, β2, and γ1 subunits of AMPK before and after leptin stimulation (Fig. 4C). In addition, leptin induced β1 expression at the mRNA and protein levels after stimulation for 6 h. We constructed siRNAs for β1, β2, and γ1 subunits and showed that these siRNAs effectively depleted C2C12 cells of the corresponding mRNAs (Fig. 4D). The siRNA for β1 inhibited the relocalization of α2 to the cytoplasm normally apparent at 6 h after leptin stimulation, whereas that for β2 inhibited the nuclear translocation of α2 at 1 or 6 h (Fig. 4E). The siRNA for β2 also abolished the leptin-induced phosphorylation of α2 on Thr172 at 1 h, at which time it was the only β subunit expressed in these cells. These results indicated that β1 mediates the cytoplasmic relocalization of α2 apparent at 6 h after leptin stimulation, whereas β2 contributes to the nuclear translocation of α2. In addition, the siRNA for γ1, which is the only γ subunit expressed in C2C12 cells, abolished both Thr172 phosphorylation (Fig. 4F) and nuclear translocation (Fig. 4G) of α2 in response to leptin. Both Thr172 phosphorylation and βγ regulatory subunits are thus essential for the leptin-induced nuclear translocation of α2. Furthermore, β1 and β2 appear to determine the subcellular localization of α2AMPK in leptin-treated cells.
The NH2-terminal myristoylation site of the β1 subunit is necessary for cytoplasmic anchoring of α2AMPK and persistent ACC phosphorylation.
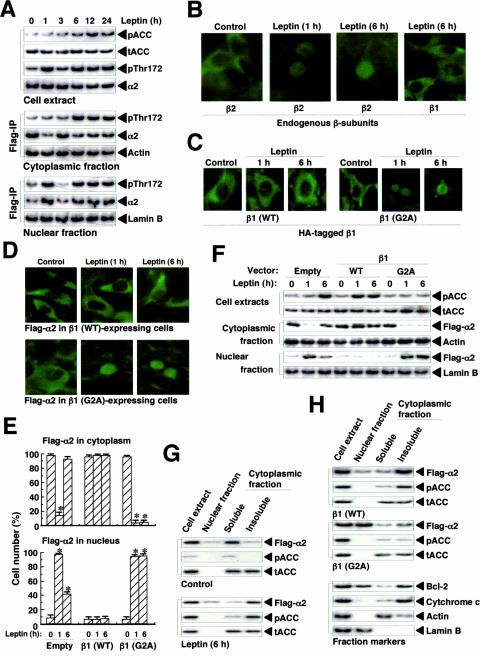
To explore the role of the β subunits of AMPK in the regulation of fatty acid oxidation, we examined the relationship between ACC phosphorylation (on Ser79) and the subcellular localization of the α2, β1, and β2 subunits of AMPK in leptin-treated cells. In parallel with the phosphorylation of cytoplasmic α2 on Thr172, the level of ACC phosphorylation was increased from 6 to 24 h after leptin stimulation (Fig. 5A). Endogenous β1 was present in the cytoplasm at 6 h after leptin stimulation, whereas β2 had translocated to the nucleus at both 1 and 6 h (Fig. 5B). These results suggested that α2AMPK containing β1 mediates ACC phosphorylation in the cytoplasm during the late phase of α2AMPK activation in response to leptin.
FIG. 5.
Role of the β1 subunit in cytoplasmic anchoring of α2AMPK and persistent ACC phosphorylation in C2C12 cells. (A) Cells stably expressing Flag-α1 or Flag-α2 were treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was subjected to immunoprecipitation (IP) with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated α subunits of AMPK and to α2. The cytoplasmic and nuclear fractions were also subjected to immunoblot analysis with antibodies to β-actin and to lamin B, respectively. In addition, cell extracts were subjected to immunoblot analysis with antibodies to phosphorylated or total ACC as well as with those to Thr172-phosphorylated α subunits of AMPK and to α2. (B) Cells were treated with leptin for 0, 1, or 6 h, fixed, and immunostained with antibodies to β1 or to β2. (C) Cells transiently expressing HA-tagged β1(WT) or β1(G2A) were treated with leptin for 0, 1, or 6 h, fixed, and immunostained with anti-HA. (D) Cells stably expressing Flag-α2 were transfected with vectors for HA-tagged β1(WT) or β1(G2A), stimulated with leptin, fixed, and immunostained with anti-Flag. (E) Cells stably expressing Flag-α2 were transfected with vectors for β1(WT) or β1(G2A) or with the corresponding empty vector. They were then treated with leptin, fixed, and immunostained with anti-Flag, and the cells in which Flag-α2 was detected in the cytoplasm or in the nucleus were counted (total of 500 cells per well). Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero. (F) Cells stably expressing Flag-α2 were transfected with vectors for β1(WT) or β1(G2A) or with the corresponding empty vector. They were then treated with leptin, after which cell lysates were separated into cytoplasmic and nuclear fractions. Cell extracts were subjected to immunoblot analysis with antibodies to phosphorylated or total ACC, whereas the cytoplasmic and nuclear fractions were subjected to immunoblot analysis with antibodies to Flag, to β-actin, or to lamin B. (G) Cells stably expressing Flag-α2 were incubated in the absence (control) or presence of leptin for 6 h, after which cell lysates were separated into nuclear and cytoplasmic fractions and the cytoplasmic fraction was further separated into soluble and insoluble portions. Cell extracts and subcellular fractions were subjected to immunoblot analysis with antibodies to Flag and to phosphorylated or total ACC. (H) Cells stably expressing Flag-α2 were transfected with vectors for β1(WT) or β1(G2A) and then treated with leptin for 1 h. Cell lysates were separated into nuclear and cytoplasmic fractions, and the cytoplasmic fraction was further separated into soluble and insoluble portions. Cell extracts and subcellular fractions were subjected to immunoblot analysis with antibodies to Flag and to phosphorylated or total ACC. The blots were also probed with antibodies to cytochrome c, to β-actin, to lamin B, and to Bcl-2 as markers for mitochondria, the cytoplasm, the nucleus, and both the nucleus and mitochondria, respectively.
The β1 subunit of AMPK contains an NH2-terminal consensus sequence for myristoylation, with Gly at position 2 and Ser at position 6 (residues 1 to 6: MGNTSS) (23, 37). To examine the role of this myristoylation site of β1 in the cytoplasmic localization of α2AMPK, we constructed wild-type and G2A mutant versions of mouse β1 with an HA tag at the COOH terminus. β1(WT) was detected in the cytoplasm constitutively before and after leptin stimulation, whereas β1(G2A) had translocated from the cytoplasm to the nucleus at 1 and 6 h after leptin stimulation (Fig. 5C). Furthermore, the expression of β1(WT) inhibited the nuclear translocation of α2 at 1 and 6 h, whereas that of β1(G2A) promoted this process (Fig. 5D to F). The expression of β1(WT) also increased ACC phosphorylation at both 1 and 6 h after leptin stimulation, whereas the expression of β1(G2A) inhibited ACC phosphorylation (Fig. 5F). Whereas α2AMPK (Flag-α2) was present in the soluble portion of the cytoplasmic fraction before leptin stimulation, it was detected in the insoluble portion of this fraction 6 h after leptin stimulation (Fig. 5G). Consistent with this change in the subcellular localization of α2, phosphorylated ACC was detected in the insoluble portion of the cytoplasmic fraction, not in the soluble portion, at this time. Furthermore, the expression of β1(WT) resulted in the localization of α2 in the insoluble portion of the cytoplasmic fraction 1 h after leptin stimulation, in contrast with the nuclear localization of α2 apparent at this time in cells expressing β1(G2A) (Fig. 5H). The amount of phosphorylated ACC was also increased in the insoluble portion of the cytoplasmic fraction at this time in cells expressing β1(WT) but not in those expressing β1(G2A). The NH2-terminal myristoylation site of β1 thus appears to be necessary both for anchoring α2AMPK to organelles in the cytoplasm such as mitochondria and for persistent ACC phosphorylation during the late phase of α2AMPK activation in response to leptin.
Nuclear α2AMPK induces PPARα gene expression.
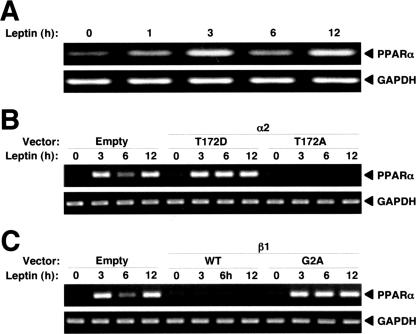
Leptin and AMPK stimulate the expression of genes whose products contribute to the regulation of fatty acid oxidation (18, 20, 42). Among these genes, we found that leptin increased the amount of PPARα mRNA in C2C12 cells in a biphasic manner (Fig. 6A). The expression of Flag-α2(T172D) enhanced this effect of leptin, whereas the expression of Flag-α2(T172A) blocked it (Fig. 6B). Furthermore, the expression of β1(WT) suppressed the up-regulation of PPARα mRNA by leptin, whereas the expression of β1(G2A) promoted it (Fig. 6C). The induction of PPARα gene expression thus correlated well with the nuclear localization of α2AMPK in leptin-stimulated cells.
FIG. 6.
Nuclear α2AMPK induces PPARα gene expression in C2C12 cells. (A) Cells were exposed to leptin for the indicated times, after which total RNA was isolated and subjected to RT-PCR analysis of PPARα and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs. (B) Cells were transiently transfected with vectors for Flag-α2(T172D) or Flag-α2(T172A) or with the corresponding empty vector and were then treated with leptin for the indicated times. Total RNA was isolated and subjected to RT-PCR analysis of PPARα and GAPDH mRNAs. (C) Cells were transiently transfected with vectors for β1(WT) or β1(G2A) or with the corresponding empty vector and were then treated with leptin for the indicated times, after which total RNA was subjected to RT-PCR analysis of PPARα and GAPDH mRNAs.
DISCUSSION
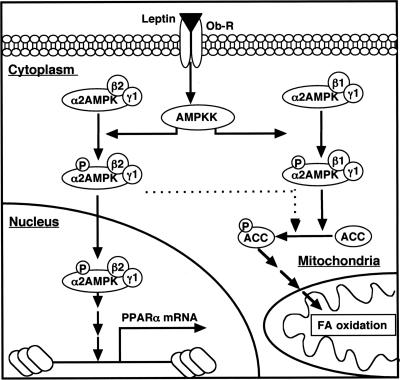
We have shown that changes in the subcellular localization of α2AMPK mediated by the α2 and β1 and β2 regulatory subunits play an important role in the metabolic effects of leptin in C2C12 cells. Previous studies demonstrated that both leptin and adiponectin activate only α2AMPK, whereas glucose deprivation activates only α1AMPK and AICAR activates both forms (2, 21, 22, 41). Furthermore, we have now shown that leptin-induced activation of α2AMPK occurs in a biphasic manner in C2C12 cells. At early time points after leptin stimulation, the α2 subunit of AMPK bound to the β2 subunit translocates to the nucleus in a manner dependent on an NLS that is present in α2 but not in α1; the α2β2 form of AMPK in the nucleus then rapidly induces transcription of the PPARα gene. The exposure of the cells to leptin for 6 h triggered transcription of the gene for the β1 subunit of AMPK, and the encoded protein, together with α2, became localized to an insoluble fraction of the cytoplasm as a result of a myristoylation sequence at its NH2 terminus. The α2β1 form of AMPK then phosphorylated ACC and stimulated fatty acid oxidation. Our data thus indicate that α2β1 and α2β2 forms of AMPK play distinct roles in mediating the metabolic actions of leptin. The mechanism by which leptin activates the expression of the β1-subunit gene remains unclear. However, the exposure of C2C12 cells to adiponectin or AICAR also induced β1 gene expression (data not shown), suggesting that α2AMPK may contribute to this effect. The anchoring of some α2β1 molecules to the outer mitochondrial membrane through the myristoylation of β1 might be expected to result in highly efficient depletion of malonyl-CoA, the activation of carnitine palmitoyltransferase 1, and the stimulation of fatty acid oxidation in mitochondria. Our proposed model for the mechanism by which α2AMPK mediates the stimulatory effect of leptin on fatty acid oxidation is summarized in Fig. 7.
FIG. 7.
Proposed model for the signaling pathway by which leptin stimulates fatty acid oxidation and PPARα gene expression in C2C12 cells. Long-term stimulation with leptin induces a biphasic activation of α2AMPK. Leptin first activates α2AMPK containing β2 and γ1 subunits, resulting in the stimulation of fatty acid (FA) oxidation through the phosphorylation of ACC and depletion of malonyl-CoA. However, this promotion of fatty acid oxidation is transient because α2AMPK containing β2 and γ1 rapidly undergoes translocation to the nucleus (within 1 h) as a result of an NLS in the α2 subunit. The active α2AMPK in the nucleus then induces the transcription of the PPARα gene. Stimulation with leptin for at least 6 h triggers a second wave of α2AMPK activation, in part attributable to the induction of transcription of the gene for the β1 subunit. Active α2AMPK containing β1 and γ1 is retained in the cytoplasm as a result of myristoylation of β1; it phosphorylates ACC, thereby stimulating fatty acid oxidation in mitochondria. Active α2AMPK containing β2 and γ1 again translocates to the nucleus and increases PPARα gene expression.
We previously showed that leptin activates α2AMPK in the soleus muscle in a biphasic manner in vivo (22). We injected leptin in a bolus through an intravenous catheter, which resulted in a transient increase in the concentration of leptin in plasma (22). This transient increase in plasma leptin level resulted in an early transient activation of α2AMPK in the soleus by a direct action of leptin, with the second phase of activation being mediated through the hypothalamus and the sympathetic nervous system. Our present data suggest that long-term exposure to leptin is necessary for a biphasic activation of α2AMPK mediated by a direct action of the hormone. Indeed, short-term treatment (1 h) with leptin did not induce a second phase of α2AMPK activation in C2C12 cells, but it did activate β1 gene expression at 6 h (data not shown).
Changes in the subcellular localization of α2AMPK in response to specific stimuli appear to be conserved from yeast to mammals. The Snf1 kinase complex is an AMPK homolog in yeast that is required for the cellular response to glucose limitation (4, 9). The Snf1 kinase complex is also activated by upstream kinases (11) and comprises a catalytic α subunit (Snf1), one of three related β subunits (Gal83, Sip1, or Sip2) (14), and a γ subunit (Snf4) (15). The three β subunits show distinct patterns of localization to the nucleus, vacuole, and cytoplasm, respectively. Gal83 is required for the nuclear localization of Snf1 in a glucose-regulated manner (35). Consistent with our present findings and previous observations with the Snf1 kinase complex, a recent study showed that the activation of α2AMPK is accompanied by its translocation to the nucleus in skeletal muscle cells in vivo (31). Our data also show that both AICAR and adiponectin induce the nuclear translocation of α2AMPK. Changes in the subcellular localization of α2AMPK that are dependent on the β subunits of this enzyme thus appear to explain, at least in part, the multifunctional effects of AMPK on metabolism. The expression of the β1 and β2 subunits and the myristoylation of β1 vary among different types of skeletal muscle and other tissues (5, 6, 23, 37). The activation of AMPK may elicit distinct metabolic effects in tissues and cells depending on the expression of the different β- and α-subunit isoforms.
Our present study also revealed that α2AMPK that has translocated to the nucleus induces transcription of the PPARα gene. It is possible that this effect of nuclear α2AMPK is mediated by the Sp1-CRSP complex. The 5′ flanking region of the PPARα gene (including that in mouse) contains several putative Sp1 binding sites (7). The CRSP complex (also known as CRSP130, SUR2, MED23, and DRIP130) directs transcriptional initiation by the RNA polymerase II apparatus and is required for efficient transcriptional activation by Sp1 (26, 28). Our preliminary data show that siRNAs for Sp1 or CRSP3, both of which are expressed in C2C12 cells, suppressed leptin-induced expression of the PPARα gene in these cells and that α2AMPK phosphorylates CRSP3 in vitro (unpublished data). The role of the Sp1-CRSP complex in the regulation of PPARα gene expression by α2AMPK thus warrants further investigation.
In conclusion, our data indicate that the metabolic effects of leptin are controlled by changes in the subcellular localization of α2AMPK. As in yeast, the subcellular localization of α2AMPK in mammalian cells appears to be determined by the regulatory β subunits. Our results provide new insight into the molecular mechanism by which energy metabolism is regulated by AMPK in response to leptin and other specific stimuli.
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) (16390062) (to Y.M.), a Grant-in-Aid for Exploratory Research (17659069) (to Y.M.), a Grant-in-Aid for Young Scientists (A) (18689023) (to A.S.), and a Grant-in-Aid for Young Scientists (B) (18790629) (to S.O.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a research award from Diko Foundation (to Y.M.); the Smoking Research Foundation (to Y.M.); and the Japan Foundation for Applied Enzymology (to Y.M.).
We thank H. Esumi and T. Ogura for expression plasmids, K. Kameda (Ehime University, Japan) for a constitutively active form of the α1 subunit of AMPK used as a positive control for the AMPK activity assay, and the Center for Analytical Instruments at the National Institute for Basic Biology (Okazaki) for DNA sequencing.
Footnotes
Published ahead of print on 9 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., K. Sakamoto, and J. R. Bayascas. 2006. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75:137-163. [DOI] [PubMed] [Google Scholar]
- 2.Andreelli, F., M. Foretz, C. Knauf, P. D. Cani, C. Perrin, M. A. Iglesias, M. B. Pillot, A. Bado, F. Tronche, G. Mithieux, S. Vaulont, R. Burcelin, and B. Viollet. 2006. Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 147:2432-2441. [DOI] [PubMed] [Google Scholar]
- 3.Bjørbaek, C., and B. B. Kahn. 2004. Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 59:305-331. [DOI] [PubMed] [Google Scholar]
- 4.Carlson, M. 1999. Glucose repression in yeast. Curr. Opin. Microbiol. 2:202-207. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Z. P., J. Heierhorst, R. J. Mann, K. I. Mitchelhill, B. J. Michell, L. A. Witters, G. S. Lynch, B. E. Kemp, and D. Stapleton. 1999. Expression of the AMP-activated protein kinase β1 and β2 subunits in skeletal muscle. FEBS Lett. 460:343-348. [DOI] [PubMed] [Google Scholar]
- 6.Culmsee, C., J. Monnig, B. E. Kemp, and M. P. Mattson. 2001. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J. Mol. Neurosci. 17:45-58. [DOI] [PubMed] [Google Scholar]
- 7.Gearing, K. L., A. Crickmore, and J. A. Gustafsson. 1994. Structure of the mouse peroxisome proliferator activated receptor α gene. Biochem. Biophys. Res. Commun. 199:255-263. [DOI] [PubMed] [Google Scholar]
- 8.Hardie, D. G., S. A. Hawley, and J. W. Scott. 2006. AMP-activated protein kinase—development of the energy sensor concept. J. Physiol. (London) 574:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensor of the eukaryotic cell? Annu. Rev. Biochem. 67:821-855. [DOI] [PubMed] [Google Scholar]
- 10.Hawley, S. A., D. A. Pan, K. J. Mustard, L. Ross, J. Bain, A. M. Edelman, B. G. Frenguelli, and D. G. Hardie. 2005. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2:9-19. [DOI] [PubMed] [Google Scholar]
- 11.Hedbacker, K., S. P. Hong, and M. Carlson. 2004. Pak1 protein kinase regulates activation and nuclear localization of Snf1-Gal83 protein kinase. Mol. Cell. Biol. 24:8255-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodel, M. R., A. H. Corbett, and A. E. Hodel. 2001. Dissection of a nuclear localization signal. J. Biol. Chem. 276:1317-1325. [DOI] [PubMed] [Google Scholar]
- 13.Hurley, R. L., K. A. Anderson, J. M. Franzone, B. E. Kemp, A. R. Means, and L. A. Witters. 2005. The Ca++/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280:29060-29066. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, R., and M. Carlson. 1996. Glucose regulates protein interactions within the yeast SNF1 protein kinase complex. Genes Dev. 10:3105-3115. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, R., and M. Carlson. 1997. The Snf1 protein kinase and its activating subunit, Snf4, interact with distinct domains of the Sip1/Sip2/Gal83 component in the kinase complex. Mol. Cell. Biol. 17:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp, B. E., K. I. Mitchelhill, D. Stapleton, B. J. Michell, Z. P. Chen, and L. A. Witters. 1999. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 24:22-25. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto, A., T. Ogura, and H. Esumi. 2006. A pull-down assay for 5′ AMP-activated protein kinase activity using GST-fused protein. Mol. Biotechnol. 32:17-21. [DOI] [PubMed] [Google Scholar]
- 18.Lee, W. J., M. Kim, H. S. Park, H. S. Kim, M. J. Jeon, K. S. Oh, E. H. Koh, J. C. Won, M. S. Kim, G. T. Oh, M. Yoon, K. U. Lee, and J. Y. Park. 2006. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem. Biophys. Res. Commun. 340:291-295. [DOI] [PubMed] [Google Scholar]
- 19.Lee, Y., X. Yu, F. Gonzales, D. J. Mangelsdorf, M. Y. Wang, C. Richardson, L. A. Witters, and R. H. Unger. 2002. PPARα is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. USA 99:11848-11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leff, T. 2003. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem. Soc. Trans. 31:224-227. [DOI] [PubMed] [Google Scholar]
- 21.Minokoshi, Y., T. Alquier, N. Furukawa, Y.-B. Kim, A. Lee, B. Xue, J. Mu, F. Foufelle, P. Ferre, M. J. Birnbaum, B. J. Stuck, and B. B. Kahn. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569-574. [DOI] [PubMed] [Google Scholar]
- 22.Minokoshi, Y., Y.-B. Kim, O. D. Peroni, L. G. Fryer, C. Muller, D. Carling, and B. B. Kahn. 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339-343. [DOI] [PubMed] [Google Scholar]
- 23.Mitchelhill, K. I., B. J. Michell, C. M. House, D. Stapleton, J. Dyck, J. Gamble, C. Ullrich, L. A. Witters, and B. E. Kemp. 1997. Posttranscriptional modifications of the 5′-AMP-activated protein kinase β1 subunit. J. Biol. Chem. 272:24475-24479. [DOI] [PubMed] [Google Scholar]
- 24.Momcilovic, M., S. P. Hong, and M. Carlson. 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 281:25336-25343. [DOI] [PubMed] [Google Scholar]
- 25.Muoio, D. M., and G. Lynis Dohm. 2002. Peripheral metabolic action of leptin. Best Pract. Res. Clin. Endocrinol. Metab. 16:653-666. [DOI] [PubMed] [Google Scholar]
- 26.Näär, A. M., D. J. Taatjes, W. Zhai, E. Nogales, and R. Tjian. 2002. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 16:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruderman, N., and M. Prentki. 2004. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 3:340-351. [DOI] [PubMed] [Google Scholar]
- 28.Ryu, S., S. Zhou, A. G. Ladurner, and R. Tjian. 1999. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature 397:446-450. [DOI] [PubMed] [Google Scholar]
- 29.Salt, I., J. W. Celler, S. A. Hawley, A. Prescott, A. Woods, D. Carling, and D. G. Hardie. 1998. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing α2 isoform. Biochem. J. 334:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokal, R. R., and F. F. Rohlf. 1987. Introduction to biostatistics. W. H. Freeman and Company, New York, NY.
- 31.Steinberg, G. R., M. J. Watt, S. L. McGee, S. Chan, M. Hargreaves, M. A. Febbraio, D. Stapleton, and B. E. Kemp. 2006. Reduced glycogen availability is associated with increased AMPKα2 activity, nuclear AMPKα2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl. Physiol. Nutr. Metab. 31:302-312. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, A., G. Kusakai, A. Kishimoto, Y. Shimojo, T. Ogura, M. F. Lavin, and H. Esumi. 2004. IGF-1 phosphorylates AMPK-α subunit in ATM-dependent and LKB1-independent manner. Biochem. Biophys. Res. Commun. 324:986-992. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, A., G. Kusakai, Y. Shimojo, J. Chen, T. Ogura, M. Kobayashi, and H. Esumi. 2005. Involvement of TGF-β1 signaling in hypoxia-induced tolerance to glucose starvation. J. Biol. Chem. 280:31557-31563. [DOI] [PubMed] [Google Scholar]
- 34.Turnley, A. M., D. Stapleton, R. J. Mann, L. A. Witters, B. E. Kemp, and P. F. Bartlett. 1999. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 72:1707-1716. [DOI] [PubMed] [Google Scholar]
- 35.Vincent, O., R. Townley, S. Kuchin, and M. Carlson. 2001. Subcellular localization of the Snf1 kinase is regulated by specific β subunits and a novel glucose signaling mechanism. Genes Dev. 15:1104-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, M. Y., L. Orci, M. Ravazzola, and R. H. Unger. 2005. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: implications for treatment of human obesity. Proc. Natl. Acad. Sci. USA 102:18011-18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warden, S. M., C. Richardson, J. J. O'Donnell, D. Stapleton, B. E. Kemp, and L. A. Witters. 2001. Post-translational modifications of the β-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem. J. 354:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witters, L. A., B. E. Kemp, and A. R. Means. 2006. Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem. Sci. 31:13-16. [DOI] [PubMed] [Google Scholar]
- 39.Woods, A., K. Dickerson, R. Heath, S. P. Hong, M. Momcilovic, S. R. Johnstone, M. Carlson, and D. Carling. 2005. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2:21-33. [DOI] [PubMed] [Google Scholar]
- 40.Woods, A., I. Salt, J. Scott, D. G. Hardie, and D. Carling. 1996. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 397:347-351. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi, T., J. Kamon, Y. Minokoshi, Y. Ito, H. Waki, S. Uchida, S. Yamashita, M. Noda, S. Kita, K. Ueki, K. Eto, Y. Akanuma, P. Froguel, F. Foufelle, P. Ferre, D. Carling, S. Kimura, R. Nagai, B. B. Kahn, and T. Kadowaki. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8:1288-1295. [DOI] [PubMed] [Google Scholar]
- 42.Yoon, M. J., G. Y. Lee, J. J. Chung, Y. H. Ahn, S. H. Hong, and J. B. Kim. 2006. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55:2562-2570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.