Abstract
Mucosal transmission is the predominant mode of human immunodeficiency virus (HIV) infection worldwide, and the mucosal innate interferon response represents an important component of the earliest host response to the infection. Our goal here was to assess the changes in mRNA expression of innate mucosal genes after oral simian immunodeficiency virus (SIV) inoculation of rhesus macaques (Macaca mulatta) that were followed throughout their course of disease progression. The SIV plasma viral load was highest in the macaque that progressed rapidly to simian AIDS (99 days) and lowest in the macaque that progressed more slowly (>700 days). The mRNA levels of six innate/effector genes in the oral mucosa indicated that slower disease progression was associated with increased expression of these genes. This distinction was most evident when comparing the slowest-progressing macaque to the intermediate and rapid progressors. Expression levels of alpha and gamma interferons, the antiviral interferon-stimulated gene product 2′-5′ oligoadenylate synthetase (OAS), and the chemokines CXCL9 and CXCL10 in the slow progressor were elevated at each of the three oral mucosal biopsy time points examined (day 2 to 4, 14 to 21, and day 70 postinfection). In contrast, the more rapidly progressing macaques demonstrated elevated levels of these cytokine/chemokine mRNA at lymph nodes, coincident with decreased levels at the mucosal sites, and a decreased ability to elicit an effective anti-SIV antibody response. These data provide evidence that a robust mucosal innate/effector immune response is beneficial following lentiviral exposure; however, it is likely that the anatomical location and timing of the response need to be coordinated to permit an effective immune response able to delay progression to simian AIDS.
Initiating a human immunodeficiency virus (HIV) infection generally requires that the virus traverse a mucosal barrier, such as the vaginal, rectal, penile, or oral mucosa, in order to establish an infection (39, 54). Mucosal transmission via the oral route occurs in both newborns and adults (41, 42, 47, 48, 52, 56). Transmission to newborns can occur during the birthing process, likely through contact between the vaginal fluids and the oral cavity, or after birth via breast feeding, with the virus present in the milk (41, 42, 51). Oral transmission in adults can occur as a result of unprotected receptive oral intercourse in which the virus is present in the semen (9, 47, 52, 59).
The simian immunodeficiency virus (SIV) infection of rhesus macaques provides an excellent model system to assess the innate and adaptive immune responses to viral infection (6, 7, 28, 38, 50). While the analysis of HIV immunity has historically focused on HIV/SIV-specific T- and B-cell responses, more recent studies have assessed the innate cytokine and chemokine immune responses in the host. Within the blood and lymph nodes of chronically SIV-infected macaques, an increase in the expression of various innate cytokines/chemokines, including Mip-1α, Mip-1β, alpha interferon (IFN-α), and CXCL9 and CXCL10, has been observed. Interestingly, this increase in mRNA expression correlated with higher levels of viral replication (1, 31, 49), indicating that high levels of these cytokines/chemokines are unable to control viral replication in blood and lymph tissues of SIV+ monkeys. With regard to the immune events occurring at the mucosa, the reduced plasma viral loads observed for some macaques directly correlate with the markedly increased cytotoxic factors (i.e., granzyme A, lysozyme, and perforin) and proinflammatory gene transcript levels in the gut-associated lymphoid tissue (GALT; jejunum), indicating a dichotomy between inflammatory responses in the GALT and those in the lymph nodes (20). In addition, expression of certain immune response gene products (i.e., interleukin 2 [IL-2], β2 microglobulin, and SDF-1) in the GALT involved in eliciting a cytotoxic T-cell response was also upregulated in macaques with lower viral loads (20). Therefore, the effectiveness of an inflammatory immune response may depend on the timing of these innate/effector genes, as well as which genes are expressed, at different tissue sites.
Innate/effector gene expression at the site of SIV mucosal inoculation likely reflects some of the earliest host responses to SIV infection (3, 4). Following vaginal transmission, the early innate response at the vaginal mucosa is predominantly comprised of proinflammatory cytokines (4). The induction of cytokines with antiviral activity (alpha/beta interferons) was delayed and consequently was too late to prevent virus replication and dissemination. Therefore, the early cytokine response favors immune activation potentially resulting in the recruitment of additional target cells for SIV infection (4). Assessment of neonatal macaques following multiple oral exposures at 7 days postinfection has also identified a predominantly proinflammatory response and delayed interferon effector response (IFN-β, IFN-α, 2′-5′ oligoadenylate synthetase [OAS], and Mx) at the mucosal site of inoculation (3). The ability of SIV to rapidly spread from the site of transmission at the oral or vaginal mucosa to lymph nodes in as little as 1 to 2 days postinfection (3, 27, 28, 40) might indeed present a challenge to the innate immune system to respond in a timely manner to benefit the host. To determine the importance of timing of the innate immune response on disease outcome, we reasoned that assessing the expression of innate/effector genes at the mucosa would be more informative if the animals were followed throughout their disease course. Following successful SIV oral inoculation of the animals, biopsies were obtained from oral mucosal tissue (gingiva adjacent to the molars) at three time points (2 to 4, 14 to 21, and 70 days postinfection) throughout the disease course. The levels of 13 different innate/effector mRNA levels were quantified, and changes in the expression of these genes in the SIV-infected macaques were monitored. Interestingly, assessing these macaques throughout their disease courses determined that the rate of disease progression was inversely associated with the ability to increase the expression of a select group of innate/effector genes (IFN-α, IFN-γ, CXCL9, CXCL10, OAS, and IL-12) at the mucosa. These data indicate that a robust innate/effector immune response at the mucosa may be beneficial to a host confronted with a lentivirus, particularly when the response is initiated during the earliest time points and maintained throughout the disease course.
MATERIALS AND METHODS
Animal inoculations and virus stock.
The macaques used in these studies were colony-bred rhesus macaques (Macaca mulatta) housed at the California National Primate Research Center (CNPRC). Upon beginning these studies, all animals were seronegative for SIV, simian T-cell leukemia virus (STLV), and simian retrovirus (SRV), as determined by antibody enzyme immunoassay. In addition, all animals were negative for SIV, STLV, and SRV proviral DNA as determined by virus-specific PCR assays using DNA extracted from peripheral blood mononuclear cells (PBMC) (Simian Retrovirus Laboratory, CNPRC) (5, 32, 33). Animals utilized in this study had the following CNPRC designations: RM11 (33291), RM12 (32167), RM13 (32174), RM14 (32296), RM15 (33353), and RM16 (32127). All animals were cared for in accordance with National Institutes of Health guidelines, and appropriate approvals from the local Animal Care and Use Committees were obtained. Each macaque was orally inoculated with two 1 × 105 50% tissue culture infective doses (TCID50) of SIVmac251-5/98 (22, 35) 1 h apart to ensure infection. Macaques were followed throughout infection and observed for signs of simian AIDS, as previously described (57), at which time they were humanely euthanized by a pentobarbital overdose, in accordance with California National Primate Research Center and federal guidelines.
Tissue collection, processing, and assessment of cellular infiltrates.
Numerous biopsies were obtained from the macaques while they were under ketamine hydrochloride anesthesia (10 mg/kg). Mucosal biopsies were approximately 2 mm in diameter and 2 mm thick and consisted of squamous epithelium, as well as underlying connective tissue. Therefore, these biopsies represent a mixed population of cell types, including epithelial and lymphoid cells. Oral mucosal biopsies were obtained from each macaque at three time points (2 to 4, 14 to 21, and 70 days postinfection), lymph node biopsies were obtained at four time points (7 to 15, 21 to 28, 45 to 56, and 85 days postinfection), and each biopsy was placed in RNAlater (Ambion, Inc., Austin, TX) and then stored at −20°C for RNA isolation. In addition, rectal mucosal biopsies were placed in Streck tissue fixative buffer (Streck Laboratories, Inc.) and were then paraffin embedded. Day 0 mucosal biopsies were not acquired from the six study animals due to concerns that the biopsies would alter the mucosal integrity and affect the outcome of the study. Instead, similar biopsies were obtained from four age-matched, uninfected macaques to achieve baselines. Assessment of cellular infiltrates in the mucosa was performed on standard hematoxylin-and-eosin-stained tissue sections by a pathologist. Microscopy was performed using a Zeiss microscope and PASCAL version 3.2 image software (512-by-512-pixel resolution) (Carl Zeiss, Oberkochen, Germany).
Quantification of plasma viral RNA.
Viral RNA in the plasma was quantified by a Chiron Corporation branch DNA (bDNA) signal amplification assay, version 4.0, specific for SIV (57). Viral load in the plasma is reported as copies of viral RNA per milliliter of plasma. The limit of detection of the bDNA assay is 125 copies of viral RNA per milliliter of plasma.
Quantitative real-time PCR analysis of immune effector genes.
Total RNA was extracted from the mucosal and lymph node biopsies as previously described, utilizing mechanical homogenization, followed by Trizol extraction (2). Real-time PCRs utilizing gene-specific primer/probe were performed on an ABI 7700 or ABI 7300 (Applied Biosystems) sequencer, utilizing the default settings as described previously (1, 2). Changes in expression of 13 innate immune genes (IFN-α, IFN-β, IFN-γ, IL-4, IL-6, IL-10, IL-12, CXCL9, CXCL10, tumor necrosis factor alpha [TNF-α], Mip1α, Mx, and OAS) and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene were calculated as previously described, utilizing delta cycle threshold (ΔCT) values (2). Briefly, the GAPDH CT value was subtracted from the CT value of the target gene, thereby generating a ΔCT value. For the four uninfected macaques, an average of the ΔCT values was derived, and this average ΔCT value was then subtracted from the ΔCT value of a target gene to achieve the ΔΔCT value. Change (n-fold) was then determined by the following formula: 2−ΔΔCT (User Bulletin no. 2; ABI Prism 7700 Sequence Detection System; Applied Biosystems). In the event that the ΔΔCT value was positive, indicating that the change was <1-fold (a negative fold change), the negative fold-change value was calculated by the following formula: −1/fold-change value. For example, a ΔΔCT value of 3 would result in a fold change of 2−3, equal to 0.125 or a negative fold change of −1/(0.125) or −8-fold change. An average fold change and a standard deviation of the target gene were calculated for the uninfected macaques. Changes in mRNA expression of a target gene in an infected macaque were deemed either increased or decreased if its fold change was greater than 2 standard deviations of the average of the four uninfected controls.
SIV envelope-specific antibody endpoint titer and avidity.
Antibody responses to native SIV envelope were measured as previously described utilizing a concanavalin A (ConA) enzyme-linked immunosorbent assay (ELISA) (17). Briefly, detergent-disrupted SIV envelope proteins from SIVsmB7 captured on the ConA plate were exposed for 1 h at room temperature to plasma antibodies, monoclonal antibodies, or plasma from SIV-negative control macaques. To determine endpoint titers, the plates were washed with phosphate-buffered saline (PBS) and developed using peroxidase-labeled goat anti-monkey immunoglobulin G antibody and TM blue (Serologicals Corp., Gaithersburg, Md.) as the substrate. Endpoint titers represent the last twofold dilution with an optical density at 450 nm (OD450) that is twice that of the SIV-negative control animals. The avidity of antibody binding was determined by measuring the stability of antibody-antigen binding in the presence of 8 M urea. The percentage of antibody avidity was calculated as follows: (OD450 of urea-treated wells/OD450 of PBS-treated wells) × 100. The results are averages of at least two independent experiments, with variation in individual antibody avidity values of less than 10%.
Statistical analysis.
A Spearman nonparametric correlation test was performed to determine whether mRNA gene expression correlated with viral load, antibody titers, or disease progression. To compare the number of upregulated genes in the oral gingiva to those in the rectal mucosa, an adjusted chi-square test was performed. All calculations were performed utilizing Prism statistical software, version 4.0c (GraphPad Software, Inc.), and a P value of less than 0.05 was considered to be significant.
RESULTS
Oral inoculation of SIV: plasma viral load and innate/effector gene levels.
These studies were initiated via a nontraumatic oral inoculation of SIVmac251 to the cheek pouch of six macaques, and each macaque became infected and developed peak viremia at 1 to 2 weeks postinfection (Fig. 1). As is commonly observed following an SIV infection, there was a variable rate in disease progression, including one rapid progressor (RM11 developed simian AIDS in 14 weeks), four intermediate progressors (RM12 and RM15 developed simian AIDS in 21 and 36 weeks, respectively), and one slow progressor (RM16 developed signs of simian AIDS after 106 weeks of infection) (Table 1). Similar to results from previous studies, a slower rate of disease progression was associated with lower plasma viral loads (P = 0.0538) (13, 14, 25, 37, 44, 55). The decrease in viral load following the acute peak was most dramatic in the slow progressor, in which the set point viral load (weeks 2 to 4 postinfection) was 37-fold lower (106 to 106.5 copies of viral RNA per milliliter of plasma) than the average 5.8-fold decrease in the other five macaques (107 to 108 copies of viral RNA) (Fig. 1). Over the course of this study, five of the macaques developed opportunistic infections of the respiratory (i.e., Klebsiella pneumoniae, Moraxella spp., and Cryptosporidium spp.) and/or intestinal (i.e., Candida albicans, Blastocystis hominis, and Blantidium coli) tract, representing the onset of simian AIDS (Table 1).
FIG. 1.
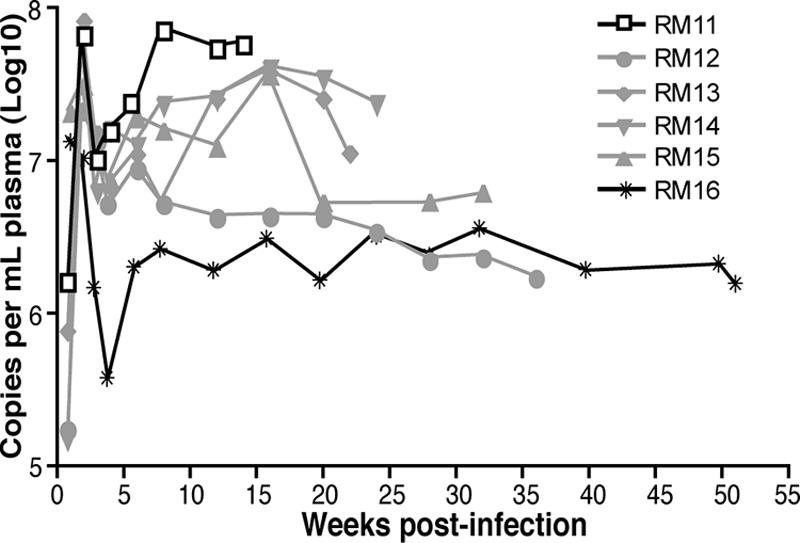
Plasma viral RNA copies per milliliter of plasma were quantified by the bDNA signal amplification assay specific for SIV (limit of detection is 125 copies per milliliter of plasma). Peak viral loads occurred at 1 to 2 weeks postinfection, and set point viral loads were established by 8 weeks postinfection. Results for the rapid progressor RM11 are shown with open boxes, for the four intermediate progressors in gray lines, and for the slow progressor RM16 in the solid black line.
TABLE 1.
Clinical and pathological findings following SIV infection
| Animal subject no. | Days postinfection | Clinical findings | Necropsy findings |
|---|---|---|---|
| RM11 | 63 | Diarrhea, Campylobacter coli | Peritonitis |
| 70 | Diarrhea, esophageal Candida albicans | Gastritis, enteritis, colitis, cystitis, glomerulonephritis | |
| 85 | Diarrhea, wt loss | Encephalitis, focal cerebral hematoma | |
| 95 | Diarrhea, oral Candida albicans | Lymphoid depletion: mucosa-associated lymphoid tissue, lymph nodes, thymus | |
| 99 | Oral candidiasis, diarrhea, wt loss, euthanasia | ||
| RM12 | 224 | Increased heart respiratory rates, labored breathing | Colitis, multifocal pneumonia, generalized lymphadenopathy, severe urinary bladder distention |
| 227 | Pneumonia, scrotal edema, euthanasia | ||
| RM13 | 54 | Diarrhea, Campylobacter coli, trichomonas, Blastocystis hominis, cryptosporidium | Cholecystitis and choledochitis, colitis, enlarged spleen |
| 85 | Diarrhea, Iodamoeba butschlii, trichomonas | ||
| 89 | Diarrhea, Balantidium coli, trichomonas | ||
| 155 | Diarrhea | ||
| 163 | Diarrhea | ||
| 178 | Diarrhea, colitis, wt loss, euthanasia | ||
| RM14 | 116 | Diarrhea | Thymic atrophy |
| 139 | Nasal discharge | ||
| 151 | Weak, unsteady in cage, wt loss, euthanasia | ||
| RM15 | 196 | Microcytic anemia | Pneumonia |
| 238 | Nasal discharge: coagulase and Staphylococcus spp. | Gastroenterocolitis | |
| 249 | Weight loss, nasal discharge: Moraxella spp. | Choledochocystitis, hydronephrosis | |
| 255 | Dehydration, wt loss, euthanasia | Lymphadenopathy, splenomegaly |
Throughout SIV infection, pinch biopsies were obtained from oral mucosa, where the virus was administered, and from rectal mucosa to determine how innate/effector gene mRNA levels compared at these different mucosal sites. Quantitative real-time PCR analysis of 13 immune response genes and one housekeeping gene (GAPDH) for the purpose of normalization between samples was undertaken with each of the biopsies obtained. In general, within the 13 innate/effector gene mRNA levels assessed (listed in Materials and Methods), a significantly higher percentage (34%) of the genes assessed in the oral mucosa (gingiva adjacent to teeth) were increased beyond the standard deviation range of ±2 from four uninfected macaques than that of rectal mucosa (27%) during acute infection (Table 2) (adjusted chi-square test, P = 0.026). The increased numbers of upregulated innate/effector genes at the oral mucosa could possibly be due to the oral route of inoculation utilized here. Progression to simian AIDS at different rates was associated with distinctions in the innate/effector gene levels when these were compared to levels from four uninfected control macaques. Of the six macaques, the slow progressor, RM16, had the largest number of genes (8 of 12) upregulated at the earliest (2 to 4 days postinfection) time point assessed postinfection (Table 2). In contrast, the rapid progressor, RM11, had the largest number of genes (6 of 13) downregulated at this earliest (2 to 4 days postinfection) time point (Table 2). Assessment of the mRNA levels of these genes was complicated by the fact that some genes did not achieve efficient PCR amplification with every biopsy obtained and remained at levels below detection (Table 2). Therefore, we have focused on a careful assessment of six immune response genes that were efficiently amplified in the majority of samples, including three cytokines (IFN-α, IFN-γ, and IL-12), two chemokines (CXCL9 and CXCL10), and one interferon-stimulated intracellular antiviral gene (OAS) product for the in-depth analysis presented here.
TABLE 2.
Fold change in expression of 13 innate/effector genes following oral SIV inoculationa
| Mucosal site | Infection time point (day) | Fold change in the expression of the following innate/effector gene products relative to the indicated control value
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-α | IFN-β | Mx | OAS | IFN-γ | CXCL10 | CXCL9 | IL-12 | IL-6 | TNF-α | MIP-1a | IL-4 | IL-10 | ||
| Control | 9.36 | 1.7 | 2.15 | 3.44 | 5.63 | 7.99 | 3.82 | 1.82 | 1.78 | 2.68 | 2.76 | 3.8 | 1.91 | |
| Gingiva | ||||||||||||||
| RM11 | 2 | −1.75 | −100 | −20 | −1.22 | −10 | −1.79 | −3.57 | −8.33 | −2.86 | 2.71 | −1.92 | −50 | −1.56 |
| RM12 | 4 | 9.47 | −5.56 | −10 | 21.74 | 4.55 | −1.75 | −6.25 | −6.67 | 1.29 | ||||
| RM13 | 4 | 6.58 | −1.64 | −1.3 | 4.85 | 1.56 | 4.6 | 9.49 | −1.01 | −4.76 | 2.96 | 5.19 | −1.16 | −1.61 |
| RM14 | 2 | 15.45 | −1.33 | −1.92 | 1.69 | −2.94 | 1.82 | 1.18 | 2.66 | 1.69 | 1.76 | −1.37 | −1.89 | |
| RM15 | 2 | 19.49 | 1 | 1.5 | 4.28 | 3.36 | −1.2 | 1.71 | 2.09 | |||||
| RM16 | 4 | 16.37 | 1.04 | 1.75 | 33.91 | 1.75 | 36.03 | 11.25 | 8.93 | 1.52 | 11.59 | 20.28 | 3.91 | |
| RM11 | 14 | 4.83 | 11.11 | 1.05 | 2.29 | −5.56 | 8.6 | 1.08 | −9.09 | −2.5 | 9.35 | 3 | ||
| RM12 | 21 | 2.8 | −20 | −7.14 | 4.65 | −20 | 1.22 | −1.85 | −3.7 | −33.3 | 5.88 | 1.18 | ||
| RM13 | 21 | 4.4 | −1.45 | 7.28 | 24.7 | 1 | 5.71 | 5.12 | 1.79 | −2.22 | 1.03 | 1.31 | −1.25 | −2.7 |
| RM14 | 14 | 27.71 | −1.19 | 1.21 | 3.58 | 1.19 | 11.84 | 6.75 | −2.22 | 3.34 | 6.17 | 1.95 | 2.16 | |
| RM15 | 14 | 13.83 | 2.34 | 1.56 | 9.27 | 4.08 | 12.19 | 21.27 | 1.94 | −1.18 | 7.96 | 6.34 | −1.41 | 2.39 |
| RM16 | 21 | 11.95 | −1.05 | −7.69 | 3.21 | 5.27 | 14.16 | 25.55 | 1.61 | 1.15 | 3.76 | −1.15 | −2.7 | −1.15 |
| RM11 | 70 | 9.37 | −5.88 | −1.15 | 3.68 | 1.72 | −3.57 | 3.06 | ||||||
| RM12 | 70 | 11.07 | −4.76 | 14.29 | 1.96 | −9.09 | −1.32 | −10 | −2.17 | −1.96 | 3.27 | −1.35 | −12.5 | −1.19 |
| RM13 | 70 | 39.24 | ||||||||||||
| RM14 | 70 | 18.51 | −1.33 | 5.03 | 17.25 | 20.7 | 1.38 | 18.6 | 1.3 | 7.99 | ||||
| RM15 | 70 | 3.86 | −1.22 | 8.74 | 12.47 | −1.15 | 2.1 | −2.86 | −1.59 | 1.54 | 2.37 | −2.08 | 1.42 | |
| RM16 | 70 | 32.83 | 2.32 | −1.96 | 7.58 | 37.5 | 145.72 | 730.12 | 4.18 | 1.01 | 8.11 | 17.03 | 4.98 | 4.27 |
| Control | 2.44 | 2.85 | 2.91 | 1.24 | 1.26 | 2.86 | 1.17 | 1.19 | 3.69 | 1.38 | 2.17 | 1.42 | 1.96 | |
| Rectum | ||||||||||||||
| RM11 | 2 | 16.67 | −25 | −25 | −1.3 | −20 | −11.1 | −11.1 | −33.3 | −3.23 | −4 | −5.56 | ||
| RM12 | 4 | −33.3 | −50 | −4.55 | −2.44 | −200 | −10 | −50 | −100 | 14.29 | −10 | −33.3 | −33.3 | 16.67 |
| RM13 | 4 | −3.33 | 1.1 | −1.59 | −1.49 | 1.53 | 3.52 | 6.34 | 1.93 | 10.56 | 2.46 | 1.89 | 3.16 | 1.92 |
| RM14 | 2 | 1.8 | −1.22 | −2.22 | 4.46 | 6.86 | 6.39 | 5.08 | 5.14 | 1.41 | −1.75 | 1.93 | 1.41 | 1.78 |
| RM15 | 2 | −11.1 | −2.44 | 3.6 | −1.2 | −3.03 | −1.14 | −2.44 | −1.59 | 1.33 | −1.37 | −6.67 | −4.17 | −2.27 |
| RM16 | 4 | −1.61 | −1.54 | 1.95 | 8.01 | 2.12 | 5.58 | 1.34 | −1.28 | 5.37 | −1.02 | −1.19 | −1.85 | 1.17 |
| RM11 | 14 | −2.56 | −4.76 | 2.51 | −1.33 | 2.38 | 2.06 | −1.3 | −11.1 | −1.64 | 1.64 | 4.52 | −3.03 | 1.25 |
| RM12 | 21 | −2.5 | −8.33 | −2.5 | −2.86 | −3.57 | 1.65 | −3.23 | −100 | 2.16 | 1.84 | 3.3 | 1.59 | 1.11 |
| RM13 | 21 | −2 | −1.67 | 9.34 | 1.73 | −1.72 | 3.62 | 1.24 | 1.35 | −1.41 | −1.89 | 1.97 | 1.58 | 1.07 |
| RM14 | 14 | −1.61 | −1.18 | 4.64 | 2.01 | 2.26 | 2.75 | 7.61 | −1.06 | 4.3 | −1.54 | 6.06 | −1.72 | 1.13 |
| RM15 | 14 | −3.33 | −2.33 | −1.82 | −1.59 | −3.7 | 1.65 | −1.64 | −2.17 | 1.13 | −2.38 | 1.14 | −2 | −1.22 |
| RM16 | 21 | −5.26 | −2.94 | −6.67 | 1.82 | 1.57 | 6 | 2.98 | 1.89 | 2.6 | −1.79 | 1.85 | 1.57 | −1.22 |
| RM11 | 70 | −7.69 | 14.29 | 2.96 | 1.06 | −50 | −1.69 | −50 | 16.67 | −7.14 | −8.33 | −16.67 | −20 | |
| RM12 | 70 | −25 | −50 | 16.67 | −5.26 | −500 | −50 | −25 | −500 | −5.56 | −3.7 | −50 | ||
| RM13 | 70 | −1.64 | −1.39 | 11.82 | 3.38 | −1.82 | 4.45 | 1.56 | −1.02 | 2.4 | −2.5 | −1.61 | 2.04 | −1.54 |
| RM14 | 70 | 1.31 | −2.5 | 16.94 | 3.89 | −1.47 | 3.01 | 5.44 | −1.32 | 3.06 | −1.52 | 3.06 | −1.89 | 1.13 |
| RM15 | 70 | −50 | −100 | 2.07 | −1.45 | −100 | −14.29 | −33.3 | −50 | −33.3 | −5.88 | −100 | −50 | |
| RM16 | 70 | −1.61 | 1.17 | −9.09 | 1.59 | 5.75 | 7.61 | 36.78 | 2.48 | 2.49 | −2.08 | 1.72 | 5.3 | −1.09 |
Summary of the normalized fold change in expression of 13 innate/effector genes assessed in the oral and rectal mucosa following oral SIV inoculation. The six genes discussed in detail in the text are in bold. Values of increased expression are shown in bold with underlining, while values of reduced expression are shown in bold italics.
Assessment of the changes in mucosal cytokine/chemokine mRNA levels during acute SIV infection (days 2 to 21).
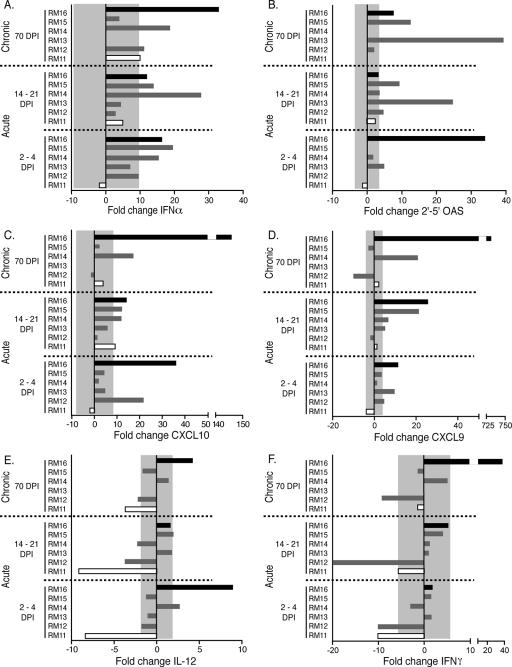
Early events postinfection were assessed at two distinct phases of acute infection: 2 or 4 days postinfection (d.p.i.) represents time points prior to the initiation of the adaptive immune response, whereas 14 or 21 d.p.i. represents time points during the initiation of the adaptive immune response. As the virus was administered orally, the innate/effector gene mRNA levels at the oral mucosa were of particular interest. Of the six mRNA levels that we focused on in detail, type I interferons (represented by IFN-α) are known to be induced at very early times following a viral infection (reviewed in reference 8). Indeed, an increase in IFN-α expression in the gingiva was observed at the 2-to-4 and 14-to-21 d.p.i. time points in three macaques that progressed relatively more slowly to disease (RM14, RM15, and the slow progressor RM16) compared to the uninfected controls (Fig. 2A). Interestingly, in the rapid progressor (RM11) as well as RM13, expression of IFN-α was not elevated at either the 2-to-4 or the 14-to-21 d.p.i. time points (Fig. 2A). The type I interferon-stimulated gene product OAS degrades viral and cellular mRNA, thereby limiting viral replication and spread to other cells. RM13 and the slow progressor RM16 had elevated levels (5-fold and 34-fold, respectively) of OAS mRNA expression in the gingiva at 2 to 4 d.p.i. (Fig. 2B). These levels dropped to within normal ranges in the gingiva at 14 to 21 d.p.i. in the slow progressor RM16. Levels of OAS expression in RM12, RM14, and RM15 were delayed until 14 to 21 d.p.i. or never increased as in the rapid progressor RM11. Interestingly, there appears to be a trend between a delay in OAS expression and higher acute and set point plasma viral loads with these macaques (Fig. 1). The mRNA level of the proinflammatory cytokine IL-12 was increased in the gingiva of RM14 and the slow progressor RM16 at 2 to 4 d.p.i. (Fig. 2E), while the rapid progressor RM11 exhibited decreased gingival IL-12 expression at 2 to 4 d.p.i. that remained decreased at 14 to 21 d.p.i. (Fig. 2E). IFN-γ expression at 2 or 4 d.p.i. in the gingiva of RM13, RM14, RM15, and the slow progressor RM16, however, was within normal ranges compared to that of SIV-negative macaques and reduced in RM12 and the rapid progressor RM11 (Fig. 2F). This trend was maintained at 14 to 21 d.p.i. in all six macaques (Fig. 2F). The mRNA expression levels of two interferon-inducible chemokines that are genetically and functionally similar, CXCL9 and CXCL10, were also assessed. Increases at 2 to 4 d.p.i. in the expression of both CXCL9 and CXCL10 in the gingiva occurred in two macaques (RM12 and the slow progressor RM16), while CXCL9 expression alone was increased in RM13 (Fig. 2C and D). By 14 to 21 d.p.i., five of the six macaques exhibited elevated expression levels of at least one of the chemokines above the levels observed for SIV-negative macaques (Fig. 2C and D). The observation that the expression of interferon-independent genes, such as IL-4 and IL-10 (Table 2), did not appear to follow the same patterns of expression as the interferon-related genes provides further evidence that the mucosal immune response during acute SIV infection is primarily driven by interferon and interferon-responsive genes.
FIG. 2.
The changes (n-fold) in mRNA expression of immune response genes in the gingiva of six orally infected macaques at 2 to 4, 14 to 21, and 70 days postinfection are shown. The rapid progressor (RM11) is shown as an open bar, the slow progressor (RM16) is shown in solid black, and the intermediate progressors are shown in gray. The mRNA levels shown are reported as n-fold changes with regard to mRNA levels in matched gingival samples of four uninfected macaques. The shaded area represents the averages ± 2 standard deviations of expression in uninfected macaques. Bars extending beyond the gray shaded area represent samples that are increased or decreased with regard to the uninfected controls.
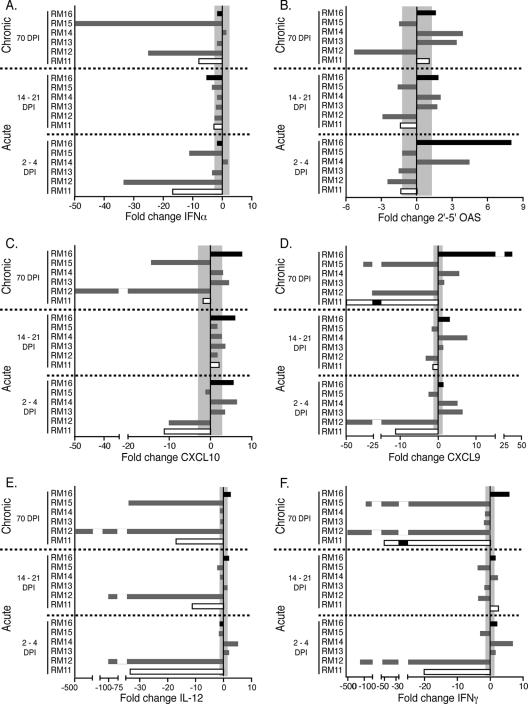
To determine if the innate/effector gene changes at the oral mucosa were reflected at other mucosal sites, we also assessed the rectal mucosa and observed both similarities and differences compared to those of the oral mucosa. Similarities included higher levels of OAS, CXCL9, and CXCL10 at the rectal mucosa during the 2-to-4-day and 14-to-21-day time points for the slow progressor RM16 and decreased levels of these genes in the rapidly progressing macaque (RM11) (Fig. 2 and Fig. 3). Differences included IFN-α expression that was increased in the slow progressor RM16 and intermediate progressors RM14 and RM15 in the gingiva but remained within normal ranges or decreased in the rectal tissues of all six macaques (Fig. 2F and Fig. 3F). Additionally, IFN-γ expression levels differed among mucosal sites as none of the macaques exhibited increased IFN-γ mRNA levels in the gingiva; however, RM11, RM13, RM14, and the slow progressor RM16 each exhibited an increased level at the 2-to-4-day or 14-to-21-day time points in the rectal mucosa. These differences are understandable as these sites represent distinct regions of the digestive tract. However, identifying similar mRNA levels for some of the genes analyzed (encoding OAS, CXCL9, and CXCL10) is interesting as the oral and rectal mucosal sites may be reacting to SIV infection of the host in a similar manner, even at these earliest time points.
FIG. 3.
The changes (n-fold) in mRNA expression of immune response genes in the rectal tissue of six orally infected macaques at 2 to 4, 14 to 21, and 70 days postinfection are shown. The rapid progressor (RM11) is shown as an open bar, the slow progressor (RM16) is shown in solid black, and the intermediate progressors are shown in gray. The mRNA levels shown are reported as n-fold changes with regard to mRNA levels in matched rectal samples of four uninfected macaques. The shaded area represents the averages ± 2 standard deviations of expression in uninfected macaques. Bars extending beyond the gray shaded area represent samples that are increased or decreased with regard to the uninfected controls.
Assessment of the mucosal cytokine/chemokine mRNA levels at 70 days after SIV infection.
Following acute infection, the mucosal immune system is impacted by the depletion of CD4+ T cells (reviewed in references 10 and 23) that provides an opportunity for opportunistic infections to establish themselves, resulting in many of the diseases commonly associated with the onset of AIDS (e.g., thrush, pneumocystis, etc.). The day 70 time point was chosen to represent the chronic stage of the disease, as by this time, macaques have undergone the depletion of CD4 cells at the mucosa (11, 24, 30, 34, 36, 45, 58) and have established their viral set point (Fig. 1). Furthermore, the presence of opportunistic infections at these various mucosal sites was monitored at this later time point (Table 1) and assessed with regard to changes in innate/effector gene mRNA levels. The oral gingival biopsies obtained at 70 d.p.i. exhibited a pattern of expression that was similar to the acute time points for the genes encoding IFN-α, OAS, IL-12, and IFN-γ (Fig. 2). In general, the rapid- and intermediate-progressing macaques exhibited declines or similar levels of these genes compared to those of uninfected macaques; however, the slowest-progressing macaque, RM16, exhibited elevated levels (similar to those at the acute time points). The most remarkable finding at day 70 in the oral mucosa was the increased expression levels of interferon-inducible chemokines CXCL9 and CXCL10 in the slow progressor RM16 (730- and 145-fold for CXCL9 and CXCL10 expression, respectively [Fig. 2C and D]).
In general, the assessment of rectal mucosal biopsies resulted in similar patterns of gene expression as those observed for the gingiva of the slowest progressor, with increases in five of the six gene products (OAS, IFN-γ, IL-12, CXCL9, and CXCL10), and neither RM12 nor the rapid progressor RM11 showed increased levels of expression of any of the six genes at 70 days postinfection (Fig. 3). Also of interest was the observation that IFN-α expression in the rectal tissue was either decreased (RM11, RM12, and RM15) or within normal ranges (RM13, RM14, and RM16) of SIV-negative macaques, which contrasted with observations for the gingiva (Fig. 2A and Fig. 3A). Similar to levels observed for the gingiva, increased levels of the chemokines CXCL9 and CXCL10 were observed for the rectal tissue of three of the macaques, including the slow progressor RM16 (Fig. 3C and D).
Although the gingival biopsies did not yield sufficient tissue to assess for cellular infiltrates, we were able to assess the levels of lymphocyte infiltration at the rectal mucosa at some time points. Indeed, hematoxylin and eosin staining indicated that the elevated levels of cells were likely lymphocytes and macrophages in the rectal biopsies of the slow progressor RM16 at 70 d.p.i. (data not shown). In summary, elevated IL-12 and IFN-γ expression levels in the gingival and rectal tissues of the slow progressor RM16 were associated with high CXCL9 and CXCL10 expression levels as well as more immune cells at the mucosa, which may indicate a heightened mucosal immune defense that might aid in preventing opportunistic infections.
Assessment of the interferon-inducible chemokine responses in lymph nodes.
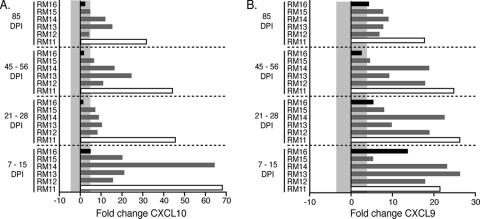
A number of studies have been undertaken that establish a direct correlation between elevated cytokine/chemokine levels in lymph nodes of SIV-infected macaques and disease progression (1, 31, 49). Here, we focused on the chemokines CXCL9 and CXCL10, as these were highly elevated in the mucosae of the slow-progressing macaques and have been observed to correlate with a poor prognosis during SIV infection (1, 31, 49). Assessment of the chemokines CXCL9 and CXCL10 in the macaques in this study revealed strikingly different expression patterns when lymph node samples (Fig. 5) were compared to mucosal samples (Fig. 2 and Fig. 3). Similar to the assessment of mucosal biopsies, lymph node biopsies were assessed at multiple time points postinfection, including day 7 to 15, 21 to 28, 45 to 56, and at day 85. CXCL9 and CXCL10 were highly expressed in the lymph node of the rapid progressor (RM11) at all time points analyzed (Fig. 4A and B). The intermediate progressors exhibited increased expression of both CXCL9 and CXCL10; however, these levels were generally lower than those observed for the rapid progressor (Fig. 4A and B). In the slow-progressing macaque (RM16), the levels of CXCL10 generally remained within levels normally observed in healthy macaques (Fig. 4A); however, CXCL9 expression was increased during the acute infection before dropping to within normal levels of expression at 45 to 56 d.p.i. (Fig. 4B). These data indicate that increased immune/effector gene expression at mucosal sites is associated with slower disease progression, whereas increased effector gene expression (CXCL9/CXCL10) at secondary lymphoid sites is associated with increased rates of disease progression.
FIG. 5.
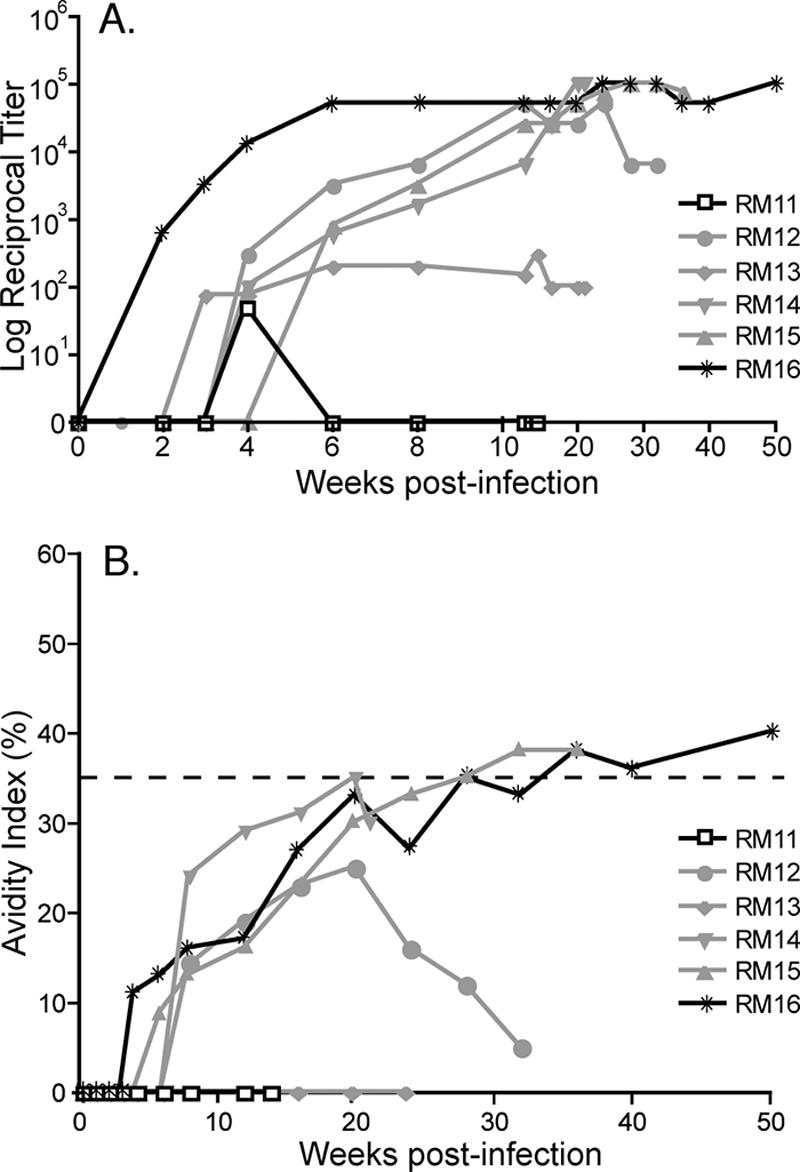
(A) Serum antibody endpoint titers were analyzed for reactivity to SIVsmB7 envelope proteins, using the ConA ELISA. Endpoint titers were determined to be the last twofold dilution with an OD450 of twice that of normal monkey serum and are reported as the log10 of the reciprocal endpoint titer. (B) Maturation of SIV envelope-specific antibody avidity following oral inoculation. Antibody avidity was determined by measuring the stability of the antigen-antibody complexes to 8 M urea and is expressed as the (OD of wells washed with 8 M urea/OD of wells washed with PBS) × 100. The rapid progressor RM11 is shown with open boxes, the intermediate progressors with gray lines, and the slow progressor RM16 with solid black lines. Avidity indexes of ≥35% are mature SIV Env-specific antibodies (15, 16).
FIG. 4.
The changes (n-fold) in mRNA expression of the chemokines CXCL9 (B) and CXCL10 (A) in the lymph nodes of six orally infected macaques at 7 to 15, 21 to 28, 45 to 56, and 85 days postinfection are shown. The rapid progressor (RM11) is shown as an open bar, the slow progressor (RM16) is shown in solid black, and the intermediate progressors are gray. The mRNA levels shown are reported as n-fold changes with regard to mRNA levels in matched lymph node samples of four uninfected macaques. The shaded area represents the averages ± 2 standard deviations of expression in uninfected macaques. Bars extending beyond the gray shaded area represent samples that are increased or decreased with regard to the uninfected controls.
SIV Env-specific antibody response following oral inoculation.
To investigate the association between increased innate immune responses and the induction or maturation of adaptive immune responses, we assessed anti-SIV Env-specific antibody titers and avidity. We observed very low levels and poor maturation of SIV-specific antibodies in the rapid progressor RM11 (Fig. 5A), consistent with other studies assessing rapidly progressing SIV+ macaques (18, 26, 43, 61). In contrast, the slow progressor RM16 exhibited Env-specific antibodies as early as 2 weeks postinfection and achieved maximal, steady-state endpoint titers by 6 weeks postinfection (Fig. 5A). Furthermore, these levels were maintained throughout the study period of 50 weeks in the slow progressor. Interestingly, the intermediate progressors (RM12, RM13, RM14, and RM15) developed SIV Env-specific antibody responses, but their levels were delayed by 2 to 3 weeks compared to that of the slow progressor (Fig. 5A). Antibody avidity assesses the strength of an antibody-antigen interaction and can be utilized as a qualitative assessment of antibody maturation. Based on previous studies (15, 16), avidity indexes greater than 35% have been determined to be consistent with a mature antibody response of the host to the SIV Env protein. The very low levels of SIV-specific antibodies precluded our abilities to assess SIV antibody avidity indexes in the most rapidly progressing macaque, RM11 (Fig. 5A). In contrast, the normal progressor RM15 and the slowest progressing macaque RM16 had increasing avidity indexes that reached levels indicative of a mature SIV-specific antibody response at 28 and 36 weeks postinfection, respectively. Delayed or insufficient maturation of Env-specific antibodies was observed in the intermediate-progressing macaques, RM12, RM13, and RM14 (Fig. 5B). These data are in agreement with previous studies indicating that slower disease progression is associated with a more robust SIV-specific antibody response (18, 26, 43, 61); however, our findings also identify a significant correlation between the induction of certain innate/effector genes at the mucosa and a more rapid triggering (by 3 weeks postinfection) of a mature SIV-specific antibody response (P = 0.0333).
DISCUSSION
The ability of HIV to replicate in and kill CD4+ T cells has made the understanding of HIV pathogenesis a difficult and complex task. While CD4+ T cells are required for initiating a successful adaptive immune response that could potentially clear the HIV infection, activation of CD4+ T cells also creates optimum virus replication factories, thereby perpetuating the HIV infection. The innate immune response plays a key role in inhibiting HIV infection, although the exact cytokines, chemokines, and immune cells needed to impact HIV replication are not known. The innate immune system responds to a variety of pathogen-associated molecular patterns (PAMPs), as exemplified in the Toll-like receptors, which are present on different immune cells (dendritic cells, macrophages, gamma-delta T cells, NK cells, etc.). To date, the data from a number of laboratories, including ours, support a model of mucosal transmission in which CD4+ T cells, macrophages, and dendritic cells are the first cells infected, followed by rapid dissemination of SIV to draining and peripheral lymph nodes (27, 28, 40, 46). Studies designed to assess the innate immune response to this initial infection have assessed fixed time points following necropsy of the monkeys in order to acquire the necessary tissues for analysis (1, 3, 4, 20, 53). A recent study assessed innate immune responses at 7 d.p.i. in a model that utilized multiple low-dose SIV exposures (to reflect infection following breast-feeding) in neonatal rhesus macaques (3). Although immune responses varied in the innate/effector genes expressed (gingival mucosa was less prone to inducing type I interferon responses than lymphoid tissues), an overall increased proinflammatory innate/effector response was observed following infection for all tissues examined (3). Similarly increased levels of innate/effector genes were previously obtained from vaginal mucosa following vaginal transmission (4). The study results shown here are distinct from those of previous studies because mucosal tissues were acquired for analysis while following the macaques until they progressed to simian AIDS. Unfortunately, this study design limited the number of macaques that could be assessed; however, the diverse range of outcomes permitted an analysis of macaques that progressed to disease rapidly or at a relatively normal rate (intermediate progressors), as well as one slow progressor. This is the first study, to our knowledge, that combines an assessment of mucosal and lymphatic innate/effector genes, SIV-specific adaptive immunity, and disease outcome such that associations between these factors can be identified.
Comparing cytokine/chemokine expression patterns with SIV-specific antibody responses and rates of disease progression permitted assessment of multiple events occurring in the orally inoculated SIV macaques throughout the acute and chronic phases of infection. The rapid progressor RM11 upregulated only TNF-α expression 2 days postinfection in the oral mucosa, while the slow progressor RM16 upregulated eight genes (encoding IFN-α, OAS, CXCL9, CXCL10, IL-12, IL-10, TNF-α, and MIP-1a) (Table 2). The intermediate progressors showed an increase in two to four of the gene mRNA levels assessed in the oral mucosa at 2 to 4 days postinfection and were associated with intermediate set point viral loads (Fig. 1). These intermediate progressors contained a phenotype that was intermediate between those of the contrasting rapid and slow progressors, and therefore provided additional confirmation that these findings reflect a phenomenon that will be observed in future SIV/macaque studies. The lower gastrointestinal tract, including the rectal mucosa, has been a site of intense investigation due to the rapid, extensive depletion of CD4+ T cells from this mucosal site (11, 24, 30, 34, 36, 45, 58). It is interesting to note in this study that different mucosal sites within the same macaque can respond similarly to infection with regard to a subset of innate/effector genes. Similar increases in CXCL9 and CXCL10 observed here at the oral and rectal mucosa have also been observed in the lung tissues of acutely and chronically SIV-infected macaques (53). In contrast, a disparate regulation of IFN-α expression was observed at these two sites as increased expression at the oral gingiva of the slower-progressing macaques occurred at times when the rectal biopsies exhibited similar or decreased IFN-α levels compared to those of the uninfected macaques. The reduced expression of IFN-α in the rectal tissue may be indicative of an early innate immune dysfunction possibly contributing to the rapid depletion of CD4+ T cells from the gut or may simply indicate an inherent distinction between the different mucosal sites. In contrast, studies assessing lymph nodes and PBMC innate immune responses in SIV-infected macaques determined that increased levels of cytokine/chemokine expression correlate with a poor disease prognosis (typically measured by increased viral loads) (1, 31, 49). High levels of both CXCL9 and CXCL10 in the lymph nodes were generally associated with higher viral loads, a poor SIV Env-specific antibody response, and a faster rate of disease progression. These data indicate that the rapid progressor was likely responding to the infection by producing proinflammatory chemokines in the lymph nodes; however, this response was not effective. Among the cell types potentially recruited by CXCL9 and CXCL10 are activated CD4+ T cells that might serve as additional target cells for the virus, providing an explanation for the higher plasma viral load in these macaques (RM11, RM12, RM13, RM14, and RM15). In contrast, high levels of CXCL9/CXCL10 expression at the mucosal sites may help to slow the spread of the virus from the portal of entry to secondary lymphoid tissues, allowing the host time to mount a more effective SIV-specific immune response, as was observed in the slow progressor RM16. These data indicate that the timing and the anatomical location of proinflammatory cytokine/chemokine expression during acute infection may impact the levels of viral replication, SIV-specific immune responses, and rate of disease progression.
During chronic HIV/SIV infection, the onset of AIDS is generally associated with opportunistic infections (e.g., oral candidiasis, Pneumocystis pneumonia, and enteric cryptosporidiosis) of mucosal tissues. We hypothesized that maintaining mucosal expression of cytokines and chemokines during chronic infection would be beneficial by preventing the onset of opportunistic infections. Indeed, our findings indicate that the rapid progressor RM11 developed an opportunistic infection at 70 d.p.i. (Table 1), at which time levels of many of the cytokines/chemokines, including CXCL9 and CXCL10, were similar to or lower than levels observed for uninfected macaques. The potential benefit of elevated cytokine/chemokine levels at mucosal sites was determined to be statistically significant due to a correlation of higher gene expression in gingival (P = 0.0333) and rectal (P = 0.0167) mucosae during chronic infection (70 d.p.i.) and delayed onset of AIDS-related death. In contrast, these and other studies have observed a poor prognosis associated with high levels of proinflammatory cytokine/chemokine expression in the lymph nodes during chronic infection (1, 31, 49). These data indicate that if the site where high levels of CXCL9 and CXCL10 are expressed is a replication site for HIV/SIV, then recruitment of additional activated CD4+ cells may be detrimental; however, if the site is important for inhibiting opportunistic infections (mucosal tissues), then the recruitment of activated CD4+ T cells, as well as of other effector cells such as NK cells, may be beneficial. This dichotomy of elevated chemokine levels between the different anatomical sites provides a rationale for assessing these chemokines in future studies.
These data highlight a potentially important role for innate/effector molecules during the first few days postexposure and may explain why mucosal transmission of HIV is a relatively rare event in humans (19, 21). Our findings suggest that the timing and magnitude of the innate immune response at the site of inoculation (oral gingiva) play potentially important roles in eliciting the anti-SIV immune response and slowing progression to simian AIDS. In this light, these data provide a rationale for upregulating interferons and interferon-stimulated innate/effector gene expression to increase mucosal efficacy of HIV vaccines. Indeed, providing PAMPs, such as CpG motifs, during a vaccine administration has been demonstrated in some studies to boost both innate and adaptive mucosal immune responses and prevent infection following SIV challenge (12, 29). However, mucosal immune activation would likely need to be undertaken in a careful manner, as the application of imiquimod (Toll-like receptor 9 agonist) or CpGs (Toll-like receptor 7 agonist) to the vaginal mucosa 30 min prior to SIV administration resulted in increased plasma viral loads, indicating that the immune activation favored viral replication (60). Our studies also suggest that disease progression may be inhibited during the chronic stages of SIV infection due to sustained expression of mucosal cytokines/chemokines, particularly the chemokines CXCL9 and CXCL10. Additional studies in the SIV+ macaques and HIV+ humans are needed to more definitively address whether the cytokines/chemokines assessed here (e.g., CXCL9 and CXCL10) are important for maintaining the proper threshold of CD4+ effector T cells at the mucosa as described by Picker et al. (45), potentially illuminating new approaches to recruit T cells back to the severely depleted mucosal sites. In addition, our data suggest that assessing mucosal sites by assessing expression levels of the innate/effector genes products including IFN-α, CXCL9, CXCL10, OAS, IL-12, and IFN-γ may be useful as an indicator of immunologic health. As such, these studies provide insights as to the direction of future studies to further assess the role of mucosal and lymphoid cytokine/chemokine responses in disease progression following SIV/HIV infections.
Acknowledgments
These studies were supported by NIH grant P51 RR00169 to the Immunology Core of the California National Primate Research Center (CNPRC), by base operating grant RR00169 to CNPRC, and by NIH grants DE12926 and AI35522 (to D.L.S.).
We thank Marta Marthas for help with the animal study design and undertaking of these experiments. Also, we acknowledge the excellent animal care and veterinary staff at the California National Primate Research Center, where the macaque experiments were performed. We also acknowledge Kristina Abel and the Immunology Core of the California National Primate Research Center for undertaking the innate/effector mRNA expression analysis. We also thank Alagar Muthukumar, David Kosub, Amanda Leone, and Kiran Mir for careful readings of the manuscript.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Abel, K., M. J. Alegria-Hartman, K. Rothaeusler, M. Marthas, and C. J. Miller. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-α/β) and IFN-α/β-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel, K., B. Pahar, K. K. Van Rompay, L. Fritts, C. Sin, K. Schmidt, R. Colon, M. McChesney, and M. L. Marthas. 2006. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J. Virol. 80:6357-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel, K., D. M. Rocke, B. Chohan, L. Fritts, and C. J. Miller. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 79:12164-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade, M. R., J. Yee, P. Barry, A. Spinner, J. A. Roberts, P. H. Cabello, J. P. Leite, and N. W. Lerche. 2003. Prevalence of antibodies to selected viruses in a long-term closed breeding colony of rhesus macaques (Macaca mulatta) in Brazil. Am. J. Primatol. 59:123-128. [DOI] [PubMed] [Google Scholar]
- 6.Baba, T. W., J. Koch, E. S. Mittler, M. Greene, M. Wyand, D. Penninck, and R. M. Ruprecht. 1994. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res. Hum. Retrovir. 10:351-357. [DOI] [PubMed] [Google Scholar]
- 7.Baba, T. W., A. M. Trichel, L. An, V. Liska, L. N. Martin, M. Murphey-Corb, and R. M. Ruprecht. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486-1489. [DOI] [PubMed] [Google Scholar]
- 8.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. [DOI] [PubMed] [Google Scholar]
- 9.Bratt, G. A., T. Berglund, B. L. Glantzberg, J. Albert, and E. Sandstrom. 1997. Two cases of oral-to-genital HIV-1 transmission. Int. J. STD AIDS 8:522-525. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7:235-239. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 13.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti, L., M. C. Cumont, L. Montagnier, and B. Hurtrel. 1994. Variable course of primary simian immunodeficiency virus infection in lymph nodes: relation to disease progression. J. Virol. 68:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clements, J. E., R. C. Montelaro, M. C. Zink, A. M. Amedee, S. Miller, A. M. Trichel, B. Jagerski, D. Hauer, L. N. Martin, R. P. Bohm, and M. Murphey-Corb. 1995. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J. Virol. 69:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole, K. S., J. D. Steckbeck, J. L. Rowles, R. C. Desrosiers, and R. C. Montelaro. 2004. Removal of N-linked glycosylation sites in the V1 region of simian immunodeficiency virus gp120 results in redirection of B-cell responses to V3. J. Virol. 78:1525-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehghani, H., B. A. Puffer, R. W. Doms, and V. M. Hirsch. 2003. Unique pattern of convergent envelope evolution in simian immunodeficiency virus-infected rapid progressor macaques: association with CD4-independent usage of CCR5. J. Virol. 77:6405-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vincenzi, I., et al. 1994. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. N. Engl. J. Med. 331:341-346. [DOI] [PubMed] [Google Scholar]
- 20.George, M. D., S. Sankaran, E. Reay, A. C. Gelli, and S. Dandekar. 2003. High-throughput gene expression profiling indicates dysregulation of intestinal cell cycle mediators and growth factors during primary simian immunodeficiency virus infection. Virology 312:84-94. [DOI] [PubMed] [Google Scholar]
- 21.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 22.Greenier, J. L., C. J. Miller, D. Lu, P. J. Dailey, F. X. Lu, K. J. Kunstman, S. M. Wolinsky, and M. L. Marthas. 2001. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J. Virol. 75:3753-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 24.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holterman, L., H. Niphuis, P. J. ten Haaft, J. Goudsmit, G. Baskin, and J. L. Heeney. 1999. Specific passage of simian immunodeficiency virus from end-stage disease results in accelerated progression to AIDS in rhesus macaques. J. Gen. Virol. 80:3089-3097. [DOI] [PubMed] [Google Scholar]
- 27.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joag, S. V., I. Adany, Z. Li, L. Foresman, D. M. Pinson, C. Wang, E. B. Stephens, R. Raghavan, and O. Narayan. 1997. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J. Virol. 71:4016-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang, S. M., and R. W. Compans. 2003. Enhancement of mucosal immunization with virus-like particles of simian immunodeficiency virus. J. Virol. 77:3615-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F. J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116:1115-1123. [DOI] [PubMed] [Google Scholar]
- 31.LaFranco-Scheuch, L., K. Abel, N. Makori, K. Rothaeusler, and C. J. Miller. 2004. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J. Virol. 78:6399-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerche, N. W., and K. G. Osborn. 2003. Simian retrovirus infections: potential confounding variables in primate toxicology studies. Toxicol. Pathol. 31(Suppl):103-110. [DOI] [PubMed] [Google Scholar]
- 33.Lerche, N. W., W. M. Switzer, J. L. Yee, V. Shanmugam, A. N. Rosenthal, L. E. Chapman, T. M. Folks, and W. Heneine. 2001. Evidence of infection with simian type D retrovirus in persons occupationally exposed to nonhuman primates. J. Virol. 75:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 35.Marthas, M. L., D. Lu, M. C. Penedo, A. G. Hendrickx, and C. J. Miller. 2001. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retrovir. 17:1455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 37.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, Jr., J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122:573-579. [DOI] [PubMed] [Google Scholar]
- 38.Miller, C. J., N. J. Alexander, S. Sutjipto, A. A. Lackner, A. Gettie, A. G. Hendrickx, L. J. Lowenstine, M. Jennings, and P. A. Marx. 1989. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milman, G., and O. Sharma. 1994. Mechanisms of HIV/SIV mucosal transmission. AIDS Res. Hum. Retrovir. 10:1305-1312. [DOI] [PubMed] [Google Scholar]
- 40.Milush, J. M., D. Kosub, M. Marthas, K. Schmidt, F. Scott, A. Wozniakowski, C. Brown, S. Westmoreland, and D. L. Sodora. 2004. Rapid dissemination of SIV following oral inoculation. AIDS 18:2371-2380. [PubMed] [Google Scholar]
- 41.Mofenson, L. M. 1997. Interaction between timing of perinatal human immunodeficiency virus infection and the design of preventive and therapeutic interventions. Acta Paediatr. Suppl. 421:1-9. [DOI] [PubMed] [Google Scholar]
- 42.Nduati, R., G. John, D. Mbori-Ngacha, B. Richardson, J. Overbaugh, A. Mwatha, J. Ndinya-Achola, J. Bwayo, F. E. Onyango, J. Hughes, and J. Kreiss. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167-1174. [DOI] [PubMed] [Google Scholar]
- 43.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 75:4448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantaleo, G., S. Menzo, M. Vaccarezza, C. Graziosi, O. J. Cohen, J. F. Demarest, D. Montefiori, J. M. Orenstein, C. Fox, L. K. Schrager, et al. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 332:209-216. [DOI] [PubMed] [Google Scholar]
- 45.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 47.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 48.Ruprecht, R. M., T. W. Baba, V. Liska, S. Ayehunie, J. Andersen, D. C. Montefiori, A. Trichel, M. Murphey-Corb, L. Martin, T. A. Rizvi, B. J. Bernacky, S. J. Buchl, and M. Keeling. 1998. Oral SIV, SHIV, and HIV type 1 infection. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S97-S103. [PubMed] [Google Scholar]
- 49.Sarkar, S., V. Kalia, M. Murphey-Corb, R. C. Montelaro, and T. A. Reinhart. 2003. Expression of IFN-gamma induced CXCR3 agonist chemokines and compartmentalization of CXCR3+ cells in the periphery and lymph nodes of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. J. Med. Primatol. 32:247-264. [DOI] [PubMed] [Google Scholar]
- 50.Sauermann, U. 2001. Making the animal model for AIDS research more precise: the impact of major histocompatibility complex (MHC) genes on pathogenesis and disease progression in SIV-infected monkeys. Curr. Mol. Med. 1:515-522. [DOI] [PubMed] [Google Scholar]
- 51.Scarlatti, G. 2004. Mother-to-child transmission of HIV-1: advances and controversies of the twentieth centuries. AIDS Rev. 6:67-78. [PubMed] [Google Scholar]
- 52.Schacker, T., A. C. Collier, J. Hughes, T. Shea, and L. Corey. 1996. Clinical and epidemiologic features of primary HIV infection. Ann. Intern. Med. 125:257-264. [DOI] [PubMed] [Google Scholar]
- 53.Schaefer, T. M., C. L. Fuller, S. Basu, B. A. Fallert, S. L. Poveda, S. K. Sanghavi, Y. K. Choi, D. E. Kirschner, E. Feingold, and T. A. Reinhart. 2006. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect. 8:1839-1850. [DOI] [PubMed] [Google Scholar]
- 54.Smith, P. D., and S. M. Wahl. 2005. Immunobiology of mucosal HIV-1 infection, p. 1199-1203. In J. Mestecky, J. Bienenstock, M. E. Lamm, L. Mayer, J. McGhee, and W. Strober (ed.), Mucosal immunology, 3rd ed. Elsevier Science, San Diego, CA.
- 55.Smith, S. M., B. Holland, C. Russo, P. J. Dailey, P. A. Marx, and R. I. Connor. 1999. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res. Hum. Retrovir. 15:1691-1701. [DOI] [PubMed] [Google Scholar]
- 56.Trichel, A. M., E. D. Roberts, L. A. Wilson, L. N. Martin, R. M. Ruprecht, and M. Murphey-Corb. 1997. SIV/DeltaB670 transmission across oral, colonic, and vaginal mucosae in the macaque. J. Med. Primatol. 26:3-10. [DOI] [PubMed] [Google Scholar]
- 57.Van Rompay, K. K., R. P. Singh, L. L. Brignolo, J. R. Lawson, K. A. Schmidt, B. Pahar, D. R. Canfield, R. P. Tarara, D. L. Sodora, N. Bischofberger, and M. L. Marthas. 2004. The clinical benefits of tenofovir for simian immunodeficiency virus-infected macaques are larger than predicted by its effects on standard viral and immunologic parameters. J. Acquir. Immune Defic. Syndr. 36:900-914. [DOI] [PubMed] [Google Scholar]
- 58.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 59.Vittinghoff, E., J. Douglas, F. Judson, D. McKirnan, K. MacQueen, and S. P. Buchbinder. 1999. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am. J. Epidemiol. 150:306-311. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Y., K. Abel, K. Lantz, A. M. Krieg, M. B. McChesney, and C. J. Miller. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79:14355-14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, J. Y., L. N. Martin, E. A. Watson, R. C. Montelaro, M. West, L. Epstein, and M. Murphey-Corb. 1988. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J. Infect. Dis. 158:1277-1286. [DOI] [PubMed] [Google Scholar]