Abstract
Kaposi's sarcoma-associated herpesvirus encodes two highly related membrane-associated, RING-CH-containing (MARCH) family E3 ubiquitin ligases, K3 and K5, that can down regulate a variety of cell surface proteins through enhancement of their endocytosis and degradation. In this report we present data that while K5 modulation of major histocompatibility complex class I (MHC-I) closely mirrors the mechanisms used by K3, alternative molecular pathways are utilized by this E3 ligase in the down regulation of intercellular adhesion molecule 1 (ICAM-1) and B7.2. Internalization assays demonstrate that down regulation of each target can occur through increased endocytosis from the cell surface. However, mutation of a conserved tyrosine-based endocytosis motif in K5 resulted in a protein lacking the ability to direct an increased rate of MHC-I or ICAM-1 internalization but still able to down regulate B7.2 in a ubiquitin-dependent but endocytosis-independent manner. Further, mutation of two acidic clusters abolished K5-mediated MHC-I degradation while only slightly decreasing ICAM-1 or B7.2 protein destruction. This same mutant abolished detectable ubiquitylation of all targets. These data indicate that while K5 can act as an E3 ubiquitin ligase to directly mediate cell surface molecule destruction, regulation of its targets occurs through multiple pathways, including ubiquitin-independent mechanisms.
Ubiquitin plays a number of roles within the cell, ranging from receptor down regulation and misfolded protein degradation to chromosome silencing (reviewed in references 1, 20, and 23). Addition of ubiquitin to lysines within a protein can lead to degradation in either a proteasome- or lysosome-dependent manner (23). Alternatively, ubiquitin modification can also deliver a protein into the endosomal recycling pathway, allowing down regulation of signaling events without the loss of receptor protein (6). The mechanisms underlying the fate of ubiquitinylated proteins are governed by a number of factors, including the length of the ubiquitin side chain added and the intraubiquitin lysine used in polymerization (18). Target specificity is imparted on this process by the particular E3 ligase that is involved and by cellular factors that are recruited to newly modified protein.
The RING-CH domain-containing E3 ubiquitin ligases, in general, are thought to act as scaffolds, bringing target proteins in close proximity to the ubiquitin-charged E2 catalytic region. However, it is clear that the E3 ligases play additional roles through binding of other cellular factors. A clear example of this comes from studies of c-Cbl, a RING-CH E3 ubiquitin ligase that down regulates the epidermal growth factor receptor (EGFR) (13). After ligand binding, c-Cbl is recruited to the receptor by growth factor receptor binding protein 2 (17). The EGFR becomes ubiquitylated but also phosphorylates c-Cbl, allowing for binding of the ligase to CIN85 and endophilin, resulting in receptor endocytosis (21). Mutation of the CIN85 binding region results in loss of EGFR down regulation, indicating that ubiquitylation is not sufficient for receptor modulation (21).
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes two type IIIb membrane proteins, K3 and K5 (also termed MIR1 and MIR2, respectively), which are members of the MARCH (membrane-associated RING-CH-containing) family of E3 ligases. Like all family members, they contain a specific zinc binding domain, termed a RING-CH (really interesting new gene) domain with a nonclassical C4HC3 conformation (2, 3, 9-11, 22). This RING-CH domain is found in a large number of cellular E3 ubiquitin ligases and, indeed, when the K5 RING-CH domain is expressed as a fusion protein with glutathione S-transferase, it can mediate self-ubiquitylation (5). K3 and K5 are responsible for decoying host cytotoxic T-cell, natural killer, and T helper cell responses (3, 4, 11, 12). On a phenotypic level, this immune avoidance is caused by a down regulation of a number of cell surface immunomodulatory proteins, including major histocompatibility class I (MHC-I), intercellular adhesion molecule 1 (ICAM-1; CD54), and B7.2 (CD86) (3, 4, 5, 11, 12). These two viral proteins are not equal in their abilities to target each of these surface molecules. While K3 has been shown to efficiently modulate multiple MHC-I alleles, including HLA-A, -B and -C, K5 is only strongly active against HLA-A (11). In contrast, however, K5 can strongly down regulate ICAM-1 and B7.2, while K3 is not capable of modulating these proteins (5, 12). The molecular mechanisms underlying down regulation of these proteins are not completely clear but have been thought to depend on the ability of these proteins to act as E3 ubiquitin ligases. Elegant work by Duncan et al. demonstrated that K3 is able to mediate lysine-63-linked polyubiquitination of MHC-I through interaction with two separate E2 ubiquitin-conjugating enzymes (8). MHC-I, ICAM-1, and B7.2 have all been shown to undergo an increased rate of endocytosis from the cell surface in the presence of K5. The K3-mediated internalization of MHC-I has been shown to proceed through an epsin 1- and clathrin-dependent, but AP-2-independent, mechanism (8). Various studies have shown that following endocytosis the internalized target proteins are degraded in an endolysosomal compartment (5, 8, 11, 14, 16, 19). Both K3 and K5 proteins contain a number of putative protein-protein interaction motifs. These domains include a tyrosine-based endocytosis motif, a stretch of four residues (NTRV) conserved between K3 and K5, a proline-rich potential SH3 binding domain (SH3B), and two stretches of acidic amino acids (16). In K3, the tyrosine-based motif and stretches of acidic residues play a critical role in the down regulation of MHC-I (16). Mutagenesis of these motifs in K3 revealed a multistep mechanism involving an increased rate of endocytosis and relocalization of protein from the cell surface into a TGN46-positive compartment followed by a second translocation and degradation by the lysosomes (16). The comparative study of these two viral E3 ligases holds the promise of giving greater insight into the mechanisms by which the cellular E3 ligases function.
Given the degree of conservation of the RING-CH domain and other motifs between K3 and K5, we hypothesized that these sequences would be critical to K5 function. To clarify the mechanism by which the K5 protein functions in the down regulation of MHC class-I, ICAM-1, and B7.2, we have produced and tested a series of K5 constructs containing mutations of each motif. Examination of target protein endocytosis, ubiquitylation, and degradation demonstrates that the mechanisms of down modulation differ for each target protein, with K5 mediating ubiquitin-dependent and -independent target degradation.
MATERIALS AND METHODS
Cell culture, transfection, and pulse-chase.
BJAB, 293T, and A7 cells were grown in RPMI plus 10% fetal calf serum (FCS), Dulbecco's modified Eagle's medium plus 10% FCS, or minimal essential medium plus 2% FCS and 10% newborn calf serum, respectively. For transient assays, expression vectors were introduced into BJAB B cells by electroporation at 275 V with one pulse for 10 ms in antibiotic-free RPMI 1640 plus 10% fetal bovine serum medium. To establish stable cell lines, V5 His-tagged K5 alleles were cloned into the retroviral vector pLXSN. Vesicular stomatitis virus G protein (VSV-G)-pseudotyped retroviral stocks were prepared by cotransfection of 293T cells with the pLXSN construct (obtained from Dusty Miller, McGill University, Montreal, Canada), pCL-Eco (obtained from Walther Mothes, Yale University), and pVSV-G (obtained from John Rose, Yale University). Following infection with retroviral stocks, BJAB cells were selected with 1 mg of G418 per ml for 6 weeks to generate BJAB K5 cell lines. The 293T cell line was transfected using Transfectin (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's recommendations. Finally, Fugene (Roche Applied Science, Indianapolis, IN) transfection was used for introduction of expression plasmids into the A7 cell line following the manufacturer's recommendations. For pulse-chase experiments, BJAB cells were starved in methionine-cysteine-free medium supplemented with 5% dialyzed fetal bovine serum at 106 cells per ml for 1 h at 37°C followed by a 30-min label and wash. Cells were subsequently chased in normal complete medium for 0 h, 0.5 h, 1 h, or 3 h. For MHC-I experiments, 2 × 106 cells were labeled for each time point using 500 μCi/ml of Express protein labeling mix (Perkin-Elmer, Wellesley, MA) at a density of 106 cells per 50 μl label medium. For B7.2 and ICAM-1 experiments, 5 × 106 cells were labeled for each time point using 250 μCi/ml of Express protein labeling mix (Perkin-Elmer) at a density of 1 ×106 cells per 50 μl label medium.
Mutant plasmid construction.
All K5 mutant constructs used in this paper were generated using oligonucleotide-directed mutagenesis (7). Briefly, an overlapping set of opposite orientation internal primers containing the desired mutation were used for an initial round of PCR along with primers at either the 3′ or 5′ end of K5, depending on the internal primer used. The first-round PCR products were then “stitched” together to create a full-length, mutated product in a second round of PCR containing only the initial PCR products without additional primers. Each of the mutated constructs was introduced into vectors pEGFP-N1 (Clontech, Mountain View, CA), pEF-1/V5 His C (Invitrogen Corp., Carlsbad, CA), or pLXSN and completely sequenced to verify the presence of the mutation. The amino acid sequence of each mutant is indicated below in Fig. 1.
FIG. 1.
K5 and mutant constructs. (A) The K5 protein is made up of three main modules as schematically represented in the center, with single-letter amino acid sequences shown for selected regions. At the N termini there is a C4HC3 RING-CH domain followed by two membrane-spanning domains (Tm). Carboxy terminal from the TMs is a CR. This conserved region is made up of a tyrosine-based motif (Tyr motif), a CM, a potential SH3B, and two stretches of acidic amino acids (DE1 and DE2). Numbering indicates the position within the K5 amino acid sequence, and letters above and below indicate residues that were mutated and the residue to which they were changed. Bars above or below the residues indicate mutant forms that contain mutations of multiple residues. (B) Expression of wild-type K5 proteins or each of the constructs was examined in stable BJAB cells. Normalized lysates were produced from each cell line and subjected to SDS-PAGE followed by Western blotting with an antibody against the V5 epitope tag encoded at the carboxy terminus of each construct.
Flow cytometry analysis and antibodies.
Cells, 5 × 105 per sample, were washed with RPMI medium containing 10% fetal calf serum and incubated with the indicated conjugated monoclonal antibodies for 30 min at 4°C. After washing, each sample was fixed with 3% paraformaldehyde solution and flow cytometry analysis was performed with a FACSCalibur (Becton Dickinson Co., Franklin Lakes, NJ). Phycoerythrin (PE)- or allophycocyanin-conjugated anti-human antibodies to CD54 (Becton-Dickinson Co.), CD86 (Becton-Dickenson Co.), and MHC class I (clone W6/32; Dako, Carpinteria, CA) and fluorescein isothiocyanate-, PE-, or allophycocyanin-conjugated isotype control antibodies (Simultest; Becton-Dickinson Co.) were used for flow cytometry.
Immunoblot assays, immunoprecipitation, and PNGase F treatment.
BJAB cells were lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS] in phosphate-buffered saline [PBS]) containing protease inhibitors (Complete; Roche) or in NP-40 buffer (0.5% NP-40, 0.15 M NaCl, and 50 mM HEPES, pH 7.5) containing protease inhibitors (Complete; Roche) for immunoprecipitation. Insoluble components were removed from lysates by centrifugation at 16,000 × g at 4°C for 15 min. For normalization, the protein concentration in cell lysates was determined using a bicinchoninic acid protein assay (Pierce). Immunoprecipitations were performed with 2 μg of anti-MHC I (W6/32; Dako), anti-ICAM-1 (H-200; Santa Cruz Biotechnology, Santa Cruz, CA), or anti-B7.2 (BU-63; Santa Cruz Biotechnology) antibodies. Immune complexes were recovered by adsorption to protein A-agarose (Santa Cruz, Biotechnology) and washed three time with lysis buffer. Immunoprecipitated proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Immobilon-P membrane filter (Millipore, Billerica, MA) using a semidry unit (Bio-Rad). For PNGase F treatments, immunoprecipitated samples were resuspended in denaturation buffer (5% SDS, 10% β-mercaptoethanol in PBS) and heated to 100°C for 10 min. After cooling on ice, samples were brought up to 0.05 M sodium phosphate, pH 7.5, 10% NP-40, and 2,000 U PNGase F (New England Biolabs, Beverly, MA) were added. Samples were then incubated for 1 h at 37°C followed by separation by SDS-PAGE and blotting onto Immobilon-P membrane, as described above. Membrane filters were then blocked for 1 hour with PBS, 0.05% Tween 20, 5% nonfat dry milk. Antibodies were diluted in blocking buffer according to the manufacturer's recommendation: anti-ICAM-1 (H103; Santa Cruz Biotechnology), anti-B7.2 (BU-63; Santa Cruz Biotechnology), anti-MHC-I (HC10; obtained from Hidde Ploegh), and antiubiquitin (P4D1; Santa Cruz Biotechnology). Filters were incubated with the appropriate primary antibody for either 1 hour at room temperature or at 4°C overnight. Subsequently, filters were washed and incubated with horseradish peroxidase-conjugated antibody for 45 min at room temperature. Proteins were detected by enhanced chemiluminescence using a LAS 3000 camera (FujiFilm, Stamford, CT). Radioactive protein gels were fixed in 5% acetic acid and analyzed by phosphorimaging (BAS 1800 II; FujiFilm).
Endocytosis assay.
Ninety minutes after electroporation, BJAB cells (6 × 106) were stained on ice for 30 min in 300 μl complete medium with 6 μl W6/32 (Dako), 7 μl anti-ICAM-1 (H-200; Santa Cruz Biotechnology), or 12 μl anti-B7.2 (BU-63; Santa Cruz Biotechnology). After washing with medium, cells were resuspended in medium at a density of 1 × 106 cells per ml. An aliquot of the cells was kept on ice as staining controls, and the remainder of the cells was incubated at 37°C. At the indicated times, 0.5 × 106 cells were transferred to ice and sodium azide was added to a final concentration of 0.05% to stop endocytosis. Following collection at all time points, all samples were incubated with a PE-conjugated goat anti-mouse antibody (Beckton Dickinson Co.) together with unstained cells for 30 min on ice. Washed cells were fixed in 3% paraformaldehyde solution, and flow cytometry analysis was performed with a FACSCalibur (Becton Dickinson Co.).
RESULTS
Exploration of the importance of K5 conserved domains to target protein down regulation.
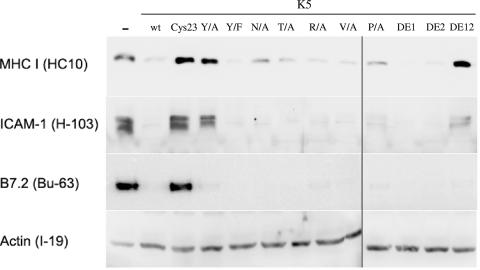
K3 and K5 share approximately 40% homology, much of it localized to a conserved region (CR) just downstream of the second membrane-spanning domain. This stretch of amino acids contains a number of potential protein trafficking or protein-protein interaction motifs that we have previously shown to be important for the down regulation of MHC class I by K3. In order to address their importance in K5, site-directed mutagenesis was performed substituting alanines, phenylalanines, or valines for the crucial residues in each motif. The K5 Cys23 mutant contains a substitution of four alanines in the central RING-CH region at residues 29, 31, 39, and 42, while the initial tyrosine of the Tyr motif was changed to either an alanine or phenylalanine to create the K5 Y156/A and Y156/F mutants, respectively (Fig. 1, upper panel). Each of the residues in the conserved motif (CM) was mutated individually to alanines, resulting in the N160/A, T161/A, R162/A, and V163/A mutants. The P/A mutant contains mutations in the proline-rich region of the potential SH3B just downstream of the CM at residues 167, 169, 171, and 174. Finally, the DE1, DE2, and DE12 mutants contain mutations of the charged residues in the first stretch (residues 178, 179, and 182), second stretch (residues 194, 195, 196, and 199), or both stretches of acidic residues, respectively. The resulting mutants were cloned into the pLXSN vector, sequence verified, and used to produce VSV-G-pseudotyped retrovirus stocks. These stocks were then used to infect BJAB B cells, which were selected for antibiotic resistance. Normalized lysates were harvested from each of the cell lines and examined by Western blotting, revealing equivalent expression levels of each of the mutants, comparable to wild-type K5 (Fig. 1, bottom panel). Next, all of the stable cell lines were stained for cell surface levels of MHC-I, B7.2, or ICAM-1 using PE-conjugated antibodies. The stained cells were then examined by flow cytometry, and the amount of PE channel fluorescence was measured. The PE fluorescence for each sample was normalized to cells transduced with the empty pLXSN vector. As expected, wild-type K5 was able to decrease the levels of cell surface MHC-I to less than 20% of empty vector-expressing cells (Fig. 2A). Levels of ICAM-1 and B7.2 in the wild-type K5-expressing cells were reduced by 94% and 97%, respectively (Fig. 2B and C). In contrast, cells expressing K5 with a mutation of the RING-CH domain (Cys23) showed only slight reductions in the cell surface levels of each target protein, even though the K5 Cys23 protein was expressed to a level comparable to wild type (Fig. 2 and data not shown). The ability of K5 to down regulate MHC-I cell surface levels was also dramatically affected by two other mutations. Mutation of the initial tyrosine of the Tyr motif to an alanine abolished down regulation of MHC-I, while a more conservative mutation of this same residue to a phenylalanine had little effect on down regulation ability (Fig. 2A). This indicates that for down regulation of MHC-I, as previously shown for K3, this motif is acting to govern protein endocytosis and degradation rather than acting as an SH2 domain, since the phenylalanine cannot be phosphorylated. Additionally, mutation of both stretches of acidic residues in the CR (DE12) resulted in a loss of MHC-I down regulation (Fig. 2A). Interestingly, both of these mutant forms were only partially reduced in their ability to modulate ICAM-1 cell surface levels (Fig. 2B) and were comparable to wild-type K5 in the ability to down regulate B7.2 (Fig. 2C). For each of the other K5 mutants tested, there was little change in the ability to down regulate ICAM-1 or B7.2; however, MHC-I down regulation was generally decreased, but not abolished, by the remainder of the mutants. These results demonstrate that K5 down regulation of MHC-I mimics the mechanisms used by K3. However, the mechanisms of ICAM-1 and B7.2 modulation both differ from the mechanisms used for MHC-I down regulation by K5 and K3.
FIG. 2.
Cell surface levels of MHC-I, ICAM-1, and B7.2 in stable K5-expressing BJAB cell lines. BJAB B cells stably expressing empty vector, wild-type K5, or K5 mutants, as indicated along the x axes, were stained with antibodies against MHC-I (A), ICAM-1 (B), or B7.2 (C) and analyzed by flow cytometry for mean channel fluorescence levels. Relative cell surface expression of each protein was determined by normalizing to the amount of fluorescence in empty vector cells. The data represent the averages of three separate experiments, with error bars indicating standard deviations.
The conserved motifs of K5 regulate its ability to induce target protein degradation.
It has been previously published that, like K3, K5 is able to induce the degradation of MHC-I. However, the ability of K5 to degrade ICAM-1 and B7.2 has only been examined by flow cytometry. To examine the relative ability of each of the K5 mutants to degrade these three target proteins, we produced normalized lysates from each of the stable BJAB B cell lines. The samples were then subjected to Western blotting to determine the level of target proteins. As expected and previously published, wild-type K5 is able to induce the degradation of MHC class I (Fig. 3, top panel). Additionally, K5 is able to induce the degradation of both ICAM-1 and B7.2 (Fig. 3, middle two panels). For all of the mutants, the steady-state protein levels were directly reflected by the cell surface presentation of the protein. K5 Cys23 was not able to down regulate cell surface levels of any of these three targets, and it also did not cause protein degradation (Fig. 2 and 3, respectively). As was observed by flow cytometry, there were marked differences in the abilities of K5 Y/A and DE12 to alter protein levels of the three targets, with both of them losing the ability to regulate MHC-I but neither losing B7.2 regulation. Again, this suggests that the mechanisms of degradation of MHC class I, ICAM-1, and B7.2 differ on a molecular level.
FIG. 3.
Steady-state levels of target proteins in stable BJAB B-cell lines. Normalized lysates were prepared from BJAB B cells stably expressing empty vector, wild-type K5, or K5 mutants as indicated above each lane. For each cell line, 30 μg of total cell lysate was subjected to SDS-PAGE followed by Western blotting with the indicated antibody.
The kinetics of degradation for each target protein is similar.
Since the target proteins were all behaving in a similar manner with respect to wild-type K5 surface down regulation and degradation, we next wanted to know if the kinetics of degradation were similar for each. Stable BJAB B cell lines were labeled with [35S]methionine-cysteine for 30 min, washed, and resuspended in normal whole medium. At various time points, cells were harvested and subjected to immunoprecipitation with either the W6/32 monoclonal antibody that recognizes MHC-I only when it is in complex with β2-microglobulin, an ICAM-1 or B7.2 antibody. After proteins were resolved by SDS-PAGE, the gels were subjected to autoradiography. Examination of the autoradiographs showed the presence of slower-migrating forms after MHC class I immunoprecipitation only from cells expressing wild-type K5 or mutants that cause MHC class I degradation (Fig. 4A and data not shown). No lower-mobility forms were seen in cells expressing the K5 Cys23, K5 Y/A, or K5 DE12 constructs (Fig. 4A). Presumably, these bands represent ubiquitylated forms of MHC-I. These forms were not observed in either ICAM-1 or B7.2 autoradiographs, but this was likely due to the high degree of glycosylation of each of these proteins, making discrete band visualization difficult (Fig. 4A). In an attempt to visualize these products, the experiment shown in Fig. 4A was repeated, but the immunoprecipitates were treated with PNGase F prior to SDS-PAGE to remove N-linked glycosylation; however, no additional bands were observed (Fig. 4B). Quantitation of the radiographs demonstrated that cells transduced with empty vector showed only a minimal level of MHC-I degradation, reflecting the normal turnover of MHC-I protein (Fig. 5). In contrast, wild-type K5 more than quadrupled the rate of MHC-I degradation, with a rate of 0.36% of labeled protein degraded per minute, compared with vector, which only induced 0.08% degradation per minute. In general, degradation by pulse-chase analysis reflected what was observed for steady-state levels of protein (Fig. 3 versus 5). The rates of degradation in the cell lines expressing K5 Cys23, Y/A, and DE12 mutants were equivalent to the rate of endogenous turnover, while the rate in the K5 Y/F cell line was close to wild type (Fig. 5).
FIG. 4.
Pulse-chase analysis of target protein degradation. (A) Empty vector, K5 wild-type, K5 Cys23, K5 Y/A, and K5 DE12 cells were serum starved for 12 h followed by a 30-min labeling with [35S]methionine-cysteine. After washing with complete, cold medium, cells were transferred to 37°C for the indicated amounts of chase time before lysis in RIPA buffer. Lysates were then subjected to immunoprecipitation with antibodies against MHC-I (clone W6/32), ICAM-1 (H-103), or B7.2 (BU-63). After washing, precipitated proteins were subjected to SDS-PAGE followed by autoradiography using a Fuji LAS-1000 phosphorimager. Stars, lower-mobility products; M, mature form; P, precursor form. (B) Empty vector, K5 wild-type, K5 Cys23, K5 Y/A, and K5 DE12 cells were labeled and immunoprecipitated as described for panel A. After washing, the samples were heated to 100°C for 10 min in denaturation buffer, followed by addition of NP-40 to compete out excess SDS. The denatured proteins were then treated with 2,000 U PNGase F (New England Biolabs) for 1 h at 37°C followed by SDS-PAGE and autoradiography.
FIG. 5.
Quantitation of MHC-I degradation in BJAB B-cell lines. As described for Fig. 4, cells were labeled with [35S]methionine-cysteine and chased for the indicated amount of time, followed by immunoprecipitation with an anti-MHC-I antibody (clone W6/32). Precipitated proteins were subjected to SDS-PAGE and autoradiography followed by quantitation using a Fuji LAS-1000 phosphorimager. The amount of signal in each lane representing both the precursor and lower-mobility forms was normalized to the amount of signal in the time zero chase sample for each cell line. Data are representative of three separate experiments.
The kinetics of ICAM-1 and B7.2 degradation were only examined for selected mutants of K5. Quantification of the pulse-chase data collected from the empty vector-expressing cells showed that for both ICAM-1 and B7.2, the rate of endogenous degradation was less than is seen with MHC-I, with almost no detectable ICAM-1 degradation and only 5% B7.2 degradation over the observed period (Fig. 6A and B). Wild-type K5, in contrast, was able to degrade 50% of the labeled ICAM-1 in 127 min, while 50% of B7.2 was degraded in only 83 min. This is equivalent to degradation rates of 0.39% per min for ICAM-1, approximately the same as MHC-I, and 0.60% per min for B7.2. Similar rates were measured when samples were first deglycosylated with PNGase F prior to SDS-PAGE and autoradiography (data not shown). The kinetics of ICAM-1 and B7.2 degradation for each of the K5 mutants examined mirrored what was seen in Western blot assays of the same cells (Fig. 6A and B versus 3). Notably, as was seen in steady-state measurements, K5 DE12 was still capable of degrading both ICAM-1 and B7.2; however, the kinetics were slower than with wild-type. Additionally, the K5 Y/A construct was able to mediate B7.2 degradation, but again with slower kinetics than wild-type K5.
FIG. 6.
Quantitation of ICAM-1 and B7.2 degradation and export. Empty vector, K5 wild-type, K5 Cys23, K5 Y/A, K5 P/A, and K5 DE12 cells were treated as in described in the legend for Fig. 5; however, the lysates were immunoprecipitated with either an ICAM-1 (H-103)-specific (A) or B7.2 (BU-63)-specific (B) antibody. The total amount of signal corresponding to both the slowly (mature) and rapidly migrating (precursor) forms in each lane was normalized to the amount of signal for the time zero chase sample for each cell line. (C) The loss of the higher-mobility B7.2 immature form (labeled P) was quantitated from the same gels and normalized to the amount of precursor product at time zero. Data in each panel are representative of three separate experiments.
Previously, K5 was shown to induce the endocytosis and degradation of these three target proteins from the surface of cells. However, we also sought to confirm that K5 was not affecting ICAM-1 and B7.2 protein production or export from the endoplasmic reticulum. To accomplish this, we quantitated the amount of rapidly migrating precursor protein (Fig. 4). The relative levels of both proteins at time zero and the rate of precursor disappearance were equal amongst all examined K5 constructs and vector-expressing cells (Fig. 6C and data not shown). This indicates that K5 does not alter B7.2 or ICAM-1 export from the endoplasmic reticulum and acquisition of higher-molecular-weight carbohydrate modifications. Together, these data indicate that the distinct mechanisms of down regulation of MHC I, ICAM-1, and B7.2 are employed by K5, with down regulation of MHC-I most closely resembling K3.
B7.2 degradation is not dependent on increased protein endocytosis.
K5 has been previously shown to increase the endocytosis rate of MHC-I, B7.2, and ICAM-1. Further, the K3 DE12 mutant has been shown to induce MHC-I endocytosis but not degradation. To establish if any of the K5 mutants affected protein endocytosis, we first attempted to perform assays using the stable cell lines. However, due to the low surface levels of each of the target proteins in many of the lines, this failed. Instead, endocytosis rates were measured by electroporating parental BJAB cells with constructs expressing enhanced green fluorescent protein (EGFP)-tagged K5 constructs. At 90 min postelectroporation, cells were transferred to ice and stained with unconjugated antibodies against each of the three targets. Cells were then washed and transferred back to 37°C for various amounts of time. Harvested cells were placed on ice and treated with sodium azide to prevent further endocytosis. The amount of cell surface antibody remaining in GFP-expressing versus nonexpressing cells was then quantitated by flow cytometry after staining with the corresponding secondary antibody. As expected, wild-type K5 induced increased rates of MHC class I, B7.2, and ICAM-1 endocytosis (Fig. 7A). As previously observed for K3, the DE12 mutant of K5 was still capable of increasing the rate of MHC-I endocytosis and was also capable of inducing ICAM-1 and B7.2 endocytosis. Surprisingly, although the steady-state levels of cell surface B7.2 protein were greatly reduced in the K5 Y/A cell lines (Fig. 2C), the endocytosis rate was very low following transient transfection and comparable to cells expressing an unrelated viral protein (Fig. 7C). Approximately 3% of the labeled B7.2 was internalized over the time course of the assay, while wild-type K5 and the K5 DE12 mutant were both able to mediate a greater-than-90% decrease. To determine whether changes in cell surface expression of B7.2 were an artifact seen only in the stable cell lines, we next performed an experiment where cells were electroporated with constructs expressing EGFP-tagged K5 wild type, K5 Y/A, or an unrelated viral protein. However, in this instance, cells were incubated at 37°C for various amounts of time followed by staining for cell surface levels of each target. This differs from the previous experiment in that the levels of total cell surface protein were determined at each time point rather than following the fate of individual molecules over time. This allowed us to be able to differentiate between altered molecular endocytosis rates as measured in the previous assay and prevention of cell surface expression. Transfection of wild-type K5, as expected, caused a decrease in the cell surface expression of all three target proteins (Fig. 7D, E, and F). MHC-I had the slowest rate of cell surface loss, while B7.2 had the highest rate after wild-type K5 expression. No reduction of either MHC-I or ICAM-1 was seen in the presence of the K5 Y/A mutant while, in contrast, B7.2 cell surface levels were reduced by 57% over the time course compared to only an 18% decrease in cells electroporated with a construct expressing the unrelated gH protein (Fig. 7D and E versus F). This indicates that the degradation of B7.2 can occur through an endocytosis-independent mechanism, while the degradation of MHC-I and ICAM-1 are endocytosis dependent.
FIG. 7.
Endocytosis and down regulation of targets following electroporation of K5 or mutants. (A, B, and C) BJAB B cells were electroporated with constructs expressing GFP-fusion proteins of K5 wild-type, K5 Y/A, K5 DE12, or an unrelated viral protein, gH. At 90 min postelectroporation, cells were placed on ice and stained with unconjugated antibodies against MHC-I (A), ICAM-1 (B), or B7.2 (C) for 30 min. Cells were then transferred to 37°C at time zero, and samples were taken at the indicated time points. For each time point, cells were stained with the appropriate secondary antibody and subjected to flow cytometry. The resulting mean channel fluorescence (MCF) in the non-GFP-expressing population was used to normalize the MCF in the GFP-expressing population and displayed as the relative surface expression. (D, E, and F) In a parallel set of experiments, cells were electroporated with constructs expressing GFP-fusion proteins of K5 wild-type, K5 Y/A, or an unrelated viral protein, gH, and then incubated at 37°C for various amounts of time. At each time point cells were harvested onto ice, stained with conjugated antibodies against MHC-I (D), ICAM-1 (E), or B7.2 (F) for 30 min and then examined by flow cytometry. The resulting MCF in the non-GFP-expressing population was used to normalize the MCF in the GFP-expressing population and displayed as the relative surface expression. The data in each panel are representative of at least three separate experiments.
Target protein degradation can occur by ubiquitylation-dependent and -independent mechanisms.
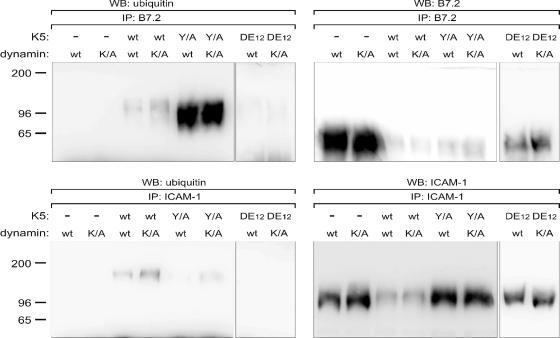
As shown in Fig. 4, MHC-I protein precipitated from wild-type K5-expressing cells contained slower-migrating species, while no such bands were observed for the ICAM-1 and B7.2 immunoprecipitation samples. To address this difference and to determine whether the K5 Y/A mutant was causing degradation of B7.2 in a ubiquitin-dependent or -independent manner, we next performed immunoprecipitation of either B7.2 or ICAM-1 from pLXSN, wild-type K5, K5 Y/A, or K5 DE12 cells followed by examination of samples for ubiquitylation by Western blotting. One potential difficulty with this method is the rather rapid turnover of ubiquitylated proteins within the cell. Positing that ubiquitylation of target proteins by K5 likely occurs prior to endocytosis or transport to a degradatory compartment, we electroporated K44A mutant dynamin to prevent endocytosis following ubiquitylation, which has been previously shown to block K5-mediated down regulation of ICAM-1 and B7.2, or wild-type dynamin as control (4). As shown in Fig. 8, both B7.2 and ICAM-1 immunoprecipitated from wild-type K5-expressing cells contained lower-mobility forms reactive with the ubiquitin antibody that were absent from cells containing empty vector (Fig. 8). The amount of ubiquitylated product in K5-expressing cells increased approximately 10-fold in the presence of dominant negative dynamin (Fig. 8). Surprisingly, the amounts of ubiquitylated B7.2 dramatically increased in cells expressing the K5 Y/A mutant compared to wild-type K5-expressing cells, but unlike wild-type K5-expressing cells, there was little effect of the dominant negative dynamin (Fig. 8). Equally surprising was a lack of detectable ubiquitin addition to either B7.2 or ICAM-1 in the K5 DE12-expressing cells (Fig. 8). Given the competent degradation of these two targets by K5 DE12, this indicates that K5 is capable of directing both endocytosis and degradation in an ubiquitin-independent fashion.
FIG. 8.
Ubiquitylation of B7.2, ICAM-1, and MHC-I in stable BJAB B-cell lines. Stable BJAB B-cell lines expressing empty vector, K5 wild-type, K5 Y/A, or K5 DE12 were electroporated with constructs expressing wild-type (wt) or dominant negative K44A mutant dynamin (K/A). At 48 h postelectroporation, live cells were purified on a Ficoll gradient and then subjected to immunoprecipitation with an antibody against B7.2 or ICAM-1, as indicated, followed by Western blotting for either ubiquitin (left panels) or the precipitated proteins (right panels).
DISCUSSION
Parallel examination of MHC class I, ICAM-1, and B7.2 down regulation by the K5 E3 ubiquitin ligase has allowed observation of differential mechanisms governing the modulation of these three molecules. Regulation of all three targets is abolished by mutation of the C4HC3 RING-CH domain. However, the targets differ in sensitivity to down modulation by K5 with mutations in the Tyr motif (K5 Y/A) or in the two stretches of acidic amino acids (K5 DE12) (Fig. 2). As with K3, the Tyr motif seems to act as an endocytosis motif, rather than an SH2 domain, because mutation of the tyrosine to a phenylalanine has little effect on down regulation of any of these three targets (Fig. 2). If the motif were acting primarily as an SH2 domain, the K5 Y/F mutant would be expected to be nonfunctional, since tyrosine phosphorylation could not take place. Interestingly, introduction of an alanine at this position results in a loss of MHC-I and ICAM-1 endocytosis and degradation (Fig. 7A and B and 3). The ability of K5 Y/A to mediate an increased rate of B7.2 endocytosis is also blocked (Fig. 7C), but degradation still occurs, albeit with slightly slower kinetics (Fig. 3 and 6). Interestingly, the degree of ubiquitylation of B7.2 is greater in cells expressing the K5 Y/A mutant than in cells expressing wild-type K5. Whether this is due to a slower rate of degradation, allowing accumulation of ubiquitylated products, or an altered kinetic of ubiquitin addition is unclear. Equally interesting is the fact that ubiquitylated B7.2 does not accumulate to a greater degree in cells expressing a dominant negative mutant of dynamin (Fig. 8). This indicates, along with the transient endocytosis data (Fig. 7), that K5 can degrade B7.2 in an endocytosis-independent fashion. This seems to be in stark contrast to K3, which has thus far been shown to mediate target protein down regulation through increased endocytosis.
K5 has been shown to direct the down modulation of several other targets, including CD31 (15). The modulation of CD31 occurs through two separate mechanisms. Mature molecules can be endocytosed and degraded from the cell surface, while newly synthesized molecules can be degraded by an endocytosis-independent mechanism. This mechanism is reliant on interactions between the K5 acidic stretches and PACS-2 (phosphofurin acidic cluster sorting protein 2). In contrast, degradation of MHC class I was shown not to be dependent on PACS-2 (15). In this report we show that MHC-I degradation, but not endocytosis, is reliant on the acidic stretches (Fig. 3 and 7A). The high levels of MHC-I on the surface of K5 DE12-expressing BJAB B cells are likely due to a recycling of MHC-I in the absence of degradation, although this requires formal proof (Fig. 2). With respect to ICAM-1 and B7.2, both can be endocytosed and degraded by K5 DE12 (Fig. 3 and 7). In both cases, endocytosis and degradation are taking place in the absence of detectable ubiquitylation (Fig. 8). Presumably, in this case, K5 is acting as an adaptor bringing together target protein with the cellular endocytosis and degradation machinery. However, it is completely unclear why K5 is not able to perform this same type of ubiquitin-independent degradation of MHC-I, although it may be due to the normal pathways of cellular degradation for each of these targets. Finer mapping of the cellular proteins required for MHC-I versus B7.2 or ICAM-1 degradation is under way to clarify these results.
Taken together, the data presented in this paper indicate that the KSHV K5 protein is able to direct the modulation of MHC-I, ICAM-1, and B7.2 through multiple mechanisms. These mechanisms include endocytosis-dependent and -independent as well as ubiquitylation-dependent and -independent pathways. To explain these data, we propose a model where the interaction of K5 with specific cellular proteins dictates localization and function. These interactions include a binding of cellular trafficking proteins through the Tyr motif and the stretches of acidic amino acids. Overall, K5 has pirated the targeting and cargo transport functions of the cellular ubiquitylation system rather than just E3 enzymatic activity directly for modulation of important immunomodulatory proteins. The extent to which cellular E3 ubiquitin ligases are also regulating target proteins in this multifactorial manner is crucial to our understanding of this key pathway of cellular regulation.
Acknowledgments
We thank T. Kirchhausen for the wild-type and dominant negative dynamin 1 pEGFP clones, Hidde Ploegh for the HC10 antibody, Walter Mothes for the pCL-Eco plasmid, Jack Rose for the VSV-G expression vector, Jack Rose and Michael Robek for technical discussions, and Meisha Bynoe for critical reading.
This work was supported in part by a National Cancer Institute grant to R. E. M. (R21 CA102535).
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 2.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. [DOI] [PubMed] [Google Scholar]
- 3.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d'Azzo, A., A. Bongiovanni, and T. Nastasi. 2005. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6:429-441. [DOI] [PubMed] [Google Scholar]
- 7.Du, Z., D. A. Regier, and R. C. Desrosiers. 1995. Improved recombinant PCR mutagenesis procedure that uses alkaline-denatured plasmid template. BioTechniques 18:376-378. [PubMed] [Google Scholar]
- 8.Duncan, L. M., S. Piper, R. B. Dodd, M. K. Saville, C. M. Sanderson, J. P. Luzio, and P. J. Lehner. 2006. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 25:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma- associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 82:1175-1180. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt, E. W., L. Duncan, D. Mufti, J. Baker, P. G. Stevenson, and P. J. Lehner. 2002. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 21:2418-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 13.Levkowitz, G., L. N. Klapper, E. Tzahar, A. Freywald, M. Sela, and Y. Yarden. 1996. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene 12:1117-1125. [PubMed] [Google Scholar]
- 14.Lorenzo, M. E., J. U. Jung, and H. L. Ploegh. 2002. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 76:5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansouri, M., J. Douglas, P. P. Rose, K. Gouveia, G. Thomas, R. E. Means, A. V. Moses, and K. Fruh. 2006. Kaposi's sarcoma herpesvirus K5 eliminates CD31/PECAM from endothelial cells. Blood 108:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Means, R. E., S. Ishido, X. Alvarez, and J. U. Jung. 2002. Multiple endocytic trafficking pathways of MHC class I molecules induced by a herpesvirus protein. EMBO J. 21:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meisner, H., and M. P. Czech. 1995. Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J. Biol. Chem. 270:25332-25335. [DOI] [PubMed] [Google Scholar]
- 18.Pickart, C. M., and D. Fushman. 2004. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8:610-616. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez, D. J., L. Coscoy, and D. Ganem. 2002. Functional organization of MIR2, a novel viral regulator of selective endocytosis. J. Biol. Chem. 277:6124-6130. [DOI] [PubMed] [Google Scholar]
- 20.Sayeed, A., and D. T. Ng. 2005. Search and destroy: ER quality control and ER-associated protein degradation. Crit. Rev. Biochem. Mol. Biol. 40:75-91. [DOI] [PubMed] [Google Scholar]
- 21.Soubeyran, P., K. Kowanetz, I. Szymkiewicz, W. Y. Langdon, and I. Dikic. 2002. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416:183-187. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 97:8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbé, S. 2005. Ubiquitin and endocytic protein sorting. Essays Biochem. 41:81-98. [DOI] [PubMed] [Google Scholar]