Abstract
Despite the well-recognized importance of CD4 T-cell help in the induction of antibody production and cytotoxic-T-lymphocyte responses, the regulation of CD4 T-cell responses is not well understood. Using mice deficient for TNF receptor I (TNFR I) and/or TNFR II, we show that TNFR I and TNFR II play redundant roles in down regulating the expansion of CD4 T cells during an acute infection of mice with lymphocytic choriomeningitis virus (LCMV). Adoptive transfer experiments using T-cell-receptor transgenic CD4 T cells and studies with mixed bone marrow chimeras indicated that indirect effects and not direct effects on T cells mediated the suppressive function of TNF on CD4 T-cell expansion during the primary response. Further studies to characterize the indirect effects of TNF suggested a role for TNFRs in LCMV-induced deletion of CD11chi dendritic cells in the spleen, which might be a mechanism to limit the duration of antigenic stimulation and CD4 T-cell expansion. Consequent to enhanced primary expansion, there was a substantial increase in the number of LCMV-specific memory CD4 T cells in the spleens of mice deficient for both TNFR I and TNFR II. In summary, our findings suggest that TNFRs down regulate CD4 T-cell responses during an acute LCMV infection by a non-T-cell autonomous mechanism.
It is well established that CD4 T cells play a central role in defense against viral infections. During viral infections, not only are CD4 T cells required for inducing effective antibody responses, effector CD4 T cells can exert direct antiviral effects by producing cytokines like gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (5, 9, 18, 19, 29, 62). Additionally, CD4 T cells are required for the elicitation and/or maintenance of optimal CD8 T-cell responses. While primary CD8 T-cell responses to lymphocytic choriomeningitis virus (LCMV) and ectromelia virus do not require CD4 T-cell help, induction of CD8 T-cell responses to vesicular stomatitis virus, influenza virus, and herpes simplex virus are CD4 T-cell dependent (2, 17, 35, 59). In contrast to acute viral infections, CD4 T cells are uniformly essential for sustaining CD8 T-cell responses during chronic viral infections (8, 35, 46, 47, 59). During chronic viral infections, CD4 T-cell deficiency leads to rapid loss of CD8 T-cell activity and uncontrolled viral replication (19, 35, 46). In acute viral infections like LCMV, although the initial induction of CD8 T-cell responses is independent of CD4 T cells, the long-term maintenance of functional memory CD8 T cells requires CD4 T-cell help (4, 10, 51, 54). Taken together, these reports provide unequivocal evidence that CD4 T cells play a critical role in orchestrating all facets of the antiviral humoral and cellular immune responses. Thus, it is of paramount importance to understand the mechanisms that regulate the antiviral CD4 T-cell response for purposes of rational design of vaccines and development of immunotherapeutic modalities.
In viral infections, although effector CD4 T cells seem to produce predominantly IFN-γ, interleukin-2 (IL-2), and TNF-α, CD4 T cells that secrete IL-4 and IL-5 are often detectable (3, 13, 22, 44, 57, 58). There is accumulating evidence that effector molecules like IFN-γ and TNF-α might have important roles in regulation of mature T-cell homeostasis (23). In chronic intracellular bacterial infections, IFN-γ and TNF-α have been shown to down regulate CD4 T-cell responses (15, 60), while deficiency of IFN-γ or TNF-α leads to enhanced expansion of CD4 T cells and exacerbation of experimental autoimmune encephalitis in mice (11, 26). TNF-α also exerts potent immunosuppressive effects in protection against T-cell-dependent autoimmune pathologies (14). However, the mechanism(s) underlying the regulation of CD4 T-cell responses by TNF is not well understood. In this study, we have investigated the role of TNF in regulating various phases (expansion, contraction, and memory phases) of the CD4 T-cell response in vivo by use of a well-characterized mouse model of acute LCMV infection. We show that TNF receptors (TNFRs) play a negative role in regulating expansion of CD4 T cells and the subsequent development of CD4 T-cell memory. Mechanistic studies suggested that the down regulatory functions of TNFRs on CD4 T-cell expansion are mediated at least in part by indirect effects on accessory cells, like dendritic cells and macrophages. Thus, modulation of TNF-mediated effects may be a viable approach to improve the magnitude of T-cell memory during vaccinations or down regulate CD4 T-cell-dependent immunopathology.
MATERIALS AND METHODS
Mice.
p55R-deficient (p55−/−) mice on the C57BL/6 background (45) and wild-type (wt) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). p75R-deficient (p75−/−) mice and p55R- and p75R-deficient (double knockout [DKO]) mice on the C57BL/6 background (43) were provided by Jacques J. Peschon (Immunex Corporation, Seattle, WA). TNF-deficient (TNF−/−) mice on the C57BL/6 background were provided by Lloyd J. Old (Memorial-Sloan Kettering Cancer Center, NY) (34). SMARTA T-cell-receptor transgenic (TCR Tg) mice (40, 41) were provided by Pamela Ohashi (University of Toronto, Canada), with permission from Hans Hengartner and Annette Oxenius (University of Zurich, Switzerland). SMARTA transgenic mice were backcrossed to the Thy1.1 background at the University of Wisconsin. Congenic Thy1.1 (B6.PL-Thy1a) mice and Rag1-deficient (Rag1−/−) mice on the C57BL/6 background were purchased from Jackson Laboratories. Experimental mice were used at 6 to 8 weeks of age. All animal experiments were performed according to the strict guidelines of the Institutional Animal Care and Use Committee.
Virus.
Mice were injected intraperitoneally with 2 × 105 PFU of the Armstrong strain of LCMV to induce an acute infection. Infectious LCMV in the tissues was quantitated by a plaque assay done with Vero cells, as described previously (1).
Flow cytometry.
Single-cell suspensions of splenocytes were stained with anti-CD4, anti-CD44, anti-Thy1.1, and anti-Thy1.2 antibodies in fluorescence-activated cell sorting buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0.1% sodium azide) for 30 min at 4°C. Following staining, cells were fixed in 2% paraformaldehyde and data were acquired using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Fluorescence-activated cell sorting data were analyzed using CellQuest (Becton Dickinson) or FlowJo (Tree Star, Inc.) software. All antibodies were purchased from BD-Pharmingen (San Diego, CA) unless mentioned otherwise.
Quantification of LCMV-specific CD4 T cells by intracellular cytokine staining.
In LCMV-infected C57BL/6 mice, CD4 T cells recognize the amino acid residues 61 to 80 in the LCMV glycoprotein (here designated GP61) presented by the major histocompatibility complex class II molecule I-Ab. The number of GP61-specific CD4 T cells in the spleen was enumerated by intracellular cytokine staining, as described elsewhere (58). Briefly, splenocytes were stimulated with GP61 peptide for 5 h in the presence of brefeldin A. After the 5-h stimulation, cells were stained for cell surface CD4 and intracellular IFN-γ, TNF-α, and IL-2 by use of a Cytofix/Cytoperm kit (BD-Pharmingen). In some experiments, cells were also stained for cell surface Thy1.1, Thy1.2, CD44, CD11a, and CD127 (eBioscience, San Diego, CA) molecules. Stained cells were fixed in 2% paraformaldehyde and acquired on a flow cytometer (FACSCalibur; Becton Dickinson), and data were analyzed using CellQuest or FlowJo software.
Adoptive transfer of transgenic CD4 T cells.
Single-cell suspensions of splenocytes from SMARTA TCR Tg mice, containing 105 TCR Tg T cells, were adoptively transferred (52) into wt and DKO mice by intravenous injection. Twenty-four hours after cell transfer, mice were infected with LCMV, and the expansion of transgenic T cells was assessed on the eighth day after infection. In some experiments, T cells purified from naïve SMARTA TCR Tg mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) and adoptively transferred into mice that were infected with LCMV 3 days before cell transfer. The proliferation of adoptively transferred SMARTA TCR Tg cells was assessed 72 h later by flow cytometry.
Isolation and characterization of dendritic cells in the spleen.
Single-cell suspensions of splenocytes were prepared by treatment with collagenase D (Roche Applied Science, Indianapolis, IN), as described previously (39). Splenocytes were stained with anti-CD11c and anti-B220 antibodies and analyzed by flow cytometry as described above.
Staining for apoptotic cells by TUNEL.
Formaldehyde-fixed sections from the spleens of LCMV-infected wt and DKO mice were stained for apoptotic nuclei by use of a fluorescein in situ cell death detection kit (Roche Applied Science, Indianapolis, IN) per the recommendations of the manufacturer. To colocalize apoptotic nuclei and dendritic cells, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining (green) was followed by incubations with biotinylated anti-mouse CD11c (eBioscience) and then streptavidin-Alexa 568 conjugate (Molecular Probes). Nonspecific binding of antibodies was blocked with bovine serum albumin and mouse serum before staining for CD11c was done.
Construction of bone marrow chimeric mice.
Bone marrow cells were prepared by flushing femurs from wt and DKO mice with RPMI media and depleted of T cells by use of anti-CD5 microbeads (Miltenyi Biotec, Auburn, CA). A 1:1 mixture of 15 × 106 T-cell-depleted bone marrow cells from wt (Thy1.1) and DKO (Thy1.2) mice was adoptively transferred into lethally irradiated (900 rads) Rag1−/− mice. Bone marrow-reconstituted Rag1−/− mice were treated with neomycin (0.025 mg/ml) and polymyxin B (0.013 mg/ml; Sigma, St. Louis, MO) in drinking water for up to 4 weeks. The reconstitution of the lymphoid system by the adoptively transferred bone marrow cells was assessed 4 to 6 weeks after cell transfer.
Statistical analysis.
Commercially available software (SYSTAT, version 10.2; Chicago, IL) was used to analyze data.
RESULTS
Primary CD4 T-cell response to LCMV is enhanced in TNFR-deficient mice.
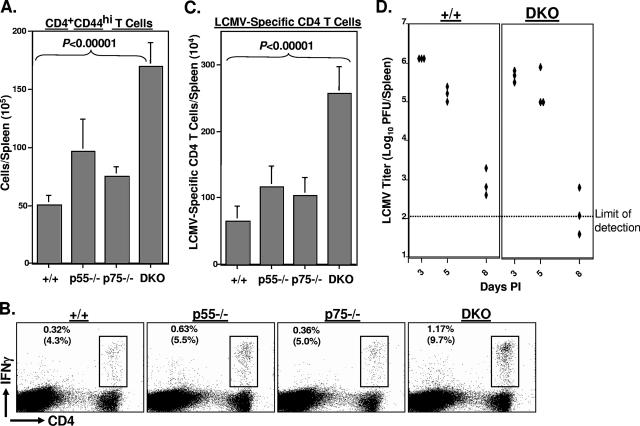
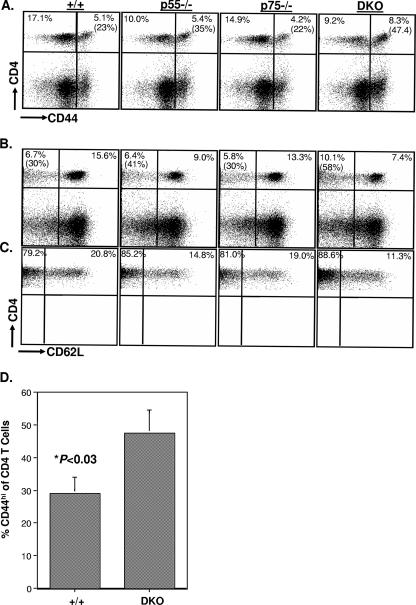
Infection of immunocompetent mice with the Armstrong strain of LCMV elicits a potent primary CD8 and CD4 T-cell response (37, 53), and the virus is cleared in 8 to 10 days. To examine the role of TNFRs in regulating primary CD4 T-cell responses during an acute viral infection, groups of wt, p55−/−, p75−/−, and DKO mice were infected with LCMV. On day 8 postinfection (p.i.), we assessed virus-induced activation of CD4 T cells in the spleen by quantifying the number of CD4+ CD44hi T cells by flow cytometry. As shown in Fig. 1A, the absolute numbers of activated CD4 T cells in the spleens of p55−/− or p75−/− mice were slightly higher than those in the spleens of wt mice. Strikingly, spleens of DKO mice contained a significantly (P < 0.00001) larger number of activated CD4 T cells than the spleens of wt, p55−/−, and p75−/− mice. These data indicate that TNFRs suppress activation of CD4 T cells during an acute viral infection.
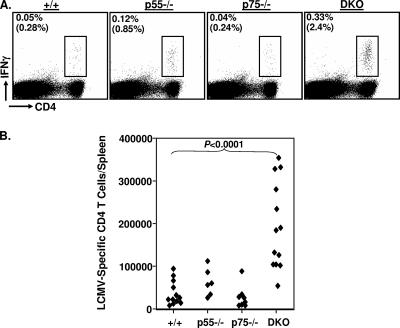
FIG. 1.
Primary CD4 T-cell responses to LCMV in TNFR-deficient mice. Eight days after infection with LCMV, CD4 T-cell responses were quantitated in the spleens of wt (+/+), p55−/−, p75−/−, and DKO mice. (A) Splenocytes were stained with anti-CD4 and anti-CD44 antibodies, and the numbers of CD4+ CD44hi cells were quantitated by flow cytometry. (B and C) Splenocytes were stimulated with the GP61 peptide for 5 h ex vivo and stained for cell surface CD4 and intracellular IFN-γ. The numbers of LCMV-specific, IFN-γ-producing CD4 T cells were quantitated by flow cytometry. The dot plots in panel B are gated on splenocytes, and the values are the percentages of IFN-γ-producing CD4 T cells among splenocytes. The values in parentheses are the percentages of GP61-specific T cells of total CD4 T cells. The data in panels A and C are the averages ± standard deviations from three to six mice/group and are representative of two to three independent experiments. (D) On the indicated days after LCMV infection, viral titers in the spleen were measured by a plaque assay using Vero cell monolayers; each symbol represents viral titer in the spleen of an individual mouse. +/+, wild type.
Next, we compared primary CD4 T-cell responses to the I-Ab-restricted LCMV epitope GP61 between wt and TNFR-deficient mice by intracellular cytokine staining (Fig. 1B and C). As shown in Fig. 1B, frequencies of GP61-specific CD4 T cells in the spleens of p55−/− and DKO mice were higher than those in the spleens of wt and p75−/− mice. The data in Fig. 1C show the absolute numbers of GP61-specific CD4 T cells in the spleens of wt and TNFR-deficient mice on day 8 p.i. The absolute numbers of GP61-specific CD4 T cells in the spleens of p55−/− and p75−/− mice were each <2-fold greater than those in the spleens of wt mice. In striking contrast, the total numbers of GP61-specific CD4 T cells in the spleens of DKO mice were fourfold higher (P < 0.00001) than those in the spleens of wt mice. The enhanced CD4 T-cell activation seen with DKO mice was not likely to be induced by higher viral load in the tissues, since TNFR deficiency did not affect either the magnitude or the kinetics of LCMV replication (Fig. 1D).
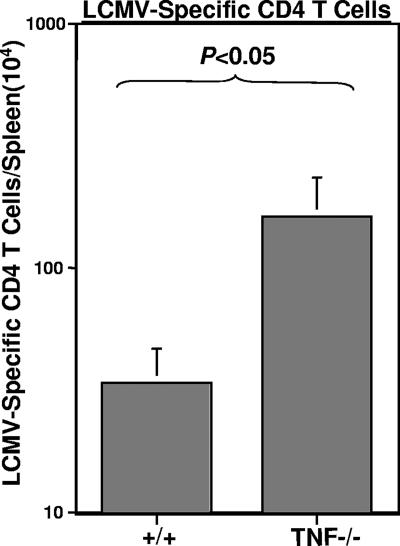
The two ligands that are known to interact with TNFRs are TNF-α and lymphotoxin alpha (56). To determine whether the enhanced primary CD4 T-cell expansion in DKO mice was due to a lack of TNF-α-mediated effects, we infected wt and TNF−/− mice with LCMV and assessed primary LCMV-specific CD4 T-cell responses in the spleens on day 8 p.i. As shown in Fig. 2, total numbers of GP61-specific CD4 T cells in the spleens of TNF−/− mice were fourfold higher (P < 0.05) than those in the spleens of wt mice. These findings, along with data shown in Fig. 1A to C, suggested that TNF-α/TNFR interactions down regulate the expansion of antigen-specific CD4 T cells during an acute LCMV infection.
FIG. 2.
CD4 T-cell responses in TNF-deficient mice. Eight days after infection with LCMV, splenocytes from wild-type (+/+) and TNF-deficient (TNF−/−) mice were stimulated with the GP61 peptide for 5 h ex vivo, and the numbers of LCMV-specific, INF-γ-producing CD4 T cells were quantitated by flow cytometry. Data are from three mice per group and are representative of results from two independent experiments.
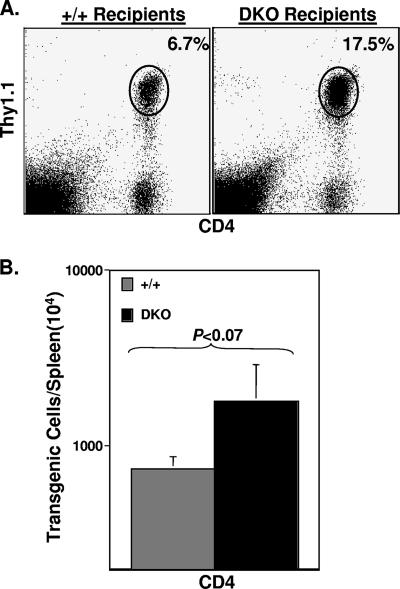
TNFR-expressing transgenic CD4 T cells expand more in a TNFR-deficient lymphoid environment.
The data presented above showed a greater expansion of LCMV-specific CD4 T cells in DKO mice than in wt mice. Here we have investigated the importance of TNFRs on CD4 T cells versus non-CD4 T cells in down regulation of virus-specific CD4 T-cell responses. To this end, we adoptively transferred 105 TNFR-sufficient naïve SMARTA CD4 T cells that express the transgenic class II-restricted TCR specific to the GP61 epitope of LCMV into wt and DKO mice. Following adoptive transfer, recipients were infected with LCMV, and the expansion of transgenic CD4 T cells was assessed on day 8 p.i. The adoptively transferred transgenic SMARTA CD4 T cells were distinguished from the host CD4 T cells based on Thy1.1 expression: while host T cells were Thy1.2+, the donor transgenic T cells were Thy1.1+. As shown in Fig. 3, the frequencies and total numbers of Thy1.1+ GP61-specific transgenic CD4 T cells were >2.5-fold higher (P < 0.07) in DKO recipients than in wt recipients. The numbers of SMARTA CD4 T cells in the spleens of control uninfected wt and DKO mice were comparable (data not shown). Thus, the activation and expansion of TNFR-sufficient CD4 T cells were greater in a TNFR-deficient lymphoid environment. These findings indicated that TNFR signaling in non-CD4 T cells could suppress the expansion of virus-specific CD4 T cells during an acute viral infection.
FIG. 3.
Expansion of TNFR-expressing SMARTA TCR Tg CD4 T cells in TNFR-deficient mice. LCMV GP61-specific Thy1.1+ SMARTA TCR Tg CD4 T cells were adoptively transferred into wt/Thy1.2 and DKO/Thy1.2 recipient mice. One day after cell transfer, recipient mice were infected with LCMV, and the numbers of transgenic CD4 T cells in the spleens were quantitated on day 8 p.i. Splenocytes were stained with anti-Thy1.1, anti-Thy1.2, and anti-CD4 antibodies, and the numbers of Thy1.1+ CD4 T cells were quantitated by flow cytometry. The dot plots in panel A are gated on total splenocytes, and the values are the percentages of transgenic CD4 T cells of splenocytes. The data in panel B are the means ± standard deviations from five mice/group and are representative of two independent experiments. +/+, wild type.
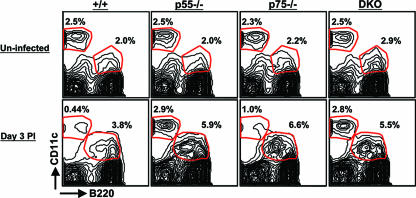
A role for TNFRs in the depletion of CD11chi non-pDCs in LCMV-infected mice.
It has been shown previously that TNF can inhibit the responses and/or viability of dendritic cells in vitro (6, 42). Therefore, it is possible that enhanced expansion of LCMV-specific CD4 T cells in TNFR-deficient mice could be due to a lack of TNF-induced inhibitory effect on the dendritic cells. To address this issue, we characterized the effect of TNFR deficiency on the dynamics of LCMV-induced alterations in the CD11c+ dendritic cell populations. As illustrated in Fig. 4, spleens of uninfected wt, p55−/−, p75−/−, and DKO mice contained comparable percentages of plasmacytoid dendritic cells (pDCs) (CD11clo B220+) and nonplasmacytoid dendritic cells (non-pDCs) (CD11chi B220−). As reported by Montoya et al. (36), the percentages of non-pDCs showed a profound drop on day 2 p.i. (data not shown) and day 3 p.i. in wt mice compared to the percentages of non-pDCs in uninfected mice; the percentages of non-pDCs in wt mice dropped from ∼2.5% (uninfected mice) to 0.37% ± 0.06% on day 3 p.i. In striking contrast, the percentages of non-pDCs in the spleens of LCMV-infected p55−/− (2.56% ± 1%) and DKO (2.6% ± 0.32%) mice were comparable to those in the respective uninfected mice. Importantly, the percentages of non-pDCs in the spleens of LCMV-infected wt mice on day 3 p.i. were significantly (P = 0.001) lower than those in the spleens of DKO mice. Compared to levels for wt mice, LCMV-induced deletion of non-pDCs was modestly affected in p75−/− mice. Unlike for non-pDCs, TNFR deficiency did not significantly (P < 0.05) alter the LCMV-induced increase in the frequencies of pDCs in the spleen. However, especially on day 3 p.i., a larger proportion of pDCs in DKO mice than in wt mice tended to express lower levels of B220. We also examined the effect of TNFR deficiency on the relative proportions of CD11b+ CD11clow and CD11b+ CD11c− macrophages in the spleens on day 3 p.i. At this time point, the percentages of CD11b+ CD11clow (3.9% ± 0.8%) and CD11b+ CD11c− (3.7% ± 0.3%) macrophages in the spleens of DKO mice were two- to threefold higher than those in the spleens of wt mice; the observed differences in the percentages of macrophages between wt and DKO mice reflect an LCMV infection-induced increase in the percentages of macrophages in DKO mice and not a depletion of these cell types in wt mice. Unlike for macrophages and non-pDCs, TNFR deficiency did not affect the relative proportions of mature B cells in the spleen; the percentages of B cells in the spleens of wt and DKO mice were 59% ± 1.6% and 52.5% ± 1.4%, respectively.
FIG. 4.
Effect of TNFR deficiency on dendritic cell subpopulations in the spleens of LCMV-infected mice. Groups of wt (+/+), p55−/−, p75−/−, and DKO mice were infected with LCMV or were left uninfected (controls). Single-cell suspensions of splenocytes from uninfected and LCMV-infected mice (day 3 p.i.) were stained with anti-CD11c and anti-B220 antibodies. The numbers of CD11chi B220− cells (non-pDCs) and CD11clo B220+ cells (pDCs) were quantitated by flow cytometry. The values are the percentages of the subsets of dendritic cells of total splenocytes. The data are derived from three to four mice/group and are representative of two independent experiments.
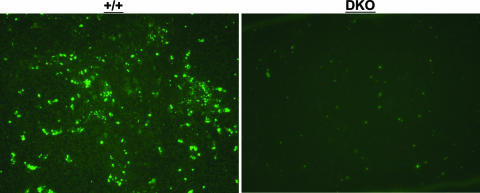
Previous work has shown that LCMV infection induces apoptosis of splenocytes during the first 3 days after infection, and, importantly, a substantial fraction of these apoptotic cells appear to be CD11c+ dendritic cells (36). Furthermore, it was recently reported that T-cell-derived TNF induced apoptosis of dendritic cells in vitro (6). To determine the effect of TNFR deficiency on the apoptosis of splenocytes in LCMV-infected mice, we performed TUNEL staining on spleen sections from wt and DKO mice on day 3 p.i. Very few TUNEL+ cells were detected in the spleens of uninfected wt and DKO mice (data not shown). Figure 5 shows staining for TUNEL+ cells in the spleens of LCMV-infected wt and DKO mice on day 3 p.i. The micrographs in Fig. 5 illustrate that the number of TUNEL+ cells in the spleens of DKO mice were considerably lower than those in the spleens of wt mice. We quantitated TUNEL+ apoptotic cells by counting five random fields in the spleen sections from day 3-infected wt and DKO mice. The numbers of TUNEL+ cells in the DKO mice (130 ± 28) were substantially less than those in the wt mice (443 ± 115). Next, by costaining for CD11c, we examined whether dendritic cells were among the TUNEL+ cells in the spleen. Approximately 37% of the TUNEL+ cells were CD11c+ in the spleens of wt mice. In the DKO mice, not only were the numbers of TUNEL+ cells fewer than the numbers in wt mice (Fig. 5), only ∼6% of these cells were CD11c+. These data suggest that TNF/TNFR interactions might be linked causally to the occurrence of cellular apoptosis in the spleen during the early phase of an acute LCMV infection. In summary, based on the data presented in Fig. 4 and 5, we theorize that TNFRs might down regulate antigen-driven expansion of CD4 T cells by limiting the life span of dendritic cells (25).
FIG. 5.
Apoptosis of splenocytes in LCMV-infected wt (+/+) and TNFR-deficient mice. wt and DKO mice were infected with LCMV, and on the third day after infection, spleens were harvested and fixed in neutral buffered formalin. Formalin-fixed sections were stained for TUNEL+ apoptotic cells (green fluorescence) as described in Materials and Methods. The data are representative of three to four mice/group.
Increased proliferation of transgenic CD4 T cells in LCMV-infected TNFR-deficient mice.
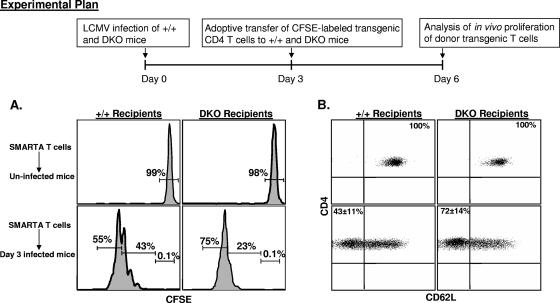
The data presented in Fig. 4 and 5 suggested that TNFR deficiency resulted in increased survival of the non-pDCs in LCMV-infected mice. It is well established that CD11c+ dendritic cells are primarily but not exclusively responsible for presenting antigen to naïve T cells in viral infections (16). Here we examined whether higher percentages of non-pDCs in DKO mice on day 3 p.i. were associated with enhanced antigen-induced proliferation of LCMV-specific CD4 T cells. We adoptively transferred 5 × 105 CFSE-labeled SMARTA Tg T cells into groups of wt and DKO mice on day 3 p.i. As controls, we transferred CFSE-labeled SMARTA Tg T cells into uninfected wt and DKO mice. The adoptively transferred SMARTA Tg CD4 T cells were distinguished from the host CD4 T cells based on Thy1.1 expression; while the host T cells were Thy1.2+, the donor T cells were Thy1.1+. Proliferation of Thy1.1+ donor transgenic cells was assessed 72 h after T-cell transfer by quantitating CFSE fluorescence by flow cytometry. As shown in Fig. 6A, donor CD4 T cells remained CFSEhi and did not proliferate upon transfer into uninfected wt or DKO mice. Data in Fig. 6A also show that SMARTA TCR Tg CD4 T cells proliferated in both LCMV-infected wt and DKO mice. However, SMARTA Tg cells proliferated more in DKO mice than in wt mice; in the DKO mice, 76% ± 2% of SMARTA CD4 T cells underwent >5 divisions, compared to 58% ± 5% in wt mice. Consequently, a larger percentage (40% ± 5%) of SMARTA CD4 T cells underwent <5 divisions in wt mice than in DKO mice (21% ± 2%). These data show that enhanced cellular proliferation was at least in part responsible for increasing the number of LCMV-specific CD4 T cells during the primary response to LCMV.
FIG. 6.
Increased proliferation of TCR Tg CD4 T cells in LCMV-infected, TNFR-deficient mice. Groups of Thy1.2/wt and Thy1.2/DKO mice were infected with LCMV. On the third day after infection, Thy1.1+ CFSE-labeled SMARTA TCR Tg CD4 T cells were transferred into these mice. Seventy-two hours after cell transfer, CFSE fluorescence of the donor transgenic T cells was analyzed by flow cytometry. (A) Splenocytes from uninfected and LCMV-infected wt (+/+) and DKO recipients were stained with anti-CD4, anti-Thy1.1, and anti-Thy1.2 antibodies, and CFSE fluorescence of the transferred SMARTA TCR Tg T cells was quantitated by flow cytometry. (B) Splenocytes from uninfected and infected wt and DKO recipients were stained with anti-CD4, anti-Thy1.1, anti-Thy1.2, and anti-CD62L antibodies. The data in panels A and B are gated on Thy1.1+ CD4 T cells. The data are the averages from three mice/group and are representative of two independent experiments.
Next, we examined the activation-induced down regulation of CD62L on the donor transgenic CD4 T cells transferred into wt and DKO recipients. As expected, donor Thy1.1+ cells transferred into uninfected wt and DKO recipients remained CD62Lhi. Upon transfer into infected wt mice, 43% ± 11% of the donor TCR Tg cells lost CD62L expression, compared to 72% ± 14% in DKO mice (Fig. 6B). Collectively, data in Fig. 6A and B suggest that antigen-driven proliferation and differentiation of naïve CD4 T cells into effectors are enhanced in a TNFR-deficient environment.
Indirect regulation of CD4 T cells by TNFRs during an acute LCMV infection.
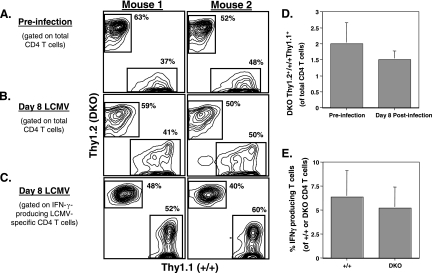
Data presented in Fig. 3, 4, and 6 indicate that TNFRs could down regulate expansion of CD4 T cells by indirect effects on non-CD4 T cells. To study whether TNFRs regulate CD4 T-cell responses by direct effects on T cells, we generated mixed bone marrow chimeras by reconstituting lethally irradiated Rag1−/− mice with bone marrow cells from wt/Thy1.1 and DKO/Thy1.2 mice at a 1:1 ratio. Six weeks after bone marrow transfer, we examined the reconstitution of the lymphoid system in Rag1−/− recipients. The wt and DKO T cells in the peripheral blood were distinguished based on expression of the congenic markers Thy1.1 and Thy1.2, respectively. The dot plots in Fig. 7A show the relative proportions of wt/Thy1.1 and DKO/Thy1.2 CD4 T cells in the peripheral blood samples from two representative chimeric mice out of eight examined. These chimeric mice were infected with LCMV, and the CD4 T-cell responses were examined 8 days later. As shown in Fig. 7B and D, on day 8 p.i., the relative proportions of wt/Thy1.1 and DKO/Thy1.2 CD4 T cells mirrored preinfection ratios. Additionally, the relative proportions of wt Thy1.1+ and DKO Thy1.2+ LCMV-specific CD4 T cells (Fig. 7C) did not differ substantially from preinfection values for CD4 T cells. It is possible that there could be differences in precursor frequencies of LCMV-specific CD4 T cells in the wt and DKO T-cell repertoires. We addressed this issue by quantitating the percentages of LCMV-specific CD4 T cells among wt/Thy1.1 and DKO/Thy1.2 CD4 T cells. The data shown in Fig. 7E clearly indicate that the percentages of LCMV-specific CD4 T cells among wt and DKO CD4 T cells were comparable. These data suggest that TNFR-deficient CD4 T cells might not have a selective advantage over wt T cells during the expansion phase of LCMV. Thus, TNF does not appear to regulate CD4 T-cell responses by direct effects on T cells during an acute LCMV infection.
FIG. 7.
Indirect regulation of CD4 T-cell responses by TNFRs. Mixed bone marrow chimeric mice were generated by reconstituting irradiated Rag1−/− mice with a 1:1 mixture of bone marrow cells from wt/Thy1.1 and DKO/Thy1.2 mice. (A) Six weeks after bone marrow transfer, peripheral blood mononuclear cells were stained with anti-CD4, anti-Thy1.1, and anti-Thy1.2 antibodies. (B and C) Approximately 7 weeks after bone marrow transfer, these bone marrow chimeric mice were infected with LCMV. On day 8 p.i., CD4 T-cell responses were quantitated; splenocytes were stained with anti-CD4, anti-Thy1.1, and anti-Thy1.2 antibodies. (C) GP61-specific CD4 T cells in the spleens were quantitated by intracellular cytokine staining as described in Materials and Methods. The dot plots in panels A and B are gated on total CD4 T cells, and the values are the percentages of Thy1.1+ (wt [+/+]) and Thy1.2+ (DKO) cells of total CD4 T cells. The dot plots in panel C are gated on IFN-γ-producing CD4 T cells, and the values are the percentages of Thy1.1+ and Thy1.2+ cells among IFN-γ-producing CD4 T cells. (D) Six weeks after cell transfer and on day 8 p.i., peripheral blood mononuclear cells were stained with anti-CD4, anti-Thy1.1, and anti-Thy1.2 antibodies and the ratios of DKO Thy1.2+ cells to wt Thy1.1+ cells among CD4 T cells were calculated from eight bone marrow chimeras. (E) Eight days p.i., peripheral blood mononuclear cells were stimulated with GP61 peptide for 5 h and stained for surface CD4, Thy1.1, Thy1.2, and intracellular IFN-γ. The percentages of LCMV-specific, IFN-γ-producing cells among wt Thy1.1+ and DKO Thy1.2+ cells were quantitated. The data in panels A, B, and C are representative of studies conducted using eight individual bone marrow chimeric mice. The data in panels D and E are the averages ± standard deviations from eight bone marrow chimeric mice.
Memory CD4 T-cell homeostasis in LCMV-infected TNFR-deficient mice.
To investigate the effect of TNFR deficiency on overall CD4 T-cell homeostasis, groups of wt, p55−/−, p75−/−, and DKO mice were infected with LCMV. Between 55 and 60 days after infection, we assessed the relative proportions of naïve and activated/memory cells in the CD4 T-cell compartment by using the well-studied cell surface markers CD44 and CD62L. The dot plots in Fig. 8A show that the relative proportion of activated/memory (CD44hi) cells among CD4 T cells is increased in DKO mice compared to the level in wt mice. The ratios of naïve (CD44lo) to activated/memory (CD44hi) CD4 T cells in the spleens of wt, p55−/−, p75−/−, and DKO mice were 3:1, 2:1, 3:1, and 1:1, respectively (Fig. 8A); 23% and 47% of the CD4 T cells were of the activated/memory phenotype in wt and DKO mice, respectively. Interestingly, phenotypic analysis using CD62L also indicated that the percentage of activated (CD62Llo) cells among all CD4 T cells in the DKO mice was higher than that in wt mice (Fig. 8B). Memory T cells can be classified into central memory and effector memory subpopulations based on expression of CD62L; while central memory T cells are CD62Lhi, effector memory T cells are CD62Llo (52). We examined the proportions of central and effector memory cells among memory phenotype (CD44hi) CD4 T cells in LCMV-infected wt and TNFR-deficient mice. Note that the dot plots in Fig. 8C are gated on CD44hi CD4 T cells. The data shown in Fig. 8C indicate that the majority of the CD44hi CD4 T cells were of the CD62Llo phenotype in all four groups of mice and that the percentages of effector memory CD4 T cells in the memory T-cell pool were slightly higher in the p55−/− and DKO mice. The alterations in the ratio of naïve (CD44lo) to activated/memory (CD44hi) CD4 T cells in the DKO mice not only were evident in the early phases of memory (∼day 60 p.i.) (Fig. 8A) but were sustained for at least up to 150 days p.i. (Fig. 8D). The total numbers of naïve CD4 T cells in uninfected DKO mice were comparable to those in wt mice at 8 to 10 weeks of age but dropped considerably by ∼8 months of age (data not shown). Taken together, these data suggest that TNFRs p55R and p75R might help maintain CD4 T-cell homeostasis in uninfected and LCMV-immune mice by regulating the sizes of the naïve and memory T-cell pools within the CD4 T-cell compartment.
FIG. 8.
Effect of TNFR deficiency on naïve and memory CD4 T-cell homeostasis in LCMV-immune mice. (A, B, and C) Between 55 and 60 days after LCMV infection, splenocytes from wt (+/+), p55−/−, p75−/−, and DKO mice were stained with anti-CD4, anti-CD44, and anti-CD62L antibodies and analyzed by flow cytometry. The dot plots in panels A and B are gated on total splenocytes, and the values are the percentages of cells among splenocytes; the values in parentheses are the percentages of CD44hi (A) or CD62lo (B) cells among CD4 T cells. The dot plots in panel C are gated on CD4+ CD44hi cells, and the values are the percentages of CD62Llo and CD62Lhi cells of CD4+ CD44hi cells. The data in panel D show the relative proportions of naïve (CD44lo) and activated/memory (CD44hi) cells of CD4 T cells in the spleens of wt and DKO mice on day 150 p.i.
Enhanced LCMV-specific CD4 T-cell memory in TNFR-deficient mice.
Recovery from an acute LCMV infection is associated with the development of potent T-cell memory (37, 53, 58). Here, we examined the effect of TNFR deficiency on the number of virus-specific memory CD4 T cells in LCMV-immune mice. Between days 200 and 250 p.i., the numbers of GP61-specific memory CD4 T cells in the spleens of wt, p55−/−, p75−/−, and DKO mice were quantified using intracellular cytokine staining for IFN-γ (Fig. 9). The data shown in Fig. 9A indicate that GP61-specific memory CD4 T cells were detected readily in all groups of mice. The absence of p55R was associated with a modest increase (twofold) in the frequency and total number of GP61-specific memory CD4 T cells in the spleens compared to levels in the spleens of wt mice. Deficiency of p75R alone had no detectable effect on CD4 T-cell memory to LCMV. Notably, there was a striking increase in the total number of GP61-specific memory CD4 T cells in DKO mice compared to the number in wt mice; the number of GP61-specific memory CD4 T cells in the spleens of DKO mice was up to sixfold higher (P < 0.0001) than the number in the spleens of wt mice (Fig. 9B). Thus, TNFRs have a role in limiting the number of memory CD4 T cells during an acute viral infection.
FIG. 9.
Enhancement of LCMV-specific memory CD4 T cells in TNFR-deficient mice. Groups of wt (+/+), p55−/−, p75−/−, and DKO mice were infected with LCMV. Between days 200 and 250 p.i., the numbers of GP61-specific memory CD4 T cells in the spleens were quantitated by intracellular staining for IFN-γ. The dot plots in panel A are gated on total splenocytes, and the values represent the percentages of IFN-γ-producing CD4 T cells of splenocytes. The values in parentheses are the percentages of IFN-γ-producing cells of CD4 T cells. Panel B shows the total number of GP61-specific, IFN-γ-producing memory CD4 T cells, and each data point represents an individual mouse from one of three experiments.
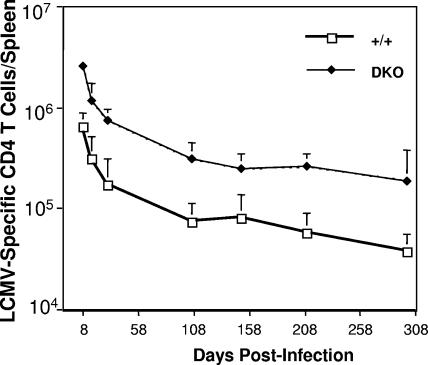
Typically, T-cell responses can be divided into three phases: (i) expansion phase, when naïve T cells are activated by an antigen to undergo clonal expansion and differentiation into effector T cells; (ii) contraction phase, when up to 90% of the expanded T cells are lost, presumably by apoptosis; and (iii) memory phase, when a finite number of memory T cells are maintained by cytokine-driven proliferative renewal (48). The number of memory T cells generated at the termination of an immune response is a function of the extent of primary clonal expansion and the magnitude of the ensuing contraction phase. Therefore, alterations in the expansion and/or the contraction phase can affect the magnitude of T-cell memory. To better understand how TNFRs regulate CD4 T-cell memory, we examined in detail the kinetics of the CD4 T-cell responses in wt and DKO mice following an acute LCMV infection (Fig. 10). Consistent with the data shown in Fig. 1, the expansion of GP61-specific CD4 T cells (day 8 p.i.) was higher in DKO mice than in wt mice. Between days 8 and 30 p.i., GP61-specific CD4 T cells in wt and DKO mice showed similar degrees of contraction; approximately 75% of GP61-specific CD4 T cells were lost by contraction in both wt and DKO mice. Between day 30 p.i. and day 106 p.i., there was an additional ∼60% drop in the numbers of GP61-specific CD4 T cells in both wt and DKO mice. Taken together, these data indicate that TNFRs have a minimal role in regulating the contraction phase of the anti-LCMV CD4 T-cell response. Following expansion, the rates of decrease in the GP61-specific CD4 T cell numbers were the same for DKO mice and wt mice (Fig. 10). Thus, the significant increase in total GP61-specific CD4 T cells in DKO mice was likely a direct result of the greater clonal burst size (primary expansion) in the absence of TNFR signaling. The increase in the number of GP61-specific memory CD4 T cells in DKO mice was not associated with detectable alterations in the expression of cell surface molecules (CD44, CD11a/CD18, and CD127) or cytokine (IFN-γ, TNF-α, and IL-2) production ex vivo (data not shown). In conclusion, TNFR deficiency enhanced the “quantity” of CD4 T-cell memory without adversely affecting the “quality” of memory CD4 T cells.
FIG. 10.
Kinetics of CD4 T-cell response to LCMV in TNFR-deficient mice. Groups of wt (+/+) and DKO mice were infected with LCMV, and the numbers of GP61-specific CD4 T cells in the spleens were quantitated at different days p.i. by intracellular cytokine staining for IFN-γ. The data are the mean values ± standard deviations for at least three mice/group at each time point.
DISCUSSION
Elicitation and maintenance of potent CD4 T-cell responses are of critical importance for defense and development of durable immunity against viral infections. However, the immune regulatory mechanisms that govern CD4 T-cell responses are not well understood. In this study, we have documented for the first time that TNF/TNFR interactions play a negative role in regulating virus-specific CD4 T-cell responses during an acute viral infection. Furthermore, we show that the negative regulatory effects of TNFRs on CD4 T cells are mediated at least in part by indirect effects, possibly via dendritic cells. Importantly, mice deficient in both TNFR I and TNFR II develop substantially greater numbers of memory CD4 T cells without affecting their cell surface phenotype or function. These findings should have clear implications in improving vaccines and treatment of T-cell-dependent immunopathologies.
Previous work that studied the role of TNF/TNFR interactions in regulating T-cell proliferation has provided equivocal results. While one study reported that TNFR I is important for optimal proliferation of alloreactive T cells, another study showed that TNF promotes alloantigen-induced proliferation of CD4 T cells (7, 24). It has also been suggested that TNFR II signaling provides a costimulatory signal during T-cell activation (27, 28). In contrast to these studies, TNF exposure seems to reduce proliferation of T cells in vitro due to the inhibition of TCR-induced calcium influx (12). Studies with mycobacterium infection and experimental autoimmune encephalomyelitis have shown that TNF has a negative role in regulating T-cell responses in vivo (26, 60). Here, we show that TNFRs I and II have redundant roles in restricting the primary expansion of virus-specific CD4 T cells during an acute LCMV infection. During LCMV infection, the antiproliferative effect of TNF is not restricted to CD4 T cells because primary CD8 T-cell responses are also enhanced in LCMV-infected, TNFR-deficient mice (49). Thus, depending on the experimental model and the context in which T cells are activated, TNFR signaling might have opposing effects on T-cell proliferation. It is noteworthy that TNF is not required to clear an acute LCMV infection and that the kinetics of LCMV resolution in TNFR-deficient mice is similar to that in wt mice. Therefore, the increased expansion of GP61-specific CD4 T cells in DKO mice is not likely due to a higher viral load.
How do TNFRs suppress expansion of activated CD4 T cells during an acute viral infection? Regulation of CD4 T-cell responses by TNFRs could occur by direct and/or indirect effects. In LCMV-infected mixed bone marrow chimeras, the activation and expansion of TNFR-deficient CD4 T cells were comparable to levels for wt CD4 T cells. Thus, at least in the LCMV model, we found no evidence for direct regulation of CD4 T-cell expansion by TNF. In contrast, TNFR-expressing monoclonal transgenic CD4 T cells expanded more in TNFR-deficient mice than in wt mice, which indicated that TNFR signaling could down regulate CD4 T-cell responses by indirect effects. The magnitude of the T-cell response is dependent upon the duration of antigenic stimulation, which in turn is governed by the life span of dendritic cells (25). In an acute LCMV infection, cross-priming by CD11chi dendritic cells is crucial for stimulating CD8 T-cell responses (16) and, quite possibly, CD4 T-cell responses. During an acute LCMV infection, these CD11chi dendritic cells are rapidly depleted by type I interferons, thereby possibly limiting the duration of T-cell activation (36). Notably, in our studies, TNFR deficiency reduced the apoptosis of splenocytes and protected against interferon-dependent depletion of CD11chi dendritic cells in the spleen. Additionally, antigen-driven expansion of adoptively transferred SMARTA T cells was enhanced in TNFR-deficient mice. Our in vivo results are consistent with a recent report that T cell-derived TNF might down regulate dendritic cell viability in vitro (6). In summary, our results favor the hypothesis that TNFR signaling might regulate the expansion of CD4 T cells by controlling the life span of dendritic cells.
In the context of antiviral immunity, we find that TNFR deficiency results in a marked increase in the number of memory CD4 T cells following an acute LCMV infection. What is the underlying mechanism(s) of enhanced CD4 T-cell memory in DKO mice? The two critical determinants of T-cell memory are (i) the size of the primary response, termed clonal burst size, and (ii) the magnitude of the loss of effector cells during contraction. Our data clearly show that TNFR deficiency increased the clonal burst size of GP61-specific CD4 T cells, with minimal effects on the magnitude of contraction. Additionally, the increase (four- to fivefold) in primary expansion correlates well with the increase in the number of GP61-specific memory CD4 T cells in DKO mice. Although these findings suggest that the primary determinant of enhanced CD4 T-cell memory in DKO mice is the larger clonal burst size, an additional role for TNF-induced apoptosis cannot be excluded (50, 55, 61). A comprehensive analysis of the rates of proliferation and apoptosis of antigen-specific CD4 T cells in vivo throughout the T-cell response will clarify the mechanism(s).
What are the implications of these findings? In chronic viral infections with hepatitis B virus and hepatitis C virus, viral persistence and disease are associated with poor T-cell responses (30, 31, 33). Interestingly, the hepatic and/or circulating levels of TNF are high in people with active hepatitis B virus- and hepatitis C virus-induced disease (20, 32, 38). It is possible that high levels of TNF in these patients might suppress the antiviral T-cell response; therefore, anti-TNF therapies might be beneficial to counteract this suppression. Since the inability to induce a potent T-cell memory might lead to vaccination failures, modulation of TNF activity might improve vaccine-induced T-cell memory and protective immunity. Anti-TNF therapy is commonly used to treat autoimmune disorders, like rheumatoid arthritis (21). Along with published reports supporting a protective role for TNF in autoimmune disorders (14), the data presented in this paper urge that caution be exercised before instituting therapies to block the effects of TNF in diseases like rheumatoid arthritis.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AI48785) and the National Multiple Sclerosis Society (RG3092A1/T) to M. Suresh.
We thank Erin Hemmila, Yumi Nakayama, Katie Skell, Kavita Tewari, and Jane Walent for help with the experiments.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, R., L. D. Butler, and L. Bhatti. 1988. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J. Virol. 62:2102-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso, K., P. Pontiggia, R. Medenica, and S. Rizzo. 1997. Cytokine patterns in adults with AIDS. Immunol. Investig. 26:341-350. [DOI] [PubMed] [Google Scholar]
- 4.Bevan, M. J. 2004. Helping the CD8 (+) T-cell response. Nat. Rev. Immunol. 4:595-602. [DOI] [PubMed] [Google Scholar]
- 5.Biron, C. A. 1994. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 6:530-538. [DOI] [PubMed] [Google Scholar]
- 6.Brehm, M. A., K. A. Daniels, and R. M. Welsh. 2005. Rapid production of TNF-alpha following TCR engagement of naïve CD8 T cells. J. Immunol. 175:5043-5049. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G. R., E. L. Lee, and D. L. Thiele. 2003. TNF enhances CD4+ T cell alloproliferation, IFN-gamma responses, and intestinal graft-versus-host disease by IL-12-independent mechanisms. J. Immunol. 170:5082-5088. [DOI] [PubMed] [Google Scholar]
- 8.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisari, F. V. 1996. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr. Top. Microbiol. Immunol. 206:149-173. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, J. P., O. Marker, and A. R. Thomsen. 1994. The role of CD4+ T cells in cell-mediated immunity to LCMV: studies in MHC class I and class II deficient mice. Scand. J. Immunol. 40:373-382. [DOI] [PubMed] [Google Scholar]
- 11.Chu, C. Q., S. Wittmer, and D. K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church, L. D., J. E. Goodall, D. A. Rider, P. A. Bacon, and S. P. Young. 2005. Persistent TNF-alpha exposure impairs store operated calcium influx in CD4+ T lymphocytes. FEBS Lett. 579:1539-1544. [DOI] [PubMed] [Google Scholar]
- 13.Cohen, J. 1993. T cell shift: key to AIDS therapy? Science 262:175-176. [DOI] [PubMed] [Google Scholar]
- 14.Cope, A. P. 1998. Regulation of autoimmunity by proinflammatory cytokines. Curr. Opin. Immunol. 10:669-676. [DOI] [PubMed] [Google Scholar]
- 15.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.den Haan, J. M., S. M. Lehar, and M. J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty, P. C. 1993. Virus infections in mice with targeted gene disruptions. Curr. Opin. Immunol. 5:479-483. [DOI] [PubMed] [Google Scholar]
- 18.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 19.Doherty, P. C., R. A. Tripp, A. M. Hamilton-Easton, R. D. Cardin, D. L. Woodland, and M. A. Blackman. 1997. Tuning into immunological dissonance: an experimental model for infectious mononucleosis. Curr. Opin. Immunol. 9:477-483. [DOI] [PubMed] [Google Scholar]
- 20.Fang, J. W., W. W. Shen, A. Meager, and J. Y. Lau. 1996. Activation of the tumor necrosis factor-alpha system in the liver in chronic hepatitis B virus infection. Am. J. Gastroenterol. 91:748-753. [PubMed] [Google Scholar]
- 21.Feldmann, M., and R. N. Maini. 2001. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 19:163-196. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura, T., R. Yamanashi, M. Masuzawa, Y. Fujita, K. Katsuoka, S. Nishiyama, M. Mitsuyama, and K. Nomoto. 1997. Conversion of the CD4+ T cell profile from T(H2)-dominant type to T(H1)-dominant type after varicella-zoster virus infection in atopic dermatitis. J. Allergy Clin. Immunol. 100:274-282. [DOI] [PubMed] [Google Scholar]
- 23.Harty, J. T., and V. P. Badovinac. 2002. Influence of effector molecules on the CD8(+) T cell response to infection. Curr. Opin. Immunol. 14:360-365. [DOI] [PubMed] [Google Scholar]
- 24.Hill, G. R., T. Teshima, V. I. Rebel, O. I. Krijanovski, K. R. Cooke, Y. S. Brinson, and J. L. Ferrara. 2000. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J. Immunol. 164:656-663. [DOI] [PubMed] [Google Scholar]
- 25.Hou, W. S., and L. Van Parijs. 2004. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5:583-589. [DOI] [PubMed] [Google Scholar]
- 26.Kassiotis, G., and G. Kollias. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, E. Y., and H. S. Teh. 2001. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J. Immunol. 167:6812-6820. [DOI] [PubMed] [Google Scholar]
- 28.Kim, E. Y., J. J. Priatel, S. J. Teh, and H. S. Teh. 2006. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J. Immunol. 176:1026-1035. [DOI] [PubMed] [Google Scholar]
- 29.Koszinowski, U. H., M. J. Reddehase, and S. Jonjic. 1991. The role of CD4 and CD8 T cells in viral infections. Curr. Opin. Immunol. 3:471-475. [DOI] [PubMed] [Google Scholar]
- 30.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 32.Lio, D., C. Caruso, R. Di Stefano, G. Colonna Romano, D. Ferraro, L. Scola, A. Crivello, A. Licata, L. M. Valenza, G. Candore, A. Craxi, and P. L. Almasio. 2003. IL-10 and TNF-alpha polymorphisms and the recovery from HCV infection. Hum. Immunol. 64:674-680. [DOI] [PubMed] [Google Scholar]
- 33.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montoya, M., M. J. Edwards, D. M. Reid, and P. Borrow. 2005. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type IFN. J. Immunol. 174:1851-1861. [DOI] [PubMed] [Google Scholar]
- 37.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, D. R., H. L. Lim, C. G. Marousis, J. W. Fang, G. L. Davis, L. Shen, M. S. Urdea, J. A. Kolberg, and J. Y. Lau. 1997. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig. Dis. Sci. 42:2487-2494. [DOI] [PubMed] [Google Scholar]
- 39.Nonacs, R., C. Humborg, J. P. Tam, and R. M. Steinman. 1992. Mechanisms of mouse spleen dendritic cell function in the generation of influenza-specific, cytolytic T lymphocytes. J. Exp. Med. 176:519-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9:449-457. [DOI] [PubMed] [Google Scholar]
- 41.Oxenius, A., M. F. Bachmann, R. M. Zinkernagel, and H. Hengartner. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390-400. [DOI] [PubMed] [Google Scholar]
- 42.Palucka, A. K., J. P. Blanck, L. Bennett, V. Pascual, and J. Banchereau. 2005. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc. Natl. Acad. Sci. USA 102:3372-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 44.Peterson, J. D., C. Waltenbaugh, and S. D. Miller. 1992. IgG subclass responses to Theiler's murine encephalomyelitis virus infection and immunization suggest a dominant role for Th1 cells in susceptible mouse strains. Immunology 75:652-658. [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 KD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 47.Saha, K., and P. K. Wong. 1992. Protective role of cytotoxic lymphocytes against murine leukemia virus-induced neurologic disease and immunodeficiency is enhanced by the presence of helper T cells. Virology 188:921-925. [DOI] [PubMed] [Google Scholar]
- 48.Seder, R. A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835-842. [DOI] [PubMed] [Google Scholar]
- 49.Suresh, M., A. Singh, and C. Fischer. 2005. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J. Virol. 79:202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sytwu, H. K., R. S. Liblau, and H. O. McDevitt. 1996. The roles of Fas/APO-1 (CD95) and TNF in antigen induced programmed cell death in T cell receptor transgenic mice. Immunity 5:17-30. [DOI] [PubMed] [Google Scholar]
- 51.Thomsen, A. R., J. Johansen, O. Marker, and J. P. Christensen. 1996. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J. Immunol. 157:3074-3080. [PubMed] [Google Scholar]
- 52.Unsoeld, H., S. Krautwald, D. Voehringer, U. Kunzendorf, and H. Pircher. 2002. CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 169:638-641. [DOI] [PubMed] [Google Scholar]
- 53.Varga, S. M., and R. M. Welsh. 1998. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J. Immunol. 161:367-374. [PubMed] [Google Scholar]
- 54.von Herrath, M. G., M. Yokoyama, J. Dockter, M. B. Oldstone, and J. L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, J., S. A. Stohlman, and G. Dennert. 1994. TCR cross-linking induces CTL death via internal action of TNF. J. Immunol. 152:3824-3832. [PubMed] [Google Scholar]
- 56.Ware, C. F. 2005. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 23:787-819. [DOI] [PubMed] [Google Scholar]
- 57.Wasik, T. J., P. P. Jagodzinski, E. M. Hyjek, J. Wustner, G. Trinchieri, H. W. Lischner, and D. Kozbor. 1997. Diminished HIV-specific CTL activity is associated with lower type 1 and enhanced type 2 responses to HIV-specific peptides during perinatal HIV infection. J. Immunol. 158:6029-6036. [PubMed] [Google Scholar]
- 58.Whitmire, J. K., M. S. Asano, K. Murali-Krishna, M. Suresh, and R. Ahmed. 1998. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72:8281-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zganiacz, A., M. Santosuosso, J. Wang, T. Yang, L. Chen, M. Anzulovic, S. Alexander, B. Gicquel, Y. Wan, J. Bramson, M. Inman, and Z. Xing. 2004. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J. Clin. Investig. 113:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng, L., G. Fisher, R. E. Miller, J. Peschon, D. H. Lynch, and M. J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature 377:348-351. [DOI] [PubMed] [Google Scholar]
- 62.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]