Abstract
Rotaviruses have been implicated as a possible viral trigger for exacerbations in islet autoimmunity, suggesting they might modulate type 1 diabetes development. In this study, the ability of rotavirus strain RRV to infect the pancreas and affect insulitis and diabetes was examined in nonobese diabetic (NOD) mice, an experimental model of type 1 diabetes. Mice were inoculated either orally or intraperitoneally as infants or young adults. In infant mice inoculated orally, rotavirus antigen was detected in pancreatic macrophages outside islets and infectious virus was found in blood cells, pancreas, spleen, and liver. Extraintestinal RRV spread and pancreatic presence of infectious virus also occurred in intraperitoneally inoculated infant and adult mice. The initiation of insulitis was unaltered by infection. The onset of diabetes was delayed in infant mice inoculated orally and infant and adult mice inoculated intraperitoneally. In contrast, adult mice inoculated orally showed no evidence of pancreatic RRV, the lowest rate of detectable RRV replication, and no diabetes modulation. Thus, the ability of RRV infection to modulate diabetes development in infant and young adult NOD mice was related to the overall extent of detectable virus replication and the presence of infectious virus extraintestinally, including in the pancreas. These studies show that RRV infection of infant and young adult NOD mice provides significant protection against diabetes. As these findings do not support the hypothesis that rotavirus triggers autoimmunity related to type 1 diabetes, further research is needed to resolve this issue.
Type 1 diabetes mellitus is an autoimmune disease resulting from the destruction of pancreatic β cells by autoreactive T lymphocytes. The disease is characterized by a long preclinical phase marked by the accumulation of islet-reactive T cells within the pancreas and production of systemic autoantibodies to a number of well-defined β-cell antigens (2). Disease susceptibility is partly determined by genetics, with the major genes predisposing humans to disease located in the HLA region (43, 76). Environmental factors including climate, dietary factors, and exposure to pathogens (particularly viruses) also have been implicated in disease pathogenesis. Viruses proposed to be associated with type 1 diabetes development in humans include rubella virus, mumps virus, herpesviruses (cytomegalovirus and Epstein-Barr virus), enteroviruses (coxsackieviruses), and rotaviruses (12, 27, 39, 79). However, attempts to establish a causal link between viral infections and diabetes have been largely unsuccessful due to the lag time between infection and onset of diabetic symptoms and increasing evidence that viruses can abrogate as well as enhance the autoimmune process (12, 27, 48, 79).
Rotaviruses, the major cause of severe acute gastroenteritis in humans and many animals worldwide, have been implicated as a possible viral trigger for progression of children to type 1 diabetes (20, 39-41). In children genetically at risk for diabetes, seroconversion in rotavirus-specific immunoglobulin A (IgA) and IgG was significantly associated with a substantial increase in autoantibodies to tyrosine phosphatase-like islet antigen (IA-2), glutamic acid decarboxylase (GAD), and/or insulin within the same 6-month period (40). Islet autoantibodies to IA-2 and GAD also were detected in acute- and/or convalescent-phase serum from 3 of 10 children with no family history of diabetes hospitalized with rotavirus gastroenteritis (40). Molecular mimicry was proposed as a possible mechanism, based on sequence similarities between the immunodominant T-cell epitope in IA-2 (amino acids [aa] 805 to 820) and rotavirus outer capsid protein VP7 (aa 41 to 49) and between GAD (aa 117 to 128) and VP7 (aa 18 to 30) (40, 41). A later study in Finnish children failed to confirm an association between rotavirus seroconversion and islet autoantibody levels (4, 56). A lack of sensitivity in seroconversion detection due to failure to measure rotavirus IgA and the use of a nonhuman G6 rotavirus strain may have contributed to the differing results. Further studies in Finnish children suggested that rotavirus infection can enhance immune responses to insulin (57). Possible associations with severe rotavirus gastroenteritis also have been reported for nonketotic hyperglycemic syndrome in one child and pancreatitis in two children, one of whom showed islet autoantibodies in acute-phase serum (22, 63, 70). Rotavirus infection and disease also may play a role in the induction of non-islet autoimmune responses, as a high frequency of rotavirus infections was associated with an increased risk of celiac disease autoimmunity in genetically predisposed children and a subset of autoantibodies in celiac patients has been reported to recognize rotavirus VP7 protein (74, 86). In rodents, the rhesus monkey rotavirus strain RRV has been associated with autoimmune responses to bile duct epithelium at the time of RRV-induced biliary atresia and linked to autoimmune uveitis (55, 83).
Rotaviruses infect differentiated enterocytes of the small intestine but also can spread extraintestinally. In humans, infectious rotavirus was detected in the liver and kidneys of children with severe combined immunodeficiency and rotavirus particles were seen in a liver abscess (28, 34, 72). Rotavirus antigen and/or RNA was detected in the serum, central nervous system, lungs, liver, kidneys, heart, spleen, testes, bladder, and pancreas of small numbers of children severely ill following rotavirus infection or at autopsy (42, 45, 46, 49, 53, 54, 58, 64, 67). The presence of serum antigenemia and/or RNA is now considered to be a typical finding during rotavirus infection in children (5, 7, 11, 25, 69). Rotavirus viremia has been detected in mice, rats, and calves (3, 6, 7, 21, 24, 68, 78). Infectious RRV and murine rotavirus were detected in the livers of infant rodents, and RRV was found in the pancreas of a neonatal rat (21, 24, 78). RRV causes hepatitis in mice (78).
The nonobese diabetic (NOD) mouse is the favored animal model for human type 1 diabetes. NOD mice show many features of the human disease, including genetic susceptibility and environmental modulation. The development of diabetes in the female NOD mouse occurs as a series of well-choreographed events. From 3 to 4 weeks of age, mononuclear infiltrates consisting of CD4+ and CD8+ T cells, macrophages, dendritic cells, and B cells begin to accumulate around the islets, in a process termed peri-islet insulitis. These infiltrates then expand in cell number and invade the islets, producing intraislet insulitis. Most mice demonstrate a noticeable degree of intraislet insulitis by 10 weeks of age (1). Several viruses, including mouse hepatitis virus, lactate dehydrogenase-elevating virus, encephalomyocarditis virus, lymphocytic choriomeningitis virus, and reovirus, prevent or delay the development of diabetes in NOD mice (37, 65, 75, 81, 82). Reoviruses and rotaviruses are both members of the Reoviridae family. Interestingly, group B coxsackievirus (CVB) infection has been shown to either delay or accelerate diabetes development in NOD mice, depending on mouse age and degree of insulitis at the time of infection (23, 73, 77).
Islets isolated from NOD mice support the replication of monkey rotavirus strains RRV and SA11 in vitro. In addition, islets isolated from pigtailed macaques facilitate replication of RRV, SA11, and two human rotavirus strains (20). This shows that rotavirus infection of pancreatic islets in vivo might be possible. Asymptomatic infection of NOD mice by RRV at 4 weeks of age has been demonstrated. Most infected mice seroconverted to RRV and excreted viral antigen in stools for up to 10 days postinfection (20). We have built on these findings to examine gastrointestinal disease, pancreatic involvement, and extraintestinal spread in NOD mice infected with RRV as infants or young adults. Mice were inoculated orally to mimic the natural route of infection and allow comparison with previous murine studies. Additionally, intraperitoneal inoculation was used to bypass any gastrointestinal barrier to extraintestinal spread, as described previously (61). Using RRV, the ability of rotavirus infection to modulate the development of pancreatic insulitis and diabetes in an animal model of human diabetes has been determined in this study for the first time.
MATERIALS AND METHODS
Viruses.
The origins, cultivation in MA104 cells, purification, and infectivity titration of monkey rotavirus RRV serotype P5B[3], G3 have been described previously (14, 16).
Mice.
NOD/Lt (NOD hereafter) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and were bred in the animal facility of the Department of Microbiology and Immunology at the University of Melbourne under specific-pathogen-free conditions. Control and inoculated mice of all ages were housed in sterilized microisolator cages in the same room and fed autoclaved standard food and water ad libitum. Microisolator cages were opened only within a class 2 biological safety cabinet, and control mice were handled and inoculated before infected mice. Prior to rotavirus inoculation, all mice were screened for rotavirus antibodies in serum by enzyme immunoassay (EIA), as previously described, with partially purified RRV as the capture antigen (15, 18). All sera collected from these mice showed negative reciprocal EIA titers of <5 × 101. All procedures were conducted in accordance with protocols approved by the Animal Ethics Committee of The University of Melbourne.
Mouse inoculation with rotavirus.
NOD mice aged 5 days (infants) were inoculated by oral gavage or oral feeding (oral) or by intraperitoneal (i.p.) injection, as indicated, with sterile Dulbecco's modified Eagle's medium (DMEM) as a control or 6 × 106 fluorescent cell-forming units (FCFU) of RRV in DMEM. This dose was 4 times that given to infant CD-1 mice to analyze extraintestinal spread (60, 61) and is equivalent to a 1 × 103 RRV 50% infective dose in infant BALB/c mice (24). NOD mice aged 4 to 6 weeks (young adults) were inoculated orally or i.p. with 2 × 107 FCFU of RRV in DMEM or with DMEM alone as a control. This dose was considered high in previous studies in adult BALB/c mice (59). A dose of 8 × 106 FCFU infected at least 90% of adult NOD mice in a previous study (20). Doses were given in volumes of 50 μl (infants), 100 μl (adults, i.p.), and 200 μl (adults, oral). At 20 min prior to RRV or mock inoculation by oral gavage, mice underwent anesthesia and stomach acid neutralization with 100 μl (adults) or 50 μl (infants) of 10% (wt/vol) NaHCO3 by oral gavage. The presence of diarrhea was recorded for 8 days after inoculation. Diarrhea was defined as the production of very fluid bright yellow stool following very gentle abdominal palpation of the mouse, combined with fecal material on the skin and around the anus.
Sample collection and processing.
Samples were always taken from mock-infected mice before RRV-infected mice. Mouse pups that were less than 10 days of age were decapitated, and blood was collected by immediate drainage of the decapitation site. Older mice were euthanized by CO2/O2 inhalation. The mouse body was rinsed in 80% (vol/vol) ethanol. Blood was collected from older mice by cardiac puncture. Tissues from all mice were collected as required in the following order: pancreas, spleen, liver, and small intestine. The small intestine was collected from mouse pups (aged 6 to 18 days) only by cutting 0.5 cm distal of the stomach and 1.0 cm above the join to the cecum. Organs were frozen at −70°C, processed as described below, and again frozen at −70°C prior to analysis. Organ extracts were prepared as 20% (wt/vol) homogenates by gently grinding the thawed organs between the frosted ends of glass microscope slides in 50 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 5 mM CaCl2 (TSC). This method was shown to give similar results to tissue homogenization, was less labor-intensive, and reduced cross-contamination between samples. At a maximum of 1 h after collection, serum was separated from whole blood by centrifugation at 7,000 × g for 6 min. Extracts of the cell pellets remaining after serum separation from several subgroups of NOD mice were prepared as 20% (wt/vol) homogenates as described above. Serum and cell pellet extracts were frozen at −70°C.
Stools were collected from adult mice by gentle palpation of the abdomen. Stools were prepared as 10% (wt/vol) homogenates in TSC, which were clarified by centrifugation at 1,000 × g for 1 min and stored at −70°C (19).
Pancreases collected from subsets of NOD mice at 1 to 29 weeks after inoculation were embedded in Optimal cutting temperature (OCT) compound (Sakura), snap-frozen in an isopentane/dry ice bath, and stored at −70°C for immunohistochemistry (IHC) or collected into Bouin's solution (Amber Scientific, Perth, Australia) for histopathological examination.
Detection of infectious rotavirus.
The presence of infectious rotavirus in mouse samples was determined by culture amplification of virus followed by assay of rotavirus antigen in cultures by capture EIA, which was a modification of the rotavirus growth assay described previously (38, 51). Dilutions in DMEM of organ and blood cell extracts (1 in 4), stool extracts (1 in 4), and sera (1 in 10 for infant mice or 1 in 4 for adult mice) were treated with 10 μg/ml porcine trypsin (Sigma) for 20 min at 37°C to activate rotavirus infectivity. Samples were incubated for 1 h at 37°C in 5% (vol/vol) CO2-95% (vol/vol) air with washed confluent monolayers of MA104 cells in 24-well plates (Nunc). The inoculum was removed and replaced with DMEM, and cells were incubated for 72 h at 37°C in 5% (vol/vol) CO2-95% (vol/vol) air. Initial experiments determined that 72 h was optimal for detection of infectious rotavirus. Virus was released from cells by two cycles of freezing and thawing. Rotavirus antigen was assayed in these harvests by capture EIA. As negative controls, organs and samples from one mock-infected mouse were included for every four rotavirus-infected mice tested. Diluted purified RRV was included as a positive control.
The titers of RRV in tissues and samples collected from mice were determined by a modification of a method described previously (14). Dilutions in DMEM of intestine suspensions and stool homogenates (1 in 20), liver suspensions (1 in 128), and pancreas suspensions (1 in 32) were treated with trypsin as described above. These dilutions were determined to be optimal, as they represented the highest sample concentration at which the cell monolayer integrity was preserved for the duration of the experiment. Confluent MA104 cell monolayers in 96-well plates were inoculated with serial dilutions of these trypsin-treated samples as described above for culture amplification. Inoculated cells were incubated for 15 h prior to acetone fixation and detection of infectious rotavirus by indirect immunofluorescent staining, as described previously (16, 38).
EIA for detection of rotavirus antigen.
The presence of rotavirus antigen in culture assay samples, organ extracts, stool homogenates, and sera was determined by antigen capture EIA as described previously, with rabbit hyperimmune antiserum to RRV as a capture antibody and monoclonal antibody RVA to VP6 as a detector antibody (19). The anti-RRV serum was raised by immunization with purified RRV as described previously (50), neutralized RRV to a reciprocal titer of 8.0 × 104, and reacted with all of the RRV structural proteins by Western blotting. Samples were tested at a dilution of 1 in 4 (culture assay samples, organ extracts, or stool samples) or 1 in 10 (sera). Samples were reacted in duplicate with capture antibody and with wells coated with the same dilution of rotavirus antibody-negative rabbit serum as a negative control. RRV rotavirus EIA antigen and antigen prepared from MA104 cell extracts as described previously (19) were included as positive and negative controls, respectively. A sample was considered positive if its mean optical density at 450 nm was ≥0.1 and at least twice that of the same sample reacted with negative control rabbit serum. All organs and samples collected from mock-infected mice were negative by antigen capture EIA carried out directly on the sample and by culture amplification followed by antigen capture EIA. The detection limit of this assay for purified RRV was determined to be 1.4 × 104 infectious virions per ml.
Assay for antibodies to rotavirus.
Sera collected from all mice were analyzed for rotavirus antibodies by EIA, as previously described (15, 18). Capture antigens were EIA antigen prepared from RRV and EIA antigen similarly prepared from mock-infected MA104 cells as a control. These have been described previously (19). Pooled sera from uninfected NOD mice and rotavirus antibody-positive NOD mice (infected with RRV) were used as negative and positive controls, respectively. Monoclonal antibody RVA diluted to give a low positive reading was also included as a positive control. The titer of antirotavirus antibodies was determined as the highest dilution of serum showing an optical density at 450 nm to RRV EIA antigen that was at least twice that of the same dilution reacted with control EIA antigen.
IHC and histology.
For IHC, serial 5-μm cryostat sections were cut, collected onto SuperFrostPlus glass slides (Menzel-Glaser, Germany), air dried, and stored at −20°C prior to fixing and staining at room temperature. Tissues were fixed in acetone for 5 min, washed in phosphate-buffered saline (PBS), and blocked with 5% (vol/vol) fetal bovine serum (JRH Biosciences, KS) in PBS (FBS-PBS) for 30 min. For detection of cells containing RRV antigen, sections were reacted with rabbit antiserum to RRV diluted 1 in 500 in FBS-PBS or similarly diluted rotavirus antibody-negative rabbit serum as a control. Bound antibody was detected with Texas red-conjugated goat anti-rabbit IgG (Invitrogen Corp.) diluted 1:200 in FBS-PBS. Insulin-producing β cells were stained by reaction with guinea pig antibody to swine insulin, which cross-reacts with mouse insulin (Dako, Glostrup, Denmark) at 1 in 200 in FBS-PBS, and fluorescein isothiocyanate-conjugated goat anti-guinea pig IgG (Jackson ImmunoResearch Laboratories, PA) diluted 1 in 100 in FBS-PBS. Macrophages were stained with rat anti-mouse F4/80 (Caltag, CA) diluted 1 in 60 in FBS-PBS followed by fluorescein isothiocyanate-conjugated swine anti-rat Ig (mouse adsorbed; Chemicon, CA) diluted 1 in 100 in FBS-PBS. In double-stained sections, primary and secondary antibodies were each mixed and reacted with sections as a cocktail. To ensure staining specificity and control for antibody cross-reactivity, sections were reacted with mismatched cocktails of primary and secondary antibodies, each primary and each secondary antibody alone, and cocktails of primary antibodies alone or secondary antibodies alone.
For histopathological analysis of islets within fixed pancreases, each pancreas was washed in 70% (vol/vol) ethanol to remove excess Bouin's solution, processed, and embedded in paraffin. Sections (5 μm) were cut 200 μm apart at four levels and stained with hematoxylin and eosin. The total number of islets per section was determined, and the degree of insulitis was scored for each islet as follows: no islet infiltrate, peri-islet infiltrate (up to 30% of islet infiltrated with accumulation of cells around the edge of the islet), or intraislet infiltrate (31 to 100% of islet infiltrated). At least 20 islets were scored for each pancreas.
Diabetes and glucose homeostasis monitoring.
In groups of NOD mice inoculated as infants or young adults, glucose levels were monitored 1 day prior to RRV or control inoculation and on days 3, 5, 7, 9, and 11 after infection. Infant mice were screened for glycosuria using Diastix reagent strips (Bayer Healthcare, Mishawaka, IN). Blood glucose levels in adult mice were monitored with an Accu-check Advantage II blood glucose meter and strips (Roche Diagnostics, Mannheim, Germany). For construction of diabetes survival curves, glucose levels in mice were monitored weekly after inoculation. Urine screening was alternated with measurement of blood levels. Blood glucose levels in mice showing elevated urinary glucose levels were determined immediately. Consecutive blood glucose levels of >13 mmol/liter on two occasions >2 days apart were considered to indicate type 1 diabetes development.
Statistical analysis.
Student's t test was used to assess the statistical significance of differences in insulitis, ages at diabetes onset, and antibody titers to rotaviruses. Differences in diabetes incidences were evaluated using Kaplan-Meier life-table analysis and GraphPrism software (San Diego, CA). Significance was set at the 95% level. On graphs, error bars represent the standard deviation of the mean.
RESULTS
Clinical gastrointestinal disease was caused by RRV in infant but not adult NOD mice.
RRV infection by oral or i.p. inoculation of 5-day-old infant NOD mice resulted in diarrhea in approximately 90% of mice, which began 1 to 2 days after infection and lasted for 2 to 5 days. Young adult mice infected orally or i.p. with RRV and control mice of all ages inoculated by any route did not show diarrhea. These findings are consistent with previous reports of the age dependency of rotavirus disease in other mouse and small animal models (13, 80). All RRV-inoculated mice seroconverted to RRV by 14 days after infection, whereas all control mice showed negative reciprocal antibody titers of <5 × 101 (data not shown). Serum antirotavirus antibody titers were determined in a subgroup of mice at least every second day (6 to 9 mice/day) for 11 days after infection. Mice seroconverted by day 2 (i.p.) or day 5 (oral) after infection (data not shown).
Infectious RRV and/or RRV antigen were detected in the intestine, liver, pancreas, serum, blood cells, and spleen of infant NOD mice.
In order to determine the degree of rotavirus infection intestinally and if virus disseminated to other organs, the presence of rotavirus antigen and infectious RRV was analyzed in a range of tissues. The relative sensitivities of direct assay for rotavirus antigen by capture EIA (direct EIA), RRV titers measured by inoculation of MA104 cells with serial dilutions (direct culture), and culture amplification of RRV followed by rotavirus antigen assay in cultures by capture EIA (culture then EIA) were determined. These assays were compared using organs collected at days 2, 3, 4, and 5 after infection from infant NOD mice inoculated orally or i.p. with RRV. Culture then EIA was the most sensitive method, detecting RRV in 89% (85/96) of intestines and 41% (37/91) of livers. Direct culture and direct EIA were of lower sensitivity, finding RRV in 64% (28/44) and 55% (53/96) of intestines and 46% (20/44) and 25% (24/96) of livers, respectively. The proportions of these organs containing RRV on each day after infection are shown in Table 1. Infectious RRV was detected in 67 to 100% of intestines, and rates did not differ between mice inoculated orally or i.p. However, infectious RRV was detected more often in liver after inoculation i.p. (50 to 83%) than orally (13 to 33%). Infectious RRV was detected in the pancreas at day 5 after oral inoculation and was present in at least 25% of mice. Rotavirus antigen but not infectious RRV was found by direct EIA in the pancreas at day 2 after oral inoculation in 7 to 60% of mice. Infectious virus was found in the pancreas of 7 to 50% of mice inoculated i.p., at day 3 after infection only (Table 1).
TABLE 1.
Detection of RRV by culture and then EIA in organs from RRV-infected infant NOD mice
| Day postinfection and inoculation | % Positive (no. positive/no. tested) in:
|
||
|---|---|---|---|
| Intestine | Liver | Pancreasa | |
| 2 | |||
| Oral | 87 (13/15) | 27 (4/15) | 0 (0/15)b |
| i.p. | 100 (7/7) | 83 (5/6) | 0 (0/7) |
| 3 | |||
| Oral | 100 (16/16) | 33 (5/15) | 0 (0/16) |
| i.p. | 67 (10/15) | 71 (10/14) | 7-50 (1/14-7/14)a,c |
| 4 | |||
| Oral | 92 (12/13) | 0 (0/13) | 0 (0/13) |
| i.p. | 93 (13/14) | 67 (8/12) | 0 (0/14) |
| 5 | |||
| Oral | 88 (7/8) | 13 (1/8) | 25-100 (2/8-8/8)a,d |
| i.p. | 88 (7/8) | 50 (4/8) | 0 (0/8) |
Pancreases were assayed as pools of four to eight organs due to their small size in infant mice. Thus, a range from a single pancreas to all pancreases in the pool could be positive for RRV. This range is provided as a number and percentage.
From 7 to 60% (1/15 to 8/15) of these pancreases contained RRV antigen.
RRV antigen was not detected in these pancreases.
All of these pancreases (8/8) contained RRV antigen.
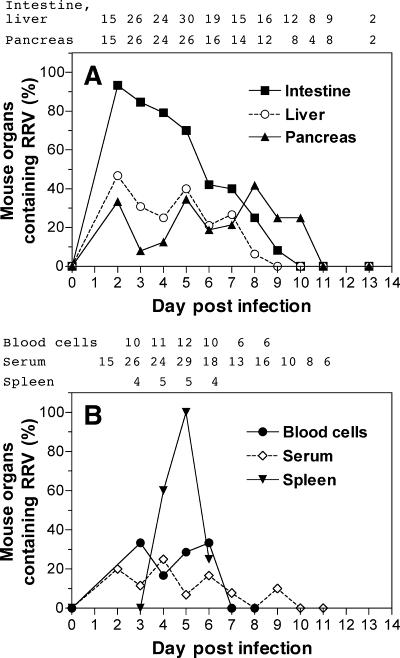
The proportion of organs from orally infected infant NOD mice that contained virus after RRV infection was further studied (Fig. 1). The kinetics of infectious RRV production intestinally was monophasic, and virus was detected for 10 days after infection (Fig. 1A). Detection of intestinal replication peaked at day 3 after infection (93% of mice). The proportions of livers and pancreases containing infectious RRV were lower and more variable than those of the intestines over this period. RRV antigen or infectious RRV was detected in 33% of pancreases at day 2 after infection, and infectious rotavirus was detected in 13 to 42% of pancreases at days 4 to 10 after infection (Fig. 1A). RRV detection in the pancreas peaked at day 8. Infectious RRV was detected in blood cells from 17 to 33% of mice at days 3 to 6 after infection. Virus was associated with leukocytes, as removal of red blood cells with red cell lysis buffer (Sigma) did not affect virus detection (data not shown). Rotavirus antigen was detected in serum from days 2 to 9 after infection in up to 25% of mice, but infectious RRV was not detected in these sera by culture then EIA (Fig. 1B), probably due in part to the rapidly increasing titer of serum antibodies to RRV from day 2 onwards. Detection of infectious RRV in blood cells indicates that virus present in tissues could be due to some degree to residual blood cells in the tissue, as mice were not perfused prior to tissue collection. Infectious RRV was detected in the spleen in 25 to 100% of mice at days 4 to 6 postinfection (Fig. 1B). Treatment with red cell lysis buffer (data not shown) indicated that non-red-cell splenocytes harbored this virus. For each inoculation route, all mock-inoculated mice (1 to 2 per time point) showed negative reciprocal antirotavirus antibody titers of <5 × 101 on euthanasia at days 2 to 9, 11, and 13 after inoculation and no organs contained detectable infectious RRV or rotavirus antigen (data not shown).
FIG. 1.
Detection of rotavirus in the intestine, liver, and pancreas (A) and in blood cells, serum, and spleen (B) of male and female infant NOD mice inoculated orally with RRV. RRV was detected by culture followed by EIA and by direct EIA. In panel B, infectious RRV was detected by culture then EIA in blood cells and spleen. Blood cells that contained infectious RRV also were positive for RRV antigen by direct EIA, and no other blood cell samples contained RRV antigen detectable by EIA. Rotavirus antigen was detected in serum by direct EIA, but infectious RRV was not detected in serum by culture then EIA. The number of mice examined at each day postinfection for each sample type (when these differed between sample types) is indicated above each panel.
Infectious RRV showed limited spread to liver and pancreas in adult NOD mice.
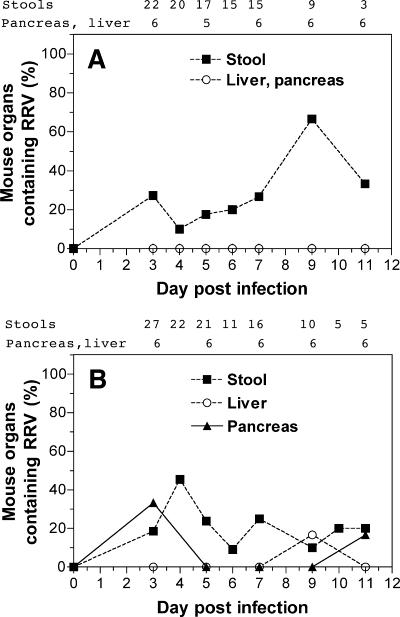
The organs containing RRV also were analyzed in RRV-infected adults (Fig. 2). Intestinal rotavirus replication was detected in 16/22 (73%) mice inoculated orally and 21/27 (78%) mice inoculated i.p. Asymptomatic rotavirus infection in mice of this age is a typical finding (20, 80). The proportions of stools from orally and i.p.-inoculated mice containing infectious RRV at days 3 to 11 postinfection were similar, at 10 to 67% and 9 to 45%, respectively (Fig. 2). However, no mice infected orally at this age and ≤30% of mice inoculated i.p. showed infectious RRV or rotavirus antigen in their pancreas or liver. Infectious RRV was detected in the pancreas at day 3 after infection in two mice inoculated i.p. that did not show detectable virus in stools. Thus, 23/27 (85%) adult mice inoculated i.p. showed evidence of rotavirus replication at any site. For each inoculation route, all mock-infected mice (1 to 2 per time point) showed negative reciprocal antirotavirus antibody titers in serum of <5 × 101 on euthanasia at days 3 to 11 after inoculation and their pancreas, liver, stools, and serum contained no detectable RRV or rotavirus antigen (data not shown). Rotavirus antigen was not detected by direct EIA in any sera from RRV-infected young adult mice (data not shown). Insufficient serum was available for culture then EIA. Blood cells were not assayed for infectious virus.
FIG. 2.
Detection of rotavirus in stools and organs of young adult NOD mice inoculated with RRV. Approximately equal numbers of female and male mice were infected orally (A) or i.p. (B). RRV was detected by culture followed by EIA and by direct EIA. The number of mice examined at each day postinfection for each sample type (when these differed between sample types) is indicated above the panel.
RRV titers in the intestine, liver, and pancreas of infant and adult NOD mice.
The titers of RRV were determined by direct culture of the intestine or stool, liver, and pancreas collected at days 2 to 11 after infection from infant and young adult mice inoculated orally or i.p. with RRV (Table 2). Within each age group, the geometric mean titers of RRV in the gastrointestinal tracts of mice were similar irrespective of RRV inoculation route. Also, titers in the intestine in infants were not significantly different from titers in stools of adults (P = 0.28), ranging from 1.4 × 104 to 3.3 × 104 FCFU/ml in infants and 4.5 × 103 to 1.5 × 104 FCFU/ml in adults. RRV titers in the livers of infant mice also were independent of inoculation route. Overall, i.p. inoculation of infants with RRV was more likely to result in detection of infectious virus in the liver than oral inoculation, but the titers of hepatic virus were similar after oral or i.p. infection. Infectious rotavirus was detected in the pancreas after infection of infants by either route (Table 1 and Fig. 1A). In adults, a low titer of infectious virus in the pancreas or liver occasionally was detected when virus was delivered i.p. but not orally.
TABLE 2.
Titers of infectious RRV present in intestinal and extraintestinal sites of infant and adult NOD mice inoculated with RRV
| Mouse age, sex, and inoculation route | Sample or organ | Geometric mean titer of RRV on day postinfection (no. of samples tested)a:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 9 | 11 | ||
| Infant | |||||||||
| Female/male, oral | Intestine | ND | 1.8 × 104 (2) | ND | 3.3 × 104 (2) | ND | - | ND | ND |
| Liver | ND | 4.7 × 104 (2) | ND | 5.6 × 104 (1) | ND | 1.8 × 105 (2) | ND | ND | |
| Pancreas | ND | - | ND | 6.8 × 103 (1) | ND | - | ND | ND | |
| Female/male, i.p. | Intestine | 1.4 × 104 (5) | 3.4 × 104 (7) | 1.6 × 104 (10) | 3.4 × 104 (6) | ND | ND | ND | ND |
| Liver | 2.0 × 104 (2) | 3.0 × 104 (8) | 3.0 × 104 (6) | 3.9 × 104 (4) | ND | ND | ND | ND | |
| Adult | |||||||||
| Female, oral | Stool | ND | 1.2 × 104 (5) | 7.6 × 103 (2) | 1.0 × 104 (2) | 1.4 × 104 (1) | 7.9 × 103 (1) | 1.5 × 104 (4) | 7.8 × 103 (2) |
| Female, i.p. | Stool | ND | 6.7 × 103 (5) | 9.0 × 103 (10) | 9.7 × 103 (5) | 4.5 × 103 (1) | 1.4 × 104 (4) | 1.2 × 104 (1) | 8.2 × 103 (2) |
| Liver | ND | - | - | - | - | - | 2.3 × 103 (1) | - | |
| Pancreas | ND | - | - | - | - | - | - | 2.3 × 103 (1) | |
Titers are expressed as the number of FCFU/ml in the supernatant fluid of a 20% (wt/vol) organ homogenate or a 10% (wt/vol) stool homogenate. A minority of samples positive for virus by culture and then EIA contained virus levels below the titration assay limits of detection, which were 4.0 × 102 FCFU/ml (intestine and stool homogenates) and 2.0 × 103 (liver and pancreas homogenates). Two mice per day were tested on days 3, 5, and 7 after oral RRV infection of infant mice. Total numbers of samples screened are indicated in Table 1 for infant mice infected i.p. and in Fig. 2 for adult mice infected orally or i.p. ND, not done. -, no samples tested contained detectable infectious RRV.
RRV antigen was detected in macrophages outside islets in the pancreas of infant NOD mice.
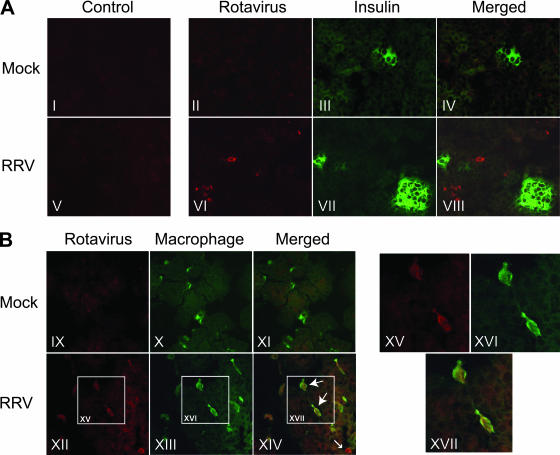
Infectious RRV was detected in the pancreas of a large proportion of orally infected infant NOD mice (Fig. 1A). The cells containing RRV were visualized by IHC in pancreatic sections obtained from RRV-infected and mock-infected mice that were littermates of the mice described in Fig. 1, at days 5 to 8 after infection. By IHC, 1/4 (25%) pancreases collected from RRV-infected mice at days 5 and 6 after infection contained cells staining for rotavirus antigen, whereas antigen was not detected in pancreases collected on days 7 (n = 4) and 8 (n = 4) (data not shown). None of the four mock-infected mice analyzed (one on each of days 5, 6, 7, and 8 after infection) showed rotavirus antigen staining in the pancreas by IHC (data not shown). The lower detection rate in IHC compared with culture then EIA probably reflects the lower IHC sensitivity. As shown in Fig. 3, a minority of cells in pancreatic sections from RRV-infected mice contained rotavirus antigen, consistent with the relatively low titers of infectious virus present (Table 2). To determine if the RRV-containing cells were islet β cells, pancreatic sections from mock- and RRV-infected mice were double stained with antibodies against insulin and RRV. The insulin-producing β cells were clearly stained with the anti-insulin antibody, showing the characteristic ball shape of pancreatic islets (Fig. 3A). The RRV-containing cells did not colocalize with these islets, demonstrating that RRV does not infect the pancreatic islets of infant NOD mice in vivo when inoculated by oral gavage (Fig. 3A). The cells containing rotavirus antigen showed a macrophage-like distribution within the tissue. To determine the identity of these cells, pancreatic sections were double stained with antibodies directed to RRV and the macrophage marker F4/80 (also known as BM8; Fig. 3B). F4/80 is considered to be the best marker for murine macrophages, including those in the NOD mouse pancreas (9, 29, 44). Both resident and nonresident macrophages (within blood vessels) were detected with F4/80 staining in pancreatic sections from mock- and RRV-infected mice. An occasional rotavirus antigen-containing cell did not stain as a macrophage. However, the majority of pancreatic cells containing RRV antigen also costained with F4/80 (Fig. 3B), so these cells were identified as resident and nonresident macrophages.
FIG. 3.
RRV antigen was detected in cells outside islets in the pancreas of infant NOD mice and colocalized with resident and nonresident macrophages. Pancreases were dissected at 5 days postinfection from mice infected orally at 5 days of age. In panel A, OCT-fixed, frozen pancreatic sections were stained with rotavirus antibody-negative control rabbit serum (I and V), rabbit antiserum to RRV (II and VI), or anti-insulin antibody (III and VII). The merged images of panels II and III and VI and VII, provided in panels IV and VIII, respectively, show no colocalization of rotavirus antigen and insulin. In panel B, pancreatic sections were stained with rabbit antiserum to RRV (IX and XII) or rat antibody to the mouse macrophage marker F4/80 (X and XIII). The merged images of panels IX and X and XII and XIII are shown in panels XI and XIV, respectively. The large arrows show examples of macrophages containing rotavirus antigen, and the small arrow indicates a cell containing rotavirus antigen that did not stain as a macrophage. Panels XV, XVI, and XVII are magnifications at ×2.2 of the boxed areas in panels XII, XIII and XIV, respectively. Original magnification, ×400.
No mice showed glucosuria or abnormal blood glucose levels in the 11 days after infection with rotavirus.
Groups of RRV-inoculated mice were monitored for alterations in urinary or blood glucose levels prior to infection and at 2-day intervals for 11 days after infection. Infant mice inoculated with RRV either orally or i.p. showed urinary glucose levels within the normal range that were similar to control mice, both prior to and after infection (data not shown). Blood glucose levels in these mice could not be measured due to the small size of the animals. Adult female mice inoculated by either route showed blood glucose levels that were within the normal range and indistinguishable from levels in control mice prior to infection and for the 11 days after infection (Table 3). The earliest age that any inoculated mice developed elevated blood glucose levels was 11 weeks, which was >4 weeks after inoculation.
TABLE 3.
Blood glucose levels in RRV-infected adult mice remain in the normal range during period of virus detection
| Day postinfectiona | Blood glucose level (mmol/liter) after RRV or mock infectionb
|
|||
|---|---|---|---|---|
| Oral
|
i.p.
|
|||
| RRV | Mock | RRV | Mock | |
| 0 | 6.1 ± 0.5 (17) | 6.3 ± 1.1 (7) | 6.3 ± 0.7 (18) | 6.2 ± 0.7 (17) |
| 3 | 6.3 ± 1.1 (6) | 5.4 ± 2.1 (3) | 6.2 ± 0.6 (6) | 5.3 ± 2.4 (3) |
| 5 | 6.3 ± 1.0 (8) | 5.5 ± 2.1 (3) | 6.6 ± 0.6 (6) | 6.7 ± 0.8 (3) |
| 7 | 6.7 ± 0.9 (9) | 6.3 ± 1.7 (3) | 6.7 ± 1.2 (6) | 6.4 ± 1.0 (3) |
| 9 | 5.2 ± 1.3 (9) | 5.1 ± 1.0 (3) | 6.1 ± 0.8 (6) | 6.8 ± 1.6 (3) |
| 11 | 5.4 ± 0.4 (6) | 5.2 ± 2.1 (3) | 6.6 ± 0.7 (6) | 6.3 ± 0.6 (3) |
Measurements on day 0 were made 4 to 6 h prior to RRV inoculation.
Values are means ± standard deviations. The number of sera tested is given in parentheses. Values between 3 and 13 mmol/liter were considered to be within the normal range.
RRV infection of infant NOD mice by either route delayed diabetes onset, whereas young adult mice were protected from diabetes following i.p. but not oral inoculation.
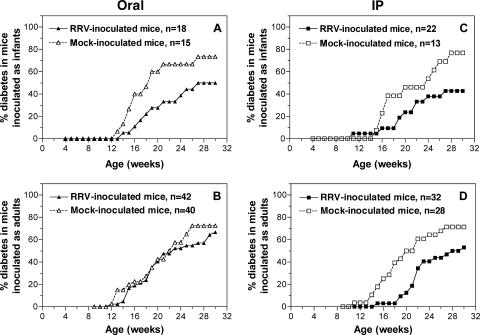
To determine if RRV infection modulates the timing and incidence of diabetes development, groups of RRV- and mock-infected female mice were monitored for hyperglycemia until 30 weeks of age (Fig. 4). All RRV-inoculated mice seroconverted to rotavirus, showing reciprocal EIA titers in convalescent-phase sera of 4 × 102 to 5.1 × 104 (data not shown). Mock-infected mice showed negative reciprocal antirotavirus antibody titers of <1 × 102 at approximately 6-week intervals during experimentation and on euthanasia (data not shown). Infant mice infected with RRV orally demonstrated a significant delay in diabetes development compared with mock-inoculated mice (Kaplan-Meier survival analysis; P = 0.042). Following RRV infection, the proportion of mice developing diabetes was reduced and their time to hyperglycemia onset increased (Fig. 4A). In contrast, there was no significant difference in the diabetes curves between control- and RRV-inoculated mice inoculated orally as young adults (P = 0.50; Fig. 4B). Infant and young adult mice infected i.p. showed a significant reduction in diabetes incidence (P = 0.040 for infants; P = 0.010 for adults) compared with mock-infected mice (Fig. 4C and D, respectively). As the ages at which mice first began to develop diabetes were similar in all groups of RRV-inoculated and control mice (11 to 14 weeks), the penetrance of the effect of RRV infection on diabetes development was incomplete. However, it is clear that rotavirus infection delayed the timing of diabetes onset in mice infected as infants by either route and in mice infected i.p. as young adults.
FIG. 4.
Modulation of diabetes development by RRV infection in infant and young adult NOD mice. Female mice were infected with RRV or mock infected as infants (A and C) or young adults (B and D) orally (A and B) or i.p. (C and D) and monitored for diabetes development until 30 weeks of age. The number of mice in each experimental group (n) is indicated in the legend to each panel. As assessed by seroconversion to RRV, all mock-infected mice were free of rotavirus infection during the course of the experiment. All RRV-inoculated mice seroconverted to RRV.
Analysis of the occurrence and timing of diabetes onset in relation to intestinal rotavirus replication and serum antibody titers to rotavirus.
The effect of intestinal rotavirus replication on diabetes development was assessed in the adult mice described in Fig. 4 by relating RRV shedding in stools (detected by culture then EIA) to age at diabetes onset. This analysis was restricted to adults as all infant mice shed RRV intestinally. The diabetes rates at 16, 22, and 30 weeks of age were 13%, 47%, and 80%, respectively, in mice that shed virus and 16%, 45%, and 55% in mice without detectable excretion. Mice that excreted virus in stools showed a median age at diabetes onset of 27 weeks, compared to 23 weeks in mice without stool virus (Kaplan-Meier analysis, P = 0.40; data not shown). Thus, detection of intestinal RRV replication was not associated with delayed diabetes onset in young adult mice.
The magnitude of the immune response generated might affect diabetes incidence. Antibodies to islet antigens are indirect markers of diabetes (40). Therefore, the geometric mean titers of antibodies to rotavirus in convalescent-phase serum from RRV-infected mice that had become diabetic by 30 weeks of age were compared with those in infected mice that were nondiabetic at this age (data not shown). There were no significant differences between titers in diabetic and nondiabetic mice for any age of inoculation or inoculation route (P > 0.05), indicating that diabetes development was unrelated to serum antibody titers to RRV. This suggests that RRV infection was not a deciding factor in precipitating diabetes. The effect of the magnitude of the immune response against rotavirus on the timing of diabetes onset was analyzed by relating titers of serum antirotavirus antibodies in mice to their age at diabetes onset. Mice were classified by antibody titer as either moderate or strong responders. There were no significant differences (0.07 < P < 0.98) between the mean ages of diabetes onset, with the exception of infant mice inoculated orally. Strong responders in this group (reciprocal titers of 1.3 × 104 to 5.1 × 104) developed diabetes at a significantly older age than mock-infected mice (P = 0.035). As the number of strong responders was small (n = 3), this indicates a possible relationship between the magnitude of the serum antibody response to RRV and the delay in diabetes development.
RRV infection had little if any effect on development of pancreatic insulitis.
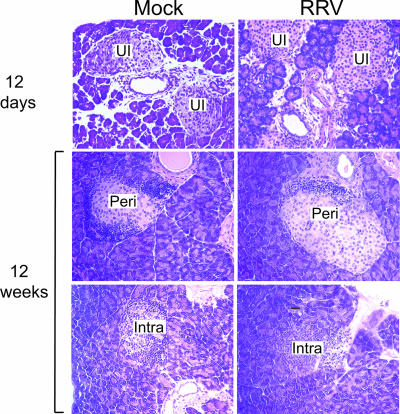
In order to determine the effect of RRV infection on pancreatic morphology and insulitis development, pancreases collected from female NOD mice at 12 days and 12 weeks of age, after oral inoculation at 5 days of age, were scored for the presence of peri-islet and intraislet insulitis (Fig. 5). Pancreatic morphology was normal and no insulitis was present in either the mock- or RRV inoculated mice at 12 days of age. This was consistent with the lack of glucosuria and indicated that pancreatitis and pancreatic damage were not features of this acute rotavirus infection. Peri- and intraislet infiltrates were present in a minority of islets in the RRV-infected and control mice at 12 weeks of age (Fig. 5). A possible trend toward a reduction in peri- and intraislet insulitis in RRV-infected mice compared with controls was observed, which was not significant (Table 4; P = 0.18). Thus, insulitis was not prevented by rotavirus infection of infant mice, and little if any reduction in the degree of insulitis occurred.
FIG. 5.
Effect of oral RRV infection on pancreatic insulitis development in female infant NOD mice. The histopathology in representative sections of pancreas dissected from mice 12 days or 12 weeks after oral inoculation at 5 days of age is illustrated. Pancreases from RRV-infected and control mice that had not developed hyperglycemia at these times were fixed in Bouin's solution, sectioned, and stained with hematoxylin and eosin. UI, uninfiltrated islet; Peri, peri-islet infiltrate; Intra, intraislet infiltrate. Definitions of these states are given in Materials and Methods. Pancreatitis or pancreatic damage was not seen in any section examined. Original magnification, ×200.
TABLE 4.
Effects of RRV infection of female NOD mice on development of pancreatic islet insulitis
| Age at inoculation and route | Age at insulitis analysis (wk) | Inoculum | % of total islets in pancreas with cellular infiltratea:
|
||
|---|---|---|---|---|---|
| None | Peri-islet | Intraislet | |||
| Infant | |||||
| Oral | 12 | RRV | 79 ± 21b | 12 ± 12 | 9 ± 9 |
| 12 | Mock | 58 ± 32 | 21 ± 16 | 21 ± 19 | |
| 30 | RRV | 48 ± 7 | 33 ± 8 | 19 ± 12 | |
| 30 | Mock | 67 ± 29 | 15 ± 13 | 18 ± 17 | |
| i.p. | 30 | RRV | 40 ± 33 | 38 ± 23 | 22 ± 14 |
| 30 | Mock | 48 ± 46 | 14 ± 13 | 38 ± 34 | |
| Young adult | |||||
| Oral | 30 | RRV | 88 ± 19c | 6 ± 6 | 6 ± 6 |
| 30 | Mock | 46 ± 13 | 18 ± 6 | 26 ± 10 | |
| i.p. | 30 | RRV | 41 ± 40 | 24 ± 24 | 35 ± 32 |
| 30 | Mock | 63 ± 9 | 19 ± 6 | 18 ± 4 | |
Four sections at 200-μm intervals within the pancreas of each of 3 to 7 mice were scored for the degree of islet insulitis as described in Materials and Methods. The total number of islets was counted for each section. The sum of the scores for each degree of insulitis for all sections was expressed as a percentage of the total number of islets detected in each pancreas. Values are means ± standard deviations.
Orally infected infant mice showed a trend toward reduced insulitis at 12 weeks of age (P = 0.18).
Orally infected young adult mice showed significantly reduced insulitis at 30 weeks of age (P = 0.034).
The effect of RRV infection on insulitis development in mice that were diabetes free at 30 weeks was examined. Mice inoculated orally or i.p. with RRV as infants or i.p. as young adults did not differ from mock-infected mice in the level of peri- or intraislet infiltrate present (Table 4). This demonstrates that RRV infection did not alter the long-term development of insulitis in these mice, including orally infected infant mice that had showed a trend toward insulitis reduction at 12 weeks. Compared with mock-inoculated mice, there was a significant reduction in the peri- and intraislet infiltrate in mice surviving to 30 weeks of age following oral RRV infection as young adults (Table 4; P = 0.034). However, the diabetes curves for these mice (Fig. 4B) indicate that this possible reduction in insulitis did not manifest in altered diabetes development.
Summary of findings on the effects of RRV infection in infant and young adult NOD mice.
The overall findings of this study are summarized in Table 5. Extraintestinal spread and pancreatic presence of infectious virus occurred only in infant mice, unless virus was given i.p. The detection rates for the presence of infectious virus at any site were as follows: infant mice (100%) > older mice inoculated i.p. (83%) > older mice inoculated orally (73%). Pancreatic insulitis development was not prevented by infection. The onset and incidence of diabetes were delayed in infant mice and in adult mice inoculated i.p. Therefore, mice inoculated orally as young adults showed no evidence of pancreatic or hepatic RRV, the lowest rate of detectable rotavirus replication at any site, and no significant diabetes delay. In contrast, rotavirus replicated and was detected extraintestinally, including in the pancreas, in all infant mice and in many adult mice inoculated i.p., and these groups of mice showed evidence of delayed diabetes development. Thus, the ability of RRV infection to delay diabetes development was related to the presence of infectious virus extraintestinally, including in the pancreas.
TABLE 5.
Summary of the effects of RRV infection in infant and young adult NOD mice
| Infection or disease parameter | Result for:
|
||
|---|---|---|---|
| Infants | Young adults
|
||
| Oral | i.p. | ||
| Gastrointestinal disease | Yes | No | No |
| Mice with detectable intestinal rotavirus replication (%) | 100 | 73 | 78 |
| Extraintestinal rotavirus | Yes | No | Yes |
| Pancreatic rotavirus | Low titer, antigen in macrophages outside islets | No | Low titer |
| Insulitis | |||
| 12 wk of age | Trend toward reduction for oral, not tested for i.p. | Not tested | Not tested |
| 30 wk of age | No alteration | Possible reduction | No effect |
| Diabetes onset | Delayed | No effect | Delayed |
| Diabetes incidence at 30 wk of age | Reduced | No effect | Reduced |
| Relationship between antibody titer and diabetes delay | Yes for oral, no for i.p. | No | No |
DISCUSSION
The possible association of childhood rotavirus infection with modulation of islet autoantibodies is controversial, and no studies addressing this issue in animal models of type 1 diabetes have been reported to date. Here we provide direct experimental evidence that rotavirus infection of NOD mice can modulate type 1 diabetes development. Infectious RRV was detected extraintestinally in blood cells, splenocytes, the pancreas, and the liver in infant mice with gastroenteritis and the pancreas and liver in a minority of asymptomatically infected older mice. In the pancreas of infant mice, morphology was normal and rotavirus antigen was present in resident and nonresident macrophages outside islets. RRV infection did not prevent insulitis development. The data obtained from this model indicate that RRV rotavirus infection of infant and young adult NOD mice delays diabetes onset and reduces incidence by a mechanism related to the presence of virus outside the gut, in organs including the pancreas and liver.
Our findings that infectious RRV is present in the small intestine, liver, pancreas, and spleen in NOD mice extend previous studies. Infectious RRV has been detected in the small intestine, liver, lungs, and kidneys of neonatal BALB/c mice after oral inoculation, using a strand-specific, quantitative reverse transcription-PCR (24). In orally inoculated neonatal rats, RRV antigen was found in the liver, lungs, kidneys, stomach, heart, bladder, thymus, and pancreas and one sample of each of the three latter sites contained infectious virus (21). In infant and adult NOD mice, infectious virus did reach the intestine and infect intestinal cells after i.p. inoculation, as these mice excreted virus in their stools for >8 days, irrespective of age. However, in adult mice, fewer stools from mice inoculated i.p. than orally contained infectious virus (Fig. 2). Interestingly, infectious virus was detected occasionally in the liver and pancreas of adult mice after i.p. but not oral infection. This suggests that following i.p. inoculation of adults, rotavirus gained access to these organs from the abdominal cavity rather than the intestine, bypassing any gut barrier to extraintestinal spread. Intestinal and hepatic infection occurred in 100% and 40% of neonatal CD-1 mice, respectively, following i.p. inoculation with RRV (61). This indicates some similarity in the organs targeted by RRV between infant NOD and CD-1 mice. These demonstrations of extraintestinal spread in NOD mice add further weight to the growing evidence of rotavirus escape from the intestine during acute infection.
The presence of rotavirus antigen in sera from RRV-infected infant NOD mice supports the recent consensus that antigenemia is a common sequela of rotavirus disease (5-7, 11, 25, 69). However, infectious rotavirus was not detected in sera diluted 1 in 4 to 1 in 10 from NOD mice by culture amplification of RRV and then EIA. Infectious RRV has been detected in serum from RRV-infected neonatal rats (21). Also, rotavirus-positive sera from mice and piglets infected with viruses ECwt and Wa, respectively, transferred infection to recipient animals with high efficiency (3, 6). However, a 1-in-10 serum dilution greatly reduced murine transfer, and the copy number of viral RNA was >4 log10 lower in serum than in stools (6). Host species-specific factors, virus strain differences affecting the level of viremia in serum, and methodological differences would explain the lack of detection of infectious virus in sera from NOD mice.
In contrast to serum, blood cells from infant NOD mice were shown by culture and then EIA to contain infectious rotavirus. Supporting this finding, a small amount of minus-strand RNA, indicating the presence of infectious rotavirus, was detected in leukocytes from EC-infected, infant BALB/c mice (24). Although viremia following ECwt inoculation of adult CD-1 mice has been associated with the plasma fraction of blood rather than cells, analysis of cells from infant mice for infectious virus was not reported (6). Detection in the red blood cell-depleted spleens and blood cells demonstrated that infectious virus was associated with leukocytes in infant NOD mice. Several potential receptors and entry cofactors for RRV and other rotaviruses can be expressed on leukocytes, including terminal sialic acids and the integrins α2β1, αxβ2, αvβ3, α4β1, and α4β7. Involvement of these receptors in extraintestinal rotavirus spread has been suggested (17, 30-33, 36, 38, 52). Supporting this, productive RRV infection of myeloid dendritic cells from human peripheral blood has been hypothesized to relate to preferential expression of α4 integrin (62). A lymphatic mechanism for RRV extraintestinal spread in the neonatal mouse has been proposed, involving RRV exit from the gut to the mesenteric lymph nodes and then movement to other organs (24, 60, 61). Consistent with this, viremia was present prior to splenic rotavirus in NOD mice, and the pattern observed suggests virus may have been transported to the spleen via blood cells 3 days after infection.
Our in vivo demonstration that RRV antigen in the pancreas is present only in cells outside islets rules out direct β-cell infection as a mechanism by which RRV could modulate diabetes development in infant NOD mice. RRV replicated to high titer in purified islets isolated from the pancreas of adult NOD mice (20) but was rarely found in the pancreas of RRV-infected adult NOD mice. This may relate to the inoculation dose of RRV or the absence from isolated islets of host factors that control pancreatic RRV replication in vivo. By analogy with CVB infection in the pancreas, these might include interferons (10, 23, 26). Most RRV-infected pancreatic cells were identified as resident and nonresident macrophages on the basis of F4/80 expression. It is possible that the few F4/80-negative, rotavirus-infected cells were nonresident macrophages that had yet to up-regulate F4/80 expression (29). Alternatively, other invading immune cells or exocrine cells within the pancreas might be infected by RRV. Our data are consistent with macrophage recruitment to the pancreas in response to infection of an occasional resident pancreatic cell. Additionally, macrophages have been suggested as a means for rotavirus dissemination (8), and replicating RRV was detected in infiltrating alveolar macrophages and adjacent pneumocytes in neonatal rat lung sections (21). The importance of rotavirus uptake into macrophages for dissemination to other organs requires further study.
Clearly the route of inoculation and NOD mouse age at the time of infection affected the degree of diabetes modulation by RRV. Diabetes development in newborn NOD mice orally infected with RRV was significantly delayed, demonstrating a protective effect of this infection. Although a possible trend toward reduced development of insulitis at 12 weeks of age was seen in these mice, by 30 weeks of age RRV-inoculated and control mice showed similar degrees of insulitis. Intraperitoneal inoculation resulted in delayed diabetes irrespective of the age of the mice. Overall, the ability of RRV infection to delay diabetes development was related to the extent of detectable virus replication and the presence of infectious virus extraintestinally, including in the pancreas. These findings suggest that immune tolerance was induced in these mice. Reovirus and RRV might have similar effects on diabetes development in infant NOD mice, as reovirus infection delayed diabetes and did not prevent insulitis by 30 weeks of age (81). Infection of the thymus and possibly in the periphery leading to loss of autoreactive lymphocytes and passive tolerance was one mechanism proposed for this diabetes delay by reovirus (66, 81). The abilities of reovirus to modulate diabetes development in young adult NOD mice and of RRV to infect the NOD mouse thymus have not been determined. However, infectious RRV was present in the thymus of neonatal rats (21). It is possible that RRV induces passive tolerance through thymic or peripheral infection and the destruction of β-cell-specific lymphocytes in infant NOD mice and young adult NOD mice inoculated i.p. RRV infection in NOD mice also might delay diabetes onset through induction of active tolerance, particularly through the expansion of CD4+ CD25+ regulatory T cells. The delay in diabetes development in these RRV-infected NOD mice also might relate to immune deviation, through adjuvant-like induction of tumor necrosis factor alpha, interleukin-4, and/or gamma interferon, for example (1).
In the NOD mouse, destructive autoreactive T cells invading the pancreas express high levels of α4β7 integrin and require expression of the α4β7 ligand MAdCAM-1 on the pancreatic peri-islet venules for invasion (35, 71, 84). MAdCAM-1 blockade also might prevent the development of autoreactive T cells in the gut-associated lymphoid tissue, prior to homing and infiltration into the pancreas, as the interactions of naïve lymphocytes with MAdCAM-1 in the gut-associated lymphoid tissue could be important in breaking self-tolerance to islet β-cell antigens (35). In mice, rotavirus-specific B and T cells strongly express α4β7 and effective immune responses to rotavirus depend on MAdCAM-1 recognition by lymphocytes expressing α4β7 (47, 85). In addition, RRV binds recombinant α4β7 via the same α-subunit domains as MAdCAM-1 (30). Any interplay between these potentially interacting components during RRV infection in NOD mice could delay the progression of the diabetic process. For example, any RRV interaction with α4β7 might (i) temporarily prevent the interaction of naïve lymphocytes with MAdCAM-1 and the break in self-tolerance required for diabetes onset or (ii) inhibit immune cell extravasation into the pancreas. These could account for the incomplete protection against diabetes development we observed. In the latter case, restoration of interactions between autoreactive lymphocytes and MAdCAM-1 after virus clearance might eventually lead to diabetes, at a later age than in uninfected mice.
Similarly to RRV and reovirus, CVB infection in infant NOD mice reduces diabetes development, by an unknown mechanism. However, in contrast to reovirus, RRV and CVB do not infect pancreatic islets in infant NOD mice, although some CVB strains cause pancreatitis (77). The mechanisms by which these viruses delay diabetes in infant NOD mice may be related but distinguishable. It will be important to determine if rotavirus strains other than RRV have a similar tropism and effect on diabetes development in infant and young adult NOD mice.
Our findings in these NOD mice do not support the hypothesis that rotavirus infection in children may trigger or exacerbate pancreatic islet autoimmunity (40). However, further exploration of the effects of infection with RRV and other rotavirus strains on NOD mice is warranted before a role for rotavirus in assisting the diabetic process can be ruled out. It is noteworthy that CVB infection in NOD mice aged >12 weeks that have well-developed insulitis results in diabetes acceleration, provided the virus is able to replicate rapidly to high titer in the pancreas (23). Our current studies are aimed at establishing if rotavirus can infect the pancreas and modulate diabetes onset in NOD mice that are fully insulitic at the time of infection.
Acknowledgments
We are most grateful to Stacey Fynch for expert technical assistance, R. Frank Ramig for advice on mouse inoculation with rotavirus by gavage, Nicole La Gruta for advice on splenocyte analysis, and Faye Docherty for preparation of paraffin sections. David Taylor, Rhiannon Hall, and other staff members of the animal facility of the Department of Microbiology and Immunology provided excellent support through their mouse breeding and husbandry.
This work was supported by project grant 208900 and research fellowship grants 172305, 251546, 299861, and 350253 (B.S.C.) from the National Health and Medical Research Council of Australia.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Anderson, M. S., and J. A. Bluestone. 2005. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23:447-485. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, M. A., and G. S. Eisenbarth. 2001. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221-229. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo, M. S., L. Yuan, K.-I. Jeong, A. Gonzalez, T. V. Nguyen, S. Pouly, M. Gochnauer, W. Zhang, A. Azevedo, and L. J. Saif. 2005. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J. Virol. 79:5428-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomqvist, M., S. Juhela, S. Erkkila, S. Korhonen, T. Simell, A. Kupila, O. Vaarala, O. Simell, M. Knip, and J. Ilonen. 2002. Rotavirus infections and development of diabetes-associated autoantibodies during the first 2 years of life. Clin. Exp. Immunol. 128:511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blutt, S. E., and M. E. Conner. 2007. Rotavirus: to the gut and beyond! Curr. Opin. Gastroenterol. 23:39-43. [DOI] [PubMed] [Google Scholar]
- 6.Blutt, S. E., M. Fenaux, K. L. Warfield, H. B. Greenberg, and M. E. Conner. 2006. Active viremia in rotavirus-infected mice. J. Virol. 80:6702-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 8.Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. [DOI] [PubMed] [Google Scholar]
- 9.Charre, S., J. G. Rosmalen, C. Pelegri, V. Alves, P. J. Leenen, H. A. Drexhage, and F. Homo-Delarche. 2002. Abnormalities in dendritic cell and macrophage accumulation in the pancreas of nonobese diabetic (NOD) mice during the early neonatal period. Histol. Histopathol. 17:393-401. [DOI] [PubMed] [Google Scholar]
- 10.Chehadeh, W., J. Kerr-Conte, F. Pattou, G. Alm, J. Lefebvre, P. Wattré, and D. Hober. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in β cells. J. Virol. 74:10153-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiappini, E., C. Azzari, M. Moriondo, L. Galli, and M. de Martino. 2005. Viraemia is a common finding in immunocompetent children with rotavirus infection. J. Med. Virol. 76:265-267. [DOI] [PubMed] [Google Scholar]
- 12.Christen, U., and M. G. von Herrath. 2005. Infections and autoimmunity—good or bad? J. Immunol. 174:7481-7486. [DOI] [PubMed] [Google Scholar]
- 13.Ciarlet, M., M. E. Conner, M. J. Finegold, and M. K. Estes. 2002. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J. Virol. 76:41-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulson, B. S., K. J. Fowler, R. F. Bishop, and R. G. H. Cotton. 1985. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J. Virol. 54:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 30:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulson, B. S., and C. Kirkwood. 1991. Relation of VP7 amino acid sequence to monoclonal antibody neutralization of rotavirus and rotavirus monotype. J. Virol. 65:5968-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulson, B. S., S. L. Londrigan, and D. J. Lee. 1997. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 94:5389-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulson, B. S., J. M. Tursi, W. J. McAdam, and R. F. Bishop. 1986. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology 154:302-312. [DOI] [PubMed] [Google Scholar]
- 19.Coulson, B. S., L. E. Unicomb, G. A. Pitson, and R. F. Bishop. 1987. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J. Clin. Microbiol. 25:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulson, B. S., P. D. Witterick, Y. Tan, M. J. Hewish, J. N. Mountford, L. C. Harrison, and M. C. Honeyman. 2002. Growth of rotaviruses in primary pancreatic cells. J. Virol. 76:9537-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford, S. E., D. G. Patel, E. Cheng, Z. Berkova, J. M. Hyser, M. Ciarlet, M. J. Finegold, M. E. Conner, and M. K. Estes. 2006. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J. Virol. 80:4820-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De La Rubia, L., M. I. Herrera, M. Cebrero, and J. C. De Jong. 1996. Acute pancreatitis associated with rotavirus infection. Pancreas 12:98-99. [DOI] [PubMed] [Google Scholar]
- 23.Drescher, K. M., K. Kono, S. Bopegamage, S. D. Carson, and S. Tracy. 2004. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381-394. [DOI] [PubMed] [Google Scholar]
- 24.Fenaux, M., M. A. Cuadras, N. Feng, M. Jaimes, and H. B. Greenberg. 2006. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J. Virol. 80:5219-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer, T. K., D. Ashley, T. Kerin, E. Reynolds-Hedmann, J. Gentsch, M. A. Widdowson, L. Westerman, N. Puhr, R. M. Turcios, and R. I. Glass. 2005. Rotavirus antigenemia in patients with acute gastroenteritis. J. Infect. Dis. 192:913-919. [DOI] [PubMed] [Google Scholar]
- 26.Flodstrom, M., A. Maday, D. Balakrishna, M. M. Cleary, A. Yoshimura, and N. Sarvetnick. 2002. Target cell defense prevents the development of diabetes after viral infection. Nat. Immunol. 3:373-382. [DOI] [PubMed] [Google Scholar]
- 27.Fujinami, R. S., M. G. von Herrath, U. Christen, and J. L. Whitton. 2006. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 19:80-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 29.Gordon, S. 1999. Macrophage-restricted molecules: role in differentiation and activation. Immunol. Lett. 65:5-8. [DOI] [PubMed] [Google Scholar]
- 30.Graham, K. L., F. E. Fleming, P. Halasz, M. J. Hewish, H. S. Nagesha, I. H. Holmes, Y. Takada, and B. S. Coulson. 2005. Rotaviruses interact with α4β7 and α4β1 integrins by binding the same integrin domains as natural ligands. J. Gen. Virol. 86:3397-3408. [DOI] [PubMed] [Google Scholar]
- 31.Graham, K. L., P. Halasz, Y. Tan, M. J. Hewish, Y. Takada, E. R. Mackow, M. K. Robinson, and B. S. Coulson. 2003. Integrin-using rotaviruses bind α2β1 integrin α2 I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J. Virol. 77:9969-9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham, K. L., Y. Takada, and B. S. Coulson. 2006. Rotavirus spike protein VP5* binds α2β1 integrin on the cell surface and competes with virus for cell binding and infectivity. J. Gen. Virol. 87:1275-1283. [DOI] [PubMed] [Google Scholar]
- 33.Graham, K. L., W. Zeng, Y. Takada, D. C. Jackson, and B. S. Coulson. 2004. Effects on rotavirus cell binding and infection of monomeric and polymeric peptides containing α2β1 and αxβ2 integrin ligand sequences. J. Virol. 78:11786-11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunow, J. E., S. F. Dunton, and J. L. Waner. 1985. Human rotavirus-like particles in a hepatic abscess. J. Pediatr. 106:73-76. [DOI] [PubMed] [Google Scholar]
- 35.Hanninen, A., I. Jaakkola, and S. Jalkanen. 1998. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J. Immunol. 160:6018-6025. [PubMed] [Google Scholar]
- 36.Haselhorst, T., H. Blanchard, M. Frank, M. J. Kraschnefski, M. J. Kiefel, A. J. Szyczew, J. C. Dyason, F. Fleming, G. Holloway, B. S. Coulson, and M. von Itzstein. 2007. STD NMR spectroscopy and molecular modeling investigation of the binding of N-acetylneuraminic acid derivatives to rhesus rotavirus VP8* core. Glycobiology 17:68-81. [DOI] [PubMed] [Google Scholar]
- 37.Hermitte, L., B. Vialettes, P. Naquet, C. Atlan, M. J. Payan, and P. Vague. 1990. Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur. J. Immunol. 20:1297-1303. [DOI] [PubMed] [Google Scholar]
- 38.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honeyman, M. C., B. S. Coulson, and L. C. Harrison. 2000. A novel subtype of type 1 diabetes mellitus. N. Engl. J. Med. 342:1835. [DOI] [PubMed] [Google Scholar]
- 40.Honeyman, M. C., B. S. Coulson, N. L. Stone, S. A. Gellert, P. N. Goldwater, C. E. Steele, J. J. Couper, B. D. Tait, P. G. Colman, and L. C. Harrison. 2000. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49:1319-1324. [DOI] [PubMed] [Google Scholar]
- 41.Honeyman, M. C., N. L. Stone, and L. C. Harrison. 1998. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol. Med. 4:231-239. [PMC free article] [PubMed] [Google Scholar]
- 42.Hongou, K., T. Konishi, S. Yagi, K. Araki, and T. Miyawaki. 1998. Rotavirus encephalitis mimicking afebrile benign convulsions in infants. Pediatr. Neurol. 18:354-357. [DOI] [PubMed] [Google Scholar]
- 43.Hyttinen, V., J. Kaprio, L. Kinnunen, M. Koskenvuo, and J. Tuomilehto. 2003. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 52:1052-1055. [DOI] [PubMed] [Google Scholar]
- 44.Inoue, T., D. Plieth, C. D. Venkov, C. Xu, and E. G. Neilson. 2005. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 67:2488-2493. [DOI] [PubMed] [Google Scholar]
- 45.Iturriza-Gómara, M., I. A. Auchterlonie, W. Zaw, P. Molyneaux, U. Desselberger, and J. Gray. 2002. Rotavirus gastroenteritis and central nervous system (CNS) infection: characterization of the VP7 and VP4 genes of rotavirus strains isolated from paired fecal and cerebrospinal fluid samples from a child with CNS disease. J. Clin. Microbiol. 40:4797-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keidan, I., I. Shif, G. Keren, and J. H. Passwell. 1992. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr. Infect. Dis. J. 11:773-775. [PubMed] [Google Scholar]
- 47.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on α4β7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894-1902. [DOI] [PubMed] [Google Scholar]
- 48.Lammi, N., M. Karvonen, and J. Tuomilehto. 2005. Do microbes have a causal role in type 1 diabetes? Med. Sci. Monit. 11:RA63-RA69. [PubMed] [Google Scholar]
- 49.Li, N., and Z. Y. Wang. 2003. Viremia and extraintestinal infections in infants with rotavirus diarrhea. Di Yi Jun Yi Da Xue Xue Bao 23:643-648. [PubMed] [Google Scholar]
- 50.Londrigan, S. L., K. L. Graham, Y. Takada, P. Halasz, and B. S. Coulson. 2003. Monkey rotavirus binding to α2β1 integrin requires the α2 I domain and is facilitated by the homologous β1 subunit. J. Virol. 77:9486-9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Londrigan, S. L., M. J. Hewish, M. J. Thomson, G. M. Sanders, H. Mustafa, and B. S. Coulson. 2000. Growth of rotaviruses in continuous human and monkey cell lines that vary in their expression of integrins. J. Gen. Virol. 81:2203-2213. [DOI] [PubMed] [Google Scholar]
- 52.Ludert, J. E., N. Feng, J. H. Yu, R. L. Broome, Y. Hoshino, and H. B. Greenberg. 1996. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J. Virol. 70:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch, M., B. Lee, P. Azimi, J. Gentsch, C. Glaser, S. Gilliam, H. G. Chang, R. Ward, and R. I. Glass. 2001. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin. Infect. Dis. 33:932-938. [DOI] [PubMed] [Google Scholar]
- 54.Lynch, M., W. J. Shieh, K. Tatti, J. R. Gentsch, T. Ferebee-Harris, B. Jiang, J. Guarner, J. S. Bresee, M. Greenwald, S. Cullen, H. D. Davies, C. Trevenen, S. R. Zaki, and R. I. Glass. 2003. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 37:1327-1333. [DOI] [PubMed] [Google Scholar]
- 55.Mack, C. L., R. M. Tucker, B. R. Lu, R. J. Sokol, A. P. Fontenot, Y. Ueno, and R. G. Gill. 2006. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology 44:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makela, M., J. Marttila, O. Simell, and J. Ilonen. 2004. Rotavirus-specific T-cell responses in young prospectively followed-up children. Clin. Exp. Immunol. 137:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makela, M., O. Vaarala, R. Hermann, K. Salminen, T. Vahlberg, R. Veijola, H. Hyoty, M. Knip, O. Simell, and J. Ilonen. 2006. Enteral virus infections in early childhood and an enhanced type 1 diabetes-associated antibody response to dietary insulin. J. Autoimmun. 27:54-61. [DOI] [PubMed] [Google Scholar]
- 58.Morrison, C., T. Gilson, and G. J. Nuovo. 2001. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum. Pathol. 32:216-221. [DOI] [PubMed] [Google Scholar]
- 59.Moser, C. A., S. Cookinham, S. E. Coffin, H. F. Clark, and P. A. Offit. 1998. Relative importance of rotavirus-specific effector and memory B cells in protection against challenge. J. Virol. 72:1108-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mossel, E. C., and R. F. Ramig. 2003. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J. Virol. 77:12352-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narváez, C. F., J. Angel, and M. A. Franco. 2005. Interaction of rotavirus with human myeloid dendritic cells. J. Virol. 79:14526-14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nigro, G. 1991. Pancreatitis with hypoglycemia-associated convulsions following rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 12:280-282. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura, S., H. Ushijima, S. Nishimura, H. Shiraishi, C. Kanazawa, T. Abe, K. Kaneko, and Y. Fukuyama. 1993. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 15:457-459. [DOI] [PubMed] [Google Scholar]
- 65.Oldstone, M. B. 1988. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science 239:500-502. [DOI] [PubMed] [Google Scholar]
- 66.Onodera, T., T. Taniguchi, T. Tsuda, K. Yoshihara, S. Shimizu, M. Sato, A. Awaya, and T. Hayashi. 1991. Thymic atrophy in type 2 reovirus infected mice: immunosuppression and effects of thymic hormone. Thymic atrophy caused by reo-2. Thymus 18:95-109. [PubMed] [Google Scholar]
- 67.Pager, C., D. Steele, P. Gwamanda, and M. Driessen. 2000. A neonatal death associated with rotavirus infection—detection of rotavirus dsRNA in the cerebrospinal fluid. S. Afr. Med. J. 90:364-365. [PubMed] [Google Scholar]
- 68.Ramig, R. F. 2004. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 78:10213-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ray, P., M. Fenaux, S. Sharma, J. Malik, S. Subodh, S. Bhatnagar, H. Greenberg, R. I. Glass, J. Gentsch, and M. K. Bhan. 2006. Quantitative evaluation of rotaviral antigenemia in children with acute rotaviral diarrhea. J. Infect. Dis. 194:588-593. [DOI] [PubMed] [Google Scholar]
- 70.Rother, K. I., and W. F. Schwenk II. 1995. An unusual case of the nonketotic hyperglycemic syndrome during childhood. Mayo Clin. Proc. 70:62-65. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz-Velasco, N., M. Guerrero-Esteo, M. J. Briskin, and J. Teixido. 2000. The α4 integrin subunit Tyr(187) has a key role in α4β7-dependent cell adhesion. J. Biol. Chem. 275:7052-7059. [DOI] [PubMed] [Google Scholar]
- 72.Saulsbury, F. T., J. A. Winkelstein, and R. H. Yolken. 1980. Chronic rotavirus infection in immunodeficiency. J. Pediatr. 97:61-65. [DOI] [PubMed] [Google Scholar]
- 73.Serreze, D. V., C. Wasserfall, E. W. Ottendorfer, M. Stalvey, M. A. Pierce, C. Gauntt, B. O'Donnell, J. B. Flanagan, M. Campbell-Thompson, T. M. Ellis, and M. A. Atkinson. 2005. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. J. Virol. 79:1045-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stene, L. C., M. C. Honeyman, E. J. Hoffenberg, J. E. Haas, R. J. Sokol, L. Emery, I. Taki, J. M. Norris, H. A. Erlich, G. S. Eisenbarth, and M. Rewers. 2006. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am. J. Gastroenterol. 101:2333-2340. [DOI] [PubMed] [Google Scholar]
- 75.Takei, I., Y. Asaba, T. Kasatani, T. Maruyama, K. Watanabe, T. Yanagawa, T. Saruta, and T. Ishii. 1992. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J. Autoimmun. 5:665-673. [DOI] [PubMed] [Google Scholar]
- 76.Thomson, G., W. P. Robinson, M. K. Kuhner, S. Joe, M. J. MacDonald, J. L. Gottschall, J. Barbosa, S. S. Rich, J. Bertrams, M. P. Baur, et al. 1988. Genetic heterogeneity, modes of inheritance, and risk estimates for a joint study of Caucasians with insulin-dependent diabetes mellitus. Am. J. Hum. Genet. 43:799-816. [PMC free article] [PubMed] [Google Scholar]
- 77.Tracy, S., K. M. Drescher, N. M. Chapman, K.-S. Kim, S. D. Carson, S. Pirruccello, P. H. Lane, J. R. Romero, and J. S. Leser. 2002. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76:12097-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Werf, N., F. G. Kroese, J. Rozing, and J. L. Hillebrands. 2007. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab. Res. Rev. 23:169-183. [DOI] [PubMed] [Google Scholar]
- 80.Ward, R. L., M. M. McNeal, and J. F. Sheridan. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wetzel, J. D., E. S. Barton, J. D. Chappell, G. S. Baer, M. Mochow-Grundy, S. E. Rodgers, Y. Shyr, A. C. Powers, J. W. Thomas, and T. S. Dermody. 2006. Reovirus delays diabetes onset but does not prevent insulitis in nonobese diabetic mice. J. Virol. 80:3078-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilberz, S., H. J. Partke, F. Dagnaes-Hansen, and L. Herberg. 1991. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34:2-5. [DOI] [PubMed] [Google Scholar]
- 83.Wildner, G., and M. Diedrichs-Mohring. 2003. Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein and retinal S-antigen. Eur. J. Immunol. 33:2577-2587. [DOI] [PubMed] [Google Scholar]
- 84.Yang, X. D., N. Karin, R. Tisch, L. Steinman, and H. O. McDevitt. 1993. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc. Natl. Acad. Sci. USA 90:10494-10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Youngman, K. R., M. A. Franco, N. A. Kuklin, L. S. Rott, E. C. Butcher, and H. B. Greenberg. 2002. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J. Immunol. 168:2173-2181. [DOI] [PubMed] [Google Scholar]
- 86.Zanoni, G., R. Navone, C. Lunardi, G. Tridente, C. Bason, S. Sivori, R. Beri, M. Dolcino, E. Valletta, R. Corrocher, and A. Puccetti. 2006. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 3:1637-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]