Abstract
Mature dendritic cells (DCs) are the most potent antigen-presenting cells within the human immune system. However, Herpes simplex virus type 1 (HSV-1) is able to interfere with DC biology and to establish latency in infected individuals. In this study, we provide new insights into the mechanism by which HSV-1 disarms DCs by the manipulation of CD83, a functionally important molecule for DC activation. Fluorescence-activated cell sorter (FACS) analyses revealed a rapid downmodulation of CD83 surface expression within 6 to 8 h after HSV-1 infection, in a manner strictly dependent on viral gene expression. Soluble CD83 enzyme-linked immunosorbent assays, together with Western blot analysis, demonstrated that CD83 rapidly disappears from the cell surface after contact with HSV-1 by a mechanism that involves protein degradation rather than shedding of CD83 from the cell surface into the medium. Infection experiments with an ICP0 deletion mutant demonstrated an important role for this viral immediate-early protein during CD83 degradation, since this particular mutant strain leads to strongly reduced CD83 degradation. This hypothesis was further strengthened by cotransfection of plasmids expressing CD83 and ICP0 into 293T cells, which led to significantly reduced accumulation of CD83. In strong contrast, transfection of plasmids expressing CD83 and a mutant ICP0 defective in its RING finger-mediated E3 ubiquitin ligase function did not reduce CD83 expression. Inhibition of the proteasome, the cellular protein degradation machinery, almost completely restored CD83 surface expression during HSV-1 infection, indicating that proteasome-mediated degradation and HSV-1 ICP0 play crucial roles in this novel viral immune escape mechanism.
Dendritic cells (DCs) are the most potent antigen-presenting cells known (41). They have the unique ability to prime naive CD4+ and CD8+ T cells and thereby induce a primary immune response. As sentinels of the immune system, they lie in wait in an immature state in almost all peripheral tissues (1). Upon encountering diverse products of infectious agents, they begin to mature and lose their ability to take up antigens (57). In order to activate antigen-specific T cells, mature DCs (mDCs) must migrate from the areas of antigen uptake to the areas of antigen presentation, primarily the T-cell zones of the secondary lymphoid organs (1). During maturation, DCs undergo significant functional and phenotypic changes; for example, they develop the ability to migrate into T-cell areas (25); they respond to different CC and CXC chemokines, compared to immature DCs (iDCs); and they strongly upregulate the surface molecule CD83 together with other costimulatory molecules such as CD80 and CD86 (73).
With respect to its strong upregulation, CD83 is well known as one of the best cell surface markers for human mDCs (1). Recently, it has been demonstrated that a precursor form of CD83 can be found inside monocytes, macrophages, and iDCs (6). However, CD83 is only stably expressed on mDCs (6) and some activated T cells and B cells (68). Interestingly, two different isoforms of CD83 have been described, a membrane-bound form (mCD83) (71, 72) and a soluble form (sCD83) (30, 31). The latter is most probably generated by proteolytic shedding of the mCD83 isoform, but the precise mechanism is still unknown (31). Increasing amounts of sCD83 have been detected by Hock and coworkers in a number of patients suffering from hematological malignancies, including patients with chronic lymphocytic leukemia and mantle cell lymphoma (30). These data indicate that sCD83 might play an important role during the downmodulation of immune responses, and indeed this was demonstrated in vitro by using mixed-lymphocyte reaction assays. Interestingly, sCD83 inhibited DC-mediated allogeneic T-cell stimulation in a dose-dependent manner (34). These observations were further strengthened with models of autoimmune diseases (75). Therefore, the effect of sCD83 was analyzed in vivo by using the murine experimental autoimmune encephalomyelitis model. It was found that sCD83 was very effective in a prophylactic, as well as in a therapeutic, application, underlining its high immunosuppressive potential also in vivo (75).
It is noteworthy that several viruses influence CD83 surface expression and thereby prevent the activation of T cells. Sénéchal and coworkers reported that sCD83 is shed from the surface of mDCs after infection with human cytomegalovirus (HCMV), a member of the Herpesviridae family (63). In the case of herpes simplex virus type 1 (HSV-1), an effect on CD83 surface expression has been detected for iDCs, as well as for mDCs. HSV-1 is able to initiate infection of both types of DCs efficiently (32, 42). Infection of iDCs with HSV-1 led to significant cytopathic effects (20 to 45% of the cells die within 24 to 48 h), generation of infectious viral particles, and failure of DC maturation (42, 47). Furthermore, CD83 upregulation was almost completely blocked during maturation (60). This elimination of antigen-presenting cells might represent an efficient way to avoid antiviral immune responses.
Kruse and coworkers reported that, in contrast to iDCs, HSV-1 infection of mDCs does not lead to infectious particles. At a multiplicity of infection (MOI) of 1, HSV-1 did not induce cell death but, surprisingly, Western blot analysis of total cell lysates revealed that the CD83 molecule was completely degraded within 24 h and was already present in reduced amounts on the cell surface after 10 h (32), while other costimulatory surface molecules such as CD80 or CD86 were unaffected. However, data concerning the underlying mechanism, the kinetics of downregulation, and the identities of viral gene products which may be responsible for this effect have not been presented until now.
A great deal of attention has been drawn to the immediate-early (IE) proteins of HSV-1, which are of crucial importance for the regulation of viral gene expression and virus-host cell interactions (45). Five IE genes are encoded by the viral genome, and their transcription is activated by the viral transactivator VP16 (69). These five proteins are infected cell protein 0 (ICP0), ICP4, ICP22, ICP27, and ICP47. In addition to VP16, another key regulatory protein, the virion host shutoff (vhs) protein, is a component of the virus particle and thus is delivered into the cell at the very beginning of infection. The vhs protein has been characterized as a viral RNase that degrades mRNAs (cellular mRNAs, as well as viral mRNAs) (23), and it has been suggested to be one of the main mediators of HSV-1 immune evasion responses (64).
ICP47 has also been directly associated with an immune escape mechanism; this viral IE protein complexes with and thereby inhibits the transporter associated with antigen presentation, which leads to a lack of major histocompatibility complex (MHC) class I peptide complex assembly (70) and to an inhibition of CD8+ T-cell-mediated protection (26).
Although it is not essential for HSV-1 replication in cultured cells infected at a high multiplicity (15), IE ICP0 plays a very special role during virus infection. In the absence of ICP0, the lytic replication cycle is initiated very poorly because the viral genome becomes subject to repression. Such repressed HSV-1 genomes in cultured cells can be reactivated by later expression of ICP0, and in animal models, ICP0-null mutant viruses reactivate poorly from latency (for reviews, see references 14, 16, 28, and 56). Several groups have reported that ICP0 inhibits the expression of interferon-stimulated genes that occurs in response to HSV-1 infection and might thereby inhibit cellular antiviral responses (13, 46). These and other functions of ICP0 are dependent on the presence of its so-called RING finger domain, which confers on the protein ubiquitin E3 ligase activity whose consequence is the degradation of a number of cellular proteins via the ubiquitin-proteasome pathway (5, 28, 29). Several cellular proteins are degraded during HSV-1 infection in an ICP0-dependent manner, including the promyeolytic leukemia (PML) protein (4, 19), SUMO-modified Sp100 (7, 27, 48), DNA-PK (54), and CENP-C (18). Thus, several cellular proteins are targets of the ubiquitin E3 ligase activity of ICP0, and inhibition of this activity with proteasome inhibitors or by mutation of the RING finger domain of ICP0 results in a significant reduction of viral genome expression, particularly at low MOIs, and as a consequence an increased likelihood of the virus entering into a quiescent or latent state of infection (16, 21).
In this study, we provide new insights into how HSV-1 disarms mDCs by the downmodulation of CD83 expression. By fluorescence-activated cell sorter (FACS) analyses, we were able to show that CD83 is downregulated with very fast kinetics after infection with HSV-1. This loss of CD83 from the cell surface is due not to a virus-induced shedding mechanism but rather to protein degradation. Infection with an ICP0 deletion mutant, together with immunofluorescence data and transfection experiments, revealed an important role for this IE protein during virus-induced CD83 degradation. Inhibition of proteasome function by a specific inhibitor of the cellular degradation machinery provided further evidence that this IE protein of HSV-1 is, either directly or indirectly, responsible for this effect. In summary, we provide here, for the first time, evidence of the molecular mechanism by which CD83 is degraded during HSV-1 infection.
MATERIALS AND METHODS
Virus strains, virus preparation, and virus titration.
HSV-1 strain 17+ was the wild-type (WT) laboratory strain used in these studies, from which the ICP0 deletion mutant virus dl1403 (HSV-1 ΔICP0) was derived (66). HSV-1/17+/CMV-EGFP/ UL43 (HSV-1 WT EGFP) contains the enhanced green fluorescent protein (EGFP) marker gene. This reporter is driven by the CMV promoter and has been inserted into the gene for UL43. As the gene for UL43 has been described as a nonessential gene (10, 61) which has previously been shown not to affect the kinetics of HSV reactivation and latency (42), this virus closely resembles the WT virus. The HSV-1/pR20.5/vhs (HSV-1 Δvhs) strain contains a cassette expressing EGFP and LacZ and was described previously (38). Virus stocks were prepared and the virus titer was determined as previously described (65). UV-inactivated particles were generated by irradiation with UV light (1,500 J/cm2) in a Vilber Luormat (Biometra, Göttingen, Germany).
Plasmids.
The expression plasmid pcDNA3-CD83, containing the CD83 coding sequence under the control of a CMV promoter, has been described previously (32). Plasmids pCI-110 and pCI-FXE express full-length ICP0 and RING finger deletion mutant ICP0, respectively (20). Plasmid pEG110 contains full-length ICP0 fused to EGFP (39).
Generation of DCs.
Peripheral blood mononuclear cells were isolated from different healthy donors by sedimentation with Lymphoprep (Nycomed Pharma AS, Oslo, Norway) and cultured in RPMI 1640 medium (BioWhittaker, Verviers, Belgium) supplemented with 1% autologous serum, 10 mM HEPES (pH 7.5; Sigma-Aldrich, Deisenhofen, Germany), 2 mM l-glutamine (Cambrex BioScience, Verviers, Belgium), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma). Mononuclear cells were seeded into standard tissue culture flasks (Nunc, Wiesbaden, Germany) and incubated for 1 h. The nonadherent fraction was washed off after 1 h with pure RPMI 1640 medium. iDCs were generated in RPMI 1640 medium supplemented with 1% autologous serum, 10 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 800 U/ml granulocyte-macrophage colony-stimulating factor (GMCSF; Wyeth), and 250 U/ml interleukin-4 (IL-4; Strathmann, Hamburg, Germany). Nonadherent cells (i.e., iDCs) were collected after 4 days of cultivation, counted, and transferred into new flasks. Maturation was induced by adding 10 ng/ml tumor necrosis factor alpha (Strathmann), 1 μg/ml prostaglandin E2 (Sigma), 200 U/ml IL-1β (Strathmann), 40 U/ml GMCSF, 1,000 U/ml IL-6 (Strathmann), and 250 U/ml IL-4 to the medium. Maturation was complete 2 days later.
To ensure the purity of the mDCs obtained, the cell population was analyzed by FACS for contamination with T cells (CD3 antibody; BD, Heidelberg, Germany), B cells (CD19 antibody, clone S725-C1; Caltag, Hamburg, Germany), and NK cells (CD56 antibody, clone MEM-188; Immunotools, Friesoythe, Germany) together with the respective isotype controls immunoglobulin G1 (IgG1) and IgG2a, clone G155-178 (BD, Heidelberg, Germany). Normally, mDCs showed a purity of 85 to 90%.
Infection procedure.
Cells (3 × 106) were infected with the respective virus strain in a total volume of 300 μl of infection medium containing pure RPMI medium and 20 mM HEPES (pH 7.5; Cambrex BioScience, Verviers, Belgium) at an MOI of 1. The infection procedure was carried out for 1 h at 37°C in a shaking heating block (Eppendorf, Hamburg, Germany) at 300 rpm. After this, the cells were transferred to DC medium at a concentration of 0.5 × 106/ml until they were harvested for further experiments (DC medium was RPMI 1640 medium containing 1% autologous serum, 10 mM HEPES [pH 7.5], 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 40 U/ml GMCSF, and 250 U/ml IL-4).
FACS analysis.
The cell surface phenotype was analyzed by FACS analysis. The monoclonal antibodies (MAbs) used were anti-CD83 (clone HB15e), anti-CD80 (clone L307.4), anti-CD86 (clone 2331 [FUN-1]), and anti-MHC class II [clone G46-6(L243)], together with the respective isotype controls IgG1 (clone MOPC-21), IgG2a (clone G155-178), and IgG2b (clone 27-35). All antibodies were obtained from BD Biosciences, Heidelberg, Germany, and used according to the manufacturer's instructions. All antibodies were phycoerythrin (PE) labeled.
For calculation of relative CD83 surface expression (rseCD83), an infection procedure was carried out as described above without adding virus. The number of CD83-positive cells determined by FACS was set as 100%. rseCD83 shows surface expression compared to that in the uninfected population.
CHX-ActD chase experiment.
A cycloheximide (CHX)-actinomycin D (ActD) chase experiment was performed essentially as described before (49). In brief, cells were mock infected or infected with HSV-1 at an MOI of 1 in the presence of 100 μg/ml CHX and incubated at 37°C and 300 rpm on a thermo-mixer for 1 h. Afterwards, the cells were transferred into culture medium containing 100 μg/ml CHX. After an additional 4 h, cells were washed twice in RPMI medium containing either 5 μg/ml ActD or dimethyl sulfoxide (DMSO; 5 μl/ml) and then transferred into culture medium with DMSO or ActD, respectively. Another 16 h later, cells were harvested and analyzed for surface expression of CD80 and CD83 by flow cytometry. The efficiency of the block of the early and late genes was verified by reverse transcription (RT)-PCR for ICP27 (IE), UL39 (early), and gG (late).
Immunofluorescence microscopy.
The following antibodies and stain were used for immunofluorescence microscopy: anti-ICP0 MAb 11060 (1:2,000), anti-CD83 MAb HB15e (Immunotech/Beckman Coulter GmbH, Krefeld, Germany), and 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes/Invitrogen, Karlsruhe, Germany). DCs were centrifuged onto polylysine-coated microslides (Menzel-Glaeser, Mainz, Germany) for 30 s at 400 rpm with a Cytospin 3 centrifuge (Shandon, Pittsburgh, PA). The cells were then fixed with 2% paraformaldehyde (Merck, Darmstadt, Germany) and subsequently permeabilized with 0.1% Triton X-100 (Sigma, Schnelldorf, Germany) for 4 min and blocked with 1% bovine serum albumin (Sigma) for 30 min. The cells were then stained for 2 h at room temperature with the respective antibodies. Following extensive washing steps in phosphate-buffered saline (PBS), cells were incubated for 2 h at room temperature with appropriate secondary antibodies conjugated to Cy2- or Cy3-conjugated fluorophores (Rockland, Gilbertsville, PA). Samples were washed in PBS and mounted in Mowiol (Calbiochem, Bad Soden, Germany).
Samples were analyzed with a Leica DM IRB microscope (Leica, Bensheim, Germany). Images were recorded with a cooled MicroMax charge-coupled device camera (Princeton Instruments) and processed with the OpenLab software (Improvision, Tübingen, Germany) and Adobe Photoshop software.
ELISA for sCD83.
The levels of sCD83 were determined with a sandwich enzyme-linked immunosorbent assay (ELISA) essentially as described before (31), with MAbs HB15a (CD83, IgG2b) and TP1.55.3 (CD69, IgG2b) (Coulter Immunotech, Marseilles, France). The ELISA was measured in a Multiskan Plus microplate reader (Labsystems).
RNA isolation and RT.
Cells were harvested and washed with PBS. Total RNA was isolated with the RNeasy Mini Kit and QIAshredder spin columns (QIAGEN, Hilden, Germany). Traces of genomic DNA were removed by DNase digestion with the RNase-free DNase set (QIAGEN). Subsequently, 1 μg of each RNA was reverse transcribed into a single-stranded cDNA with avian myeloblastosis virus reverse transcriptase as specified by the manufacturer, together with oligo(dT) primers as supplied by the manufacturer (Promega, Heidelberg, Germany).
RT-PCR.
The following PCR primers were used for RT-PCR: CD83 sense (5′-GTTATTGGAGGGTGGTGAAGAGAGG) and antisense (5′-GTGAGGAGTCACTAGCCCTAAATGC), ICP0 sense (5′-ACTCTGAGGCGGAGACCGAA) and antisense (5′-TTGCACAGCGGGCAGGTGTT), ICP4 sense (5′-CGACACGGATCCACGACCC) and antisense (5′-GATCCCCCTCCCGCGCTTCG), ICP22 sense (5′-GCTCACGAGCTCTCCCGATC) and antisense (5′-AACAAGGAAGCTTGCACACG), ICP27 sense (5′-CGAGACCAGACGGGTCTCCTGG) and antisense (5′-GCAGACACGACTCGAACACTCCTG), ICP47 sense (5′-ACTCTGAGGCGGAGACCGAA) and antisense (5′-GAAATGGCGGACACCTTCCT), UL39 sense (5′-GACAGCCATATCCTGAGC) and antisense (5′-ACTCACAGATCGTTGACGACCG), glycoprotein G (gG) sense (5′-CATGCCAAGTATTGGACTGGAGGAG) and antisense (5′-CACAGGTGTGTCGCCATCGCAC), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sense (5′-CACCACCATGGAGAAGGCTGG) and antisense (5′-GAAGTCAGAGGAGACCACCTG). The following PCR cycling profile (30 cycles) was used: 94°C for 60 s, 60°C for 60 s, and 72°C for 2 min. The reaction products were visualized by ethidium bromide staining on 2% agarose gels.
Transfection of 293T cells.
293T cells were transfected with the following plasmids in various combinations: 0.02 μg of pcDNA3-CD83; 1.0 μg of pCI-FXE; 0.75 μg of pcDNA3; and 0.5, 0.75, or 1.0 μg of pCI-110 and the pCI-110GFP plasmid. Transfection was accomplished by the DEAE-dextran method in accordance with a previously published protocol (11). Cells were harvested at 24 h posttransfection, lysed, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Western blotting.
The following primary antibodies were used for Western blot analysis: anti-CD83 MAb (clone 1G11 [33], 1:500), anti-CD86 MAb (clone 2331 [FUN-1]), anti-β-actin MAb (clone AC-74 [Sigma-Aldrich], 1:1,000), and anti-ICP0 MAb (clone 11060 [17], 1:2,000). Lysates were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with the primary antibody for 1 h at room temperature to overnight at 4°C, depending on the antibody used. After incubation with the appropriate horseradish peroxidase-labeled secondary antibody, detection was performed with ECL Western blotting substrate (Pierce, Rockford, IL).
Statistical methods.
If not otherwise stated, results are shown as means ± standard deviations (SD). To determine the significance of variance in the experimental results obtained, data were analyzed with the Student t test. Significance was accepted if P was <0.05.
RESULTS
CD83 is downregulated from the surface of infected mDCs with fast kinetics and in a vhs-independent manner.
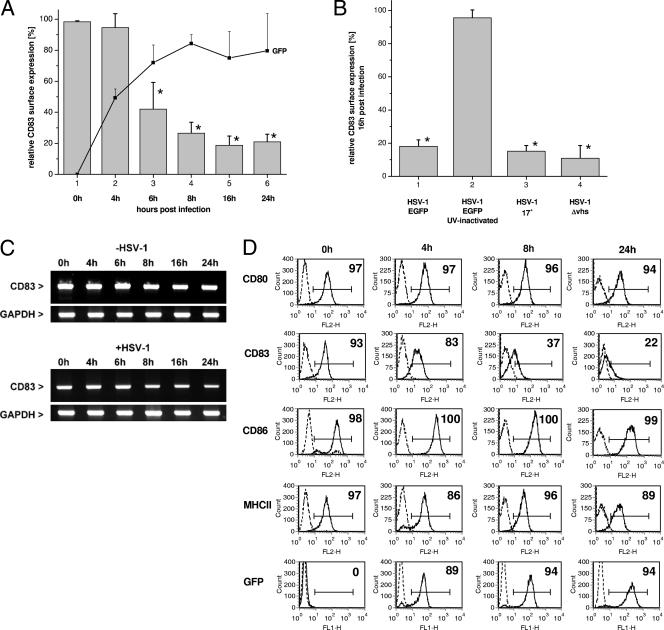
mDCs were infected at an MOI of 1 with an HSV-1 strain expressing EGFP under the control of a CMV promoter (HSV-1 WT EGFP). This allowed easy determination of infection efficiency by FACS analysis. To additionally confirm the data reported by Kruse and coworkers (32) and to provide more detailed information regarding the efficiency and kinetics of HSV-1-mediated CD83 downregulation, the surface expression of this molecule was determined together with the respective EGFP expression at various time points. Figure 1A shows the CD83 expression profile of infected mDCs (rseCD83). As early as 6 h after the first contact of viral particles with the cells, a clear and strong loss of CD83 from the cell surface was detected: only approximately 50% of the cells were still CD83 positive compared with uninfected cells. This effect increased over time, resulting in populations with only 20% CD83 expression after 16 and 24 h. It is noteworthy that CD83 downregulation goes hand in hand with increasing EGFP expression, indicating ongoing transcription from the viral genome, and that this rapid downmodulation was observed only for CD83 and not other cell surface markers. As previously reported, the expression of CD80, CD86, and MHC class II was not influenced by HSV-1 (Fig. 1D). Finally, it should be noted that infection of mDCs with HSV-1 does not lead to rapid induction of apoptosis; thus, the observed effects are not a consequence of cell death (32, 55).
FIG. 1.
CD83 is downregulated from the infected mDC surface with fast kinetics by a vhs-independent mechanism, independently of other costimulatory molecules. (A) rseCD83 on HSV-1 WT EGFP-infected mDCs (compared to uninfected cells). With the increase in viral gene expression (indicated by increasing EGFP expression; thin black line), CD83 was downregulated. A significant loss of CD83 expression from the surface was detected as early as 6 hpi (only ∼20% of the cells were still positive for CD83). The EGFP graph shows the mean infection efficiency profile of at least three different DC isolations from different donors ± the SD. (B) rseCD83 at 16 hpi with different viruses. Column 2 shows the rseCD83 of mDCs infected with UV-inactivated HSV-1 WT EGFP. Column 3 shows infection with a WT laboratory strain carrying no EGFP insertion. Column 4 shows infection with a Δvhs mutant virus. The columns represent surface expression on infected DCs from three independent experiments with cells from different donors (i.e., mean rseCD83 ± SD). Significant changes compared with uninfected DCs are marked with asterisks (P < 0.05). (C) RT-PCR analysis of CD83 mRNA levels. The lower part demonstrates CD83 expression in the case of HSV-1 WT EGFP infection over time, while the upper part shows CD83 mRNA levels of uninfected cells. This panel is representative of three independent experiments with cells from different donors. (D) Histograms demonstrating the effect of HSV-1 infection on the surface expression of the costimulatory molecules CD80 (first lane), CD86 (third lane), and MHC class II (fourth lane) at 4 (column 2), 8 (column 3), and 24 (column 4) hpi. While the surface expression of these markers is not influenced, CD83 (second lane) is dramatically reduced over time. EGFP expression (fifth lane) demonstrates the efficiency of cell infection. This panel is representative of three independent experiments with cells from different donors.
To provide evidence that the mechanism depends on active viral gene expression and was not simply a result of inserting an EGFP-expressing cassette into the viral genome, we performed the following control experiment. Viral particles were inactivated with UV radiation, and mDCs were infected according to our standard infection procedure. As shown in Fig. 1B, column 2, UV-inactivated virus was no longer able to downmodulate CD83. In contrast to the inactivated virions, a WT laboratory HSV-1 strain and the EGFP-expressing virus strain were equally able to induce CD83 downregulation (Fig. 1B, columns 1 and 3).
The vhs protein has been discussed as one possible mediator of immune escape mechanisms induced by HSV-1. It functions as a viral RNase and is able to degrade both viral and cellular mRNAs (23, 52, 64). To exclude the possibility that degradation of host mRNAs was the reason for CD83 downmodulation, we infected mDCs with an HSV-1 mutant carrying a deletion of vhs (Δvhs) at an MOI of 1 and determined the CD83 surface expression at 16 h postinfection (hpi). As shown in Fig. 1B, column 4, the Δvhs virus induced CD83 downregulation to an extent comparable to that of the WT strain. To further strengthen these results, we isolated RNA from infected and uninfected cells at different time points. The RNA was reverse transcribed, and a PCR for the CD83-encoding cDNA was carried out. Figure 1C demonstrates that the observed CD83 protein downregulation is not due to a reduction of the cognate mRNA. Although HSV-1 infection caused a slight reduction in CD83 mRNA levels at later time points (Fig. 1C, bottom), the loss of cell surface expression of CD83 occurred at a much faster rate (Fig. 1B, column 1).
Finally, we provide additional information on the surface expression of other costimulatory molecules. Therefore, mDCs were infected with the EGFP-expressing WT virus and at 0, 4, 8, and 24 h after infection cells were analyzed by FACS for surface expression of CD80, CD83, CD86, and MHC class II. Figure 1D shows the histogram plots of CD80 (first lane), CD83 (second lane), CD86 (third lane), and MHC class II (fourth lane) at 4 h (column 2), 8 h (column 3), and 24 h (column 4) after infection. While the surface expression of these molecules is not influenced, CD83 expression is dramatically reduced over time. EGFP expression (fifth lane) demonstrates efficiency of cell infection.
CD83 is not shed in a soluble form after infection with HSV-1 and is also not trapped inside the cell.
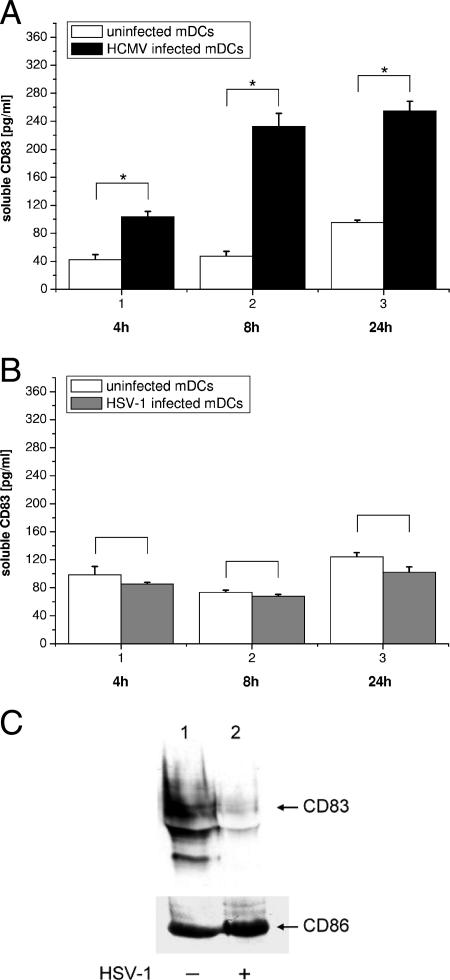
Sénéchal and coworkers reported that infection of monocyte-derived mDCs with HCMV increased the amount of the soluble isoform of CD83 in the culture medium by a factor of 3 by 1 day after infection. Thus, in the case of HCMV, mCD83 is converted into a soluble form by an undefined virus-induced shedding mechanism (63).
In order to investigate whether a similar mechanism was in operation during HSV-1 infection, mDCs were either left uninfected or infected with HCMV strain AD169 (Fig. 2A) or an HSV-1 WT EGFP strain (Fig. 2B). At the indicated time points, aliquots of the cell culture medium were collected and the concentration of sCD83 was determined. Figure 2A essentially confirms the data reported by the Sénéchal group; at 24 hpi with HCMV (black bars), an approximate threefold increase in sCD83 could be detected, compared with uninfected cells (white bars). However, infection with HSV-1 (Fig. 2B, infected cells, gray bars) did not lead to increased levels of sCD83 in the culture supernatant. Even at 24 hpi with HSV-1, no increased sCD83 levels were observed (Fig. 2B, column 3). These results demonstrate that, rather than shedding, another mechanism must be responsible for the observed HSV-1-mediated CD83 downmodulation.
FIG. 2.
CD83 is not shed from the cell surface after HSV-1 infection and is also not trapped inside the cell. (A) Release of sCD83 after HCMV infection. mDCs were infected with HCMV AD169 at an MOI of 1, and after 4 h (column 1), 8 h (column 2), and 24 h (column 3), aliquots of the medium were analyzed. Infected cells are represented by black bars, and uninfected cells are represented by white bars. (B) Infection of mDCs with HSV-1 did not promote the release of sCD83 into the culture medium. This experiment was performed essentially as described for panel A, except that the cells were infected with WT HSV-1 (gray bars). While in HCMV-infected cells release of sCD83 into the medium could be detected, no increase in sCD83 levels was observed after HSV-1 infection. (C) Western blot analysis of HSV-1-infected or mock-infected cells. After 16 h, cells were harvested and equal amounts were separated by 12% PAGE. The upper part of the panel shows the total cellular amount of the highly glycosylated CD83 molecule in mock-infected (right column) and HSV-1 WT EGFP-infected (left column) cells. The lower part of the panel shows reprobing of the membrane with a CD86 antibody, indicating no effect on CD86 cellular levels and serving as a loading control. These results are representative of three independent experiments with cells derived from different donors. Significant changes (P < 0.05) are indicated by asterisks.
To provide further evidence for virally induced degradation of CD83, we performed Western blot experiments. Thus, mDCs were either mock infected or infected with HSV-1 WT EGFP and 16 h after the infection, the cells were harvested and the lysate was resolved by 12% PAGE. After transfer, the membrane was stained for CD83 (Fig. 2C, top) and reprobed for CD86 (Fig. 2C, bottom). While mock-infected cells showed high levels of glycosylated CD83 (33) (Fig. 2C, top, lane 1), HSV-1-infected cells had strongly reduced levels of CD83 (Fig. 2C, top, lane 2). In strong contrast, CD86 levels (Fig. 2C, bottom) were not affected by HSV-1 infection. These data confirm that CD83 is not retained inside the cell after infection with HSV-1 but is degraded independently from other costimulatory molecules.
Inhibition of the cellular proteasome in HSV-1-infected mDCs strongly reduces CD83 downregulation from the cell surface.
Proteasome-mediated degradation is the eventual fate of the majority of proteins inside a eukaryotic cell. Usually, the process of selective degradation by this nanomachine is divided into three main steps, (i) finding the target protein for degradation, (ii) marking the target protein by the attachment of ubiquitin moieties in a polyubiquitin chain (ubiquitination), and (iii) degradation inside the proteasome with recycling of the ubiquitin (for detailed information about the proteasome and ubiquitination, see references 2, 8, and 67).
For this reason, we investigated whether manipulation of the cellular degradation machinery influences the virus-induced degradation of CD83. The peptide aldehyde MG-132 is a specific and reversible inhibitor of the proteasome (35, 53) that blocks the 26S subunit of the proteasome without influencing its ATPase or isopeptidase activity (35). Furthermore, MG-132 does not generally affect cell viability for up to 20 h (59).
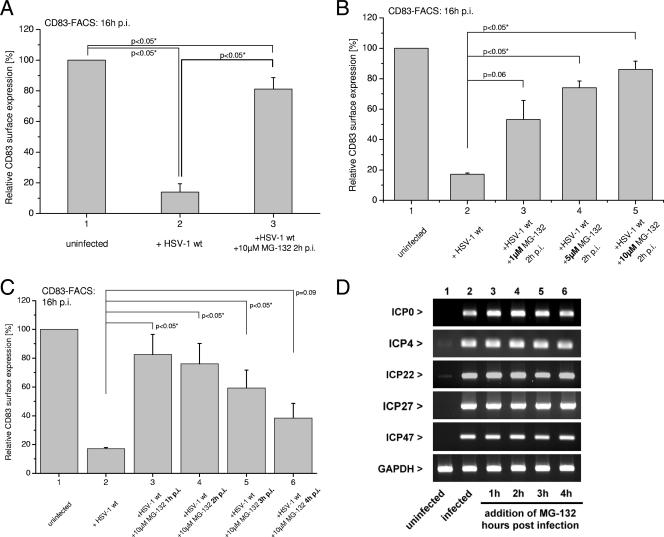
First, we tested the effect of MG-132 on CD83 downregulation by comparing levels of FACS-detectable surface CD83 in HSV-1-infected and uninfected mDCs in the presence or absence of MG-132 at 16 hpi. Figure 3A demonstrates that MG-132 inhibited the loss of CD83 surface expression from infected mDCs. Without addition of the proteasome inhibitor, only 15% of the cells were CD83 positive (Fig. 3A, column 2), whereas in the presence of 10 μM MG-132, CD83 surface expression was retained in 80% of the cells (Fig. 3A, column 3). To exclude the possibility that a toxic effect of MG-132 was responsible for our observations, we repeated the experiment but this time stained with propidium iodide and gated on propidium iodide-negative cells (see Fig. S1 in the supplemental material). In order to provide evidence for the specificity of our observations, we next infected mDCs with HSV-1 and transferred the cells 2 h later to medium containing different concentrations of MG-132. Figure 3B shows the relative surface expression of CD83 at 16 hpi. When only a 1 μM concentration of the drug was added, the number of cells that retained CD83 surface expression increased from 15% in the absence of the drug (Fig. 3B, column 2) to 50% (Fig. 3B, column 3). With higher concentrations of MG-132 (Fig. 3B, column 4, 5 μM MG-132, and column 5, 10 μM MG-132), retention of CD83 surface expression increased to a level of 80% of the cells. These data confirm that the proteasome plays a crucial role in CD83 degradation and that MG-132 inhibits the virus-induced loss of CD83 expression in a concentration-dependent manner.
FIG. 3.
Inhibition of the cellular proteasome by MG-132 prevents CD83 degradation after infection of mDCs with HSV-1 in a time- and concentration-dependent manner. (A) MG-132 prevents downregulation of CD83 after infection with HSV-1. mDCs were infected with WT HSV-1 at an MOI of 1 (middle and right columns) or left untreated (left column). At 2 hpi, 10 μM MG-132 was added to the culture medium (left column), and at 16 hpi, cells were analyzed for CD83 surface expression by FACS. The uninfected DCs were set as 100%, and compared to infected DCs, only approximately 15% of the cells were positive for CD83. However, blocking the cellular proteasome at 2 hpi restored CD83 surface expression significantly (80% positive cells). (B) Prevention of CD83 degradation by MG-132 is concentration dependent. MG-132 was added to HSV-1-infected cells at 2 hpi in different concentrations (column 3, 1 μM; column 4, 5 μM; column 5, 10 μM). With 1 μM, 50% of the cells were CD83 positive; with 5 μM, 70% of the cells were CD83 positive; and with 10 μM, >80% of the cells were CD83 positive. (C) Prevention of CD83 degradation depends on the time point of MG-132 addition. Addition of 10 μM MG-132 at 1 hpi (column 3) restored rseCD83 up to 80% when analyzed at 16 hpi. The later MG-132 was added to the DC medium, the less prominent was the effect (column 4, 2 hpi, 75%; column 5, 3 hpi, 60%; column 6, 4 hpi, 40%), strongly indicating that an IE protein is most likely responsible for or involved in the mediation of CD83 degradation. (D) Addition of MG-132 did not influence the expression of HSV-1 IE genes. mDCs were infected with WT HSV-1 or left untreated (column 1). At different time points postinfection, 10 μM MG-132 was added to the culture medium (columns 3 to 6) and RT-PCR analyses of IE mRNAs were performed. GAPDH served as an internal loading control. The graphs represent the CD83 expression of infected DCs from three independent experiments with cells from different donors (i.e., mean relative surface expression compared to uninfected cells ± SD). Significant changes (P < 0.05) are indicated.
To investigate whether specific HSV-1 proteins, particularly of the IE class, were involved in CD83 degradation, we next performed kinetic experiments. mDCs were infected with WT HSV-1, and MG-132 was added 1, 2, 3, or 4 hpi. Figure 3C shows that the observed effect of blocking CD83 downmodulation was strictly dependent on the time point of addition of MG-132 to the infected cells. When the proteasome inhibitor was added at 1 hpi, CD83 expression was retained in approximately 80% of the cells at 16 hpi, whereas only 40% of the cells remained CD83 positive when MG-132 was added later, at 4 hpi. Thus, the effect of MG-132 is time and concentration dependent. Furthermore, a delay of about 2 h until the drug unfolds its full action could be observed (see Fig. S2 in the supplemental material). These results imply that a viral protein expressed during the first 4 h of infection is directly or indirectly responsible.
Zhu and coworkers reported that ubiquitin-mediated proteolytic processing is involved in transcriptional activation by the viral VP16 transactivation domain. They provided further evidence that a subunit of the proteasome (SUG-1) is recruited for transcription activation, as well as for degradation of this transactivator, indicating a role for the proteasome during HSV-1 IE transcription (74). To ensure that application of MG-132 did not simply prevent the transcription of viral IE genes, we performed RT-PCR for all five IE mRNAs at 6 hpi. Figure 3D shows the mRNA levels for uninfected (column 1) and infected (column 2) cells that had been incubated with MG-132 at different time points (columns 3 to 6). We observed that addition of MG-132 to the infected cells did not lead to a reduction of IE mRNA expression, indicating that the observed effect on CD83 is not due to a variation of IE gene transcription caused by MG-132. The cellular GAPDH mRNA served as a loading control in these experiments.
An IE gene product is responsible for the downmodulation of CD83.
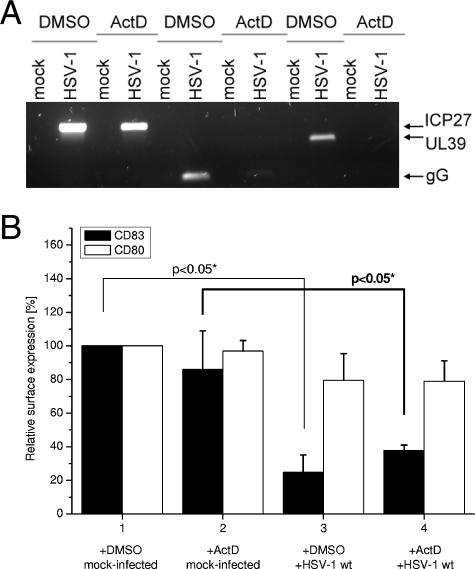
Next, we wanted to confirm our observations described above that an IE gene product is responsible for downmodulation of CD83 from the surface. Therefore, we performed a classical CHX-ActD block chase experiment essentially as described before (49). In brief, mDCs were infected with HSV-1 in the presence of CHX (100 μg/ml) in order to prevent viral protein translation but allow transcription of the viral IE genes. After 4 h, the CHX was washed out and the cells were cultured for a further 16 h in the presence of DMSO (5 μl/ml) or 5 μg/ml ActD, allowing the translation of mRNAs already present but preventing the transcription of new mRNAs. Afterward, cells were harvested and analyzed by FACS for CD80 and CD83 surface expression or total cellular RNA was isolated. To confirm a complete block of the expression of early and late gene products, we first performed RT-PCR experiments with the isolated total cellular mRNAs. Figure 4A demonstrates that the IE gene product ICP27 could be detected efficiently in both DMSO- and ActD-treated cells. In contrast, the UL39 mRNA (representing an early gene product) and the gG mRNA (representing a late gene product) could not be detected. This confirms a complete block of viral early and late gene expression.
FIG. 4.
An IE gene product is responsible for initiation of CD83 downmodulation. A CHX-ActD block chase experiment demonstrates that an IE gene product is involved in downmodulation of CD83 from the cell surface. (A) An RT-PCR was performed to analyze the expression of viral genes from each of the three phases of replication of HSV-1. The gene for IE ICP27 was expressed in HSV-1-infected mDCs after addition of DMSO and ActD (5 μg/ml). The mRNAs for the early gene for UL39 and the late gene for gG, however, were only present in the DMSO-treated samples. This experiment demonstrates that the block of early and late gene transcription was efficient. (B) Surface expression of CD83 and CD80 on mock-infected or HSV-1-infected mDCs was analyzed by FACS. Mock-infected and DMSO-treated cells were set to 100%, and relative surface expression is shown. In CHX- and DMSO-treated cells, infection with HSV-1 resulted in a significant reduction of CD83 levels. The same is true for CHX- and ActD-treated mDCs, indicating a major role for an IE gene product in this process. Surface expression of CD80 was not significantly influenced under any condition.
Next, we analyzed the cells for surface expression of CD80 and CD83. Figure 4B demonstrates the relative surface expression (+DMSO mock infected set as 100%) of cells that were mock infected or HSV-1 infected and either treated with ActD (columns 2 and 4) or left untreated (columns 1 and 3). While CD80 was again not affected by the HSV-1 treatment, cells that were blocked by ActD for the production of early and late gene products (column 4) also showed significantly reduced surface levels of CD83. This additionally supports our data that an IE protein directly or indirectly catalyzes CD83 downmodulation.
An ICP0 deletion mutant of HSV-1 shows diminished CD83 downmodulation.
HSV-1 infection has been reported to result in the proteasome-mediated degradation of several cellular proteins. One of its IE proteins, ICP0, is a ubiquitin E3 ligase that stimulates the formation of polyubiquitin chains, and this activity either directly or indirectly leads to the degradation of a number of cellular proteins (3, 4, 7, 18, 19).
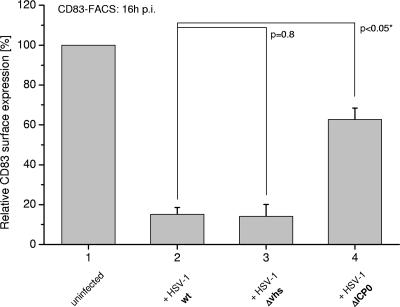
To investigate whether ICP0 was involved in virus-mediated CD83 degradation, mDCs were infected at equal MOIs with the WT and the Δvhs and ΔICP0 mutant strains of HSV-1. To exclude the possibility that the effects observed could be due to delayed expression of viral genes in the absence of ICP0, we performed RT-PCR experiments as a control (see Fig. S3 in the supplemental material). As shown in Fig. 5, in WT-infected (column 2) and Δvhs-infected (column 3) cells, similar levels of CD83 degradation were observed. In contrast, the ICP0 deletion mutant-infected cells showed significantly higher CD83 surface expression compared with WT and Δvhs virus-infected cells (from 15 to 60%). These data suggest that ICP0 plays a role in the observed degradation of CD83. However, some loss of CD83 surface expression occurred in ΔICP0-infected cells, indicating that the loss of CD83 is not dependent on ICP0 exclusively and that other (cellular and/or viral) factors may also be involved. Nevertheless, the effect of deletion of ICP0 was indeed evident.
FIG. 5.
Infection of mDCs with a ΔICP0 mutant virus results in reduced CD83 degradation. Column 2 shows CD83 surface expression on WT HSV-1-infected cells compared to uninfected mDCs (column 1), mDCs infected with a Δvhs mutant strain (column 3), and cells infected with a ΔICP0 mutant strain (column 4). To compare DCs from different donors, the surface expression of uninfected cells at the indicated time point was set as 100%; the columns represent the expression on infected DCs from three independent experiments with cells from different donors (i.e., mean relative surface expression compared to uninfected cells ± SD). Significant changes compared with WT HSV-1-infected DCs are marked with asterisks (P < 0.05).
In mDCs, ICP0 localizes predominantly to the cytoplasm and its expression is accompanied by CD83 downmodulation.
Upon infection, newly synthesized ICP0 predominantly accumulates within cellular nuclear substructures known as ND10 or PML nuclear bodies during the early stages of infection of fibroblast cells. The main component of these structures is the PML protein. The ubiquitin ligase activity of ICP0 leads to degradation of the PML protein and disruption of ND10, a process that correlates with the efficiency of progression of the infection to the stage of DNA replication and the formation of replication compartments. Once replication compartments form in these types of cultured cells, ICP0 begins to accumulate in the cytoplasm (reviewed in references 14 and 28). Since proteasome-mediated degradation of CD83 takes place in the cytoplasm of the infected cell, we next investigated whether ICP0 localizes mainly to the nucleus or to the cytoplasm in HSV-1-infected DCs. If the latter, it would present the possibility that ICP0 is involved in the degradation of CD83.
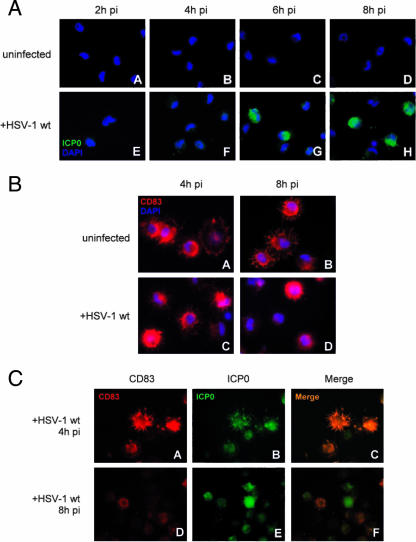
Therefore, mDCs were infected with the WT HSV-1 strain and at 2, 4, 6, or 8 hpi the cells were fixed and stained for both ICP0 and DAPI. Figure 6A demonstrates that in infected mDCs ICP0 is predominantly located in the cytoplasm right from the beginning of the infection and does not preferentially enter the nucleus (Fig. 6A, parts E to H). A predominantly cytoplasmic localization of ICP0 has also been described for certain other cell types (43). As shown in Fig. 6B, parts A to D, CD83 is located, as expected, in the cytoplasm of uninfected, as well HSV-1-infected, cells (Fig. 6B, parts A to D).
FIG. 6.
Immunofluorescent staining of WT HSV-1-infected mDCs. (A) Directly from the start of infection, ICP0 is localized mainly in the cytoplasm. At 2, 4, 6, or 8 hpi, cells (uninfected, parts A to D; WT HSV-1 infected, parts E to H) were fixed and stained for intracellular ICP0; the nucleus was stained with DAPI. (B) At 4 or 8 hpi, cells (uninfected, parts A and B; WT HSV-1 infected, parts C and D) were fixed and stained for CD83. Additionally, the nuclei of the cells were identified with DAPI. (C) ICP0 expression correlates with loss of CD83. At the 4-hpi time point, CD83 and ICP0 accumulate in similar compartments (parts A to C). At 8 hpi, CD83 is already strongly degraded and mainly ICP0 staining is still present (parts D to F).
To determine the localization of both CD83 and ICP0 during the course of infection, costaining experiments for both proteins were performed (Fig. 6C). Noteworthy was the observation that, at 8 hpi, the higher the intensity of the ICP0 staining, the lower the intensity of that of CD83. These data are consistent with our earlier data demonstrating that a large proportion of CD83 is degraded by 8 hpi of DC with HSV-1 (Fig. 1A). At 4 hpi, CD83 and ICP0 could be observed in similar cellular compartments, underlining the potential role of ICP0 in CD83 degradation.
The presence of ICP0 strongly reduces levels of CD83 in transfected 293T cells.
To provide further evidence of a direct influence of ICP0 on CD83 degradation, additional transfection experiments were performed with 293T cells. A plasmid expressing CD83 was transfected together with plasmids encoding WT or mutant ICP0 (all under the control of the CMV promoter). The mutant ICP0 bears a deletion inside the RING finger domain and is thus no longer able to function as an E3 ubiquitin ligase and therefore cannot polyubiquitinate proteins and thus mark them for degradation (5). At 24 h after transfection, cells were lysed and analyzed by SDS-PAGE. Specific antibodies were used to detect CD83, ICP0, or β-actin as a loading control.
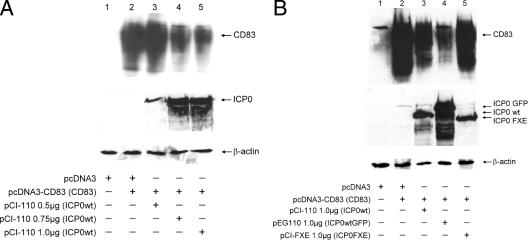
Transfection of CD83 together with the pcDNA3 plasmid (Fig. 7A, lane 2) resulted in large amounts of detectable CD83. By cotransfecting increasing amounts of pCI-110, which encodes WT ICP0, a clear reduction in the accumulation of CD83 was observed, in a manner that was proportional to the amount of the ICP0 expression plasmid used (Fig. 7A, lanes 3 to 5). To test whether the levels of ICP0 correspond to the amount of transfected pCI-110 plasmid, we also probed the membranes for ICP0, and indeed, ICP0 levels increased with rising amounts of plasmid. Furthermore, equal loading was verified by reprobing the membranes with an anti-β-actin MAb.
FIG. 7.
The presence of ICP0, but not ICP0 FXE, strongly reduces CD83 levels in transfected 293T cells. (A) 293T cells were transfected with plasmids expressing CD83 (pcDNA3-CD83, 0.02 μg) and WT ICP0 (pCI-110; ICP0 WT, 0.5, 0.75, and 1.0 μg). Transfection of 0.75 μg of pcDNA3, either alone or together with pcDNA3-CD83, was used as a control. At 24 h after transfection, cells were harvested and lysates were screened for the presence of CD83. In the absence of ICP0, high levels of CD83 could be detected (lane 2, upper row). Cotransfection of rising amounts of WT ICP0 resulted in a dose-dependent reduction of detectable CD83 levels (lanes 3 to 5, upper row). Equal loading was verified by comparing β-actin levels (lower row). Levels of WT ICP0 are shown in the middle row. (B) A 0.02-μg sample of the CD83 expression construct was cotransfected with either 1.0 μg of pCI-110 (ICP0 wt), 1.0 μg of pEG110 (ICP0 GFP), or 1.0 μg of pCI-FXE (ICP0 FXE). For WT ICP0, as well as for ICP0 GFP, once more a reduction of CD83 levels was observed (lanes 3 and 4, upper row). The mutant form, however, did not influence CD83 levels (lane 5, upper row). Transfection of pcDNA3 once again served as a control. Equal loading of the gel was again verified by reprobing with an anti-β-actin antibody (lower row). Each of the transfection experiments was conducted at least three times, and representative data are shown.
To investigate what property of multifunctional ICP0 is involved in the reduction of CD83, we repeated the experiment by including the RING finger mutant mentioned above. Again, a reduction in CD83 levels in the presence of WT ICP0 was observed (Fig. 7B, lanes 3 and 4). The mutant form of ICP0, however, did not have any impact on detectable CD83 (Fig. 7B, lane 5). A transfection experiment without the CD83-expressing plasmid served as a control and demonstrated the specificity of the antibody used (Fig. 7A and B, lane 1). These results strengthen the hypothesis that ICP0 is mechanistically involved in HSV-1-mediated CD83 degradation and that the E3 ligase activity of ICP0 is necessary for this effect.
DISCUSSION
Viruses are highly successful in terms of escaping the host's immune response. In this respect, DCs often come into focus as several viruses influence the function and immunostimulatory capacity of this particular cell type. Examples can be found within all virus families (for an overview, see reference 58). Human immunodeficiency virus type 1, for example, exploits DCs as transport vehicles to reach T-cell-rich areas (36). Furthermore, some human immunodeficiency virus type 1 proteins influence specific functions of DCs. Vpr prevents expression of the costimulatory molecules CD80, CD83, and CD86 at the transcriptional level without altering normal cellular transcription (40); glycoprotein gp120 induces an impaired ability to secrete cytokines or chemokines, and as a consequence, T-cell stimulation is reduced (24).
Varicella-zoster virus (VZV), a member of the Herpesviridae family, is able to infect both iDCs and mDCs. Infection of iDCs does not significantly change DC function but leads to transmission of the virus to T lymphocytes. However, infection of mDCs leads to the downmodulation of several surface markers, including MHC class I, CD80, CD83, and CD86 (44). In contrast, in the case of HSV-1, the downregulation of CD83 in mDCs occurs with much faster kinetics and is specific for CD83 since costimulatory molecules such as CD80 or CD86 are not affected (32). We demonstrate here that, as early as 6 hpi, a significant loss of CD83 from the cell surface can be detected (Fig. 1), whereas with VZV a significant loss of CD83 could be detected only after 96 hpi (44). Another major difference between HSV-1 and VZV is the mode of CD83 downregulation. While VZV-mediated reduction of CD83 surface expression was a consequence of retention of the protein in cytoplasmic vesicles (44), we have demonstrated by Western blotting (Fig. 2C) and immunofluorescence (Fig. 5B, C, and D) that in the case of HSV-1, CD83 is completely degraded.
For another member of the Herpesviridae family, HCMV, an additional and yet new mechanism to influence DC function by modulating the CD83 molecule has been reported (63). Infection of mDCs with HCMV leads to the disappearance of CD83 from the cell surface. Again, as in the case of VZV, this process is much slower than the very fast CD83 degradation induced by HSV-1. At 1 day postinfection, HCMV-infected mDCs show only very low surface expression of CD83 but the molecule is still expressed intracellularly (63). By Western blotting, we have shown that by 16 hpi only very low levels of CD83 are left in the whole-cell lysate (Fig. 2C). However, the most interesting difference between HSV-1 and HCMV is the mode of action. Sénéchal and coworkers reported that HCMV induces shedding of CD83, whereby mCD83 is converted into a soluble form (63). sCD83 can now function as an immunosuppressive agent (12, 34, 62) which inhibits stimulation of T-cell proliferation (63). In contrast, with an ELISA for sCD83, we found that HSV-1 infection of mDCs does not lead to shedding of sCD83 (Fig. 2A and B). These data were confirmed by immunofluorescence, which demonstrated that CD83 disappears from inside the cell, as well as from the cell surface (Fig. 6B).
These observations raise the question of how HSV-1 mediates CD83 degradation. Several HSV-1 proteins have been suggested to be mediators of immune escape mechanisms, for example, the viral RNase vhs protein (64). Accordingly, we tested whether CD83 mRNA levels are influenced by the virus. Although a slight effect of HSV-1 on the CD83 mRNA level could be detected, this occurs slowly and thus cannot be responsible for the loss of surface-bound CD83 from mDCs (Fig. 1C). On the other hand, Samady et al. reported a role for the HSV-1 vhs protein during the HSV-1-induced inhibition of DC maturation. In these experiments, DCs were induced to mature by treatment with lipopolysaccharide (LPS) at the time of infection. In this situation, while LPS-stimulated DCs infected with a WT virus were not able to mature and upregulate CD83, LPS-stimulated DCs infected with a vhs deletion mutant retained the ability to upregulate surface CD83 to a high level (61). In strong contrast with these results, we detected downregulation of CD83 by the Δvhs mutant to an extent similar to that seen with WT HSV-1 (Fig. 1B, columns 1, 3, and 4). Thus, although vhs is important for the block of LPS-induced maturation of iDCs, it seems not to be of importance for CD83 degradation in mDCs.
Controlled degradation of intracellular proteins is of vital importance during a broad range of cellular processes, such as the cell cycle, cell division, and transcription. The fundamental discovery that proteolysis is mainly regulated not by lysosomal degradation but by a complex interplay of a degradation machinery called the proteasome and degradation signals such as ubiquitination was finally honored in 2004 with the Nobel Prize in chemistry (reviewed in reference 9). Several low-molecular-weight inhibitors have been shown to block certain functions of the proteasome (50). By the use of MG-132, we were able to show that blocking the proteasome leads to significantly reduced CD83 downregulation after HSV-1 infection (Fig. 3), in a strictly time- and concentration-dependent manner. Recently, Nencioni and coworkers reported that treatment of DCs with proteasome inhibitors promotes apoptosis. However, this was only observed when inhibitors were added to differentiating monocytes for longer than 24 h (51), which was never the case in our experimental setting, in which the maximum contact with the inhibitor was 16 h. Additionally, we were able to exclude an influence of MG-132 on viral IE gene transcription by RT-PCR experiments (Fig. 3D). In order to exploit the proteasome for its own purposes, the virus must either have an opportunity to manipulate the specific components of the proteasome or have the ability to label proteins for proteasomal degradation (e.g., by the addition of polyubiquitin chains [67]).
Ubiquitination (i.e., the covalent attachment of ubiquitin to specific lysine residues in target proteins) is a stepwise procedure catalyzed by specialized enzymes: activation of ubiquitin (E1), conjugation of ubiquitin (E2), and substrate-specific ligation of ubiquitin to target proteins (E3). One class of E3 ligases, which are responsible for substrate recognition, contains a common zinc-binding RING finger motif (9).
Considering the kinetics of the CD83 degradation experiment, as well as the CHX-ActD block chase experiment, there is clear evidence that an IE gene product is involved in this process. Viral IE protein ICP0, itself a RING finger protein, has been identified as a mediator of the proteasome-dependent degradation of several cellular proteins, consistent with its ability to induce the accumulation of colocalizing conjugated ubiquitin in vivo (5, 22). To investigate the involvement of ICP0 in virus-induced CD83 degradation, we infected mDCs at the same MOI with both a WT and a ΔICP0 strain (Fig. 5). With this ICP0 mutant, CD83 downmodulation was significantly reduced. However, an additional unknown mechanism might still be involved since CD83 expression was not completely restored.
Newly synthesized ICP0 is usually located in the nucleus during the early stages of infection of fibroblasts and other common cultured cell types. Once viral replication compartments begin to form, ICP0 accumulates in increasing amounts in the cytoplasm (28). Thus, in order to interact at the observed early stages with CD83, both expressed on the cell surface and located inside the cytoplasm, ICP0 would have to be present in the cytoplasm of DCs during the early stages of infection. Indeed, we found that in mDCs, ICP0 is present in the cytoplasm even at the earliest stages of infection (Fig. 6A) and therefore, in principle, it has the potential to mediate effects on CD83.
In 293T cotransfection experiments, levels of detectable CD83 were strongly reduced in the presence of a WT ICP0-expressing plasmid. This reduction occurred in a dose-dependent fashion, suggesting a major role for ICP0 in this process (Fig. 7A). This effect was completely abolished when a mutant form of ICP0 defective in ubiquitin E3 ligase activity was used (Fig. 7B). The fact that ICP0 is indeed able to induce the degradation of molecules from the cell surface has been reported by Liang and coworkers for the epidermal growth factor receptor (37).
Taken together, our data suggest an important role for ICP0 in the degradation of CD83. The mechanism seems to be controlled, to a large extent, by ICP0 and requires its E3 ligase activity, as well as a functional cellular proteasome.
Supplementary Material
Acknowledgments
We thank Kerstin Zander for critical reading of the manuscript.
This work was supported by the ELAN-Fonds of the University Hospital Erlangen, grant 04.08.08.2; by the Deutsche Forschungsgemeinschaft, SFB 643, grant A4; by the Bundesministerium für Bildung und Forschung (BMBF)—Nationales Genomforschungsnetz-2 (NGFN-2), grant NIE-S10T02; and by the Interdisziplinaeres Zentrum für Klinische Forschung, IZKF, University Hospital Erlangen, grant B6.
Footnotes
Published ahead of print on 11 April 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Neriah, Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3:20-26. [DOI] [PubMed] [Google Scholar]
- 3.Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 4.Boutell, C., A. Orr, and R. D. Everett. 2003. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77:8686-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, W., S. H. Lee, and J. Lu. 2005. CD83 is preformed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem. J. 385:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanover, A. 2005. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 6:79-87. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover, A. 2005. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture). Angew. Chem. Int. Ed. Engl. 44:5944-5967. [DOI] [PubMed] [Google Scholar]
- 10.Coffin, R. S., A. R. Maclean, D. S. Latchman, and S. M. Brown. 1996. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 3:886-891. [PubMed] [Google Scholar]
- 11.Cullen, B. R. 1987. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 152:684-704. [DOI] [PubMed] [Google Scholar]
- 12.Dudziak, D., F. Nimmerjahn, G. W. Bornkamm, and G. Laux. 2005. Alternative splicing generates putative soluble CD83 proteins that inhibit T cell proliferation. J. Immunol. 174:6672-6676. [DOI] [PubMed] [Google Scholar]
- 13.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 15.Everett, R. D. 1999. A surprising role for the proteasome in the regulation of herpesvirus infection. Trends Biochem. Sci. 24:293-295. [DOI] [PubMed] [Google Scholar]
- 16.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett, R. D., A. Cross, and A. Orr. 1993. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197:751-756. [DOI] [PubMed] [Google Scholar]
- 18.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D., M. Meredith, and A. Orr. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantuzzi, L., C. Purificato, K. Donato, F. Belardelli, and S. Gessani. 2004. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J. Virol. 78:9763-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores-Romo, L. 2001. In vivo maturation and migration of dendritic cells. Immunology 102:255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldsmith, K., W. Chen, D. C. Johnson, and R. L. Hendricks. 1998. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 187:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hock, B. D., L. F. Haring, A. Steinkasserer, K. G. Taylor, W. N. Patton, and J. L. McKenzie. 2004. The soluble form of CD83 is present at elevated levels in a number of hematological malignancies. Leukemia Res. 28:237-241. [DOI] [PubMed] [Google Scholar]
- 31.Hock, B. D., M. Kato, J. L. McKenzie, and D. N. J. Hart. 2001. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int. Immunol. 13:959-967. [DOI] [PubMed] [Google Scholar]
- 32.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechmann, M., E. Kremmer, H. Sticht, and A. Steinkasserer. 2002. Overexpression, purification, and biochemical characterization of the extracellular human CD83 domain and generation of monoclonal antibodies. Protein Expr. Purif. 24:445-452. [DOI] [PubMed] [Google Scholar]
- 34.Lechmann, M., D. J. E. B. Krooshoop, D. Dudziak, E. Kremmer, C. Kuhnt, C. G. Figdor, G. Schuler, and A. Steinkasserer. 2001. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 194:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8:397-403. [DOI] [PubMed] [Google Scholar]
- 36.Lekkerkerker, A. N., Y. van Kooyk, and T. B. Geijtenbeek. 2006. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr. HIV Res. 4:169-176. [DOI] [PubMed] [Google Scholar]
- 37.Liang, Y., A. Kurakin, and B. Roizman. 2005. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc. Natl. Acad. Sci. USA 102:5838-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilley, C. E., F. Groutsi, Z. Han, J. A. Palmer, P. N. Anderson, D. S. Latchman, and R. S. Coffin. 2001. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75:4343-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumder, B., M. L. Janket, E. A. Schafer, K. Schaubert, X. L. Huang, J. Kan-Mitchell, C. R. Rinaldo, Jr., and V. Ayyavoo. 2005. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J. Virol. 79:7990-8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 42.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishige, N., J. V. Jester, J. Naito, N. Osorio, A. Wahlert, C. Jones, R. D. Everett, S. L. Wechsler, and G. C. Perng. 2006. Herpes simplex virus type 1 ICP0 localizes in the stromal layer of infected rabbit corneas and resides predominantly in the cytoplasm and/or perinuclear region of rabbit keratocytes. J. Gen. Virol. 87:2817-2825. [DOI] [PubMed] [Google Scholar]
- 44.Morrow, G., B. Slobedman, A. L. Cunningham, and A. Abendroth. 2003. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J. Virol. 77:4950-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller, D. B., M. J. Raftery, A. Kather, T. Giese, and G. Schonrich. 2004. Frontline: induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur. J. Immunol. 34:941-951. [DOI] [PubMed] [Google Scholar]
- 48.Müller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulvey, M., C. Arias, and I. Mohr. 2006. Resistance of mRNA translation to acute endoplasmic reticulum stress-inducing agents in herpes simplex virus type 1-infected cells requires multiple virus-encoded functions. J. Virol. 80:7354-7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myung, J., K. B. Kim, and C. M. Crews. 2001. The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 21:245-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nencioni, A., A. Garuti, K. Schwarzenberg, G. Cirmena, B. G. Dal, I. Rocco, E. Barbieri, P. Brossart, F. Patrone, and A. Ballestrero. 2006. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur. J. Immunol. 36:681-689. [DOI] [PubMed] [Google Scholar]
- 52.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 54.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prechtel, A. T., N. M. Turza, D. J. Kobelt, J. I. Eisemann, R. S. Coffin, Y. McGrath, C. Hacker, X. Ju, M. Zenke, and A. Steinkasserer. 2005. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J. Gen. Virol. 86:1645-1657. [DOI] [PubMed] [Google Scholar]
- 56.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 57.Reis e Sousa, C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476-483. [DOI] [PubMed] [Google Scholar]
- 58.Rinaldo, C. R., Jr., and P. Piazza. 2004. Virus infection of dendritic cells: portal for host invasion and host defense. Trends Microbiol. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 59.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761-771. [DOI] [PubMed] [Google Scholar]
- 60.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 61.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholler, N., M. Hayden-Ledbetter, A. Dahlin, I. Hellstrom, K. E. Hellstrom, and J. A. Ledbetter. 2002. Cutting edge: CD83 regulates the development of cellular immunity. J. Immunol. 168:2599-2602. [DOI] [PubMed] [Google Scholar]
- 63.Sénéchal, B., A. M. Boruchov, J. L. Reagan, D. N. Hart, and J. W. Young. 2004. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood 103:4207-4215. [DOI] [PubMed] [Google Scholar]
- 64.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67(Pt. 12):2571-2585. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson, K. D. 2000. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11:141-148. [DOI] [PubMed] [Google Scholar]
- 68.Wolenski, M., S. O. Cramer, S. Ehrlich, C. Steeg, B. Fleischer, and A. von Bonin. 2003. Enhanced activation of CD83-positive T cells. Scand. J. Immunol. 58:306-311. [DOI] [PubMed] [Google Scholar]
- 69.Wysocka, J., and W. Herr. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 28:294-304. [DOI] [PubMed] [Google Scholar]
- 70.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]
- 71.Zhou, L. J., R. Schwarting, H. M. Smith, and T. F. Tedder. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 149:735-742. [PubMed] [Google Scholar]
- 72.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3835. [PubMed] [Google Scholar]
- 73.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu, Q., J. Yao, G. Wani, J. Chen, Q. E. Wang, and A. A. Wani. 2004. The ubiquitin-proteasome pathway is required for the function of the viral VP16 transcriptional activation domain. FEBS Lett. 556:19-25. [DOI] [PubMed] [Google Scholar]
- 75.Zinser, E., M. Lechmann, A. Golka, M. B. Lutz, and A. Steinkasserer. 2004. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J. Exp. Med. 200:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.