Abstract
The thymidine kinase (TK) encoded by Epstein-Barr virus (EBV) differs not only from that of the alphaherpesviruses but also from that of the gamma-2 herpesvirus subfamily. Because cellular location is frequently a determinant of regulatory function, to gain insight into additional role(s) of EBV TK and to uncover how the lymphocryptovirus and rhadinovirus enzymes differ, the subcellular localizations of EBV TK and the related cercopithecine herpesvirus-15 TK were investigated. We show that in contrast to those of the other family members, the gamma-1 herpesvirus TKs localize to the centrosome and even more precisely to the periphery of the centriole, tightly encircling the tubulin-rich centrioles in a microtubule-independent fashion. Centrosomal localization is observed in diverse cell types and occurs whether the protein is expressed independently or in the context of lytic EBV infection. Surprisingly, analysis of mutants revealed that the unique N-terminal domain was not critical for targeting to the centrosome, but rather, peptide sequences located C terminal to this domain were key. This is the first herpesvirus protein documented to reside in the centrosome, or microtubule-organizing center, an amembranous organelle that regulates the structural biology of the cell cycle through control of chromosome separation and cytokinesis. More recently, proteasome-mediated degradation of cell cycle regulatory proteins, production and loading of antigenic peptides onto HLA molecules, and transient homing of diverse virion proteins required for entry and/or egress have been shown to be coordinated at the centrosome. Potential implications of centrosomal localization for EBV TK function are discussed.
Many large DNA viruses encode enzymes that salvage nucleic acid substrates that are required for virus replication (42). Increasing evidence suggests that the nucleotide pools that are generated by these enzymes may also be important for orchestration of the cell cycle during virion replication. The virus-regulated substrates can provide direct and/or feedback control to cellular proteins that are then utilized to stall the virus-infected cell, blocking completion or normal exit from the cell cycle (50). This can ensure adequate cellular resources and increase the amount of time available for virion production. Certain viruses express several nucleoside kinases, whereas in other viruses (e.g., the alphaherpesviruses), a single enzyme serves as a polynucleoside kinase (13). The enzyme most consistently expressed by the large DNA viruses is thymidine kinase (TK) (2, 30). In contrast to that of other nucleosides, de novo synthesis of thymidine requires a single cellular protein that is regulated in a complex manner responsive to TMP pools and other cell cycle cues (63). This is believed to account for why large DNA viruses preferentially encode orthologs of cellular thymidine/thymidylate kinases or in some cases even thymidylate synthases, to ensure adequate thymidine nucleotides for virion replication and to prevent the rapid onset of “thymineless” cell death (1). In vivo, loss of normal TK expression is associated with the diminished abilities of different viruses to establish infection, replicate, and produce new virions (15).
Most viral TKs are composed of a single domain consisting of a nucleotide and a substrate binding site(s); the individual domains may associate to form dimers or tetramers. Derivation from cellular precursors is often apparent (poxviruses, cytosolic TK1; herpesviruses, mitochondrial TK2) on the basis of conserved sequences (2, 30). These enzymes are generally expressed early in the lytic cycle, and most, though not all, localize to the intracellular compartments where virion replication is ongoing.
Compared with other viral TK enzymes, the TKs of the gamma subfamily of herpesviruses display several unique features. Although the C-terminal kinase domain is similar to those of other herpesvirus family members and contains five and one-half of six conserved sites described by Balasubramaniam et al. (4, 30), compared with alphaherpesvirus TKs, the gammaherpesvirus TKs are much more limited in their substrate specificities (thymidine alone) and kinase activities (35, 36, 46, 65). Furthermore, both the gamma-1 and the gamma-2 herpesvirus TKs contain additional N-terminal domains of unknown functions. For Epstein-Barr virus (EBV) TK, removal of the N-terminal domain does not significantly alter its known enzymatic properties (35). Also of interest, Kaposi's sarcoma-associated herpesvirus (KSHV) TK and to a lesser degree EBV TK have been detected in the tegument of the purified virion (6, 38, 70), suggesting that they may function upon egress and/or entry of virus. Although in recent studies KSHV and related gamma-2 herpesvirus TKs were found to be constitutively tyrosine phosphorylated, to localize to a novel cytoplasmic network, and to produce dramatic alteration of cell adhesion and morphology when expressed individually, somewhat surprisingly the related gamma-1 herpesvirus protein EBV TK shared none of these properties (31). Furthermore, in a recent microarray analysis, EBV TK was transcribed with a significant delay compared with what was found for other early-class RNAs that encode the enzymes responsible for virion replication (69). Perhaps most interestingly, transcripts of EBV TK were detected in cells treated with the ribonucleotide reductase inhibitor hydroxyurea, which causes nucleotide pool depletion and genotoxic stress/replication stalling, even though the EBV lytic cycle was not induced (51). Thus, to gain insight into the additional function(s) of EBV TK and to uncover how the lymphocryptovirus TKs differ from those of the rhadinovirus family, the subcellular localization of EBV TK and also the related cercopithecine herpesvirus-15 TK (CeHV-15 TK) was studied, as cellular location is a frequent determinant of regulatory function.
In this report, we demonstrate that in contrast to the rhadinoviruses, the gamma-1 herpesvirus TKs (EBV TK and CeHV-15 TK) localize to the centrosome, and we identify peptide sequences required for strict pericentriolar targeting. Although several viral proteins, most prominently oncoproteins, have been demonstrated to alter the behavior of centrosomes and to uncouple the centrosome cycle from the cell cycle and from cytokinesis (11, 19, 22, 25, 39, 52, 61), few of these proteins reside within this small, highly specialized, membrane-free organelle. Herein, we identify an EBV protein that resides in the microtubule-organizing center (MTOC), or centrosomal compartment, separate from other EBV immediate-early (IE) and early (E) proteins that localize to replication compartments within the nucleus. We query as to how and why EBV TK localizes to the centrosome and how this might affect the pathobiology of EBV infection.
MATERIALS AND METHODS
Expression constructs.
All EBV TK- and CeHV-15-derived gene constructs were amplified with Pfu polymerase (Stratagene) and confirmed by sequencing. CeHV-15 TK was generously provided by Fred Wang, Brigham and Women's Hospital/Harvard Medical School, Boston, MA. The mammalian expression clones listed below were generated by amplifying either full- or partial-length genes (mutants) using the forward and reverse primers displayed in Table 1 and cloned into pEGFP-C2, -C3, or -N2 (Clontech) using the restriction sites underlined in Table 1. KSHV TK and herpes simplex virus type 1 (HSV-1) TK were cloned as previously described (31). Monomeric red fluorescent protein (mRFP1)-centrin was constructed as a fusion of mRFP1 (8), generously provided by R. Y. Tsien, University of California, San Diego, CA, and human centrin 2 (NM_004344), which was amplified from human HeLa cells and sequenced to ensure fidelity.
TABLE 1.
Primer sequences for generation of egfp-TK expression clones
| Clonea | Primer | Primer sequenceb (restriction site) |
|---|---|---|
| C2-EBV TK1-607 | MG161 | F, 5′ TGCTCGAGCATGGCTGGATTTCCAGGAAAGG 3′ (XhoI) |
| MG158 | R, 5′ GGAATTCCTAGTCCCGATTTCC3′ (BamHI) | |
| N2-EBV TK1-607 | MG155 | F, 5′ TGCTCGAGA CCATGGCTGGATTTCCAGGAAAGG 3′ (XhoI) |
| MG156 | R, 5′ GGAATTCGTCCCGATTTCCCCTCTCAAAATCAG 3′ (EcoRI) | |
| C2- EBV TK1-243 | MG161 | As stated |
| MG162 | R, 5′ GGAATTCCTACACACTCCCACTTAGCCGCTTCAGC 3′ (EcoRI) | |
| C2-EBV TK244-561 | MG157 | F, 5′ TGCTCGAGCATGAATGTTCTGAATCTGG 3′ (XhoI) |
| MG264 | R, 5′ CCGGAATTCCTATGAGAGGTCCACAAGC 3′ (EcoRI) | |
| C2-EBV TK244-607 | MG157 + MG158 | As stated |
| C2-EBV TK266-607 | MG159 | F, 5′ TGCTCGAGCATGCGGGTTCCAATTGTGACC 3′ (XhoI) |
| MG158 | As stated | |
| C2-EBV TK271-607 | MG160 | F, 5′ TGCTCGAGCGTGACCCACCTAACAAATCATCTACC 3′ (XhoI) |
| MG158 | As stated | |
| C2-EBV TK290-607 | MG193 | F, 5′ CCGCTCGAGCGAAGGTGCCCCTGGTGTGG 3′ (XhoI) |
| MG158 | As stated | |
| C2-EBV TK266-561 | MG159 + MG264 | As stated |
| C2-EBV TK271-561 | MG160 + MG264 | As stated |
| C2-EBV TK285-607 | MG263 | F, 5′ CCGCTCGAGTGTTCCCTATTTTTGGAAGG 3′ (XhoI) |
| MG264 | As stated | |
| C2-EBV TK267-270 (RVPI) | MG114 | F, 5′ GTTTTGGCACCAAAATCAACG 3′ |
| MG177 | R, 5′ CTACTAAATTGGAACCCGCTTGTACAGCTCGTCC 3′ | |
| C2-CeHV-15 TK | MG346 | F, 5′ CCGCTCGAGCATGGCAGGATATCCTGGAAAAGG 3′ (XhoI) |
| MG347 | R, 5′ GGGGTACCCTAGTCACGACTGCCCCTCTC 3′ (KpnI) | |
| C2-HSV1 TK | MG163c | F, 5′ TGCTCGAGCATGGCTTCGTACCCCTGCCATCAACACG 3′ (XhoI) |
| MG164c | R, 5′ GGAATTCTCAGTGAGCCTCCCCCATCTCC 3′ (EcoRI) | |
| C2-KSHV TK | MG165c | F, 5′ TGCTCGAGCATGGCAGAAGGCGGTTTTGGAGC 3′ (XhoI) |
| MG166c | R, 5′ GGAATTCAGGCTAGACCCTGCATGTCTCC 3′ (EcoRI) |
The egfp-EBV TK, CeHV-15 TK, HSV-1 TK, and KSHV TK expression clones were generated by amplification of the corresponding viral cDNA with Pfu polymerase (Stratagene) using the indicated sense and antisense primers and cloned into either pEGFP-C2 (C2) or pEGFP-N2 (N2) (Clontech). Restriction enzyme sequences are underlined. Superscript numbers indicate amino acid positions. CeHV-15TK (GenBank accession no. AAK95465) was a generous gift from Fred Wang, Brigham and Women's Hospital.
F, sense primer; R, antisense primer.
Primer previously reported (31).
Cell culture and transfections. (i) Cell lines.
293 human embryonic kidney cells immortalized by sheared adenovirus (ATCC), 293T human embryonic kidney cells immortalized by sheared adenovirus and stably transfected with simian virus 40 (SV40) large T antigen (ATCC), and 143B TK−, a human rhabdomyosarcoma cell line that lacks human TK-1 expression (ATCC), were all grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Chinese hamster ovary K1 (CHO-K1) cells (ATCC) were grown in F-12 medium and similarly supplemented. B95-8, an EBV-B-lymphoblastoid cell line of marmoset origin (ATCC), was grown in RPMI medium and supplemented with RPMI 10% heat-inactivated calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were transfected with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions.
(ii) Primary cells.
Primary human oral keratinocytes were grown in complete keratinocyte serum-free medium (keratinocyte serum-free medium supplemented with 30 μg/ml bovine pituitary extract, 0.2 ng/ml epidermal growth factor, and 0.3 mM CaCl2) (Invitrogen) as previously described (31, 59). The cells were plated at 5 × 104 in a six-well culture dish 1 or 2 days prior to transient transfection using FuGene transfection reagent according to the manufacturer's directions (Invitrogen).
Cell staining and imaging.
Twelve to 24 h posttransfection, cells expressing the different enhanced green fluorescent protein (egfp)-TKs and/or expressing mRFP1-centrin were counterstained with Hoechst 33342 (Molecular Probes) and fixed in 3.7% paraformaldehyde-bovine serum albumin for 20 m. All cells were imaged at ×100 magnification using oil immersion on a NIKON E600 microscope with a SPOT2 digital camera using SPOTcam v.3.5.5. software (Digital Instruments, Inc.).
Antibody preparation.
Recombinant EBV TK was expressed in bacteria and purified as described in reference 35 and for Fig. 6A. Preimmune rabbit serum was collected, and rabbits were immunized with a recombinant protein provided by a commercial vendor (Harlan Bioproducts). Serum was harvested at different time intervals, and antibodies from both preimmune and immune sera were purified by protein A chromatography (Pierce) and analyzed for titer and specificity.
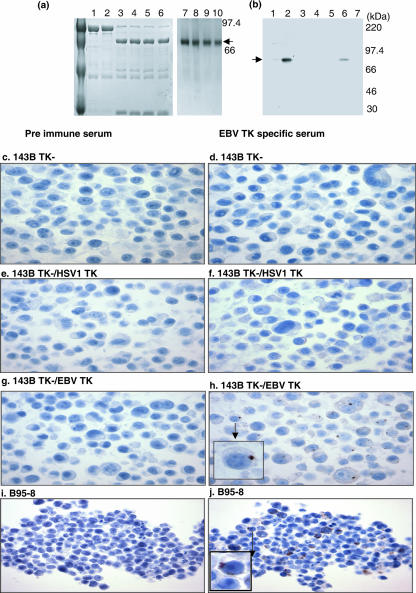
FIG. 6.
Detection of recombinant and native EBV TKs in centrosomes. (a) Purification of recombinant EBV TK for immunization of rabbits (top left). EBV TK expressed in bacteria as a fusion with glutathione S-transferase (lanes 1 and 2) was cleaved with PreScission protease to remove the glutathione S-transferase moiety (lanes 3 to 6) and then repurified (lanes 7 to 10) as demonstrated by SDS-polyacrylamide gel electrophoresis followed by Coomassie staining. MM markers are indicated at right. (b) Characterization of rabbit anti-EBV TK by immunoblot analysis (top right). Rabbit anti-EBV TK detected minimal protein in uninduced B95-8 cells (lane 1), whereas in lytically induced B95-8 cells (treated with 20 ng/ml phorbol myristate acetate for 24 h), native protein was readily detected (lane 2). In addition, recombinant protein was specifically detected in 143B TK− cells stably expressing EBV TK (lane 6) but not in cells stably expressing HSV-1 TK (lane 3), KSHV TK (lane 4), or vaccinia TK (lane 5) or in 143 TK− cells alone (lane 7). MM markers are indicated at right. (c to h) Immunohistochemical analysis of 143B TK− cells expressing herpesviral TKs with rabbit anti-EBV TK (middle panels). No protein was detected by preimmune rabbit serum in 143B TK− cells alone (c) or in cells expressing either HSV-1 TK (e) or EBV TK (g). In contrast, rabbit anti-EBV TK serum detected protein localized to large perinuclear dot-like structures (centrosomes) in EBV TK transfected 143B TK− cells (h) but not in cells transfected with HSV-1 TK (f) or in 143B TK− cells alone (d). (i and j) Immunohistochemical analysis of induced B95-8 cells with rabbit anti-EBV TK. (i) No protein was detected by preimmune rabbit serum. In contrast, multiple perinuclear dot light structures consistent with centrosomes were visualized with rabbit anti-EBV TK (j) and supernumerary centrosomes were evident (j, inset).
Immunoblot analysis.
Cell lysates were prepared from stably transfected cells as previously described (31). Briefly, cells were centrifuged and 1 ml of preboiled lysis buffer (50 mM Tris-1% sodium dodecyl sulfate [SDS]-0.5% beta-mercaptoethanol) was added to 107 cells. The cell lysates were sonicated and boiled for 5 min and the protein concentration in each lysate was quantified (Bio-Rad). Ten percent SDS-polyacrylamide gel electrophoresis was performed using 30 μg total protein/lane, and the separated protein was transferred to nitrocellulose by electroblotting. The membrane was incubated with rabbit anti-EBV TK at a 1:500 dilution, extensively washed, and then reincubated with goat anti-rabbit horseradish peroxidase (A5420; Sigma-Aldrich) for 30 min at room temperature, washed, and developed with a chemiluminescence substrate (ECL; Amersham) according to the directions of the manufacturer. After detection with specific antibody, beta-tubulin content was assessed as a further loading control.
Immunohistochemistry.
Cell lines were centrifuged to create a pellet, formalin fixed, and then processed as tissue sections. Immunohistochemistry was performed using 5-μm-thick paraffin cell block sections. Briefly, slides were soaked in xylene, passed through graded alcohols, and placed in distilled water. Slides were then pretreated with 10 mM citrate, pH 6.0 (Zymed, South San Francisco, CA), in a steam pressure cooker (decloaking chamber; BioCare Medical, Walnut Creek, CA) according to the manufacturer's instructions, followed by a wash in distilled water. All further steps were performed at room temperature with a hydrated chamber. Slides were pretreated with peroxidase block (DAKO USA, Carpinteria, CA) for 5 min to quench endogenous peroxidase activity. Primary rabbit anti-EBV TK (or a control prebleed) antibody was applied (1:1,000) in DAKO diluent for 1 h. The slides were washed in 50 mM Tris-Cl, 0.05% Tween 20, pH 7.4, and detected with an anti-rabbit Envision+ kit (DAKO) according to the manufacturer's instructions. After a further wash, immunoperoxidase staining was developed using a DAB chromogen (DAKO) and counterstained with hematoxylin.
RESULTS
Divergence of the gamma-1 and gamma-2 herpesvirus TKs.
Although the respective N-terminal domains of EBV TK and KSHV TK contain stretches of proline-rich sequence and display enhanced DE/G/S content, direct similarity is limited compared with that between their C-terminal kinase domains (Fig. 1A). In light of reports of functional differences between KSHV TK and EBV TK (31), a phylogenetic tree was constructed utilizing 12 published gammaherpesvirus TK sequences (Fig. 1B) to assess whether these differences reflect variation among the individual EBV and KSHV proteins or divergence between the gamma-1 and gamma-2 TK subfamily enzymes. From the results obtained, it can be seen that the lymphocryptovirus enzymes EBV TK and CeHV-15 TK (56) cluster together in a subgroup distinct from the rhadinovirus TKs (KSHV, herpesvirus saimiri, herpesvirus ateles, and both of the rhesus rhadinoviruses) (Fig. 1B). Bovine herpesvirus-3 TK is equally related to the gamma-1 and gamma-2 subfamilies and interestingly is most similar to murine herpesvirus-4 TK. Direct sequence alignment further illustrates that although EBV TK and CeHV-15 TK are 89% identical overall, even among these closely related lymphocryptoviruses, similarity remains greatest in the C-terminal kinase domain (Fig. 1C).
FIG. 1.
(A) Comparison of the primary amino acid sequence of the N terminus (top) contrasted with that of the C-terminal kinase (bottom) of the human gammaherpesviruses EBV (lymphocryptovirus, top line) and KSHV (rhadinovirus, middle line) using MultiAlign (4). Similar sequences are indicated in red. Dashed lines represent gaps. A consensus sequence is displayed on the bottom line. (B) Comparative evolution of gammaherpesvirus TKs. A phylogenetic tree was constructed using the TreeTop phylogenetic tree prediction system (http://www.genebee.msu.su/services/phtree_reduced.html) based on 12 published gammaherpesvirus TK sequences: alcelaphine herpesvirus 1 (AlHV-1) (GenBank accession no. AAC58068), ateline herpesvirus 3 (AtHV-3) (AAC95545), bovine herpesvirus 4 (BoHV-3) (AAK07940), callitrichine herpesvirus 3 (CalHV-3) (AAK38221), equine herpesvirus 2 (EHV-2) (AAC13808), human herpesvirus-4 (HHV-4) (CAA24799), CeHV-15 (AAK95465), human herpesvirus-8 (HHV-8) (AAC57102), Macaca mulatto rhadinovirus 17577 (RRV/17577) (AAD21347), Macaca mulatta rhadinovirus 26-95 (RRV/26-95) (AAF59999), murid herpesvirus 4 (MuHV-4) (AAF19286), and Saimiriine herpesvirus 2 (SaHV-2) (CAA45643) TKs. (C) Comparison of the primary amino acid sequences of EBV TK (CAA24799, top line) and CeHV-15 TK (AAK95465, middle line) using MultiAlign. Nonconserved amino acids are indicated in blue. Divergent peptides are additionally underlined. A consensus sequence is displayed on the bottom line.
EBV TK fused to egfp localizes to centrosomes.
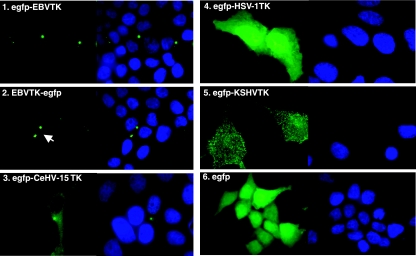
The IE and most of the E proteins that regulate EBV DNA replication colocalize to discrete sites within the nucleus known as replication compartments (17). Thus, it was predicted that EBV TK would also localize to the nucleus and that detection of EBV TK in the purified virion likely reflected transit of assembling virus through this compartment (38). Interestingly, although many viral TKs reside within or close to the site(s) of virion replication, this is not uniformly the case, as shown for some members of the alphaherpesvirus family (18) and recently for the rhadinovirus TKs (31). Thus, after phosphorylation, salvaged nucleotides appear able to transit to sites of viral replication irrespective of where they are synthesized. To visualize the subcellular localization of EBV TK, two expression clones in which EBV TK cDNA (BXLF1) was placed either upstream or downstream of egfp were generated (Table 1). Surprisingly, when expressed in 293 cells, a human epithelial cell line permissive for EBV infection, EBV TK, specifically localized to one, sometimes two, well-defined dot present not within the nucleus but in the perinuclear region of the cell, characteristic of the appearance of centrosomes (Fig. 2). This localization was observed irrespective of whether the fusion peptide was placed upstream or downstream of the enzyme (Fig. 2, panels 1 and 2) and was clearly distinct from that obtained upon expression of egfp-tagged HSV-1 TK (Fig. 2, panel 4) (a nuclear protein), KSHV TK (Fig. 2, panel 5) (a cytoplasmic protein), or egfp alone (Fig. 2, panel 6) (a protein that distributes uniformly throughout the cell).
FIG. 2.
Subcellular localization of egfp-tagged (green) EBV TK and CeHV-15 TK in 293 cells compared with HSV-1 TK, KSHV TK, and egfp alone. Cells transfected under identical conditions with the respective expression vectors (see Materials and Methods) were visualized. Panel 1, egfp-EBV TK; panel 2, EBV TK-egfp; panel 3, egfp-CeHV-15 TK; panel 4, egfp-HSV-1 TK; panel 5, egfp-KSHV TK; panel 6, egfp alone (control). Corresponding nuclei were revealed with Hoechst stain (blue). Cells were imaged at ×100 magnification using oil immersion on a NIKON E600 microscope with a SPOT2 digital camera using SPOTcam v.3.5.5. software (Digital Instruments, Inc.).
CeHV-15 TK also localizes to the centrosome.
Because CeHV-15 TK is closely related to EBV TK, its intracellular localization was investigated to determine whether residence in the centrosome was a conserved property of the gamma-1 herpesviruses or was in some manner unique to EBV. A vector consisting of CeHV-15 TK fused to the C terminus of egfp was constructed (Table 1) and similarly expressed in 293 cells. As shown in Fig. 2, panel 3, CeHV-15 TK also localized to the centrosome. However, compared to EBV TK, the primate enzyme could also be partially detected in the cytoplasm (Fig. 2, panel 3). Although EBV and CeHV-15 TK are very similar (Fig. 1C), several regions of sequence variation do occur (at the N-terminal regions comprising amino acids [aa] 42 to 53, 86 to 120, and 191 to 200; at the interface of the two domains comprising aa 253 to 266; and in the penultimate C-terminal region consisting of aa 589 to 607 [Fig. 1C, underlined regions]), suggesting that one or a combination of these peptide sequences might be important for stringent homing to the centrosome.
Centrosome localization is independent of cellular origin and occurs in cells with supernumerary centrosomes.
The precise localization of EBV TK to centrosomes in 293 cells was unique among the herpesvirus proteins described to date. However, 293 cells contain sheared adenovirus DNA, express E1A and E1B proteins, and are immortalized (34), raising the possibility that aberrant expression of another viral or cellular protein(s) altered the normal targeting of EBV TK. To assess whether centrosomal localization was a consistent feature of EBV TK biology, egfp-EBV TK was transfected into a variety of cell types, including 143B TK− human rhabdomyosarcoma cells, CHO cells, and normal primary human oral keratinocytes. As shown in Fig. 3, left panels, EBV TK was detected in the centrosome irrespective of cell type and also irrespective of species. In addition, transiently transfected egfp-EBV TK localized to the centrosome in B95-8, an EBV-immortalized marmoset cell line, both before and after induction of lytic replication (not shown).
FIG. 3.
Centrosomal localization of egfp-EBV TK in diverse cell types. (Left) egfp-EBV TK (green) transiently transfected into rhabdomyosarcoma tumor cells (143B TK−), immortalized CHO cells, and normal primary human oral keratinocytes localized to single centrosomes. (Right) egfp-EBV TK (green) transiently transfected into 293 human embryonic kidney cells immortalized with sheared adenovirus and stably expressing SV40 T antigen (293T cells) localized to supernumerary centrosomes. All cells were counterstained with Hoechst to reveal corresponding nuclei (blue). 143B TK− cells and CHO cells were additionally stained with tetramethyl rhodamine isocyanate-phalloidin (red), displaying the distribution of actin. The cells were imaged as described for Fig. 2.
The 293T cell line is derived from 293 clones that also express SV40 large T antigen. These cells efficiently take up and express exogenous DNA. When egfp-EBV TK was transiently transfected into 293T cells, the enzyme was also detected in supernumerary centrosomes (Fig. 3, right). This observation was duplicated when the EBV TK homolog CeHV-15 TK (not shown) was expressed in these cells. Expression of certain tumor virus oncoproteins, including E1A of adenovirus, E7 of human papillomavirus (8-10, 22, 25), HBx of hepatitis B virus (13), Tax of human T-cell leukemia virus type 1, and the small and large T antigens of SV40, causes centrosomal abnormalities (increased numbers, expanded and/or abnormal protein content) (19, 22, 24, 25, 28, 45, 52). The contents of supernumerary centrosomes often differ among cells, reflecting distinct cell cycle perturbations that predispose to their development. Although supernumerary centrosomes were not routinely observed in standard 293 cells, 293T cells may form these structures more often. Therefore, further work will be required to resolve whether expression of EBV TK contributes to development of supernumerary centrosomes in 293T and certain other cells, does so cooperatively with endogenous oncoproteins, or simply transits to these aberrant structures.
EBV TK is a microtubule-independent component of the pericentriolar matrix.
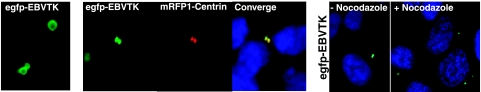
Examination of egfp-EBV TK in 293 cells under high-power magnification (Fig. 4, left) revealed that EBV TK specifically localized to the periphery of the perinuclear dot and was absent from the central core of this structure (Fig. 4, left, shows an enlargement of the intracellular dots designated by the arrow in Fig. 2, panel 2). This finding suggested that EBV TK was not only positioned within the centrosomal matrix, which typically appears more diffuse, but specifically located in close proximity to the mature centriole, the major constituent of this amembranous organelle. To confirm its pericentriolar localization, egfp-EBV TK was coexpressed in 293 cells with mRFP1 fused to centrin, producing mRFP1-centrin 2, which is a well-established centriole-associated protein (58). As seen in Fig. 4, middle, both egfp-EBV TK and mRFP1-centrin localized in the vicinity of centrioles where the distribution of the two proteins overlapped, as highlighted upon the merging of both images (Fig. 4, middle panel, right). Because the centriole is highly enriched in microtubules though also composed of many microtubule-associated and some microtubule-independent proteins, to gain insight into whether EBV TK directly associated with the major centriolar component, tubulin, or a microtubule-associated protein (MAP), enzyme-expressing cells were challenged with the microtubule-depolymerizing reagent nocodazole. As shown in Fig. 4, right panels, EBV TK did not change its relative position within the centrosome after nocodazole exposure, indicating that the tight association of EBV TK with the centriole was microtubule independent.
FIG. 4.
Microtubule-independent localization of EBV TK to the pericentriole. (Left) High-magnification view (×100) of the image (egfp-EBV TK expressed in 293 cells) indicated by the arrow displayed in Fig. 2, panel 2. (Middle) Visualization of 293 cells expressing egfp-EBV TK (green) and mRFP1-centrin (red) and merging of egfp-EBV TK/mRFP1-centrin images (yellow). (Right) 293 cells expressing egfp-EBV TK before and after treatment with 40 μM nocodazole.
Centrosomal targeting of EBV TK occurs through C-terminal sequences.
As previously described, wild-type EBV TK1-607 is composed of a novel N terminus (aa 1 to 243) of an unknown function and a conserved C-terminal domain (aa 244 to 607) that encompasses the active kinase (aa 244 to 561). Although the kinase domain can be identified on the basis of secondary structure analysis, the overall similarity between the different herpesvirus TK domains is modest (approximately 20 to 25% identity), with many unique amino acids interspersed between the conserved sequences that define the core enzyme (35, 37). In fact, EBV TK contains two potential internal short open reading frames encoding the C-terminal kinase (SORF1, EBV TK244-607, and SORF2, EBV TK266-607). Each SORF begins with a methionine that marks the potential start of the C-terminal domain. To determine which regions of EBV TK mediate targeting to the pericentriolar region, a series of egfp-EBV TK truncation mutants were generated (Fig. 5, top). Surprisingly, when the N-terminal domain (EBV TK1-243) alone was expressed, this fragment localized exclusively to the cytoplasm (Fig. 5, panel 3). In contrast, a truncated C-terminal kinase domain (EBV TK244-561) retained centriolar localization, though some additional nuclear and cytoplasmic accumulation was evident (Fig. 5, panel 4). Notably, when the entire C-terminal domain (EBV TK244-607), including the penultimate amino acid sequences, was expressed, this fragment again localized predominantly to the centriole, although unlike the wild-type protein (Fig. 5, panel 2), the truncated protein radiated away from the centriole, forming elongated blade-like structures within the centrosome (Fig. 5, panel 5) rather than forming a tight pericentriolar ring as observed when wild-type protein was expressed.
FIG. 5.
Identification of EBV TK sequences required for pericentriolar localization. A schematic diagram of N- and C-terminal EBV TK truncation mutants is shown at the top. Panels 2 to 11 display 293 cells expressing the corresponding egfp-EBV TK vectors (green). Results are shown for EBV TK1-607 or the wild type (panel 2), EBV TK1-243 (panel 3), EBV TK244-561 (panel 4), EBV TK244-607 (panel 5), EBV TK266-607 (panel 6), EBV TK271-607 (panel 7), EBV TK290-607 (panel 8), EBV TK266-561 (panel 9), EBV TK271-561 (panel 10), EBV TK285-561 (panel 11), and EBV TK267-270 (panel 12). The cells were counterstained with Hoechst to reveal corresponding nuclei (blue) and imaged as described for Fig. 2. The egfp-EBV TK truncation clones were generated as outlined in Table 1.
Because the EBV TK244-607 mutant remained closely associated with the centriole, to more precisely define the critical sequence(s) required for centriolar targeting, three further N-terminal truncation mutants, consisting of EBV TK266-607 (which coincides with SORF2), EBV TK271-607, and EBV TK290-607, were generated. When expressed, EBV TK266-607 was clearly visible at the centrosome, where it radiated from the body of the centriole similarly to EBV TK244-607 (Fig. 5, panel 6 and inset). In contrast, EBV TK290-607 only minimally associated with centrioles, although the egfp enzyme was abundantly detected in the cytoplasm and nucleus (Fig. 5, panel 8). Strikingly, when five additional amino acids were removed from the N-terminal region producing the C-terminal fragment EBV TK271-607 (Fig. 5, panel 7), centrosomal localization was again abolished and the overall distribution of the resulting truncation mutant became indistinguishable from that of EBV TK290-607. Because elimination of the RVPI peptide (aa 267 to 270) significantly altered EBV TK localization, an egfp-RVPI (EBV TK267-270) fusion protein was synthesized to determine whether this short sequence could by itself localize EBV TK to the pericentriole. However, when expressed alone, EBV TK267-270 was distributed throughout the entire cell (Fig. 5, panel 12). Thus, although this short peptide appears to play a critical role in EBV TK localization, the four amino acids are not the sole determinants of targeting to the pericentriolar region, suggesting that flanking sequences and/or more distal sequences within the C terminus of the enzyme are also relevant. Therefore, to further investigate the role of the distal C-terminal amino acids (aa 562 to 607) in subcellular localization, three additional C-terminal truncation clones were examined: EBV TK266-561, EBV TK271-561, and EBV TK285-561. In contrast to the pericentriolar localization observed upon expression of EBV TK266-607, the C-terminal truncation mutants either were further distributed throughout the nucleus and the cytoplasm (EBV TK266-561, EBV TK271-561) (Fig. 5, panels 9 and 10) or showed predominant nuclear staining (EBV TK285-561) (Fig. 5, panel 11), indicating an additional role for sequences at the end of the C-terminal domain in pericentriolar localization or protein stabilization.
In summary, analysis of both N- and C-terminal egfp truncation mutants showed that two amino acid sequences comprising both ends of the C-terminal domain, aa 244 to 270 and 562 to 607, were required to strictly target EBV TK to the centriole. While it remains likely that pericentriolar localization is mediated through association with other resident proteins, the observation that nocodazole did not disrupt localization (Fig. 4, right) suggested that neither microtubules nor MAPs directly interact with EBV TK. Furthermore, although the novel N-terminal domain of EBV TK1-243 was not per se critical for centriolar targeting, removal of this domain did alter the overall structure of centrosome association, as the EBV TK244-607 truncation mutant radiated away from the centriole core, forming elongated blades that were morphologically distinct from the tight rings observed when wild-type protein was expressed.
Immunohistochemical detection of EBV TK confirms the centrosomal localization of native enzyme.
The precision and consistency of pericentriolar localization, coupled with loss of centrosomal targeting upon expression of specific mutants, provided credible evidence that EBV TK was a resident of the centrosome. Nevertheless, there remained some possibility that the observed compartmentalization was a consequence of fusion to egfp, causing the protein to become misfolded. Unfolded/misfolded proteins can form aggresomes, dynamic structures composed of aggregates of poorly folded protein inadequately degraded by the proteasome. Aggresomes frequently form at the centrosome, which is the subcellular site where many components of the ubiquitin proteasome system also converge (16, 29). However, although aggresomes produce perinuclear aggregates, they do not typically localize with precision to the pericentriolar component of the centrosome. Still, to further confirm that native EBV TK localized to this organelle, a monospecific rabbit antiserum to full-length recombinant EBV TK (long open reading frame), synthesized in bacteria and purified by affinity chromatography (Fig. 6A), was generated. As shown by immunoblot analysis (Fig. 6B), the antiserum recognized a protein of the predicted molecular mass (MM) of EBV TK, ∼70,000 kDa, in B95-8 cells (Fig. 6B, lane 1), which markedly increased after lytic cycle induction (Fig. 6B, lane 2). The antiserum was highly specific, as it recognized recombinant egfp-EBV TK expressed following stable transfection of 143B TK− cells (Fig. 6B, lane 6) but did not detect KSHV TK, HSV-1 TK, vaccinia TK (Fig. 6B, lanes 3 to 5), or any other protein expressed in 143B TK− cells (Fig. 6B, lane 7). Next, to reveal the subcellular localization of native EBV TK in cells, an immunohistochemical analysis was performed (Fig. 6) using the purified rabbit antibody. Whereas preimmunization rabbit serum failed to detect enzyme in EBV TK-transfected or in virus-infected cells (Fig. 6c, e, g, and i), the anti-EBV TK serum detected protein in discrete perinuclear dot-like structures clearly resembling those observed upon expression of egfp-EBV TK. Specific staining was apparent in EBV TK-transfected cells (Fig. 6h) but not in HSV-1 TK-transfected cells (Fig. 6f) or in 143B TK− cells alone (Fig. 6d). Significantly, following lytic cycle induction of B95-8 cells with phorbol myristate acetate, rabbit anti-EBV TK serum (Fig. 6j), but not preimmune serum (Fig. 6i), detected native EBV TK protein in structures resembling centrosomes. Moreover, in several cells, multiple (supernumerary) centrosomes were evident (Fig. 6j). No significant intranuclear or intracytoplasmic staining was observed.
DISCUSSION
Because of its unique appearance, the centrosome, or MTOC, was among the first organelles identified by microscopists (21, 55). An amembranous structure located adjacent to the nucleus, it is composed of approximately 100 proteins distributed between the pericentriolar matrix and a pair of core centrioles, highly structured filaments of polymerized tubulin. As the centrosome regulates the microtubular component of the cytoskeleton, it is a major determinant of cell morphology and intracellular movement during interphase. Coordinate with the onset of DNA replication, the two centrioles begin to duplicate in a semiconservative manner at the G1/S boundary of the cell cycle, generating daughter centrioles. In early mitosis, the centrosome, consisting of two pairs of linked mother-daughter centrioles and the pericentriolar matrix, splits to form the two spindle pole bodies that then govern chromosome separation and cytokinesis. In addition to its role in the physical work of cell division, in recent years the centrosome was discovered to regulate the cell cycle through control of protein turnover. Many proteins, including the major regulators of cell cycle progression and DNA repair, reside in or shuttle between the centrosome and other intracellular locales. Moreover, transit to the centrosome is mandatory for normal functioning of these proteins, as a major component of the proteasome, the master regulator of intracellular protein homeostasis, is located here (67).
We report that EBV TK, and also the related gamma-1 herpesvirus CeHV-15 TK, is a constitutive resident of the centrosome, colocalizing with centrin-2 in the immediate vicinity of centrioles. Homing to the centrosome was evident under every condition tested and was detected in tumor and immortalized lines that were derived from diverse lineages and different mammalian species as well as in normal primary human keratinocytes. Expressed recombinant enzymes fused at either the N or the C terminus to egfp as well as native EBV TK induced following stimulation of the lytic cycle were consistently identified at this site. Strikingly, when viewed under high-power magnification, EBV TK could be seen to form a circumferential ring with a dull internal core within the centrosomal matrix. This suggested that the enzyme might attach directly to the centriole or perhaps to a MAP. However, when cells were treated with nocodazole to depolymerize tubulin, although the centriole became disorganized, the circumferential sheath formed by EBV TK remained intact, suggesting that direct contact with microtubules or with MAPs was unlikely, even though the enzyme associated with a structure that enveloped the tubulin-rich centriole. Certain A-kinase anchor proteins can form a pericentriolar lattice (20). In addition, the Skp1 protein, a component of the SCF ubiquitin ligase that regulates cell cycle progression through G1/S, is distributed about centrioles in a manner almost identical to that observed with egfp-EBV TK and is similarly unaltered when cells are treated with nocodazole (26). Thus, it is plausible that EBV TK interacts directly or indirectly with the proteasome.
A review of serial photomicrographs revealed that in many though not all cells, EBV TK fluorescence was confined to a single centrosomal dot. These preliminary observations suggested that EBV TK might preferentially target one centrosome or alternatively that the enzyme might alter the abilities of centrosomes to efficiently duplicate. In contrast, in certain lines, such as 293T, EBV TK could be visualized within multiple or supernumerary centrosomes. Supernumerary centrosomes are a hallmark of cell cycle dysregulation and often appear coordinate with oncoprotein activation, as they are associated with development of abnormal cytokinesis, aneuploidy, and ultimately tumorigenesis (27, 43). Though ostensibly discrepant, the different observations are not exclusive, as the content of supernumerary centrosomes often differs from that of normal centrosomes. Thus, it is possible that proteins that normally associate with only one centrosome may be found indiscriminately in supernumerary structures. Importantly, the homing of EBV TK to supernumerary centrosomes in certain cell lines and the detection of supernumerary centrosomes in the EBV-immortalized lymphoblastoid cell line B95-8 raise questions about whether EBV TK simply localizes to these structures or whether when expressed alone or together with putative oncoproteins EBV TK actively stimulates centrosome duplication and amplification. The centrosome performs multiple highly coordinated tasks, though it contains only approximately 100 proteins; therefore, minimal perturbations are believed to potentially alter its function (21). As EBV TK is a lytic cycle protein, induction of centrosome abnormalities would be predicted to be of little consequence, perhaps even accelerating cell death after completion of virus replication. However, if abortive replication occurs, or if EBV TK is briefly delivered upon initial infection (38), or if the enzyme is selectively induced during latency by transient ribonucleotide reductase inhibition (51) or other forms of genotoxic stress that cause replication stalling (5), then transient centrosome dysregulation could predispose to the development of aneuploidy, a constant feature, for example, of EBV-positive Hodgkin's lymphoma (3) and certain other EBV-associated malignancies (10, 62).
Because the consistency with which EBV TK localized to the MTOC and its distinctive location suggested that interaction with a widely expressed and conserved cellular protein(s) mediated transit to and/or retention by the centrosome, we investigated the peptide sequences in EBV TK that were involved in centrosomal localization by serial egfp-EBV TK deletion mutagenesis. Identification of protein-derived targeting signals often provides important clues about probable ligand interaction, uncovering information about functional activity. Somewhat unexpectedly, our study showed that sequences at the extremes of the C-terminal domain were responsible for localization of the enzyme, rather than the unique N-terminal sequence. Because these sequences potentially encompass the C-terminal kinase, further experiments are in progress to clarify whether kinase function and centrosomal localization are separable. A molecular model of the C-terminal kinase constructed from the known structure of HSV-1 TK (A. Prota, personal communication) was analyzed, as no crystal structure is available. This model locates the RVPI peptide in an unstructured region N terminal to the classic kinase domain; thus, we cautiously speculate that elimination of this sequence (and therefore tight centrosomal localization) would not be likely to alter the protein fold or the function of the C-terminal kinase.
Although centrosomal targeting signals (single and bipartite) have been reported for several cellular proteins (AKAP450, pericentrin A and B, ninein, cyclin E, and others) (12, 32, 48) as well as for viral proteins, including some retroviral Gag proteins (60, 68), current data are limited, and to date no global motif that predicts centrosome homing has been identified. A directed BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/) revealed limited similarity with the centrosomal proteins pericentrin A and B and ninein; however, these sequences did not overlap the EBV TK regions implicated in centrosome localization. Surprisingly, though, a search of protein databases for the short amino acid sequence RVPI, implicated in TK localization, uncovered fewer matches than expected for a short query sequence, repeatedly identifying human p53 and related proteins. A further comparison of sequences flanking RVPI revealed some local similarity to p53 (Fig. 7), and a search for the pattern MXRXPI(LIV)TX(LIV)T retrieved relatively few proteins that were unrelated to p53 and its orthologs. Furthermore, subsequent comparison of full-length p53 and EBV TK detected additional similarity in the proline-rich N termini of the respective proteins as shown in Fig. 7, which was notable because of the relatively conserved alignment of the two N-terminal regions. The overall spacing between the N- and C-terminal regions of similarity within each protein was also somewhat preserved (EBV TK, 136 aa, and p53, 157 aa, respectively), thereby raising the possibility of a shared function. As a master regulator of the cell cycle and guardian of the genome, p53 transits between the nucleus, cytoplasm, and centrosome, and it is well known that protein interactions that prevent correct spatial and temporal localization of p53 alter its normal functions (27, 33). Recently, direct localization of p53 to the MTOC was shown to be critical for regulation of centrosome duplication, as the aberrant retention of p53 in the cytoplasm caused by overexpression of the hsp70 family protein mortalin blocked normal centrosomal function, independent of transactivation (40, 47, 66). Curiously, the mortalin binding domain of p53 maps between aa 253 and 282 of p53, partially overlapping the motif shared with EBV TK, and thereby also raises speculation that the presence of EBV TK in the centrosome could compete with p53 or with other cell cycle regulators to alter their functions in the course of virion replication.
FIG. 7.
Comparison of the primary amino acid sequences of EBV TK (top) and p53 (bottom) (GenBank accession no. P04637). Identical amino acids are highlighted in bold text.
Events that might be affected by EBV TK expression during the virus replicative cycle include abolition of licensing of cellular DNA replication despite a G1/S- and cyclin-rich cell environment (9, 23, 44), prevention of rapid apoptosis despite p53- and cyclin-dependent kinase inhibitor expression (64), and sequestration of ubiquitinylating enzymes (i.e., from latent cycle proteins, thereby aborting some of their functions [41]) as well as many others. Controlled degradation of EBV TK itself could also prompt temporally appropriate apoptosis sped by the depletion of TTP (1). Consistent with a centrosomal function affecting cell cycle regulation, peptide motifs present in EBV TK predict polo-like kinase 1 (aa 309 to 315 and aa 345 to 351) and cyclin-dependent kinase (aa 357 to 363) phosphorylation (ELM search; http://elm.eu.org/links.html).
The cytoplasmic localization and behavior of gamma-2 herpesvirus TKs are entirely distinct from those of EBV/CeHV-15 TK, with gamma-2 TK expression accompanied by dramatic loss of cell matrix adhesion and proliferation in the absence of cell death. Such global reorganization of the cytoskeleton is likely to involve major suppression of integrin activity. Recently, an inactivating mutation of the beta-1 integrin cytoplasmic domain was shown to profoundly alter centrosome function (54), potentially providing a mechanistic link to a centrosomal function shared by the different TKs. It has been previously noted that whereas the gamma-2 herpesviruses frequently modulate cellular gene expression through stepwise processes, the more compact gamma-1 herpesviruses encode master regulators to achieve similar ends (49).
On a separate note, much work in recent years has demonstrated a critical role for the centrosome as a physical platform for disassembly of incoming retrovirions (foamy virus) (53, 57) and as a way station for the assembly of capsids prior to budding (Mason-Pfizer monkey virus and other D- and B-type retroviruses [foamy virus]) (14, 60, 68). The centrosomal targeting of Gag following viral entry occurs through a conserved coiled-coil motif and is required for foamy virus to access the nucleus (53). During egress, another conserved 18-aa sequence in the matrix domain of the Gag polyprotein called the cytoplasmic targeting/retention signal mediates centrosome attachment (14). An N-terminal proline-rich motif conserved among retroviral Gag proteins that can also be found in many other viruses is important for efficient retrovirus budding, though the precise mechanism is not known (7). Part of the motif, the sequence PTAP consistently binds the E2-like ubiquitinating enzyme TSG101 (7), though its role is also unclear. Of note, EBV TK also contains a PTAP motif within an N-terminal proline-rich environment (see above). Therefore, in light of the relatively delayed synthesis of EBV TK during lytic replication, its homing to the centrosome, and its detection in the virion tegument, it may be that this enzyme also functions in entry and/or egress of the virion.
In summary, virion proteins are often multifunctional. At present, we do not know why EBV TK localizes to the centrosome, nor is the role(s) that EBV TK plays in facilitating virion production beyond its modest ability to replenish thymidine nucleotides clear. However, based on clues provided by several recent studies, together with our current results, we speculate that the enzyme contributes to cell cycle regulation during lytic replication (and perhaps sporadically during latency) and/or that it provides a platform for virion entry and/or egress at the centrosome.
Acknowledgments
We thank Fred Wang (Channing Laboratory, Brigham and Women's Hospital, Boston, MA) for the generous gift of CeHV-15 TK, Jo-Ellen Murphy for providing primary keratinocytes, and Jim Rheinwald for free and unlimited access to the microscope facility (Department of Dermatology, Brigham and Women's Hospital, Boston, MA). In addition, we thank German Pihan (Beth Israel Deaconess Medical Center, Boston, MA) and Andrea Prota (Paul Scherrer Institute, Villigen, Switzerland) for helpful discussions.
This work was supported by NIH grants R01 DE12186 and K24 CA085083 and an American Heart Association grant-in-aid.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Ahmad, S. I., S. H. Kirk, and A. Eisenstark. 1998. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu. Rev. Microbiol. 52:591-625. [DOI] [PubMed] [Google Scholar]
- 2.Al-Madhoun, A. S., W. Tjarks, and S. Eriksson. 2004. The role of thymidine kinases in the activation of pyrimidine nucleoside analogues. Mini Rev. Med. Chem. 4:341-350. [DOI] [PubMed] [Google Scholar]
- 3.Atkin, N. B. 1998. Cytogenetics of Hodgkin's disease. Cytogenet. Cell Genet. 80:23-27. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramaniam, N. K., V. Veerisetty, and G. A. Gentry. 1990. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J. Gen. Virol. 71:2979-2987. [DOI] [PubMed] [Google Scholar]
- 5.Balczon, R., L. Bao, W. E. Zimmer, K. Brown, R. P. Zinkowski, and B. R. Brinkley. 1995. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechtel, J. T., R. C. Winant, and D. Ganem. 2005. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:4952-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, W. Y., A. B. Chan, A. Y. Liu, J. H. Chow, E. K. Ng, and S. S. Chung. 2001. Chromosome 11 copy number gains and Epstein-Barr virus-associated malignancies. Diagn. Mol. Pathol. 10:223-227. [DOI] [PubMed] [Google Scholar]
- 11.Chang, F., F. Re, S. Sebastian, S. Sazer, and J. Luban. 2004. HIV-1 Vpr induces defects in mitosis, cytokinesis, nuclear structure, and centrosomes. Mol. Biol. Cell 15:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C. H., S. L. Howng, T. S. Cheng, M. H. Chou, C. Y. Huang, and Y. R. Hong. 2003. Molecular characterization of human ninein protein: two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem. Biophys. Res. Commun. 308:975-983. [DOI] [PubMed] [Google Scholar]
- 13.Chen, M. S., W. P. Summers, J. Walker, W. C. Summers, and W. H. Prusoff. 1979. Characterization of pyrimidine deoxyribonucleoside kinase (thymidine kinase) and thymidylate kinase as a multifunctional enzyme in cells transformed by herpes simplex virus type 1 and in cells infected with mutant strains of herpes simplex virus. J. Virol. 30:942-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi, G., S. Park, B. Choi, S. Hong, J. Lee, E. Hunter, and S. S. Rhee. 1999. Identification of a cytoplasmic targeting/retention signal in a retroviral Gag polyprotein. J. Virol. 73:5431-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman, H. M., B. de Lima, V. Morton, and P. G. Stevenson. 2003. Murine gammaherpesvirus 68 lacking thymidine kinase shows severe attenuation of lytic cycle replication in vivo but still establishes latency. J. Virol. 77:2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corboy, M. J., P. J. Thomas, and W. C. Wigley. 2005. Aggresome formation. Methods Mol. Biol. 301:305-327. [DOI] [PubMed] [Google Scholar]
- 17.Daikoku, T., A. Kudoh, M. Fujita, Y. Sugaya, H. Isomura, N. Shirata, and T. Tsurumi. 2005. Architecture of replication compartments formed during Epstein-Barr virus lytic replication. J. Virol. 79:3409-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degrève, B., M. Johansson, E. De Clercq, A. Karlsson, and J. Balzarini. 1998. Differential intracellular compartmentalization of herpetic thymidine kinases (TKs) in TK gene-transfected tumor cells: molecular characterization of the nuclear localization signal of herpes simplex virus type 1 TK. J. Virol. 72:9535-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca, A., R. Mangiacasale, A. Severino, L. Malquori, A. Baldi, A. Palena, A. M. Mileo, P. Lavia, and M. G. Paggi. 2003. E1A deregulates the centrosome cycle in a Ran GTPase-dependent manner. Cancer Res. 63:1430-1437. [PubMed] [Google Scholar]
- 20.Dictenberg, J. B., W. Zimmerman, C. A. Sparks, A. Young, C. Vidair, Y. Zheng, W. Carrington, F. S. Fay, and S. J. Doxsey. 1998. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doxsey, S., W. Zimmerman, and K. Mikule. 2005. Centrosome control of the cell cycle. Trends Cell Biol. 15:303-311. [DOI] [PubMed] [Google Scholar]
- 22.Duensing, S., L. Y. Lee, A. Duensing, J. Basile, S. Piboonniyom, S. Gonzalez, C. P. Crum, and K. Munger. 2000. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA 97:10002-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duursma, A., and R. Agami. 2005. p53-dependent regulation of Cdc6 protein stability controls cellular proliferation. Mol. Cell. Biol. 25:6937-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauth, C., M. J. O'Hare, G. Lederer, P. S. Jat, and M. R. Speicher. 2004. Order of genetic events is critical determinant of aberrations in chromosome count and structure. Genes Chromosomes Cancer 40:298-306. [DOI] [PubMed] [Google Scholar]
- 25.Forgues, M., M. J. Difilippantonio, S. P. Linke, T. Ried, K. Nagashima, J. Feden, K. Valerie, K. Fukasawa, and X. W. Wang. 2003. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol. Cell. Biol. 23:5282-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freed, E., K. R. Lacey, P. Huie, S. A. Lyapina, R. J. Deshaies, T. Stearns, and P. K. Jackson. 1999. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13:2242-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukasawa, K. 2005. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 230:6-19. [DOI] [PubMed] [Google Scholar]
- 28.Gaillard, S., K. M. Fahrbach, R. Parkati, and K. Rundell. 2001. Overexpression of simian virus 40 small-T antigen blocks centrosome function and mitotic progression in human fibroblasts. J. Virol. 75:9799-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Mata, R., Z. Bebok, E. J. Sorscher, and E. S. Sztul. 1999. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell Biol. 146:1239-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentry, G. A. 1992. Viral thymidine kinases and their relatives. Pharmacol. Ther. 54:319-355. [DOI] [PubMed] [Google Scholar]
- 31.Gill, M. B., J. E. Murphy, and J. D. Fingeroth. 2005. Functional divergence of Kaposi's sarcoma-associated herpesvirus and related gamma-2 herpesvirus thymidine kinases: novel cytoplasmic phosphoproteins that alter cellular morphology and disrupt adhesion. J. Virol. 79:14647-14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillingham, A. K., and S. Munro. 2000. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Lazaro, M., F. J. Fernandez-Gomez, and J. Jordan. 2004. p53: twenty five years understanding the mechanism of genome protection. J. Physiol. Biochem. 60:287-307. [DOI] [PubMed] [Google Scholar]
- 34.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 35.Gustafson, E. A., A. C. Chillemi, D. R. Sage, and J. D. Fingeroth. 1998. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob. Agents Chemother. 42:2923-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafson, E. A., R. F. Schinazi, and J. D. Fingeroth. 2000. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J. Virol. 74:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holton, R. H., and G. A. Gentry. 1996. The Epstein-Barr virus genome encodes deoxythymidine kinase activity in a nested internal open reading frame. Intervirology 39:270-274. [DOI] [PubMed] [Google Scholar]
- 38.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouvenet, N., and T. Wileman. 2005. African swine fever virus infection disrupts centrosome assembly and function. J. Gen. Virol. 86:589-594. [DOI] [PubMed] [Google Scholar]
- 40.Kaul, S. C., S. Aida, T. Yaguchi, K. Kaur, and R. Wadhwa. 2005. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J. Biol. Chem. 280:39373-39379. [DOI] [PubMed] [Google Scholar]
- 41.Knight, J. S., N. Sharma, and E. S. Robertson. 2005. Epstein-Barr virus latent antigen 3C can mediate the degradation of the retinoblastoma protein through an SCF cellular ubiquitin ligase. Proc. Natl. Acad. Sci. USA 102:18562-18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knipe, D., P. Howley, D. Griffin, R. Lamb, M. Martin, B. Roizman, and S. Straus. 2001. Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
- 43.Kops, G. J., B. A. Weaver, and D. W. Cleveland. 2005. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 5:773-785. [DOI] [PubMed] [Google Scholar]
- 44.Kudoh, A., M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi. 2003. Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication. J. Virol. 77:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavia, P., A. M. Mileo, A. Giordano, and M. G. Paggi. 2003. Emerging roles of DNA tumor viruses in cell proliferation: new insights into genomic instability. Oncogene 22:6508-6516. [DOI] [PubMed] [Google Scholar]
- 46.Lock, M. J., N. Thorley, J. Teo, and V. C. Emery. 2002. Azidodeoxythymidine and didehydrodeoxythymidine as inhibitors and substrates of the human herpesvirus 8 thymidine kinase. J. Antimicrob. Chemother. 49:359-366. [DOI] [PubMed] [Google Scholar]
- 47.Ma, Z., H. Izumi, M. Kanai, Y. Kabuyama, N. G. Ahn, and K. Fukasawa. 2006. Mortalin controls centrosome duplication via modulating centrosomal localization of p53. Oncogene 25:5377-5390. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto, Y., and J. L. Maller. 2004. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 306:885-888. [DOI] [PubMed] [Google Scholar]
- 49.Moore, P. S., and Y. Chang. 2003. Kaposi's sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu. Rev. Microbiol. 57:609-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordlund, P., and P. Reichard. 2006. Ribonucleotide reductases. Annu. Rev. Biochem. 75:681-706. [DOI] [PubMed] [Google Scholar]
- 51.Pan, Y. R., C. Y. Fang, Y. S. Chang, and H. Y. Chang. 2005. Analysis of Epstein-Barr virus gene expression upon phorbol ester and hydroxyurea treatment by real-time quantitative PCR. Arch. Virol. 150:755-770. [DOI] [PubMed] [Google Scholar]
- 52.Peloponese, J. M., Jr., K. Haller, A. Miyazato, and K. T. Jeang. 2005. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc. Natl. Acad. Sci. USA 102:18974-18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petit, C., M. L. Giron, J. Tobaly-Tapiero, P. Bittoun, E. Real, Y. Jacob, N. Tordo, H. De The, and A. Saib. 2003. Targeting of incoming retroviral Gag to the centrosome involves a direct interaction with the dynein light chain 8. J. Cell Sci. 116:3433-3442. [DOI] [PubMed] [Google Scholar]
- 54.Reverte, C. G., A. Benware, C. W. Jones, and S. E. LaFlamme. 2006. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder, C. L., S. Faruki, and A. Khodjakov. 2001. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11:413-419. [DOI] [PubMed] [Google Scholar]
- 56.Rivailler, P., H. Jiang, Y. G. Cho, C. Quink, and F. Wang. 2002. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein-Barr virus animal model. J. Virol. 76:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saïb, A., F. Puvion-Dutilleul, M. Schmid, J. Périès, and H. de Thé. 1997. Nuclear targeting of incoming human foamy virus Gag proteins involves a centriolar step. J. Virol. 71:1155-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salisbury, J. L., K. M. Suino, R. Busby, and M. Springett. 2002. Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12:1287-1292. [DOI] [PubMed] [Google Scholar]
- 59.Schon, M., J. Benwood, T. O'Connell-Willstaedt, and J. G. Rheinwald. 1999. Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J. Cell Sci. 112:1925-1936. [DOI] [PubMed] [Google Scholar]
- 60.Sfakianos, J. N., R. A. LaCasse, and E. Hunter. 2003. The M-PMV cytoplasmic targeting-retention signal directs nascent Gag polypeptides to a pericentriolar region of the cell. Traffic 4:660-670. [DOI] [PubMed] [Google Scholar]
- 61.Si, H., and E. S. Robertson. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 80:697-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sugimoto, M., H. Tahara, M. Okubo, T. Kobayashi, M. Goto, T. Ide, and Y. Furuichi. 2004. WRN gene and other genetic factors affecting immortalization of human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Genet. Cytogenet. 152:95-100. [DOI] [PubMed] [Google Scholar]
- 63.Tai, N., J. C. Schmitz, J. Liu, X. Lin, M. Bailly, T. M. Chen, and E. Chu. 2004. Translational autoregulation of thymidylate synthase and dihydrofolate reductase. Front. Biosci. 9:2521-2526. [DOI] [PubMed] [Google Scholar]
- 64.Tang, Y., J. Luo, W. Zhang, and W. Gu. 2006. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell 24:827-839. [DOI] [PubMed] [Google Scholar]
- 65.Tung, P. P., and W. C. Summers. 1994. Substrate specificity of Epstein-Barr virus thymidine kinase. Antimicrob. Agents Chemother. 38:2175-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker, C., S. Bottger, and B. Low. 2006. Mortalin-based cytoplasmic sequestration of p53 in a nonmammalian cancer model. Am. J. Pathol. 168:1526-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wigley, W. C., R. P. Fabunmi, M. G. Lee, C. R. Marino, S. Muallem, G. N. DeMartino, and P. J. Thomas. 1999. Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 145:481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu, S. F., S. W. Eastman, and M. L. Linial. 2006. Foamy virus capsid assembly occurs at a pericentriolar region through a cytoplasmic targeting/retention signal in Gag. Traffic 7:966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan, J., E. Cahir-McFarland, B. Zhao, and E. Kieff. 2006. Virus and cell RNAs expressed during Epstein-Barr virus replication. J. Virol. 80:2548-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]