Abstract
In this study we compared a prime-boost regimen with two serologically distinct replication-defective adenovirus (Ad) vectors derived from chimpanzee serotypes C68 and C1 expressing Gag, Pol, gp140, and Nef of human immunodeficiency virus type 1 with a regimen in which replication-defective Ad vectors of the human serotype 5 (AdHu5) were given twice. Experiments were conducted in rhesus macaques that had or had not been preexposed to antigens of AdHu5. There was no significant difference in T-cell responses tested from peripheral blood of the different groups, although responses were overall highest in nonpreexposed animals immunized with the chimpanzee Ad vectors. Preexisting immunity to AdHu5 completely inhibited induction of transgene product-specific antibodies by the AdHu5 vectors without affecting antibody responses to the chimpanzee vectors. Upon euthanasia, T-cell responses were tested from a number of tissues. Preexisting immunity to AdHu5, commonly found in humans, changed the homing pattern of vaccine-induced T cells. In AdHu5-preexposed animals vaccinated with the chimpanzee Ad vectors, frequencies of transgene-specific T cells were higher in spleens than in blood, and in most preexposed animals vaccinated either with AdHu5 vectors or chimpanzee adenovirus vectors, frequencies of such T cells were exceptionally high in livers. The latter results indicate that analysis of T-cell responses solely from blood mononuclear cells of vaccine recipients may not suffice to compare the potencies of different vaccine regimens.
A multitude of different vaccine prototypes for human immunodeficiency virus type 1 (HIV-1) have undergone early-stage clinical trials, and more are in preclinical testing (5, 7, 10, 11, 18). Among those, E1-deleted adenoviral (Ad) vectors of the human serotype 5 (AdHu5) are promising candidates that have been tested in phase II trials (16). E1-deleted Ad recombinants of common human serotypes, such as AdHu5, were initially developed for gene therapy (3, 15). Their advantages as gene transfer vehicles are numerous. They are well characterized, easy to grow, infect a wide range of cell types including resting cells, and induce high levels of transgene expression. Due to deletion of E1, which renders the virus replication defective, the constructs are well tolerated unless given at excessive doses. The disadvantages of such constructs for permanent replacement of missing or faulty genes include the hosts’ vigorous immune responses against both the Ad antigens and the transgene products (32, 33). Furthermore, preexposure to the homologous serotypes of Ad, many of which infect nearly all humans during childhood, induces serotype-specific virus-neutralizing antibodies (VNAs) (30), which interfere with successful delivery of the gene therapy vehicles (31, 32).
The high immunogenicity of E1-deleted Ad recombinants of the common AdHu5 disallowed their successful use for sustained gene therapy but led to their development as vaccine carriers. E1-deleted AdHu5 vaccines have been shown to induce unsurpassed B- and CD8+ T-cell responses in experimental animals, including rodents (13, 28, 31), canines (27), and primates (17, 24, 25), and they are currently being tested in human clinical trials for antigens of HIV-1 by Merck. Ad vectors are being tested in additional clinical trials by the Vaccine Research Center using DNA vaccine priming regimens followed by AdHu5 vector booster immunizations.
However, the same problems that plagued gene therapists using E1-deleted AdHu5 recombinants may hamper the use of such constructs as vaccines in humans, especially in humans residing in developing countries (2). Most humans are preimmune to common human serotypes of Ad, such as serotypes 2, 4, 5, 7, or 12. In the United States, depending on the age of the study population and the sensitivity of the assay, 40 to 60% of humans carry readily detectable VNAs to AdHu5 virus (29). Seroprevalence rates to AdHu5 virus are markedly higher in human populations from developing countries (29). In animal models, including nonhuman primates (NHPs), VNAs generated upon preexposure to AdHu5 virus have been shown previously to strongly impair the B- and T-cell responses to the transgene product of a vaccine based on an E1-deleted Ad of the same serotype (1, 6, 20). A similar impairment was observed in human volunteers that were tested by Merck in a clinical trial with their AdHu5 vaccine to HIV-1 (16).
To circumvent impairment of vaccine efficacy by preexisting neutralizing antibodies, we developed vaccine vectors based on Ads that had been isolated from chimpanzees. Vectors described here were derived from two different viruses, i.e., AdC68 and AdC1, that represent two distinct serotypes (21, 22). AdC1 was constructed as a chimera with some of the early genes of AdC5, a chimpanzee virus that is related to AdC68, to allow for its growth on cell lines that provide the E1 of AdHu5. AdC68 has close sequence homology with AdHu4 virus and thus belongs to subgroup E of the Adenovirideae. AdC1 virus does not bind the coxsackievirus-adenovirus receptor that is used by most human Ad serotypes but attaches to CD46 (26), which is used by the B2 subgroup of human Ad viruses, such as AdHu11 and AdHu35 (8, 23).
Here we tested prime-boost regimens using a mixture of vectors expressing Gag, Pol, Nef, and gp140 of HIV-1 clade B in small groups of AdHu5-preexposed and nonpreexposed NHPs. We compared a heterologous prime-boost regimen using AdC68 vectors followed by AdC1/C5 vectors to a homologous prime-boost regimen in which AdHu5 vectors were used twice. The data show that preexisting immunity to AdHu5 vectors alters homing of transgene product-specific T cells elicited by chimpanzee and human Ad vectors. Furthermore, the data show that preexposure to AdHu5 vectors abrogates the antibody responses to the transgene product expressed by AdHu5 but not by the chimpanzee Ad vectors.
MATERIALS AND METHODS
Production of vectors.
Ad vectors were derived from AdHu5 or chimpanzee serotypes 68 (AdC68) or 1 (AdC1). Vectors were E1 or E1 and E3 deleted and generated from viral molecular clones by viral rescue on HEK 293 cells. The AdC1 vector could not be propagated on HEK 293 cells that provide the E1 of AdHu5 virus in trans. To allow for growth of this vector on HEK 293 cells, a chimeric vector was constructed between AdC1 (ATCC VR-20) and AdC5 (a chimpanzee adenovirus that is related to AdC68 [ATCC VR-591]). In the chimeric AdC1/C5 vector, map units 1 to 7937 (left-handed inverted terminal repeat) and 32651 to 35524 (right-handed inverted terminal repeat) were replaced with sequences 1 to 7948 and 33547 to 36462 of AdC5 virus. E1-deleted Ads were grown on HEK 293 cells and purified by two rounds of buoyant density ultracentrifugation on CsCl gradients followed by column purification (Bio-Gel P-6DG). Vectors were diluted in phosphate-buffered saline (PBS) supplemented with 10% glycerol and stored at −80°C.
Titration of vectors.
Content of virus particles (VP) was determined by spectrophotometry at 260 nm and 280 nm, with the latter determining purity of the preparation.
Quality control of vectors.
Vector batches were checked for replication-competent Ad on A549 cells. None of the batches used had detectable contamination with replication-competent Ad. Batches were tested for sterility and lipopolysaccharide contamination (Limulus test).
Vector inserts.
An AdHu5 vector expressing alpha-1-antitrypsin (A1AT) was used for preexposure of animals. The vaccine vectors were constructed to express a codon-optimized Gag of HIV-1 clade B (generous gift from G. Pavlakis at the NIH NCI, Fredrick, MD), a codon-humanized HIV-1 clade B gp140 (provided by G. Nabel at the NIH Vaccine Research Center, Bethesda, MD), the 5′ end of polymerase of HIV-1 clade B encoding amino acids 1 to 650 and a fusion of HIV-1 Nef (containing a tetradecanoyl phorbol acetate [TPA] leader sequence and a G2A mutation), and the 3′ end of polymerase (encoding the C-terminal 368 amino acids). Both HIV5′pol and TPAnef-3′pol were synthesized using human codons by Geneart (Regensburg, Germany). Vectors expressing the rabies virus glycoprotein (rab.gp) were used as controls. Vectors expressing enhanced green fluorescent protein were used for neutralization assays. A list of vectors and their pertinent features is shown in Table 1.
TABLE 1.
Ad vectors used in the study
| Vector name | Deletion | Promotera | Insert | VP/TCID50b |
|---|---|---|---|---|
| AdHu5A1AT | E1, E3 | CMV | A1AT | 21 |
| AdHu5HIVpol650 | E1, E3 | CMV | HIV 5′ Pol | 15 |
| AdHu5HIVgag55 | E1, E3 | CMV | HIV Gag | 77 |
| AdHu5HIVgp140 | E1, E3 | CMV | HIV Env | 312 |
| AdHu5HIVTPAnefpol | E1, E3 | CMV | HIV Nef + 3′ Pol | 12 |
| AdC68HIVpol650 | E1, E3 | CMV | HIV 5′ Pol | 67 |
| AdC68HIVgag55 | E1 | CMV | HIV Gag | 244 |
| AdC68HIVgp140 | E1, E3 | CMV | HIV Env | 40 |
| AdC68HIVTPAnefpol | E1, E3 | CMV | HIV Nef + 3′ Pol | 17 |
| AdC1/C5HIVpol650 | E1, E3 | CMV | HIV 5′ Pol | 176 |
| AdC1/C5HIVgag55 | E1 | CMV | HIV Gag | 273 |
| AdC1/C5HIVgp140 | E1, E3 | SV40 | HIV Env | 205 |
| AdC1/C5HIVTPAnefpol | E1, E3 | CMV | HIV Nef + 3′ Pol | 124 |
| AdC68rab.gp | E1 | CMV | Rabies virus gp | 533 |
| AdC1/C5rab.gp | E1 | CMV | Rabies virus gp | 795 |
CMV, cytomegalovirus; SV40, simian virus 40.
VP/TCID50 is the ratio of virus particles to infectious units (50% tissue culture infective dose [TCID50]) of the batches that were used for the NHP study.
Nonhuman primates.
Two- to 3-year-old Chinese origin Macaca mulatta were purchased from PrimateProducts Inc. and housed at the Richards Primate Facility at the University of Pennsylvania. Sera were prescreened for neutralizing antibodies to AdHu5, AdC68, and AdC1 virus. Animals that were selected for the study did not have detectable titers of neutralizing antibodies to these viruses.
Immunization of nonhuman primates.
The 16 animals were divided into five groups (Table 2). Animals of group 2 and 4 were preexposed to AdHu5 vector. These animals were injected with 2 × 1011 VP of AdHu5 expressing A1AT. Animals were bled 4 weeks later, and neutralizing antibody titers to AdHu5 were determined. Thirty-four days after preexposure, animals were vaccinated. Animals of groups 1 and 2 were immunized with AdC68 vectors expressing Gag, gp140, 5′pol, or TPAnef-3′pol. Animals of groups 3 and 4 were immunized with the corresponding AdHu5 vectors. Each vector was given at 2.5 × 1010 VP/animal, and thus each NHP received a total of 1011 VP of vector. Vectors expressing HIV Gag and HIV 5′pol were inoculated into one leg, and those expressing HIV nef-3′pol and HIV gp140 were inoculated into the opposite leg. The control animal of group 5 was primed with a total of 1011 VP of AdC68rab.gp vector (5 × 1010 VP per leg). NHPs were boosted 120 days later with a second dose of vector. Animals of groups 1 and 2 were boosted with AdC1/C5 vectors expressing Gag, gp140, 5′pol, or TPAnef-3′pol. Animals of groups 3 and 4 were boosted with the corresponding AdHu5 vectors. Vectors expressing HIV Gag and HIV 5′pol were inoculated into the left leg, and HIV TPAnef-3′pol and HIV gp140 were inoculated into the right leg. Again, each vector was given at 2.5 × 1010 VP/animal. The control animal of group 5 was boosted with 1011 VP of AdC1/C5rab.gp vector. All vaccinations were performed by injecting animals with vector diluted in 1 ml of saline into the right or left quadriceps muscle.
TABLE 2.
Vaccination schedule for the NHP study
| Group no. | Animal ID/no. (sex) | Material administered
|
Time of euthanasia (day) | ||
|---|---|---|---|---|---|
| Preexposure, day −28 | 1st vaccination, day 0 | 2nd vaccination, day 120 | |||
| 1 | R0001128/1 (F) | None | AdC68 HIV Pol650 | AdC1/C5 HIV Pol650 | NA |
| R0002175/2 (M) | None | AdC68 HIV Gag55 | AdC1/C5 HIV Gag55 | 195 | |
| R0004048/3 (F) | None | AdC68 HIV gp140 | AdC1/C5 HIV gp140 | 217 | |
| R0005087/4 (M) | None | AdC68 HIV TPAnefpol | AdC1/C5 HIV TPAnefpol | 195 | |
| 2 | R0006046/5 (F) | AdHu5 | AdC68 HIV Pol650 | AdC1/C5 HIV Pol650 | 236 |
| R0007052/6 (F) | AdHu5 | AdC68 HIV Gag55 | AdC1/C5 HIV Gag55 | 252 | |
| R0104059/7 (M) | AdHu5 | AdC68 HIV gp140 | AdC1/C5 HIV gp140 | 229 | |
| R0105047/8 (M) | AdHu5 | AdC68 HIV TPAnefpol | AdC1/C5 HIV TPAnefpol | 266 | |
| 3 | R0001006/9 (F) | None | AdHu5 HIV Pol650 | AdHu5 HIV Pol650 | 217 |
| R0103015/10 (M) | None | AdHu5 HIV Gag55 | AdHu5 HIV Gag55 | 266 | |
| R0105066/11 (F) | None | AdHu5 HIV gp140 AdHu5 HIV TPAnefpol | AdHu5 HIV gp140 AdHu5 HIV TPAnefpol | 175 | |
| 4 | R0002151/12 (M) | AdHu5 | AdHu5 HIV Pol650 | AdHu5 HIV Pol650 | 236 |
| R0004064/13 (F) | AdHu5 | AdHu5 HIV Gag55 | AdHu5 HIV Gag55 | 224 | |
| R0105080/14 (F) | AdHu5 | AdHu5 HIV gp140 | AdHu5 HIV gp140 | 229 | |
| R0109015/15 (M) | AdHu5 | AdHu5 HIV TPAnefpol | AdHu5 HIV TPAnefpol | 252 | |
| 5 | R0108033/16 (M) | None | AdC68 rab.gp | AdC1/C5 rab.gp | 175 |
aNA, not applicable.
Sample collection.
Animals were bled from the cephalic vein under light anesthesia. Blood was collected into Vacutainer cell collection tubes (CPT) with or without heparin (BD Biosciences). Upon euthanasia animals were exsanguinated. The peritoneal cavity was rinsed with 300 ml of Hanks’ buffered saline solution (HBSS; Cellgro) to isolate lymphoid cells. Tissues, including lymph nodes, spleen, liver, and peritoneal lavage fluid, were collected into HBSS.
Isolation of PBMCs.
Lymphocytes were collected in CPT tubes. The plasma layer was frozen at −80°C. Peripheral blood mononuclear cells (PBMCs) were treated with ACK lysis buffer (Amersham) for 5 min at room temperature and washed twice with HBSS and twice with RPMI complete medium (RPMIc; RPMI 1640 supplemented with 10% fetal bovine serum [FBS; Tissue Culture Biological], 10 mM HEPES, penicillin-streptomycin, and gentamicin [Cellgro]).
Isolation of lymphocytes from tissues.
Lymphocytes were isolated from peritoneal lavage fluid by passing the lavage fluid through a 70-μm nylon filter and then washing cells two times with HBSS and two times with RPMIc. Cells were isolated from spleens and lymph nodes by dissecting the tissues into small sections and grinding them against a stainless steel mesh. Cells were filtered through a 70-μm nylon filter (BD Falcon) and washed. Liver lymphocytes were isolated by grinding the tissue in HBSS supplemented with 2% FBS. The resulting suspension was spun at 400 rpm for 10 min at 4°C. The supernatant was isolated and spun at 1,500 rpm for 5 min. The resulting pellet was washed twice with PBS plus 2% FBS. Lymphocytes were obtained using Percoll gradient centrifugation and washed twice with RPMI.
Preservation of lymphocytes.
Cells were tested immediately after isolation by enzyme-linked immunospot (ELISPOT) assays. Remaining cells were frozen in 90% FBS and 10% dimethyl sulfoxide (Sigma), first at −80°C and then cryopreserved in liquid nitrogen. When using frozen PBMCs, cells were thawed in a 37°C water bath, diluted 1:10 in RPMI, and then washed twice with RMPI plus 2 units/ml of RNase-free DNase (Roche). Intracellular cytokine staining (ICS) was conducted on cells that had been frozen.
Synthetic peptides.
HIV clade B consensus sequence Gag, Pol, Nef, and Env peptides, 15-mers overlapping by 11 amino acids, were obtained from the NIH Research and Reference Reagents Program and pooled into either 7 or 10 peptide pools for ICS and ELISPOT assays, respectively. For ICS, this resulted in two pools for the Gag peptides, four pools for Pol peptides, one pool for Nef, and three pools for Env (corresponding to gp140). For ELISPOT assays one pool was used for Gag, two pools for Pol, one pool for Nef, and three pools for Env (gp140).
ELISPOT.
The ELISPOT assay was conducted as described elsewhere (19). Briefly, lymphocytes were added to wells of MultiScreen-IP white plates (Millipore) at 2.5 × 105 cells per well. For each tissue, pools were tested in triplicate (except where cell number was limiting). Each 15-mer peptide was used at a final concentration of 2 μg/ml. Plates were incubated either with or without peptide for 16 to 18 h at 37°C, 5% CO2. Spots were counted using the CTL series 3A analyzer and ImmunoSpot 3.2 (Cellular Technology Ltd., Cleveland, OH). The minimum spot size was set to 0.0014 mm2, and the maximum spot size was set to 0.0607 mm2. Data had been summarized as spots per 106 cells, and so a dilution factor was used to multiply the raw values obtained from the ImmunoSpot count. There were three criteria for positive samples: (i) for every 106 cells stimulated with peptides, at least 55 spots had to be detected; (ii) the number of spots in peptide-stimulated wells had to be at least three times the number of spots in unstimulated control wells; (iii) the number of spots in peptide-stimulated wells had to be 3 standard deviations above the mean of the control wells. Data shown on graphs represent values of peptide-stimulated wells from which background values have been subtracted.
ICS.
PBMCs (106 cells) were stimulated in RPMI medium for 6 h with anti-CD28, anti-CD49d, and brefeldin A (10 μg/ml each), with or without 1 μg/ml/peptide of the HIV-1 peptide pools at 37°C, 5% CO2. After incubation, cells were stained with anti-human-CD8-perchloridinin chlorophyll a protein-Cy5.5 for 30 min at 4°C. After washing, fixing, and permeabilization, cells were stained with anti-human gamma interferon (IFN-γ)-allophycocyanin, anti-human interleukin-2 (IL-2)-phycoerythrin, and anti-monkey CD3-fluorescein isothiocyanate for 30 min at 4°C. Cells were washed twice, resuspended in 1% formalin in PBS, and analyzed by fluorescence-activated cell sorting (FACS). Cells were acquired on a Cyan LX flow cytometer (DakoCytomation), and FACS data were analyzed by using Summit software (DakoCytomation). All antibodies were purchased from BD Bioscience except for anti-CD3, which was purchased from Biosource.
Titration of antibodies to Gag.
Nunc Immunoplates (MaxiSorb F96; Denmark) were coated with 100 μl of a solution containing 1 μg of Gag/ml in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.8) overnight at 4°C. Plates were blocked overnight with PBS supplemented with 3% bovine serum albumin. Plates were washed with PBS, and serial dilutions of monkey sera were added in borate buffer (0.1 M boric acid, 47 mM sodium borate, 75 mM NaCl, 0.05% [vol/vol] Tween 20) with 3% bovine serum albumin and incubated at 37°C for 2 h. Wells were washed with PBS, and 80 μl of a 1:200 dilution of alkaline phosphatase-conjugated, goat anti-monkey immunoglobulin G (Sigma Chemical Company, St. Louis, MO) was added and incubated at 37°C for 2 h. Wells were washed and incubated with 100 μl of substrate buffer containing p-nitrophenylphosphate disodium hexahydrate in diethanolamine. After 30 min at room temperature, absorbance was read at 405 nm.
Adenovirus neutralization assay.
Heat-inactivated (30 min, 56°C) NHP plasma was tested on HEK 293 cells for neutralization of Ads using vectors expressing enhanced green fluorescent protein in a plaque reduction assay starting with a 1:20 dilution of sample (30).
Statistics.
Significance was determined by one-tailed Student's t tests and analysis of variance performed with Microsoft Excel. Significance was set at a P level of ≤0.05.
RESULTS
Prescreening, preexposure, and immunization of animals.
A trial was conducted in 2- to 3-year-old Chinese Macaca mulatta animals to assess the immunogenicity of replication-defective chimpanzee origin Ad vectors in comparison to replication-defective AdHu5 vectors. NHPs were tested prior to purchase for VNAs to the Ad vectors. Sixteen animals that were negative were selected. One of those animals (R001006) had antibodies that by enzyme-linked immunosorbent assay bound to AdHu5, AdC68, and AdC1/C5. Eight animals were immunized intramuscularly with 2 × 1011 VP of an AdHu5A1AT vector. Animals were bled 2 weeks later and tested for VNA titers to AdHu5 virus. Animals had VNA titers ranging from 1:80 to 1:640. It should be pointed out that in human adults, average titers to AdHu5 virus range from 1:100 to 1:300 using the assay system that was employed for studies presented here.
Vaccination of NHPs.
All of the animals were vaccinated 4 weeks after injection of the AdHu5A1AT vector (Table 2). Each of the vaccines to HIV-1 was composed of an equal mixture of four vectors expressing different sequences of HIV-1 clade B. Specifically, vectors expressed Gag, gp140, the 5′ part of Pol, and a fusion polypeptide composed of Nef and the 3′ part of Pol. Two groups of animals, i.e., groups 1 and 2, were primed with vaccines based on the chimpanzee origin AdC68 virus. These animals were boosted with the AdC1/C5 chimpanzee Ad vector expressing the same transgenes. The other two experimental groups were immunized twice with AdHu5 vectors expressing the same transgenes. This immunization schedule was chosen to allow for comparison with results from other research teams that have focused on the use of AdHu5 vectors and that have used such vectors in homologous prime-boost regimens (1). The control animal was immunized with 1011 VP of chimpanzee Ad vectors expressing the rabies virus glycoprotein. All of the vectors prior to their use in NHPs were tested in vitro for expression of the transgene product (except for the vector expressing HIVTPAnefpol3, for which no suitable antibody was available). In addition, all of the vectors (of the same batch that was used in NHPs) were tested in dose escalation experiments in mice to confirm their immunogenicity (data not shown).
HIV-1-specific T-cell responses in blood.
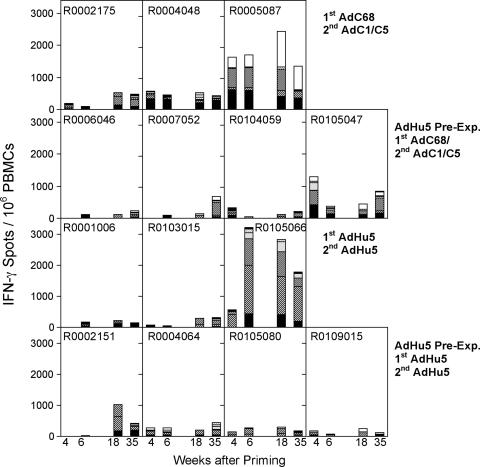
PBMCs were collected twice before vaccination and tested for T-cell responses to the different HIV-1 peptide pools by ELISPOT and ICS. Specific responses could not be detected by either assay. After each vaccine dose, animals were bled at different time points, and frequencies of T cells were determined by an ELISPOT for IFN-γ (Fig. 1) and by ICS for CD3, CD4, CD8, IL-2, and IFN-γ (Fig. 2). By 4 weeks after priming, four of four animals of group 1 that were not preexposed and received the AdC68 vectors responded, and three animals had frequencies above 200 spots/106 PBMCs (Fig. 1). In group 2, which received the AdC68 vectors after AdHu5 preexposure, two of four NHPs developed T-cell frequencies that were comparable to those of group 1. In group 3, which received the AdHu5 vectors without preexposure, two of three animals were tested and only one developed a response; CD8+ T-cell frequencies in this animal were below frequencies achieved by the high responders of the two groups of NHPs that were vaccinated with the AdC68 vectors. From group 4, which was vaccinated with AdHu5 after preexposure, three of four animals were tested and all of them had low but detectable frequencies of HIV antigen-specific IFN-γ-producing cells. Animals were retested 6 weeks after priming by ELISPOT assay. T-cell responses could be detected by 6 weeks after priming in four of four animals of group 1 (AdC68 vaccinated), one of four animals in group 2 (AdHu5 preexposed, AdC68 vaccinated), two of three animals in group 3 (AdHu5 vaccinated), and two of four animals in group 4 (AdHu5 preexposed, AdHu5 vaccinated). Responses in one of two animals of group 3 and two of three animals of group 4, the two groups that were vaccinated with the AdHu5 vectors, were barely above background by week 6 (55 spots for a given peptide pool).
FIG. 1.
Two groups of four NHPs were injected with 2 × 1011 VP of AdHu5 expressing A1AT. Thirty-four days later, preexposed and nonpreexposed NHPs were vaccinated. Groups 1 and 2 (upper two panels) were immunized with AdC68 vectors expressing Gag, gp140, 5′pol, or TPAnef-3′pol. Animals of groups 3 and 4 (lower two panels) were immunized with the corresponding AdHu5 vectors. The control animal (R0108033) was primed with an AdC68rab.gp vector. T-cell responses from blood were tested 4 and 6 weeks after vaccination by ELISPOT for IFN-γ. NHPs were boosted 120 days later with a second dose of vector. Animals of groups 1 and 2 were boosted with AdC1/C5 vector expressing Gag, gp140, 5′pol, or TPAnef-3′pol. Animals of groups 3 and 4 were boosted with the corresponding AdHu5 vector. The control animal of group 5 was boosted with AdC1/C5rab.gp vector. T-cell responses were tested from blood 2 and 17 weeks after the boost. The graph shows responses of individual animals against seven different pools of peptides. Background data (no peptide) were subtracted. Data for the control animal are not shown. PBMCs from this animal showed the following cumulative spots (i.e., sum of spots obtained with all of the peptide pools minus background spots)/106 cells: week 4, 18; week 6, 0; week 18, 96; week 35, 61. Responses to individual pools were <55 spots/106 PBMCs and thus failed to meet our criteria for a positive response. The different patterns on the bars show responses to different peptide pools. Solid black, Gag peptides; bold stripes, two pools of Pol peptides; dots, Nef peptides; thin stripes and no pattern, three pools of Env peptides.
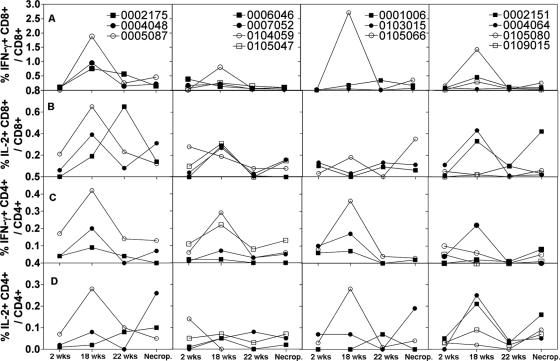
FIG. 2.
The same animals described in Fig. 1 were tested for T-cell responses by ICS of CD3+ CD8+ or CD3+ CD4+ T cells for IFN-γ or IL-2 using 10 pools of peptides. PBMCs were tested 2 weeks after priming, 2 and 6 weeks after the boost, and at the time of necropsy. The background data from the control animal were subtracted. The graphs show the sum of frequencies of CD3+ CD8+ cells secreting IFN-γ (A), CD3+ CD8+ cells secreting IL-2 (B), CD3+ CD4+ cells secreting IFN-γ (C), and CD3+ CD4+ cells secreting IL-2 (D) obtained by adding frequencies obtained with individual peptide pools.
Approximately 110 days after priming, one of the animals (R0001128) of group 1 (AdC68 vaccinated) died (see below for further details). The surviving NHPs were boosted, and their PBMCs were retested 2 weeks later. All of the animals of groups 1 to 4 scored positive by ELISPOT after booster immunization, but only some animals showed an increase in frequencies compared to peak frequencies after the first immunization. After the AdC1/C5 boost of AdC68-primed NHPs, frequencies were lower in the AdHu5-preexposed group 2 compared to nonpreexposed animals of group 1, suggesting that preexposure to AdHu5 had a negative effect on the recall immune response to the chimpanzee vectors. Nevertheless, this difference was not statistically significant. It was of interest that animals of groups 3 and 4 that had received two doses of the AdHu5 vectors developed mainly T cells to Gag and Pol, and only animals of group 1 and to a lesser extent group 2 developed strong T-cell responses to peptides of Env. Responses to Nef peptides were low in all of the animals. PBMCs were retested 15 weeks later and then again at the time of necropsy. In animals that had been vaccinated with the chimpanzee Ad vectors, one of three animals of the nonpreexposed group 1 showed a decrease in frequencies over time, while frequencies remained stable in the other two NHPs. In AdHu5-preexposed group 2, frequencies increased in all four animals. Upon the homologous booster immunization with AdHu5 vectors, frequencies decreased in two of three animals of the nonpreexposed group 3 and three of four animals in the AdHu5-preexposed group 4. In general, frequencies of specific T cells in blood did not decrease markedly between weeks 2 and 17 after the boost.
Results obtained by ICS (Fig. 2 and Table 3) showed a similar, although not identical, pattern to those obtained by ELISPOT assays. Two weeks after priming, most animals had low frequencies (≤0.2%) of CD8+ IFN-γ-producing T cells and only one animal of group 2, which had been preexposed prior to vaccination with AdC68 vectors, had frequencies of >0.2% (Fig. 2). After booster immunization, frequencies increased to above 0.2% in all of the nonpreexposed animals vaccinated with the chimpanzee Ad vectors (group 1) and in three of four animals that were preexposed prior to vaccination with the same vectors (group 2). In AdHu5-vaccinated animals, frequencies above 0.2% were seen in two of three of the nonpreexposed animals (group 3) and in two of four of the preexposed (group 4). Frequencies declined by 6 weeks after booster immunization, and at the time of necropsy only one animal of each group but for the group that was preexposed prior to vaccination with the chimpanzee Ad vectors retained frequencies of >0.2%. As had been observed using ELISPOT assays, responses were mainly directed against Gag and Pol peptides, and responses against Env were mainly detected after priming in nonpreexposed chimpanzee Ad vector-vaccinated NHPs (not shown).
TABLE 3.
Summary of ICS dataa
| Cell population and group no. | Avg frequency ± SD (no. positive/no. NHPs analyzed)
|
||
|---|---|---|---|
| 1st immunization | 2nd immunization | At necropsy | |
| CD8+ IFN-γ+ | |||
| Group 1 | 0.07 ± 0.05 (0/3) | 1.19 ± 0.60 (3/3) | 0.27 ± 0.16 (1/3) |
| Group 2 | 0.15 ± 0.17 (1/4) | 0.34 ± 0.31 (3/4) | 0.03 ± 0.03 (0/4) |
| Group 3 | 0.007 ± 0.01 (0/3) | 0.97 ± 1.51 (2/3) | 0.14 ± 0.2 (1/3) |
| Group 4 | 0.08 ± 0.06 (0/4) | 0.55 ± 0.61 (2/4) | 0.10 ± 0.10 (1/3) |
| CD8+ IL-2+ | |||
| Group 1 | 0.09 ± 0.10 (1/3) | 0.41 ± 0.23 (2/3) | 0.19 ± 0.10 (1/3) |
| Group 2 | 0.15 ± 0.17 (1/4) | 0.27 ± 0.05 (3/4) | 0.10 ± 0.07 (0/4) |
| Group 3 | 0.09 ± 0.05 (1/3) | 0.07 ± 0.10 (0/3) | 0.19 ± 0.15 (1/3) |
| Group 4 | 0.04 ± 0.05 (0/4) | 0.20 ± 0.21 (2/4) | 0.10 ± 0.10 (1/4) |
| CD4+ IFN-γ+ | |||
| Group 1 | 0.08 ± 0.08 (0/3) | 0.24 ± 0.17 (1/3) | 0.07 ± 0.07 (0/3) |
| Group 2 | 0.05 ± 0.05 (0/4) | 0.15 ± 0.13 (2/4) | 0.06 ± 0.05 (0/4) |
| Group 3 | 0.08 ± 0.02 (0/3) | 0.20 ± 0.15 (1/3) | 0.02 ± 0.01 (0/3) |
| Group 4 | 0.05 ± 0.04 (0/4) | 0.08 ± 0.10 (1/4) | 0.03 ± 0.04 (0/4) |
| CD4+ IL-2+ | |||
| Group 1 | 0.03 ± 0.03 (1/3) | 0.12 ± 0.14 (1/3) | 0.14 ± 0.11 (1/3) |
| Group 2 | 0.05 ± 0.06 (0/4) | 0.04 ± 0.03 (0/4) | 0.04 ± 0.02 (0/4) |
| Group 3 | 0.03 ± 0.04 (1/3) | 0.11 ± 0.14 (1/3) | 0.08 ± 0.10 (0/3) |
| Group 4 | 0.02 ± 0.02 (0/4) | 0.14 ± 0.11 (2/4) | 0.09 ± 0.05 (0/4) |
The results shown were obtained for the different groups 2 weeks after the first and second immunizations and at the time of necropsy. The numbers in parentheses show the number of animals that responded (i.e., had cumulative frequencies of >0.2% or frequencies against a single peptide pool of >0.1%) over all animals of this group. The bold numbers highlight responses of >0.2%. Lymphocytes isolated from prebleeds were assayed for all four parameters, and cumulative frequencies were determined to be below 0.1% with the exception of animals R0001006 and R0004064, which had cumulative frequencies of 0.24% for CD8+ IL-2+ and 0.22% for CD8+ IFN-γ+ cells, respectively. These two animals failed to mount immune responses after immunization.
Frequencies of CD8+ T cells producing IL-2 were lower than those of CD8+ T cells producing IFN-γ (Fig. 2A). After the second immunization with the heterologous chimpanzee Ad vector, two of three of the nonpreexposed animals (group 1) and three of four of the AdHu5-preexposed animals (group 2) had CD8+ IL-2+ frequencies above 0.2% (Fig. 2B), while after AdHu5 vaccination none of the nonpreexposed (group 3) and two of four of the preexposed animals (group 4) scored positive above 0.2%. Frequencies of CD4+ T cells producing IFN-γ or IL-2 (Fig. 2C and D) were low, and only one or two animals of each of the different groups developed detectable CD4+ T-cell responses after priming or booster immunization. Overall, although nonpreexposed NHPs vaccinated with the two chimpanzee Ad vectors mounted the highest CD8+ T-cell responses for IFN-γ and IL-2, differences between the groups did not reach significance.
T-cell responses in different tissues.
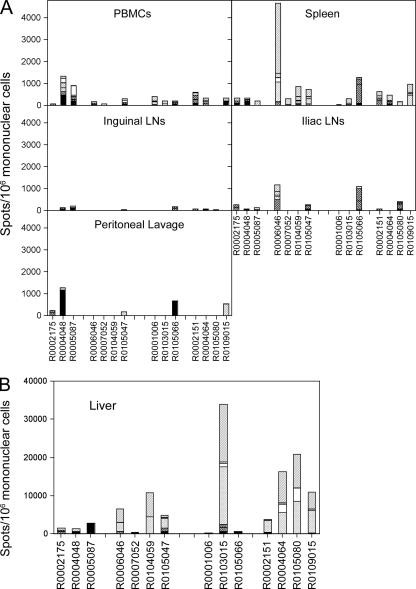
Animals were euthanized 25 to 38 weeks after the initial priming, and two animals were euthanized approximately every second week. Animals were chosen randomly from the different groups. Lymphocytes were harvested from a number of compartments and tested by ELISPOT assays (Fig. 3A and B). From most animals, specific T cells could be isolated from the spleens. It was remarkable that frequencies in spleens failed to correlate with frequencies in blood. For example, the mean frequencies of responses from PBMCs upon vaccination with the chimpanzee Ad vectors were higher in nonpreexposed than in preexposed animals, suggesting that preexisting immunity affected the T-cell response to the chimpanzee Ad vectors. In contrast, in splenocytes mean frequencies were higher in preexposed animals. Neither difference reached statistical significance. Of nonpreexposed animals vaccinated with the chimpanzee Ad vectors, two of three and three of three showed detectable frequencies of cells producing IFN-γ in inguineal and iliac lymph nodes, respectively. Such responses were seen in one of four and two of four preexposed chimpanzee Ad vector-vaccinated animals from groups 2 and 4, respectively. Upon AdHu5 vaccination, one of three in group 1 and one of three in group 3 of the nonpreexposed animals and three of four in group 2 and two of four in group 4 of the preexposed animals responded. Some animals showed responses in one but not the other set of lymph nodes. HIV-1 antigen-specific T cells could only be detected in two of three animals of the nonpreexposed, chimpanzee Ad vector-vaccinated group and in one animal of each of the other three groups in the peritoneal lavage (Fig. 3A). Lymphocytes isolated from the liver showed extraordinarily high frequencies of HIV-1-specific IFN-γ-producing T cells, ranging from 3,000 to 34,000 cells/106 lymphocytes (Fig. 3B). Frequencies were especially high in animals that had been preexposed to the AdHu5 A1AT vector, i.e., preexposed animals vaccinated either with the chimpanzee Ad vectors or with AdHu5 vectors showed in general higher frequencies than nonpreexposed animals given either vaccine regimen. Two animals did not follow this pattern, i.e., one nonpreexposed AdHu5-vaccinated animal (R0103015) had a very high response in the liver, and one preexposed animal vaccinated with the chimpanzee Ad vectors (R0007052) had only a low response in the liver. Overall, T-cell frequencies in liver were higher in preexposed animals upon AdHu5 vaccination than upon vaccination with the chimpanzee Ad vectors. The majority of cells isolated from liver responded to the Nef and Env peptide pools. Comparing the groups, none of the responses in spleen, blood, or lymph node tissues reached a statistically significant difference (P > 0.05) except for the response in liver comparing nonpreexposed, chimpanzee Ad vector-immunized animals with those that had been preexposed prior to vaccination with AdHu5 vectors (P = 0.025).
FIG. 3.
Lymphocytes from different tissues from the same NHPs described in Fig. 1 were tested at the time of necropsy for T cells producing IFN-γ by ELISPOT. (The codes for the animal numbers and the group designations are shown in Table 2.) The shading of the bars, which indicates the cumulative response to the different pools of peptides, is identical to that in Fig. 1. Data for the control animal (group 5) are not shown. (A) Results for cells from blood, spleens, lymph nodes, and peritoneal lavage. For these tissues the control animal showed the following numbers of spots for all peptide pools/106 mononuclear cells: blood, 54; inguinal lymph nodes, 8; iliac lymph nodes, 36; peritoneal lavage, 46; spleen, 51. All responses to individual pools were <55 spots/106 cells and thus failed to meet our criteria for a positive response. (B) Results for cells isolated from the liver, shown on a different scale. Cells isolated from the liver of the control monkey showed 0 spots per 106 mononuclear cells.
Serum antibody responses.
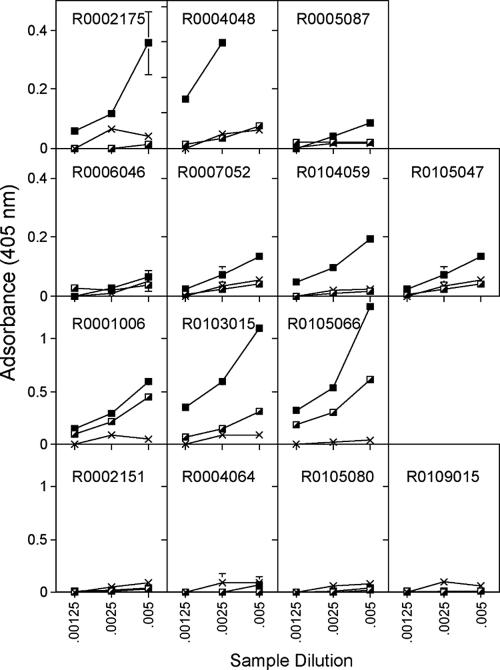
Sera were tested before vaccination and 2 weeks before and 2 weeks after booster immunization for antibodies to Gag. After priming, nonpreexposed animals vaccinated with the AdHu5 vectors showed the highest antibody titers; titers were low to undetectable in animals that were vaccinated with AdC68 or that had been preexposed to AdHu5 prior to vaccination with the AdHu5 vector (Fig. 4). After booster immunization two of three and three of four of the chimpanzee Ad vector-vaccinated animals that had not or had been preexposed to AdHu5 showed a significant increase in titers, and there was no significant difference in the antibody response between the two groups, although mean titers of preexposed animals were lower than those of animals that had not been preexposed. In AdHu5-vaccinated animals that had not been preexposed, two of three showed an increase in antibodies to Gag after the boost, while none of the animals that had been preexposed to AdHu5 developed antibodies to Gag after two immunizations with the AdHu5 vectors. Animals were tested for neutralizing antibodies to HIV-1 to SF162 and BAL (D. Montefiori, Duke University, Durham, NC). All samples (before and after boost) were negative. Animals were also tested for binding antibodies to gp120. Only three of the animals responded, i.e., one of those that was preexposed to AdHu5 and then immunized with the chimpanzee Ad vectors (R0006046), one of the AdHu5-immunized animals that had not been preexposed (R0103015), and one of the AdHu5-preexposed animals vaccinated with the AdHu5 vectors (R0105080). Responses were low, and although there was no significant difference in the magnitude of antibody titers between the responders of the different groups, animals immunized with the chimpanzee Ad vectors developed detectable titers after one immunization while those immunized with the AdHu5 vectors only generated detectable titers after the second immunization (data not shown).
FIG. 4.
Sera from the same NHPs shown in Fig. 1 were tested for antibodies to Gag before and after each vaccine dose. The graphs, which are organized in the same fashion as for Fig. 1, show mean absorbance ± standard deviations at three serum dilutions. The Xs indicate sera harvested before vaccination, half-filled squares indicate sera harvested after the first immunization, and filled squares indicate sera harvested after the boost. The control animal did not develop antibodies to Gag (not shown).
Animals were tested for neutralizing antibody titers to the Ad vectors used for the experiment, and upon preexposure and immunization, animals developed antibodies to the Ad vectors (Table 4). Antibodies to AdHu5 were boosted in preexposed animals after priming and in all of the AdHu5-vaccinated animals after the boost with AdHu5 vectors. Immunization with AdC68 or AdC1/C5 vectors failed to increase antibody titers to AdHu5, again confirming lack of cross-reactivity of neutralizing antibodies to these vectors. In addition, animals were analyzed after preexposure and after the first immunization for binding antibodies to the Ad vector by an enzyme-linked immunosorbent assay (data not shown). In the AdHu5-preexposed groups, six of eight animals developed antibodies that cross-reacted between the three Ad vectors; two animals (R005087 and R0109015) developed antibodies that only bound to AdHu5. In the nonpreexposed groups, one of three animals that had been vaccinated with AdC68 vector developed antibodies that also bound to AdHu5 and AdC1/C5, and the other two animals of this group had antibodies that only bound to AdC68. The control animal that was vaccinated with an AdC68 vector expressing an irrelevant antigen showed a response that was specific for AdC68. In the AdHu5-preexposed group, all animals upon AdC68 vaccination showed an increase in titers to AdC68; animal R0105047 remained negative for antibodies to AdC1. In the nonpreexposed group that was vaccinated with the AdHu5 vectors, two of three animals developed antibodies that showed cross-reactive binding between AdHu5, AdC68, and AdC1, while one animal (R0105066) had antibodies that bound to AdHu5 and AdC1 but not to AdC68. The pattern of serum reactivity in the AdHu5-preexposed group did not change after vaccination with AdHu5 expressing HIV antigens. These data show that while there is a lack of neutralizing antibody cross-reactivity among the human- and chimpanzee-derived adenoviruses, binding antibodies cross-react extensively.
TABLE 4.
Titers of neutralizing antibodies to AdHu5, AdC68, and AdC1/C5 in individual animals after preexposure, priming, and booster immunization
| Group no. | Animal ID | Anti-AdHu5 titer after:
|
Anti-AdC68 titer after:
|
Anti-AdC1/C5 titer after boosting | |||
|---|---|---|---|---|---|---|---|
| Preexposure | Priming | Boosting | Priming | Boosting | |||
| 1 | R0001128 | <1:10 | NTa | NT | NT | NT | NT |
| R0002175 | <1:10 | <1:10 | <1:10 | 1:80 | 1:40 | <1:10 | |
| R0004048 | <1:10 | <1:10 | <1:10 | 1:80 | 1:40 | 1:20 | |
| R0005087 | <1:10 | <1:10 | <1:10 | 1:80 | 1:40 | 1:20 | |
| 2 | R0006046 | 1:640 | 1:160 | 1:20 | 1:40 | 1:160 | 1:10 |
| R0007052 | 1:160 | 1:160 | 1:640 | 1:20 | 1:20 | 1:80 | |
| R0104059 | 1:320 | 1:160 | 1:160 | 1:40 | 1:80 | 1:10 | |
| R0105047 | 1:80 | 1:20 | 1:20 | 1:160 | 1:160 | 1:10 | |
| 3 | R0001006 | <1:10 | 1:40 | >1:1280 | <1:10 | <1:10 | <1:10 |
| R0103015 | <1:10 | 1:160 | >1:1280 | <1:10 | <1:10 | <1:10 | |
| R0105066 | <1:10 | 1:80 | >1:1280 | <1:10 | <1:10 | <1:10 | |
| 4 | R0002151 | 1:160 | >1:1280 | >1:1280 | <1:10 | <1:10 | <1:10 |
| R0004064 | 1:160 | >1:1280 | >1:1280 | <1:10 | <1:10 | <1:10 | |
| R0105080 | 1:640 | 1:640 | >1:1280 | <1:10 | <1:10 | <1:10 | |
| R0109015 | 1:80 | >1:1280 | >1:1280 | <1:10 | <1:10 | <1:10 | |
| Control | R0108033 | <1:10 | <1:10 | <1:10 | 1:160 | 1:40 | 1:20 |
NT, not tested.
Adverse reactions.
During sample collection 10 weeks after priming, one of the AdC68-vaccinated animals (R0001128 in group 1) developed hypothermia and died the following day. Data for this animal are not shown in any of the figures. During anesthesia, serum had been collected and the animal's glucose level was found to be very low (<25 mg/ml). A necropsy followed, and histological analyses of tissue sections did not reveal any tissue pathology that could be associated with the vaccine vectors. It was concluded that the death of the animal was not related to the vaccine but rather to anesthesia-related stress.
DISCUSSION
We developed Ad vectors of chimpanzee origin to prevent a potential dampening effect of preexisting VNAs to common human serotypes of Ad on the potency of Ad vector-based vaccines. We generated vectors from several different serotypes to allow for heterologous prime-boost regimens. Here, we tested two chimpanzee Ad vectors, i.e., AdC68, which belongs to subfamily E of the adenovirideae, and AdC1/C5, in which AdC1 that belongs to subfamily B2 encodes the structural viral antigens. Adenoviruses of the B2 subfamily use CD46 as the attachment receptor. This receptor is also used by the measles vaccine, which causes a transient immunosuppression and a shift towards Th2 responses (12, 14), neither of which have been observed upon immunization of animals with CD46 binding adenovirus vectors (4, 26).
Experiments were conducted in nonhuman primates that had or had not been preexposed to antigens of AdHu5. Additional animals were immunized twice with AdHu5 vectors. Animals were preexposed with a high dose of an AdHu5 vector expressing an irrelevant antigen. As in previously described studies, AdHu5 vectors were given intramuscularly (1, 20). This route of immunization elicits strong VNA responses to AdHu5 and presumably T cells, including CD8+ T cells, to antigens of AdHu5 (6). Animals were preexposed to AdHu5 virus rather than a chimpanzee adenovirus to mimic humans that commonly carry neutralizing antibodies to AdHu5 but show low prevalence rates of neutralizing antibodies to the chimpanzee adenoviruses (30). While neutralizing antibodies generated upon postexposure were highly specific to AdHu5, binding antibodies showed extensive cross-reactivity between the different Ad vectors we used in this study (not shown).
All of the chimpanzee Ad vectors we generated had been tested previously in comparison with AdHu5 vectors in naïve mice and in mice that had been preexposed to AdHu5 (6, 26, 29). The results showed that in naïve mice the AdC68 vector induced transgene product-specific CD8+ T cells that were comparable or, depending on the insert, slightly higher than those induced by AdHu5 vectors. AdC1/C5 vectors were less immunogenic. In AdHu5-preexposed animals CD8+ T cells induced by the chimpanzee Ad vectors were slightly reduced, and this was caused by CD8+ T cells that cross-reacted between antigens of human and chimpanzee Ad vectors (6). In contrast, CD8+ T-cell responses to AdHu5 vectors were strongly diminished or even abolished in mice preexposed to antigens of AdHu5. AdHu5 vectors induced higher antibody responses than chimpanzee Ad vectors in naïve mice, but again such responses were strongly reduced upon preexposure of animals to AdHu5, which had no effect on the induction of transgene product-specific antibodies by the chimpanzee vectors (29).
In the NHP study described here, preexposure of rhesus macaques to antigens of AdHu5 had an apparent effect on induction of transgene product-specific CD8+ T cells by the chimpanzee Ad vectors when PBMCs were analyzed. Preexposed animals mounted a less vigorous CD8+ T-cell response based on comparison of PBMCs from nonpreexposed animals to those that had been preexposed. Although this difference did not reach statistical significance due to the small number of animals, there was a clear trend that could be observed by ELISPOT assays (which fail to discriminate between T-cell subsets) and by ICS. For example, animal R0006046, which had the highest prevaccination antibody titers to AdHu5 (1:640) of this group, developed the poorest T-cell response, while animal R0105047, which had low antibody titers to AdHu5 (1:80) prior to vaccination, mounted a more robust T-cell response. Animal R0104059, which had anti-AdHu5 antibody titers of 1:320 prior to vaccination, also failed to develop a strong T-cell response in blood. Nevertheless, testing of lymphocytes from different lymphatic and nonlymphatic tissues upon euthanasia showed that R0006046 had very high frequencies of HIV-1 antigen-specific T cells in its spleen (>4,500 spots/106 splenocytes), and both R0006046 and R0104059 had high frequencies of such T cells in the liver. Overall, responses in the spleen and liver were higher in three of four NHPs in the AdHu5-preexposed group than in nonpreexposed NHPs vaccinated with the chimpanzee vectors, suggesting that preexposure to AdHu5 vectors affected a change in the homing pattern of the responding T cells.
T-cell responses to the AdHu5 vectors given twice were indistinguishable in blood and spleens between NHPs that had or had not been preexposed to AdHu5. In both groups, a second immunization with AdHu5 vectors increased frequencies of HIV-1 antigen-specific CD8+ T cells producing IFN-γ in some but not all of the animals. It should be pointed out that animal R0105080, which had the highest titers of antibodies upon preexposure (1:640), had very low frequencies of specific T cells in its spleen at the time of euthanasia, indicating that in AdHu5-vaccinated NHPs, unlike those vaccinated with the chimpanzee Ad vectors, preexposure to AdHu5 did not cause a shift in distribution of vaccine-induced T cells towards the spleen. Preexposure resulted in a marked increase in T cells that homed to the liver in AdHu5-vaccinated NHPs. The mechanism of the pronounced homing of T cells towards the liver in AdHu5-preimmunized animals vaccinated either with AdHu5 vectors (four of four) or the chimpanzee Ad vectors (three of four) is unclear. Ad vectors readily infect hepatocytes, and some of the vectors may have entered the bloodstream upon intramuscular application and reached the liver, thus attracting and retaining a T-cell infiltrate. Adenoviruses acquired by natural infections persist for years, mainly in T cells (9). We showed recently that E1-deleted Ad vectors persist for more than 1 year mainly at the site of inoculation and in T cells (submitted for publication). In mice, the Ad vector genome can be detected in livers of intramuscularly immunized mice transiently for a few days after vector inoculation and is then cleared. We tested NHP liver sections harvested at the time of euthanasia for the presence of vector genomes by a nested PCR. Although we could detect vector sequences in some of the samples, there was no correlation between their presence and the frequency of transgene product-specific T cells in these livers, suggesting expression of the transgene through persisting Ad vectors within the liver tissue was unlikely to have contributed to the recruitment or retention of the specific T cells (data not shown). Also, we would like to point out that the PCR analyses were conducted using unfractionated liver sections, which did not allow us to distinguish between vector genomes present in liver cells or in infiltrating lymphocytes.
Neutralizing or nonneutralizing antibodies directed to antigens of adenovirus may have further promoted targeting of Fc receptor-positive hepatocytes. One of the AdHu5-vaccinated animals that had not been preexposed to antigens of AdHu5 developed very high frequencies of T cells in the liver (>30,000 spots/106 mononuclear cells). This animal developed high neutralizing antibody titers to AdHu5 after the first vaccine dose, and the shifted migration of T cells to the liver could have been caused by these antibodies. Nevertheless, as there was no correlation between the presence of cross-reactive binding antibodies to the Ad vectors and frequencies and migration patterns of transgene product-specific T cells, retargeting of vectors through antibodies fails to explain our data.
The AdHu5 vectors induced a more potent transgene product-specific antibody response than the chimpanzee Ad vectors, reproducing previous findings in mice (29). Nevertheless, such a Gag-specific antibody response could not be elicited in AdHu5-preexposed animals even after a second application of the AdHu5 vector. Immunization with a single moderate dose of the AdC68 vector expressing Gag failed to elicit detectable antibodies to Gag. Such antibodies were induced in two of the three nonpreexposed NHPs and in three of the four AdHu5-preexposed NHPs after booster immunization with the AdC1/C5 vector. Levels of antibodies were slightly higher in the nonpreexposed group; nevertheless, this difference failed to reach statistical significance. None of the animals developed HIV-1 neutralizing antibodies after immunization (data not shown; assays most kindly conducted by D. Montefiori, Duke University, Durham, NC).
In summary, the presented study allows for three conclusions. First, preexposure to an Ad virus, which induces CD8+ T cells and nonneutralizing antibodies that widely cross-react with other Ad serotypes, including chimpanzee Ads, affects the homing pattern of transgene product-specific T cells induced by a heterologous Ad vector. In preexposed animals vaccinated with heterologous Ad serotypes, T cells home preferentially to the spleen, and fewer T cells circulate through the blood. In clinical phase I/II trials, the potency of a vaccine is established by testing PBMCs for T cells to the vaccine antigen. Data presented here indicate that results obtained with PBMCs may be misleading. Second, in NHPs, preexisting immunity to antigens of Ad results in a pronounced enrichment of T cells in the liver, induced by vaccination with homologous or heterologous Ad vectors. This had not been observed in mice (unpublished observation), and the underlying mechanism remains elusive. Third, preexisting immunity to antigens of AdHu5 virus completely inhibits the transgene product-specific antibody response to an AdHu5 vaccine vector. It is one of the major goals of ongoing research efforts to design Env immunogens that are capable of eliciting broadly cross-reactive neutralizing antibodies to HIV-1. Although AdHu5 vectors can induce very potent antibody responses, data presented here conclusively show that their efficacy as B-cell-inducing vaccines will most likely be strongly impaired in humans with even moderate titers of preexisting VNAs to AdHu5 virus.
Acknowledgments
This work was supported by a program project grant administered through the National Institutes of Health (P01HL078810).
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, P. 2006. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Rep. 10:1-5. [PubMed] [Google Scholar]
- 3.Crystal, R. G. 1992. Gene therapy strategies for pulmonary disease. Am. J. Med. 92:44S-52S. [DOI] [PubMed] [Google Scholar]
- 4.DiPaolo, N., S. Ni, A. Gaggar, R. Strauss, S. Tuve, Z. Y. Li, D. Stone, D. Shayakhmetov, N. Kiviat, P. Toure, S. Sow, B. Horvat, and A. Lieber. 2006. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 13:756-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durier, C., O. Launay, V. Meiffredy, Y. Saidi, D. Salmon, Y. Levy, J. G. Guillet, G. Pialoux, and J. P. Aboulker. 2006. Clinical safety of HIV lipopeptides used as vaccines in healthy volunteers and HIV-infected adults. AIDS 20:1039-1049. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 7.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 8.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 9.Garnett, C. T., D. Erdman, W. Xu, and L. R. Gooding. 2002. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 76:10608-10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goepfert, P. A., H. Horton, M. J. McElrath, S. Gurunathan, G. Ferrari, G. D. Tomaras, D. C. Montefiori, M. Allen, Y. L. Chiu, P. Spearman, J. D. Fuchs, B. A. Koblin, W. A. Blattner, S. Frey, M. C. Keefer, L. R. Baden, and L. Corey. 2005. High-dose recombinant canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J. Infect. Dis. 192:1249-1259. [DOI] [PubMed] [Google Scholar]
- 11.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahm, B., N. Arbour, and M. B. Oldstone. 2004. Measles virus interacts with human SLAM receptor on dendritic cells to cause immunosuppression. Virology 323:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Z., A. P. Wlazlo, D. W. Kowalczyk, J. Cheng, Z. Q. Xiang, W. Giles-Davis, and H. C. Ertl. 2000. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology 270:146-161. [DOI] [PubMed] [Google Scholar]
- 14.Kerdiles, Y. M., C. I. Sellin, J. Druelle, and B. Horvat. 2006. Immunosuppression caused by measles virus: role of viral proteins. Rev. Med. Virol. 16:49-63. [DOI] [PubMed] [Google Scholar]
- 15.Kozarsky, K. F., and J. M. Wilson. 1993. Gene therapy: adenovirus vectors. Curr. Opin. Genet. Dev. 3:499-503. [DOI] [PubMed] [Google Scholar]
- 16.Kresge, K. J. 2005. Renewed promise. Annual AIDS vaccine meeting highlights recent data from clinical trials and lessons on recruitment and retention of volunteers. IAVI Rep. 9:18-20. [PubMed] [Google Scholar]
- 17.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan, M. J., N. D. Russell, C. Celum, J. Kahn, E. Noonan, D. C. Montefiori, G. Ferrari, K. J. Weinhold, J. M. Smith, R. R. Amara, and H. L. Robinson. 2006. Excellent safety and tolerability of the human immunodeficiency virus type 1 pGA2/JS2 plasmid DNA priming vector vaccine in HIV type 1 uninfected adults. AIDS Res. Hum. Retrovir. 22:678-683. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Sandoval, A., J. C. Fitzgerald, R. Grant, S. Roy, Z. Q. Xiang, Y. Li, G. P. Gao, J. M. Wilson, and H. C. J. Ertl. 2004. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 78:7392-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 21.Roy, S., G. Gao, D. S. Clawson, L. H. Vandenberghe, S. F. Farina, and J. M. Wilson. 2004. Complete nucleotide sequences and genome organization of four chimpanzee adenoviruses. Virology 324:361-372. [DOI] [PubMed] [Google Scholar]
- 22.Roy, S., Y. Zhi, G. P. Kobinger, J. Figueredo, R. Calcedo, J. R. Miller, H. Feldmann, and J. M. Wilson. 2006. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J. Gen. Virol. 87:2477-2485. [DOI] [PubMed] [Google Scholar]
- 23.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatsis, N., A. Blejer, M. O. Lasaro, S. E. Hensley, A. Cun, L. Tesema, Y. Li, G. P. Gao, Z. Q. Xiang, D. Zhou, J. M. Wilson, and H. C. Ertl. 2007. A CD46-binding chimpanzee adenovirus vector as a vaccine carrier. Mol. Ther. 15:608-617. [DOI] [PubMed] [Google Scholar]
- 27.Tims, T., D. J. Briggs, R. D. Davis, S. M. Moore, Z. Xiang, H. C. Ertl, and Z. F. Fu. 2000. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine 18:2804-2807. [DOI] [PubMed] [Google Scholar]
- 28.Wang, D., A. L. Schmaljohn, N. U. Raja, C. M. Trubey, L. Y. Juompan, M. Luo, S. B. Deitz, H. Yu, J. Woraratanadharm, D. H. Holman, K. M. Moore, B. M. Swain, W. D. Pratt, and J. Y. Dong. 2006. De novo syntheses of Marburg virus antigens from adenovirus vectors induce potent humoral and cellular immune responses. Vaccine 24:2975-2986. [DOI] [PubMed] [Google Scholar]
- 29.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang, Z., Y. Li, A. Cun, W. Yang, S. Ellenberg, W. M. Switzer, M. L. Kalish, and H. C. Ertl. 2006. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 12:1596-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 32.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 33.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]