Abstract
The human hepatitis B virus (HBV) X protein (HBx) plays a crucial role(s) in the viral life cycle and contributes to the onset of hepatocellular carcinoma (HCC). HBx caused the mitochondrial translocation of Raf-1 kinase either alone or in the context of whole-viral-genome transfections. Mitochondrial translocation of Raf-1 is mediated by HBx-induced oxidative stress and was dependent upon the phosphorylation of Raf-1 at the serine338/339 and Y340/341 residues by p21-activated protein kinase 1 and Src kinase, respectively. These studies provide an insight into the mechanisms by which HBV induces intracellular events relevant to liver disease pathogenesis, including HCC.
Hepatitis B virus (HBV) infection results in a broad range of clinical symptoms, from mild, inapparent disease to fulminant hepatitis. Infection with this virus remains a major worldwide public health problem. It is estimated that there are about 500 million chronic carriers worldwide. Although the sequence of events remains poorly defined, a significant correlation has been made between long-term carriage of the virus and the development of hepatocellular carcinoma (4, 6). Among the HBV proteins encoded by the four open reading frames (S, C, P, and X), the X protein (HBx) plays a crucial role in the pathogenesis of hepatocellular carcinoma (6, 17). Like several viral oncoproteins, the HBx protein is implicated in a wide variety of cellular functions: as a trans-activator of transcription, in deregulation of cell cycle checkpoints, as a participant in the cellular signal transduction pathway, and in apoptosis (2, 6, 17). HBx regulates a series of cell-signaling cascades involving most notably the Ras- and Raf-induced mitogen-activated protein kinase pathways (reviewed in reference 2).
The Raf serine/threonine kinases are involved in the Ras-induced mitogen-activated protein kinase pathway (1, 15). They act downstream of Ras and are activated in a significant number of human malignancies (1, 15). There are three isoforms of Raf, A-Raf, B-Raf, and C-Raf, each displaying distinct expression profiles (1, 13, 15). C-Raf is ubiquitously expressed in many tissues, whereas A-Raf and B-Raf display tissue-specific expression (1, 15). Only A-Raf and C-Raf have been shown to translocate to mitochondria and regulate apoptosis (1). C-Raf, also known as Raf-1, exists in the cytoplasm as a multiprotein complex of 300 to 500 kDa consisting of heat shock protein 90 and dimeric protein 14-3-3. Binding of Ras to Raf displaces the 14-3-3 complex and unmasks amino acid residues critical for its activation. Mitochondrially localized Raf-1 protects cells from stress-mediated apoptosis. Raf-1 contains a central activation domain whose phosphorylation is activated by p21-activated protein kinase (PAK) at amino acids Ser338 and Ser339 or Src kinase at amino acid residues Y340 and Y341 (5, 8). B-Raf does not contain these tyrosine residues.
The results of this study demonstrate that HBx can stimulate the mitochondrial translocation of Raf-1 via oxidative stress. We previously showed that HBx itself targets to mitochondria and directly interacts with voltage-dependent anion channel 3 (VDAC3) (7, 10, 11, 12). HBx expression induces oxidative stress via calcium signaling and activates cellular kinases, which leads to the activation of transcription factors NF-κB and STAT-3 and others via phosphorylation (2, 3, 14). We observed that HBV-induced oxidative stress also stimulated the translocation of Raf-1 to mitochondria. This activation involves both the Src- and the PAK-mediated phosphorylation of the activation domain of Raf-1. Src inhibitors and dominant-negative PAK mutants abolished HBx-mediated Raf-1 mitochondrial translocation.
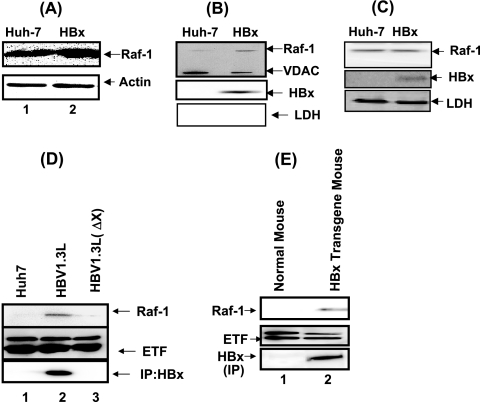
To demonstrate the role of HBx protein in regulating Raf-1 translocation, we first examined Raf-1 expression in Huh-7 cell lysates transfected with pCMVXF, which encodes the X gene placed under the transcriptional control of a cytomegalovirus (CMV) promoter containing a Flag sequence. The Western blot results show similar levels of Raf-1 expression in both untransfected and pCMVXF-transfected Huh-7 cellular lysates. (Fig. 1A). We next examined the association of Raf-1 with mitochondria. The results of Western blot analysis of mitochondria prepared according to a detailed procedure (9) from Huh-7 and pCMVXF cells (presented in Fig. 1B) demonstrate that both HBx protein and Raf-1 are associated with mitochondria. VDAC is used as a mitochondrial marker. To ensure that the Raf-1 and HBx signals were not due to cytoplasmic contamination, the lysates were also blotted for a cytoplasmic marker, lactate dehydrogenase (LDH). LDH expression was not observed in these mitochondrial preparations. The cytoplasmic fractions were also prepared and analyzed by a Western blot assay using anti-Raf, anti-Flag (which detects HBx), and anti-LDH (Fig. 1C). Raf-1 kinase levels were again similar to those described for Fig. 1A, indicating that not all Raf-1 kinase translocates to mitochondria, consistent with previous studies (1, 15). pCMVXF-transfected cellular lysates showed HBx expression, and LDH, used here as a cytoplasmic protein marker, was positive for both lysates. The vectors used thus far contained HBx under the transcriptional control of a CMV enhancer/promoter. To verify the role of HBx when expressed under the transcriptional control of the native promoter/enhancer in the context of the whole HBV genome, which recapitulates the viral life cycle, longer-than-genome-length HBV constructs were transfected into Huh-7 cells (6, 16). Analysis of these cells showed Raf-1 mitochondrial translocation, whereas the whole-genome construct, defective in HBx expression, failed to induce mitochondrial translocation of Raf-1, as did the untransfected cells (Fig. 1D). We further examined this phenomenon in transgenic mice harboring the HBx gene expressed under the control of its native promoter/enhancer (16). Mitochondria fractionated from liver tissues of HBx-transgenic mice were examined by a Western blot assay. As shown in Fig. 1D, Raf-1 also translocated to mitochondria in HBx-transgenic mice, as did HBx. This is the first report showing the association of the HBx with mitochondria in vivo using a transgenic-mouse model. We and others have previously shown that HBx targets to mitochondria using a variety of in vitro experimental strategies (7, 11, 12). In normal mouse liver tissue, Raf-1 association with mitochondria was not observed (Fig. 1E). These studies collectively provide evidence that HBx is associated with mitochondria and causes Raf-1 translocation to mitochondria both in cells transfected with HBx alone and in the context of the whole HBV genome. Moreover, a similar association was also seen in transgenic mice expressing HBx under the native promoter/enhancer (16).
FIG. 1.
HBx protein induces Raf-1 mitochondrial translocation. (A) Raf-1 levels in untransfected and pCMVXF-transfected Huh-7 cellular lysates. Western blot analysis was carried out using anti-Raf-1 kinase antibody. Anti-actin was used as a protein loading control. (B) Mitochondrial preparations from untransfected and pCMVXF-transfected Huh-7 cells were used for Western blot analysis using anti-Raf-1 kinase antibody. VDAC serves as a mitochondrial marker. Anti-Flag was used to monitor HBx expression, and anti-LDH was used to monitor for cytoplasmic contamination. (C) Cytoplasmic fractions from untransfected and pCMVXF-transfected Huh-7 cells were analyzed by Western blot assays using anti-Raf-1, anti-Flag (which detects HBx), and anti-LDH. LDH is used here as a cytoplasmic marker. (D) Mitochondria were fractionated (9) from untransfected Huh-7 cells and cells transfected with whole-HBV-genome plasmids (HBV1.3L) and an X-defective mutant plasmid of the HBV genome [HBV1.3L (ΔX)] (gift from J. Ou, USC). Western blot analysis was carried out using anti-Raf-1 kinase antibody and anti-electron transport factor (anti-ETF; a mitochondrial marker) antibody. HBx protein expression was monitored by first immunoprecipitating with anti-HBx antibody (16), followed by immunoblotting with the same antiserum (16). (E) Raf-1 mitochondrial translocation (9) in the HBx-transgenic mouse. Mitochondria were fractionated from normal and HBx-transgenic mice (gift from James Ou). HBx was expressed under its native promoter/enhancer (16). Western blot analysis was performed on the mitochondrial preparation. Lane 1, normal mouse liver tissue; lane 2, HBx-transgenic mouse liver Western blots using anti-Raf-1. ETF is used as a mitochondrial marker. HBx expression in the HBx-transgenic mice was determined using anti-HBx antibody by immunoprecipitation (IP), followed by immunoblot analysis with the same antibody (gift from Betty Slagle) (16).
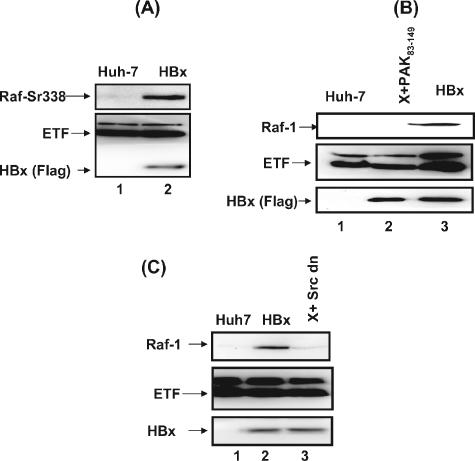
Mitochondrial translocation of Raf-1 requires phosphorylation of Ser and Tyr residues in its activation domain (5, 7). To investigate the role of HBx in phosphorylating Raf-1 at Ser338 residues, mitochondrial preparations from untransfected and pCMVXF-transfected Huh-7 cells were used for Western blot analysis using antiserum that recognizes Ser338 residues of Raf-1. The results show that indeed HBx mediates the phosphorylation of Raf at Ser338 residues (Fig. 2A). Serine phosphorylation of Raf-1 is catalyzed by PAK-1 (8). Infection of untransfected and pCMVXF-transfected Huh-7 cells with a retrovirus encoding the autoinhibitory domain (PAK83-149) of Pak-1 abrogated mitochondrial translocation of Raf-1, whereas in cells expressing HBx alone, Raf-1 migrated to mitochondria (Fig. 2B). Tyrosine340,341 phosphorylation of Raf-1 is known to be catalyzed by Src kinase (5). Huh-7 cells coexpressing HBx and dominant-negative Src kinase (kinase-dead pM5Hmet295; gift from Richard Jove [Moffat Cancer Center]) also failed to cause Raf-1 mitochondrial translocation (Fig. 2C). Together, these results indicate that HBx must induce the activation of Src and Pak-1 kinases to phosphorylate Raf-1 at the Tyr and Ser residues, respectively, to trigger its mitochondrial translocation. HBx activates both Tyr and Ser/Thr kinases (reviewed in reference 2).
FIG. 2.
HBx protein activates both Src-dependent tyrosine and PAK-1-dependent serine phosphorylation of Raf-1 and causes its mitochondrial translocation. (A) HBx activates serine phosphorylation of mitochondrial Raf-1. Western blot analysis was performed on isolated mitochondria fractionated from untransfected Huh-7 cells and those transiently transfected with the pCMV4X plasmid using the anti-Raf-1 Ser338 monoclonal antibody, which recognizes serine338-phosphorylated Raf-1 kinase (Upstate Biotechnologies). Anti-electron transport factor (anti-ETF) was used to detect ETF, a mitochondrial marker. HBx was monitored using anti-Flag antibody. (B) Pak-1 is required for Raf-1 translocation. Results are shown for Western blot analysis of isolated mitochondria prepared from untransfected Huh-7 cells (lane 1), Huh-7 cells transiently transfected with the pCMV4X plasmid (lane 2), Huh-7 cells cotransfected with pCMV4X-Flag (HBx), and dominant-negative Pak-1 protein (the PAK-1 inhibitory domain containing PAK83-149) (lane 2) using anti-Raf-1 antibody. ETF serves as a mitochondrial marker. HBx expression was monitored using anti-Flag antibody (an internal control). (C) Western blot analysis was carried out as described for panel B, except that a Src dominant-negative mutant (gift from R. Jove) was used for cotransfection along with the pCMV4X (HBx) plasmid (lane 2).
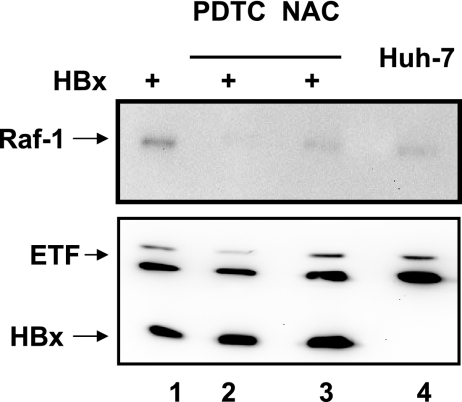
To examine whether HBx-induced reactive oxygen species play a role in the Raf-1 activation process, HBx (pCMVXF transfected)-expressing cells were treated with antioxidants N-acetyl cysteine and pyrrolidine dithiocarbamate. The results revealed that antioxidants prevented mitochondrial translocation of Raf-1 (Fig. 3). Cells treated with calcium chelators (BAPTA-AM and TMB-8) did not interfere with Raf-1 mitochondrial translocation in either HBx or whole-HBV-genome-transfected cells (data not shown). The role of Ca2+ signaling needs to be further characterized.
FIG. 3.
HBx induces Raf-1 mitochondrial translocation via oxidative stress. Western blot analysis of isolated mitochondria prepared from untransfected Huh-7 cells (lane 4) and Huh-7 cells transiently transfected with pCMV4X (lanes 1 to 3) is shown. Cells were either untreated (lanes 1 and 4) or treated with antioxidants pyrrolidine dithiocarbamate (PDTC; 50 μM) (lane 2) and N-acetyl cysteine (NAC; 30 mM) (lane 3) for 6 h each and subjected to Western blot assays using Raf-1 antibody. Electron transport factor (ETF) serves as a mitochondrial marker. Anti-Flag was used to detect HBx (internal control).
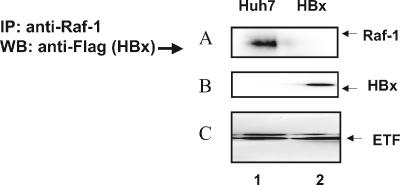
Since both HBx and Raf-1 proteins are localized to mitochondria, we next investigated whether there is physical interaction between these proteins. Mitochondrial preparations from untransfected Huh-7 cells (Fig. 4A, lane 2) and those transfected with pCMVXF (Fig. 4A, lane 1) were immunoprecipitated with anti-Raf-1 antibody, followed by immunoblotting with anti-Flag antibody (Flag tagged to HBx). The presence of Raf-1 in the immunoblot indicated that Raf-1 and HBx formed a complex (Fig. 4A). Expression of HBx-transfected cells is shown in Fig. 4B. Immunoprecipitation of lysates with an unrelated antibody (anti-HCV core) did not show any bands in the immunoblots (data not shown).
FIG. 4.
HBx protein directly binds mitochondrially associated Raf-1. (A) Mitochondrial preparations from untransfected and pCMV4X-transfected Huh-7 cells were first immunoprecipitated with anti-Raf-1, followed by immunoblotting with anti-Flag (which detects HBx) (lane 1) antibodies. (B and C) Western blot analysis of the mitochondrial preparations with anti-Flag (HBx) and anti-electron transport factor (anti-ETF) (a mitochondrial marker).
These studies collectively demonstrate the ability of HBx to induce the mitochondrial translocation of cytoplasmic Raf-1, and while resident in mitochondria, HBx forms a protein-protein complex with Raf-1 kinase. The functional significance of this interaction may be that it reinforces the antiapoptotic program in infected hepatocytes. We previously noted the absence of cytochrome c release in HBx-expressing cells (unpublished results). Mitochondrial Raf-1 participates in the survival program (13), but the exact mechanism behind the antiapoptotic role of Raf-1 remains to be investigated. HBx-activated Raf-1 may contribute to HBV-associated liver oncogenesis.
Acknowledgments
This work was supported by NIH grant CA64415 to A.S.
We thank James Ou for transgenic mice and HBV1.3L (ΔHBx) vectors and B. Slagle for anti-HBx sera.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Beeram, M., A. Patnaik, and E. Rowinsky. 2005. Raf: a strategic target for therapeutic development against cancer. J. Clin. Oncol. 23:6771-6790. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard, M. J., and R. J. Schneider. 2004. The enigmatic X gene of hepatitis B virus. J. Virol. 78:12725-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchard, M. J., L. H. Wang, and R. J. Schneider. 2001. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science 294:2376-2378. [DOI] [PubMed] [Google Scholar]
- 4.Cougot, D., C. Neuveut, and M. A. Buendia. 2005. HBV-induced carcinogenesis. J. Clin. Virol. 34(Suppl. 1):875-878. [DOI] [PubMed] [Google Scholar]
- 5.Fabian, R., I. O. Daar, and D. K. Morrison. 1998. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13:7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganem, D., and R. Schneider. 2001. The molecular biology of the hepatitis B viruses, p. 2923-2970. In D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, B. Roizman, and S. Strauss (ed.), Fields virology, 4th edition, vol. 2. Lippicott Willams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7.Henkler, F. F., J. Hoare, N. Waseem, R. Golin, M. McGarvy, and R. Koshy. 2001. Intracellular localization of the HBV HBx protein. J. Gen. Virol. 64:871-882. [DOI] [PubMed] [Google Scholar]
- 8.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-184. [DOI] [PubMed] [Google Scholar]
- 9.Lapidus, R. G., and P. Sokolove. 1993. Spermine inhibition of the permeability transition isolated rat live mitochondria: an investigation of mechanism. Arch. Biochem. Biophys. 306:246-253. [DOI] [PubMed] [Google Scholar]
- 10.Lee, Y. I., J. M. Hwang, J. H. Im, Y. I. Lee, N. S. Kim, D. G. Kim, D. Y. Yu, H. B. Moon, and S. K. Park. 2004. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J. Biol. Chem. 279:15460-15471. [DOI] [PubMed] [Google Scholar]
- 11.Rahmani, Z., K. W. Huh, R. Lasher, and A. Siddiqui. 2000. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 74:2840-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada, S., Y. Shirakata, N. Kaneniwa, and K. Koike. 1999. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene 18:6965-6973. [DOI] [PubMed] [Google Scholar]
- 13.Troppmair, J., and U. R. Rapp. 2003. Raf and the road to cell survival: a tale of bad spells, ring bearers and detours. Biochem. Pharmacol. 66:1341-1345. [DOI] [PubMed] [Google Scholar]
- 14.Waris, G., K. W. Huh, and A. Siddiqui. 2001. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol. Cell. Biol. 21:7721-7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellbrock, C., M. Karasarides, and R. Marais. 2004. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5:875-885. [DOI] [PubMed] [Google Scholar]
- 16.Xu, Z., T. S. B. Yen, L. Wu, C. Madden, W. Tan, B. Slagle, and J. Ou. 2002. E HBV replication by its X protein in transgenic mice. J. Virol. 76:2579-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen, T. S. 1996. Hepadnaviral X protein: review of recent progress. J. Biomed. Sci. 3:20-30. [DOI] [PubMed] [Google Scholar]