Abstract
The development of anti-human immunodeficiency virus (anti-HIV) neutralizing antibodies and the evolution of the viral envelope glycoprotein were monitored in rhesus macaques infected with a CCR5-tropic simian/human immunodeficiency virus (SHIV), SHIVSF162P4. Homologous neutralizing antibodies developed within the first month of infection in the majority of animals, and their titers were independent of the extent and duration of viral replication during chronic infection. The appearance of homologous neutralizing antibody responses was preceded by the appearance of amino acid changes in specific variable and conserved regions of gp120. Amino acid changes first appeared in the V1, V2, C2, and V3 regions and subsequently in the C3, V4, and V5 regions. Heterologous neutralizing antibody responses developed over time only in animals with sustained plasma viremia. Within 2 years postinfection the breadth of these responses was as broad as that observed in certain patients infected with HIV type 1 (HIV-1) for over a decade. Despite the development of broad anti-HIV-1 neutralizing antibody responses, viral replication persisted in these animals due to viral escape. Our studies indicate that cross-reactive neutralizing antibodies are elicited in a subset of SHIVSF162P4 infected macaques and that their development requires continuous viral replication for extended periods of time. More importantly, their late appearance does not prevent progression to disease. The availability of an animal model where cross-reactive anti-HIV neutralizing antibodies are developed may facilitate the identification of virologic and immunologic factors conducive to the development of such antibodies.
Most patients infected with the human immunodeficiency virus (HIV) gradually develop homologous serum neutralizing antibody responses (37), but only a small fraction of patients develop broadly reactive serum neutralizing antibody responses capable of neutralizing diverse heterologous primary HIV isolates (29). Monoclonal antibodies (MAbs), isolated from HIV-infected patients, with broad neutralizing activity were shown to inhibit the infection of diverse HIV type 1 (HIV-1) strains in vitro (2), protect nonhuman primates from experimental infection with simian/human immunodeficiency viruses (SHIVs) (1, 26, 34, 42), and delay the rebound of HIV-replication in patients undergoing structured antiretroviral treatment interruption (43). It is hoped that the development of broad and potent neutralizing antibody responses by vaccination will protect the vaccinees from infection by HIV.
Why only a small percentage of HIV-1-infected patients develop broadly reactive anti-HIV neutralizing antibodies (NAbs) is not currently understood. Specifically, the features of the immune system of the infected patient and of the phenotype of the infecting virus that are conducive to the development of broadly reactive NAbs are unknown. For example, it is possible that the development of broadly reactive NAbs during HIV infection is related to the duration of viral replication, the extent of viral replication, the degree of viral env evolution during infection, or the appearance during infection of specific Env forms. These possibilities (which are not mutually exclusive) are not easily addressable by studying HIV infection in humans, since the time of infection is not known in most cases, and thus little information is available regarding the early infecting virus. Also, humans who have been infected with HIV for extended periods of time typically undergo antiretroviral therapy, which limits viral replication and may hinder the development of NAbs by reducing antigenic stimulation.
The SHIV/macaque animal model of infection has been used extensively as a surrogate for the human/HIV infection to examine transmission and pathogenesis, as well as for the testing of vaccination and therapeutic methodologies. In this model, both the time of infection and the phenotypic properties of the infecting virus are known. The vast majority of these studies have been conducted with SHIVs that utilize the CXCR4 rather than CCR5 coreceptor utilized by HIV in most cases of transmission (8, 16, 18, 24, 36). CXCR4-tropic SHIVs rapidly induce a profound and sustained depletion of CD4+ T lymphocytes. As a result, disease develops usually within 12 months after infection and during this short time, cross-reactive anti-HIV NAbs are not readily developed (11, 28). Therefore, an animal model where cross-reactive anti-HIV neutralizing antibody responses are generated has not yet been described.
Here we monitored plasma viremia levels, peripheral CD4+ T-cell numbers, the amino acid changes introduced in the viral Env during the course of infection and the development of homologous and heterologous NAbs in rhesus macaques infected with the CCR5-tropic SHIV, SHIVSF162P4 (15). Macaques infected with SHIVSF162P4 do not experience a rapid and sustained loss of their peripheral CD4+ T lymphocytes within a few weeks after infection and do not display any obvious signs of disease for extended periods of time, often many years. Because of the extended length of the asymptomatic phase of infection in these latter animals, it is feasible to monitor for extended periods of time the longitudinal development of NAb responses, as well as the evolution of the viral env. Here, we report that SHIVSF162P4-infected macaques can develop broad anti-HIV NAb responses during infection. The availability of an animal model where cross-reactive anti-HIV NAbs are developed may allow us to address more systematically some of the questions related to the development of broadly reactive NAbs in HIV-infected humans.
MATERIALS AND METHODS
Animals and virus challenge.
Rhesus macaques of Indian origin were housed at the Tulane and Washington National Primate Research Centers. Animals were challenged by the intra venous route with 100 50% tissue culture infective doses of cell-free SHIVSF162P4 virus. Plasma viremia was monitored by the b-DNA assay (Bayer), and the CD4+ T lymphocyte numbers were determined by standard techniques (5). The animals described here served as nonimmunized controls for three independent studies (5, 10, 45), and therefore they were not challenged at the same time and were not monitored for the same period of time. Some of these animals (AT54, A141, and C640) were euthanized when they developed AIDS, whereas others were euthanized at earlier time points due to the specifics of the study these animals were part of.
HIV-1-infected patients.
The long-term nonprogressor cohort includes HIV-1-infected patients recruited at the Fred Hutchinson Cancer Research Center HIV Vaccine Trials unit since 1997. Criteria for enrollment included documented seropositivity for more than 10 years and CD4+ T-cell counts of either ≥600 cells/μl or >500 cells/μl with slope zero or positive over the previous 2 years.
PCR amplification, cloning, and sequencing of the viral envelope from PBMC.
Genomic DNA was extracted from 5 × 106 peripheral blood mononuclear cells (PBMC), and the gp120 or gp140 portion of the viral envelope was amplified by nested PCR: the first reaction used the primers E0 (5′-TAGAGCCCTGGAAGCATCCAGGAAGT CAGCCTA-3′) and env 6R (5′-CTTGCCCACTTATCCAATTC-3′) or E0 and env 8R (5′-CACAATCCTCGCTGCAATCAAG-3′) and the second reaction used the primers gp160F (5′-GGACCATAGTGTACATAGAATACAG-3′) and Gmb (5′-CTCTCTTCTCTTTGCCTT-3′). The products were then cloned into the pCR3.1 eukaryotic TA bidirectional expression vector (Invitrogen) or the pCR2.1-TOPO vector (Invitrogen) and transformed into chemically competent DH5α cells, and the resulting clones were screened for the presence of inserts. Plasmid DNA was prepared by using the QIAGEN miniprep kit, and the V1 to V5 region of the clones was sequenced with the primers Gmb and 216 (5′-AACCCACAAGAAATAGTATTG-3′).
PCR amplification, cloning, and sequencing of viral envelope from plasma.
Viral RNA was isolated from plasma and reverse transcribed by using oligo(dT) and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). Transcripts were amplified via two rounds of PCR with AccuPrime Pfx SuperMix (Invitrogen). First-round PCR was done with the primers E0 and E01 (5′-TCCAGTCCCCCCTTTTCTTTTAAAAA-3′), followed by nested PCR with the primers Nhe162F (5′-GGGATGTTGATGATCTGTGCTAGCGTAGAAAAATTGTGGGTCAC) and Cla160R (5′-GACCACTTGCCATCGATGTTATAGCAAAGCCC).
Subcloning of the HIV Env into a DNA expression vector and generation of single round competent viral particles.
The method used to generate viral particles expressing Envelopes from viruses circulating in the blood of infected macaques during the course of infection was similar to that previously reported by Richman et al. (37). The above-mentioned PCR products were double digested with NheI and ClaI and purified from agarose gel using a QIAquick gel extraction kit (QIAGEN), and the fragments were cloned into the pEMC* vector (35). The inserted env was sequenced with primers 216 (forward primer, 5′-AACCCACAAGAAATAGTATTG-3′) and Env6R (reverse primer, 5′-CTTGCCC ACTTATCCAATTC-3′). pEMC* vectors expressing the various Envs were used to generate single-round replication-competent viruses as previously described (40).
MAbs.
The anti-V3 MAb 447D, which binds to the V3 loop of gp120, was provided by M. Gorny and S. Zolla-Pazner (NYU Medical Center) (9, 13, 14). The immunoglobulin G1b12 MAb, which binds to an epitope overlapping the CD4-binding site (7, 33), was obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health/National Institute of Allergy and Infectious Disease). MAb 2G12, which binds to an epitope formed by mannose residues (39, 41), and MAb 2F5, which binds to an epitope in the extracellular region of gp41 (47), were purchased from Polymun Scientific. The anti-V1 loop MAb P3C8 and the anti-V3 loop MAb P3E1 were recently isolated by our group from mice immunized with SF162-derived gp140 proteins (N. R. Derby et al., unpublished data).
Determination of serum neutralizing activity and viral escape.
Neutralization assays using replication-competent SHIVSF162P4 (grown in human PBMC) and activated human PBMC as target cells were performed as previously described (27). Neutralization assays using single-round competent viruses expressing different HIV-1 Env and TZM-bl cells as targets were performed as previously described (10, 40). Escape from serum antibody neutralization was evaluated by incubating sera collected at different times during infection, or known MAbs, with single-round competent virions expressing Env cloned from virus present in the plasma. To evaluate the neutralization susceptibility of the virus circulating in the animals, several Env clones were amplified from different times during infection. Single-round viruses were generated with all amplified Env clones, and the viruses were tested as a pool against the sera or MAbs.
RESULTS
Viral replication in SHIVSF162P4-infected macaques.
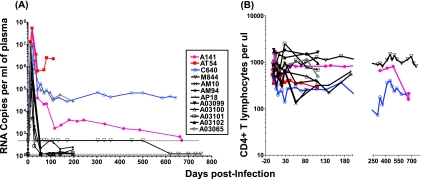
The replication kinetics of SHIVSF162P4 in rhesus macaques challenged intravenously with cell-free virus are summarized in Fig. 1A. These animals (12 total) were used as immunized controls is several immunization studies (5, 10, 45). They were challenged at different times and were monitored for different periods of time. High viral load titers (5 × 106 to 5 × 107 RNA copies per ml of plasma) were observed in all animals during primary infection. However, after acute infection, approximately half of the animals effectively controlled viral replication in the periphery. The remaining animals (colored lines) displayed distinct levels of plasma viremia during chronic infection. Animal AT54 had sustained high viremia (above 106 RNA copies per ml) and developed AIDS within 110 days postinfection (dpi) (5). Animal C640 had persistent plasma viremia (approximately 105 RNA copies per ml) for the duration of the observation and was euthanized at 643 dpi due to AIDS (5). Animal A141 also had detectable plasma viremia for an extended period of time, but at levels that gradually decreased over time. It was euthanized at 670 dpi, at a time when it experienced a significant drop of CD4+ T-cell numbers (Fig. 1B) in the periphery and in lymph nodes (data not shown). A fourth animal, A03065, also had high plasma viremia after acute infection but was only monitored for 196 dpi, during which period the peripheral CD4+ T-lymphocyte numbers remained relatively stable. Animal A03102 had very low levels of plasma viremia (between 200 and 500 RNA copies per ml) after acute infection. The levels of SHIVSF162P4 replication did not appear to be related to the presence of HLA genotypes that have been associated with disease protection in previous studies (31, 46). For example, animal C640 was MamuA*01, animal A141 was MamuA*08/MamuB*01, while animals AT54 and M844 were MamuA*08.
FIG. 1.
Plasma viremia and CD4+ T-lymphocyte numbers in SHIVSF162P4-infected rhesus macaques. (A) Plasma viremia was determined by the b-DNA assay (Bayer). In most cases, the limit of detection was 125 RNA copies per ml of plasma (dashed line). For animals AT54, A141, C640, and M844 the limit of detection for the first 500 days of infection was 500 RNA copies per ml of plasma (dashed line). (B) The numbers of CD3+ CD4+ T lymphocytes in the blood of these animals were determined longitudinally throughout infection.
Development of homologous neutralizing antibodies.
Anti-SHIVSF162P4 NAbs developed in all animals, with the exception of animal AT54 (Table 1) . Animals C640, A141, and A03065, which were three of four animals with persistent high (above 103 RNA copies per ml) plasma viremia levels, developed very high titers of homologous NAbs against the challenge virus within a month or two after infection. Animal M844 had undetectable plasma viremia levels during chronic infection (up to 852 dpi) and had low but persistent titers of homologous NAbs. However, a clear relationship between the titer of homologous NAbs and the levels of plasma viremia during chronic infection was not obvious. For example, animal A03102 had very low levels of plasma viremia (below 103 RNA copies per ml) between days 56 and 197 (day of euthanasia) after infection, and yet it developed higher titers of homologous NAbs than animals C640, A141, and A03065, which all had higher plasma viremia levels during chronic infection. On the other hand, animal AT54, which had the highest levels of plasma viremia after acute infection (Fig. 1A) and which rapidly progressed to AIDS, did not develop anti-SHIVSF162P4 NAbs.
TABLE 1.
Development of homologous neutralizing antibody responses
| dpi | Serum dilutiona in animal:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M844 | AT54 | C640 | A141 | A03065 | A03099 | A03100 | A03101 | A03102 | AM10 | AM94 | AP18 | |
| 0 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| 21 | 300 | |||||||||||
| 28 | (-) | 370 | 2,861 | 585 | 438 | 1,596 | 517 | |||||
| 56 | 794 | (-) | 12,836 | 6,326 | 8,143 | 1,174 | 4,628 | 1,589 | 5,831 | 18,130 | 5,600 | 3,675 |
| 107 | 16,400* | |||||||||||
| 111 | (-)* | 27,117 | 939 | 4,533 | 1,360 | >72,900 | ||||||
| 140 | 14,147 | 1,468 | 5,653 | 1,705 | >72,900 | |||||||
| 145 | 18,876 | |||||||||||
| 171 | 204 | 7,723 | ||||||||||
| 197 | 14,510* | 720* | 58,159* | 1,547* | >72,900* | 4,250* | 1,650* | |||||
| 215 | 27,737 | |||||||||||
| 304 | 13,568 | |||||||||||
| 332 | 244 | |||||||||||
| 643 | 3,190* | |||||||||||
| 670 | 9,070* | |||||||||||
| 852 | 250* | |||||||||||
The values represent the serum dilutions at which 50% inhibition of SHIVSF162P4 infection (or HIV-1SF612 in the case of animals AM10, AM94, and AP18 neutralization) was recorded. (-), Neutralization was not detected at the highest serum concentration tested (1:10); *, Time of euthanasia.
Development of heterologous neutralizing antibodies.
We next examined whether sera collected from the above-mentioned animals contained antibodies that could block infection of heterologous HIV-1 isolates. A panel of single-round competent viruses expressing 23 heterologous HIV-1 Env was used (Table 2). This panel was chosen because it represents the diverse neutralization susceptibilities of primary HIV-1 clade B isolates to polyclonal sera and MAbs (2, 23). SHIVSF162P4 expresses an Env whose amino acid sequence is similar to that of HIV-1SF162 (data not shown) and, as a result, both HIV-1SF162 and SHIVSF162P4 are equally highly susceptible to neutralization by several neutralizing antibodies and heterologous HIV-positive sera, as well as sera from macaques immunized with SF162 Env-derived immunogens (2, 6). Therefore, for these experiments we used SF162 as an internal control (i.e., homologous neutralization).
TABLE 2.
Development of heterologous neutralizing antibody responses
| Virus | % Inhibition (SD)a
|
|||||
|---|---|---|---|---|---|---|
| C640
|
A141
|
A03065 (197 dpi) | AT54 (111 dpi) | |||
| 304 dpi | 643 dpi | 214 dpi | 670 dpi | |||
| SF162 | 98 (0.5) | 99 (0.5) | 99 (0.5) | 97 (3) | 96.5 (3) | 32.5 (4) |
| ADA | 61.5 (7) | 81 (6) | 23 (10.5) | 28 (3) | 25 (10) | 11 (10) |
| JRFL | 99 (0.5) | 95 (4) | 37 (18) | 42 (4) | 23 (12) | 2 (2) |
| YU2 | 53 (7) | 87 (2) | 34 (26) | 34 (2) | 5 (5) | 22 (2) |
| 89.6 | 71 (4) | 71 (15) | 71 (12) | 90 (2) | 84 (5) | 3 (3) |
| HxB2 | 93 (3) | 98 (0) | 94 (3) | NT | 97 (2) | NT |
| 3988 | 12 (32) | 65 (9) | 5 (40) | 45 (2.5) | 3 (2) | 22 (5) |
| 6101 | 10 (6) | 53 (13) | 5 (8) | 57.0 (6.5) | 5 (8) | NT |
| 6535 | 63 (14) | 95 (3) | 70 (5) | 70 (8) | 66.5 (4) | 5 (8) |
| PVO.4 | 42.5 (11) | 65 (6) | 14 (21) | 37 (5) | 6 (8) | 38 (10) |
| QH0692.42 | 38.5 (12) | 59 (10) | 29 (17.5) | 75 (30) | 9 (14) | 12 (0.5) |
| REJO.67 | 21 (10) | 53 (22) | 24 (31) | 68 (3) | 8 (12) | 13.5 (1.5) |
| SC422661.8 | 4 (35) | 76 (2) | 1 (55) | 47.0 (6.0) | 3 (2) | 30 (9) |
| SS1196.1 | 86 (6.5) | 77 (17) | 62 (10) | 91 (0.5) | 97 (2) | 4 (8) |
| TRO11 | 20.5 (33) | 66 (6) | 3 (47) | 53 (6.5) | 6 (2) | 8.5 (5) |
| TRJO | 23.5 (26) | 56 (9) | 58 (28) | 28.0 (18.5) | 5 (5) | 10 (6) |
| 5768 | 4.0 (27) | 44 (15) | 8 (49) | 23 (5) | 7 (9) | 6 (7) |
| 7165 | 6 (9) | 40 (16) | 20 (33) | 63 (31) | 5 (5) | 6 (10) |
| AR29 | 15 (13) | 28 (5) | 27 (26) | 37 (7) | 6 (1) | 16 (7) |
| BG1168 | 10 (18) | 40 (8) | 8 (28) | 38 (1) | 4 (7) | 38 (4) |
| CAAN.A2 | 20 (16) | 38 (0.5) | 4 (16) | 35 (4) | 7 (5) | 3 (10) |
| QH0515 | 40 (25) | 40 (9) | 14 (45) | 37 (4) | 8 (10) | NT |
| RHPA | 25 (20) | 25 (17) | 2 (27) | 32 (7) | 6 (12) | 8 (8) |
| WITO | 19 (13) | 41 (7) | 30 (28) | 16 (12) | 15 (12) | 5 (5) |
| MLV | 10 (23) | 9 (21) | 15 (20) | 21 (16) | 3 (28) | 3.5 (17) |
The values are the percent inhibition of infection recorded at a single (1:20) serum dilution. The mean percent inhibition from two independent experiments is shown. Murine leukemia virus (MLV) was used as a control for nonspecific neutralization. Neutralization was considered significant (values in boldface) when the value was at least twice that obtained with the unrelated MLV virus and greater than 50%. *, Day of euthanasia; NT, not tested.
With the exception of animals C640, A141, and A03065, none of the other animals developed detectable heterologous NAbs (Table 2 and data not shown). Sera collected from animal C640 at 304 dpi neutralized seven heterologous isolates, while sera collected 643 dpi neutralized 15 heterologous isolates, an indication that the breadth of neutralization was increasing during infection in this animal. Sera from animal A141 collected at 214 dpi neutralized four heterologous isolates, while sera collected at 670 dpi could neutralize eight heterologous isolates. Therefore, an increase in the breadth of neutralization over time was also observed in animal A141, although the breadth of this cross-neutralizing response was narrower than that seen in animal C640. Animal A03065 was only monitored for 197 days, but sera collected at that time neutralized four heterologous isolates. Therefore, cross-reactive NAbs were beginning to be generated in this animal.
The breadth of serum NAbs present in animals C640 and A141 at the time of their death (643 and 670 dpi, respectively) was compared to that of seven chronically infected HIV-1 patients (Table 3). These patients participate in a long-term nonprogressor study. The median viral load in these patients ranged from undetectable to 5.1 log10. The breadth of neutralization was evaluated against the same panel of heterologous isolates described above. Plasma from animals C640 and A141 neutralized 65 and 30%, respectively, of the heterologous viruses tested. Plasma from only one of the seven patients neutralized more than 50% of the heterologous isolates tested (patient P4 neutralized 62% of the heterologous isolates tested). Plasma from the remaining six patients neutralized between 10 and 38% of the heterologous viruses tested. Therefore, the breadth of the NAbs developed by these two animals within 2 years of SHIVSF162P4 infection was similar (P = 0.3333; 95% confidence interval; unpaired t test with Welch correction) to that developed by the patients studied here which were infected with HIV-1 for over a decade.
TABLE 3.
Serum cross-reactive neutralizing antibody responses in SHIVSF162P4-infected macaques and HIV-1 chronically infected humansa
| Parameter | SHIV-infected macaques
|
HIV-infected humans
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| C640 | A141 | P2 | P5 | P10 | P23 | P11 | P4 | P14 | |
| Approximate duration (in yr) of infection | 2 | 2 | 20 | 21 | 18 | 19 | 17 | 23 | 20 |
| Median viral load (log10) | 5 | 3.2 | 3.3 | UDc | UD | UD | 5.1 | 4.1 | 3.3 |
| % Neutralization for virus | |||||||||
| SF162 | 99 | 97 | 85 | 78 | 93 | 99 | 93 | 95 | 99 |
| ADA | 81 | (-) | (-) | 40 | (-) | 93 | 74 | 95 | (-) |
| JRFL | 95 | (-) | (-) | (-) | (-) | (-) | 99 | 86 | (-) |
| YU2 | 87 | (-) | (-) | (-) | (-) | 55 | 73 | 80 | (-) |
| HXB2 | 98 | (-) | 95 | 80 | 97 | 98 | 99 | 95 | |
| 3988 | 65 | (-) | (-) | (-) | (-) | 95 | (-) | 99 | (-) |
| 6101 | 53 | 57 | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| PVO.4 | 65 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | |
| QH 0692 | 59 | 75 | (-) | (-) | (-) | (-) | 80 | (-) | (-) |
| REJO.67 | 53 | 68 | (-) | (-) | (-) | (-) | (-) | 82 | (-) |
| SC422661 | 76 | (-) | (-) | (-) | (-) | (-) | (-) | 94 | (-) |
| SS 1196 | 77 | 91 | (-) | 52 | 99 | 97 | 72 | 99 | (-) |
| TRO11 | 66 | 53 | (-) | 95 | (-) | (-) | (-) | 80 | (-) |
| TRJO | 56 | (-) | (-) | (-) | (-) | (-) | 80 | 93 | (-) |
| 5768 | (-) | (-) | (-) | (-) | (-) | 76 | (-) | 85 | (-) |
| 7165 | (-) | 63 | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| AR29 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| BG 1168 | (-) | (-) | 75 | (-) | 68 | 78 | (-) | 80 | (-) |
| CAAN.A2 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| QH 0515 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| RHPA | (-) | (-) | (-) | (-) | (-) | (-) | (-) | 98 | (-) |
| Ratiod | 13/20 | 6/20 | 3/21 | 5/21 | 4/21 | 8/21 | 8/21 | 13/21 | 2/21 |
Sera from the indicated macaques (C640 and A141) and humans (P2, P5, P10, P23, P11, P4, and P14) were tested at a 1:20 dilution against the indicated viruses. The percent neutralization values were recorded.
b-, neutralization was not detected.
UD, undetectable viral load (<25 copies/ml).
The numbers at the bottom of the table indicate the numbers of heterologous isolates tested that were susceptible to neutralization by each serum/total number of isolates tested.
In a separate experiment the potency of the broadly reactive NAbs present in sera isolated from animals C640 and A141 at the day of their death (643 dpi for animal C640 and 670 dpi for animal A141) was evaluated against a second panel of heterologous isolates, which included two laboratory-adapted isolates (MN and NL4-3) (Table 4). Sera from both animals displayed comparable neutralization potency to those of an HIV-infected patient (N16), which was used as internal control during these experiments due to its broad serum anti-HIV neutralizing antibody responses (2). Most of the isolates tested were susceptible to neutralization by both C640 and A141 sera although the titers against the two laboratory-adapted isolates were higher than those against the primary isolates. Although A141 and C640 sera had similar titers of NAbs against MN and NL4-3, the C640 sera had higher titers of NAbs against all of the other isolates tested.
TABLE 4.
Relative titers of cross-reactive NAbs in macaque and human sera
| Virus | Serum dilutiona
|
||
|---|---|---|---|
| C640 (643 dpi) | A141 (670 dpi) | N16b | |
| 92HT593 | 89 | 19 | 157 |
| 92HT594 | 50 | 28 | 20 |
| 92US712 | 221 | 116 | 152 |
| BAL | 476 | 131 | 305 |
| BX08 | 706 | 366 | 403 |
| JRCSF | 69 | <10 | 234 |
| MN | 2,889 | 4,056 | 1,622 |
| NL43 | 1,072 | 1,142 | 2,086 |
| QZ5489 | 488 | 111 | 197 |
| MLV | 24 | <10 | 14 |
The values indicate the serum dilution that resulted in 50% inhibition of infection. MLV was used as control for nonspecific neutralization. Neutralization was considered significant (values in boldface) when the value was at least three times the value obtained with the unrelated MLV virus. The dpi was the day at which the animals were euthanized.
N16 sera was from a chronically HIV-1-infected nonprogressor that developed broadly reactive anti-HIV-1 NAbs (2).
Viral escape from broadly reactive neutralizing antibodies.
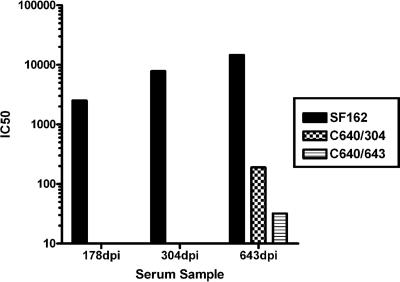
Animal C640 developed the broadest anti-HIV neutralizing antibody responses out of all of the animals tested (Tables 2 and 3), even though it did not develop the highest titers of homologous neutralizing antibody responses (Table 1). Despite the development of broadly reactive NAbs, viral replication continued unabated in this animal (Fig. 1A). This suggests that the virus replicating in this animal was escaping from the action of serum NAbs. To examine this, we amplified 10 Env clones from the plasma of animal C640 at 304 dpi and nine Env clones at 643 dpi (time of death). Single-round competent viruses expressing these Envs were generated, termed C640/304 and C640/643, and their neutralization susceptibility to longitudinally collected autologous sera was evaluated (Fig. 2). As seen in humans infected with HIV (44), SHIVSF162P4 escape from the contemporaneous serum neutralization was evident. Thus, C640/304 and C640/643 were less susceptible than SF162 to serum neutralization, irrespective of the time of serum collection. C640/304 was resistant to neutralization by sera collected prior to its isolation but was susceptible to neutralization by sera collected at 643 dpi. C640/643 was similarly resistant to neutralization by sera collected at 178 and 304 dpi and only minimally susceptible to sera collected 643 dpi.
FIG. 2.
Viral escape from serum neutralization. Sera from animal C640, collected at 304 and 643 dpi, were tested for their neutralizing potential against SF162, and viruses isolated at 304 and 643 dpi (C640/304 and C640/643, respectively). Serum dilutions at which 50% inhibition of viral infection occurred (IC50) are shown.
The C640/304 and C640/643 viruses were resistant to neutralization by anti-V3 MAbs (447D and P3E1) and to the anti-V1 MAb P3C8, whereas the SF162 virus was highly susceptible to neutralization by these antibodies. Also, a gradual increase in resistant to the anti-CD4-binding site MAb b12 was evident, so that C640/304 was more resistant than SF162 and C640/643 was more resistant than C640/304. In contrast, there was no evidence that C640/304 or C640/643 were more resistant to neutralization by MAb 2G12 (which binds a complex glycan epitope on gp120) and to MAb 2F5 (which binds to gp41). These results suggest that the reduced susceptibility to serum neutralization of viruses circulating at 304 and 643 dpi (C640/304 and C640/643, respectively) was due to escape from antibodies that recognize distinct Env such as the V1 and V3 loops, and the CD4-binding site regions (Table 5). However, additional experiments need to be performed in order to clearly define the role in viral escape of the anti-V1 and V3 NAbs present in these sera.
TABLE 5.
Neutralization phenotype of “early” and “late” viruses from animal C640
| MAb | MAb concna (μg/ml)
|
|||
|---|---|---|---|---|
| Epitopeb | SF162 | C640/304 | C640/643 | |
| 2G12 | Glc | 1 | 0.05 | 0.05 |
| 447D | V3 loop | 0.1 | (-) | (-) |
| P3E1 | V3 loop | 0.3 | (-) | (-) |
| P3C8 | V1 loop | 0.3 | (-) | (-) |
| IgG1b12 | CD4-bs | 0.005 | 0.7 | 10 |
| 2F5 | MPER | 2 | 1.5 | 1 |
The values indicate the MAb concentration at which a 50% inhibition of infection was observed. (-), Inhibition of infection was not recorded at the highest MAb concentration tested (10 μg/ml).
Glc, glycan epitope; CD4-bs, CD4 binding site.
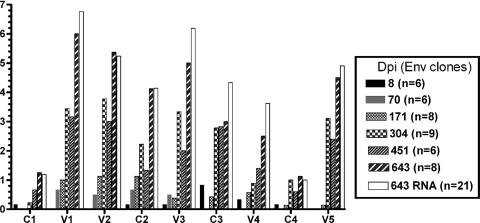
Viral Env evolution in SHIVSF162P4-infected macaques.
The results presented above suggested that the viral env evolved in these animals in ways that allowed escape from the action of diverse NAbs. Longitudinal amino acid sequence analysis of the viral Env gp120 in animals C640 and A141 revealed the stepwise appearance of changes at several gp120 regions (Fig. 3, 4, and http://www.sbri.org/research/stamatatos_pubs_supplemental-figure_JV2007.asp). These changes initially appeared in the C2, V1, V2, and V3 regions of gp120 and subsequently in the C3, V4, and V5 regions (Fig. 3). Some of the changes persisted over time. The early changes observed in the C2, V1, V2, and V3 regions appeared concomitantly with the emergence of homologous neutralizing antibody responses (Table 1), and therefore it is possible that the initial neutralizing antibody response to SHIVSF162P4-infection targets these four envelope regions, as has been previously reported in the case of SIVsm-infected macaques (38). This would also explain the escape of the C640/304 and C640/643 viruses from anti-V3 and anti-V1 antibodies (Table 5).
FIG. 3.
Temporal changes in specific gp120 Env regions. The number of amino acid changes in the conserved and variable regions of gp120 in animal C640 is shown. The number of Env clones amplified sequenced (in parentheses) from PBMC isolated at the indicated time points during infection (dpi) are indicated. The number of changes at each Env region per dpi is the average from all of the Env clones sequenced. Env was also sequenced from plasma RNA (□). Here we show the comparison of the number of AA changes in PBMC and plasma (RNA) at 643 dpi.
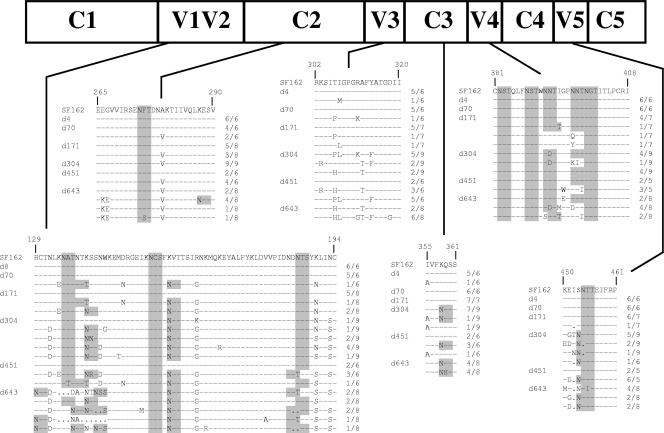
FIG. 4.
Env evolution in animal C640. The amino acid changes in viral Env gp120 over time in animal C640 are indicated. The numbering is based on the SF162 Env sequence. The numbers shown on the right of each sequence indicate the number of Env clones with that particular sequence, compared to SF162 Env, out of the indicated total number of Env clones amplified from PBMC and sequenced from the indicated day of infection. Sequence changes over time are compared to the HIV-1 SF162 Env sequence, which is similar to that of SHIVSF162P4. Highlighted in gray are the positions of potential N-linked glycosylation sites.
Several amino acid changes altered the glycosylation pattern of gp120 (Fig. 4 and unpublished data). In addition to allowing the virus to escape the action of NAbs, some of the amino acid changes may have allowed the virus to alter its cellular tropism. For example, the Ala-to-Val change at position 279 (numbering based on the SF162 Env) (A279V) in C2 affects the binding of gp120 to CD4 (21) and glycosylation changes in V1V2 can alter the interaction of Env with receptor and coreceptor molecules (19, 25, 30). Also, the K158N change was previously shown to be involved in the interaction of HIV-1 SF162 with DCs (17).
The amino acid changes in Env gp120 recorded in animal A141 also appeared first in C2, V1, V2, and V3 regions of gp120 and subsequently in the C3, V4, and V5 regions (http://www.sbri.org/research/stamatatos_pubs_supplemental-figure_JV2007.asp) and were located in the same Env regions as those in animal C640, such as the base of the V1V2 region and the central part of the V3 loop. Some of the changes, such as the A279V change in C2 and the Lys-to-Asn change at position 158 (K158N) in V2, occurred at the same exact Env position in viruses from both animals. However, in contrast to what we observed in animal C640, where a gradual increase in Env diversification was recorded over time, the viral Env in animal A141 evolved toward a single species, that is, the majority of Env clones isolated from this animal at the day of its death (670 dpi) had the same amino acid sequence. Whether this is related to the gradual decrease of plasma viremia over time in this animal (Fig. 1A) is not yet known.
In animal AT54, which displayed high plasma viremia and rapidly progressed to AIDS, amino acid changes in Env occurred primarily in the C2, V1, and V2 regions (http://www.sbri.org/research/stamatatos_pubs_supplemental-figure_JV2007.asp). Interestingly, the A279V change in C2 and the K158N change in V2 substitutions occurred in this animal, as in C640 and A141, in the absence of neutralizing antibody responses (Table 1). Therefore, the emergence of these two changes in animals C640 and A141 may not be due to pressure exerted by NAbs. As mentioned above, these changes may allow the virus to expand its cellular tropism.
Finally, in animal M844, which had undetectable plasma viremia for the duration of observation (852 dpi) (Fig. 1A) the viral Env did not sustain alterations (data not shown).
DISCUSSION
In this study we report that rhesus macaques infected with the CCR5-tropic SHIVSF162P4 virus can develop broad anti-HIV NAb responses. Our results are in general agreement with those from human studies that broadly neutralizing antibody responses are not elicited during acute HIV infection (22, 37), although autologous NAbs develop as soon as a month after infection. The development of broadly reactive anti-HIV NAbs has not been previously reported in the SHIV/macaque animal model. One reason for this may be that the vast majority of SHIV/macaque studies have been conducted with CXCR4-tropic and infection by such viruses results in the rapid and irreversible elimination of CD4+ T-cell lymphocytes and progression to disease within months after infection, during which time broadly reactive neutralizing antibody responses are undetectable. Homologous and autologous NAbs develop in SHIV89.6P-infected macaques (3). However, broad neutralizing antibody responses have not been generated in CXCR4-tropic SHIV-89.6-, SHIV-89.6PD-, or SHIV-HXB2-infected macaques over the time periods analyzed (28).
In addition to the duration of infection, another factor that may influence the development of broadly reactive NAbs appears to be the extent of viral replication. This may not be the case for homologous NAbs, since high titers of homologous neutralizing responses can develop in the absence of robust viral replication (animal A03102, Fig. 1A). Therefore, the development of strong homologous neutralizing antibody responses and the development of broad heterologous neutralizing antibody responses may not be linked, as studies conducted with HIV-infected humans have suggested (12, 20, 29).
The extent and duration of viral replication influences the extent and degree of viral Env evolution. This sustained evolution of the viral env may gradually focus the humoral immune response to Env epitopes that are conserved among diverse HIV isolates. Alternatively, a sustained viral env evolution may result in the emergence of Envs with specific structural features which are conducive to the development of broadly reactive NAbs. In addition, host genetic factors may also play an important role in the development of both autologous and heterologous NAbs.
Our results obtained in the SHIVSF162P4/macaque model are in agreement with those obtained in humans infected with HIV-1, in that the viral Env can sustain significant changes but remain functional and allow the virus to escape the action of neutralizing antibodies (44). In addition, our results suggest that the HIV Env can evolve to simultaneously escape the action of several NAbs, including several broadly reactive NAbs. The fact that, despite the development of broadly reactive neutralizing antibody responses, the animals examined here eventually experienced an irreversible loss of their CD4+ T lymphocytes in the periphery and lymph nodes (Fig. 1) (5) suggests that the development of broadly neutralizing antibody responses during the chronic phase of HIV-1 infection may not protect from progression to disease.
Our studies indicate that the virus evolved in the animals examined here to escape the action of broadly reactive NAbs such as b12. However, b12-like antibodies were not detected in these animals (10). Most likely, the changes that occurred in the CD4-binding site of Env may have indirectly altered the b12 epitope. Also, we have no evidence that 4E10 or 2F5 antibodies were generated in the animals examined here. The sequence of both epitopes remained unaltered during the course of infection (data not shown). In contrast, low titers of 2G12-like antibodies were present in sera from animal C640 (10). Recently, another group reported that 2G12-like antibodies are generated in HIV-infected humans (4). What protective roles (if any) do 2G12-like antibodies play during HIV (or SHIV) infection is not currently known. Our future efforts will focus on better defining the epitope specificity of the broadly reactive NAbs generated in the animals described here and in examining how their development affects viral replication kinetics in the host.
We expect that some of the Env changes observed not only altered the neutralization susceptibility of the circulating virus but also may have altered its cellular tropism (19, 25, 32). As discussed above, some of the changes observed in the animals that developed broad neutralizing antibody responses were also observed in an animal that did not develop NAbs; therefore, they did not emerge in response to antibody-mediated immune pressure. Some of these changes have been reported to alter the interaction of Env with cell surface receptors. The observation that amino acid changes initially emerged in the V1, V2, C2, and V3 regions and subsequently in the C3, V4, and V5 regions could therefore be explained in different ways. It is possible that the initial antibody responses targeted the V1-V3 region of Env. Alternatively, changes in the V1-V3 region, but not in the C3-V5 region, assisted the virus to expand its cellular tropism.
In our attempts to better understand how HIV and the immune system of the infected hosts interact, it would therefore be important to distinguish the env changes that allow the virus to escape antibody-mediated neutralization from those that alter the cellular tropism of the virus.
In summary, we believe that the identification of an animal model where broadly reactive anti-HIV NAbs are developed provides us with the opportunity to examine how such antibodies are developed, to identify factors that are conducive to their development and potentially to develop methodologies to elicit such antibodies by vaccination. However, since SHIVSF162P4 infection results in persistent viral replication only in a subset of infected animals and since the development of broadly NAbs appears to depend on the extent and duration of viral replication in the host, it becomes imperative to develop CCR5-tropic SHIVs with better replicative potentials.
Acknowledgments
This study was supported by NIH grants R01 AI47708 and R01 AI51217 (L.S.). We also received financial support from the M. J. Murdock Charitable Trust and the J. B. Pendleton Charitable Trust.
We thank all those individuals who contributed reagents for this study.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blay, W. M., S. Gnanakaran, B. Foley, N. A. Doria-Rose, B. T. Korber, and N. L. Haigwood. 2006. Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques. J. Virol. 80:999-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braibant, M., S. Brunet, D. Costagliola, C. Rouzioux, H. Agut, H. Katinger, B. Autran, and F. Barin. 2006. Antibodies to conserved epitopes of the HIV-1 envelope in sera from long-term non-progressors: prevalence and association with neutralizing activity. AIDS 20:1923-1930. [DOI] [PubMed] [Google Scholar]
- 5.Buckner, C., L. G. Gines, C. J. Saunders, L. Vojtech, I. Srivastava, A. Gettie, R. Bohm, J. Blanchard, S. W. Barnett, J. T. Safrit, and L. Stamatatos. 2004. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology 320:167-180. [DOI] [PubMed] [Google Scholar]
- 6.Burke, B., N. R. Derby, Z. Kraft, C. J. Saunders, C. Dai, N. Llewellyn, I. Zharkikh, L. Vojtech, T. Zhu, I. K. Srivastava, S. W. Barnett, and L. Stamatatos. 2006. Viral evolution in macaques coinfected with CCR5- and CXCR4-tropic SHIVs in the presence or absence of vaccine-elicited anti-CCR5 SHIV neutralizing antibodies. Virology 355:138-151. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thorton, P. H. I. Parren, L. S. W. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. I. Barbas. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 8.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley, A. J., M. K. Gorny, J. A. Kessler, 2nd, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 80:8745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etemad-Moghadam, B., G. B. Karlsson, M. Halloran, Y. Sun, D. Schenten, M. Fernandes, N. L. Letvin, and J. Sodroski. 1998. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J. Virol. 72:8437-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost, S. D., T. Wrin, D. M. Smith, S. L. Kosakovsky Pond, Y. Liu, E. Paxinos, C. Chappey, J. Galovich, J. Beauchaine, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. USA 102:18514-18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorny, M. K., A. J. Conley, S. Karwowska, A. Buchbinder, J.-Y. Xu, E. A. Emini, S. Koenig, and S. Zolla-Pazner. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J. Virol. 66:7538-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny, M. K., J.-Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 15.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 16.Harouse, J. M., R. C. Tan, A. Gettie, P. Dailey, P. A. Marx, P. A. Luciw, and C. Cheng-Mayer. 1998. Mucosal transmission of pathogenic CXCR4-utilizing SHIVSF33A variants in rhesus macaques. Virology 248:95-107. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, M., J. M. Harouse, A. Gettie, C. Buckner, J. Blanchard, and C. Cheng-Mayer. 2003. Increased mucosal transmission but not enhanced pathogenicity of the CCR5-tropic, simian AIDS-inducing simian/human immunodeficiency virus SHIV(SF162P3) maps to envelope gp120. J. Virol. 77:989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi, T., O. K. Donau, H. Imamichi, M. J. Dumaurier, R. Sadjadpour, R. J. Plishka, A. Buckler-White, C. Buckler, A. F. Suffredini, H. C. Lane, J. P. Moore, and M. A. Martin. 2003. Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages. J. Virol. 77:13042-13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostrikis, L. G., Y. Cao, H. Ngai, J. P. Moore, and D. D. Ho. 1996. Quantitative analysis of serum neutralization of human immunodeficciency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J. Virol. 70:445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, B., J. M. Decker, R. W. Johnson, F. Bibollet-Ruche, X. Wei, J. Mulenga, S. Allen, E. Hunter, B. H. Hahn, G. M. Shaw, J. L. Blackwell, and C. A. Derdeyn. 2006. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J. Virol. 80:5211-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Z. Q., S. Muhkerjee, M. Sahni, C. McCormick-Davis, K. Leung, Z. Li, V. H. Gattone II, C. Tian, R. W. Doms, T. L. Hoffman, R. Raghavan, O. Narayan, and E. B. Stephens. 1999. Derivation and biological characterization of a molecular clone of SHIV(KU-2) that causes AIDS, neurological disease, and renal disease in rhesus macaques. Virology 260:295-307. [DOI] [PubMed] [Google Scholar]
- 25.Ly, A., and L. Stamatatos. 2000. V2 loop Glycosylation of the human Immunodeficiency Virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV- 1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 78:3279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collman, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 serotypes. J. Virol. 70:427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabatov, A. A., G. Pollakis, T. Linnemann, A. Kliphius, M. I. Chalaby, and W. A. Paxton. 2004. Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies. J. Virol. 78:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 77:9029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogert, R. A., M. K. Lee, W. Ross, A. Buckler-White, M. A. Martin, and M. W. Cho. 2001. N-linked glycosylation sites adjacent to and within the V1/V2 and the V3 loops of dualtropic human immunodeficiency virus type 1 isolate DH12 gp120 affect coreceptor usage and cellular tropism. J. Virol. 75:5998-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantophlet, R., E. Ollmann Saphire, P. Poignard, P. W. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77:642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Planelles, V., A. Haislip, E. S. Withers-Ward, S. A. Stewart, Y. Xie, N. P. Shah, and I. S. Chen. 1995. A new reporter system for detection of retroviral infection. Gene Ther. 2:369-376. [PubMed] [Google Scholar]
- 36.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expresing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rybarczyk, B. J., D. Montefiori, P. R. Johnson, A. West, R. E. Johnston, and R. Swanstrom. 2004. Correlation between env V1/V2 region diversification and neutralizing antibodies during primary infection by simian immunodeficiency virus sm in rhesus macaques. J. Virol. 78:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 79:9069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 43.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 44.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 45.Xu, R., I. K. Srivastava, L. Kuller, I. Zarkikh, Z. Kraft, Z. Fagrouch, N. L. Letvin, J. L. Heeney, S. W. Barnett, and L. Stamatatos. 2006. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 349:276-289. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Z. Q., T. M. Fu, D. R. Casimiro, M. E. Davies, X. Liang, W. A. Schleif, L. Handt, L. Tussey, M. Chen, A. Tang, K. A. Wilson, W. L. Trigona, D. C. Freed, C. Y. Tan, M. Horton, E. A. Emini, and J. W. Shiver. 2002. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J. Virol. 76:12845-12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]