Abstract
Lassa virus causes thousands of deaths annually in western Africa and is considered a potential biological weapon. In an attempt to develop a small nonhuman primate model of Lassa fever, common marmosets were subcutaneously inoculated with Lassa virus strain Josiah. This inoculation resulted in a systemic disease with clinical and morphological features mirroring those in fatal human Lassa infection: fever, weight loss, high viremia and viral RNA load in tissues, elevated liver enzymes, and severe morbidity between days 15 and 20. The most prominent histopathology findings included multifocal hepatic necrosis with mild inflammation and hepatocyte proliferation, lymphoid depletion, and interstitial nephritis. Cellular aggregates in regions of hepatocellular necrosis were largely composed of HAM56-positive macrophages, devoid of CD3-positive and CD20-positive cells, and characterized by marked reductions in the intensity of HLA-DP, DQ, DR staining. A marked reduction in the major histocompatibility complex class II expression was also observed in the lymph nodes. Immunophenotypic alterations in spleen included reductions in overall numbers of CD20-positive and CD3-positive cells and the disruption of lymphoid follicular architecture. These findings identify the common marmoset as an appropriate model of human Lassa fever and present the first experimental evidence that replication of Lassa virus in tissues is associated with alterations that would be expected to impair adaptive immunity.
Lassa virus is the causative agent of Lassa fever, a fatal disease affecting more than 300,000 people a year in western Africa (33). The overall instance of fatality is 1 to 2%, but it can be as high as 15 to 30% in hospitalized Lassa fever patients. Lassa virus, a member of the Arenaviridae family (Old World or Lassa lymphocytic choriomeningitis virus [LCMV] serogroup) (41), is transmitted from a natural rodent reservoir, Mastomys natalensis, to humans via contaminated rodent excreta or by close contact with infected individuals (33). Following an incubation period of 7 to 18 days, the disease is marked by a gradual onset of symptoms, including fever, weakness, and malaise. As the disease progresses, nausea, vomiting, diarrhea, and abdominal pain are often observed. Hemorrhage on mucosal surfaces, such as conjunctival hemorrhages or gastrointestinal or vaginal bleeding, occurs in less than 20% of cases. Late stages of the disease are marked by shock, seizures, and coma, culminating in death. Lassa virus disease severity, imported cases of disease from patients that traveled to areas of endemicity, and the potential use of this agent as a biological weapon underscore the need to understand its viral pathogenesis as well as to develop intervention strategies (6, 27, 31, 33).
Several models, including guinea pigs and nonhuman primates, have been used to study the disease (8, 17, 18, 39, 45, 46). The strain 13 guinea pig has become a useful model for the study of Lassa fever pathogenesis and shares characteristics consistent with human disease (18, 39). This model has been successfully used for the evaluation of the efficacy of Lassa fever vaccine candidates (2, 9, 25, 40). However, Lassa virus is treated differently by the immune systems of guinea pigs and nonhuman primates, and vaccine studies in guinea pigs are not necessarily predictive for human vaccines or therapeutics (13). Because the immune systems of nonhuman primates are similar to those of humans, nonhuman primates are relevant models to study the pathogenesis of many human infectious diseases and are generally good predictors of efficacy in vaccine development and intervention strategies. The rhesus macaque has been used to study Lassa virus and has proven to be a superior model in pathology. It is hypothesized to be more predictive than the guinea pig model in the development of intervention strategies (8, 17, 45, 46). Unfortunately, there is now a shortage of rhesus macaques available for biomedical research in the United States (38). The development of alternative nonhuman primate models in which to study potential bioterrorism agents and emerging infections is therefore warranted.
The common marmoset (Callithrix jacchus) is a small anthropoid primate that generally weighs between 320 to 450 g when kept in captivity. Because of the need for maximum biocontainment housing of Lassa virus-infected animals, the use of these small-bodied primates would be valuable and would allow the use of technologies already developed for large rodents and guinea pigs. Marmosets have been successfully used to characterize a number of viral diseases, including arenavirus infections caused by Junin virus, LCMV, severe acute respiratory syndrome coronavirus, and GB virus B as well as other human syndromes (3, 15, 16, 19, 30, 36).
Here we report that subcutaneous inoculation of common marmosets with Lassa virus resulted in a systemic viral disease with fatal outcomes and histological features similar to those described for fatal disease in humans. Using this model, we have demonstrated for the first time that the virus induces alterations in target tissues that would be expected to impair adaptive immune responses. These findings support the observations of immunosuppression contributing to Lassa disease progression in humans (28).
MATERIALS AND METHODS
Biosafety.
Lassa virus is an agent belonging to risk group 4 (Centers for Disease Control and Prevention [CDC]) and category A (National Institutes of Health) (6). All experiments with this virus were performed within a biosafety level 4 facility at the Southwest Foundation for Biomedical Research (SFBR, San Antonio, TX) certified by the CDC. Personnel wore appropriate protective equipment (biosafety suits). Experimental animal protocols were approved by the Institutional Animal Care and Use Committee and the Institutional Biohazards Committee of the SFBR.
Virus.
The Josiah strain of Lassa virus was provided by Tom Ksiazek (CDC, Atlanta, GA). The Lassa virus stock was grown in Vero E6 cells within a 150-mm tissue culture flask with Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) containing 4,500 mg/liter d-glucose and l-glutamine and supplemented with 10% heat-inactivated fetal calf serum at 37°C, 5% CO2, and 85% humidity. The multiplicity of infection was 0.002, and the virus was harvested at 7 days after infection. The infectious titer of the virus stock as determined by conventional virus plaque assay was 5 × 106 PFU/ml. Cells and virus stocks were free of mycoplasma contamination.
Experimental animal and virus inoculation.
Four adult marmosets, aged 2.5 to 4 years and ranging in weight from 368 to 425 g, were obtained from the Southwest National Primate Research Center at the SFBR. Two additional animals were used as a control group. One week before the start of the study, animals were transferred to the biosafety level 4 facility at SFBR and housed individually in caging specifically developed for marmoset work. For inoculation at day 0, two animals were subcutaneously injected with 1 × 103 PFU of virus diluted in saline, while the remaining two animals received 1 × 106 PFU of the virus in the same volume (0.5 ml). Two control animals received diluted conditioned medium of Vero E6 cells. At predetermined time intervals, animals were sedated and blood samples were collected for hematology, chemistries, and virus titration. When animals became moribund they were euthanized, and tissue was harvested for histological examination and immunohistochemistry.
Virus quantification.
Virus titer was determined by conventional plaque titration. Briefly, serial virus dilutions were made in Dulbecco's modified Eagle's medium and then transferred to six-well tissue culture plates containing a confluent monolayer of Vero E6 cells. After a 1-h adsorption period, the virus mixture was removed and a 0.5% agar overlay containing Eagle's minimum essential medium with 2% fetal calf serum (BioWhittaker, Inc., Walkersville, MD) was transferred to each well and allowed to solidify. Plates were incubated at 37°C and 5% CO2. At day 6 after infection, a secondary overlay containing neutral red (0.2 g/liter) was added and then plates were incubated at 37°C for an additional 24 h. Virus plaques were counted at day 7 after infection.
In addition to plaque assay, viremia and viral load in tissues were measured by quantitative reverse transcription-PCR (qRT-PCR). For the detection of viral RNA in the blood of experimental animals, viral RNA was extracted from 500 μl EDTA-treated blood using the RiboPure blood kit according to the manufacturer's recommendations (Ambion, Austin, TX). Total RNA was extracted from tissue samples that were stored in RNAlater. Briefly, 100 mg tissue was transferred to a screw-top microcentrifuge tube containing TRIzol reagent (Invitrogen) and a 5-mm stainless steel bead (QIAGEN Inc., Valencia, CA). The tissue was then homogenized using the TissueLyser (QIAGEN) homogenization system as per the manufacturer's recommendations. RNA was then extracted from the homogenate by following the TRIzol reagent protocol described by Invitrogen. Real-time qRT-PCR was performed using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) and the RNA UltraSense one-step real-time qRT-PCR system (Invitrogen) according to the manufacturers' recommendations. The primer/probe set for Lassa virus strain Josiah RNA targeted a GPC region of the S segment using 36E2 and 80F2 primers (11). The qRT-PCR contained 5 μM of each primer and 2.5 μM of the probe for each primer/probe set in a final reaction volume of 25 μl. The reaction conditions were 50°C for 20 min, 95°C for 2 min, and then 40 cycles alternating between 60°C for 1 min and 95°C for 15 s. Standards used in qRT-PCR were generated from serial 10-fold dilutions of RNA isolated from Lassa virus strain Josiah virus stock (10−1 PFU/ml to 10−7 PFU/ml) that were enumerated in triplicate by conventional plaque assay as previously described (25). Validation experiments revealed the specificity and sensitivity of qRT-PCR, allowing the detection of 10 PFU/ml blood or 10 PFU/g tissue. We have seen good correlation between viral RNA and infectious titers. The ratios of RNA to PFU range between 80 and 200, which are consistent with a ratio that has been previously reported (1). The utility of the qRT-PCR method has been described recently (8a).
Hematology and blood chemistries.
The biochemical analysis of plasma samples was performed using a mammalian liver enzyme profile rotor on a VetScan analyzer (Abaxis, Inc., Union City, CA). Complete blood counts were performed using a VetScan HMT machine (Abaxis, Inc., Union City, CA).
Histology (H&E) and immunohistochemistry.
Samples of aseptically removed tissues were fixed in phosphate-buffered (pH 7.2) 4% paraformaldehyde and embedded in paraffin for histology. Paraffin-embedded tissues were cut in 5-μm sections, deparaffinized, and stained with hematoxylin and eosin (H&E). For immunohistochemistry analysis, deparaffinized tissue sections were stained for CD3, CD20, HAM56, Ki67, and the major histocompatibility complex class II (MHC-II) antigen HLA-DQ, DP, DR (catalog no. M0775; DAKO, Carpenteria, CA) and visualization of positive cells was completed by an avidin-biotin-horseradish peroxidase complex technique with diaminobenzidine chromogen as previously described (16).
RESULTS
Clinical observations.
All animals appeared normal until 8 days after infection, when weight loss was observed. After day 10, animals lost nearly 10% of their body weights. Coinciding with weight loss, behavioral changes were observed. Animals became depressed, had reduced stool production, became partially anorexic, and developed low-grade fevers just prior to euthanasia. Beginning at 8 days after infection, blood chemistries were marked by elevated serum alanine and aspartate aminotransferases, which are markers of hepatocellular necrosis, and these parameters peaked by the day of euthanasia (Fig. 1). Total bilirubin, gamma-glutamyltransferase, and alkaline phosphatase values, which are commonly used measures for the evaluation of excretory liver functions (26), were in normal ranges in plasma samples collected on day −7 to day 14 after infection. However, on day 18, plasma levels of alkaline phosphatase in monkeys infected with high doses of Lassa virus were significantly elevated (average, 447 U/liter). No jaundice was observed in the infected monkeys. Levels of albumin in plasma, one of the markers of the synthetic capacity of the liver (10), decreased after day 8 and dropped almost twofold on the day of euthanasia (Fig. 1). We did not see significant differences in red or white blood cell counts or hemoglobulin levels in infected marmosets. However, there was a gradual reduction in platelet numbers in both the low-dose-infected animals and the high-dose-infected animals over the course of infection (not shown).
FIG. 1.
Lassa virus infection in common marmosets. (A) alanine aminotransferase (ALT) and (B) albumin (ALB) levels in plasma of four monkeys infected with Lassa virus. (C) Gross pathology indicates an enlarged liver with pale foci in animal 17280, which was infected with 1 × 106 PFU of Lassa virus. (D) Viral load in tissues of four infected monkeys was measured by qRT-PCR as described in Materials and Methods and was expressed as means + standard deviations. Error bars indicate standard deviations. LN, lymph nodes.
Viremia and viral burden in tissues.
The virulence of Lassa virus in humans and experimental animals is related to the level of viremia, suggesting that the level of virus replication is a primary factor of pathogenesis. In humans, a viremia level of ≥103.6 50% tissue culture infective doses/ml in Lassa fever patient admission was associated with a case fatality rate of 76% (34). In different species of nonhuman primates experimentally infected with Lassa virus, fatal infection was associated with levels of viremia greater than or equal to 4 log10 PFU/ml (39).
In Lassa virus-infected marmosets, we detected the virus by conventional plaque assay and by RT-PCR by day 8. The virus replicated exponentially in marmoset tissues, resulting in viremia levels of >5 to 7 log10 PFU/ml on day 14 and on the day of necropsy. Using a real-time qRT-PCR assay, we measured Lassa virus S RNA in tissues and expressed viral burden as PFU equivalents (Fig. 1). All tested tissues contained significant amounts of viral RNA comparable with levels of viremia. On the day of necropsy, we did not see significant differences in viral RNA loads in the tissues of animals infected with low (1 × 103 PFU) or high (1 × 106 PFU) doses of Lassa virus. In reticuloendothelial tissue, such as the liver, spleen, and lymph nodes, viral loads exceeded viremia levels, suggesting that virus targets these tissues.
Gross pathology.
Upon necropsy, an enlarged liver was observed in each experimentally inoculated marmoset. In those animals that received a high dose of virus, pale foci were observed throughout the liver. Another finding consistent with all animals was lung abnormalities, primarily hemorrhage, in most lobes. The spleens of three of four animals were enlarged, while the kidney of one animal given a high dose was pale red in appearance. No significant pathological lesions were observed in other tested tissues (Table 1).
TABLE 1.
Microscopic findings in Lassa virus-infected marmosets
| Tissue/pathology | Finding for indicated dose and animala
|
|||
|---|---|---|---|---|
| 103 PFU
|
106 PFU
|
|||
| 17102 | 18164 | 17280 | 18810 | |
| Liver | ||||
| Hepatitis | ++ | ++ | + | ++ |
| Eosinophilic inclusions | + | + | + | + |
| Biliary stasis | + | + | − | + |
| Hepatic necrosis | + | + | + | + |
| Portal infiltrates | − | + | − | + |
| Lymphoid and hematopoietic | ||||
| Lymph node lymphadenopathy | + | + | + | + |
| Spleen, lymphoid depletion | + | + | + | + |
| Bone marrow | NSF | NSF | NSF | NSF |
| Kidney | ||||
| Interstitial nephritis | ++ | + | + | + |
| Mesangial thickening | + | + | + | + |
| Protein casts | + | + | + | + |
| Gastrointestinal system | ||||
| Stomach | NSF | NSF | NSF | NSF |
| Salivary gland | NE | NSF | NSF | NSF |
| Pancreas | NSF | NE | NSF | NSF |
| Large intestine | NSF | NSF | NSF | NSF |
| Small intestine | NSF | NSF | NSF | NSF |
| Lung | ||||
| Interstitial pneumonitis | − | − | + | + |
| Pulmonary edema | − | − | + | + |
| Mesothelial hypertrophy | − | − | + | + |
| Other tissues | ||||
| Necrosis in adrenal gland | NE | + | NE | + |
| Brain | NSF | NSF | NSF | NSF |
| Heart | NSF | NSF | NSF | NE |
| Skin | NSF | NSF | NSF | NSF |
+, mild to moderate; ++, moderate to severe; −, absent; NE, not examined; NSF, no significant findings.
Histology.
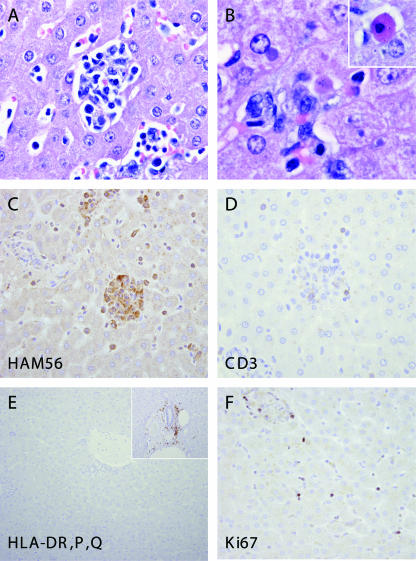
In general, histological changes were mild compared with the extensive viral burden of visceral tissues and were located predominantly in tissues of the reticuloendothelial system, such as the liver, spleen, and lymph nodes (Table 1; Fig. 2A and B). Hepatic lesions were observed in all animals and characterized by multifocal random hepatic necrosis. Areas of necrosis varied from individual hepatocytes, which appeared rounded with eosinophilic cytoplasm and pyknotic nuclei (Councilman bodies), to small clusters of necrotic hepatocytes infiltrated by macrophages and lymphocytes. Degenerative hepatocytes often contained single or multiple rounded eosinophilic to amphophilic inclusions of 3 to 8 μm in diameter (not shown). A green to brown granular pigment indicative of bile and biliary stasis was evident within many hepatocytes throughout the evaluated hepatic sections and within some macrophages found in necrotic foci. Portal infiltrates were observed in two animals and were mild.
FIG. 2.
Hepatic pathology in Lassa-inoculated common marmosets. Multifocal random hepatic necrosis accompanied by a mixed inflammatory cell infiltrate consisting of macrophages and lymphocytes (A). Degenerate hepatocytes contained well-circumscribed eosinophilic to amphophilic cytoplasmic inclusions, and individual hepatocyte necrosis was apparent (inset, Councilman body) (B). Cellular aggregates in regions of hepatocellular necrosis were largely composed of HAM56 macrophages and devoid of (C) CD3-positive and (D) CD20-positive cells. A marked reduction in the intensity of HLA-DP, DQ, DR staining (E) was observed relative to normal control tissue (inset). Increased numbers of cells positive for the proliferation marker Ki67/MIB1 were observed (F).
A mild to moderate multifocal interstitial pneumonitis was evident in the lungs of two animals infected with a high dose of Lassa virus (Fig. 3). In these animals, septal thickening was accompanied by the presence of proteinaceous exudates within the alveolar spaces and increased numbers of macrophages and lymphocytes. Vascular changes were also noted in these animals and were characterized by multifocal edema and thickening of the periarterial space to 5 to 10 times the normal diameter. Occasionally, these edematous areas contained increased numbers of lymphocytes and macrophages. Finally, in the affected lungs, multifocally, the pleurae contained hypertrophied and eosinophilic mesothelial cells.
FIG. 3.
Pulmonary pathology in common marmosets infected with Lassa virus. A multifocal interstitial pneumonitis was observed in two of four animals (Table 1), as evidenced by septal thickening, alveolar edema, and increased numbers of inflammatory cells (A). In these animals, multifocal periarterial edema (B) and multifocal hypertrophy of pleural mesothelial cells (C) were observed.
A mild to moderate lymphoid depletion was observed in the spleen in the periarteriolar sheath in each animal. The normal dense outer mantle surrounding the sheath was absent, but evidence of ongoing necrosis was minimal. A lymphadenopathy was observed in each animal and was characterized by a marked enlargement of lymph nodes to two to three times the normal size, with concurrent lymphoid depletion. Lymph nodes were infiltrated by increased numbers of histiocytes that were present within dilated sinuses, medullary cords, and paracortical spaces, giving the tissues an overall eosinophilic and depleted appearance. Well-developed follicles were rarely observed. In several nodes, extensive ongoing necrosis of lymphocytes was evidenced by the presence of extensive foci of pyknotic nuclei within the mantle region of B-cell follicular areas. Rare multinucleated syncytial cells were observed in some sections. Lymph node capsules were multifocally thickened and contained infiltrating inflammatory cells.
A mild multifocal lymphocytic interstitial nephritis was observed in all animals. In addition, mesangial thickening was observed and was accompanied by proteinaceous material within Bowman's space and dilated cortical tubules. However, rare sclerotic glomeruli were evident, suggesting that this nephritis represents a preexisting condition.
The adrenal glands were available from only two marmosets. In these animals, mild to moderate multifocal necrosis was observed within the adrenal cortex. In one animal, larger areas of degeneration within the adrenal cortex were observed and characterized by the vacuolization of cells, individual cell necrosis, and infiltration by histiocytes.
Immunophenotyping of cells in target tissues.
Immunohistochemical analysis revealed that cellular aggregates noted by H&E stains in areas of liver necrosis were composed primarily of HAM56-positive macrophages (Fig. 2C). These areas recruited just a few CD3-positive (Fig. 2D) or CD20-positive cells (data not shown), and overall, there were decreased numbers of both T and B cells within the hepatic parenchyma of Lassa virus-inoculated animals. Immunostaining for the MHC-II antigen HLA-DP, DQ, DR (Fig. 2E) revealed that in spite of macrophage infiltration, there was marked, decreased MHC-II antigen expression in areas of necrosis and throughout the hepatic parenchyma and portal areas. We have shown previously that in Lassa virus-like hepatitis in rhesus macaques, necrotic foci were associated with areas of strong hepatocyte proliferation (22, 23). For the detection of proliferating cells in the livers of Lassa virus-infected marmosets, tissue sections were stained for Ki67 nuclear antigen. This staining revealed increased numbers of Ki67-positive cells (Fig. 2F), both within areas of necrosis and in the adjacent hepatic parenchyma. These cells were most frequently observed in hepatic sinusoids, perisinusoidal spaces, or portal regions.
As in the liver, in the lymph nodes of infected marmosets, there was marked, decreased expression of HLA-DP, DQ, DR (Fig. 4). The staining of HAM56 antigen revealed a marked increase in macrophages in lymph nodes and the disruption of normal nodal architecture. The discrete HAM56 expression found in normal nodes was replaced by a more diffuse expression. B-cell follicles with well-defined germinal centers and mantle zones were absent or remarkably reduced in number and found intermixed throughout the node rather than confined to the cortical regions. CD3-positive cells were found throughout the cortex and medulla, and Ki67 staining revealed a marked proliferation of cells (not shown).
FIG. 4.
Immunophenotypic alterations in lymph node during experimental Lassa infection. A marked reduction in the intensity of HLA-DR staining was observed in lymph nodes obtained from Lassa-infected animals (B) compared to that seen in lymph nodes from controls (A). Increased numbers of HAM56-positive cells were noted in the infected animals (D) compared with controls (C), and the discrete staining observed in normal lymph node was largely absent.
In the spleen, immunohistochemical analysis revealed a depletion of CD3-positive lymphocytes, particularly in the periarteriolar sheaths, which were reduced in size and number (Fig. 5). Overall reductions in CD20-positive B cells were also evident, although lymphoid follicles were present in splenic white pulp. As in the lymph node, these changes were accompanied by increased numbers of HAM56-positive macrophages throughout the red pulp and a marked decreased expression of HLA-DR.
FIG. 5.
Immunophenotypic alterations in spleen of marmosets infected with Lassa virus. Overall numbers of CD20-positive B lymphocytes were reduced in Lassa virus-infected animals (B) compared with that in controls (A), and lymphoid follicles lacked their normal architecture. Similarly, CD3-positive T cells within the periarteriolar sheath were reduced in number or absent from the infected animals (D) compared to controls (C).
DISCUSSION
The relatively small size of marmosets, lower caging and feeding costs, and ease of handling in a biosafety environment represent substantial benefits compared with the use of macaques. Experimental infection of common marmosets with Lassa virus resulted in a systemic viral disease with high viremia and high viral RNA load in all tested tissues, elevated liver enzymes, decreased levels of albumin in plasma, weight loss, and severe morbidity 15 to 20 days after inoculation. Morphological features mirrored those described for human cases of fatal Lassa fever and included hepatic and adrenal necrosis, lymphoid depletion, and interstitial nephritis.
Postmortem histological studies of Lassa fever patients and Lassa virus-infected experimental animals indicate that the liver is one of the most affected organs participating in a systemic breakdown (32, 43, 49). Three categories of morphological changes were found in the livers of patients that died from Lassa virus hepatitis: active hepatocellular injury, continued damage and early recovery, and a regenerative phase with high mitotic activity of hepatocytes. Researchers observed a macrophage response to cellular damage, but they did not find evidence of lymphocytic infiltration in infected hepatic tissues (32). Despite the substantial liver dysfunction, the degree of hepatic damage was not sufficient to implicate hepatic failure as the primary cause of death. The relationship between liver damage and hematological and endothelial dysfunctions leading to shock and death remains unclear.
Guinea pigs and mice are susceptible to experimental Lassa virus infection (39). In mice, the outcome of the infection depends on the MHC background, the age of the animal, and the route of inoculation (20, 21). Intracerebral inoculation of Lassa virus into adult mice induces a fatal convulsive disorder resembling a classical immunopathology caused by LCMV, the prototype arenavirus (37, 41). The strain 13 guinea pig is the most sensitive to Lassa virus and the most commonly used model for elucidating pathogenesis and for developing effective treatment and protective regimens for Lassa fever (7, 18, 25, 39, 40). However, the evaluation of vaccine candidates in guinea pigs and primates has produced conflicting results (9, 12).
Common marmosets were successfully used for pathogenesis and protection studies with Junin virus, a member of the New World arenaviruses (Tacaribe serogroup) and a causative agent of Argentine hemorrhagic fever (14, 47, 48). An infection of Callithrix jacchus with the prototype strain of Junin virus produced a fatal disease with multifocal hemorrhages and characteristic microscopic lesions, such as meningoencephalitis, interstitial pneumonia, lymphocytic depletion of lymphatic tissue, hepatocytic necrosis, and a variable decrease in bone marrow cellularity. High virus concentrations correlated with lesions and with the presence of virus antigen.
Feeding callitrichids neonatal mice that were infected with LCMVCH caused outbreaks of callitrichid hepatitis in captive marmosets and tamarins in zoos and animal parks in the United States and Europe (1, 35, 36, 42). Pathologies of both experimental and natural outbreaks of callitrichid hepatitis in common marmosets and tamarins clearly established the liver as a principal target for LCMVCH. Callitrichid hepatitis was characterized by elevated levels of liver aminotransferases and bilirubin in plasma, random foci of hepatocellular degeneration associated with a mild mononuclear inflammatory cell infiltrate, and a few neutrophils. A characteristic finding was acidophilic structures resembling apoptotic, Councilman-like bodies seen in the sinusoids but also occasionally within Kupffer cells (35). LCMV antigen was observed in hepatic foci as well as in nondegenerating areas in lung, kidney, urinary bladder, brain, and testes. Interestingly, pygmy marmosets, which died 5 to 14 days later than the common marmosets and tamarins, had minimal hepatocellular necrosis but an intensive portal mononuclear inflammatory cell infiltration, suggesting the involvement of immune-mediated reactions in fatal hepatitis in these animals (35).
In our experiments, Lassa virus infection of common marmosets resulted in a fatal disease with morphological features similar to those described for patients dying from Lassa disease (32, 49). The most prominent morphological features of Lassa virus-inducible hepatitis in common marmosets were (i) multifocal hepatic necrosis with mild inflammation presented predominantly by HAM56-positive macrophages; (ii) the near absence of CD20-, CD8-, or CD3-positive lymphocytes in necrotic foci; (iii) the complete lack of expression of MHC-II antigen; and (iv) hepatocyte proliferation as judged by positive Ki67 staining.
Hepatocyte proliferation was a prominent feature of fatal Lassa virus hepatitis in humans (32) and of LCMV-inducible fatal hepatitis in rhesus macaques (22-24). However, in the LCMV model, the proliferation response was much stronger and it was associated with significant up-regulation of such markers of liver regeneration as Ki67, interleukin-6, soluble IL-6 receptor, and soluble tumor necrosis factor receptor. MHC-II antigens were not described for that model. Striking findings in Lassa virus-infected marmosets were the marked reduction of staining for the MHC-II antigen HLA-DP, DQ, DR and poor T-cell responses. These findings contrast with other forms of viral hepatitis in marmosets caused by GB virus B (16) and by severe acute respiratory syndrome coronavirus (15), in which hepatic inflammation is associated with a marked increase in MHC-II expression. These findings suggest evasion of the normal immune response as a virulence factor in the development of Lassa virus-induced hepatitis.
Multifocal interstitial pneumonitis was observed in two animals and was characterized by edema, septal thickening, and inflammatory infiltrates. Pulmonary arterial lesions have previously been recognized in rhesus macaque but not in human tissues (8, 17, 44, 46). In the Lassa virus-infected marmosets, mild to moderate periarterial edema was recognized, but pulmonary arteritis as found in rhesus macaques was not observed.
Adrenal necrosis is a characteristic feature of fatal Lassa virus infection in humans and nonhuman primates (8, 17, 44, 46). Adrenal glands from only two animals were available for evaluation and mild, multifocal adrenal necrosis and adrenalitis were observed. Degenerate cells occasionally contained eosinophilic spherical inclusions.
The significance of the renal lesions remains to be determined. A mild multifocal lymphocytic interstitial nephritis was observed in all animals and is compatible with that described for humans and rhesus macaques.
As described for humans (44), lymphoid depletion was also observed in the spleen and lymph nodes of Lassa virus-infected marmosets. These changes were most pronounced in lymph nodes in which the normal architecture was largely effaced through loss of follicles and infiltration by large numbers of histiocytes. Lymphocytic necrosis was occasionally observed, suggesting that this was the primary process and that infiltrates were secondary to cell death.
In addition to that in liver tissues, a marked reduction in the intensity of HLA-DR staining was also observed in the lymph nodes of Lassa virus-infected marmosets. Immunophenotypic alterations in the spleen included overall numbers of CD3- and CD20-positive lymphocytes that were reduced in infected animals compared to the numbers in controls and the disruption of the architecture of lymphoid follicles. Previously, the immunosuppressive phenotype of Lassa virus infection was based on detection of proinflammatory cytokines and immunomodulatory molecules in culture medium of human cells infected in vitro (4, 5, 26, 29), in plasma of experimentally infected animals (22), or in Lassa fever patients (28). Here we have presented the first experimental evidence that the replication of Lassa virus in target tissues of a nonhuman primate, the common marmoset, is associated with alterations that would be expected to impair adaptive immunity.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) for Regional Centers of Excellence for Biodefense and Emerging Infectious Diseases (grants U54 AI057156 and U54 AI57168) and by an NIH laboratory construction grant (1C06RR12087). We acknowledge the New England Primate Center (grant P51RR00168-45) for histopathology support.
We thank Robert Geiger, Donna Layne, Michelle Reynolds, Laurie Condel, Stacey Perez, Carmen Moreira, and Gia Marotta for technical support and helpful discussions. We also thank Maria Messenger and April Hopstetter of the SFBR for assistance with manuscript preparation.
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Asper, M., P. Hofmann, C. Osmann, J. Funk, C. Metzger, M. Bruns, F. J. Kaup, H. Schmitz, and S. Gunther. 2001. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology 284:203-213. [DOI] [PubMed] [Google Scholar]
- 2.Auperin, D. D., J. J. Esposito, J. V. Lange, S. P. Bauer, J. Knight, D. R. Sasso, and J. B. McCormick. 1988. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 9:233-248. [DOI] [PubMed] [Google Scholar]
- 3.Avila, M., S. R. Samoilovich, R. P. Laguens, M. S. Merani, and M. C. Weissenbacher. 1987. Protection of Junin virus-infected marmosets by passive administration of immune serum: association with late neurologic signs. J. Med. Virol. 21:67-74. [DOI] [PubMed] [Google Scholar]
- 4.Baize, S., D. Pannetier, C. Faure, P. Marianneau, I. Marendat, M. C. Georges-Courbot, and V. Deubel. 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 8:1194-1202. [DOI] [PubMed] [Google Scholar]
- 5.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. [DOI] [PubMed] [Google Scholar]
- 6.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat for the Working Group on Civilian Biodefense. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391-2405. [DOI] [PubMed] [Google Scholar]
- 7.Bredenbeek, P. J., R. Molenkamp, W. J. Spaan, V. Deubel, P. Marianneau, M. S. Salvato, D. Moshkoff, J. Zapata, I. Tikhonov, J. Patterson, R. Carrion, A. Ticer, K. Brasky, and I. S. Lukashevich. 2006. A recombinant yellow fever 17D vaccine expressing Lassa virus glycoproteins. Virology 345:299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callis, R. T., P. B. Jahrling, and A. DePaoli. 1982. Pathology of Lassa virus infection in the rhesus monkey. Am. J. Trop. Med. Hyg. 31:1038-1045. [DOI] [PubMed] [Google Scholar]
- 8a.Carrion, R., Jr., et al. 27 February 2007, posting date. A ML29 reassortant virus protects guinea pigs against a distantly related Nigerian strain of Lassa virus and can provide sterilizing immunity. Vaccine. doi: 10.1016/j.vaccine.2007.02.038. [DOI] [PMC free article] [PubMed]
- 9.Clegg, J. C., and G. Lloyd. 1987. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guinea pigs against Lassa fever. Lancet ii:186-188. [DOI] [PubMed] [Google Scholar]
- 10.Davern, T., and B. F. Scharschmidt. 1998. Biochemical liver tests, p. 345-367. In M. Feldman, B. F. Scharschmidt, and M. H. Sleisenger (ed.), Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology/diagnosis/management. Saunders, Philadelphia, PA.
- 11.Demby, A. H., J. Chamberlain, D. W. Brown, and C. S. Clegg. 1994. Early diagnosis of Lassa fever by reverse transcription-PCR. J. Clin. Microbiol. 32:2898-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher-Hoch, S. P., L. Hutwagner, B. Brown, and J. B. McCormick. 2000. Effective vaccine for Lassa fever. J. Virol. 74:6777-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher-Hoch, S. P., and J. B. McCormick. 2004. Lassa fever vaccine. Expert Rev. Vaccines 3:189-197. [DOI] [PubMed] [Google Scholar]
- 14.González, P. H., R. P. Laguens, M. J. Frigerio, M. A. Calello, and M. C. Weissenbacher. 1983. Junin virus infection of Callithrix jacchus: pathologic features. Am. J. Trop. Med. Hyg. 32:417-423. [DOI] [PubMed] [Google Scholar]
- 15.Greenough, T. C., A. Carville, J. Coderre, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, and K. Mansfield. 2005. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 167:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob, J. R., K. C. Lin, B. C. Tennant, and K. G. Mansfield. 2004. GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J. Gen. Virol. 85:2525-2533. [DOI] [PubMed] [Google Scholar]
- 17.Jahrling, P. B., R. A. Hesse, G. A. Eddy, K. M. Johnson, R. T. Callis, and E. L. Stephen. 1980. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 141:580-589. [DOI] [PubMed] [Google Scholar]
- 18.Jahrling, P. B., S. Smith, R. A. Hesse, and J. B. Rhoderick. 1982. Pathogenesis of Lassa virus infection in guinea pigs. Infect. Immun. 37:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludlage, E., C. L. Murphy, S. M. Davern, A. Solomon, D. T. Weiss, D. Glenn-Smith, S. Dworkin, and K. G. Mansfield. 2005. Systemic AA amyloidosis in the common marmoset. Vet. Pathol. 42:117-124. [DOI] [PubMed] [Google Scholar]
- 20.Lukashevich, I. S. 1987. Human pathogens of the Arenaviridae family. Sov. Med. Rev. E 2:133-186. [Google Scholar]
- 21.Lukashevich, I. S. 1985. Lassa virus lethality for inbred mice. Ann. Soc. Belg. Med. Trop. 65:207-209. [PubMed] [Google Scholar]
- 22.Lukashevich, I. S., I. Tikhonov, J. D. Rodas, J. C. Zapata, Y. Yang, M. Djavani, and M. S. Salvato. 2003. Arenavirus-mediated liver pathology: acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J. Virol. 77:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukashevich, I. S., J. D. Rodas, I. I. Tikhonov, J. C. Zapata, Y. Yang, M. Djavani, and M. S. Salvato. 2004. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch. Virol. 149:2319-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukashevich, I. S., J. D. Rodas, I. Tikhonov, J. C. Zapata, Y. Yang, M. Djavani, and M. S. Salvato. 2003. Arenavirus-mediated liver pathology in rhesus macaques, p. 490-491. In Proceedings of the 11th International Symposium on Viral Hepatitis and Liver Disease. International Medical Press Ltd., Sydney, Australia.
- 25.Lukashevich, I. S., J. Patterson, R. Carrion, D. Moshkoff, A. Ticer, J. Zapata, K. Brasky, R. Geiger, G. H. Hubbard, J. Bryant, and M. S. Salvato. 2005. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J. Virol. 79:13934-13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukashevich, I. S., R. Maryankova, A. S. Vladyko, N. Nashkevich, S. Koleda, M. Djavani, D. Horejsh, N. Voitenok, and M. S. Salvato. 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-α gene expression. J. Med. Virol. 59:552-560. [PMC free article] [PubMed] [Google Scholar]
- 27.Macher, A. M., and M. S. Wolfe. 2006. Historical Lassa fevers reports and 30-year clinical update. Emerg. Infect. Dis. 12:835-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahanty, S., D. G. Bausch, R. L. Thomas, A. Goba, A. Bah, C. J. Peters, and P. E. Rollin. 2001. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J. Infect. Dis. 183:1713-1721. [DOI] [PubMed] [Google Scholar]
- 29.Mahanty, S., K. Hutchinson, S. Agarwal, M. McRae, P. E. Rollin, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170:2797-2801. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield, K. 2003. Marmoset models commonly used in biomedical research. Comp. Med. 53:383-392. [PubMed] [Google Scholar]
- 31.McCormick, J. B., P. A. Webb, J. W. Krebs, K. M. Johnson, and E. S. Smith. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437-444. [DOI] [PubMed] [Google Scholar]
- 32.McCormick, J. B., D. H. Walker, I. J. King, P. A. Webb, L. H. Elliott, S. G. Whitfield, and K. M. Johnson. 1986. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am. J. Trop. Med. Hyg. 35:401-407. [DOI] [PubMed] [Google Scholar]
- 33.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 34.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Craven, K. M. Johnson, L. H. Elliott, and R. Belmont-Williams. 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 35.Montali, R. J., B. M. Connolly, D. L. Armstrong, C. A. Scanga, and K. V. Holmes. 1995. Pathology and immunohistochemistry of callitrichid hepatitis, an emerging disease of captive New World primates caused by lymphocytic choriomeningitis virus. Am. J. Pathol. 147:1441-1449. [PMC free article] [PubMed] [Google Scholar]
- 36.Montali, R. J., C. A. Scanga, D. Pernikoff, D. R. Wessner, R. Ward, and K. V. Holmes. 1993. A common-source outbreak of callitrichid hepatitis in captive tamarins and marmosets. J. Infect. Dis. 167:946-950. [DOI] [PubMed] [Google Scholar]
- 37.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 263:83-117. [DOI] [PubMed] [Google Scholar]
- 38.Patterson, J. L., and R. Carrion, Jr. 2005. Demand for nonhuman primate resources in the age of biodefense. ILAR J. 46:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters, C. J., P. B. Jahrling, C. T. Liu, R. H. Kenyon, K. T. McKee, Jr., and J. G. Barrera Oro. 1987. Experimental studies of arenaviral hemorrhagic fevers. Curr. Top. Microbiol. Immunol. 134:5-68. [DOI] [PubMed] [Google Scholar]
- 40.Pushko, P., J. Geisbert, M. Parker, P. Jahrling, and J. Smith. 2001. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J. Virol. 75:11677-11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvato, M. S., J. C. S. Clegg, M. J. Buchmeier, R. N. Charrel, J.-P. Gonzalez, I. S. Lukashevich, C. J. Peters, R. Rico-Hesse, and V. Romanowski. 2005. Arenaviridae, p. 725-733. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy, VIIIth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom.
- 42.Stephensen, C. B., J. Y. Park, and S. R. Blount. 1995. cDNA sequence analysis confirms that the etiologic agent of callitrichid hepatitis is lymphocytic choriomeningitis virus. J. Virol. 69:1349-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tandon, B. N., and S. K. Acharya. 1987. Viral diseases involving the liver. Bailliere's Clin. Gastroenterol. 1:211-230. [DOI] [PubMed] [Google Scholar]
- 44.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]
- 45.Walker, D. H., H. Wulff, J. V. Lange, and F. A. Murphy. 1975. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull. W. H. O. 52:523-534. [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, D. H., K. M. Johnson, J. V. Lange, J. J. Gardner, M. P. Kiley, and J. B. McCormick. 1982. Experimental infection of rhesus monkeys with Lassa virus and a closely related arenavirus, Mozambique virus. J. Infect. Dis. 146:360-368. [DOI] [PubMed] [Google Scholar]
- 47.Weissenbacher, M. C., C. E. Coto, M. A. Calello, S. N. Rondinone, E. B. Damonte, and M. J. Frigerio. 1982. Cross-protection in nonhuman primates against Argentine hemorrhagic fever. Infect. Immun. 35:425-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissenbacher, M. C., M. A. Calello, M. S. Merani, J. B. McCormick, and M. Rodriguez. 1986. Therapeutic effect of the antiviral agent ribavirin in Junin virus infection of primates. J. Med. Virol. 20:261-267. [DOI] [PubMed] [Google Scholar]
- 49.Winn, W. C., Jr., T. P. Monath, F. A. Murphy, and S. G. Whitfield. 1975. Lassa virus hepatitis. Observations on a fatal case from the 1972 Sierra Leone epidemic. Arch. Pathol. 99:599-604. [PubMed] [Google Scholar]