Abstract
Open reading frame 24 (ORF24) of murine gammaherpesvirus 68 (MHV-68) is conserved among beta- and gammaherpesviruses; however, its function in viral replication has not been defined. Using MHV-68 as a model, we have identified ORF24 as being essential for viral replication. An ORF24-null virus was generated and shown to be defective in late gene expression. Expression of early genes, as well as viral genome replication, was not affected. Furthermore, the defect in late gene expression was likely due to a deficiency in transcription. Thus, we have identified an MHV-68 protein, ORF24, that is essential for the expression of viral late proteins yet dispensable for viral DNA replication.
Murine gammaherpesvirus 68 (MHV-68) has been used to study the replication cycle of gammaherpesvirus due to its ability to lytically infect various cell lines, including those of human and murine origins (6, 17, 20-23). Open reading frame 24 (ORF24) is conserved among all beta- and gammaherpesviruses. Both ORF24 of MHV-68 and its human cytomegalovirus homolog (UL87) have previously been identified as being essential for lytic replication by genome-wide mutagenesis (7, 19, 25). ORF24 of MHV-68 is 39% and 26% identical to the Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus homologs, respectively (23). However, it does not have any significant homology to any cellular proteins. The gene product of ORF24 was found to be associated with MHV-68 virions, but its function is unknown (4). Previously, array studies did not provide conclusive information regarding the kinetic class of ORF24 due to the lack of sensitivity of the arrays for less abundant transcripts (8, 13). However, one group has classified ORF24 as being an early gene (1).
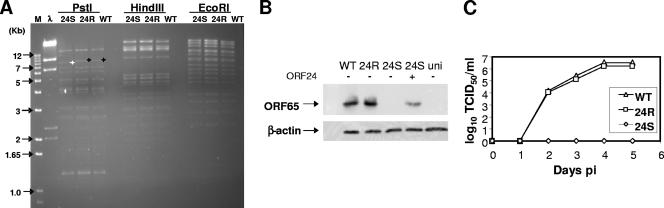
To characterize this viral gene, an ORF24-null virus, 24S, was generated by the insertion of a triple stop codon with a PstI restriction site into the N-terminal region (nucleotide [nt] 40056) of the ORF24 coding sequence on the wild-type (WT) MHV-68 bacterial artificial chromosome (BAC) by allelic exchange as described previously (3, 10, 18). A revertant virus (24R) was subsequently generated using allelic exchange of the 24S BAC plasmid with a FLAG-tagged WT ORF24 shuttle plasmid. Digestion with three restriction enzymes confirmed the correct genomic structure of the three viruses (Fig. 1A).
FIG. 1.
Restriction digest pattern of 24S, 24R, and WT DNA. (A) BAC DNA was digested with PstI, HindIII, or EcoRI, and the DNA fragments were separated on an agarose gel. A DNA fragment from 24S is shifted to 7.6 kb (white asterisk) from the WT and the 24R 7.8-kb fragment (black asterisk) due to the inserted PstI site. (B) Rescue of 24S virus by ORF24 trans-complementation. The 24S BAC plasmid was cotransfected with (+) or without (−) the complementing FLAG-tagged ORF24 expression vector into BHK-21 cells. WT and 24R BAC were also transfected as controls. At 5 days posttransfection, viral lytic antigen expression was analyzed by Western blotting using rabbit polyclonal antibody against capsid protein ORF65. M, marker; uni, uninfected. β-Actin antibody was used as a loading control. (C) Multiple-step growth kinetics of 24S. BHK-21 cells were infected with WT (triangles), 24R (squares), or 24S (diamonds) virus at an MOI of 0.01. Total infected cells were harvested at indicated time points postinfection, and the viral titer, the 50% tissue culture infectivity dose (TCID50), was determined in a tetracycline-inducible ORF24 cell line (FT293-ORF24) by limiting dilution.
Transfection of 24S BAC DNA into BHK-21 cells did not result in any detectable productive viral infection when surveyed for 7 days. However, when a FLAG-tagged ORF24 expression plasmid was cotransfected along with the 24S BAC DNA, a virally induced cytopathic effect was observed by 5 days posttransfection, similar to transfection with WT or 24R BAC DNA. Viral replication was further confirmed by examining the expression of capsid protein ORF65 in the transfected cells (Fig. 1B). No capsid protein was detected in the transfection of 24S DNA alone. However, capsid protein expression was restored in the 24S transfection by complementation with an ORF24 expression plasmid. To reconstitute the 24S virus, 24S BAC DNA was transfected into the complementing cell line 293FT-ORF24. 293FT-ORF24 is a stable cell line derived from Flp-In 293 Trex cells (Invitrogen) and expresses FLAG-tagged ORF24 upon tetracycline induction. A stock of the virus was generated by passaging virus produced from the transfection of 24S DNA onto the complementing cell line multiple times.
To determine the replication capacity of the ORF24 recombinant viruses, a multiple-step growth curve was determined. BHK-21 cells were infected with WT, 24S, or 24R viruses at a multiplicity of infection (MOI) of 0.01, and the supernatants were harvested at various time points. The viral titer (50% tissue culture infective dose/ml) of the lysates was determined by limiting dilution on complementing cells (Fig. 1C). The 24S mutant was unable to undergo productive viral replication and did not produce any infectious particles at any time point. This indicates that the level of WT-like revertant viruses is below our limit of detection. The WT and 24R viruses grew with similar kinetics and to similar titers. Taken together, the ability of an ORF24-expressing plasmid to complement 24S, and the restored growth kinetics of the 24R virus from 24S, confirms that the mutation in 24S is ORF24 specific and that the lack of productive infection is due to the deficiency in ORF24.
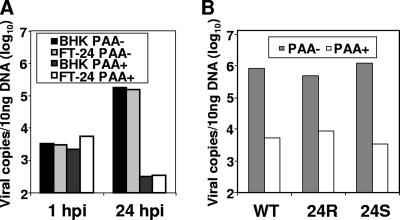
To determine the step in which 24S viral replication was inhibited, we examined DNA replication of the viral genome. Noncomplementing BHK-21 cells or complementing 293 FT-ORF24 cells were infected with 24S at an MOI of 2. After 1 h, the inoculum was removed, and total DNA was harvested at this time as well as at 24 h postinfection (hpi). The viral genome copy number was determined by quantitative real-time PCR using primers that amplified a region of the ORF57 promoter. At 1 hpi, there were approximately 3,000 copies of the viral genome per 10 ng of total DNA, and 24 h later, the amount of viral DNA increased to over 150,000 copies. Treatment with the viral polymerase inhibitor phosphonoacetic acid (PAA) resulted in a decrease in viral genome copy numbers to a level below the input amount (Fig. 2A). The amount of DNA was similar in both complementing and noncomplementing cells at early as well as late time points. This indicates the there were no differences in either the input amount of viral DNA or viral genome amplification between complemented virus and noncomplemented virus. To compare the viral DNA replications in cells infected with the recombinant viruses, BHK-21 cells were infected with WT, 24S, or 24R virus at an MOI of 3 in the presence or absence of PAA, and total DNA was harvested at 24 hpi. We found that the level of viral DNA replication was similar for all three viruses (Fig. 2B). Similar to previous experiments, treatment with PAA resulted in a 2- to 3-log decrease in viral genome copy numbers, and this is consistent with previously characterized genome replication levels (3). Similar genome replication was observed in noncomplementing cells transfected with 24S (data not shown). Based on these data, we determined that ORF24 is not required for viral DNA replication.
FIG. 2.
Quantitation of viral genome replication. (A) BHK-21 or FT-24 cells were infected with 24S at an MOI of 2 with (+) or without (−) PAA. Total DNA was harvested at 1 and 24 hpi, and the viral genome copy number in 10 ng total DNA was determined by quantitative PCR. (B) BHK-21 cells were infected with WT, 24R, or 24S virus at an MOI of 3 with (+) or without (−) the viral DNA polymerase inhibitor PAA. The total infected cellular DNA was harvested at 24 hpi, and the viral genome copy number in 10 ng total DNA was determined by quantitative PCR.
Since we observed no defect in viral genome replication, we next looked for the expression of late antigens during the 24S infection of BHK-21 cells. At 24 hpi, expression of the capsid protein ORF26 can be detected in WT and 24R infections; however, this expression is abolished in 24S infection (Fig. 3A). This defect in late antigen expression was also seen when ORF65 expression was examined. PAA treatment confirmed that these two proteins are truly late proteins. To confirm the infectivity of 24S, 293FT-ORF24 cells were infected with WT, 24S, or 24R virus. Lytic protein expression was restored in 24S during infection of the complementing cell line (Fig. 3A).
FIG. 3.
Lytic viral gene expression. (A) The noncomplementing BHK-21 and ORF24-complementing FT293-ORF24 cell lines were infected with WT, 24R, or 24S virus at an MOI of 3 in the presence (+) or absence (−) of PAA. At 24 hpi, the infected cell lysate was harvested, and the expression of MHV-68 lytic antigens was determined by Western blotting using antibodies against viral capsid proteins ORF26 and ORF65. (B) BHK-21 cells were infected with WT or 24S virus. Total RNA was harvested at 24 hpi and subjected to Northern blotting. The blots were probed with ORF65 (nt 93962 to 94519), ORF52 (nt 71013 to 71364), ORF39 (nt 56048 to 56949), ORF17 (nt 28832 to 29879), ORF57 (nt 76650 to 77139), ORF54 (nt 71820 to 72602), M3 (nt 6060 to 7277), or β-actin. UI, uninfected.
To further look at the gene expression pattern of 24S infections, we analyzed the expression of several viral transcripts. RNA from WT- or 24S-infected cells along with RNA from uninfected cells were harvested, and transcripts were analyzed by Northern blotting (Fig. 3B). We first looked at late gene expression by probing with the PCR products of ORFs 65, 52, 39, and 17. In all cases, the deficiency in ORF24 resulted in no expression of the late transcripts. Next, we probed the membranes with early and early-late genes. Consistent with the viral DNA replication data, expression of the early genes ORF54 and ORF57 was not affected in 24S infection, as the levels of these two transcripts were similar in both infections. Furthermore, we looked at the expression of the early-late gene M3. Expression of M3 was present during 24S infection but at a reduced level. To control for the even loading of RNA between the two infections, the membrane was probed with β-actin.
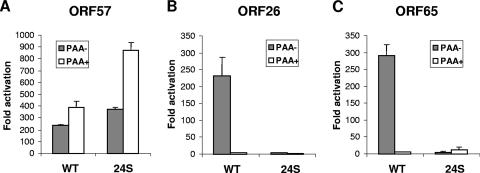
To further explore this mechanism, we examined viral promoter activity during 24S infection using a reporter assay. We chose to analyze the activation of promoters from both early and late genes. The early gene reporter plasmid for ORF57 (57pLuc) was a kind gift from S. Speck (Emory University) (12), and the late gene reporters for ORF65 (pORI/ORF65 promoter-LUC) and ORF26 (pORI/ORF26 promoter-LUC) were described previously (3). BHK-21 cells were transfected individually with each reporter plasmid and then subsequently infected with WT or 24S virus. PAA treatment was included to assay the dependence on DNA replication. The early ORF57 promoter was activated several hundredfold in both infections, and this activation was not dependent on DNA replication (Fig. 4A). The late gene promoters (ORF26 and ORF65) were activated several hundredfold in WT infection but minimally induced by 24S infection (Fig. 4B and C). The activation of the late gene promoters was dependent on DNA replication as well as ORF24. The promoter assay along with Northern analysis of viral transcripts support that ORF24 is essential for the transcription of only late genes and not early genes.
FIG. 4.
Activation of viral promoters. The viral promoter of ORF57 (A), ORF26 (B), or ORF65 (C) driving firefly luciferase reporter constructs was transfected into BHK-21 cells. Twenty-four hours posttransfection, the cells were infected with WT or 24S virus in the presence (+) or absence (−) of PAA. At 24 hpi, cells were lysed, and the luciferase activity was measured. The values were normalized against Renilla luciferase internal control, and activation (n-fold) was calculated by comparing the normalized values of infection to those obtained from uninfected samples.
In summary, we have identified an MHV-68 protein, ORF24, that is essential for the expression of viral late proteins yet dispensable for viral DNA replication. Given that ORF24 is involved in the late stages of viral replication, previous assessments of ORF24 as being an early gene are further supported. Although many groups examined the requirements for late gene expression of herpesviruses, the mechanism of its regulation is still not clear (2, 5, 9, 11, 14, 16). Our data demonstrate that the expression of late proteins can be controlled at a stage after viral DNA replication. Furthermore, we have shown that ORF24 functions to facilitate late gene expression at the level of transcription initiation or elongation. This differs from other herpesviral proteins, such as herpes simplex virus type 1 ICP22 and human cytomegalovirus IE2 40, which have been demonstrated to be involved in late gene expression at the level of mRNA stabilization and protein translation, respectively (15, 24). Since ORF24 does not have any obvious DNA binding domain, we speculate that it might interact with another viral or cellular protein(s) to regulate gene expression. Identifying such proteins will be critical for studying the mechanism of viral late gene regulation.
Acknowledgments
We thank Vaithi Arumugaswami for the critical reading of the manuscript.
This work was partially supported by NIH grants CA91791, DE14153, and DE15752 and the Stop Cancer Foundation.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Ahn, J. W., K. L. Powell, P. Kellam, and D. G. Alber. 2002. Gammaherpesvirus lytic gene expression as characterized by DNA array. J. Virol. 76:6244-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amon, W., U. K. Binne, H. Bryant, P. J. Jenkins, C. E. Karstegl, and P. J. Farrell. 2004. Lytic cycle gene regulation of Epstein-Barr virus. J. Virol. 78:13460-13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugaswami, V., T.-T. Wu, D. Martinez-Guzman, Q. Jia, H. Deng, N. Reyes, and R. Sun. 2006. ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus. J. Virol. 80:9730-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, J., and D. Ganem. 2000. On the control of late gene expression in Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8). J. Gen. Virol. 81:2039-2047. [DOI] [PubMed] [Google Scholar]
- 6.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 7.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi, B., B. M. Dutia, K. L. Roberts, J. J. Garcia-Ramirez, P. Dickinson, J. P. Stewart, P. Ghazal, D. J. Roy, and A. A. Nash. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 84:99-109. [DOI] [PubMed] [Google Scholar]
- 9.Gao, M., and D. M. Knipe. 1991. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J. Virol. 65:2666-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia, Q., T. T. Wu, H. I. Liao, V. Chernishof, and R. Sun. 2004. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J. Virol. 78:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler, C. P., J. A. Kerry, M. Carter, V. P. Muzithras, T. R. Jones, and R. M. Stenberg. 1994. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J. Virol. 68:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, S., I. V. Pavlova, H. W. Virgin IV, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavromara-Nazos, P., and B. Roizman. 1987. Activation of herpes simplex virus 1 gamma 2 genes by viral DNA replication. Virology 161:593-598. [DOI] [PubMed] [Google Scholar]
- 15.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serio, T. R., J. L. Kolman, and G. Miller. 1997. Late gene expression from the Epstein-Barr virus BcLF1 and BFRF3 promoters does not require DNA replication in cis. J. Virol. 71:8726-8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 18.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song, M. J., S. Hwang, W. H. Wong, T. T. Wu, S. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. USA 102:3805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 21.Stewart, J. P., N. Micali, E. J. Usherwood, L. Bonina, and A. A. Nash. 1999. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus-induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine 17:152-157. [DOI] [PubMed] [Google Scholar]
- 22.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 23.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White, E. A., C. J. Del Rosario, R. L. Sanders, and D. H. Spector. 2007. The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus. J. Virol. 81:2573-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]