Abstract
The assembly of herpesvirus remains incompletely defined due to the structural complexity of these viruses. Although the assembly of the capsid of these large DNA viruses is well studied and reasonably well conserved for all members of this diverse family of viruses, the cytoplasmic processes of tegumentation and envelopment are not well understood. The virion of the largest human herpesvirus, human cytomegalovirus (HCMV), contains over 70 virus-encoded proteins that are incorporated during a nuclear and cytoplasmic phase of assembly. Envelopment of this virus requires the function of at least one tegument protein, pp28, the product of the UL99 open reading frame. However, the role of pp28 in the envelopment of HCMV remains undefined. We have generated a pp28 mutant virus that encodes only the first 50 amino acids (aa) of this 190-aa virion protein. This virus is replication impaired and is defective in virus assembly. Characterization of both intracellular and extracellular virions from cells infected with this viral mutant indicated that the decrease in production of infectious virus was secondary to a defect in envelopment and the accumulation of tegumented, noninfectious intracellular particles. Image analysis using fluorescence recovery after photobleaching indicated that the pp28 mutant protein encoded by this virus failed to efficiently accumulate in the virus assembly compartment (AC). Our results suggest that pp28 must accumulate in the AC for efficient envelopment of the particle and provide evidence for a direct role of this tegument protein in the late stages of assembly, such as envelopment.
Infection with human cytomegalovirus (HCMV) has been associated with acute and chronic disease in both healthy and immunocompromised populations (7, 21, 28). Although a considerable body of knowledge about HCMV replication has been gathered, particularly about the regulation of viral gene expression and interactions with cellular transcription programs during infection, a similarly detailed description of the assembly of HCMV has not been accomplished. Like more well described alphaherpesviruses such as herpes simplex virus (HSV) and pseudorabies virus (PRV), DNA replication, encapsidation, and capsid assembly take place in the nuclei of infected cells (19). The processes of nuclear egress, cytoplasmic envelopment, and virion release from the infected cell have not been completely defined for HSV or PRV, and these processes remain even less well defined for HCMV because of the increased structural complexity of this prototypic member of the betaherpesvirus family (2, 11-13, 16, 18, 19, 30). Moreover, the infectious particle of HCMV contains proteins that have no homologous proteins in HSV or PRV and, based on the eclipse period in permissive cells, is assembled with significantly delayed kinetics compared to either HSV or PRV (8, 19). Additional findings highlight the potential differences in the assembly pathways of HCMV and HSV or PRV, including the presence of tegumented particles in the nuclei of HCMV-infected cells (27). Furthermore, several tegument and envelope proteins that have been shown to be dispensable for the assembly of infectious HSV or PRV particles have homologous proteins in HCMV that are essential for the assembly of infectious virus (5, 15, 17, 18, 24, 26, 27). More recent studies have also argued that HCMV could be enveloped in a different cellular compartment than has been proposed for HSV or PRV (10, 18). These observations have led investigators to postulate that the cytoplasmic assembly of HCMV could share some features of the pathways proposed for HSV but also differs fundamentally in many aspects of virus assembly.
Several approaches for the study of HCMV have been reported, but the most informative has been the characterization of viral mutants that exhibit phenotypic defects in assembly. Utilizing genetic systems introduced by Messerle and Koszinowski, several laboratories have described the phenotypes of viral mutants with defined mutations in single open reading frames (ORFs) (4, 5, 24, 26). The deletion of essential envelope glycoproteins such as glycoprotein B (gB), gH, or gM resulted in the loss of virus infectivity (14, 17). Similarly, the deletion of capsid proteins also prevented the recovery of infectious virus (14). In contrast to these findings, viral mutants with deletions of tegument proteins have exhibited more-variable phenotypes in terms of virus replication (9, 32). One such tegument protein that has been studied by several laboratories is pp28, the product of the UL99 ORF. This protein has been shown to be essential for the production of infectious virus, and deletion of this ORF resulted in a viral mutant that could not assemble enveloped particles (5, 15, 26). A more recent report has described the intercellular spread of pp28-negative virions, although the relevance of this finding to the in vivo behavior of HCMV is unclear at this time (25). pp28 is a true late protein that is membrane linked by myristoylation and by palmitoylation (W. J. Britt, unpublished data) and is localized to the cytoplasmic compartment designated the virus assembly compartment (AC) (22, 23). This compartment is thought to be a modified compartment of the cellular secretory system and distal to the trans-Golgi network (TGN), possibly a compartment derived from late endosomes (22, 24). Localization of pp28 to the AC is required for virus assembly, and mutations that impair its localization in this compartment also limit the production of infectious virus (24). Two different studies have shown that only the first 60 amino acids (aa) of this 190-aa phosphoprotein are required for the generation of viruses that exhibit a phenotype of wild-type virus replication (15, 24). Furthermore, these same studies demonstrated that infectious virus was not recovered following the deletion of a 16-aa cluster of acidic amino acids between aa 44 and 59, suggesting that this stretch of amino acids is essential for the assembly of infectious virus (15, 24). The role of this acidic cluster in the function of pp28 in the assembly of infectious HCMV is unknown, although potential roles in the interactions with cellular adaptor proteins have been proposed (15). Regardless of the contribution of this domain of pp28 to its function in virus assembly, pp28 is an essential structural protein, and thus far, available data point to its critical role in the envelopment of the infectious particle.
To further define the role of pp28 in the assembly of HCMV, we have characterized the phenotype of a replication-impaired virus mutant generated by the insertion of a translational stop codon at nucleotide position 151, resulting in a virus encoding only the first 50 aa of pp28. The phenotype of this mutant virus, pp28STOP50, was compared to that of a previously described viral mutant (pp28STOP61) that has been shown to exhibit a wild-type phenotype in terms of virus replication, localization of the pp28 mutant protein to the AC, and assembly of infectious enveloped particles (24). The phenotype of the pp28STOP50 viral mutant was particularly informative because, although replication impaired, this mutant produced infectious particles that could be characterized during assembly without the necessity of complementation, such as the expression of pp28 in trans. Our results indicated that cells infected with the pp28STOP50 mutant virus assembled intracellular particles that contained similar quantities of viral DNA, the virus major capsid protein (MCP), and at least one viral tegument protein, yet were significantly less infectious than particles produced by cells infected with the pp28STOP61 virus. Additional studies, including electron microscopy (EM) and quantitation of a major envelope glycoprotein, gB, in particles produced by the pp28STOP50 virus-infected cells, indicated that the majority of intracellular particles produced by this mutant virus were nonenveloped and, thus, presumably noninfectious. The pp28 mutant protein encoded by this virus appeared to contribute to this loss of envelopment by failing to accumulate in the AC of infected cells, supporting the argument that the role of pp28 in envelopment is expressed after its localization in the AC. Finally, our results also suggested that the incorporation of a threshold amount of pp28 during assembly is sufficient for the envelopment of an infectious particle and, in turn, for the release into the extracellular supernatant.
MATERIALS AND METHODS
Cells and viruses.
Human primary fibroblasts (HF) were prepared and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% newborn calf serum and penicillin and streptomycin antibiotics as described previously (24). Wild-type virus (strain AD169) was obtained by electroporation of the infectious clone HB-5 maintained in Escherichia coli into HF. This bacterial artificial chromosome (BAC) was obtained from Martin Messerle and Ulrich Koszinowski (University of Munich, Germany) and was used to generate all recombinant viruses in this study (4). The recombinant viruses pp28STOP61 and pp28STOP50 have been described previously and were constructed by the insertion of a translational stop codon at nucleotide positions 184 and 151, respectively (24).
Virus infections, infectivity titrations, and real-time PCR assays.
Monolayers of HF in 35-mm dishes were infected with a designated multiplicity of infection (MOI) for 1 h with gentle rocking, washed, and then incubated at 37°C and 5% CO2. Contents of dishes were harvested daily and either separated into cells and supernatant or frozen at −80°C until titrated for infectivity. Results are expressed as infectious units/ml. Titrations were carried out as described previously (1). Briefly, 0.2 ml of 10-fold dilutions was seeded in replicates (2, 3) in the wells of a 96-well flat-bottom plate containing HF monolayers. After a 1-h incubation, the wells were washed and the plate was incubated for 16 h. The plate was then washed with phosphate-buffered salt solution (PBS; pH 7.4) and then fixed in absolute ethanol for 20 min. The plate was then washed, a monoclonal antibody (MAb) reactive with the IE-1 protein was added, and reactivity was developed with fluorescein isothiocyanate anti-mouse immunoglobulin G antibodies (1). The number of fluorescent nuclei was counted, and results were expressed as infectious units per ml of input virus. Quantitation of the viral genome copy number was carried out using real-time PCR, as we have described previously, using UL55 as the target template (24).
EM analysis.
HF were infected with the wild-type, the pp28STOP61, or the pp28STOP50 virus at an MOI of 0.1 and processed for EM on day 5 postinfection. Cells were collected from the culture plate in 1 ml of PBS and centrifuged into pellets. The resulting pellets were then rinsed with 0.1 M PBS and fixed with 2.5% glutaraldehyde in PBS for 1 h at room temperature. After being rinsed in 0.1 M PBS three times, pellets were postfixed in 1% osmium tetroxide for 1 h at 4°C in the dark. These pellets were rinsed with 0.1 M PBS three times and with water once more. The pellets were then dehydrated in a graded series of ethanol solutions beginning with 25% and ending with 100% and embedded on a copper grid. Approximately 90-nm sections of the pellets were stained with 1% uranyl acetate and lead citrate and examined with a Hitachi 7000 series EM at an acceleration voltage of 75 kV.
Gradient separation of extracellular and intracellular virions.
Extracellular and intracellular virions were purified by centrifugation through sorbitol gradients as previously described (6). Briefly, supernatant virus was isolated by initially clarifying the supernatant by centrifugation at 3,000 × g for 15 min. Virions were pelleted by centrifugation of the clarified supernatant through a 20% sucrose cushion at 25,000 rpm in an SW28 rotor for 1 h. The pellet was then resuspended in 1 ml of Tris-buffered saline (pH 7.4) and applied to a preformed 20 to 70% sorbitol gradient (10 ml) and centrifuged at 27,000 rpm in an SW41 rotor for 1 h at 16°C. Intracellular particles were prepared from postnuclear supernatants of the same cultures of virus-infected cells by passing cells repeatedly through a 25-gauge needle followed by clarification of the cell lysate at 6,000 rpm for 10 min. This material was loaded directly onto preformed 20 to 70% sorbitol gradients and centrifuged as described above. The gradients were fractionated into 1-ml fractions from the bottom by pumping mineral oil onto the top of the gradient. The infectivity titers of the fractions were determined, and the fractions were analyzed for viral DNA by real-time PCR. Subsequently, individual fractions were analyzed for viral proteins by Western blotting as described below.
Antibodies and Western blotting.
MAbs used in this study include anti-MCP MAb 28-4, anti-pp28 MAb 41-18, anti-gB MAb 7-17, anti-gM MAb IMP, and anti-pp65 MAb 28-19 (22). Samples were analyzed by Western blotting as described in previous publications and developed using enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL).
Imaging and FRAP analysis.
Immunofluorescence assays of viral protein expression in virus-infected cells was carried out as previously described (24). Fluorescence recovery after photobleaching (FRAP) analysis was performed at day 6 postinfection in live HF in which enhanced green fluorescent protein (EGFP)-tagged pp28 protein was expressed following electroporation of a plasmid encoding the first 35 aa, 50 aa, or 61 aa of pp28 or the full length of the pp28 molecule fused at the C terminus to EGFP. Electroporated HF were infected with HCMV 48 h later at anMOI of 0.1. The infected HF were grown on a 13-mm glass coverslip at 37°C in DMEM containing 10% fetal calf serum. At day 6 postinfection, the infected live HF on a coverslip were washed with 25 mM HEPES-buffered phenol red-free DMEM, and each coverslip was mounted on a slide. A drop of this HEPES-buffered medium was added directly to the well created by a rubber gasket (Molecular Probes) on the slide. The coverslip with live cells was sealed onto the slide. Photobleaching was performed on a confocal laser scanning microscope (Leica SP2). Cells expressing EGFP fusion proteins in moderate amounts, i.e., with a sufficiently high fluorescence above background levels, were monitored using the 488 laser line of the argon laser at 25% power and bleached at 100% laser power. For AC bleaching or cytoplasm (except nucleus and AC) bleaching, the regions of interest were bleached with three subsequent bleach frames. The images were taken at a rate of 5 frames at 20-s intervals for prebleaching, 3 frames at 2.9-s intervals for bleaching, and 40 frames at 30-s intervals for postbleaching. The recovered fluorescence intensity in the region of interest was measured and normalized. The intensity in the prebleaching image was set at 100%, and the first postbleaching image was set as time point 0. The average fluorescence intensity was plotted over time. The recovery curves shown in Fig. 7 are the mean values and standard errors of the recovery curves for at least five cells (or three cells for cytoplasm bleaching experiments) and are representative of two independent experiments.
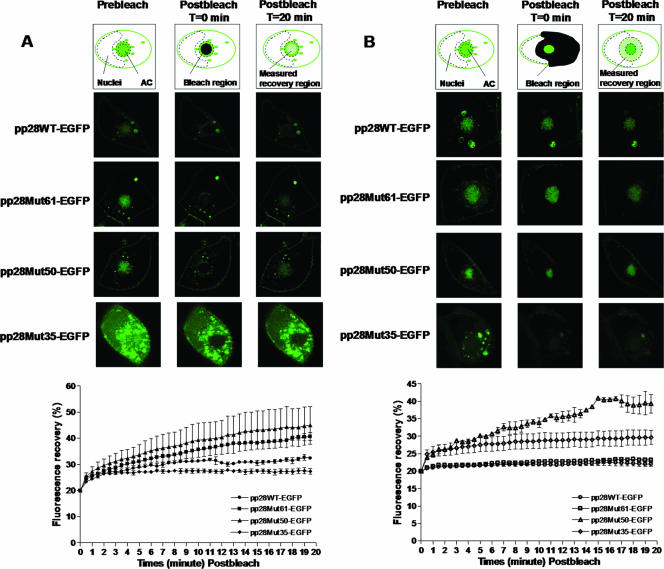
FIG. 7.
FRAP analysis of accumulation of pp28 wild type, pp28Mut61, pp28Mut50, and pp28Mut35-EGFP in the AC. HF were transfected with pp28WT-EGFP, pp28Mut61-EGFP, pp28Mut50-EGFP, or pp28Mut35-EGFP, followed by infection with HCMV at an MOI of 0.1 48 h later. At day 6 postinfection, the infected live HF were subjected to FRAP analysis as described in Materials and Methods. Illustrations at the tops of the images show the areas of FRAP, a cell prior to bleaching (left), a region selectively bleached (middle, black portion), and a measured recovery region over time (right, green-hatched portion). (A) Localization of pp28WT-EGFP, pp28Mut61-EGFP, pp28Mut50-EGFP, and pp28Mut35-EGFP to the AC. FRAP was performed using the entire AC of infected cells (n = 5). At time zero postbleaching, the fluorescence in the bleached region was normalized to 20% of the prebleaching fluorescence. Fluorescence recovery was monitored, and results were plotted over time. (B) Movement of pp28WT-EGFP, pp28Mut61-EGFP, pp28Mut50-EGFP, and pp28Mut35-EGFP out of the AC. FRAP analysis was performed with the cytoplasm of infected cells (n = 3). Fluorescence recovery was monitored, and the results were plotted over time as described above. Error bars represent standard errors of the means.
RESULTS
A replication-competent HCMV expressing only the first 50 aa of pp28 has delayed replication kinetics.
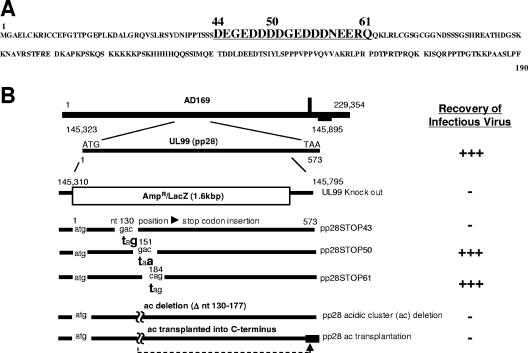
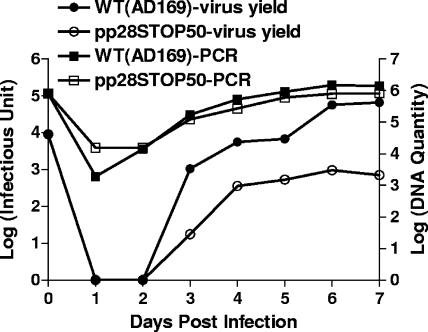
Previous studies from our laboratory and other laboratories have reported that a stretch of acidic amino acids between aa 44 and 59 of pp28 was required for the function of pp28 in the assembly of infectious virus (Fig. 1) (15, 24). In addition, the function of this cluster of acidic amino acids was context dependent in that the deletion of this cluster of acidic amino acids and the transplantation to the C terminus of pp28 failed to restore the function of the protein (24). To further define the function of this acidic domain in pp28, we generated three recombinant viruses containing translational stop codons at aa positions 44, 51, and 62 (Fig. 1). As reported previously, infectious virus could be recovered from the pp28STOP61 and the pp28STOP50 HCMV BAC but not from the pp28STOP43 BAC (24). Although infectious virus could be recovered from the pp28STOP50 BAC, the replication kinetics of this recombinant virus was delayed compared to those of both the wild-type virus and the pp28STOP61 virus, a mutant virus that replicates with kinetics similar to that of the wild-type parent virus (24). Comparison of the replication kinetics of the pp28STOP50 and pp28STOP61 viruses at low MOIs revealed that the pp28STOP50 virus-infected cells produced about 2 logs less virus than that produced by cells infected with the pp28STOP61 virus (Fig. 2). Similar levels of viral genome replication were observed in cells infected with the pp28STOP50 and pp28STOP61 viruses, indicating that the decrease in virus yield was not secondary to a defect in genome replication (Fig. 2). At higher MOIs (3-5), the differences in virus yields between the pp28STOP50 and the pp28STOP61 viruses remained but were less apparent than those previously reported for infections at low MOIs (24).
FIG. 1.
Amino acid sequence of pp28 and diagrammatic representation of recombinant HCMV BACs constructed for the generation of mutant viruses. (A) Amino acid sequence of pp28 with aa 44 to 61 shown in larger, underlined type. The 16-aa acidic cluster is contained within this sequence. (B) The structure of the HCMV genome with the UL99 ORF (nucleotides [nt] 145310 to 145795) shown in greater detail. The two viral mutants characterized in this report, the pp28STOP61 and pp28STOP50 mutants, have been previously described with the locations of the translational stop codons indicated (24).
FIG. 2.
Virus yield and viral DNA replication of HF infected with wild-type (WT) and pp28STOP50 viruses (MOI, 0.01). Cells and supernatants from 35-mm dishes infected with the wild type and the pp28STOP50 virus were combined, and titers were determined as described in Materials and Methods. Viral genome copy numbers were determined by real-time PCR as previously described and reported as a log of the copy number (24).
The defect in the replication of the pp28STOP50 virus is not related to virus entry or spread.
To further investigate the role of pp28 in the replication and assembly of HCMV, we characterized the replication phenotype of the pp28STOP50 virus. Initially, we determined whether the pp28STOP50 virus had a deficit in virus entry and/or spread within a monolayer of permissive HF. An assay of virus entry revealed that the pp28STOP50 virus entered HF as efficiently as the wild-type and the pp28STOP61 viruses, suggesting that the replication defect in the pp28STOP50 virus was not secondary to a defect in the incorporation of a protein(s) essential for virus attachment and entry (Fig. 3). Furthermore, we also analyzed the capacity of the pp28STOP50 virus to spread between cells in a monolayer of HF maintained under an agarose overlay. This assay allowed us to measure the expansion of an infectious center as a function of time and thus provided quantitation of the capacity of these viruses to spread within a monolayer, independently of extracellular virus yield. We found no differences in the rates of spread of the pp28STOP50 and the wild-type viruses, again suggesting that the defect in virus production associated with the pp28STOP50 virus was not directly related to virus entry or spread between cells (Fig. 4). Our previous findings indicated that the pp28STOP50 virus replicated levels of viral genome similar to those of the wild-type parental virus; thus, it was unlikely that the decrease in virus yield from cultures infected with the pp28STOP50 virus was secondary to a defect in viral DNA replication (Fig. 2). Taken together, the results of these experiments suggest that the deficit(s) in infectious virus production in cells infected with the pp28STOP50 virus was likely related to a defect(s) in virus assembly and/or virus release.
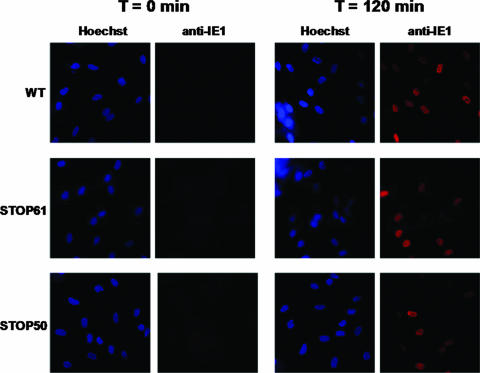
FIG. 3.
Virus entry of wild-type (WT), pp28STOP61, and pp28STOP50 viruses in HF. Monolayers of HF on 13-mm coverslips were infected at 4°C with wild-type, pp28STOP61, and pp28STOP50 viruses at an MOI of approximately 5. Following an incubation at 4°C for 90 min, the cultures were shifted to 37°C for 2 h. Coverslips were fixed at 0 min and 2 h, and then the cells were assayed for expression of IE-1 antigen by using MAb p63-27 developed with a Texas Red-conjugated anti-mouse immunoglobulin G (red). Nuclei were stained with Hoechst dye (blue).
FIG. 4.
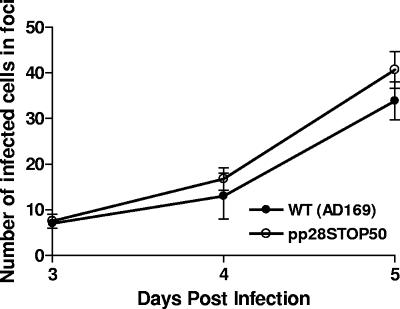
Intercellular spread of the wild type (WT) and the pp28STOP50 virus. Monolayers of HF on 13-mm coverslips were infected with the wild type or the pp28STOP50 virus at an MOI of 0.1 and overlaid with agarose to prevent extracellular virus spread. The monolayers were fixed on days 3, 4, and 6 postinfection and then stained with MAb p63-27 to detect IE-1 expression. The number of nuclei per individual focus was counted. Approximately 20 foci per time point (and virus) were counted, and the means and standard errors are shown at the various time points.
The defect in the replication of the pp28STOP50 virus is associated with a decrease in the production of enveloped virions.
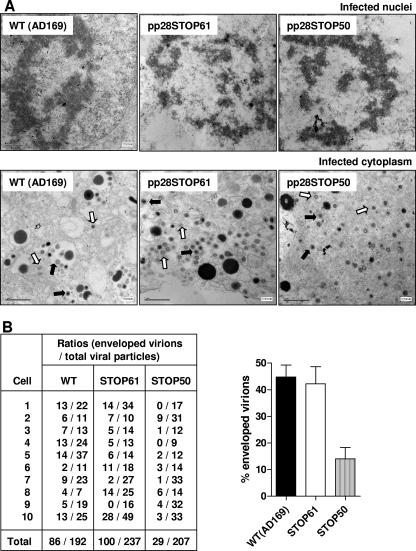
The assembly of the pp28STOP50, pp28STOP61, and wild-type viruses was investigated utilizing several assays. Initially, the assembly of enveloped virions was studied by EM. HF were infected with wild-type, pp28STOP61, and pp28STOP50 viruses at similar MOIs and fixed on day 5 postinfection. The numbers of cytoplasmic capsids, tegumented capsids, and enveloped particles were counted in multiple frames and multiple cells within each frame (Fig. 5A). The ratio of enveloped particles to the total number of viral particles indicated that about 45% of wild-type and pp28STOP61 viral cytoplasmic particles appear to have an envelope (Fig. 5B). In contrast, using the same criteria that were used to identify enveloped particles in the micrographs of the wild-type and pp28STOP61 virus-infected cells, we determined that <15% of particles in cells infected with the pp28STOP50 virus were enveloped (Fig. 5B). In all cases, similar numbers of particles could be observed in the nuclei of infected cells. This result suggested that the defect in the pp28STOP50 virus was associated with a decrease in the production of enveloped particles in the cytoplasm of infected cells.
FIG. 5.
EM analysis of virus-infected cells and estimation of frequency of enveloped particles in cytoplasm of infected cells. Monolayers of HF were infected with wild-type (WT), pp28STOP61, and pp28STOP50 viruses at an MOI of 0.1 and processed for EM at 5 days postinfection. (A) Multiple frames from each sample were imaged and photographed. Nuclear (top row) and cytoplasmic (bottom row) particles from a representative cell are shown at final magnifications of ×30,000 and ×20,000, respectively. Open and filled arrows indicate nonenveloped particles and enveloped particles, respectively. (B) The numbers of enveloped and nonenveloped particles were counted in each frame and tabulated as a ratio of enveloped particles to total particles in the cytoplasm of infected cells (n = 10). There was no significant difference between the ratios of enveloped particles to total particles for the wild type and the pp28STOP61 mutant virus (P > 0.5), whereas the pp28STOP50 virus-infected cells contained a smaller ratio of enveloped particles to total particles than either the wild-type (P < 0.001) or the pp28STOP61 (P < 0.001) virus-infected cells. Paired, one-tailed t tests were used to analyze the ratios of enveloped particles to total particles for the wild-type, pp28STOP61, and pp28STOP50 viruses.
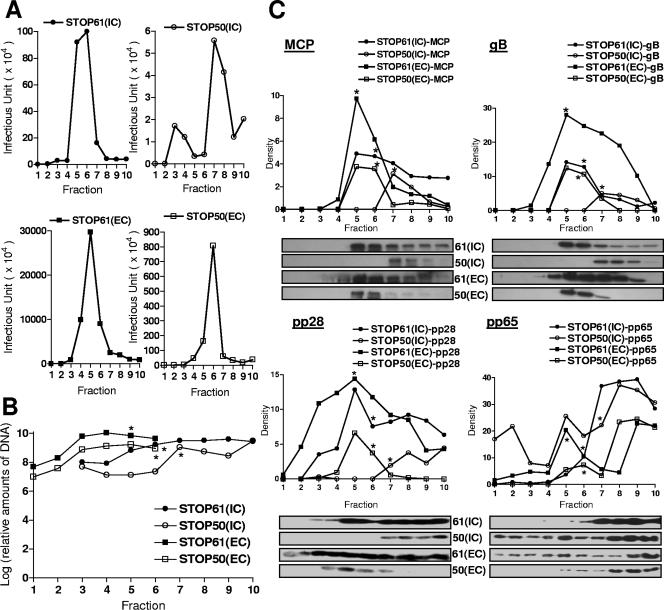
These results were confirmed using a second methodology. Virions were gradient purified from HF that had been infected at MOIs similar to those of the pp28STOP61 and pp28STOP50 viruses. In these experiments, we used the pp28STOP61 virus instead of the wild-type virus for several reasons, including (i) the finding that the pp28STOP61 virus replicated to levels similar to those of the wild-type virus, (ii) the in vitro replication phenotype of the pp28STOP61 virus was indistinguishable from that of the wild-type virus, and (iii) the pp28STOP61 virus allowed us to study the role of aa 44 to 59 of pp28 in virion assembly without the introduction of the additional structural complexity of aa 62 to 190 in the wild-type virus. Fractionation of the gradients revealed that infectious extracellular virus in cells infected with either the pp28STOP61 or the pp28STOP50 virus exhibited similar densities and that peak infectivity could be found midway down the gradient, within one fraction of one another (Fig. 6A). The differences in the levels of infectivity in these fractions was approximately 1.5 logs (Table 1), a value that was within the range of differences in viral DNA copy numbers determined for these fractions (Fig. 6B and Table 1). Thus, in this experiment, it appeared that the pp28STOP50 virus-infected HF produced infectious virions that banded with similar densities following ultracentrifugation but that the quantity of extracellular infectious particles and viral DNA-containing particles was reduced in cells infected with the pp28STOP50 virus compared to cells infected with the pp28STOP61 virus (Fig. 6A and B). Consistent with these results, total extracellular viral particles derived from HF that had been infected with the pp28STOP61 and pp28STOP50 viruses prior to application to the gradient had similar differences in the levels of infectivity (1.3 logs) and viral DNA copy numbers (l log) (Table 1). These results suggest that the decrease in infectious virus produced by cells infected with the pp28STOP50 virus could be explained by the production of fewer infectious extracellular particles and was not the result of the production of a similar number of particles but with a decreased infectivity of the individual particles.
FIG. 6.
Gradient separation and analysis of extracellular and intracellular viruses from pp28STOP61- and pp28STOP50-infected cells. (A) Intracellular (IC) and extracellular (EC) particles were prepared as described in Materials and Methods and fractionated on 20 to 70% sorbitol gradients as described previously (6). The infectivity titers of gradient fractions (1 ml) collected from the bottom of density gradients were determined as described in Materials and Methods. (B) Real-time PCR of viral genome copy numbers in fractions from gradients. Fifty microliters of each fraction was extracted using column technology (QIAGEN blood PCR kit; QIAGEN, Valencia, CA) and assayed by real-time PCR as described in Materials and Methods. Results are expressed as genome copy number per fraction. (C) Relative expression of MCP, gB, pp28, and pp65 in each fraction as detected by Western blotting with specific MAbs. Fifty microliters of each fraction was loaded on 12% polyacrylamide gels and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting as described in Materials and Methods. Following development with enhanced chemiluminescence, densitometry was carried out and the relative signal for each lane was determined. Note that densitometry was done only with fluorograms acquired during the same experiment and developed for the same time interval. The fluorograms shown for pp28 and pp65 were processed in Adobe Photoshop to remove nonspecific background signals assumed to be secondary to nonspecific binding of primary antibodies; however, densitometry was performed prior to image processing to remove background. The pp65- and pp28-specific bands were not processed. *, gradient fraction with peak infectivity.
TABLE 1.
Estimations of the levels of viral proteins, viral DNA, and infectivity in extracellular and intracellular virion particles produced in cells infected with the pp28STOP61 or pp28STOP50 viruses
| Virus and domaina | MCPb | gB | pp28 | pp65 | gB/MCP ratioc | pp28/MCP ratioc | pp65/MCP ratioc | DNA copy numberd | Infectivity leveld |
|---|---|---|---|---|---|---|---|---|---|
| pp28STOP61 | |||||||||
| IC (6) | 4.67 | 12.75 | 7.56 | 10.27 | 2.7 (0.05) | 1.6 (0.02) | 2.2 (0.10) | 1.7 × 109 (9.22) | 6.00 |
| EC (5) | 9.74 | 27.96 | 14.40 | 20.36 | 3.1 (0.10) | 1.58 (0.08) | 2.14 (0.14) | 6.9 × 109 (9.84) | 8.47 |
| pp28STOP50 | |||||||||
| IC (7) | 3.13 | 5.00 | 1.95 | 22.10 | 1.64 (0.07) | 0.65 (0.06) | 6.92 (0.23) | 1.1 × 109 (9.04) | 4.75 |
| EC (6) | 3.56 | 10.59 | 3.74 | 7.23 | 3.06 (0.07) | 1.04 (0.01) | 1.93 (0.05) | 8.9 × 108 (8.95) | 6.91 |
The numbers in parentheses represent fractions containing peak infectivity. IC, intracellular; EC, extracellular.
The densities of the corresponding proteins in peak fractions from Fig. 6C are shown.
The ratios of envelope (gB), tegument (pp28), and tegument (pp65) proteins to MCP were calculated for the peak fractions of infectivity. Values represent the means (standard errors) of the results of three to four independent experiments. Results of a single representative experiment are shown in Fig. 6C.
The viral DNA copy numbers in parentheses and infectivity levels are represented in log scale for the peak fractions of infectivity. Note that prior to application to the gradients, the total numbers of extracellular viral particles from HF (as analyzed in clarified supernatant) that had been infected with the pp28STOP61 and pp28STOP50 viruses differed by comparable levels of infectivity (1.3 logs) and viral DNA copy number (l log).
Somewhat different results were noted when intracellular viruses were analyzed following purification by ultracentrifugation through density gradients. The results of these experiments demonstrated a greater disparity between the generation of infectious particles than in the replication of viral DNA in HF infected with the pp28STOP50 mutant virus and the pp28STOP61 virus (Fig. 6A and B and Table 1). This result raised the possibility that similar numbers of DNA-containing particles were produced in pp28STOP50 virus- and pp28STOP61 virus-infected HF but that fewer infectious particles were assembled in pp28STOP50 virus-infected cells. To address the possibility that pp28STOP50 virus-infected HF failed to assemble amounts of infectious virions similar to those of cells infected with the pp28STOP61 virus, we analyzed the quantity of several viral proteins together with the DNA copy number in gradient fractions from both the extracellular and the intracellular STOP61 and STOP50 viruses. The quantity of viral proteins was measured by Western blot analysis using specific MAbs followed by densitometry (Fig. 6C). When the DNA copy number present in particles was determined from the fractions containing infectious virus, approximately 55% of the total (intracellular plus extracellular) DNA-containing particles from pp28STOP50 virus-infected cells remained intracellular, compared to only 20% of particles from pp28STOP61 virus-infected cells (Table 1). In agreement with previous findings, this result suggested that the pp28STOP50 virus had a defect in virus assembly/release into the extracellular supernatant, unlike the pp28STOP61 virus (Table 1). The amounts of the UL86 (MCP) present in the peak fractions of intracellular particles from the two viruses were similar (pp28STOP61/pp28STOP50 ratio for MCP = 1.5) as were the copy numbers of viral DNA (pp28STOP61/pp28STOP50 ratio for DNA = 1.52), suggesting that the defect in the production of extracellular particles by pp28STOP50 virus-infected cells could not be explained by differences in the synthesis of MCP or the assembly of DNA-containing particles within the infected cells (Table 1). However, the infectivity of pp28STOP50 intracellular particles (4.75 logs) was about 1.3 logs lower than the infectivity of the pp28STOP61 particles (6.00 logs), indicating that a significant number of MCP- and DNA-containing intracellular particles produced in pp28STOP50 virus-infected HF were not infectious, perhaps as a result of being nonenveloped, as suggested by the EM findings (Table 1). This interpretation was also consistent with the observation that intracellular particles from the pp28STOP50 virus-infected cells had a gB/MCP ratio (1.64) of about 50% of the gB/MCP ratio of either intracellular or extracellular particles isolated from pp28STOP61-infected HF (2.7 and 3.10, respectively), or, more interestingly, of extracellular particles from pp28STOP50-infected HF (3.06) (Table 1). Interestingly, similar calculations from only one experiment in which the ratio of gM complex to MCP was measured revealed results that were consistent with the results from the more extensive analysis of gB. In these experiments, the gM/MCP ratios for intracellular and extracellular particles from pp28STOP61 virus-infected cells were 2.99 and 3.02, respectively, and 1.89 and 2.95 for the intracellular and extracellular particles from pp28STOP50 virus-infected cells. These results suggest that only a fraction of intracellular pp28STOP50 viral particles were enveloped (gB and gM containing) and, therefore, infectious.
The deficit in the envelopment of the pp28STOP50 mutant virus could be explained by a defect in tegumentation leading to a defect in envelopment. This possibility was examined initially by assaying the quantities of a major tegument protein, pp65, in both extracellular and intracellular particles. The amount of pp65 in intracellular particles purified from the pp28STOP50 virus-infected cells was greater than the amount detected in intracellular or extracellular particles purified from the pp28STOP61-infected cells (Fig. 6C and Table 1). Extracellular pp28STOP50 virions had decreased amounts of pp65 compared to pp28STOP61 virions, consistent with the conclusion that fewer particles were released by cells infected with the pp28STOP50 virus (Fig. 6C and Table 1). Thus, it was unlikely that the deficit in envelopment of the pp28STOP50 mutant virus was initially secondary to a defect in tegumentation. In contrast to these findings, the pp28/MCP ratios from pp28STOP61 intracellular and extracellular particles were similar. However, intracellular particles from pp28STOP50 virus-infected HF had a pp28/MCP ratio of about 50% of that of pp28STOP50 extracellular particles, indicating that lesser amounts of pp28Mut50 protein were present in the population of intracellular particles (Table 1). This result also suggested that only a fraction of intracellular particles from pp28STOP50 virus-infected HF contained the mutant form of pp28 and, therefore, were enveloped during assembly. Alternatively, the possibilities that only a threshold amount of pp28 was required for the assembly of an enveloped particle and that each particle derived from pp28STOP50-infected cells contained a decreased amount of the pp28 mutant could not be ruled out. Consistent with the latter possibility was the finding that extracellular particles from pp28STOP50 virus-infected cells had a pp28/MCP ratio that was less than that of pp28STOP61 extracellular particles, arguing the possibility that only a threshold amount of pp28 was required for the successful envelopment of an infectious particle and that each particle contained limiting amounts of the pp28 mutant protein (Table 1).
The pp28 mutant protein encoded by the pp28STOP50 virus fails to concentrate in the AC.
The pp28 protein is localized and increases in amount in the AC late in infection (22). To investigate the possibility that the deficit in the production of infectious virus by the pp28STOP50 virus-infected HF was secondary to the altered localization of the pp28STOP50 mutant to the AC or a lack of the accumulation of pp28 in the AC, we analyzed the kinetics of pp28 localization to the AC by FRAP experiments. An EGFP-tagged pp28 protein was expressed in HF following electroporation of a plasmid encoding the first 35 aa, 50 aa, or 61 aa of pp28 or the full length of the pp28 molecule, each of which was fused at its C terminus to EGFP (pp28Mut35-EGFP, pp28Mut50-EGFP, pp28Mut61-EGFP, or pp28WT-EGFP). HF were electroporated with the indicated plasmid and then infected with HCMV 48 h later at an MOI of 0.1. A FRAP assay of live infected HF was carried out 6 days postinfection. The fluorescence of pp28Mut35-EGFP, pp28Mut50-EGFP, pp28Mut61-EGFP, or pp28WT-EGFP in the prebleaching compartment was saturated late in infection (Fig. 7A). We selectively bleached the entire AC and monitored the fluorescence recovery in this region (Fig. 7A). The fluorescence in the bleached region dropped to about 20% immediately after bleaching. Thus, for these FRAP experiments, the fluorescence in the bleached region was normalized to 20% at time zero postbleaching, and the recovered fluorescence was monitored for 20 min postbleaching. For pp28WT-EGPF, the fluorescence in the bleached AC recovered to 32% (Fig. 7A). Similar values were noted for both the pp28Mut50-EGFP and pp28Mut61-EGFP proteins (45% and 41%, respectively) (Fig. 7A). These values indicated that the transport of these pp28 mutants was similar to that of the wild type and that differences between the rates of localization of pp28Mut50 and wild-type pp28 (and pp28Mut61) to the AC could not explain the defect observed in the replication of the pp28Mut50-expressing recombinant virus (pp28STOP50). In contrast to these findings, the recovery of the pp28Mut35 protein in the AC reached only 25% (5% above postbleaching levels) and then appeared to decline, suggesting that the AC was less efficiently refilled by this mutant form of pp28 than was the wild type, pp28Mut61, or pp28Mut50 (Fig. 7A). This finding was consistent with our previous results using static imaging (24).
In the next series of experiments, the accumulation of pp28, pp28Mut61, pp28Mut50, and pp28Mut35 in the AC was assayed using transfected/infected cells and FRAP as described above. In these experiments, the entire cytoplasm (except the AC and nucleus) was bleached, and the recovery of the fluorescence signal in a region of cytoplasm surrounding the AC was measured over time (Fig. 7B). The fluorescence in the measured region was normalized to 20% at time zero postbleaching. We noted significant differences in the recovery rates. The fluorescence signal in wild-type pp28-EGFP-transfected, HCMV-infected cells recovered to about 23% following photobleaching of the cytoplasm (Fig. 7B). Similarly, the recovery of the fluorescence signal from the pp28Mut61-transfected, HCMV-infected cells was 24% during the measurement interval (Fig. 7B). The analysis of the pp28Mut35 protein suggested similarly inefficient refilling of this area of the cell, presumably because there was a minimal amount of the pp28Mut35 protein in the AC available to refill the cytoplasm of these previously bleached cells (Fig. 7B). In contrast to these findings, the recovery of fluorescence in the photobleached cytoplasm of pp28Mut50-transfected, HCMV-infected cells was almost 40%, indicating that the trafficking of pp28Mut50 out of the AC was significantly increased compared to that of either the pp28Mut61 mutant or the wild-type pp28 (Fig. 7B). When the rate of movement of these proteins into and out of the AC is expressed as a ratio of recovery of fluorescence at 20 min following photobleaching to a value of 20% at time zero, these differences are more apparent. The ratios of movement into the AC to movement out of the AC for wild-type pp28 and pp28Mut61 were 1.5 and 1.7, respectively, indicating that, at steady-state kinetics, the localization of these proteins in the AC was favored (Table 2). In contrast, the ratio of movement into the AC to movement out of the AC for pp28Mut50 was 1.1, indicating that, at late times following infection, nearly equivalent amounts of pp28Mut50 were exiting the AC as were trafficking into this compartment in HF (Table 2). Moreover, the recovery pattern on the plot showed that, in contrast to the wild type and pp28Mut61, the recovered fluorescence of pp28Mut50 in the cytoplasm was not saturated during the measured time (Fig. 7B). When the same ratios were calculated for the pp28Mut35 protein, the ratio of movement into the AC to movement out of the AC was 0.9, confirming our findings with static imaging carried out at equilibrium that the majority of the pp28Mut35 protein is localized outside of the AC (Table 2). Taken together, these results were consistent with the favored kinetics for the trafficking of the pp28Mut50 out of the AC and suggested that the failure of this mutant form of pp28 to accumulate in the AC could limit its concentration in the AC and, therefore, its role in the envelopment of the virion.
TABLE 2.
Recovery kinetics of the pp28 and pp28Mut proteins in FRAP assays
| Rate type | Ratio of fluorescence recoverya
|
|||
|---|---|---|---|---|
| pp28WT | pp28Mut61 | pp28Mut50 | pp28Mut35 | |
| Recovery in AC | 1.6 | 2.0 | 2.2 | 1.4 |
| Recovery in cytoplasm | 1.1 | 1.2 | 2.0 | 1.5 |
| Accumulation in AC | 1.5 | 1.7 | 1.1 | 0.9 |
Ratios of fluorescence recovery are based on the ratio of fluorescence at 20 min to the fluorescence at 0 min. For the kinetics of accumulation in AC, i.e., localization in the AC, the ratio of fluorescence recovery in the AC to the fluorescence recovery in the cytoplasm was calculated. Note that the accumulation rate of pp28Mut35 in AC was <1.0, indicating that, at equilibrium, the majority was localized outside of the AC, whereas pp28WT had an accumulation rate of 1.5.
DISCUSSION
Although pp28 has been shown to be an essential tegument protein for the assembly of an infectious HCMV particle, its precise role in assembly has not been elucidated. Cells infected with recombinant viruses in which pp28 was deleted failed to produce enveloped particles, and the analysis of cells infected with this mutant virus complemented with a retrovirus expressing pp28 suggested that pp28 has a role in the envelopment of the particle (26). However, it was unclear whether the deletion of pp28 resulted in a loss of tegument and/or envelope assembly, and because progeny virions were not produced, the protein composition of particles produced by this recombinant virus also could not be analyzed. Although the stage at which virion assembly was blocked was not determined, this report convincingly demonstrated that pp28 has a critical role in the cytoplasmic envelopment of HCMV (26). Similarly, our results also suggested that the replication-impaired phenotype of the pp28 mutant virus, pp28STOP50, resulted from a defect in the assembly of enveloped particles. Analysis of this mutant virus was particularly informative because, although it exhibited a replication-impaired phenotype, it produced sufficient amounts of both intracellular and extracellular virus for the characterization of its assembly, including the protein composition of the particle. The results of these studies indicated that the pp28STOP50 virus was replication impaired secondary to a defect in assembly based on findings that (i) the viral genome was replicated to levels similar to those of replication-competent wild-type viruses; (ii) intracellular particles produced by the pp28STOP50 mutant contained amounts of MCP and viral DNA similar to the amounts in particles purified from the replication-competent pp28STOP61 virus; (iii) the infectivity of intracellular particles produced in pp28STOP50-infected cells was about 1.5-log lower than that of particles from pp28STOP61-infected cells, suggesting that the defect in the pp28STOP50 virus was not secondary to a block in the release of infectious virus; and (iv) extracellular virions produced by cells infected with the pp28STOP50 mutant could readily enter permissive HF. Taken together with previous findings that demonstrated that pp28 was expressed late in the replicative cycle of HCMV, these studies were consistent with earlier results that proposed a key role of pp28 in the cytoplasmic assembly of infectious HCMV (5, 15, 26).
The pp28STOP50 mutant virus expressed only 7 acidic aa of the 16-aa acidic cluster present in the 18 aa between aa 44 and 61 of the pp28STOP61 viral mutant, including the sequence GEDDD that is duplicated within this 16-aa sequence. However, the production of infectious virus was impaired in the pp28STOP50 virus mutant compared to that in the pp28STOP61 virus, indicating that the remaining stretch of acidic amino acids between aa 44 and 50 in the pp28STOP50 mutant virus was insufficient to support wild-type virus assembly. Previously, we have shown that a recombinant virus encoding a pp28 mutant protein constructed by the deletion and transplantation of the 16-aa acidic cluster (aa 44 to 59) to the carboxyl terminus of pp28 was replication incompetent (24). The finding of context dependence for the function of the acidic cluster present between aa 44 and 59 in virus raised the possibility that the acidic domain of pp28 could have functions other than simply acting as an intracellular trafficking motif that directed pp28 to a specific cellular compartment. This function was previously proposed for this stretch of acidic amino acids in pp28 based on the findings from an analysis of an acidic cluster in the amino-terminal domain of HSV homolog UL11 (16). Clusters of amino acids in the cytoplasmic domains of viral glycoproteins and also present in UL11 have been reported to function in intracellular trafficking through interactions with cellular adaptor proteins such as PACS-1 (3, 20, 29, 31). Considerable variability in the sequences of amino acids found in a number of acidic clusters has been described, and in many cases, phosphorylation of adjacent amino acids has been shown to regulate interactions with PACS-1 (3). In some cases, acidic clusters of 6 to 7 aa have been reported to be sufficient for interaction with cellular adaptor proteins, and bipartite acidic clusters have been described (3, 31). Thus, we expected that the remaining seven acidic amino acids on the carboxyl terminus of pp28STOP50 should have been sufficient to interact with cellular adaptors such as PACS-1 and to support levels of replication that were similar to those of the wild-type virus. Yet the results in the current study argued that an intact acidic cluster (16 aa) within the carboxyl terminus of the pp28STOP61 mutant virus was required for levels of wild-type virus replication. A study of the proprotein convertase PC6B described two acidic clusters in the cytoplasmic domain of this protein that functioned differently in intracellular sorting (31). The carboxyl-terminal acidic cluster of this protein interacted with PACS-1 but was not required for the intracellular localization of the protein (31). The authors of this study postulated that the interactions of PC6B with PACS-1 contributed to the intracellular localization of PC6B by directing the protein to the TGN, a step required for efficient PC6B localization that was shown to be absolutely dependent on the acidic cluster of amino acids more proximal to the amino terminus of the protein (31). Additional mutations in the remaining amino acids in pp28STOP50 could determine whether the contiguous sequence of acidic amino acids between aa 44 and 59 of pp28 functions as a bipartite sorting sequence similar to the acidic amino acids in PC6B. In addition, additional mutations in the remaining acidic amino acids at positions 44 to 50 could identify the essential amino acids required for wild-type replication and perhaps provide clues to the role of this domain in pp28 in virus assembly.
Characterization of the assembly of infectious virions provided evidence consistent with a defect in the envelopment of the pp28STOP50 recombinant virus compared to that in both the wild-type virus and the pp28STOP61 mutant virus. Several findings led to this conclusion. First, EM indicated that only about 15% of intracellular particles in pp28STOP50 virus-infected cells were enveloped compared to over 40% of particles in pp28STOP61 virus-infected cells. Analysis of the genome copy number and protein composition of intracellular particles purified from pp28STOP50 virus- and pp28STOP61 virus-infected cells indicated that both viruses produced a population of particles that contained similar quantities of viral DNA and MCP. Furthermore, analysis of the intracellular particles for the tegument protein pp65 (UL83) also revealed similar amounts of this protein in intracellular particles isolated from cells infected with the two mutant viruses. In fact, intracellular particles from pp28STOP50 virus-infected cells appeared to have a greater amount of pp65 than particles from pp28STOP61 virus-infected cells, suggesting that a decrease in the acquisition of the pp28 tegument protein resulted in an accumulation of pp65 on intracellular particles, perhaps secondary to a block and/or a delay in the normal assembly pathway. Taken together, these findings suggested that the defect in the pp28STOP50 mutant virus was not associated with a generalized defect in the production of DNA-containing particles or capsids or in the tegumentation of cytoplasmic particles but rather with a defect in a late step of assembly, such as envelopment, of the infectious particle. A formal particle count was not carried out, but under identical conditions of gradient purification, similar viral DNA copy numbers and amounts of MCP were detected in the peak gradient fractions for both viruses, thus arguing that cells infected with either virus produced similar numbers of capsid-containing particles and that these particles also contained similar amounts of viral DNA. However, the intracellular particles purified from cells infected by these viruses differed significantly in level of infectivity. Cells infected with the pp28STOP61 virus produced approximately 1.3 log more infectious intracellular particles than cells infected with the pp28STOP50 mutant virus, a finding consistent with the results from EM that suggested that only about 15% of intracellular particles in cells infected with the pp28STOP50 mutant virus were enveloped. In addition, intracellular particles purified from pp28STOP50 virus-infected cells contained lesser amounts of the envelope gB (and gM) relative to the MCP than intracellular particles purified from pp28STOP61 virus-infected cells. Interestingly, the ratios of gB and gM to MCP were similar for both intracellular and extracellular particles from pp28STOP61 virus-infected cells and from extracellular particles isolated from the supernatant of pp28STOP50 virus-infected cells, suggesting that a relatively fixed stoichiometry of gB (gM) and MCP in particles was associated with the infectivity of HCMV virions. The lower gB/MCP (and gM/MCP) ratios in intracellular particles isolated from pp28STOP50 virus-infected cells suggested either that all particles in this population contained decreased amounts of gB (gM) or, more likely, that only a minor population of particles contained gB (gM) in amounts similar to those in particles from pp28STOP61 virus-infected cells and that the remaining particles were not completely enveloped. Thus, these findings were consistent with the production of nearly equivalent numbers of intracellular particles by cells infected with the pp28STOP50 virus and cells infected with the pp28STOP61 virus but a decrease in the production of infectious intracellular particles as well as a decrease in the number of infectious particles released into the extracellular supernatant. When the percentages of total infectious particles present intracellularly are compared for these two viruses, this defect was also apparent. Of the total amount of infectious virus produced late in infection, approximately 55% of that produced by cells infected with the pp28STOP50 virus remained inside the cells, whereas less than 20% of infectious virus produced by the pp28STOP61 virus was present in the cell. Taken together, our findings were most consistent with a defect in the envelopment of the pp28STOP50 virus and also raised the possibility that during infection in HF, HCMV envelopment and virus release into the supernatant could be linked, i.e., defects in envelopment appeared to reduce the efficiency of virus release from the cell.
Interestingly, the defect that resulted in the impaired replication of the pp28STOP50 virus did not result in a complete block in the production of infectious virus, suggesting that a fraction of particles contained adequate amounts of the pp28Mut50 mutant protein to support the envelopment and release of infectious particles into the extracellular media. As noted above, this conclusion was supported by the finding that infectious particles isolated from the extracellular supernatant contained a gB/MCP ratio similar to that of extracellular virus isolated from the supernatant from cells infected with the pp28STOP61 mutant virus. However, the number of particles released from cells infected with the pp28STOP50 virus was reduced significantly based on the viral DNA content and the MCP content of extracellular particles from the gradient fraction containing the peak infectivity. Although we cannot determine the specific infectivity and protein content of individual particles within this fraction, our results are compatible with a mechanism in which the majority of intracellular particles produced in cells infected with the pp28STOP50 virus mutant fail to become enveloped and are not released from the infected cell. The minority of particles that become enveloped appear to acquire similar amounts of gB. Thus, it appeared that the defect in the assembly of the pp28STOP50 mutant virus was related to the decreased efficiency of envelopment, presumably secondary to a loss in function of the pp28Mut50 mutant protein.
The loss in function of the pp28Mut50 protein that led to decreased envelopment of intracellular particles remains undefined. A failure to localize to the AC was a possibility, based on our previous studies that demonstrated a failure of pp28 mutants expressing only aa 1 to 44 to localize in the AC (24). In cells infected with the recombinant pp28STOP50 virus, static imaging revealed that the pp28Mut50 protein localized to the AC, but less efficiently than either the pp28Mut61 protein or the wild-type pp28 (24). These results were confirmed in the current study utilizing FRAP to analyze protein trafficking in living cells. These studies indicated that the pp28Mut50 protein localized to the AC as efficiently as either the pp28Mut61 or wild-type pp28 protein; however, the pp28Mut50 protein exited the AC more than twice as rapidly as either the pp28Mut61 or wild-type pp28 protein. This finding indicated that, at steady state, only a fraction of the pp28Mut50 protein localized in the AC compared to the amount of either the pp28Mut61 or the wild-type pp28 protein, a finding consistent with previous results using static imaging (24). The decreased amount of the pp28Mut50 in the AC could in turn limit the envelopment and assembly of infectious virus, particularly if the pp28 concentration in the AC was rate limiting in the assembly of infectious particles.
Although our findings provided an explanation of the decreased production of infectious virions from pp28STOP50 mutant virus-infected cells based on decreased amounts of the pp28Mut50 mutant protein in the AC, it was unclear why the pp28Mut50 mutant protein failed to accumulate in the AC. The results from studies using FRAP indicated that the mutant protein was efficiently localized to the AC; thus, it is unlikely that the interaction with adaptor proteins required for cellular trafficking in the secretory pathway was altered by this mutation. An obvious possibility was that the pp28Mut50 protein could not interact efficiently with cellular and/or viral proteins within the AC and thus exited the AC nearly as rapidly as it entered this compartment. Because this mutant was not completely defective in assembly, it was also likely that once a sufficient quantity of the pp28Mut50 mutant protein accumulated in the AC, the assembly of an infectious particle could take place. Thus, it could be argued that a threshold amount of pp28 must be present in the AC to permit envelopment and assembly. The requirement for a threshold amount of pp28Mut50 mutant protein in the AC for virion assembly could be secondary to productive interactions with other viral proteins, including multimerization with itself, or interactions with cellular proteins. A corollary of such a mechanism would be that the defect in the assembly of the pp28STOP50 virus could be overcome by delivering more mutant protein to the AC. Indeed, this appears to be the case in that monolayers infected with a high MOI of the pp28STOP50 mutant virus produce infectious virus nearly as efficiently as do monolayers infected with the wild-type virus.
Acknowledgments
We thank Albert Tousson for assistance in the imaging experiments.
This work was supported by PHS grants R01 AI35602 and R01 AI50189 from NIAID, NIH, to W.J.B.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 2.Baines, J. D., and B. Roizman. 1992. The U[infscap]l 11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 4.Borst, E.-M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt, W. J., M. Jarvis, J.-Y. Seo, D. Drummond, and J. Nelson. 2004. Rapid genetic engineering of human cytomegalovirus by using a lambda phage linear recombination system: demonstration that pp28 (UL99) is essential for production of infectious virus. J. Virol. 78:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 7.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Raven Press, Philadelphia, PA.
- 8.Britt, W. J., and S. Boppana. 2004. Human cytomegalovirus virion proteins. Hum. Immunol. 65:395-402. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of the human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218-232. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granzow, H., F. Weiland, A. Jöns, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, T. R., and S.-W. Lee. 2004. An acidic cluster of human cytomegalovirus UL99 tegument protein is required for trafficking and function. J. Virol. 78:1488-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomis, J. S., J. B. Bowzard, R. J. Courtney, and J. W. Wills. 2001. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 75:12209-12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mach, M., B. Kropff, M. Kryzaniak, and W. Britt. 2005. Complex formation by glycoproteins M and N of human cytomegalovirus: structural and functional aspects. J. Virol. 79:2160-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocarski, E. S., and C. Tan Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 20.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9:28-35. [DOI] [PubMed] [Google Scholar]
- 21.Rubin, R. 2002. Clinical approach to infection in the compromised host, p. 573-679. In R. Rubin and L. S. Young (ed.), Infection in the organ transplant recipient. Kluwer Academic Press, New York, NY.
- 22.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez, V., E. Sztul, and W. J. Britt. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-golgi-intermediate compartment. J. Virol. 74:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo, J.-Y., and W. J. Britt. 2006. Sequence requirements for localization of human cytomegalovirus tegument protein pp28 to the virus assembly compartment and for assembly of infectious virus. J. Virol. 80:5611-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva, M. C., J. Schroer, and T. Shenk. 2005. Human cytomegalovirus cell-to-cell spread in the absence of an essential assembly protein. Proc. Natl. Acad. Sci. USA 102:2081-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva, M. C., Q.-C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, J. D., and E. de Harven. 1973. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J. Virol. 12:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stagno, S., and W. J. Britt. 2006. Cytomegalovirus, chapter 23. .In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 6th ed. W. B. Saunders, Philadelphia, PA.
- 29.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 30.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang, Y., S. S. Molloy, L. Thomas, and G. Thomas. 2000. The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol. Biol. Cell 11:1257-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]