Abstract
Cervical cancer is one of the leading causes of cancer mortality in women worldwide, yet few suitable animal models currently exist for study of this disease. Virtually all cases of cervical cancer in women are caused by specific types of genital human papillomavirus (HPV). In this study, we investigated naturally occurring genital PVs in female cynomolgus macaques (Macaca fascicularis) without breeding contact for at least 3.5 years. Exfoliated cervicovaginal cells from 19 of 54 animals tested positive for at least one PV. Seven different PVs were identified, including four novel genotypes and two genotypes (RhPV-d and RhPV-a) previously identified in rhesus macaques (Macaca mulatta). Four PV types were associated with cervical intraepithelial neoplasia (CIN), which resembled human CIN by endoscopy, cervical cytology, histology, and immunohistochemistry. The presence of CIN was highly associated with PV infection (P < 0.0001). The most prevalent virus type was RhPV-d, which was identified in 60% of animals with CIN. An RhPV-d genome sequenced from a high-grade CIN lesion was found to be phylogenetically related to the highly oncogenic HPV16. Transfer of cervical cytobrush samples from donor animals naturally carrying RhPV-d resulted in new infections in 4 of 12 previously virus-free animals and abnormal cytology and histology in 1 of 4 infected animals after 18 weeks of infection. Experimental transmission was confirmed by E1^E4 reverse transcription-PCR products and RhPV-d sequence identity with the donor variant. These findings identify key similarities between macaque and human oncogenic PVs which should prove useful in the study of viral persistence, carcinogenesis, and therapeutic development.
Human papillomaviruses (HPVs) are among the most common sexually transmitted agents and the primary cause of cervical dysplasia and cancer in women (11, 14, 17, 19). Over 40 distinct genital HPV genotypes have been characterized, 10 to 15 of which are considered oncogenic (19, 27). Genital HPVs are globally widespread, with the cumulative infection rate approaching 60% in many populations of younger women (11, 14, 18). While the majority of these infections resolves spontaneously, a small fraction persists and induces cancer across an extended preinvasive stage called cervical intraepithelial neoplasia (CIN) (26). Despite recent progress in prophylactic HPV vaccine development (28), medical therapies for HPV infection and CIN currently do not exist, and HPV-related disease will continue to be an important public health burden for decades to come (23).
The induction and development of CIN requires a very specific biologic context. PVs replicate only within a maturation lineage of keratinocytes, and high-risk HPVs generally target the basal epithelial cells of the cervical squamocolumnar junction (34). This cell type has a unique and variable phenotype (4) not easily reproduced in tissue culture systems. Host determinants such as hormone and immunologic status, coinfections, and environmental cofactors may also strongly influence viral persistence and oncogenic potential (3, 32). Collectively, these complex features of HPV-related oncogenesis have proven difficult to replicate in existing preclinical models of cervical neoplasia (2, 24).
The remarkable diversity of HPVs (with over 100 types now described) has not been reported in other animal species (8). This discrepancy is likely due in large part to survey bias but may also involve particular ecologic determinants of PV evolution. The high-risk oncogenic mucosal HPVs are members of the alpha-PV group, which collectively represents over half of the known HPV types (8). Related alpha-PVs have only been identified in other Old World primates, including macaques (6), colobus monkeys (20), and a pigmy chimpanzee (30). These reports are sporadic, however, and few studies have systematically evaluated and typed genital PVs across nonhuman primate populations (6, 22). In the most extensive survey, 12 novel rhesus PVs (RhPV-a through -m) and 1 cynomolgus PV (MfPV-a) were identified in genital samples from a group of 286 rhesus macaques (Macaca mulatta) and 7 cynomolgus macaques (Macaca fascicularis) (6), strongly suggesting that diverse lineages of genital alpha-PVs exist in different nonhuman primate species.
Among natural models of HPV-related disease, macaques are the only nonhuman species in which naturally occurring PV-associated cervical neoplasia has been described (21, 33). Survey studies of spontaneous cervical dysplasias in macaques were initially reported over three decades ago (9, 12, 13), although an association with PV infection was not established until more recently. In this initial report, PV DNA was identified in a metastasis of a primary penile carcinoma in a male rhesus macaque (21). A subsequent survey of 30 female macaques, some of which had known mating contact with the infected male, revealed DNA from this PV (named RhPV-1) from cervical samples in 19 of the females and dysplastic or invasive CINs in 6 of the females (21). More recently, we demonstrated that cervical neoplasia occurs relatively frequently in female macaques and that these lesions express PV antigens and share distinctive histopathologic similarities with those found in women (33). Molecular characterization of macaque PVs has been limited, however, and transmission has never been demonstrated experimentally. The goals of the current study were to characterize cervical lesions in relation to specific macaque PV types in a group of female cynomolgus macaques and to evaluate the experimental infectivity of a high-risk oncogenic macaque PV.
MATERIALS AND METHODS
Animal subjects.
For this study, we surveyed 54 adult female cynomolgus monkeys (Macaca fascicularis) originally imported from Bogor, Indonesia. The animals were housed in all-female social groups of three to five monkeys each and had no breeding activity from the time of import. Population characteristics are provided in Table 1. All procedures were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee. The facilities and laboratory animal program of Wake Forest University are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
TABLE 1.
Survey of female cynomolgus macaques for PVsa
| Study | No. in group | % PV positive (no. positive/total no.) | Ovariectomized | Treatment | Yr old (mean ± SE) at:
|
PV types found in group at baseline survey (no. of animals with type)c | |
|---|---|---|---|---|---|---|---|
| Survey | Import | ||||||
| A | 30 | 43% (13/30) | Yes | E2b | 19.3 ± 0.5 | 14.5 ± 0.5 | RhPV-d (6)*, RhPV-a (1), MfPV-n1 (1), MfPV-n2 (1), MfPV-n3 (1)*, MfPV-n4 (1), untyped (4) |
| B | 24 | 25% (6/24) | No | None | 12.1 ± 0.6 | 8.6 ± 0.6 | RhPV-a (2)*, RhPV-d (1), MfPV-a (1)*, untyped (2) |
Animals were screened by PCR using MY09/11, GP5+/6+, and FAP primers with DNA from exfoliated cervicovaginal cells. Animals were housed in all-female social groups with no breeding contact from the time of import.
17β-Estradiol given orally in the diet at the human equivalent of 1.0 mg/day.
Asterisks indicate association with a CIN on histology. Two animals in study A had coinfections (RhPV-d/MfPV novel 1 and RhPV-d/RhPV-a).
Cervicovaginal cell sampling.
The animals were briefly anesthetized with ketamine, and a small plastic cervix brush (Cervex-brush; Rovers Medical Devices, The Netherlands) or nylon cytobrush (Surgipath Medical Industries, Richmond, IL) ∼0.3 cm in diameter was used to collect exfoliated keratinocytes. A subset of the animals in study A (Table 1) were resampled 10 weeks (n = 13) and 31 weeks (n = 15) after the initial sampling. After collection, the brush was placed in a preservative solution (SurePath, Burlington, NC) and centrifuged to concentrate the cells. A portion of the cells were placed on a slide and Papanicolaou (Pap) stained for cytologic assessment using standard procedures. The remaining cell fraction was coded for laboratory blinding and frozen at −80°C before shipment for PV testing. Pap procedures were performed by the Cytopathology Laboratory at Wake Forest University NC Baptist Medical Center.
PV DNA detection and typing.
Cervicovaginal cell samples were screened for macaque PV DNA using PCR-based testing, as described previously for HPV detection (5, 11). Briefly, preserved cells were centrifuged and digested, and DNA was precipitated with ammonium acetate. Pelleted DNA was resuspended in Tris-EDTA buffer and amplified by PCR using gold Taq polymerase (Applied Biosystems, Foster City, CA) with MY09/11, GP5+/6+, and FAP primer sets targeting conserved regions of the L1 gene (1, 5, 10). PCR products of appropriate sizes were confirmed by agarose gel electrophoresis, purified by using the Quickstep2 PCR purification kit (Edge BioSystems, Gaithersburg, MD), and submitted for direct sequencing in the Einstein Sequencing Facility.
PCR products generated using monkey-specific primers (based on macaque PV L1 open reading frames [ORFs], available from investigators) were analyzed by gel electrophoresis, transferred to nylon filters, hybridized overnight with radiolabeled generic probes used for HPVs, washed at 55°C, and exposed to X-ray film (1). To analyze positive PCR products for their PV DNA type, aliquots of the initial PCR mixture were denatured and applied to replicate filters using a Hydra96 (Robbins Scientific). The filters were individually hybridized using biotinylated type-specific oligonucleotide probes for multiple known monkey PV types (6) and others identified on our initial analyses of sequence products, as described previously (5). Samples which tested positive by the generic probe mix but negative by all type-specific probes were classified as “uncharacterized” PV types. A subset of these PCR-positive and dot blot-negative samples were sequenced to confirm novel monkey PVs and to allow the development of additional dot blot probes. Hybridization signals were recorded using a 1+ to 5+ scale for signal intensity as a semiquantitative measure of type-specific viral load.
Macaque PV classification.
Sequences of the MY09/11 region were compared with known PV sequences from the NCBI GenBank database using a BLAST searching algorithm. Viruses with MY09/11 DNA sequences less than 90% similar to previously typed PVs were numbered as novel MfPVs (8).
Tissue sampling and endoscopy.
To evaluate CIN prevalence by histology, cervical tissues were collected from the 54 survey animals either by biopsy (study A) or at necropsy (conducted for unrelated experimental reasons) (study B) (Table 1). For biopsy samples, animals were anesthetized with ketamine and butorphanol, and an endoscopy device (Stryker Endoscopy, Kalamazoo, MI) was used to remove a small pinch sample (∼25 mg) of cervical mucosa. The endoscope was also used to record video footage of CIN lesions that were grossly evident. Prior to colposcopy, 1.0 ml of dilute acetic acid (5% solution) was applied to the cervix via pipette to assist in lesion visualization. Biopsies were performed by an experienced gynecologic surgeon (B. E. Miller). For collection of necropsy samples, the animals were first sedated with ketamine and then euthanized using sodium pentobarbital (100 mg/kg, intravenously), a method consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. All tissues were fixed at 4°C in 4% paraformaldehyde for 24 h, transferred to 70% ethanol, and then processed for histology by using standard procedures. Sections were stained with hematoxylin and eosin and reviewed by a board-certified veterinary pathologist (J. M. Cline). Lesions were scored using criteria adapted from the CIN nomenclature system, which uses the following categories (in order of increasing grade): CIN1, CIN2, CIN3 (carcinoma in situ), and invasive carcinoma (34). The association between PV and CIN status was evaluated by using a two-sided Fisher's exact test.
Immunohistochemistry.
Immunohistochemical staining for markers of cell proliferation (Ki67) and PV infection (p16INK4a) was performed on fixed tissues. The following primary antibodies were used: Ki67 (MIB1; Dako, Carpinteria, CA) and p16INK4a (CMC811; Cell Marque, Hot Springs, AR). Biotinylated secondary Fc antibody was used as a linking reagent, and alkaline-phosphatase-conjugated streptavidin (Biogenex, San Ramon, CA) was applied for labeling. The chromogen used was Vector red, obtained as Vector red substrate kit no. 1 (Vector Laboratories, Burlingame, CA), diluted in Tris (pH 8.2 to 8.5) and applied to slides for 5 to 10 min at 30 to 35°C. Negative staining controls were obtained by omitting the primary antibody and substituting nonimmune serum (BioGenex, San Ramon, CA).
Experimental transmission of RhPV-d.
A subset of animals (n = 13) from the survey study was used to evaluate the infectivity of RhPV-d. At 3-week intervals for 33 weeks, animals were sedated with ketamine and exfoliated cervicovaginal cells were transferred from the donor animal(s) to the 12 RhPV-d-negative animals. At weeks 0, 3, 6, and 9, cervical cells were transferred directly via cytobrush from a single donor animal (6660) to each recipient. At the 12-week time point, three RhPV-d-positive female macaques (6643, 6645, and 6646) from the original survey group were transferred into the inoculation study as donors in place of three RhPV-d-negative animals, and a new method of cervical cell transfer was adopted in which cervical cytobrush samples were collected from the four RhPV-d-positive donor animals (6660, 6643, 6645, and 6646), vortexed briefly in 1.5 ml of physiologic saline to remove cells from each brush, and centrifuged at 1,000 × g for 1 min. The supernatant was discarded, the pellets were resuspended by vortexing in 2.0 ml saline, and the donor samples were combined into a single inoculum. An aliquot of this inoculum (0.1 ml) was then applied via pipette directly to the cervixes of the nine recipients (weeks 15 to 33). Prior to each transfer, cytobrush samples were collected for cytology evaluation and viral DNA analysis.
Spliced E1^E4 RhPV-d transcripts.
Exfoliated cervicovaginal cells collected by cytobrush at weeks 18 and 21 of the transmission study were resuspended in RNAlater RNA stabilization reagent (QIAGEN, Valencia, CA). Total RNAs were extracted by using an RNeasy mini kit (QIAGEN), treated with RNase-free DNase I (Promega, San Luis Obispo, CA), quantitated, and reverse transcribed using random hexamer primers (SuperScript first strand reverse transcription [RT]-PCR system; Invitrogen, Carlsbad, CA). PCR was performed by using a standard 45-cycle PCR thermocycling protocol with an equal mixture of AmpliTaq gold DNA (Applied Biosystems) and Pwo DNA polymerase (Gibco-BRL, Rockville, MD). The following primers were used to amplify a 180-bp RhPV-d-spliced E1^E4 fragment: 5′ ACTGCAGCAGACCTCAATCC 3′ (forward) and 5′ CCTGTGGTGTTTTCACATGC 3′ (reverse). Primers for macaque β-actin (NCBI accession no. DQ464112) were also run as an internal mRNA control. PCR products were analyzed by gel electrophoresis and stained with ethidium bromide.
RESULTS
Survey of macaque PVs.
Our initial aim was to evaluate genital PV prevalence in a closed population of female cynomolgus macaques. PV DNA was detected in 24 of a total of 82 cervical survey samples (29%) and 19 of 54 animals (35%) (Table 1). Seven different PV types were identified by sequencing of PCR-amplified viral DNA fragments. These PVs included four novel genotypes (MfPV novel 1 to 4) and two genotypes (RhPV-d and RhPV-a) previously isolated from rhesus macaques (6) (Table 1). All PV-positive macaque samples were negative for HPV DNA on dot blot assays.
Evaluation of cervical neoplasia.
Our next aim was to evaluate the relationships between specific macaque PV types and the presence of CIN, as determined by endoscopic visualization (colposcopy), cytology, and histology. In the survey study, evidence of CIN was identified by colposcopy in 7/15 animals, by histology in 6/54 animals, and by cytology in 8/54 animals (Table 2). Total lesion prevalence was 19% overall (10/54) and 53% (10/19) among PV-positive animals. All CIN lesions occurred among PV-positive animals (10/10), while no lesions were found in PV-negative animals (0/35) (P < 0.0001). Four different macaque PV types were associated with histologically confirmed CIN (RhPV-d, RhPV-a, MfPV-a, and MfPV novel type 3) (Table 2).
TABLE 2.
Association between genital PV type and CIN in female cynomolgus macaques
| Animal | PV type | Result for:
|
||
|---|---|---|---|---|
| Colposcopya | Histology | Cytology | ||
| 6660 | RhPV-d | + | + | + |
| 6646 | RhPV-d | + | + | + |
| 6637 | RhPV-d | + | − | + |
| 7297 | RhPV-a | ND | + | + |
| 6643 | RhPV-a/d | + | + | + |
| 6644 | MfPV-n3 | + | + | + |
| 6645 | RhPV-d | + | − | − |
| 6635 | RhPV-d | + | − | − |
| 7279 | MfPV-a | ND | + | + |
| 6647 | Untyped | − | − | + |
| 6656b | RhPV-d | +/− | + | + |
ND, not determined.
Cytologic, histologic, and possible colposcopic lesions were noted in animal 6656 at or after week 24 of the transmission study.
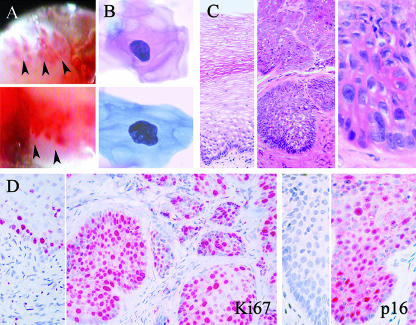
On colposcopy, cervical lesions were characterized by irregular patches of reddened mucosa, often with a cratered appearance and acetowhite staining of the margins (Fig. 1A). Cytologic abnormalities were noted on Pap samples in 42% (8/19) of naturally PV-positive animals. Cytologic changes included atypical cells with large and often irregularly shaped nuclei and a dense chromatin pattern (Fig. 1B). Histologically, CIN lesions exhibited a range of morphological changes similar to those described for human CIN (34), including nuclear atypia and enlargement, loss of polarity, perinuclear clearing (koilocytosis), and variable expansion of the spinosum (CIN1) and basal epithelium (CIN2/3) (Fig. 1C). Histologic lesions were also immunostained for several markers of PV infection. Cellular proliferation (Ki67) was diffusely increased throughout the full thickness of the squamous cervical mucosa within CIN lesions but restricted to the basal layer in adjacent normal mucosa (Fig. 1D). Nuclear expression of p16INK4a was present within all CIN lesions examined but not within adjacent normal mucosa (Fig. 1D).
FIG. 1.
Detection of naturally occurring CIN in female cynomolgus macaques. (A) Colposcopic features of CIN. Lesions showed increased vascularity, irregular cratering, and acetowhite staining of mucosa along the lesion margins (designated by arrows); the images are from animals with RhPV-d infections. (B) Cytologic features of CIN. Atypical Pap-stained keratinocytes had enlarged, irregular nuclei with increased chromatin staining; the cells are from animals with RhPV-d (upper) and MfPV-n3 (lower) infections. (C) Histologic features of CIN. The images show normal cervical mucosa with maturation strata of keratinocytes overlying a single layer of dark basal cells (left) and high-grade CIN lesions showing basal cell expansion, cellular atypia, nuclear enlargement, prominent nucleoli, and loss of polarity (middle, right); the lesions shown are from animals infected with RhPV-d. (D) Immunostaining for the proliferation marker Ki67 and the PV infection marker p16INK4a within normal cervical mucosa and CIN lesions (left and right, respectively).
Characterization of RhPV-d.
The most common type of PV identified was RhPV-d, which was found in 13% (7/54) of animals, 47% (7/15) of typed PV infections, and 60% (6/10) of animals with CIN lesions (Tables 1 and 2), suggesting that this virus is both persistent and oncogenic. RhPV-d was also associated with the highest grade lesion (CIN3) detected on histology (Fig. 1C). A full-length genome of RhPV-d was isolated from a frozen portion of this CIN3 lesion by using an overlapping PCR-based approach (29). The complete nucleotide sequence of RhPV-d was 7,935 bases in size (GC content, 48%) and was predicted to encode five early (E6, E7, E1, E2, and E4) and two late (L2 and L1) genes. Further details of the RhPV-d genome will be published elsewhere (R. D. Burk, unpublished data). The phylogenetic position of RhPV-d was inferred from the concatenated amino acids and nucleotide sequences of six ORFs by using the Bayesian method (7) (Fig. 2A). Nucleotide comparison of the L1 gene indicated that RhPV-d was most closely related to RhPV-1, which was the first nonhuman PV associated with cervical neoplasia (21), while the RhPV-d E6 ORF sequence was most closely related to HPV16. Both RhPV-d and RhPV-1 were classified as alpha-12 species, forming a monophyletic group with the alpha-9 (which includes HPV16) and alpha-11 species of genital HPVs.
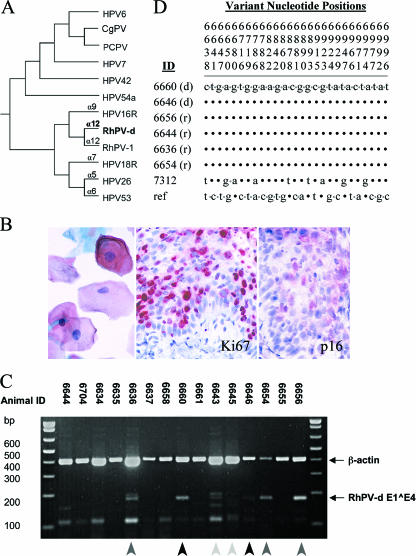
FIG. 2.
Characterization and experimental transmission of RhPV-d in female macaques. (A) Phylogenetic classification of the RhPV-d genome, showing close relationships to RhPV-1 and HPV16. CgPV, colobus monkey PV; PCPV, pygmy chimpanzee PV. (B) CIN associated with experimental RhPV-d infection in animal 6656. Atypical cervical cells were found by Pap cytology (left) after 18 weeks of RhPV-d infection, and dysplasia was seen in a cervical biopsy taken after 27 weeks of infection, shown here immunostained for proliferation marker Ki67 and PV infection marker p16INK4a. (C) Detection of spliced RhPV-d E1^E4 mRNA in female macaques by RT-PCR at week 21 of transmission study. Black arrows indicate positive samples from donor animals (6660 and 6646). Dark gray arrows indicate positive samples from three recipient animals (6654, 6656, and 6636). Interestingly, animal 6636 tested positive for RhPV-d E1^E4 mRNA but not DNA. Light gray arrows indicate samples from the other donor animals, 6643 and 6645 (inconclusive). Note also that animal 6644, which tested positive for MfPV-n3, did not test positive for the RhPV-d E1^E4 gene target. Molecular weight markers are shown on the left. β-Actin was used as an internal mRNA control. (D) Sequence analysis of RhPV-d MY09/11 fragments amplified from cervical cell DNA samples from two donors (d) (6660 and 6646); four recipients (r) at week 12 (6656 and 6644), week 21 (6636), and week 24 (6654); and a separate RhPV-d-positive animal (7312) from the initial survey. The RhPV-d reference sequence (ref) was obtained from GenBank (6). Note that the vertical numbers above the sequences represent only the nucleotide positions that varied (7), so the horizontal nucleotide sequences shown in the lower portion are not contiguous.
Experimental transmission of RhPV-d.
To evaluate the infectivity of RhPV-d, exfoliated cervicovaginal cells were transferred from RhPV-d-positive macaques to RhPV-d-negative recipients once every 3 weeks for 33 weeks. Recipient animals tested negative for RhPV-d DNA (using MY09/11, GP5+/6+, and FAP primer sets) in at least two different baseline cervical samples taken more than 2 months apart. New infections were detected by PCR (MY09/11- and RhPV-d-specific primer sets) and confirmed by dot blot methods. Experimental inoculations resulted in new RhPV-d infections in four recipients overall, with three current infections at 33 weeks (Table 3). Following 3 initial negative samples, animal 6656 became positive for RhPV-d at a 4+ or 5+ viral load in the subsequent 10 samples taken over 27 weeks. Animal 6644 (which was naturally coinfected with MfPV-n3) converted to a 2+ viral load at 6 weeks and a 3+ viral load at 12 weeks but then remained RhPV-d negative in subsequent samples through week 33. Animal 6654 converted to 3+ (18 weeks) and 5+ (21, 24, 27, and 33 weeks) RhPV-d viral loads after seven prior negative samples, while animal 6704 converted to 2+ (24 weeks), 5+ (27 weeks), and 4+ (33 weeks) viral loads after nine prior negative samples. For animal 6656, atypical cells were noted on Pap cytology after 18 weeks of infection (following nine prior negative samples) (Fig. 2B). Follow-up cervical biopsies at 33 weeks revealed low-grade CIN which stained positively for both Ki67 and p16INK4a (Fig. 2B).
TABLE 3.
Experimental transmission of RhPV-d in female macaquesa
| Screening primers and source(s) of DNA | No. RhPV-d positive/total no. in group at:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline time pointb
|
Wk
|
||||||||||||
| 1 | 2 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | |
| MY09/11 primers | |||||||||||||
| Donor (initial) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| Donor (added) | 2/3 | 3/3 | 1/3 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | |||
| Recipientsc | 1/12 | 1/12 | 0/12 | 2/12 | 2/12 | 2/9 | 2/9 | 3/9 | 3/9 | 4/9 | 4/9 | 3/9 | 4/9 |
| RhPV-d primers | |||||||||||||
| Donor (initial) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | |
| Donor (added) | 1/3 | 2/3 | 2/3 | 2/3 | 1/3 | 1/3 | 2/3 | 1/3 | |||||
| Recipientsd | 0/12 | 0/12 | 2/12 | 1/12 | 2/9 | 1/9 | 2/9 | 2/9 | 3/9 | 3/9 | 2/9 | 3/9 | |
Cervicovaginal cells were transferred from the RhPV-d-positive donor animal(s) to recipients every 3 weeks for 33 weeks. Animals were screened for PV DNA by PCR using consensus MY09/11- and RhPV-d-specific primers at two baseline time points and then at 3-week intervals. At 12 weeks, three animals that had tested positive previously for RhPV-d at BL1 and/or BL2 were transferred into the study as donor animals, replacing three of the recipients.
Donors were confirmed as RhPV-d positive based on dot blot assays and PCR product sequencing.
The one PV-positive recipient at baseline sampling was confirmed as MfPV novel 3 positive based on dot blot assays and sequencing.
Recipients testing positive for RhPV-d included 6656 (weeks 6 to 33), 6644 (weeks 6 and 12), 6654 (weeks 18 to 27 and 33), and 6704 (weeks 24, 27, and 33).
To confirm that the RhPV-d virus detected in newly infected animals was the result of active PV infection and not residual DNA, RT-PCR was used to identify spliced viral RNA. The E1^E4 gene product was confirmed in two of the RhPV-d donors (6660 and 6646) and three recipients (6656, 6654, and 6636) in cervical cells collected at 21 weeks (Fig. 2C). PCR products from these same animals, along with a third RhPV-d-positive animal from the initial survey (7312), were then sequenced. Animals 6660 and 6646 carried a variant of the MY09/11 sequence that was different from both the original rhesus sequence (6) and the RhPV-d isolate in 7312 but identical to that sequenced from other recipient animals (Fig. 2D).
DISCUSSION
These findings show that genital PVs are a diverse group of natural pathogens in female cynomolgus macaques, often associated with cervical neoplasia. The overall prevalence of genital PV infection in the survey portion of this study was 35%. All animals with CIN lesions tested positive for PV DNA, strongly suggesting that cervical neoplasia in macaques is induced by persistent PV infection. The most common type of genital PV detected was RhPV-d, which was found to be related phylogenetically to oncogenic HPVs and experimentally transmissible. This conclusion is based on the lack of RhPV-d detection in multiple samples from experimentally infected animals before conversion, followed by the presence of high amounts of RhPV-d DNA in exfoliated cervical cells after conversion; the presence of spliced RNA between the E1 and E4 viral genes in experimentally infected animals, indicative of active PV transcription; identification of the exact RhPV-d PCR product sequence in donor and experimentally infected monkeys; and, finally, the appearance of cervical neoplasia after 18 weeks of RhPV-d infection in a recipient animal.
Species specificity is considered a hallmark of PV biology. Apart from certain cutaneous bovine PVs (which have been isolated from horses and other hooved mammals), PVs are not considered to be infective across host species (2). In this study of cynomolgus macaques, we identified two genital PVs previously reported in a colony of rhesus macaques (6). Rhesus and cynomolgus macaques have only ∼0.4% divergence in their coding region sequences, compared to ∼2.2% average divergence between macaque and human coding sequences (15); still, the apparent cross-infectivity of RhPV-d and RhPV-a in separate macaque species is an unexpected finding and suggests that certain molecular boundaries restricting cross-species infectivity of primate genital PVs may be only partial in some instances. Future studies of macaque PV diversity and epidemiology should help identify these molecular barriers, as well as other determinants of viral host specificity.
Vaccines targeting particular types of oncogenic genital HPVs have been shown recently to be safe and effective in preventing persistent HPV infection and CIN lesions in young women (16, 31), representing a major landmark in cancer prevention. Nevertheless, important questions about prophylactic HPV vaccines remain, such as duration of protection, type specificity of the antibody responses, age dependence of protection, and efficacy following prior HPV exposure. Current vaccines are not effective for treatment of active HPV infections or CIN lesions, and the anticipated expense may limit their use in less-developed countries where cervical cancer is most common (25). A nonhuman primate model of PV infection and cervical neoplasia may be valuable in the development/refinement of second-generation vaccines and other antiviral therapeutics that address these limitations.
The development of a suitable animal model for cervical cancer has been a challenging problem in women's health research for many years. Current tissue culture, transgenic mouse, and natural PV infection models have led to considerable advances in our understanding of PV pathobiology (24). However, key differences between these systems and natural HPV infections (e.g., in microenvironment, mucosal tropism, viral transcription, and genomic factors) have limited their use in addressing certain important questions of HPV persistence and oncogenesis (2, 24). The findings of this study suggest that female macaques may serve as a unique and highly relevant preclinical model for the study of PV biology and CIN pathogenesis. Such a model would provide an important research resource for evaluating preventive and therapeutic strategies against HPV-related disease.
Acknowledgments
We thank Jean Gardin, Hermina Borgerink, Matt Dwyer, Diana Swaim, Leonore Accettullo, Anne Dunn, Andrew Prior, and Suzanne Leanza for technical support and Jeffry Leary for help initiating the study.
This work was supported in part by Public Health Service award K01 RR21322-02 from the National Center for Research Resources (NCRR), National Institutes of Health (NIH), and by GlaxoSmithKline (GSK).
The contents of this work are solely the responsibility of the authors and do not necessarily represent the view of the NCRR, NIH, or GSK.
Footnotes
Published ahead of print on 11 April 2007.
REFERENCES
- 1.Burk, R. D., G. Y. F. Ho, L. Beardsley, M. Lempa, M. Peters, and R. Bierman. 1996. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J. Infect. Dis. 174:679-689. [DOI] [PubMed] [Google Scholar]
- 2.Campo, M. S. 2002. Animal models of papillomavirus pathogenesis. Virus Res. 89:249-261. [DOI] [PubMed] [Google Scholar]
- 3.Castellsagué, X., and N. Muñoz. 2003. Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J. Natl. Cancer Inst. Monogr. 31:20-28. [PubMed] [Google Scholar]
- 4.Castle, P. E., J. Jeronimo, M. Schiffman, R. Herrero, A. C. Rodriguez, M. C. Bratti, A. Hildesheim, S. Wacholder, L. R. Long, L. Neve, R. Pfeiffer, and R. D. Burk. 2006. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 66:1218-1224. [DOI] [PubMed] [Google Scholar]
- 5.Castle, P. E., M. Schiffman, P. E. Gravitt, H. Kendall, S. Fishman, H. Dong, A. Hildesheim, R. Herrero, M. C. Bratti, M. E. Sherman, A. Lorincz, J. E. Schussler, and R. D. Burk. 2002. Comparisons of HPV DNA detection by MY09/11 PCR methods. J. Med. Virol. 68:417-423. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. Y., H. U. Bernard, M. Ratterree, T. A. Birkebak, A. J. Faras, and R. S. Ostrow. 1997. Genomic diversity and evolution of papillomaviruses in rhesus monkeys. J. Virol. 71:4938-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Z., M. Terai, L. Fu, R. Herrero, R. DeSalle, and R. D. Burk. 2005. Diversifying selection in human papillomavirus type 16 lineages based on complete genome analyses. J. Virol. 79:7014-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 324:17-27. [DOI] [PubMed] [Google Scholar]
- 9.DiGiacomo, R. F. 1977. Gynecologic pathology in the rhesus monkey (Macaca mulatta). II. Findings in laboratory and free-ranging monkeys. Vet. Pathol. 14:539-546. [DOI] [PubMed] [Google Scholar]
- 10.Forslund, O., A. Antonsson, P. Nordin, B. Stenquist, and B. G. Hansson. 1999. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 80:2437-2443. [DOI] [PubMed] [Google Scholar]
- 11.Herrero, R., P. E. Castle, M. Schiffman, M. C. Bratti, A. Hildesheim, J. Morales, M. Alfaro, M. E. Sherman, S. Wacholder, S. Chen, A. C. Rodriguez, and R. D. Burk. 2005. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J. Infect. Dis. 191:1796-1807. [DOI] [PubMed] [Google Scholar]
- 12.Hertig, A. T., and J. J. MacKey. 1973. Carcinoma in situ of the primate uterus: comparative observations on the cervix of the crab-eating monkey, Macaca fascicularis, the endometrium of the chimpanzee, Pan troglodytes, and on similar lesions in the human patient. Gynecol. Oncol. 1:165. [Google Scholar]
- 13.Hertig, A. T., J. J. MacKey, G. Feeley, and K. Kampschmidt. 1983. Dysplasia of the lower genital tract in the female monkey, Macaca fascicularis, the crab-eating macaque from Southeast Asia. Am. J. Obstet. Gynecol. 145:968-980. [DOI] [PubMed] [Google Scholar]
- 14.Ho, G. Y., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 15.Magness, C. L., P. C. Fellin, M. J. Thomas, M. J. Korth, M. B. Agy, S. C. Proll, M. Fitzgibbon, C. A. Scherer, D. G. Miner, M. G. Katze, and S. P. Iadonato. 2005. Analysis of the Macaca mulatta transcriptome and the sequence divergence between Macaca and human. Genome Biol. 6:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao, C., L. A. Koutsky, K. A. Ault, C. M. Wheeler, D. R. Brown, D. J. Wiley, F. B. Alvarez, O. M. Bautista, K. U. Jansen, and E. Barr. 2006. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet. Gynecol. 107:18-27. [DOI] [PubMed] [Google Scholar]
- 17.Moscicki, A. B., M. Schiffman, S. Kjaer, and L. L. Villa. 2006. Updating the natural history of HPV and anogenital cancer. Vaccine 24:S42-S51. [DOI] [PubMed] [Google Scholar]
- 18.Moscicki, A. B., N. Hills, S. Shiboski, K. Powell, N. Jay, E. Hanson, S. Miller, L. Clayton, S. Farhat, J. Broering, T. Darragh, and J. Palefsky. 2001. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 285:2995-3002. [DOI] [PubMed] [Google Scholar]
- 19.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518-527. [DOI] [PubMed] [Google Scholar]
- 20.O'Banion, M. K., J. P. Sundberg, A. L. Shima, and M. E. Reichmann. 1987. Venereal papilloma and papillomavirus in a colobus monkey (Colobus guereza). Intervirology 28:232-237. [DOI] [PubMed] [Google Scholar]
- 21.Ostrow, R. S., R. C. McGlennen, M. K. Shaver, B. E. Kloster, D. Houser, and A. J. Faras. 1990. A rhesus monkey model for sexual transmission of a papillomavirus isolated from a squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 87:8170-8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostrow, R. S., S. M. Coughlin, R. C. McGlennen, A. N. Johnson, M. S. Ratterree, J. Scheffler, N. Yaegashi, D. A. Galloway, and A. J. Faras. 1995. Serological and molecular evidence of rhesus papillomavirus type 1 infections in tissues from geographically distinct institutions. J. Gen. Virol. 76:293-299. [DOI] [PubMed] [Google Scholar]
- 23.Parkin, D. M., and F. Bray. 2006. The burden of HPV-related cancers. Vaccine 24:S11-S25. [DOI] [PubMed] [Google Scholar]
- 24.Peh, W. L., K. Middleton, N. Christenson, P. Nicholls, K. Egawa, K. Sotlar, J. Brandsma, A. Percival, J. Lewis, J. W. Liu, and J. Boorbar. 2002. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 76:10401-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden, R., and T. C. Wu. 2006. How will HPV vaccines affect cervical cancer? Nat. Rev. Cancer 6:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffman, M., and S. K. Kjaer. 2003. Natural history of anogenital human papillomavirus infection and neoplasia. J. Natl. Cancer Inst. Monogr. 31:14-19. [DOI] [PubMed] [Google Scholar]
- 27.Schiffman, M., R. Herrero, R. Desalle, A. Hildesheim, S. Wacholder, A. C. Rodriguez, M. C. Bratti, M. E. Sherman, J. Morales, D. Guillen, M. Alfaro, M. Hutchinson, T. C. Wright, D. Solomon, Z. Chen, J. Schussler, P. E. Castle, and R. D. Burk. 2005. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 337:76-84. [DOI] [PubMed] [Google Scholar]
- 28.Steinbrook, R. 2006. The potential of human papillomavirus vaccines. N. Engl. J. Med. 354:1109-1112. [DOI] [PubMed] [Google Scholar]
- 29.Terai, M., and R. D. Burk. 2001. Complete nucleotide sequence and analysis of a novel human papillomavirus (HPV 84) genome cloned by an overlapping PCR method. Virology 279:109-115. [DOI] [PubMed] [Google Scholar]
- 30.Van Ranst, M., A. Fuse, H. Sobis, W. De Meurichy, S. M. Syrjanen, A. Billiau, and G. Opdenakker. 1991. A papillomavirus related to HPV type 13 in oral focal epithelial hyperplasia in the pygmy chimpanzee. J. Oral Pathol. Med. 20:325-331. [DOI] [PubMed] [Google Scholar]
- 31.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, K. A. Ault, A. R. Giuliano, C. M. Wheeler, L. A. Koutsky, C. Malm, M. Lehtinen, F. E. Skjeldestad, S. E. Olsson, M. Steinwall, D. R. Brown, R. J. Kurman, B. M. Ronnett, M. H. Stoler, A. Ferenczy, D. M. Harper, G. M. Tamms, J. Yu, L. Lupinacci, R. Railkar, F. J. Taddeo, K. U. Jansen, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271-278. [DOI] [PubMed] [Google Scholar]
- 32.Wang, S. S., and A. Hildesheim. 2003. Viral and host factors in human papillomavirus persistence and progression. J. Natl. Cancer Inst. Monogr. 31:35-40. [DOI] [PubMed] [Google Scholar]
- 33.Wood, C. E., H. Borgerink, T. C. Register, L. Scott, and J. M. Cline. 2004. Cervical and vaginal epithelial neoplasms in cynomolgus macaques. Vet. Pathol. 41:108-115. [DOI] [PubMed] [Google Scholar]
- 34.Wright, T. C., A. F. Ferenczy, and R. J. Kurman. 1994. Precancerous lesions of the cervix, p. 229-278. In R. J. Kurman (ed.), Blaustein's pathology of the female genital tract, 4th ed. Springer-Verlag Press, New York, NY.