Abstract
Minocycline is a tetracycline derivative with antiapoptotic and anti-inflammatory properties, and the drug has been shown to have beneficial effects in a variety of models of neurological disorders. The potentially neuroprotective role of minocycline was assessed in experimental in vitro and in vivo models of rabies virus infection. In this study, 5 nM minocycline did not improve the viability of embryonic mouse cortical and hippocampal neurons infected in vitro with the attenuated SAD-D29 strain of rabies virus, based on assessments using trypan blue exclusion. Two-day-old ICR mice were inoculated in the right hind limb thigh muscle with SAD-D29, and they received daily subcutaneous injections of either 50 mg/kg minocycline or vehicle (phosphate-buffered saline). Infected minocycline-treated mice experienced an earlier onset of neurologic signs and greater mortality (83% versus 50%) than those receiving vehicle (log rank test, P = 0.002 and P = 0.003, respectively). Immunohistochemical analysis of rabies virus antigen distribution was performed at early time points and in moribund mice. There were greater numbers of infected neurons in the regional brain areas of minocycline-treated mice than in vehicle-treated mice, which was significant in the CA1 region of the hippocampus. There was less apoptosis (P = 0.01) and caspase 3 immunostaining (P = 0.0008) in the midbrains of mice treated with minocycline than in mice treated with vehicle, consistent with a neuroprotective role of neuronal apoptosis that may have had a mild effect of inhibiting viral spread. Reduced infiltration of CD3+ T cells was observed in the pons/medulla of moribund mice that received minocycline therapy (P = 0.008), suggesting that the anti-inflammatory actions of minocycline may intensify the neurologic disease. These findings indicate that minocycline has important detrimental effects in the therapy of experimental rabies. Empirical therapy with minocycline should therefore be approached with caution in cases of human rabies and possibly other viral encephalitides until more experimental data become available.
New therapeutic agents are needed for the therapy of rabies encephalitis in humans. Despite initial enthusiasm about therapy with the anesthetic agent ketamine (6), recent experimental (19) and clinical (4) evidence have raised questions about the therapeutic usefulness of this agent in human rabies. A combination of therapeutic agents is a reasonable approach for consideration in the treatment of rabies as it is used for other viral infections and diseases (6). Minocycline is a tetracycline derivative with antiapoptotic and anti-inflammatory properties, and the drug has been shown to have beneficial effects in a variety of neurological disorders (21). The efficacy of minocycline therapy has also been reported for a variety of experimental models of viral infections in animals, including neuroadapted Sindbis virus infection of mice (2), reovirus infection of neonatal mice (14), and simian immunodeficiency virus infection of pig-tailed macaques (23). In this report, we evaluate the efficacy of minocycline therapy in experimental infections of neonatal mice with rabies virus as well as in rabies virus-infected mouse embryonic neurons. We have selected a model using the SAD-D29 strain of rabies virus with peripheral inoculation in neonatal mice (5) that, with adjustment of the viral dose, produces brain infection with only about a 50% mortality rate and facilitates evaluation of either mildly beneficial or detrimental effects of the therapy. Preliminary studies of adult mice with highly virulent rabies virus (challenge virus standard-11) failed to demonstrate a beneficial effect. We have recently characterized this model and shown an encephalitis with prominent lesions in the brain stem and cerebellum (5).
MATERIALS AND METHODS
Virus.
The SAD-D29 strain of rabies virus was obtained from Teshome Mebatsion (Intervet International, Boxmeer, The Netherlands) (11). SAD-D29 was generated by replacement of arginine (R333) of the mature rabies virus glycoprotein with aspartic acid (D333) by using recombinant rabies virus L16, which contains the authentic sequence of the SAD-B19 vaccine strain (11). Baby hamster kidney (BHK) cells (clone C13) grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, Canada) were used for virus propagation.
Preparation of primary neuron cultures.
Primary cultures of mouse cortical and hippocampal neurons were prepared as described previously (1). Pregnant CD-1 mice at embryonic day 17 were anesthetized with isoflurane (Baxter Corporation, Canada), and the fetuses were immediately removed from the uterus and placed in a petri dish on ice. Under sterile conditions, the fetuses were separated from the embryonic sacs. The fetal cerebral cortices and hippocampi were collected and digested with trypsin (0.1%) and DNase I (50 μg/ml). The tissues were pooled in sterile Neurobasal medium (Invitrogen Life Technologies, Canada) supplemented with B27 (2%) and penicillin-streptomycin (100 IU/ml), triturated, and filtered through a 37-μm nylon mesh. The filtrate was centrifuged for 10 min at 1,000 rpm and resuspended in Neurobasal medium at a concentration of 1.0 × 104 cells/ml. The cells were then plated onto 24-well plates (1 ml each well) containing 12-mm-diameter round coverslips coated with poly-d-lysine (50 μg/ml) and incubated in a 5% CO2 atmosphere at 37°C. The cells were cultivated for 4 days prior to viral adsorption.
Determination of viability of infected primary neuron cultures.
Neurons plated in 24-well plates (1 ml each well; 1 ×104 neurons per well) were infected with SAD-D29 at a multiplicity of infection of 10 focus-forming units (FFU)/neuron. After viral adsorption for 1 h, the cells were washed once with phosphate-buffered saline (PBS), and fresh Neurobasal medium with or without minocycline (catalog no. M-9511; Sigma-Aldrich, St. Louis, MO) was added immediately with incubation at 37°C. The media and minocycline were not replenished. At 24, 48, and 72 h postinfection (p.i.), cells were fixed with 4% paraformaldehyde and viability was determined by trypan blue exclusion with phase-contrast microscopy at a high-power magnification (40× objective). Three separate experiments were performed. Trypan blue exclusion is a cell viability assay based on the ability of the cells to exclude the vital dye trypan blue, and cells in an early stage of apoptosis would be expected to exclude the dye and be scored as viable cells (10). Nonviable neurons failed to exclude the vital dye and showed trypan blue staining in their cytoplasm.
Viral yield assays.
The supernatants of primary neuronal cultures were assayed by fluorescent focus formation on BHK cell monolayers (7, 16).
Animals and inoculations.
Two-day-old ICR mice (Charles River Canada, St. Constant, Quebec, Canada) were inoculated in the right hind limb thigh muscle with 100 FFU of SAD-D29 in 20 μl. Uninfected controls were inoculated with the vehicle, PBS, in the same manner. Minocycline (50 mg/kg of body weight/day) diluted in 0.05 to 0.10 ml PBS was administered once daily subcutaneously for a total of 18 treatments per animal (n = 24) on days 0 to 17 postinfection, beginning within 6 h after virus inoculation. Vehicle (0.05 to 0.10 ml PBS) was given to an equal number (n = 24) of SAD-D29-infected mice. A repeat (second) experiment was performed with 60 SAD-D29-infected mice treated with vehicle or minocycline in order to verify the clinical observations and mortality data. A separate (third) experiment was performed in order to assess the number of infected neurons on days 5 and 7 p.i. in vehicle (n = 12)- and minocycline (n = 12)-treated mice.
Clinical observations.
Mice were evaluated daily for 28 days p.i. for clinical neurological signs, including hunching, limb paresis, and ataxia. Moribund animals were euthanized. Deaths occurring during the first 3 days p.i. were excluded because they were likely unrelated to the infection.
Preparation of tissue sections.
Mice were anesthetized with isoflurane and perfused with buffered 4% paraformaldehyde between days 3 and 7 p.i. or after they developed clinical rabies and became moribund. Their brains were removed and immersion fixed in the same fixative for 24 h at 4°C. Coronal tissue sections (6 μm) were prepared after dehydration and embedding in paraffin and stained with cresyl violet for light microscopic examination.
Assessment of neuronal apoptosis in mouse tissues.
Neuronal apoptosis was graded in major regional brain areas of minocycline- and vehicle-treated mice. A semiquantitative evaluation of the severity of apoptotic changes (using a 40× objective) was performed using the following scores: 0, no significant changes; 1, mild changes; 2, moderate changes; 3, severe changes; and 4, very severe changes and/or neuronal loss. The identities of all slides were masked during scoring. Rating scale scores are expressed as the mean ± standard error of the mean. Treatments with vehicle and minocycline were compared using the Mann-Whitney test.
Immunohistochemistry.
For expression of rabies virus antigen, cleaved (activated) caspase 3, and MAP-2, primary neurons were grown on poly-d-lysine-coated glass coverslips. At various times following SAD-D29 infection, neurons were fixed with 4% paraformaldehyde-PBS at 4°C overnight and analyzed. To detect rabies virus antigen, cells were washed in PBS, blocked for 20 min in 5% rabbit serum-PBS, and then incubated overnight at 4°C with a mouse anti-rabies virus nucleocapsid protein monoclonal antibody (5DF12), which was obtained from A. I. Wandeler (Centre of Expertise for Rabies, Canadian Food Inspection Agency, Nepean, Ontario, Canada) at a 1:160 dilution in 2% rabbit serum-PBS. For caspase 3, cells were washed in PBS, blocked for 20 min in 5% goat serum-PBS, and then incubated overnight at 4°C with polyclonal rabbit antibody against caspase 3 (catalog no. 9661; Cell Signaling Technology, Inc., Danvers, MA) at a 1:200 dilution in 2% goat serum-PBS. For identification of the presence of a neuronal marker, cells were washed in PBS, permeabilized in PBS containing 0.5% Triton X-100 for 10 min at room temperature, blocked for 1 h in 5% rabbit serum-PBS, and then incubated overnight at 4°C with anti-MAP-2 (Sigma-Aldrich, St. Louis, MO) at a 1:200 dilution in 5% rabbit serum-PBS. The neurons were washed twice in PBS and then incubated for 30 min with a secondary antibody, either biotinylated rabbit anti-mouse immunoglobulin G (IgG; for viral antigen and neuronal marker detection) or goat anti-rabbit IgG (caspase 3 activity; Vector Laboratories Inc., Canada), at a 1:100 dilution in 2% normal rabbit or normal goat serum-PBS, respectively. Neurons were washed twice in PBS and incubated for 30 min with 1.0% H2O2 in methanol. The ABC kit (Vector Laboratories Inc.) was used to localize the biotinylated antibody, and diaminobenzidine was used as a substrate for color development. Slides were counterstained with Gill's hematoxylin and examined using light microscopy.
For rabies virus antigen detection in tissue sections, the sections were deparaffinized and rehydrated prior to incubation with 1.0% H2O2 in methanol. Tissue was subsequently treated overnight at room temperature with mouse anti-rabies virus nucleocapsid protein monoclonal antibody 5DF12 (see above) diluted 1:160, EnVision+ System horseradish peroxidase-labeled polymer anti-mouse antibody (DakoCytomation, Denmark) for 40 min, 0.5 mg/ml 3,3-diaminobenzidine tetrachloride in 0.01% H2O2 in PBS, and 0.5% CuSO4 in 0.15 M NaCl. The slides were counterstained with hematoxylin and examined using light microscopy.
Immunohistochemical staining for activated caspase 3 was performed using a polyclonal rabbit antibody against caspase 3 (catalog no. 9661; Cell Signaling Technology, Inc.) as described previously (13); immunohistochemical staining of inflammatory T cells was done using a CD3 marker (polyclonal anti-human CD3 antibody; DakoCytomation, Glostrup, Denmark) diluted 1:400 as the primary antibody.
Statistical analyses.
Data were analyzed using t tests for the significance of the difference between the means of two counted samples and Mann-Whitney tests for the significance between two independent samples using a semiquantitative scoring system (see Fig. 3A), as well as Kaplan-Meier survival curves, and log rank test values of P < 0.05 were considered statistically significant.
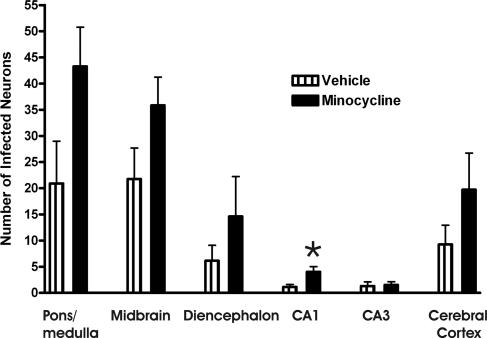
FIG. 3.
Counts of the number of infected neurons in various brain regions of moribund mice (9 to 21 days p.i.) that received daily treatment with vehicle (n = 9) or minocycline (n = 12). Slides stained for rabies virus antigen were masked, and the numbers of infected neurons in three different fields of the same brain region were counted using a high-power (40×) objective for areas with the most marked staining. *, statistical significance at a P of <0.05 determined using an unpaired t test. Error bars represent the standard errors of the means for comparisons of values for treatments with vehicle versus minocycline.
RESULTS
Characterization of primary neuron cultures.
More than 95% of the cells in the cortical and hippocampal cultures expressed the neuronal marker MAP-2 based on immunohistochemical staining (data not shown). By 72 h p.i., there was immunostaining of rabies virus antigen in 78% of SAD-D29-infected cells (data not shown), while staining was not observed in mock-infected cultures. At 24, 48, and 72 h p.i., 17%, 26%, and 28% of SAD-D29-infected cells expressed activated caspase 3, respectively (data not shown).
Dose-related effects of minocycline on the viability of primary neuronal cultures.
We initially evaluated the viability of cortical neurons with minocycline over a concentration range of 0 to 200 nM. A concentration of 5 nM was selected for further studies because it showed only small reductions in viability (5, 3, and 0% at 24, 48, and 72 h p.i., respectively) in screening toxicity studies of cultures (data not shown) and was similar to the concentration used by others using embryonic neurons (9).
SAD-D29 infection resulted in the loss of viability in primary neuronal cultures.
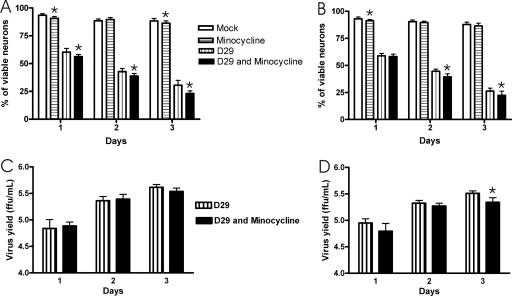
Neurons in the cortical (Fig. 1A) and hippocampal (Fig. 1B) cultures were infected with SAD-D29 or mock infected and analyzed for viability using trypan blue exclusion at days 1, 2, and 3 p.i. (Fig. 1A and B). There was a progressive loss in the viability of the cortical- and hippocampal-neuron cultures with the SAD-D29-infected neurons over time compared with the mock-infected cultures.
FIG. 1.
Viability of mock- and SAD-D29 (D29)-infected cultured cortical (A) and hippocampal (B) neurons with and without treatment with 5 nM minocycline as assessed by trypan blue exclusion. Minocycline produced small reductions (4.1 to 7.6% in cortical neurons and 0.7 to 5.2% in hippocampal neurons) in the viability of the SAD-D29-infected neurons. Viral yield assays of culture supernatants are expressed as mean titers (FFU/ml) from cortical (C) and hippocampal neurons (D). *, statistical significance at a P of <0.05 determined using an unpaired t test. Error bars represent the standard errors of the means for comparisons of values for mock infection without minocycline versus that with minocycline and of SAD-D29 infection without minocycline versus that with minocycline.
Minocycline did not exert a neuroprotective effect in SAD-D29-infected cultures.
In three separate experiments, there were small reductions in the viability of cultures with 5 nM minocycline versus cultures without minocycline (Fig. 1A and B). In this system, 5 nM minocycline did not affect the viral yield in cortical-neuron cultures (Fig. 1C) and demonstrated only a mild reduction in viral yield at 3 days p.i. in the hippocampal-neuron cultures (Fig. 1D).
Clinical observations of mice.
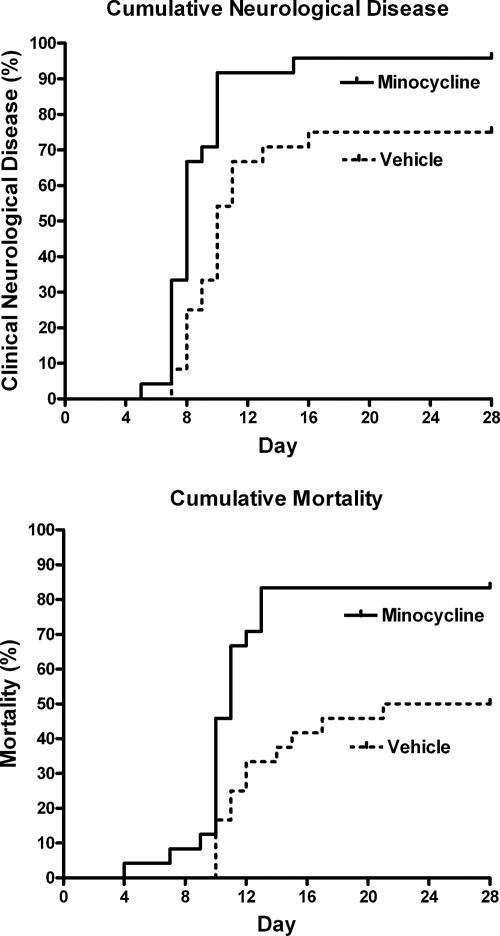
Forty-eight 2-day-old mice were inoculated in the hind limb thigh muscle with 100 FFU of SAD-D29 and were given either minocycline (50 mg/kg/day) or vehicle (PBS) subcutaneously once daily for a total of 18 treatments. Both cumulative clinical neurological disease, including paresis and ataxia, and mortality (83% versus 50% in vehicle-treated controls) were significantly more marked in the minocycline-treated mice (log rank test, P = 0.002 and P = 0.003, respectively) (Fig. 2). The larger repeat experiment (with 60 infected mice) confirmed greater cumulative clinical neurological disease and mortality in the minocycline-treated mice (log rank test, P = 0.006 and P = 0.0002, respectively) (data not shown). Clinical improvement of limb paresis occurred in a minority of mice (27%) in both treatment groups. Hence, minocycline actually aggravated the neurological disease and increased the mortality rate of the rabid mice in this model rather than providing neuroprotection.
FIG. 2.
Cumulative clinical neurological signs and cumulative mortality for vehicle (n = 24)- and minocycline (n = 24)-treated mice.
Rabies virus antigen distribution in mouse tissues.
We compared the numbers of neurons expressing rabies virus antigen in the brains of minocycline- and vehicle-treated moribund mice pooled from days 9 to 21 p.i. (Fig. 3). Generally, we found greater numbers of infected neurons in regional brain areas of the minocycline-treated mice, which reached statistical significance in CA1 pyramidal neurons, indicating only modest evidence of more-widespread infection in the brains of minocycline-treated mice. In a separate experiment, mice were assessed on day 5, before the onset of clinical neurological disease, and three of eight (38%) minocycline-treated mice and two of eight (25%) vehicle-treated mice were found to have infected neurons in the brain. On day 7, three of four (75%) minocycline-treated mice and one of four (25%) vehicle-treated mice (with right hind limb paresis) had infected neurons in the brain, predominantly in the brain stem and cerebellum.
Neuronal apoptosis in mouse tissues.
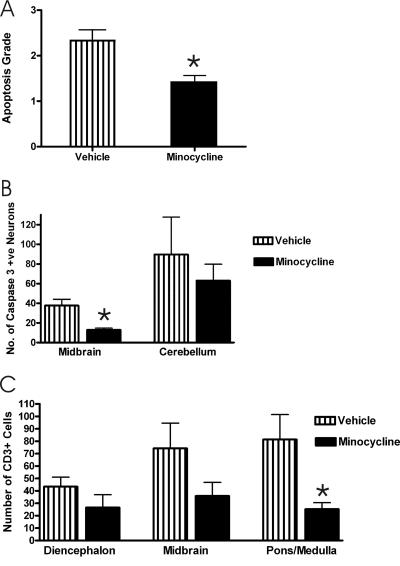
Minocycline-treated mice showed less neuronal apoptosis in the cerebellum and midbrain than vehicle-treated mice; the difference achieved statistical significance for results with the midbrain (Mann-Whitney test, P = 0.012) (Fig. 4A).
FIG. 4.
(A) Morphological changes of apoptosis were evaluated for neurons in the midbrain of moribund mice (9 to 21 days p.i.) treated with either vehicle (n = 9) or minocycline (n = 12) daily as described in Materials and Methods. (B and C) Counts of the number of neurons expressing caspase 3 in the midbrain and cerebellum (B) and of CD3-positive cells in the diencephalon, midbrain, and pons/medulla (C) of moribund mice that received daily treatment with vehicle (n = 9) or minocycline (n = 12). Slides stained for caspase 3 or CD3 were masked, and the numbers of stained neurons and CD3-positive cells in three different fields of the same brain region were counted by two independent observers using a high-power (40×) objective for areas with the most marked staining. *, statistical significance at a P of <0.05 determined using an unpaired t test. Error bars represent the standard errors of the means for comparisons of values for treatments with vehicle versus minocycline.
Immunohistochemical evaluation of activated caspase 3 and CD3 expression in mouse tissues.
Immunohistochemical evaluation of the number of neurons expressing activated caspase 3 (13), which is a downstream executioner of the apoptotic process, also showed a lower level of expression in minocycline-treated mice, and the difference was highly significant for the midbrain results (t test, P = 0.0008) (Fig. 4B). An immunohistochemical evaluation of inflammatory T cells using a CD3 marker (polyclonal anti-human CD3 antibody; DakoCytomation, Glostrup, Denmark) showed fewer CD3-positive cells in minocycline-treated mice than in vehicle-treated mice, with statistical significance in the difference in the pons/medulla results (t test, P = 0.008) (Fig. 4C).
DISCUSSION
Because of its neuroprotective antiapoptotic and anti-inflammatory properties, minocycline shows promise as a future therapy for a variety of neurological diseases, including multiple sclerosis, spinal cord injury, amyotrophic lateral sclerosis, Parkinson's disease, and Huntington's disease (21). A large phase III clinical trial of humans assessing the efficacy of minocycline therapy for amyotrophic lateral sclerosis is presently in progress (17). Some of the potential mechanisms of action of minocycline include antiapoptotic and anti-inflammatory properties, suppression of free-radical production, and inhibition of matrix metalloproteinases (21). The efficacy of minocycline therapy has been reported for a neonatal mouse model of reovirus infection (14). In this model, minocycline therapy (35 mg/kg intraperitoneally daily beginning 2 days p.i.) delayed the onset, progression, and mortality of the encephalitis by 4 days, and there was less apoptosis and greater reductions in viral titer and viral antigen expression levels in minocycline-treated mice than in controls. In neuroadapted Sindbis virus infection, minocycline (50 mg/kg intraperitoneally daily beginning at the start of infection) protected mice from the development of hind limb weakness and from virus-induced motor neuron death without effects on viral replication or spread (2). In simian immunodeficiency virus infection of pig-tailed macaques, minocycline therapy (4 mg/kg/day orally) reduced the severity of the encephalitis, suppressed the viral load in the brain, and decreased the expression of inflammatory markers in the brain (23). An effective neuroprotective agent would be an exciting advance in the therapy of viral encephalitis in humans, and there is a temptation to use the drug empirically with the hope that it might have a beneficial effect before the results of further studies in animals or humans become available. This is especially true for rabies, which is almost always fatal.
There is abundant evidence that rabies virus strains induce apoptosis of infected rodent embryonic neurons in vitro (12, 19). Furthermore, more-attenuated rabies virus strains have a greater capacity to induce neuronal apoptosis in vitro (12). We have recently shown that the highly attenuated SAD-D29 strain with a mutation at position 333 in the rabies virus glycoprotein also has a greater capacity to induce neuronal apoptosis in vivo than the less-attenuated L16 strain from which it was derived. Minocycline did not produce neuroprotection of SAD-D29-infected embryonic neurons in the present study. Although SAD-D29 infection is associated with increased expression of activated caspase 3 in the embryonic neurons, there may also be SAD-D29-induced nonapoptotic mechanisms of cell death and caspase-independent mechanisms of apoptotic cell death, as previously reported for rabies virus infection (15), accounting for the loss of viability in these primary neurons.
In the present neonatal mouse model, minocycline treatment of rabies virus-infected mice was associated with more-severe neurological disease and a higher mortality rate than treatment with vehicle. The number of infected neurons in regional brain areas of moribund mice treated with minocycline was greater than the number in those treated with vehicle, but this difference reached statistical significance only for the results with the CA1 region of the hippocampus. In mice assessed at earlier time points, including before the onset of clinical neurological disease, there was a trend towards finding more infected neurons in the brains of minocycline- than vehicle-treated mice. This suggests a slightly more efficient viral spread in the minocycline-treated mice, which could reflect effects on host defenses, including both anti-inflammatory and antiapoptotic effects.
Although minocycline did not improve the viability of SAD-D29-infected embryonic neurons in vitro, the drug exerted an antiapoptotic effect in vivo, findings similar to the observations of Richardson-Burns and Tyler (14) for neonatal reovirus infection. The neuroprotective effects in the reovirus model may have occurred because neuronal apoptosis plays a more fundamental role in the pathogenesis of the disease, rather than mainly as a mechanism for host defense by inhibiting viral spread (5, 12). There were fewer CD3-positive cells in the brain parenchyma of minocycline-treated SAD-D29-infected mice than in vehicle-treated mice, a difference which reached statistical significance for the pons/medulla results. This indicates an anti-inflammatory effect of minocycline, which may have also affected other immune effectors and impaired the host's ability to control the infection, resulting in aggravation of the rabies encephalomyelitis with a higher mortality rate. Both innate and adaptive immunities are important for recovery from rabies virus infection (8), and we feel that the anti-inflammatory effects of minocycline were likely important in aggravating the rabies encephalitis.
Minocycline has also been recognized to aggravate disease in animal models of neurodegenerative diseases. In the 3-nitropropionic acid mouse model of Huntington's disease, minocycline treatment was associated with a lower mean motor score, impaired general activity, and significantly deteriorated performances on the rotarod, pole test, and beam-traversing tasks and was associated with neuronal cell loss in the dorsal striatum that was more severe than that in untreated mice (3). The same study also found more severe clinical features of parkinsonism and greater loss of putaminal dopaminergic nerve endings in minocycline-treated monkeys than in untreated animals in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of Parkinson's disease (3). Minocycline was also found to increase MPTP-induced damage to dopaminergic neurons in mice (20). Although minocycline has been found to protect against cerebral ischemia in a rat model of transient middle cerebral artery occlusion (22), the opposite effect has been observed in a mouse model of hypoxic-ischemic brain injury (18).
Given the present finding that minocycline therapy aggravated rabies encephalitis in an experimental mouse model, we recommend that minocycline not be administered to human patients with rabies and possibly other viral encephalitides on an empirical basis, because of its potential ability to aggravate the disease. More studies are needed to understand the complex effects of minocycline in different neurological diseases, including rabies and other viral encephalitides.
Acknowledgments
This work was supported by Canadian Institutes of Health Research grant MOP-64376 and the Queen's University Violet E. Powell Research Fund (both to A. C. Jackson).
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Brewer, G. J., J. R. Torricelli, E. K. Evege, and P. J. Price. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567-576. [DOI] [PubMed] [Google Scholar]
- 2.Darman, J., S. Backovic, S. Dike, N. J. Maragakis, C. Krishnan, J. D. Rothstein, D. N. Irani, and D. A. Kerr. 2004. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J. Neurosci. 24:7566-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diguet, E., P. O. Fernagut, X. Wei, Y. Du, R. Rouland, C. Gross, E. Bezard, and F. Tison. 2004. Deleterious effects of minocycline in animal models of Parkinson's disease and Huntington's disease. Eur. J. Neurosci. 19:3266-3276. [DOI] [PubMed] [Google Scholar]
- 4.Hemachudha, T., B. Sunsaneewitayakul, T. Desudchit, C. Suankratay, C. Sittipunt, S. Wacharapluesadee, P. Khawplod, H. Wilde, and A. C. Jackson. 2006. Failure of therapeutic coma and ketamine for therapy of human rabies. J. Neurovirol. 12:407-409. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, A. C., P. Rasalingam, and S. C. Weli. 2006. Comparative pathogenesis of recombinant rabies vaccine strain SAD-L16 and SAD-D29 with replacement of Arg333 in the glycoprotein after peripheral inoculation of neonatal mice: less neurovirulent strain is a stronger inducer of neuronal apoptosis. Acta Neuropathol. 111:372-378. [DOI] [PubMed] [Google Scholar]
- 6.Jackson, A. C., M. J. Warrell, C. E. Rupprecht, H. C. J. Ertl, B. Dietzschold, M. O'Reilly, R. P. Leach, Z. F. Fu, W. H. Wunner, T. P. Bleck, and H. Wilde. 2003. Management of rabies in humans. Clin. Infect. Dis. 36:60-63. [DOI] [PubMed] [Google Scholar]
- 7.King, A. A. 1996. Cell culture of rabies virus, p. 114-130. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies. World Health Organization, Geneva, Switzerland.
- 8.Lafon, M. 2002. Immunology, p. 351-369. In A. C. Jackson and W. H. Wunner (ed.), Rabies. Academic Press, San Diego, CA.
- 9.Lee, S. M., T. Y. Yune, S. J. Kim, Y. C. Kim, Y. J. Oh, G. J. Markelonis, and T. H. Oh. 2004. Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J. Neurochem. 91:568-578. [DOI] [PubMed] [Google Scholar]
- 10.McGahon, A. J., S. J. Martin, R. P. Bissonnette, A. Mahboubi, Y. Shi, R. J. Mogil, W. K. Nishioka, and D. R. Green. 1995. The end of the (cell) line: methods for the study of apoptosis in vitro, p. 153-185. In L. M. Schwartz and B. A. Osborne (ed.), Cell death, vol. 46. Academic Press, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 11.Mebatsion, T. 2001. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 75:11496-11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto, K., D. C. Hooper, S. Spitsin, H. Koprowski, and B. Dietzschold. 1999. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J. Virol. 73:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasalingam, P., J. P. Rossiter, and A. C. Jackson. 2005. Recombinant rabies virus vaccine strain SAD-L16 inoculated intracerebrally in young mice produces a severe encephalitis with extensive neuronal apoptosis. Can. J. Vet. Res. 69:100-105. [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson-Burns, S. M., and K. L. Tyler. 2005. Minocycline delays disease onset and mortality in reovirus encephalitis. Exp. Neurol. 192:331-339. [DOI] [PubMed] [Google Scholar]
- 15.Sarmento, L., T. Tseggai, V. Dhingra, and Z. F. Fu. 2006. Rabies virus-induced apoptosis involves caspase-dependent and caspase-independent pathways. Virus Res. 121:144-151. [DOI] [PubMed] [Google Scholar]
- 16.Smith, J. S., P. A. Yager, and G. M. Baer. 1996. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody, p. 181-192. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies. World Health Organization, Geneva, Switzerland.
- 17.Traynor, B. J., L. Bruijn, R. Conwit, F. Beal, G. O'Neill, S. C. Fagan, and M. E. Cudkowicz. 2006. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology 67:20-27. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji, M., M. A. Wilson, M. S. Lange, and M. V. Johnston. 2004. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp. Neurol. 189:58-65. [DOI] [PubMed] [Google Scholar]
- 19.Weli, S. C., C. A. Scott, C. A. Ward, and A. C. Jackson. 2006. Rabies virus infection of primary neuronal cultures and adult mice: failure to demonstrate evidence of excitotoxicity. J. Virol. 80:10270-10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, L., S. Sugama, J. W. Chirichigno, J. Gregorio, S. Lorenzl, D. H. Shin, S. E. Browne, Y. Shimizu, T. H. Joh, M. F. Beal, and D. S. Albers. 2003. Minocycline enhances MPTP toxicity to dopaminergic neurons. J. Neurosci. Res. 74:278-285. [DOI] [PubMed] [Google Scholar]
- 21.Yong, V. W., J. Wells, F. Giuliani, S. Casha, C. Power, and L. M. Metz. 2004. The promise of minocycline in neurology. Lancet Neurol. 3:744-751. [DOI] [PubMed] [Google Scholar]
- 22.Yrjanheikki, J., T. Tikka, R. Keinanen, G. Goldsteins, P. H. Chan, and J. Koistinaho. 1999. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA 96:13496-13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zink, M. C., J. Uhrlaub, J. DeWitt, T. Voelker, B. Bullock, J. Mankowski, P. Tarwater, J. Clements, and S. Barber. 2005. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293:2003-2011. [DOI] [PubMed] [Google Scholar]