Abstract
We previously reported that the human immunodeficiency virus type 1 NL4-3 Nef is necessary and sufficient to induce a severe AIDS-like disease in transgenic (Tg) mice when the protein is expressed under the regulatory sequences of the human CD4 gene. We have now assayed additional Nef alleles (SF2, JR-CSF, YU10x, and NL4-3 [T71R] Nef alleles), including some from long-term nonprogressors (AD-93, 032an, and 039nm alleles) in the same Tg system and compared their pathogenicities. All these Nef alleles downregulated cell surface CD4 in human cells in vitro and also, with the exception of NefYU10x, in Tg CD4+ T cells. Depletion of double-positive and single-positive thymocytes occurred with all alleles but was less pronounced in NefYU10x Tg mice. A loss of peripheral CD4+ T cells was observed with all alleles but was minimal in NefYU10x Tg mice. In Nef032an and NefSF2 Tg mice, T-cell loss was severe despite lower levels of Tg expression, suggesting a higher virulence of these alleles. All Nef alleles except the NefYU10x and NefNL4-3(T71R) alleles induced an enhanced activated memory (CD25+ CD69+ CD44high CD45RBlow CD62Llow) and apoptotic phenotype. Also, all could interact with and/or activate PAK2 except the NefJR-CSF allele. Organ (lung and kidney) diseases were present in NefNL4-3(T71R), Nef032an, Nef039nm, and NefSF2 Tg mice, despite very low levels of Tg expression for the last strain. However, no organ disease or minimal organ disease developed in NefYU10x and NefAD-93 Tg mice and NefJR-CSF Tg mice, respectively, despite high levels of Tg expression. Our data show that important differences in the pathogenicities of various Nef alleles can be scored in Tg mice. Interestingly, our results also revealed that some phenotypes can segregate independently, such as CD4+ T-cell depletion and activation, as well as severe depletion of thymic CD4+ T cells and peripheral CD4+ T cells. Therefore, expression of Nef alleles in Tg mice under the CD4C regulatory elements represents a novel assay for measuring their pathogenicity. Because of the very high similarity of this murine AIDS-like disease to human AIDS, this assay may have a predictive value regarding the behavior of Nef in infected humans.
The development of AIDS following infection with human immunodeficiency virus type 1 (HIV-1) varies widely but occurs after a relatively long latency in most adults (median, 8 to 10 years) (8, 27, 63, 77). In children infected at birth, AIDS develops with a bimodal distribution, after either a very short incubation period (median, 4.1 months) or a longer incubation period (median, 6.1 years) (5, 90, 93). However, in some individuals, the progression of the disease course is very slow and AIDS develops after a much longer period (>12 years after infection for adults and >8 years after neonatal infection for children) (10, 78). These HIV-1-infected individuals are designated slow progressors or long-term nonprogressors (LTNP) (24). On the contrary, in other HIV-infected individuals, AIDS develops very quickly. These fast progressors are found in both pediatric (5, 80, 87, 93) and adult (43, 63, 72, 76, 77, 80) cohorts. As for many other viral diseases, it is likely that the severity and time course of AIDS development reflect individual host genetic susceptibility/resistance, virus pathogenicity, or both (26). The host genes could control levels of virus replication and immune response to HIV-1 as well as the signaling pathways with which the viral proteins may interfere. The best example of such genetic susceptibility is that of the CCR5 gene, coding for one of the major coreceptors for HIV-1 infection (73). A homologous deletion of 32 bp in CCR5 (Δ32-CCR5), found in ∼1% of the Caucasian population, confers nearly complete protection against HIV-1 infection in vivo (16, 42, 65, 89). The most evident case of low viral pathogenicity of HIV-1 in humans was documented in the Sydney Blood Bank Cohort, in which eight HIV-1-infected individuals, all LTNP, were infected with a Nef-defective HIV-1 strain by a single blood donor (15, 58). Studies of this cohort and of a few other individuals infected with HIV-1 strains with deletions or functional defects in Nef (12, 29, 53, 85, 88) strongly suggested that Nef is a major determinant of pathogenicity in humans.
Work with experimental animal models also confirmed the different pathogenicities of various simian immunodeficiency virus (SIV) nef alleles (23), as well as the low pathogenicities of nef-deleted SIV strains in macaques (51, 67) and of Nef-mutated HIV-1 strains in SCID-hu mice (2, 48). Finally, expression of HIV-1 or SIV Nef in transgenic (Tg) mice was shown to have very important pathological consequences (7, 20, 21, 35, 47, 57, 64, 94, 96).
In vitro comparison of various nef alleles and their effects on viral replication and downregulation of CD4 or major histocompatibility complex class I (MHC-I) molecules has revealed a wide range of potencies (4, 13, 14, 19, 28, 30, 69, 100, 104). A correlation could be established, although not by all groups (74), between the extent of Nef-induced CD4 downregulation and viral replication and disease progression (11, 13, 30), suggesting that differences in Nef function may have a major impact on viral pathogenicity. However, a similar head-to-head in vivo comparison has been reported for only two nef alleles in Tg mice with the same promoter (7). Moreover, the few available Tg mouse models of HIV-1 expression exhibiting immunity defects following expression of the same NefNL4-3 allele (35, 96) were constructed with different regulatory elements and expressed Nef in different cell subpopulations, making direct comparison difficult.
In another murine model of AIDS, the CD4C/HIV Tg mice expressing only the Nef protein of the HIV-1 strain NL4-3 develop a severe AIDS-like disease in the absence of virus replication (34, 35, 45). These mice express Nef in immature CD4+CD8+ and CD4+CD8− thymocytes, in peripheral CD4+ T cells, and in cells of the macrophage/dendritic-cell (DC) lineage (peritoneal, alveolar and tissue macrophages, Kupffer cells, and DCs) (34, 35, 82). The disease involves pathological changes of immunity-related structures, such as thymic and lymphoid organ atrophy, expansion of marginal zone, and decreased formation of germinal centers and follicular dendritic-cell network (35, 82, 83). Also, immune functions are significantly impaired: downregulation of CD4 cell surface, preferential loss of CD4+ T cells, increase of B-cell numbers, activation of both CD4+ T cells and B cells, hypergammaglobulinemia, loss of lymph node (LN) DCs, accumulation of CD11c+ CD11bhigh DC population, impaired DC function, and lower levels of helper CD4+ T-cell function have been documented (35, 82, 83, 84, 103). In addition, these Tg mice show an increased susceptibility to Candida albicans (18). Finally, kidney (cystic dilatation, focal segmental glomerulosclerosis, and interstitial nephritis), lung (lymphocytic interstitial pneumonitis), and heart (cardiomyocytis and cardiomyolysis) diseases develop (35, 46, 49). These pathological and functional changes are very similar to those found in humans with AIDS.
The large number of Nef-induced phenotypes which can be observed in these CD4C/HIV Tg mice represents a novel in vivo assay for Nef functions. However, it remained to be established whether Nef from other HIV strains would be equally pathogenic when expressed in Tg mice with the same CD4C regulatory elements. Here, we used this Tg mouse model to compare the pathogenicities of six additional nef alleles derived from different HIV-1-infected individuals with distinct clinical courses.
MATERIALS AND METHODS
Proviral and Nef expression constructs for in vitro assays.
For functional analysis, HIV-1 nef alleles were cloned into the biscistronic pCG expression vector (31) or a proviral HIV-1 NL4-3 construct coexpressing Nef and the enhanced green fluorescent protein (eGFP) via an internal ribosome entry site (IRES). Generation of the pCG vectors and the NL4-3 IRES-eGFP constructs carrying a defective nef reading frame or the NL4-3 nef allele has been previously described (91, 92). PCR amplification and sequencing of nef genes from three individuals with LTNP infection (032an-93, 039nm-94, and AD-93) was carried out as described previously (11, 52). The JR-CSF (56) and YU10x (61, 62) HIV-1 DNA clones were obtained from Irvin S.Y. Chen and Yoshio Koyanagi and from Beatrice Hahn and George Shaw, respectively, through the NIH AIDS Research and Reference Reagent Program. The SF2 (60) DNA clone was obtained from Louise Poulin and Jay Levy. All nef alleles were cloned into the bicistronic pCG vector, and the proviral HIV-1 NL4-3 IRES-eGFP construct by using unique XbaI (pCG vector) or HpaI (proviral construct) and MluI (both) restriction sites flanking the nef open reading frame (ORF) essentially as described previously (75, 91, 92). Sequence analysis confirmed that the recombinant proviral constructs contained the correct nef genes and verified the absence of undesired changes.
Virus stocks and in vitro infectivity.
For virus production, 293T cells were transfected by the calcium phosphate method with 10 μg of the proviral constructs, and the p24 antigen concentrations were quantified as described previously (11). Virus infectivity was determined on P4-CCR5 (81) and TZM-bl (17) cells by using the β-galactosidase screen from TROPIX as described previously (75).
Receptor modulation in vitro.
Jurkat T cells or HeLa CIITA cells were transfected with the pCG vectors or transduced with HIV-1 proviral constructs both coexpressing Nef and eGFP as described previously (54, 75, 92). Nef-mediated down- or upregulation of cellular receptors was quantified by flow cytometry, along with the expression of the eGFP reporter molecules as described previously (11, 54, 75, 92). These include CD4, MHC-I, and CD28 measured in Jurkat T cells, MHC-II, and the invariant chain (Ii) associated with immature MHC-II molecules on HeLa CIITA cells (98). Briefly, four ranges of green fluorescence representing no GFP and low, medium, and high levels of GFP and, indirectly, Nef expression were defined. For quantitative fluorescence-activated cell sorter (FACS) analysis, the mean channel numbers of red fluorescence obtained for cells transfected with a control construct expressing GFP only were divided by the corresponding numbers obtained for cells coexpressing Nef and GFP to calculate the values for down- or up-modulation, respectively. The same ranges of green fluorescence were used in all calculations.
Generation of Tg mice.
The nef fragments were amplified from the corresponding proviral DNA by PCR using primers harboring a 5′ MluI site and a 3′ NotI site, respectively, to generate unique sites for convenient cloning as follows: primers 585 (5′-ACGCGTATGGGTGGCAAGTGGTCAAAA-3′) and 584 (5′-GCGGCCGCTTAGTTCTTGTAGTACTCCGG-3′) for AD-93; primers 585 and 582 (5′-GCGGCCGCTCAGCAGTTCTTGTAGTACTC-3′) for both 032an and 039nm; primers 585 and 725 (5′-GCGGCCGCTCAGCAGTCCTTGTAGTACTC-3′) for JR-CSF; primers 1009 (5′-ACGCGTATGGCTGGATGGCCTACTGTA-3′) and 1010 (5′-GCGGCCGCTCGAGGTCATCAGTTCTTG-3′) for YU10x; and primers 585 and 583 (5′-GCGGCCGCTCAGCAGTCTTTGTAGTACTC-3′) for SF2. The threonine residue at position 71 of the HIV-1 NefNL4-3 protein was mutated to arginine (T71R). This mutation was produced using PCR site-directed mutagenesis on a SacI-BamHI HIV-1 fragment subcloned in the pBS KS vector as described previously (37, 40), using mutational primer 857 (GTACCTGAGGTCTGACTGGAA) containing C, replacing G, at nucleotide 8998 to produce the NefNL4-3(T71R) mutant. The identity of each allele and the presence of the T71R mutation were confirmed by sequencing. Each of the MluI-NotI fragments was then incorporated into the CD4C/HIVMutG DNA backbone, thus replacing NefNL4-3(WT) (the wild type). The CD4C/HIVMutG (designated CD4C/HIV-NefNL4-3) Tg DNA construct has been described previously (35). This Tg DNA harbors a complete HIV-1NL4-3 genome in which all the known ORFs, except that of Nef, have been interrupted by mutations. These Tg sequences have the ability to express only the Nef gene. Each linearized transgene was purified and inoculated into 1-day-old (C57BL/6 × C3H)F2 embryos to generate Tg mice, as described previously (34, 35). A minimum of two independent founder (F) Tg mice faithfully expressing each transgene were generated. Tg lines were established by breeding as a heterozygote for the transgene on the C3H/HeN background.
Transgene expression.
Northern blot analysis, using the whole HIV-1 genome as a probe, was used to assess HIV-1 RNA transgene expression, as previously described (34, 35, 37). The 32P-labeled bands were detected with the PhosphorImager screen and scanned with the StormImaging unit (Amersham Biosciences). Semiquantitation of the RNA bands was estimated with the ImageQuant software (Amersham Bioscience). The ratio of HIV-specific 8.8-kb signal to the actin-specific signal was obtained. In addition, transgene RNA expression was estimated by real-time quantitative reverse transcription-PCR (RT-PCR), as described previously (103). For the RT reaction, total RNA (1 to 2 μg) isolated from thymuses was used as a template in a 20-μl reaction mixture containing 1 × FIRST-strand buffer, as described by the manufacturer (Invitrogen), with Moloney murine leukemia virus reverse transcriptase and 1 mmol of pd(N)6 random hexamers/liter. After incubation at 42°C for 1.5 h, the enzyme was inactivated at 99°C for 5 min, and the product was diluted to 40 μl for amplification. Four microliters was used for quantitative PCR amplification with a Quantitect probe PCR kit (QIAGEN) with a reaction mixture containing 10 μl SYBR green PCR master mix, 1.6 μl of each primer, at 10 pmol/μl, and 4 μl template DNA (cDNA) in 2.8 μl RNase-free H2O. Quantitative PCR was performed with an MX4000 multiplex quantitative PCR instrument (Stratagene). Amplification was performed with a reaction volume of 20 μl for 40 cycles (45 s at 95°C and 45 s at 60°C). The primers used were specific for a 150-bp amplicon of fully spliced HIV-1 transcripts and a 100-bp amplicon of the S16 as internal standard. Primers used were a forward primer (oligo 77) located in exon 1 of the human CD4 gene (CCCCACTGGGCTCCTGGTTGCAGC) and a reverse primer for HIV (strain NL4-3) (CAGTCGCCGCCCCTCGCCTCTT) at nucleotide 743 upstream of the major 5′ splicing junction, and for mouse S16, forward primer oligo 2432 (AGGAGCGATTTGCTGGTGTGG) and reverse primer oligo 2433 (GCTACCAGGGCCTTTGAGATG). The specificity, sensitivity, and reproducibility of the quantitative PCR assays were verified by using cDNA prepared from the thymuses of CD4C/NefNL4-3(WT) Tg mice used as a positive control.
Detection of Nef protein was done by Western blot analysis, as described previously (34, 35), using various Nef antibodies. The rabbit anti-Nef antibody used (1:2,000) was prepared by injection of glutathione S-transferase-Nef fused protein as described previously (40). The monoclonal anti-Nef AE6 (32), EH1, and 6.2 antibodies were obtained from James Hoxie, Kai Krohn, and Vladimir Ovod, through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID.
Histological analysis.
Histological assessment on lymphoid and nonlymphoid organs was carried out essentially as previously described (34, 35). Semiquantitative assessment of the histological phenotypes was done as previously described (36). The score 1 was given to diseased kidneys with one to five glomeruli affected in their tubules, 2 was given to kidneys with 10 to 50% surface area affected, 3 was given to kidneys with >50% surface affected, and 4 was given to kidneys with almost all nephrons affected. The individual scores were added up and divided by total number of mice analyzed to obtain average scores.
Flow cytometry of primary mouse cells.
Flow cytometry was performed on a FACScan and FACSCalibur (BD Biosciences, San Jose, CA) using antibodies specific for various cell surface markers (CD4, CD8, T-cell receptor αβ, and Thy1.2 for T cells, B220 and Mac-1 for B cells and macrophages, respectively, and CD25, CD44, CD45RB, CD69, and CD62L for T-cell activation) as described previously (35, 103). Cell Quest software (BD Biosciences) was used for analysis.
Apoptosis analysis.
This procedure was performed as described previously (84). Apoptosis/death of CD4+ T cells was evaluated by Cell Quest software by using two different techniques. First, cells were stained with annexin V and propidium iodide (PI) to detect early apoptotic cells (single positive [SP] [annexin V positive and PI negative]) and dead cells (double positive [DP] [annexin V positive and PI positive]). Alternatively, 7-amino-actinomycin D (7AAD) staining (membrane-impermeable dye) was used to detect apoptotic and dead cells gating on high and intermediate 7AAD-positive cells. In both techniques, fragments and necrotic cells were excluded by gating on high and intermediate forward scatter excluding low forward scatter and low and high side scatter. The two techniques provided comparable data.
Purification of CD4+ T cells.
CD4+ T cells were purified by cell sorting by positive selection, as previously described (103). LN cells were resuspended for 20 min in blocking buffer (phosphate-buffered saline, 20% fetal bovine serum [GIBCO/BRL, Life Technologies]). Staining was performed with phycoerythrin (PE)-coupled anti-CD4 on ice for 40 min. CD4+ T cells were sorted by gating on the PE-positive population by using the MoFlo cell sorter (Dako, CO).
IVKA.
Immunoprecipitation was first performed with rabbit anti-Nef polyclonal antibodies followed by an in vitro kinase assay (IVKA) on the immunoprecipitated pellet. A detailed protocol for this technique has previously been published (102). [35S]Methionine labeling of proteins from thymocytes was described previously (102).
Statistics.
Statistical analyses (analysis of variance, Sigmastat, and Student's t test) were performed as previously described (84, 103).
RESULTS
Functional activity of primary HIV-1 nef alleles in vitro.
Eight nef alleles derived from HIV-1-infected individuals were assayed for their abilities to modulate CD4, CD28, CXCR4, MHC-I, MHC-II, and Ii surface expression. The primary AD-93, 032an-93, and 039nm-94 nef alleles were derived from individuals with long-term nonprogressive HIV-1 infection (11, 52). Four nef genes were obtained from well-characterized molecular clones NL4-3 (1), SF2 (3, 14, 60), JR-CSF (48, 56), and YU10x (61, 62, 104). SF2, JR-CSF, and YU10x HIV-1 strains were derived from AIDS patients. A variant of the NL4-3 Nef containing a substitution (T71R) at amino acid residue 71 in the conserved proline-rich region was also included in the analysis. This was done because this amino acid residue is frequently found in primary HIV-1 isolates (29) and because it has been proposed that this variation in Nef might affect T-cell activation and viral replication (25), although this could not be confirmed in a subsequent study (86). Alignment of Nef amino acid sequences showed a high conservation of known functional domains and no obvious inactivating point mutations (Fig. 1).
FIG. 1.
Alignment of Nef amino acid sequences. The NL4-3 sequence is shown in the upper panel for comparison. Some conserved sequence elements in Nef, the position of some binding (bdg) domains, and the position of the polypurine tract (PPT), as well as the start of the 3′ long terminal repeat (LTR) are indicated schematically. Dots indicate identity with the consensus sequence, and dashes indicate gaps introduced to optimize the alignment.
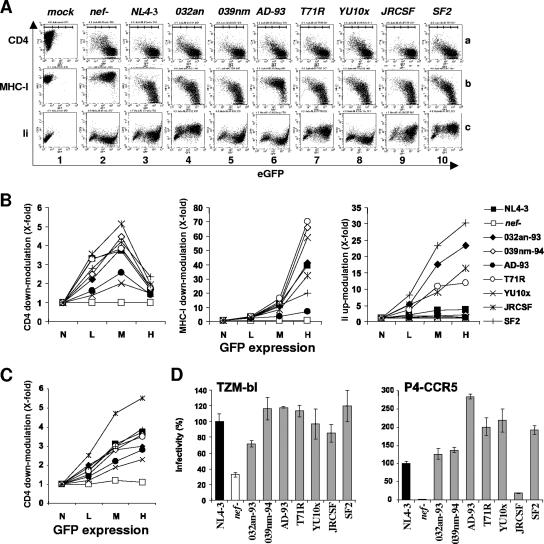
We examined the abilities of the eight nef alleles to modulate CD4, MHC-I, and Ii surface expression. The CD4- and MHC-I-downmodulating activities of Nef are well established (59, 79, 95), whereas the Ii-modulating activity of Nef has been described more recently and might enable HIV-1 to inhibit functional MHC-II antigen presentation (98). Cells transduced with the proviral NL4-3 construct containing a defective nef gene did not show significant alterations in the surface levels of MHC-I or Ii (Fig. 2A, panels 2b and c). In contrast, CD4 expression was diminished in nef-defective HIV-1 infection because Env and Vpu also down-modulate this receptor (Fig. 2A, panel 2a). At low and medium levels of eGFP and, hence, viral gene expression, however, CD4 down-modulation was more efficient in the presence of Nef (Fig. 2B, left panel). All HIV-1 nef alleles analyzed modulated the surface expression of cellular receptors, albeit with differential efficiencies. For example, the AD-93 Nef was only marginally active in modulating CD4, MHC-I, and Ii (Fig. 2A, panel 6). The YU10x Nef also showed little activity in modulating CD4 and Ii but efficiently down-modulated MHC-I (Fig. 2A, panel 8). The eight HIV-1 Nef alleles analyzed also showed differential activity in upregulating Ii. The NL4-3, 039nm, AD-93, and YU10x Nef proteins were poorly effective at enhancing Ii surface expression, whereas the SF2, 032an, and JR-CSF Nef proteins strongly upregulated its expression (Fig. 2B, right panel). Interestingly, replacing arginine by threonine at position 71 (T71R) of NefNL4-3 enhanced its Ii-upregulating activity (Fig. 2B, right panel). Vpu- and Env-mediated CD4 down-modulation to some extent complicated the quantitative analysis of this Nef function in the proviral context, particularly at high levels of viral gene expression (Fig. 2B, left panel). Therefore, we also investigated CD4 down-modulation in Jurkat cells transiently transfected with bicistronic vectors coexpressing the eight HIV-1 nef alleles and GFP. As expected from the results obtained using the proviral constructs, all nef alleles modulated CD4 surface expression, albeit with differential efficiencies (Fig. 2C). In both cases, the JR-CSF Nef showed the highest activity and the YU10x and AD-93 Nef proteins the weakest activity (compare Fig. 2B, left panel, and C). At medium levels of GFP (corresponding indirectly to Nef expression), the efficiencies of CD4 down-modulation in Jurkat cells expressing Nef alone or in the context of the HIV-1 provirus correlated very well (R2 = 0.73; P = 0.007). Finally, we also examined the effects of Nef on CXCR4 and MHC-II surface expression. Both receptors were, however, only marginally down-modulated (up to threefold) by the eight nef alleles investigated (data not shown).
FIG. 2.
Functional activity of HIV-1 nef alleles in in vitro assays. (A) Jurkat T (lanes a and b) or HeLa CIITA (lane c) cells were transduced with eGFP-expressing HIV-1 NL4-3 particles containing a disrupted nef gene (nef-) or the indicated nef alleles pseudotyped with the vesicular stomatitis virus G glycoprotein and analyzed by flow cytometric analysis. The ranges of no expression and low, medium, and high levels of eGFP expression used to calculate receptor modulation are indicated in the upper panel. (B) Quantitative effect of HIV-1 Nef proteins on CD4, MHC-I, and Ii surface expression. Values were determined as described in Materials and Methods. Shown are data derived from the transduced cells whose results are presented in panel A. Similar results were obtained in two independent experiments. The ranges of no expression (N) and low (L), medium (M), and high (H) levels of eGFP expression used to calculate receptor modulation are indicated on the x axes. (C) Quantitative presentation of CD4 down-modulation in Jurkat T cells transiently transfected with bicistronic vectors expressing GFP alone (nef-) or together with Nef. Symbols and abbreviations are as shown in panel B. (D) Infectivity of HIV-1 IRES-eGFP variants containing the indicated HIV-1 nef alleles. TZM-bl or P4-CCR5 indicator cells were infected in triplicate with 293T cell-derived virus stocks containing 1 ng p24 antigen. Infectivity is shown relative to that of the recombinant virus containing the NL4-3 nef allele. Similar results were obtained in another independent experiment.
To analyze the abilities of these HIV-1 Nef alleles to enhance virion infectivity, we generated viral particles by transient transfection of 293T cells and infected two indicator cell lines with virus stocks containing normalized quantities of the p24 antigen. All nef alleles enhanced HIV-1 infectivity for TZM-bl cells (Fig. 2D, left panel). This cell line is susceptible to both wild-type and nef-defective HIV-1 infections although Nef enhances viral infectivity about threefold. In contrast, the effects of Nef were much stronger in P4-CCR5 cells. Viral particles produced in the presence of Nef were about 40-fold more infectious for P4-CCR5 cells (Fig. 2D, right panel). All HIV-1 Nef alleles enhanced infection of P4-CCR5 cells, but the JR-CSF Nef gene was much less effective than the remaining nef genes analyzed (Fig. 2D, right panel).
Generation of CD4C/HIV-Nef Tg mice expressing different Nef alleles.
The pathogenicities of the Nef alleles studied in vitro were then assayed in vivo in Tg mice. Each of these nef alleles replaced Nef from the NL4-3 strain in the HIVMutG cassette in which all the other known ORFs except that of Nef have been interrupted by mutations (35). The nef alleles were expressed under the control of the chimeric human CD4 promoter and mouse CD4 enhancer regulatory elements (CD4C) to generate CD4C/HIV-NefAD-93, CD4C/HIV-Nef032an, CD4C/HIV-Nef039nm, CD4C/HIV-NefJR-CSF, CD4C/HIV-NefYU10x, CD4C/HIV-NefSF2, and CD4C/HIV-NefNL4-3(T71R) Tg mice (Fig. 3). For all transgenes, at least two independent founder lines faithfully expressing the transgene were established and studied.
FIG. 3.
Structure of the CD4C/HIV transgenes expressing various Nef alleles. Nef allele fragments NefAD-93, Nef032an, Nef039nm, NefJR-CSF, NefSF2, and NefYU10x, as well as the mutant NefNL4-3(T71R) were inserted within the same CD4C/HIVMutG backbone sequences replacing NefNL4-3(WT). The transgenes were constructed as described in Materials and Methods. Abbreviations: mCD4enh, mouse CD4 enhancer; hCD4 prom, human CD4 promoter; SV40, polyadenylation sequences from simian virus 40; Ex1, CD4 gene exon 1; X, interruption of the ORF of the indicated HIV-1 genes; LTR, long terminal repeat; A, restriction site AatII; Bs, restriction site BssHII; S, restriction site SstI.
Expression of the transgene was first assayed by Northern blot analysis on RNA of different organs from mice of each founder line. This analysis revealed the presence of the three major HIV-1 RNA transcripts, mainly in lymphoid tissues (thymus [Fig. 4A], spleen, and LN [data not shown]). Expression was not detected in other organs (heart, brain, liver, testis, and muscle) by this technique (data not shown), although previous work with more-sensitive techniques (18, 34, 38, 49, 82; unpublished data) has shown that these CD4C regulatory elements allow expression in tissue macrophages and T cells of almost every organ. This expression faithfully reflects the specificity of the CD4C promoter documented previously (34, 38, 39). Semiquantitative analysis showed that the levels of RNA expression differed among founder lines, as expected, most likely reflecting distinct integration sites of the transgene. This was confirmed by quantitative real-time RT-PCR on RNA extracted from the thymus of mice with each nef allele (Fig. 4A). The detection of Nef protein was done by Western blotting with various anti-Nef antibodies, using the one recognizing best each individual Nef allele. This analysis showed expression of Nef protein in the thymus of mice of at least one high-expressor Tg line of each allele (Fig. 4B). However, the use of different anti-Nef antibodies and the different sequences of each Nef allele precluded a direct comparison of the levels of Nef protein expression between these alleles. Therefore, we relied on Tg RNA expression in the thymus, as detected by Northern blot analysis, for comparison.
FIG. 4.
Expression of HIV-1 Nef in CD4C/HIV-Nefallele Tg mice. Thymuses from different founder lines with each of the CD4C/HIV-Nefallele transgenes were analyzed. (A) Northern blot analysis of HIV-1 RNA. Total RNAs from thymuses were hybridized with a 32P-labeled HIV-1-specific probe. The blots were stripped and rehybridized with a 32P-labeled actin DNA probe. Also shown below are the semiquantitative values for the levels of expression of each Nef allele relative to NefNL4-3(WT) (value of 1), measured in Northern blot or quantitative RT-PCR (Q-RT-PCR) analysis, as described in Materials and Methods. (B) Western blot analysis of protein extracts (100 μg) from thymuses of 1-month-old Tg and non-Tg littermates, using the following anti-Nef antibodies: rabbit polyclonal antibody produced in our laboratory (Lab) or monoclonal EH1 or 6.2 antibody. NefNL4-3(WT) represents the gene carried by the CD4C/HIVMutG (F27367) Tg mice previously reported (34) and used as a positive control. The membranes were stripped and reanalyzed with anti-actin antibody.
Tg mice expressing different Nef alleles, including those from LTNP, undergo CD4+ T-cell depletion associated with T-cell activation and apoptosis.
We previously reported that Tg mice expressing the NefNL4-3 allele showed a loss of thymocytes, depletion of peripheral CD4+ T cells, and downregulation of CD4 cell surface molecules (35). Similarly, all Tg mice expressing various Nef alleles at sufficient levels showed a loss of thymocytes (Table 1). This was most apparent in Tg lines expressing higher levels of the transgene, as expected. However, the loss of thymocytes was less severe in CD4C/HIVYU10x, CD4C/HIVJR-CSF, and CD4C/HIV039 nm Tg mice, despite relatively high levels of Nef expression in these lines.
TABLE 1.
Cell numbers in thymocyte subsets from Tg mice expressing different Nef alleles
| Mouse linea | Expression levelh | Absolute cell no. (106)b
|
Mean fluorescence (%)c of CD4 DP | |||
|---|---|---|---|---|---|---|
| Total | DP | SP CD4 | SP CD8 | |||
| Non-Tgd | 94 ± 21 | 80 ± 17 | 8.1 ± 1.5 | 3.9 ± 0.6 | 100 | |
| AD-93 | ||||||
| F115817 | L | 70 ± 27 | 60 ± 25 | 6.1 ± 1.4 | 1.2 ± 0.5e | 100 |
| F115820 | M | 16 ± 7e | 11 ± 6e | 4.1 ± 0.7e | 2.8 ± 0.9 | 60 ± 4e |
| 032an | ||||||
| F95581 | L | 30 ± 17e | 20 ± 12e | 3.4 ± 1.3e | 3.9 ± 3.3 | 58 ± 6e |
| F95582 | L-M | 53 ± 28f | 42 ± 21e | 5.4 ± 1.7 | 3.0 ± 0.9 | 63 ± 16f |
| 039nm | ||||||
| F75581 | M-H | 70 ± 27 | 62 ± 21 | 5.3 ± 1.9 | 1.3 ± 0.3e | 37 ± 4e |
| F51444 | M-H | 59 ± 19f | 44 ± 12e | 4.3 ± 1.8e | 2.5 ± 1.5 | 62 ± 12f |
| JR-CSF | ||||||
| F104995 | M-H | 48 ± 25e | 37 ± 20e | 2.6 ± 1.0e | 3.4 ± 3.0 | 45 ± 5e |
| F104996 | L-M | 100 ± 21 | 84 ± 19 | 4.2 ± 0.7e | 9.1 ± 3.0g | 25 ± 4e |
| YU10x | ||||||
| F92751 | H | 88 ± 18 | 75 ± 17 | 6.9 ± 1.0 | 2.1 ± 0.4 | 93 ± 21 |
| F92754 | L | 101 ± 12 | 84 ± 11 | 7.9 ± 3.3 | 3.3 ± 0.5 | 96 ± 13 |
| SF-2 | ||||||
| F92748 | L-M | 20 ± 10e | 12 ± 7e | 2.1 ± 1.0e | 2.1 ± 1.0 | 40 ± 16e |
| F92749 | L | 58 ± 19f | 47 ± 18e | 5.1 ± 1.4 | 2.1 ± 1.1 | 95 ± 4 |
| NL4-3(T71R) | ||||||
| F101376 | L | 65 ± 17f | 49 ± 13e | 8.1 ± 3.2 | 3.0 ± 1.0 | 78 ± 5f |
| F101374 | L | 66 ± 17f | 57 ± 13f | 4.9 ± 1.6e | 2.3 ± 2.3 | 69 ± 10f |
| F101371 | L | 81 ± 22 | 71 ± 20 | 4.8 ± 1.6e | 1.7 ± 0.7e | 93 ± 13 |
| F86707 | L-M | 67 ± 17f | 52 ± 13f | 3.0 ± 0.7e | 5.6 ± 1.9g | 44 ± 4e |
| Non-Tgd | 104 ± 26 | 90 ± 23 | 9.2 ± 3.1 | 2.4 ± 0.6 | 100 | |
| NL4-3(WT) | M-H | 69 ± 23e | 58 ± 20e | 2.9 ± 0.9e | 2.8 ± 0.6 | 36 ± 2e |
FACS analysis was performed on 4 to 10 mice (1.5 to 4 months old) for each Tg line, including the positive control CD4C/HIV-NefNL4-3(WT) Tg line. Data from Tg mice were always compared to those of their littermates done the same day. Older (4- to 6-month-old) Tg mice expressing Nef032an (F95582) or NefT71R (F101376) showed more severe depletion. For non-Tg mice, the total number of thymocytes is (53 ± 7.6) × 106 and the number of DP is (45 ± 5.9) × 106; for Nef032an Tg mice, the total number of thymocytes is (13.8 ± 10) × 106 and the number of DP is (11 ± 8.9) × 106; and for NefT71R Tg mice, the total number of thymocytes is (20.3 ± 9.5) × 106 and the number of DP is (17.4 ± 8.3) × 106 (six to eight mice in each group).
The absolute cell number was calculated by multiplying the total cell number in each organ by the percentage of each cell subset in this organ.
The mean fluorescences for CD4 were obtained by calculating the ratio of CD4 staining in Tg mouse thymuses relative to that of non-Tg mouse thymuses (100%) done in the same experiment, the same day. Mean values were then calculated with the values for each line.
The non-Tg control values were obtained by pooling the results of all non-Tg littermates from different lines.
Significant decrease (P < 0.01) according to Student's t test.
Significant decrease (P < 0.05) according to Student's t test.
Significant increase (P < 0.05) according to Student's t test.
L, low; M, medium; H, high; L-M, low to medium; M-H, medium to high.
Further analysis was performed by use of a FACS on thymocytes from Tg mice expressing each Nef allele. Consistent with previous data on NefNL4-3-expressing Tg mice (34, 35, 103), depletion of DP CD4+CD8+ and SP CD4+CD8− thymocytes was observed for all Tg lines expressing higher levels of the transgene, although such decrease was not as pronounced in Tg mice expressing NefYU10x, NefJR-CSF, or Nef039nm (Table 1). Interestingly, a large loss of DP and SP CD4+ thymocytes was documented even in Tg lines expressing lower levels of NefSF2, Nef032an, and NefNL4-3(T71R) RNA (Table 1), suggesting a high pathogenicity of these alleles. Intriguingly, in Tg mice expressing Nef032an or NefNL4-3(T71R), the loss of total and DP thymocytes was delayed, and it was not as marked in younger (1.5- to 2-month-old) mice as it was for mice expressing other Nef alleles but became severe as the animals got older (approximately 4 to 6 months old). The loss of thymocytes was accompanied by a downregulation of CD4 cell surface molecule in all Tg mice expressing higher levels of Nef alleles, except those expressing NefYU10x (Table 1). The lack of CD4 downregulation in CD4+ T-cell thymocytes expressing NefYU10x and the very low depletion of these cells is unlikely to reflect lack of protein expression, since mice of the higher-expressor founder were found to express reasonable protein levels relative to those detected in CD4C/HIV-NefNL4-3 Tg mice (Fig. 4B). Rather, this allele appears to be defective in the CD4 downregulation function both in human Jurkat cells (Fig. 2B) and in primary mouse cells (Table 1 and Fig. 5A).
FIG. 5.
Immunophenotypic analysis of thymic and peripheral T lymphocytes from CD4C/HIV-Nefallele Tg mice. Thymus (A) and peripheral LN (pLN) (B) cells from a representative Tg mouse with each allele (CD4C/HIV-NefAD93 [F115820], CD4C/HIV-Nef032an [F95582], CD4C/HIV-Nef039nm [F75581], CD4C/HIV-NefJR-CSF [F104995], CD4C/HIV-NefYU10x [F92751], CD4C/HIV-NefSF2 [F92748], CD4C/HIV-NefNL4-3(T71R) [F101376], and CD4C/HIV-NefNL4-3(WT) [F27367]) and from a non-Tg littermate were analyzed the same day by flow cytometry for the expression of CD4 and CD8. The percentage of cells found in the relevant quadrant is indicated on the left of the colon, while the underlined number on the right refers to the mean fluorescence intensity. Note the presence of two subsets of CD4+ T cells (CD4high and CD4low) in pLN cells expressing Nef039nm and to a lesser extent in those expressing NefSF2 and NefJR-CSF. These correspond, respectively, to cells expressing low and high levels of Nef, as previously reported for Tg mice expressing NefNL4-3(WT) (103). These data are representative of at least three independent experiments with 4 to 10 mice. (C) Three-color FACS analysis (CD4-allophycocyanin, CD8-fluorescein isothiocyanate, and CD44-PE) was performed on pLN cells from a representative mouse from CD4C/HIV-NefAD-93 (F115820), CD4C/HIV-Nef032an (F95582 and F95581 [not shown]), CD4C/HIV-Nef039nm (F75581 and F115817 [not shown]), CD4C/HIV-NefYU10x (F92751), CD4C/HIV-NefJR-CSF (F104995), CD4C/HIV-NefSF2 (F92748), or CD4C/HIV-NefNL4-3(T71R) (F101376 and F101374 [not shown]) Tg lines and from a non-Tg littermate. Isotype control antibody was used as a negative control. Data are shown only for the CD4+ T-cell population. The results shown are representative of at least two or three independent experiments. (D) Table representing ratios (Tg/non-Tg) of the percentages of cells expressing (high for CD25, CD44, and CD69) or not expressing (negative/low for CD62L and CD45RB) the indicated cell surface marker, as shown in panel C. Four to six Tg mice of each allelic line along with their respective non-Tg littermates lines were used. (E) Quantitation of apoptotic/dead peripheral CD4+ T cells from the same CD4C/HIV-Nefallele Tg mice whose analysis is shown in panel D. LN cells of Tg and non-Tg littermates were analyzed by FACS after staining with anti-CD4 monoclonal antibody and 7AAD. The data were pooled from results from Tg and non-Tg mice of each line as described for panel D and represent ratios (Tg/non-Tg) of the percentage of apoptotic/dead cells among the CD4+ T cells. Statistical analysis was performed with the Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
A similar FACS analysis on cells of peripheral lymphoid organs (mesenteric and peripheral LN) showed depletion of peripheral CD4+ T cells in Tg mice expressing higher levels of each of the Nef alleles tested, although the depletion in those expressing NefYU10x was more modest (Table 2). The downregulation of cell surface CD4 was also apparent in peripheral CD4+ T cells from all Tg mice expressing the transgene at higher levels, except those expressing NefYU10x and Nef032an (Table 2; see also the FACS profile of a representative mouse in Fig. 5B). The lack of downregulation of CD4 in peripheral cells from Nef032an Tg mice was intriguing. It was unlikely to represent an intrinsic lack of CD4 downregulation potential, since Tg thymocytes expressing this allele showed CD4 downregulation and this nef allele was capable of down-modulating CD4 in vitro (Fig. 2). It may arise from the lack of sufficient Nef expression, since expression was low in these founders. To test this possibility directly, we measured the levels of HIV-1 Tg RNA expression using quantitative RT-PCR on sorted peripheral CD4+ T cells from CD4C/HIV-Nef032an (F95581 and F95582) Tg mice relative to those from CD4C/HIV-NefNL4-3 Tg mice. This analysis showed indeed very low levels of Tg RNA in these mice (50- to 100-fold lower in both founders relative to NefNL4-3-expressing cells) (data not shown). Loss of CD8+ T cells both in the thymus and peripheral organs could also be documented at a later stage of the disease in most animals, as previously reported for NefNL4-3-expressing Tg mice (34, 35, 103). The number of B220+ (B cells) and Mac1+ (macrophages) cells were sometimes increased in the spleen of these Tg mice (data not shown).
TABLE 2.
Cell numbers in T-cell subsets of peripheral lymphoid organs from Tg mice expressing different Nef alleles
| Mouse linea | Absolute cell no. (106)b
|
CD4/CD8 ratio | Mean fluorescence (%)c of CD4 | ||
|---|---|---|---|---|---|
| Total LN | CD4 | CD8 | |||
| Non-Tgd | 21.5 ± 5.5 | 10.6 ± 2.5 | 4.3 ± 0.8 | 2.5 ± 0.4 | 100 |
| AD-93 | |||||
| F115817 | 20.1 ± 18.9 | 9.8 ± 8.0 | 3.0 ± 2.5 | 3.1 ± 0.2 | 100 |
| F115820 | 8.4 ± 5.7e | 1.8 ± 1.2e | 2.2 ± 1.6f | 1.0 ± 0.4e | 65 ± 10f |
| 032an | |||||
| F95581 | 9.7 ± 5.2f | 2.9 ± 2.0e | 1.6 ± 0.8e | 1.8 ± 0.3f | 89 ± 6 |
| F95582 | 7.8 ± 5.4e | 3.0 ± 2.6e | 1.6 ± 1.6f | 1.9 ± 1.1 | 85 ± 9 |
| 039nm | |||||
| F75581 | 5.8 ± 3.0e | 0.8 ± 0.3e | 1.1 ± 0.3e | 0.7 ± 0.1e | 25 ± 8e |
| F51444 | 12.5 ± 7.0f | 2.4 ± 1.1e | 2.1 ± 0.6e | 1.1 ± 0.2e | 84 ± 16 |
| JR-CSF | |||||
| F104995 | 6.6 ± 4.0e | 1.4 ± 1.2e | 1.9 ± 0.8e | 0.5 ± 0.2e | 71 ± 16f |
| F104996 | 15.3 ± 5.0 | 3.0 ± 1.4e | 6.1 ± 2.5 | 0.5 ± 0.2e | 11 ± 3e |
| YU10x | |||||
| F92751 | 11.3 ± 2.0e | 5.3 ± 1.0e | 3.1 ± 0.6f | 1.7 ± 0.2e | 97 ± 3 |
| F92754 | 12.9 ± 5.5f | 6.2 ± 1.8 | 2.8 ± 0.9f | 2.1 ± 0.2 | 94 ± 6 |
| SF-2 | |||||
| F92748 | 5.9 ± 4.0e | 0.3 ± 0.2e | 0.9 ± 0.1e | 0.3 ± 0.2e | 44 ± 26e |
| F92749 | 20.3 ± 15.0 | 9.3 ± 6.0 | 4.1 ± 3.8 | 2.7 ± 0.6 | 99 ± 9 |
| T71R | |||||
| F101376 | 11.5 ± 3.0f | 5.4 ± 1.0e | 1.9 ± 0.5e | 2.8 ± 0.4 | 98 ± 2 |
| F101374 | 9.3 ± 2.0e | 3.7 ± 1.0e | 2.4 ± 0.4e | 1.5 ± 0.3e | 65 ± 20f |
| F101371 | 28.4 ± 5.5 | 14.1 ± 3.0 | 5.9 ± 1.0 | 3.0 ± 0.3 | 95 ± 7 |
| F86707 | 8.8 ± 1.5e | 1.3 ± 0.3e | 2.2 ± 0.3e | 1.0 ± 0.1e | 21 ± 2e |
| Non-Tgd | 19.5 ± 3.5 | 9.3 ± 2.5 | 4.3 ± 1.0 | 2.2 ± 0.4 | 100 |
| NL4-3(WT) | 6.3 ± 3.6e | 1.2 ± 0.5e | 1.7 ± 0.9e | 0.6 ± 0.1e | 24 ± 11e |
FACS analysis was performed on 4 to 10 mice (1.5 to 4 months old) for each Tg line, including the positive control CD4C/HIV-NefNL4-3(WT) Tg line. Data from Tg mice were always compared to those of their littermates done the same day.
The absolute cell number was calculated by multiplying the total number of cells in each organ by the percentage of each cell subset in this organ.
The mean fluorescences for CD4 were obtained by calculating the ratio of CD4 staining in Tg mouse thymuses relative to that of non-Tg mouse thymuses (100%) done in the same experiment, the same day. Mean values were then calculated with the values for each line.
The non-Tg control values were obtained by pooling the results of all non-Tg littermates from different lines.
Significant decrease (P < 0.01) according to Student's t test.
Significant decrease (P < 0.05) according to Student's t test.
Since CD4+ T cells expressing some of these nef alleles are as depleted as those expressing NefNL4-3 and since the latter cells were previously reported to exhibit an activated/memory-like phenotype (103), we investigated whether CD4+ T cells expressing some Nef alleles were also activated. Triple staining of peripheral LN cells from Tg mice expressing various nef alleles was performed for CD4, CD8, and CD25, CD44, CD69, CD45RB, or CD62L cell surface markers. This FACS analysis revealed that in Tg lines expressing each nef allele tested, aside from NefYU10x and NefNL4-3(T71R), the percentage of CD4+ T cells expressing CD44high, CD45RBlow, or CD62Llow was higher than that for non-Tg mice and that a larger proportion of them expressed CD69 and CD25 (Fig. 5D; see data shown for one representative mouse in Fig. 5C). This activated/memory-like phenotype is similar to that previously reported for CD4+ T cells expressing NefNL4-3 (103). It is worth noting that the activated phenotype of Nef032an-expressing CD4+ T cells developed despite the absence of downregulation of CD4 and the very low levels of Nef expression in these cells.
As shown before, Tg CD4+ T cells expressing NefNL4-3 exhibit an enhanced apoptotic phenotype (84). A FACS analysis performed with annexin V or 7AAD labeling revealed that Tg mice expressing all these Nef alleles, except those expressing NefYU10x or Nef NL4-3(T71R) showed a significantly higher proportion of apoptotic/dead CD4+ T cells (Fig. 5E).
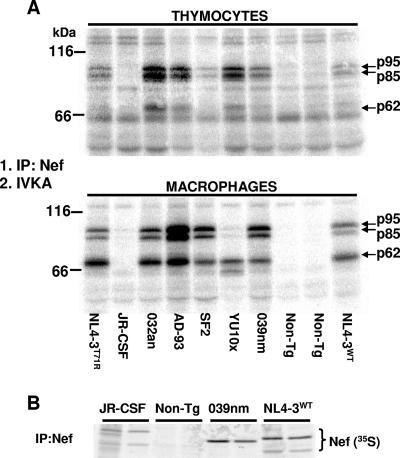
We have recently reported that NefNL4-3 binds to and activates PAK2 when expressed in various immune cell populations of Tg mice (102). A similar experiment was performed on Tg thymocytes and macrophages of Tg mice expressing each Nef allele. Anti-Nef immunoprecipitation was followed by IVKA, and the 32P-labeled substrates, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), were visualized by autoradiography. All the tested Nef alleles except the NefJR-CSF allele were found capable of binding to and activating a Nef-associated kinase (NAK) (Fig. 6). Moreover, the 32P-labeled substrates had migration patterns very similar to those obtained with NefNL4-3-expressing cells, strongly suggesting that this NAK is PAK2, as previously documented (102). The lack of mouse PAK2 activation by NefJR-CSF confirms previous results in studies of human cells in vitro (28). Interestingly, we found that PAK2 was activated reproducibly more strongly in NefSF2-expressing macrophages than in thymocytes, while the converse was true for cells expressing NefYU10x. Low expression levels may not totally explain this differential activation. Indeed, another Nef allele (the Nef032an allele) expressed at levels as low as the NefSF2 level in thymocytes induced a very strong PAK2 activation. Moreover, NefYU10x induced very weak PAK2 activation in macrophages, despite being expressed at levels equivalent to those of wild-type NefNL4-3 in these cells (see below). Together, these results suggest the presence of a cell-specific factor(s) able to module the PAK2-Nef interaction in an allele-specific manner. To investigate whether the lack of in vitro kinase observed in NefJR-CSF-expressing extracts was related to the inability of the anti-Nef antibody to immunoprecipitate this protein, we performed anti-Nef immunoprecipitation and SDS-PAGE migration on NefJR-CSF- and control NefNL4-3(WT)-expressing thymocytes labeled with [35S]methionine. This experiment revealed substantial levels of the 35S-labeled NefJR-CSF proteins, although not as high as those of NefNL4-3(WT) proteins (Fig. 6B). Thus, the inability to bind to and/or to activate PAK2 appears to be intrinsic to the JR-CSF Nef allele and may be caused by the presence of leucine at position 201 (equivalent to 191 in NefNL4-3), as shown previously (28). Therefore, this Nef-dependent PAK2 activation appears to be a conserved function among most different Nef alleles.
FIG. 6.
Interaction of various Nef alleles with NAK in Tg mice. IP, immunoprecipitation. (A) Total protein extracts from thymocytes (250 μg) and macrophages (100 μg) from CD4C/HIV-Nef Tg mice expressing the indicated Nef alleles and from their non-Tg littermates were incubated with rabbit anti-Nef polyclonal antibodies overnight and subjected to an IVKA using 10 μCi of [γ-32P]ATP for 5 min at room temperature. The phosphorylated proteins were next separated by SDS-PAGE and visualized by autoradiography. (B) Thymocytes from CD4C/HIV-NefJR-CSF, CD3C/HIV-Nef039, and CD4C/HIV-NefNL4-3(WT) Tg and non-Tg mice were metabolically labeled with [35S]methionine. Lysates were immunoprecipitated with rabbit polyclonal anti-Nef antibody produced in our laboratory. The 35S-labeled proteins were separated by SDS-PAGE and visualized by autoradiography. Note the ability of the polyclonal anti-Nef antibody used to immunoprecipitate the NefJR-CSF proteins effectively.
These results indicate that most of the tested Nef alleles in Tg mice affect CD4+ T cells in a way similar to that of the NefNL4-3 allele, which was previously reported (34, 35, 84, 102, 103). Only the NefYU10x allele appears to be significantly less pathogenic for Tg CD4+ T cells than all other alleles tested, despite being apparently expressed at levels higher than other Nef alleles.
Nef alleles differ in their abilities to induce AIDS-like lung and kidney organ diseases in Tg mice.
Tg mice and their non-Tg littermates from each line of Nef allele were monitored for clinical signs of disease (hypoactivity, ruffled hair, weakness, loss of body weight, and early death). Tg mice from different lines expressing NefNL4-3 (T71R), Nef032an, or Nef039nm all exhibited a mortality rate close to or higher than that observed for Tg mice expressing NefNL4-3(WT), while Tg mice expressing another Nef allele (the NefSF2 allele) showed minimal mortality (Fig. 7A). For these NefSF2 Tg mice, this may be related to the relatively low levels of Nef RNA expression. It is worth noting that the high mortality rate of CD4C/HIV-NefNL4-3(T71R) Tg mice occurs despite HIV RNA expression lower than that in CD4C/HIV-NefNL4-3(WT) Tg mice, suggesting a higher pathogenicity for this variant. For all Tg lines of each Nef allele that exhibited high mortality (032an, 039nm, SF2, and NL4-3 [T71R] Nef alleles), a good correlation between shorter survival and higher level of transgene HIV RNA expression in thymocytes could be observed for founders expressing the same allele. One notable exception was the Tg line F101376 expressing the NefT71R allele: mice from this line had a shorter life span than did those from the F101374 or F101371 lines despite showing equivalent or lower transgene expression in thymocytes. Since organ disease, in particular, kidney disease, largely responsible for poor survival, segregates independently of T-cell loss (36, 37, 102) (see Table 4) and can develop in absence of CD4+ T cells (103), this suggests that another subset(s) of cells critical for organ disease induction expresses the transgene. In the mouse line F101376, the site of transgene insertion may favor enhanced transcription through the CD4C regulatory elements in this particular cell subset yet to be identified.
FIG. 7.
Survival and histopathology of CD4/HIV-Nefallele Tg mice. (A) Nef-expressing strains showing shorter survival and/or pathological lesions. (Upper panel) Cumulative incidence of mortality. Mice from the indicated Tg lines were observed for a period of up to 12 months. The cumulative incidence of mortality of the different Tg founder lines from each indicated Tg mouse strain, as well as that of their non-Tg littermates, was plotted as the percentage of surviving mice. NefNL4-3(WT) represents the CD4C/HIV-NefNL4-3(WT) (F27367) Tg mice used as positive controls. The number of animals observed (n) in each indicated Tg line is shown. The number of non-Tg mice represents the sum of control mice for all founders in these lines. (Lower panel) Kidney histology of a representative Tg mouse from corresponding Tg lines. All these Tg mice exhibit the typical pathology previously observed for CD4C/HIV-NefNL4-3(WT) Tg mice. Note the tubular dilatation and atrophy, with some cystic changes, tubulointerstitial nephritis as well as focal segmental glomerulosclerosis in all Tg kidneys. (B) No or minimal lung or kidney organ disease in CD4C/HIV-NefYU10x, CD4C/HIV-NefAD-93, and CD4C/HIV-NefJR-CSF Tg mice. (Upper panel) Absence or very low mortality. Mice were observed for up to 12 months. The data are presented as in the upper panel of panel A. (Lower panel) Light microscopic analysis of kidneys from Tg mice. The histological appearance of the kidney from these Tg animals was indistinguishable (NefYU10x and NefAD-93) or showed only minimal lesions (NefJR-CSF) relative to that of non-Tg mice. (C) Semiquantitative assessment of the kidney disease in Tg mice. A score 1 to 4 was assigned to each mouse, and the average score for each group is shown.
TABLE 4.
Summary of phenotypes in CD4C/HIV Tg mice expressing different Nef alleles
| Nef allele | Phenotypea
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In vitro infectivityb | Downregulation of CD4
|
Activated/memory | Depletion of T cells
|
Lung and kidney disease | PAK activity | |||||
| In vitro | In vivo
|
LN CD4 | Thymus
|
|||||||
| Thymus DP | LN CD4+ | DP | CD4+ SP | |||||||
| NL4-3 | +++e | +++ | +++ | ++++ | Yes | ++++ | +++ | +++ | ++++ | Yes |
| NL4-3(T71R) | ++++ | +++ | +++ | ++++ | No | ++++ | +++ | +++ | ++++ | Yes |
| AD-93 | ++++ | + | ++ | ++ | Yes | ++++ | ++++ | ++ | − | Yes |
| 032an | +++ | +++ | ++ | +c | Yes | ++ | ++++ | +++ | ++++ | Yes |
| 039nm | +++ | +++ | +++ | ++++ | Yes | ++++ | +++ | ++ | ++++ | Yes |
| JR-CSF | +/− | ++++ | ++++ | ++++ | Yes | +++ | +++ | +++ | + | No |
| YU10x | ++++ | + | − | − | No | + | + | + | − | Yes |
| SF2 | +++ | +++ | +++ | +++ | Yes | ++++ | ++++ | ++++ | +d | Yes |
The most severe phenotypes observed in the higher-expressor Tg lines are shown for each Nef allele.
Measured on P4-CCR5 cells (see Fig. 2D).
The low CD4 downregulation in LN of Nef032an-expressing Tg mice is most likely related to very low levels of expression in these cells.
Lung and kidney diseases are apparent in NefSF2-expressing Tg mice, despite low levels of expression, suggesting high pathogenicity.
Each phenotype was scored as very mild (+), mild (++), severe (+++), and very severe (++++).
Histological evaluation of tissues from Nef032an, Nef039nm, NefNL4-3(T71R), or NefSF2-expressing Tg mice revealed pathological changes indistinguishable from those previously observed in CD4C/HIV-NefNL4-3 Tg mice (34, 35) (Fig. 7A; Table 3). Phenotypes include loss of architecture and atrophy of lymphoid organs, as well as kidney (interstitial nephritis, focal segmental glomerulosclerosis, and microcystic dilatation) and lung (lymphocytic interstitial pneumonitis) diseases (Fig. 7A; Table 3). These pathological lesions affect various percentages of Tg mice and were of different severities, depending on the individual allele and its level of expression (Fig. 7C). This was especially evident for NefSF2 Tg mice, which showed typical lesions despite the low levels of allele expression. Importantly, novel phenotypes not previously documented in Tg mice expressing the NefNL4-3 allele were not observed in these Tg mice.
TABLE 3.
Histopathological assessment of Tg mice expressing different Nef alleles
| Diseased organ | No. of animals with disease/total number of animalsa
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD-93
|
032an
|
039nm
|
JR-CSF
|
YU10x
|
SF2
|
NL4-3T71R
|
||||||||
| F115820 (M) | F115817 (L) | F95582 (L-M) | F95581 (L-M) | F75581 (M) | F51444 (M) | F104996 (L) | F104995 (H) | F92754 (L) | F92751 (H) | F92748 (L) | F92749 (L) | F101376 (L) | F101371 (L) | |
| Thymusb | 9/12 | 2/7 | 6/7 | 1/9 | 13/13 | 5/9 | 2/12 | 8/14 | 2/17 | 0/7 | 7/10 | 0/7 | 3/7 | 0/10 |
| LNc | 11/13 | 8/9 | 6/9 | 4/11 | 7/11 | 5/8 | 4/12 | 4/14 | 7/17 | 3/13 | 2/7 | 3/11 | 4/8 | 7/14 |
| Kidneyd | 0/14 | 0/9 | 11/11 | 4/11 | 13/13 | 9/9 | 5/12 | 2/14 | 1/18f | 0/13 | 5/13g | 3/14g | 13/13 | 8/15 |
| Lunge | 0/14 | 0/9 | 0/11 | 2/11 | 3/13 | 2/9 | 2/12 | 0/14 | 0/18 | 0/13 | 0/13 | 2/14g | 0/13 | 2/15 |
Number of animals with disease over total number of animals studied for the two founders of each Tg line showing the most severe phenotype. Non-Tg mice (n = 86) showed none of these phenotypes. The levels of RNA expression in the thymus are provided in parentheses. L, low; M, medium; H, high. Medium expression is comparable to that of NefNL4-3(WT) (F27367).
Atrophy and disorganization.
Diseases include follicle hyperplasia, fragmentation, involution, and relative enlarged paracortex with lymphocytopenia. For the 032an, 039nm, JR-CSF, and NL4-3T71R Nef alleles, the predominant change is depletion of lymphocytes throughout the LN.
Different degrees of glomerulopathy include focal segmental glomerulosclerosis and tubulointerstitial nephritis with microcystic dilation.
Lymphocytic interstitial pneumonitis.
Despite high expression levels in NefYU10x (F92751)-expressing mice, kidney disease was mild.
The low penetrance of disease in NefSF2 Tg mice is likely to be related to the low levels of expression in both founder lines relative to those of the NefNL4-3(WT) Tg mice.
Therefore, the pathological changes previously observed in Tg mice expressing the NefNL4-3 allele were not restricted to this single allele but could also be elicited with other primary Nef alleles. In fact, Tg mice expressing some nef alleles from LTNP (032an and 039nm) or other nef alleles (SF2 and NL4-3T71R) appear to be phenocopies of CD4C/HIV-NefNL4-3 Tg mice.
Intriguingly, Tg mice expressing NefJR-CSF, NefAD-93, or NefYU10x RNA at levels comparable to or even higher than that of NefNL4-3 or Nef032an behave differently. Mice from both founder lines of each of the CD4C/HIV-NefYU10x, CD4C/HIV-NefAD-93, or CD4C/HIV-NefJR-CSF Tg strain had life spans comparable to those of their non-Tg littermates, free of apparent clinical signs of disease, for the whole period of observation (up to 12 months) (Fig. 7B). Histological examination of their organs showed pathological lesions (loss of architecture and atrophy) in their lymphoid organs (LN and spleen) as severe as those observed in Tg mice expressing the NefNL4-3 allele but not in all mice (Table 3 and data not shown). However, these Tg mice were free of lung and kidney diseases (YU10x and AD-93) or had only minimal lung and kidney diseases (JR-CSF) (Fig. 7B and C), despite the fact that transgene RNA expression in the thymus was comparable to or slightly higher than that of CD4C/HIV-NefNL4-3 or CD4C/HIV-Nef032an Tg mice (Fig. 4A).
The CD4C regulatory elements used to express the different alleles have been previously shown to be specific for immature and mature CD4+ T cells and cells of the macrophage/dendritic lineage. To ascertain that the lack of development of the kidney and lung diseases in the CD4C/HIV-NefYU10x and CD4C/HIV-NefJR-CSF Tg mice was not the consequence of a lack of expression of the transgene in myeloid cells, we measured the levels of transgene RNA in macrophages of these Tg mice. Transgene RNA expression in NefYU10x (F92751) or NefJR-CSF (F104995) Tg mouse macrophages was found to be comparable to that in NefNL4-3-expressing mouse macrophages (data not shown), suggesting that the poor capability of these alleles to elicit organ diseases in Tg mice is unlikely to be related to low transgene expression in myeloid cells but rather to their low intrinsic virulence.
These results indicate that the NefYU10x and NefAD-93 alleles and the NefJR-CSF allele are, respectively, nonpathogenic or poorly pathogenic for kidney and lungs of Tg mice when expressed in CD4+ T cells and in cells of the macrophage/dendritic lineage.
A summary of the phenotypes observed in these Tg mice expressing different nef alleles is shown in Table 4.
DISCUSSION
The HIV-1 NefNL4-3 allele induces a severe AIDS-like disease in Tg mice when expressed under the control of the CD4C regulatory elements in immature and mature CD4+ T cells and in cells of the macrophage/dendritic lineage (34, 35). We show here that this property is not unique and restricted to this specific allele but is shared by other Nef alleles tested in the same in vivo assay. It is worth noting that novel phenotypes, absent in Tg mice expressing the NefNL4-3 alleles, were not observed in Tg mice expressing any of the new nef alleles tested. This strongly suggests that all the phenotypes to be elicited by various nef alleles, when expressed in their natural target cells in Tg mice, have indeed developed in NefNL4-3-expressing Tg mice. These pathological changes are quite numerous and in fact cover most of the phenotypes associated with this syndrome (AIDS) in humans or in SIV-infected primates, except for pathologies which are known to be caused by pathogens other than HIV or SIV itself.
However, although the seven new nef alleles tested induced CD4+ T-cell depletion to various degrees, they did not induce identical phenotypes, and important differences could be noted. In particular, our data clearly show that the pathogenic potentials of the alleles differ. For example, despite its robust expression, the NefYU10x allele induced only a modest loss of thymic and peripheral CD4+ T cells, by comparison with other alleles (NL4-3, 039an, and AD-93 Nef alleles). In contrast, the NefSF2 and Nef032an alleles were able to elicit significant loss of thymic and peripheral CD4+ T cells, despite their low expression levels. NefSF2 harbors at position 71 an arginine which has been shown to significantly enhance viral replication in immature DC/T-cell cocultures and to enhance association with T-cell-signaling molecules (Lck, Vav, and T-cell receptor ζ) (25). Our data also suggest that, when levels of Nef expression were taken into account, the mutated NefNL4-3(T71R) allele was more pathogenic in Tg mice than the parental NefNL4-3 wild-type allele, especially regarding survival and kidney lesions. However, an arginine at this position is not required for the development of an AIDS-like disease in Tg mice, since the Nef032an allele is highly pathogenic in Tg mice although it contains a threonine at the position corresponding to amino acid residue 71 in the NL4-3 Nef (Fig. 1). Thus, the influence of the R-to-T variation in the proline-rich region of Nef on its pathogenicity appears to be context dependent.
Further comparison between the immune phenotypes studied here also provided interesting hints regarding Nef pathogenicity. We indeed found an absence of CD4 downregulation on peripheral CD4+ T cells expressing Nef032an, most likely reflecting its low expression level in these cells, yet these cells were activated and depleted and showed enhanced apoptosis. The genetic segregation of these major phenotypes (CD4 downregulation versus activation/apoptosis/depletion) strongly suggests that CD4 downregulation itself on peripheral CD4+ T cells is not the cause of their depletion. These results are consistent with our previous observations on some mutants of NefNL4-3 (D174K and RD35/36AA mutants) impaired in their capacity for downregulating CD4 but still competent at inducing an activated/memory-like phenotype and a modest depletion of CD4+ T cells (36).
Previous studies have shown that some LTNP are infected with HIV-1 variants expressing nef alleles showing a diminished activity in CD4 downregulation (4, 68, 101). One of the three nef alleles from LTNP studied here (AD-93) also showed a diminished capacity for downregulating human CD4 relative to the that of the NL4-3 allele. We found that in CD4C/HIV-NefAD-93 Tg mice, CD4+ T-cell depletion was severe despite only modest CD4 downregulation. Also, the CD4C/HIV-Nef032an Tg mice did not exhibit much of a downregulation of murine CD4 on peripheral T cells despite showing a loss of this T-cell population and severe lung and kidney diseases. Thus, effective CD4 down-modulation does not seem sufficient for Nef to cause CD4+ T-cell depletion and severe disease in Tg mice.
A second apparently discordant finding observed with two nef alleles and not previously documented with the NefNL4-3 allele is the lack of an activated/memory-like phenotype and of detectable enhanced apoptosis of CD4+ T cells expressing NefNL4-3(T71R) (F101374 and F101376) and NefYU10x (F92751). However, the NefNL4-3(T71R)-expressing CD4+ T cells were modestly depleted to levels comparable to the levels of CD4+ T cells expressing other virulent alleles at comparable low levels (Nef032an). The genetic segregation of these two phenotypes suggests that the activated state of CD4+ T cells may not be the single major factor responsible for their Nef-induced depletion. The state of activation of the CD4+ T cells is now largely interpreted as a plausible mechanism for the CD4+ T-cell loss observed for HIV-1-infected individuals (22, 33, 41, 71, 97). Our results with this NefNL4-3(T71R) allele suggest alternative, yet unknown mechanism(s) of cell death induced by HIV-1 Nef in these Tg mice. We have already excluded Fas, FasL, interleukin-1β-converting enzyme, and tumor necrosis factor receptor I pathways by showing that depletion of NefNL4-3-expressing CD4+ T cells is not abrogated in Tg mice deficient in each of these four genes (84).
The third interesting discordant finding revealed by our study is that thymic and peripheral CD4+ T cells can be depleted independently following Nef expression. We indeed found that SP CD4+ thymocytes were depleted only mildly in most CD4C/HIV-Nef039nm Tg mice, while their peripheral CD4+ T cells were severely depleted. Similarly, in CD4C/HIV-NefAD-93 Tg mice, peripheral CD4+ T cells appeared more severely depleted than the thymic SP CD4+ T cells. Assuming that Nef expression levels are not downregulated specifically in SP CD4+ thymocytes, these results strongly suggest that Nef-mediated depletion of thymic and peripheral CD4+ T cells can occur independently and possibly through distinct pathways and that some Nef alleles (such as the Nef039nm or NefAD-93 allele) may interact more efficiently than others with effectors of peripheral CD4+ T cells than of thymic CD4+ T cells. In independent studies of Tg mice expressing some mutants of NefNL4-3 (D174K and RD35/36AA mutants), we previously reached similar conclusions (37). This may partly explain why the thymic function and regenerative capacity may be conserved better and for a longer time in some HIV-1-infected individuals (55, 70, 71).
The capacity for eliciting another organ phenotype, kidney disease (focal segmental glomerulosclerosis, microcystic dilatation, and tubulointerstitial nephritis), also distinguishes the seven new alleles tested. Three (YU10x, JR-CSF, and AD-93 Nef alleles) of the seven nef alleles were totally or almost completely defective at inducing kidney disease, even though their levels of RNA expression were comparable to or higher than the levels of other Nef alleles causing this disease, including the wild-type NefNL4-3 previously reported (35). This suggests that the molecular or cellular requirements for the induction of kidney disease are more stringent than those for the loss of CD4+ T cells, since two of these alleles (the JR-CSF and AD-93 Nef alleles) were able to induce significant CD4+ T-cell loss. A similar phenomenon was observed in studies of some mutants of NefNL4-3 in Tg mice, in which it was found that mutants had lost their capacity for eliciting kidney disease more easily than their capacity for inducing T-cell changes (37). More recently, it was reported that Tg mice expressing the whole genome of HIV-1JR-CSF under the regulation of its own long terminal repeat promoter showed only a modest depletion of peripheral CD4+ T cells but no changes in the thymocyte populations or any organ disease (99). These results are consistent with the phenotypes observed in our CD4C/HIV-NefJR-CSF Tg mice, i.e., a depletion of peripheral CD4+ T cells more severe than that of thymic CD4+ T cells and presence of only minimal organ diseases. The inability of NefJR-CSF to activate PAK2 may be responsible for some of its milder phenotypes. However, it would have to be hypothesized that such activation is not sufficient or context dependent, since another Nef allele (the YU10x Nef allele) with a similar phenotype is able to activate PAK2.
Interestingly, the three nef alleles from LTNP which were studied in Tg mice were quite pathogenic in this in vivo biological assay, while one allele from an AIDS patient (the YU10x Nef allele) had very low virulence. If this Tg assay is of any relevance for predicting pathogenicity in humans, this suggests that the slow tempo of AIDS development in the LTNP patients from which these nef genes were obtained reflects mutations in viral genes other than nef or is caused by a host genetic resistance in these individuals. It may also suggest that some of the HIV-1 variants molecularly cloned from AIDS patients may not fully represent the virulent HIV-1 population causing the disease. This is known to occur and has been well documented in the macaque model of SIV infection in which reinoculation of viruses is possible (6, 9, 44, 50, 66). The availability of such an in vivo Tg assay could then help in classifying LTNP patients in distinct subgroups and to assess the virulence of specific HIV-1 variants obtained from them and from individuals with AIDS. Obviously, such comparative analysis should take into account the specificity of the Tg system, i.e., minimal contribution of opportunistic infection, since these mice are kept in specific-pathogen-free conditions, presence of a genetic background susceptible for the development of HIV-1-associated nephropathy, and absence of virus replication.
In conclusion, our data indicate that the CD4C/HIV Tg mouse model system represents a biological in vivo assay for nef alleles. Although this assay does not score the influence of Nef on viral replication, it scores numerous other phenotypes caused by Nef expression in very distinct primary T-cell (immature and mature) and myeloid cell (macrophage/DC) subpopulations. These phenotypes consist of perturbations of immature and mature T cells as well as severe organ diseases similar to those found in human AIDS. We have shown here that very important different T-cell changes may be induced by different Nef alleles. These phenotypes could not have been scored in vitro or easily in HIV-infected humans, highlighting the need to study HIV-1 pathogenesis in primary, unstimulated T cells and the advantage to use an animal model for such an investigation. It remains to be determined whether such assay in the mouse has any predictive value regarding the behavior of Nef in infected humans.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, HIV/AIDS Research Program, to P.J. and Z.H. and from the Deutsche Forschungsgemeinschaft to F.K.
We thank Benoît Laganière, Ginette Massé, Pascale Jovert, Valérie Côté, Karina Lamarre, Stéphanie Lemay, Caroline Larocque, Marie-Eve Higgins, Eve-Lyne Thivierge, Jean-René Sylvestre, and Isabelle Corbin for their excellent technical assistance. We are grateful to Chantal Guertin for typing the manuscript.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrovandi, G. M., L. Gao, G. Bristol, and J. A. Zack. 1998. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J. Virol. 72:7032-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, S., D. C. Shugars, R. Swanstrom, and J. V. Garcia. 1993. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J. Virol. 67:4923-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 278:33912-33919. [DOI] [PubMed] [Google Scholar]
- 5.Auger, I., P. Thomas, V. De Gruttola, D. Morse, D. Moore, R. T. B. Williams, and C. E. Lawrence. 1988. Incubation periods for paediatric AIDS patients. Nature 336:575-577. [DOI] [PubMed] [Google Scholar]
- 6.Banapour, B., M. L. Marthas, R. J. Munn, and P. A. Luciw. 1991. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). Virology 183:12-19. [DOI] [PubMed] [Google Scholar]
- 7.Brady, H. J., D. J. Pennington, C. G. Miles, and E. A. Dzierzak. 1993. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. EMBO J. 12:4923-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchbinder, S. P., M. H. Katz, N. A. Hessol, P. M. O'Malley, and S. D. Holmberg. 1994. Long-term HIV-1 infection without immunologic progression. AIDS 8:1123-1128. [DOI] [PubMed] [Google Scholar]
- 9.Burns, D. P., and R. C. Desrosiers. 1991. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J. Virol. 65:1843-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 11.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casartelli, N., G. Di Matteo, C. Argentini, C. Cancrini, S. Bernardi, G. Castelli, G. Scarlatti, A. Plebani, P. Rossi, and M. Doria. 2003. Structural defects and variations in the HIV-1 nef gene from rapid, slow and non-progressor children. AIDS 17:1291-1301. [DOI] [PubMed] [Google Scholar]
- 13.Casartelli, N., G. Di Matteo, M. Potesta, P. Rossi, and M. Doria. 2003. CD4 and major histocompatibility complex class I downregulation by the human immunodeficiency virus type 1 Nef protein in pediatric AIDS progression. J. Virol. 77:11536-11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng-Mayer, C., P. Iannello, K. Shaw, P. A. Luciw, and J. A. Levy. 1989. Differential effects of nef on HIV replication: implications for viral pathogenesis in the host. Science 246:1629-1632. [DOI] [PubMed] [Google Scholar]
- 15.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 16.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, S. J. O'Brien, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 17.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, Z. Zhang, W. A. O'Brien, L. Ratner, G. M. Shaw, and E. Hunter. 2001. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virol. 75:8605-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Repentigny, L., F. Aumont, J.-S. Ripeau, M. Fiorillo, D. G. Kay, Z. Hanna, and P. Jolicoeur. 2002. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J. Infect. Dis. 185:1103-1114. [DOI] [PubMed] [Google Scholar]
- 19.de Ronde, A., B. Klaver, W. Keulen, L. Smit, and J. Goudsmit. 1992. Natural HIV-1 NEF accelerates virus replication in primary human lymphocytes. Virology 188:391-395. [DOI] [PubMed] [Google Scholar]
- 20.Dickie, P. 1996. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res. Hum. Retrovir. 12:177-189. [DOI] [PubMed] [Google Scholar]
- 21.Dickie, P. 2000. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res. Hum. Retrovir. 16:777-790. [DOI] [PubMed] [Google Scholar]
- 22.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 23.Du, Z., S. M. Lang, V. G. Sasseville, A. Lackner, P. O. Ilyinskii, M. D. J. J. Daniel, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 24.Easterbrook, P. J. 1994. Non-progression in HIV infection. AIDS 8:1179-1182. [DOI] [PubMed] [Google Scholar]
- 25.Fackler, O. T., D. Wolf, H. O. Weber, B. Laffert, P. D'Aloja, B. Schuler-Thurner, R. Geffin, K. Saksela, M. Geyer, B. M. Peterlin, G. Schuler, and A. S. Baur. 2001. A natural variability in the proline-rich motif of Nef modulates HIV-1 replication in primary T cells. Curr. Biol. 11:1294-1299. [DOI] [PubMed] [Google Scholar]
- 26.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 27.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 28.Foster, J. L., R. P. Molina, T. Luo, V. K. Arora, Y. Huang, D. D. Ho, and J. V. Garcia. 2001. Genetic and functional diversity of human immunodeficiency virus type 1 subtype B Nef primary isolates. J. Virol. 75:1672-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geffin, R., D. Wolf, R. Muller, M. D. Hill, E. Stellwag, M. Freitag, G. Sass, G. B. Scott, and A. S. Baur. 2000. Functional and structural defects in HIV type 1 nef genes derived from pediatric long-term survivors. AIDS Res. Hum. Retrovir. 16:1855-1868. [DOI] [PubMed] [Google Scholar]
- 30.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenway, A. L., D. A. McPhee, E. Grgacic, D. Hewish, A. Lucantoni, and I. A. A. Macreadie. 1994. Nef 27, but not the Nef 25 isoform of human immunodeficiency virus-type 1 pNL4.3 down-regulates surface CD4 and IL-2R expression in peripheral blood mononuclear cells and transformed T cells. Virology 198:245-256. [DOI] [PubMed] [Google Scholar]
- 33.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 34.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 36.Hanna, Z., E. Priceputu, C. Hu, P. Vincent, and P. Jolicoeur. 2006. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology 346:40-52. [DOI] [PubMed] [Google Scholar]
- 37.Hanna, Z., E. Priceputu, D. G. Kay, J. Poudrier, P. Chrobak, and P. Jolicoeur. 2004. In vivo mutational analysis of the N-terminal region of HIV-1 Nef reveals critical motifs for the development of an AIDS-like disease in CD4C/HIV transgenic mice. Virology 327:273-286. [DOI] [PubMed] [Google Scholar]
- 38.Hanna, Z., N. Rebai, J. Poudrier, and P. Jolicoeur. 2001. Distinct regulatory elements are required for faithful expression of human CD4 in T-cells, macrophages and dendritic cells of transgenic mice. Blood 98:2275-2278. [DOI] [PubMed] [Google Scholar]
- 39.Hanna, Z., C. Simard, and P. Jolicoeur. 1994. Specific expression of the human CD4 gene in mature CD4+CD8− and immature CD4+CD8+ T cells, and in macrophages of transgenic mice. Mol. Cell. Biol. 14:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanna, Z., X. Weng, D. G. Kay, J. Poudrier, C. Lowell, and P. Jolicoeur. 2001. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J. Virol. 75:9378-9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 42.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 43.Isaksson, B., J. Albert, F. Chiodi, A. Furucrona, A. Krook, and P. Putkonen. 1988. AIDS two months after primary human immunodeficiency virus infection. J. Infect. Dis. 158:866-868. [DOI] [PubMed] [Google Scholar]
- 44.Johnson, P. R., T. E. Hamm, S. Goldstein, S. Kitov, and V. M. Hirsch. 1991. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology 185:217-228. [DOI] [PubMed] [Google Scholar]
- 45.Jolicoeur, P., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and Z. Hanna. 1999. A novel mouse model of HIV-1 disease. Leukemia 13:S78-S80. [DOI] [PubMed] [Google Scholar]
- 46.Jolicoeur, P., P. Yue, Z. Hanna, M.-C. Simard, and D. G. Kay. 2004. Cardiac disease in HIV-1 Tg mice, p. 377-386. In R. R. Watson (ed.), AIDS and heart disease. Marcel Dekker Inc., New York, NY.
- 47.Kajiyama, W., J. B. Kopp, N. J. Marinos, P. E. Klotman, and P. Dickie. 2000. Glomerulosclerosis and viral gene expression in HIV-transgenic mice: role of nef. Kidney Int. 58:1148-1159. [DOI] [PubMed] [Google Scholar]
- 48.Kawano, Y., Y. Tanaka, N. Misawa, R. Tanaka, J. I. Kira, T. Kimura, M. Fukushi, K. Sano, T. Goto, M. Nakai, T. Kobayashi, N. Yamamoto, and Y. Koyanagi. 1997. Mutational analysis of human immunodeficiency virus type 1 (HIV-1) accessory genes: requirement of a site in the nef gene for HIV-1 replication in activated CD4+ T cells in vitro and in vivo. J. Virol. 71:8456-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kay, D. G., P. Yue, Z. Hanna, S. Jothy, E. Tremblay, and P. Jolicoeur. 2002. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 Nef in cells of the immune system. Am. J. Pathol. 161:321-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kestler, H. W., Y. N. Naidu, T. Kodama, N. W. King, M. D. Daniel, Y. Li, and R. C. Desrosiers. 1989. Use of infectious molecular clones of simian immunodeficiency virus for pathogenesis studies. J. Med. Primatol. 18:305-309. [PubMed] [Google Scholar]
- 51.Kestler, H. W., D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 52.Kirchhoff, F., P. J. Easterbrook, N. Douglas, M. Troop, T. C. Greenough, J. Weber, S. Carl, J. L. Sullivan, and R. S. Daniels. 1999. Sequence variations in human immunodeficiency virus type 1 Nef are associated with different stages of disease. J. Virol. 73:5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 54.Kirchhoff, F., M. Schindler, N. Bailer, G. H. Renkema, K. Saksela, V. Knoop, M. C. Muller-Trutwin, M. L. Santiago, F. Bibollet-Ruche, M. T. Dittmar, J. L. Heeney, B. H. Hahn, and J. Munch. 2004. Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors. J. Virol. 78:6864-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 56.Koyanagi, Y., S. Miles, R. T. Mitsuyasu, J. E. Merrill, H. V. Vinters, and I. S. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 57.Larsen, N. B., H. W. Kestler, and J. J. Docherty. 1998. Mice transgenic for simian immunodeficiency virus nef are immunologically compromised. J. Biomed. Sci. 5:260-266. [DOI] [PubMed] [Google Scholar]
- 58.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 59.Le Gall, S., J. M. Heard, and O. Schwartz. 1997. Analysis of Nef-induced MHC-I endocytosis. Res. Virol. 148:43-47. [DOI] [PubMed] [Google Scholar]
- 60.Levy, J. A., A. D. Hoffman, S. M. Kramer, J. A. Landis, J. M. Shimabukuro, and L. S. Oshiro. 1984. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science 225:840-842. [DOI] [PubMed] [Google Scholar]
- 61.Li, Y., H. Hui, C. J. Burgess, R. W. Price, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1992. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J. Virol. 66:6587-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li, Y. X., J. C. Kappes, J. A. Conway, R. W. Price, G. M. Shaw, and B. H. Hahn. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lifson, A. R., G. W. Rutherford, and H. W. Jaffe. 1988. The natural history of human immunodeficiency virus infection. J. Infect. Dis. 158:1360-1367. [DOI] [PubMed] [Google Scholar]
- 64.Lindemann, D., R. Wilhelm, P. Renard, A. Althage, R. Zinkernagel, and J. Mous. 1994. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J. Exp. Med. 179:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in hiv-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 66.Luciw, P. A., K. E. Shaw, R. E. Unger, V. Planelles, M. W. Stout, J. E. Lackner, E. Pratt-Lowe, N. J. Leung, B. Banapour, and M. L. Marthas. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res. Hum. Retrovir. 8:395-402. [DOI] [PubMed] [Google Scholar]
- 67.Mackay, G. A., Y. Niu, Z. Q. Liu, S. Mukherjee, Z. Li, I. Adany, S. Buch, W. Zhuge, H. M. McClure, O. Narayan, and M. S. Smith. 2002. Presence of intact vpu and nef genes in nonpathogenic SHIV is essential for acquisition of pathogenicity of this virus by serial passage in macaques. Virology 295:133-146. [DOI] [PubMed] [Google Scholar]
- 68.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mariani, R., and J. Skowronski. 1993. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl. Acad. Sci. USA 90:5549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCune, J. M. 1997. Thymic function in HIV-1 disease. Semin. Immunol. 9:397-404. [DOI] [PubMed] [Google Scholar]
- 71.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 72.McLean, K. A., D. A. Holmes, B. A. Evans, L. McAlpine, R. Thorp, J. V. Parry, and M. G. Glaser. 1990. Rapid clinical and laboratory progression of HIV infection. AIDS 4:369-371. [DOI] [PubMed] [Google Scholar]
- 73.Michael, N. L. 1999. Host genetic influences on HIV-1 pathogenesis. Curr. Opin. Immunol. 11:466-474. [DOI] [PubMed] [Google Scholar]
- 74.Michael, N. L., G. Chang, L. A. d'Arcy, C. J. Tseng, D. L. Birx, and H. W. Sheppard. 1995. Functional characterization of human immunodeficiency virus type 1 nef genes in patients with divergent rates of disease progression. J. Virol. 69:6758-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munch, J., M. Schindler, S. Wildum, E. Rucker, N. Bailer, V. Knoop, F. J. Novembre, and F. Kirchhoff. 2005. Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef alleles modulate cell surface expression of various human receptors and enhance viral infectivity and replication. J. Virol. 79:10547-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munoz, A., A. J. Kirby, Y. D. He, J. B. Margolick, B. R. Visscher, C. R. Rinaldo, R. A. Kaslow, and J. P. Phair. 1995. Long-term survivors with HIV-1 infection: incubation period and longitudinal patterns of CD4+ lymphocytes. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:496-505. [DOI] [PubMed] [Google Scholar]
- 77.Munoz, A., M. C. Wang, S. Bass, J. M. Taylor, L. A. Kingsley, J. S. Chmiel, B. F. Polk, et al. 1989. Acquired immunodeficiency syndrome (AIDS)-free time after human immunodeficiency virus type 1 (HIV-1) seroconversion in homosexual men. Am. J. Epidemiol. 130:530-539. [DOI] [PubMed] [Google Scholar]
- 78.Patarca, R., D. Sandler, K. Maher, C. Hutto, N. L. Martin, N. G. Klimas, G. B. Scott, and M. A. Fletcher. 1996. Immunological correlates of disease severity in pediatric slow progressors with human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 12:1063-1068. [DOI] [PubMed] [Google Scholar]
- 79.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 80.Pizzo, P. A., C. M. Wilfert, et al. 1995. Markers and determinants of disease progression in children with HIV infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:30-44. [PubMed] [Google Scholar]
- 81.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poudrier, J., X. Weng, D. G. Kay, Z. Hanna, and P. Jolicoeur. 2003. The AIDS-like disease of CD4C/HIV Tg mice is associated with accumulation of immature CD11bhi dendritic cells. J. Virol. 77:11733-11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poudrier, J., X. Weng, D. G. Kay, G. Paré, E. L. Calvo, Z. Hanna, M. H. Kosco-Vilbois, and P. Jolicoeur. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-γ and IL-6. Immunity 15:173-185. [DOI] [PubMed] [Google Scholar]
- 84.Priceputu, E., I. Rodrigue, P. Chrobak, J. Poudrier, T. W. Mak, Z. Hanna, C. Hu, D. G. Kay, and P. Jolicoeur. 2005. The Nef-mediated AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with increased Fas/FasL expression on T cells and T-cell death but is not prevented in Fas-, FasL-, tumor necrosis factor receptor 1-, or interleukin-1β-converting enzyme-deficient or Bcl2-expressing transgenic mice. J. Virol. 79:6377-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhodes, D. I., L. Ashton, A. Solomon, A. Carr, D. Cooper, J. Kaldor, N. Deacon, et al. 2000. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. J. Virol. 74:10581-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rucker, E., J. Munch, S. Wildum, M. Brenner, J. Eisemann, L. Margolis, and F. Kirchhoff. 2004. A naturally occurring variation in the proline-rich region does not attenuate human immunodeficiency virus type 1 nef function. J. Virol. 78:10197-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salvatori, F., S. Masiero, C. Giaquinto, C. M. Wade, A. J. Brown, L. Chieco-Bianchi, and A. De Rossi. 1997. Evolution of human immunodeficiency virus type 1 in perinatally infected infants with rapid and slow progression to disease. J. Virol. 71:4694-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salvi, R., A. R. Garbuglia, A. Di Caro, S. Pulciani, F. Montella, and A. Benedetto. 1998. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 72:3646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. J. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the ccr-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 90.Scarlatti, G. 1996. Paediatric HIV infection. Lancet 348:863-868. [DOI] [PubMed] [Google Scholar]
- 91.Schindler, M., J. Munch, and F. Kirchhoff. 2005. Human immunodeficiency virus type 1 inhibits DNA damage-triggered apoptosis by a Nef-independent mechanism. J. Virol. 79:5489-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schindler, M., S. Wurfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Munch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott, G. B., C. Hutto, R. W. Makuch, M. T. Mastrucci, T. O'Connor, C. D. Mitchell, E. J. Trapido, and W. P. Parks. 1989. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N. Engl. J. Med. 321:1791-1796. [DOI] [PubMed] [Google Scholar]
- 94.Simard, M.-C., P. Chrobak, D. G. Kay, Z. Hanna, S. Jothy, and P. Jolicoeur. 2002. Expression of simian immunodeficiency virus nef in immune cells of transgenic mice leads to a severe AIDS-like disease. J. Virol. 76:3981-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol. 64:453-463. [DOI] [PubMed] [Google Scholar]
- 96.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9:853-860. [DOI] [PubMed] [Google Scholar]
- 98.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun, J., T. Soos, V. N. Kewalramani, K. Osiecki, J. H. Zheng, L. Falkin, L. Santambrogio, D. R. Littman, and H. Goldstein. 2006. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80:1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Terwilliger, E. F., E. Langhoff, D. Gabuzda, E. Zazopoulos, and W. A. Haseltine. 1991. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 88:10971-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobiume, M., M. Takahoko, T. Yamada, M. Tatsumi, A. Iwamoto, and M. Matsuda. 2002. Inefficient enhancement of viral infectivity and CD4 downregulation by human immunodeficiency virus type 1 Nef from Japanese long-term nonprogressors. J. Virol. 76:5959-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vincent, P., E. Priceputu, D. Kay, K. Saksela, P. Jolicoeur, and Z. Hanna. 2006. Activation of p21-activated kinase 2 and its association with Nef are conserved in murine cells but are not sufficient to induce an AIDS-like disease in CD4C/HIV transgenic mice. J. Biol. Chem. 281:6940-6954. [DOI] [PubMed] [Google Scholar]
- 103.Weng, X., E. Priceputu, P. Chrobak, J. Poudrier, D. G. Kay, Z. Hanna, T. W. Mak, and P. Jolicoeur. 2004. CD4 T cells from CD4C/HIV nef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro, but are dispensable for the development of a severe AIDS-like organ disease. J. Virol. 78:5244-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zazopoulos, E., and W. A. Haseltine. 1993. Effect of nef alleles on replication of human immunodeficiency virus type 1. Virology 194:20-27. [DOI] [PubMed] [Google Scholar]