Abstract
Most humans carry Epstein-Barr virus (EBV) in circulating memory B cells as a latent infection that is controlled by an immune response. When infected by EBV, B lymphocytes in fetal cord blood are readily transformed to lymphoblastoid cell lines (LCL). It is frequently assumed that this high efficiency of transformation is due to the absence of a primary immune response. However, cord blood lymphocytes stimulated with autologous LCL yield CD4+ T cells that can completely inhibit the growth of LCL by a major histocompatibility complex-restricted cytotoxic mechanism mediated by granulysin and granzyme B. Because EBV-transformed B cells maintain the phenotype of antigen-activated B-cell blasts, they can potentially receive inhibitory or helper functions from CD4+ T cells. To assess these functions, the effect of EBV-specific CD4+ T cells on the efficiency of virus transformation of autologous B cells was assayed. Paradoxically, although the cytotoxic CD4+ T-cell lines reduced EBV B-cell transformation at a high effector/target ratio of 10:1, they caused a twofold increase in B-cell transformation at the lower effector/target ratio of 1:1. Th1-polarized CD4+ T cells were more effective at inhibiting B-cell transformation, but Th2-polarized cell lines had reduced cytotoxic activity, were unable to inhibit LCL growth, and caused a 10-fold increase in transformation efficiency. Tonsil lymphoid follicles lacked NK cells and CD8+ T cells but contained CD4+ T cells. We propose that CD4+ T cells provide helper or cytotoxic functions to EBV-transformed B cells and that the balance of these functions within tonsil compartments is critical in establishing asymptomatic primary EBV infection and maintaining a stable lifelong latent infection.
More than 90% of the human population is infected with Epstein-Barr virus (EBV). For the majority, EBV is acquired during infancy and infection is asymptomatic at this age. Thereafter, the virus is maintained as a lifelong systemic latent infection of B lymphocytes along with limited productive infection and virus shedding in the oropharynx (43). EBV infection of B cells recapitulates all the stages of normal B-cell development that occur during antigenic stimulation (27, 49, 52). Following in vitro infection of resting B cells, the expression of EBV nuclear antigens (EBNA-LP, EBNA 1, EBNA 2, and EBNA 3) along with that of the latent membrane proteins (LMP1 and LMP2) transforms the B cells into continuously proliferating CD23-positive lymphoblastoid cell lines (LCL), a phenotype similar to that of an antigen-stimulated B-cell blast. This growth program of gene expression occurs only in vivo in naive immunoglobulin D (IgD)-positive B cells within follicles of healthy tonsils (21, 51). EBV-infected IgD-negative memory B cells in the germinal center limit the expression of EBV genes to EBNA1, LMP1, and LMP2 (2, 3). Latent EBV infection is maintained in the circulating pool of memory B cells, where either LMP2 alone or no virus proteins are expressed (1). These IgD-negative B cells have undergone antibody class switching and maturation in the same way as normal memory B cells (49). The lytic cycle of virus replication within B cells occurs spontaneously in a proportion of LCL cultured in vitro and is associated with terminal differentiation of LCL into CD38 high-plasma cells (11), a phenotype which also supports lytic infection in vivo (27).
The control of EBV infection depends on a functional immune response since either immunosuppressive therapy or AIDS can lead to EBV-driven lymphoproliferative disease (14, 39). When primary EBV infection occurs during adolescence, 30 to 50% of cases develop infectious mononucleosis (34), in which there is marked clonal expansion of infected B cells within the tonsil (26) and lytic replication of Epstein-Barr virus in blood (41), with up to 10% of peripheral B cells being EBV positive (45). These findings could be interpreted as a failure to establish and regulate stable latent infection in memory B cells, which in turn stimulates the large expansion of activated EBV-specific CD8+ T cells characteristic of the clinical stage of the disease (7, 8). This delay in establishing immune regulation of EBV has long-term implications, with an increase in the subsequent risk of developing EBV-associated Hodgkin's disease to four times that of the population average (18). In EBV-associated endemic Burkitt's lymphoma, risk factors include malaria infection, in which there are also greatly increased numbers of EBV-positive B cells in the circulation (28), and elevation of serum antibody to lytic virus proteins (12), which precede the onset of symptoms. Similarly, nasopharyngeal carcinoma and EBV-associated gastric adenocarcinomas are preceded by an elevation of serum IgG and IgA antibodies to EBV antigens (9, 30). Despite the diverse etiology of these conditions, all can be linked by evidence of increased lytic EBV activity prior to the onset of disease, which may in turn reflect a loss of immune control over viral latency.
Most studies of immune control of EBV have measured the killing of EBV-transformed B cells. Major histocompatibility complex (MHC) class I-restricted CD8+ T cells specific for EBNA3 are abundant in the circulation of healthy carriers and can kill LCL in vitro (44, 55). CD8+ T cells specific for EBNA1, LMP1, and LMP2 are also found in the circulation and, although able to kill peptide-sensitized LCL in vitro, are unable to efficiently lyse LCL naturally expressing these antigens (16, 31, 37, 47, 48). We presume that in vivo EBNA1- and LMP-specific CD8+ T cells will also be unable to kill the population of IgD-negative memory B cells in tonsil follicles where EBV antigen expression is limited to these proteins. Moreover, as activated CD8+ T cells do not express homing receptors, such as CCR7, required for entry into the B-cell germinal centers (17), they can have only restricted access to the immunological compartment of the tonsil where latently infected memory B cells are found (3).
CD4+ T cells are present within B-cell follicles, where their normal function is to provide essential cognate help for class switching and B-cell memory formation following antigen stimulation (32, 53). The extent to which EBV may also require CD4+ T-cell help in establishing, maintaining, and controlling its latent infection in memory B cells is not known, but as latent memory B-cell phenotypes are not detected in LCL cultures in vitro, it would seem unlikely that they can be generated solely by the activity of virus-encoded signaling proteins. To date, the majority of studies have focused on the ability of cytotoxic EBV-specific CD4+ T cells to prevent the growth of LCL in vitro (6, 23, 24, 29, 35, 36, 56, 58, 59). The lysis of LCL by CD4+ T cells has been associated with a gamma interferon (IFN-γ)-secreting Th1-polarized phenotype in which several cytotoxic mechanisms, including Fas-FasL interactions, the perforin/granzyme pathway, and granulysin release, have been described previously (24, 36, 50, 59). Despite their potential importance in disease pathogenesis, few studies have investigated the helper functions of CD4+ T cells in EBV.
Titration of EBV by transformation of fetal cord blood has been used both to quantify virus and to assay neutralizing antibody to EBV (57). This assay can also measure primary immune responses to EBV, including the helper and cytotoxic functions of cell subsets during primary EBV infection. We have previously shown that EBV transformation of purified fetal cord blood B cells is reduced by over 90% compared to that of unfractionated cord blood lymphocytes (CBL) and that optimal virus transformation requires the presence of other cell types (58). The EBV transformation titer was also greatly reduced in cultures containing high numbers of activated NK cells and cytotoxic CD4+ T cells; however, it was not clear to what extent each cell type was responsible (58). Using fetal CBL as a source of EBV-specific CD4+ T cells, we report here an investigation of their interaction with autologous B cells during primary EBV infection and growth transformation. We have also examined the influence of Th1 and Th2 polarizations on CD4+ T cells in terms of cytotoxic and helper activities, respectively, and their subsequent effect on the efficiency of EBV B-cell transformation.
MATERIALS AND METHODS
CBL, LCL, and tonsils.
Informed consent was obtained from patients prior to elective cesarean section (United Bristol Healthcare Trust Ethics Committee project number E4971). Fifty milliliters of cord blood was aspirated from a placental vein into a heparinized syringe. Lymphocytes were separated by centrifugation through Ficoll-Paque (Amersham Biosciences), plasma was removed and stored at −20°C, and the CBL were frozen in aliquots and stored in liquid nitrogen. Cells were cultured at 37°C in 5% CO2 in RPMI supplemented with either 10% autologous plasma or 5% human AB serum (National Blood Service, South West, United Kingdom) plus 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). LCL were generated as previously described (59). Tonsils were collected from elective surgery cases (United Bristol Healthcare Trust Ethics Committee project number E 5440), snap-frozen in isopentane, and stored at −70°C until used.
Generation of CD4+ T-cell lines.
CBL (2 × 106) were stimulated with 104 irradiated (4,000 rads) LCL in 2 ml of RPMI and 10% autologous plasma, and after 10 days, 1 ml of medium was replaced with fresh medium containing 105 irradiated LCL. On day 14, the wells were replated at 106/well, with an equal number of irradiated LCL in 2 ml of medium supplemented with 20 IU/ml recombinant human interleukin-2 (IL-2; R&D Systems) (56). Once established, CD4+ T-cell lines were positively selected by magnetic cell sorting (Miltenyi Biotec), then stimulated weekly with γ-irradiated LCL, and fed biweekly with IL-2. In some cases, CD4+ T cells purified prior to culture using a Dynal CD4-negative selection kit were stimulated using either irradiated LCL alone or LCL and magnetic cell sorting-purified CD14+ monocytes at a density equivalent to that of the unfractionated cord blood. To generate Th1-polarized lines, 10 ng/ml recombinant human IFN-γ (Leinco) and 10 μg/ml rabbit anti-IL-4 (Genzyme) were added on days 1, 10, and 14 to cultures of unfractionated CBL. For Th2 lines, 20 U/ml IL-4 (BD Biosciences) and 10 μg/ml rabbit anti-IFN-γ (R&D Systems) were added. In later experiments, for Th1 polarization, anti-IL-4 and 10 U/ml IL-12, or, for Th2 lines, IL-4 and anti-IL-12 monoclonal antibody C11.12 were used on day 1 only.
Growth inhibition, cytotoxicity, and transformation assays.
Long-term LCL growth inhibition assays were carried out as previously described (56). Briefly, doubling dilutions of LCL were made in a U-bottomed 96-well plate (BD Biosciences), starting at 104 cells/well in a 100-μl volume of media. CD4+ T cells (104) were added to each well in triplicate columns so that effector/target (E:T) ratios increased from 1:1 in row A to 128:1 in row H. One hundred microliters of medium was replaced weekly and the plates photographed on day 21.
To quantify CD4+ T-cell effects on LCL proliferation, LCL were cultured either alone or with CD4+ T cells in 2-ml wells. At 24-h intervals 100-μl volumes from each 2-ml well were transferred to triplicate wells in 96-well U-bottomed plates, pulsed with [3H]thymidine (0.32 μCi/well; Amersham Biosciences) for 24 h, harvested, and counted in a Microbeta 1450 liquid scintillation counter (Wallac). 51Cr release assays were carried out as previously described (37). For blocking studies, CD4+ T cells were incubated with 5 nM concanamycin A (CMA; Sigma) for 10 min or anti-human FasL antibody (NOK-1, 10 μg/ml; BD Biosciences) or anti-human TRAIL antibody (RIK-2, 10 μg/ml; BD Biosciences) for 30 min prior to the addition of T cells. The influence of CD4+ T cells on EBV transformation of B cells was assayed by titration of B958 virus as previously described (57, 58).
Flow cytometry and immunofluorescence.
For cell surface staining, 105 cells were washed and resuspended in phosphate-buffered saline (PBS) at 106 cells/ml; 100-μl aliquots were stained for 20 min at 4°C, washed in PBS, fixed in 1% paraformaldehyde for 20 min at 4°C, and then stored at 4°C in the dark until analysis. Antibodies were obtained from the following sources: anti-CD4, -CD8, -CD19, -CD23, and -CD14 and granzyme B from Dako, anti-CD3, -CD11c, -CD28, -CD69, -CD40L, -CD80, and -CD86, MHC class I, MHC class II, intercellular adhesion molecule-1, CD40, and perforin from BD Pharmingen, and CD25 and FasL from Caltag. Cytospin slides of CD4+ T cells or sections of frozen tonsil were fixed in acetone-methanol, blocked with PBS containing 10% bovine serum albumin, and stained with primary antibodies to CD markers granulysin and granzyme B. Slides were washed and stained with an appropriate subclass-specific fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isocyanate (TRITC) conjugate (Southern Biotech). Granulysin was detected using a rabbit polyclonal antibody (Santa Cruz Biotechnology) with donkey anti-rabbit Texas Red conjugate (Jackson ImmunoResearch). Images were obtained from a Leica DMRA2 fluorescence microscope through either an HC plan Apo 20×/0.70 or an HC X plan Apo 40×/0.85-corr lens, with a Hamamatsu C4742-95 digital camera using LeicaQFluoro software. Images were cropped and resized using Adobe Photoshop.
Intracellular staining of CD4+ T-cell lines.
CD4+ T cells (5 × 105) were stimulated with 50 ng/ml phorbol myristate acetate (PMA) and 250 ng/ml ionomycin or with 5 × 105 LCL/ml in 2-ml wells of a 24-well plate. Monensin (10 μM) was added 1 hour later, and after 6 h, cells were washed and fixed by resuspension in PBS containing 1% paraformaldehyde at 4°C for 20 min. Washed cells were suspended at 106/ml in PBS-0.1% sodium azide-0.1% saponin and stained for 20 min at 4°C with FITC-conjugated mouse anti-human CD4 antibody plus phycoerythrin-conjugated mouse anti-human cytokines (IL-2, IL-4, IFN-γ, IL-10, IL-12, and IL-13; BD Pharmingen) or phycoerythrin-conjugated mouse IgG(κ). A Becton Dickinson FACScan system was used for acquisition of data, which were then analyzed using WinMDI software.
Statistical analysis.
Data were analyzed using the Spearman rank correlation coefficient or the Wilcoxon signed-rank test where appropriate.
RESULTS
Influence of monocytes and NK cells on the development of cord blood CD4+ T cells.
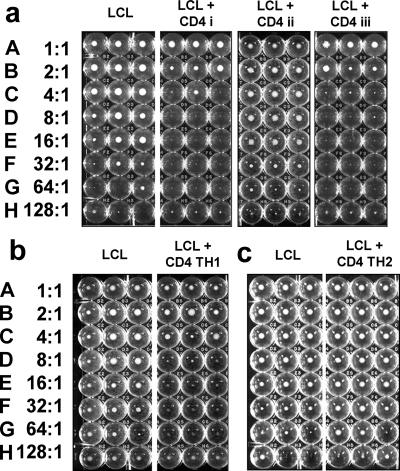
To determine the role of CD14+ monocytes and NK cells in the activation and differentiation of naïve CD4+ T cells, matched lines were generated using LCL to stimulate whole CBL, purified CD4+ T cells, or CD4+ T cells mixed with CD14+ monocytes. The ability of the CD4+ T-cell lines to regulate LCL growth was tested in a long-term growth inhibition assay in which enhanced LCL growth could also indicate T-cell helper function. The assay was validated by preliminary experiments which showed that in the absence of exogenous IL-2, the CD4+ T cells did not grow, and flow cytometry confirmed that at the end of the culture period, all live cells were CD19+ LCL. Although there was considerable variation between CD4+ T-cell lines derived from different cord blood samples, most were able to eliminate LCL growth at E:T ratios of 16:1 or greater, but none were able to eliminate LCL growth at ratios of less than 2:1. Purified CD4+ T cells from the same cord blood samples were seldom successfully cultured when directly stimulated with LCL alone, and the few lines produced in this way were unable to inhibit LCL growth. Where both LCL and CD14+ monocytes were added on day 1 of the culture period, the growth of purified CD4+ T cells was restored and, once established, could be maintained using LCL as antigen-presenting cells (APCs) with the addition of IL-2. These CD4+ T-cell lines were equally effective in inhibiting LCL growth as lines derived from whole cord blood, indicating that NK cells were not essential for the generation of inhibitory CD4+ T cells. Figure 1a shows representative results for each type of CD4+ T-cell line. CD4+ T-cell lines polarized toward a Th1 phenotype by the addition of IFN-γ and anti-IL-4 during the initiation of the culture showed no overall increase in LCL inhibition compared to nonpolarized T-cell lines (Fig. 1b). In contrast, none of the CD4+ T-cell lines polarized toward a Th2 phenotype by the addition of IL-4 and anti-IFN-γ could inhibit LCL (Fig. 1c), and some enhanced LCL growth.
FIG. 1.
Inhibition of LCL outgrowth by coculture with anti-EBV CD4+ T-cell lines. (a) (LCL) Doubling dilutions of LCL seeded in triplicate U-bottomed wells beginning at 104 cells/well in row A and decreasing to 78 cells per well in row H. The photograph on day 21 of culture shows distinct pellets of growing cells in rows A to F. LCL in rows E to H seeded with fewer cells (<624/well) grew poorly, with pellets of viable cells present in only some of the wells. (LCL + CD4 i) Triplicate wells seeded with identical dilutions of LCL and 104 CD4+ T cells/well of a line derived from unfractionated CBL stimulated with LCL as described in Materials and Methods, giving an E:T ratio from 1:1 in row A to 128:1 in row H, as indicated on the left-hand side. After 21 days, visible pellets of LCL were seen only in rows A and B; the remaining rows had no live cells, indicating complete inhibition of LCL growth. (LCL + CD4 ii) Wells seeded with LCL and a T-cell line derived from purified CD4+ CBL stimulated with LCL. No inhibition of LCL growth is seen. (LCL + CD4 iii) Wells seeded with LCL and T cells derived from purified CD4+ CBL stimulated with LCL and CD14+ monocytes. Inhibition of LCL growth is seen at E:T ratios of 2:1 or greater. (b) (LCL) Triplicate control LCL and LCL CD4+ T-cell cocultures set up as for panel a. (LCL + CD4 TH1) CD4+ T-cell lines derived from unfractionated CBL polarized toward a Th1 phenotype by the addition of IFN-γ and neutralizing antibody to IL-4. LCL growth is inhibited at E:T ratios greater that 4:1. (c) (LCL) Triplicate control LCL and LCL CD4+ T-cell cocultures set up as for panel a. (LCL + CD4 TH2) Growth of LCL cultured in the presence of CD4+ T cells polarized to a Th2 phenotype by the addition of IL-4 and neutralizing antibody to IFN-γ was not inhibited. All live cells at the conclusion of the experiments were confirmed to be LCL by flow cytometry.
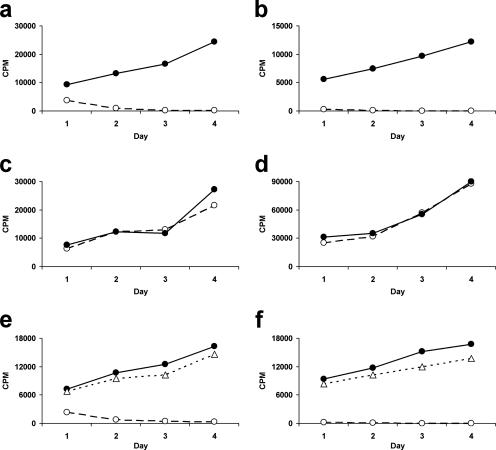
The inhibition of LCL growth by CD4+ T-cell lines was quantified using [3H]thymidine uptake to assay growth. Preliminary experiments confirmed that CD4+ T cells did not grow in cultures stimulated by irradiated LCL without additional IL-2 and showed that the majority of inhibition occurred in the first 4 days (data not shown). At an E:T ratio of 16:1, all three CBL-derived lines tested and two out of three Th1-polarized lines completely inhibited LCL growth by day 4 of culture, but none of the three Th2 lines tested were inhibitory (Fig. 2a to c). None of the inhibitory CD4+ T-cell lines tested had any effect on the growth of nonautologous LCL (Fig. 2d). Using a Transwell system, we found that the inhibition of LCL growth was contact dependent (Fig. 2e). Furthermore, stimulation of cytokine release by adding LCL to the CD4+ T-cell compartment of the Transwell had no effect on the growth of LCL on the other side of the membrane (Fig. 2f).
FIG. 2.
Inhibition of LCL proliferation by CBL-derived CD4+ T-cell lines over the short term. LCL were cultured either alone (•) or in the presence of CD4+ T cells (○) at an E:T ratio of 16:1. LCL were seeded at 5 × 103/ml in 2-ml wells of 24-well tissue culture plates. Proliferation was measured by tritiated thymidine incorporation over a 24-h period in triplicate 100-μl aliquots of the cultures taken daily from days 1 to 4. Data represent mean values of triplicates for representative CD4+ T-cell lines. (a) Inhibition of LCL growth by CBL-derived CD4+ T-cell lines. (b) Inhibition of LCL growth by Th1-polarized CD4+ cell lines. (c) No inhibition of LCL growth by Th2-polarized CD4+ cell lines. (d) No inhibition of nonautologous LCL by CBL-derived CD4+ T-cell lines. (e) Inhibition of LCL growth is contact dependent. LCL were cultured alone (•), in contact with CBL-derived CD4+ T-cell lines (○), or separately from the CD4+ T cells by a Transwell (▵). (f) T-cell-derived soluble factors do not contribute to inhibition. Cultures were set up as for panel e, but additional LCL were placed in the Transwell to stimulate cytokine production by the CD4+ T cells.
Mechanisms of CD4+ T-cell cytotoxicity.
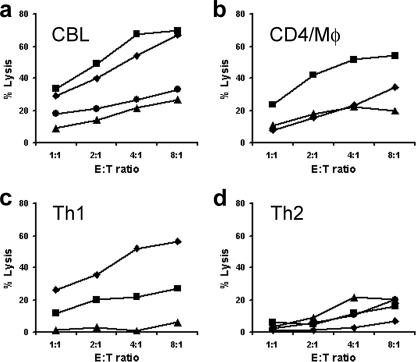
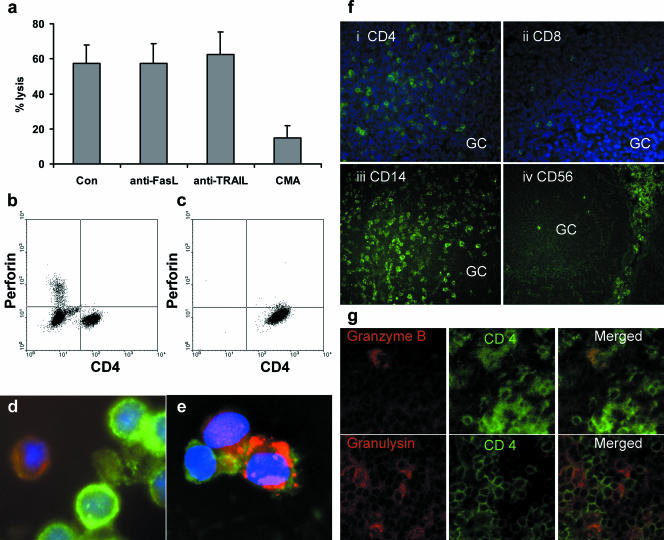
The results described above are consistent with inhibition being caused by a cytotoxic mechanism, and this was confirmed by chromium release assays. CD4+ T-cell lines generated from whole CBL or purified CD4+ T cells with monocytes were all cytotoxic (Fig. 3a and b). Th1-polarized CD4+ cells had a range of activities but were not consistently more cytotoxic than unpolarized lines (Fig. 3c). In contrast, Th2-polarized CD4+ T-cell lines were all weakly cytotoxic (Fig. 3d). No killing was seen when nonautologous target LCL were used, confirming that the cytotoxic activity was HLA restricted (not shown). Compared to typical NK cell killing of LCL in 5-h assays (58), the cytotoxic mechanism was slow with maximal lysis, occurring only after 24 h. Lysis was not inhibited by antibodies to TRAIL or FasL but was markedly reduced by the addition of CMA, indicating that cytotoxicity was perforin/granzyme dependent (Fig. 4a). In addition, we were unable to detect FasL on cytotoxic T cells by using flow cytometry, and where both anti-FasL antibody and a blocking anti-Fas antibody were used together, no inhibition of killing was observed (data not shown). Although perforin was readily detected by flow cytometry in CBL (Fig. 4b), only minimal amounts of perforin were present in either unstimulated CD4+ T cells (Fig. 4b) or CD4+ T-cell lines (Fig. 4c). Granzyme B was detected by immunofluorescence in a small proportion of cytotoxic CD4+ T-cell lines (Fig. 4d) and a larger proportion of cells stained strongly for granulysin (Fig. 4e).
FIG. 3.
Cytotoxicity of CD4+ T-cell lines toward autologous LCL. CD4+ T-cell-mediated cytotoxicity was assessed at E:T ratios between 1:1 and 16:1 by 51Cr release assays. LCL were labeled with 100 μCi of chromium for 1 h, seeded at 104/well in U-bottomed 96-well plates, and cultured with CD4+ T-cell lines over a 16-h period. Data represent specific lysis of autologous LCL by (a) CD4+ T-cell lines derived from unfractionated CBL by stimulation with autologous LCL, (b) T-cell lines derived from purified CBL CD4+ T cells and CD14+ monocytes stimulated with autologous LCL, (c) CD4+ T-cell lines derived from unfractionated CBL stimulated with autologous LCL and polarized toward a Th1 phenotype by the addition of IFN-γ and neutralizing antibody to IL-4, and (d) CD4+ T-cell lines derived from unfractionated CBL and polarized toward a Th2 phenotype by the addition of IL-4 and neutralizing antibody to IFN-γ. Different graph symbols are used simply to discriminate between different lines in the same panel. Mφ, macrophage.
FIG. 4.
Mechanism of CD4+ T-cell cytotoxicity. (a) CD4+ T-cell-mediated cytotoxicity measured by a 16-h chromium release assay at an E:T ratio of 16:1. Killing was not inhibited by neutralizing antibodies to Fas ligand or TRAIL but was markedly reduced by the addition of the granule-disrupting antibiotic CMA. Con, control. Means ± standard errors of the means for six CD4+ T-cell lines. (b and c) Flow cytometry of formalin-fixed cells, dual stained for CD4+ and intracellular perforin, showed the absence of perforin in both (b) freshly isolated resting cord blood CD4+ T cells and (c) cytotoxic CD4+ T-cell lines. (d) Acetone-fixed cytospin of cytotoxic CD4+ T-cell lines stained with CD4 (FITC) and granzyme B (TRITC). In flow cytometry using a log scale, the intensity of CD4 on the cell lines varies by nearly an order of magnitude (c). By comparison, in the linear scaling of fluorescence microscopy, these cells show the same range from very bright CD4 to dim CD4. Dual staining with granzyme B TRITC showed that a minority of CD4+ T cells contained granzyme B, and this was mainly in CD4 dim cells (d). (e) Dual staining of a cytotoxic CD4+ T-cell line with CD4 FITC and granulysin Texas Red, showing that a proportion of CD4+ T cells also contain granulysin. (f) Acetone-fixed frozen sections of adult tonsil stained with (i) anti-CD4, (ii) anti-CD8, (iii) anti-CD14, and (iv) anti-CD56. Sections i and ii are counterstained with DAPI (4′,6′-diamidino-2-phenylindole) to highlight the nuclei. The germinal center is marked GC. (g) Dual staining of interfollicular T-cell areas of the tonsil sections with anti-CD4 FITC and anti-granzyme B TRITC or anti-granulysin Texas Red. Scattered CD4+ T cells that stained for granzyme B were detected; a greater number of granulysin-positive cells were seen, but the majority of CD4+ T cells were negative.
Flow cytometry demonstrated that CD3 expression was greater on highly cytotoxic CD4+ T-cell lines than on noncytotoxic Th2 cells and was down-regulated on all cells after exposure to LCL. Also, following antigen stimulation, the increase in expression of the IL-2 receptor (CD25) and the activation marker CD69 correlated with cytotoxic activity across the cell lines (Spearman's rank correlation between the percentage of chromium release and the mean fluorescence channel: for CD25, r = 0.7714, n = 6, P ≤ 0.05; for CD69, r = 0.7714, n = 6, P ≤ 0.005). CD40L was expressed by all CD4+ T-cell lines and was down-regulated following interaction with LCL, but there was no apparent association between CD40L and cytotoxic function. Similarly, there was no consistent difference in expression of the CD28 costimulator molecule and the cytotoxic-T-lymphocyte-associated antigen 4 (CD152)-negative regulator of T-cell responses between cytotoxic and noncytotoxic lines (data not shown).
Distribution of T cells, monocytes, and NK cells within the tonsil.
In vivo, the architecture of the immune system has a critical role in governing the immune response. The distribution of cells within frozen tonsil sections confirmed that CD8+ T cells and NK cells are largely excluded from B-cell follicles and germinal centers (Fig. 4f, panels ii and iv). CD4+ T cells and CD14+ monocytes are present in these areas (Fig. 4f, panels i and iii), making it most likely that these cell types interact with newly infected B cells in the germinal center. Very few CD4+ T cells in the tonsil stained with granzyme B (Fig. 4g), and none of the CD4+ granzyme B cells were found within the follicles themselves. Granulysin staining was more widespread but was also found largely in the interfollicular areas. Again, not all CD4+ T cells were granulysin positive and many granulysin-positive cells were CD4 negative (Fig. 4g). Collectively, these results suggest that cytotoxic cells, including cytotoxic CD4+ T cells, are largely absent from B-cell follicles.
Cytokine expression by Th1- and Th2-polarized CD4+ T cells.
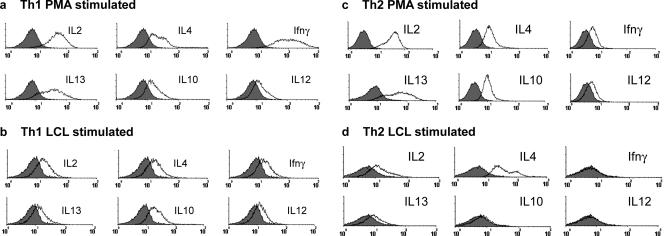
Intracellular staining detected basal levels of IL-2 and IL-4 in resting CD4+ T-cell lines (data not shown). After stimulation with PMA and ionomycin, all CD4+ T-cell lines produced the full range of cytokines assayed, including IL-2, IL-4, IL-10, IL-12, IL-13, and IFN-γ. Although the Th1 lines produced higher levels of IFN-γ than the Th2 lines (Fig. 5a and c), differences in cytokine expression between Th1 and Th2 cells became more evident when the cells were stimulated using LCL as APCs (Fig. 5b and d). Under these conditions, the Th2 cells produced markedly more IL-4 but little of any other cytokine was detected (Fig. 5d), while Th1 cells continued to secrete all cytokines. Interestingly, LCL-stimulated Th1 cells produced 10-fold less IFN-γ and IL-13 than PMA-ionomycin-stimulated cells but maintained the production of IL-10 and IL-4 (Fig. 5a and b), suggesting that LCL stimulation favors the production of Th2 cytokines, even in Th1-polarized cells.
FIG. 5.
Flow cytometric determination of intracellular cytokine profiles of CD4+ T-cell lines following stimulation with PMA and ionomycin or autologous LCL. CD4+ T cells were cultured in the presence of PMA and ionomycin or autologous LCL for 6 h and in the presence of monensin for the last 5 h. CD4+ T cells were subsequently fixed, made permeable, stained, and analyzed by flow cytometry. A gate was set on live cells according to forward and side scatter properties, and a second gate was set around the CD4+ T cells. Histograms represent staining of the gated CD4+ T cells with an isotype control antibody (shaded area) and staining for IL-2, IL-4, IFN-γ, IL-13, IL-10, and IL-12, depicted by the black line, as labeled above the appropriate histogram.
Effect of CD4+ T cells on EBV transformation of autologous B cells.
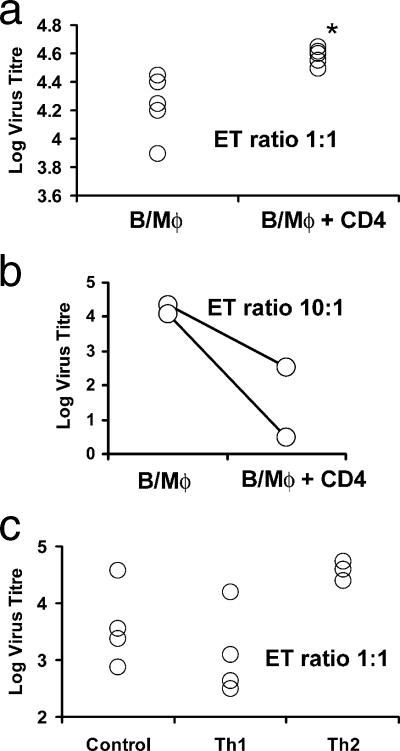
To measure the direct effect of EBV-reactive CD4+ T-cell lines on EBV transformation without confounding effects due to the presence of NK cells or CD8+ T cells, assays were set up using EBV with a high transfection titer, purified cord blood B cells, and autologous monocytes. In the first experiment, using cytotoxic CD4+ T-cell lines from five different donors, CD4+ T cells were added at a ratio of 1:1 with B cells. The transformation titer (average ± standard deviation) of virus on B cells and monocytes alone was 4.22 ± 0.205, while for all five cases, the transformation efficiency rose in the presence of CD4+ T cells to a titer of 4.58 ± 0.044 (P = 0.009, Mann-Whitney U test) (Fig. 6a). This increase of 0.36 log is equivalent to more than a doubling of the transformation efficiency. Sufficient CBL and CD4+ T cells were available in two samples to allow an additional test in which the T-cell/B-cell ratio was 10:1. In these cases, the virus transformation titer decreased by more than 1 log (Fig. 6b). We interpret this result as showing that the majority of noncytotoxic CD4+ T cells provided help for virus transformation, but where CD4+ T-cell numbers were increased, the minority of cytotoxic T cells in the population dominated the response and reduced the transformation efficiency by over 90%.
FIG. 6.
Effect of autologous cord blood-derived CD4 T-cell lines on the titer of EBV measured by transformation of B cells. (a) (B/Mφ) Titer of EBV assayed in cultures containing equal numbers of purified B cells and CD14 monocytes from five different samples of cord blood. (B/Mφ + CD4) Titration of the same samples of virus and cord blood cells in the presence of cytotoxic CD4 T cells added at a T-cell/resting B-cell ratio of 1:1. *, the median titer of virus was significantly raised after the addition of cytotoxic CD4 T cells (P = 0.009, Mann-Whitney U test). (b) (B/Mφ) Two further titrations of EBV assayed in cultures containing equal numbers of purified B cells and CD14 monocytes. (B/Mφ + CD4) The addition of cytotoxic CD4 T cells at the higher ratio of 10:1 substantially reduced the transformation efficiency in both cases. (c) (Control) Titer of EBV assayed in cultures containing equal numbers of purified B cells and CD14 monocytes from three different samples of cord blood. (Th1) Titration of the same samples of virus and cord blood cells in the presence of cytotoxic CD4 T cells derived from unfractionated CBL stimulated with LCL polarized to a Th1 phenotype by the addition of IL-12 and anti-IL-4. (Th2) Titration of the same samples of virus and cord blood cells in the presence of cytotoxic CD4 T cells derived from unfractionated CBL stimulated with LCL polarized to a Th2 phenotype by the addition of IL-4 and anti-IL-12. In every case, the addition of Th2 cells substantially raised the transformation efficiency. B, B cell; Mφ, macrophage.
Effects of Th1 and Th2 cells on virus transformation.
To investigate the effects of CD4+ Th1 and Th2 cells on virus transformation titer, Th1-polarized CD4+ T cells were generated using IL-12 and anti-IL-4 and Th2 CD4+ T cells were polarized with IL-4 and anti-IL-12. Their effects on virus transformation at an E:T ratio of 1:1 are shown in Fig. 6c. Three out of four Th1-polarized lines reduced the virus transformation titer, while the third caused an increase in transformation. In contrast, the addition of Th2 lines raised the transformation efficiency in every case by an average of 1 log, demonstrating a dominant helper effect of the Th2 cells on EBV transformation. The fourth Th2 line was not used in the transformation assay as it was found to be contaminated with LCL, which grew out rapidly and overwhelmed the culture even after sorting. This LCL contamination was probably a result of small amounts of virus released from irradiated LCL-transforming B cells in the cord blood during the induction of the Th2 cells (22). Although undesirable, this result in general supports the conclusion that Th2-polarized conditions favor efficient virus transformation.
DISCUSSION
We previously showed that during the primary EBV infection of fetal cord blood, the presence of high numbers of activated NK cells and cytotoxic CD4+ T cells was associated with a reduction of viral B-cell transformation (58). It was not clear to what extent NK cell activation was dependent on cytokine production by EBV-responsive CD4+ T cells within the cultures or whether the development of cytotoxic CD4+ T cells was consequent to NK cell activation and cytokine synthesis. The data presented here confirm that fetal cord blood can give rise to cytotoxic CD4+ T cells when stimulated by LCL in vitro and show that the presence of NK cells or the addition of exogenous IFN-γ is not required. T-cell lines were not efficiently generated from cultures of purified naïve CD4+ T cells and LCL alone, indicating that although LCL are effective APCs for the maintenance of established CD4+ T-cell lines, they were unable to effectively initiate a response for which the addition of CD14+ monocytes was required. Studies of adult blood found that neither LCL nor monocytes were effective primary APCs and that dendritic cells were required to initiate primary immune responses to EBV (5). However, the CD14+ monocytes from cord blood may act as a source of dendritic cell precursors which mature when stimulated by signals such as IL-4, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor (TNF), and CD40L (4) that can potentially be provided by the LCL in coculture (46).
When stimulated with PMA-ionomycin, the established CD4+ T-cell lines we describe here were all able to synthesize a wide range of cytokines with surprisingly little difference between the Th1- and Th2-polarized lines. As we have previously shown for adult CD4+ T-cell lines (56), the polarization of Th1 and Th2 lines is more apparent if LCL are used to stimulate cytokine production. Under these conditions, Th1 lines continued to produce a range of cytokines, although only reduced amounts of IFN-γ, IL-2, and IL-13 were detected and IL-4 continued to be secreted. IL-4 was the only cytokine up-regulated in Th2 lines. The lower production levels of cytokines following LCL stimulation may better reflect the physiological stimuli that would occur during a B-cell/T helper cell interaction in vivo. This effect could be mediated by LCL-derived cytokines, such as IL-10 and TNF, that could reduce IFN-γ secretion and T-cell receptor signaling, respectively (10, 42), which in turn may reduce the activation status of the CD4+ T cells. Alternatively, differential interactions between B7 ligands on the LCL and CD28 costimulators on the CD4+ T cells may bias cytokine secretion (54). It would be most interesting to determine whether CD4+ T cells encountering dendritic cells presenting EBV antigens display a cytokine profile similar to that of cells stimulated by PMA-ionomycin or more similar to that of cells stimulated by LCL.
The cytotoxicity of EBV-specific CD4+ T cells has been linked with a Th1 phenotype (29, 33, 58), but the cell lines used in this study and several other polyclonal CD4+ T-cell lines that have been described previously secreted both Th1 and Th2 cytokines (36, 50, 56). Although CD4+ T-cell lines biased toward a Th1 phenotype by using IFN-γ and anti-IL-4 did produce Th1 cytokines, their cytotoxic and inhibitory capacities were not always increased. In contrast, biasing the differentiation of CD4+ T cells to a Th2 phenotype consistently reduced the cytotoxic capacity of the CD4+ T-cell lines and reduced their ability to inhibit the outgrowth of autologous LCL. Perhaps the adoptive transfer of CD4+ T cells of the Th2 phenotype to humans should be avoided and the possibility of inducing Th2 CD4+ T cells in EBV vaccination should be considered. The marked inhibition of cytotoxicity by CMA and analysis of the intracellular granule content of the CD4+ T cells demonstrate that granzyme B and granulysin are the most likely candidates for mediating LCL killing by cord blood-derived CD4+ T cells, in agreement with earlier reports (24, 50). The observation that only a proportion of CD4+ T cells in cytotoxic lines contain granules, combined with the finding that complete inhibition of LCL growth occurred only at E:T ratios of greater than 4:1, suggests that even in the most cytotoxic lines, not all the CD4+ T cells are able to kill LCL.
The clear similarities between the stages of EBV infection in B cells and the stages of normal B-cell development suggest that EBV-infected B cells are potentially open to the full range of CD4+ T-cell-mediated effects, including helper functions. In vivo primary infection of naïve B cells with EBV occurs in the follicular and germinal center regions of the tonsil (21). Tonsil B-cell follicles lack both NK cells and CD8+ T cells, which do not express the homing receptors required for entry, such as CD62L and CCR7 (17). CD4+ T cells are therefore a more likely cell type to interact with newly infected follicular B cells, where they may be pivotal during the early stages of B-cell infection by EBV. Given that the majority of CD4+ T cells within the B-cell follicles did not contain cytotoxic granules and that CD4+ T cells produce mainly Th2-type cytokines when directly stimulated in vitro by an EBV-transformed B cell, it would seem likely that they will provide helper signals at this site.
Few studies have considered the effects of CD4+ T cells on EBV-infected B cells other than in terms of cytotoxicity, although the requirement for T cells in establishing lymphomas in SCID mice strongly suggests that helper functions are present (20). In clinical posttransplant lymphomas, CD4+ T cells form a close association or ring around the tumor cells and outnumber CD8+ T cells, although their direct activity within these tumors has not been established (38). CD4+ T cells have been shown to provide help for the reactivation of EBV from resting memory B cells in vitro associated with up-regulation of the lytic cycle transactivator BZLF1 (13). In addition, coculture of CD4+ T cells and established LCL has been reported to reduce proliferation, increase expression of CD38, and decrease the susceptibility of LCL cells to lysis by latent antigen-specific CD8+ cytotoxic T lymphocytes (23). Together, these results demonstrate that CD4+ T cells could act as helper cells to facilitate the reactivation and escape of virus by accelerating the transition of B cells from the latent to the lytic cycle in later stages of infection. These observations have particular resonance when they are considered in light of the strong association between increased EBV activity, elevated levels of antibody to lytic virus antigen, and subsequent tumor development (9, 12, 18, 28, 30).
The results presented here provide additional evidence for T helper cell functions in the initial stages of EBV infection and transformation. This apparently contradicts our earlier finding showing that the addition of EBV-reactive cytotoxic CD4+ T cells to whole cord blood at a ratio as low as 1:100 reduced the virus transformation titer to near zero in most cases (58). However, the prior results do not distinguish between the direct effect of CD4+ T cells on LCL and possible indirect effects such as enhanced NK cell activation. By culturing CD4+ T cells with purified B cells and CD14+ monocytes, a combination that more closely resembles the cells found within the tonsil B-cell follicles, we can now measure the direct effect of CD4+ T cells. The finding that B-cell transformation was enhanced twofold at low E:T ratios but reduced at high E:T ratios supports our conclusion that only the minority of the CD4+ T cells which contain cytotoxic granules are able to limit EBV infection with the remaining cells, providing help for the outgrowth of LCL. The effect of CD4+ T cells on B-cell transformation was strongly influenced by the Th1/Th2 phenotype. Three out of four Th1-polarized CD4+ T-cell lines inhibited transformation at the lower E:T ratio of 1:1, while all Th2-polarized lines enhanced virus transformation at least 10-fold.
The mechanism for CD4+ T-cell enhancement of EBV transformation has not been fully investigated but could be mediated by cytokine release, particularly that of IL-4, which is a potent B-cell growth factor, or by cell surface molecules such as CD40L. CD40/CD40L interactions are critical in vivo for the establishment of EBV infection, as B cells from patients with dysfunctional CD40L transform poorly with EBV (19). In normal B cells, class switching and memory formation are dependent on T-cell signaling via CD40/CD40L (32). The circulating memory B cells that maintain latent EBV have also undergone class switching and affinity maturation (49), which may in part be a result of T helper interactions. EBV LMP1 mimics CD40/CD40L signaling and was recently shown to promote class switching in vitro, which questions the need for CD4+ T-cell help in establishing latent EBV infection in the B-cell memory compartment (15). However, LMP1 itself is up-regulated by CD40/CD40L while both IL-4 and IL-10 induce LMP1 in tonsil B cells (25), consistent with a role for CD4+ T cells in establishing EBV latency in vivo.
Despite over 50 years of study, the complex sequence of stimuli that control the generation of memory B cells is not fully understood, but it is clear that T helper cells have a central role (32). EBV has evolved some autonomy from this process through LMP1 and LMP2 mimicry of CD40 and B-cell receptor signaling (15, 40). Nevertheless, there are other important interactions between B cells and T cells which govern their differentiation and may also control the entry of B cells into the circulating memory pool, with the attendant change in phenotype from proliferating germinal center blast to resting memory cell (32). Whether immune regulatory signaling affects similar changes on EBV-infected B cells is unknown, but the answer to this question could have central importance in understanding the range of EBV-associated diseases and deserves to be further researched. In particular, further in situ studies of the expression of TNF superfamily members and cytokines by T lymphocytes and EBV-infected B cells should be performed with tonsils.
Acknowledgments
This work was initiated with support from the Wellcome Trust (reference no. 055388), while G.J.M. was funded by a Ph.D. studentship from the University of Bristol.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Babcock, G. J., L. L. Decker, R. B. Freeman, and D. A. Thorley-Lawson. 1999. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 190:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. [DOI] [PubMed] [Google Scholar]
- 3.Babcock, G. J., and D. A. Thorley-Lawson. 2000. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc. Natl. Acad. Sci. USA 97:12250-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Bickham, K., and C. Munz. 2003. Contrasting roles of dendritic cells and B cells in the immune control of Epstein-Barr virus. Curr. Top. Microbiol. Immunol. 276:55-76. [DOI] [PubMed] [Google Scholar]
- 6.Bickham, K., C. Munz, M. L. Tsang, M. Larsson, J. F. Fonteneau, N. Bhardwaj, and R. Steinman. 2001. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J. Clin. Investig. 107:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 106:1251-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callan, M. F., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, W. M., K. H. Chan, H. L. Chen, R. X. Luo, S. P. Ng, W. Luk, B. J. Zheng, M. F. Ji, J. S. Liang, J. S. Sham, D. K. Wang, Y. S. Zong, and M. H. Ng. 2002. Assessing the risk of nasopharyngeal carcinoma on the basis of EBV antibody spectrum. Int. J. Cancer 97:489-492. [DOI] [PubMed] [Google Scholar]
- 10.Cope, A. P., R. S. Liblau, X. D. Yang, M. Congia, C. Laudanna, R. D. Schreiber, L. Probert, G. Kollias, and H. O. McDevitt. 1997. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J. Exp. Med. 185:1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford, D. H., and I. Ando. 1986. EB virus induction is associated with B-cell maturation. Immunology 59:405-409. [PMC free article] [PubMed] [Google Scholar]
- 12.de-Thé, G., A. Geser, N. E. Day, P. M. Tukei, E. H. Williams, D. P. Beri, P. G. Smith, A. G. Dean, G. W. Bronkamm, P. Feorino, and W. Henle. 1978. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 274:756-761. [DOI] [PubMed] [Google Scholar]
- 13.Fu, Z., and M. J. Cannon. 2000. Functional analysis of the CD4+ T-cell response to Epstein-Barr virus: T-cell-mediated activation of resting B cells and induction of viral BZLF1 expression. J. Virol. 74:6675-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottschalk, S., C. M. Rooney, and H. E. Heslop. 2005. Post-transplant lymphoproliferative disorders. Annu. Rev. Med. 56:29-44. [DOI] [PubMed] [Google Scholar]
- 15.He, B., N. Raab-Traub, P. Casali, and A. Cerutti. 2003. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J. Immunol. 171:5215-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill, A. B., S. P. Lee, J. S. Haurum, N. Murray, Q. Y. Yao, M. Rowe, N. Signoret, A. B. Rickinson, and A. J. McMichael. 1995. Class I major histocompatibility complex-restricted cytotoxic T lymphocytes specific for Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell lines against which they were raised. J. Exp. Med. 181:2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hislop, A. D., M. Kuo, A. B. Drake-Lee, A. N. Akbar, W. Bergler, N. Hammerschmitt, N. Khan, U. Palendira, A. M. Leese, J. M. Timms, A. I. Bell, C. D. Buckley, and A. B. Rickinson. 2005. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Investig. 115:2546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjalgrim, H., J. Askling, K. Rostgaard, S. Hamilton-Dutoit, M. Frisch, J. S. Zhang, M. Madsen, N. Rosdahl, H. B. Konradsen, H. H. Storm, and M. Melbye. 2003. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N. Engl. J. Med. 349:1324-1332. [DOI] [PubMed] [Google Scholar]
- 19.Imadome, K., M. Shirakata, N. Shimizu, S. Nonoyama, and Y. Yamanashi. 2003. CD40 ligand is a critical effector of Epstein-Barr virus in host cell survival and transformation. Proc. Natl. Acad. Sci. USA 100:7836-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannessen, I., M. Asghar, and D. H. Crawford. 2000. Essential role for T cells in human B-cell lymphoproliferative disease development in severe combined immunodeficient mice. Br. J. Haematol. 109:600-610. [DOI] [PubMed] [Google Scholar]
- 21.Joseph, A. M., G. J. Babcock, and D. A. Thorley-Lawson. 2000. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J. Virol. 74:9964-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keever-Taylor, C. A., B. Behn, S. Konings, R. Orentas, B. Davies, and D. Margolis. 2003. Suppression of EBV release from irradiated B lymphoblastoid cell-lines: superior activity of ganciclovir compared with acyclovir. Cytotherapy 5:323-335. [DOI] [PubMed] [Google Scholar]
- 23.Khanolkar, A., Z. Fu, L. J. Underwood, K. L. Bondurant, R. Rochford, and M. J. Cannon. 2003. CD4(+) T cell-induced differentiation of EBV-transformed lymphoblastoid cells is associated with diminished recognition by EBV-specific CD8(+) cytotoxic T cells. J. Immunol. 170:3187-3194. [DOI] [PubMed] [Google Scholar]
- 24.Khanolkar, A., H. Yagita, and M. J. Cannon. 2001. Preferential utilization of the perforin/granzyme pathway for lysis of Epstein-Barr virus-transformed lymphoblastoid cells by virus-specific CD4+ T cells. Virology 287:79-88. [DOI] [PubMed] [Google Scholar]
- 25.Kis, L. L., M. Takahara, N. Nagy, G. Klein, and E. Klein. 2006. Cytokine mediated induction of the major Epstein-Barr virus (EBV)-encoded transforming protein, LMP-1. Immunol. Lett. 104:83-88. [DOI] [PubMed] [Google Scholar]
- 26.Kurth, J., M. L. Hansmann, K. Rajewsky, and R. Kuppers. 2003. Epstein-Barr virus-infected B cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc. Natl. Acad. Sci. USA 100:4730-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam, K. M., N. Syed, H. Whittle, and D. H. Crawford. 1991. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet 337:876-878. [DOI] [PubMed] [Google Scholar]
- 29.Landais, E., X. Saulquin, E. Scotet, L. Trautmann, M. A. Peyrat, J. L. Yates, W. W. Kwok, M. Bonneville, and E. Houssaint. 2004. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 103:1408-1416. [DOI] [PubMed] [Google Scholar]
- 30.Levine, P. H., G. Stemmermann, E. T. Lennette, A. Hildesheim, D. Shibata, and A. Nomura. 1995. Elevated antibody titers to Epstein-Barr virus prior to the diagnosis of Epstein-Barr-virus-associated gastric adenocarcinoma. Int. J. Cancer 60:642-644. [DOI] [PubMed] [Google Scholar]
- 31.Levitskaya, J., M. Coram, V. Levitsky, S. Imreh, P. M. Steigerwald-Mullen, G. Klein, M. G. Kurilla, and M. G. Masucci. 1995. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature 375:685-688. [DOI] [PubMed] [Google Scholar]
- 32.McHeyzer-Williams, L. J., and M. G. McHeyzer-Williams. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487-513. [DOI] [PubMed] [Google Scholar]
- 33.Münz, C., K. L. Bickham, M. Subklewe, M. L. Tsang, A. Chahroudi, M. G. Kurilla, D. Zhang, M. O'Donnell, and R. M. Steinman. 2000. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 191:1649-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niederman, J. C., A. S. Evans, L. Subrahmanyan, and R. W. McCollum. 1970. Prevalence, incidence and persistence of EB virus antibody in young adults. N. Engl. J. Med. 282:361-365. [DOI] [PubMed] [Google Scholar]
- 35.Nikiforow, S., K. Bottomly, and G. Miller. 2001. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 75:3740-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforow, S., K. Bottomly, G. Miller, and C. Münz. 2003. Cytolytic CD4+-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 77:12088-12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong, K.-W., A. D. Wilson, T. R. Hirst, and A. J. Morgan. 2003. The B subunit of Escherichia coli heat-labile enterotoxin enhances CD8+ cytotoxic-T-lymphocyte killing of Epstein-Barr virus-infected cell lines. J. Virol. 77:4298-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera, S. M., J. A. Thomas, M. Burke, and D. H. Crawford. 1998. Analysis of the T-cell micro-environment in Epstein-Barr virus-related post-transplantation B lymphoproliferative disease. J. Pathol. 184:177-184. [DOI] [PubMed] [Google Scholar]
- 39.Piriou, E., K. van Dort, N. M. Nanlohy, M. H. van Oers, F. Miedema, and D. van Baarle. 2005. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood 106:3166-3174. [DOI] [PubMed] [Google Scholar]
- 40.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene 23:8619-8628. [DOI] [PubMed] [Google Scholar]
- 41.Prang, N. S., M. W. Hornef, M. Jager, H. J. Wagner, H. Wolf, and F. M. Schwarzmann. 1997. Lytic replication of Epstein-Barr virus in the peripheral blood: analysis of viral gene expression in B lymphocytes during infectious mononucleosis and in the normal carrier state. Blood 89:1665-1677. [PubMed] [Google Scholar]
- 42.Rennick, D. M., M. M. Fort, and N. J. Davidson. 1997. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 61:389-396. [DOI] [PubMed] [Google Scholar]
- 43.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In P. M. Howley and D. M. Knipe (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 44.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 45.Robinson, J., D. Smith, and J. Niederman. 1980. Mitotic EBNA-positive lymphocytes in peripheral blood during infectious mononucleosis. Nature 287:334-335. [DOI] [PubMed] [Google Scholar]
- 46.Rochford, R., M. J. Cannon, R. E. Sabbe, K. Adusumilli, G. Picchio, J. M. Glynn, D. J. Noonan, D. E. Mosier, and M. V. Hobbs. 1997. Common and idiosyncratic patterns of cytokine gene expression by Epstein-Barr virus transformed human B cell lines. Viral Immunol. 10:183-195. [DOI] [PubMed] [Google Scholar]
- 47.Schaft, N., B. Lankiewicz, J. Drexhage, C. Berrevoets, D. J. Moss, V. Levitsky, M. Bonneville, S. P. Lee, A. J. McMichael, J. W. Gratama, R. L. Bolhuis, R. Willemsen, and R. Debets. 2006. T cell re-targeting to EBV antigens following TCR gene transfer: CD28-containing receptors mediate enhanced antigen-specific IFNgamma production. Int. Immunol. 18:591-601. [DOI] [PubMed] [Google Scholar]
- 48.Shi, Y., K. D. Smith, M. G. Kurilla, and C. T. Lutz. 1997. Cytotoxic CD8+ T cells recognize EBV antigen but poorly kill autologous EBV-infected B lymphoblasts: immunodominance is elicited by a peptide epitope that is presented at low levels in vitro. J. Immunol. 159:1844-1852. [PubMed] [Google Scholar]
- 49.Souza, T. A., B. D. Stollar, J. L. Sullivan, K. Luzuriaga, and D. A. Thorley-Lawson. 2005. Peripheral B cells latently infected with Epstein-Barr virus display molecular hallmarks of classical antigen-selected memory B cells. Proc. Natl. Acad. Sci. USA 102:18093-18098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun, Q., R. L. Burton, and K. G. Lucas. 2002. Cytokine production and cytolytic mechanism of CD4(+) cytotoxic T lymphocytes in ex vivo expanded therapeutic Epstein-Barr virus-specific T-cell cultures. Blood 99:3302-3309. [DOI] [PubMed] [Google Scholar]
- 51.Thorley-Lawson, A. D. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75-82. [DOI] [PubMed] [Google Scholar]
- 52.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 53.Vinuesa, C. G., S. G. Tangye, B. Moser, and C. R. Mackay. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5:853-865. [DOI] [PubMed] [Google Scholar]
- 54.Wang, S., and L. Chen. 2004. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell. Mol. Immunol. 1:37-42. [PubMed] [Google Scholar]
- 55.Whitney, B. M., A. T. Chan, A. B. Rickinson, S. P. Lee, C. K. Lin, and P. J. Johnson. 2002. Frequency of Epstein-Barr virus-specific cytotoxic T lymphocytes in the blood of Southern Chinese blood donors and nasopharyngeal carcinoma patients. J. Med. Virol. 67:359-363. [DOI] [PubMed] [Google Scholar]
- 56.Wilson, A. D., J. C. Hopkins, and A. J. Morgan. 2001. In vitro cytokine production and growth inhibition of lymphoblastoid cell lines by CD4+ T cells from Epstein-Barr virus (EBV) seropositive donors. Clin. Exp. Immunol. 126:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson, A. D., and A. J. Morgan. 1998. Indirect measurement of Epstein-Barr virus neutralising antibodies by ELISA. J. Virol. Methods 73:11-19. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, A. D., and A. J. Morgan. 2002. Primary immune responses by cord blood CD4+ T cells and NK cells inhibit Epstein-Barr virus B-cell transformation in vitro. J. Virol. 76:5071-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson, A. D., I. Redchenko, N. A. Williams, and A. J. Morgan. 1998. CD4+ T cells inhibit growth of Epstein-Barr virus-transformed B cells through CD95-CD95 ligand-mediated apoptosis. Int. Immunol. 10:1149-1157. [DOI] [PubMed] [Google Scholar]