Abstract
Bluetongue virus (BTV) is a member of the Orbivirus genus within the Reoviridae family. Like those of other members of the family, BTV particles are nonenveloped and contain two distinct capsids, namely, an outer capsid and an inner capsid or core. The two outer capsid proteins, VP2 and VP5, are involved in BTV entry into cells and in the delivery of the transcriptionally active core to the target cell cytoplasm. However, very little is known about the precise mechanism of BTV entry. In this report, using RNA interference, we demonstrate that inhibition of the clathrin-dependent endocytic pathway correlates with reduced BTV internalization and subsequent replication. Furthermore, by using the ATPase inhibitor bafilomycin A1, we show that exposure of the virus to acidic pH is required for productive infection. Moreover, microscopic analysis of cells incubated with BTV indicated that the virus is internalized into early endosomes, where separation of the outer capsid and inner core occurs. Together, our data indicate that BTV undergoes low-pH-induced penetration in early endosomes following clathrin-mediated endocytosis from the plasma membrane, supporting a stepwise model for BTV entry and penetration.
Endocytosis provides pathways through which many viruses productively enter their target cells. Not only does endocytosis provide access to acidic conditions for pH-dependent viruses, but it can also bypass barriers imposed by the cell's cortical cytoskeleton and/or mediate delivery to specific sites within the target cell (see the review by Marsh and Helenius [24]). Of the multiple endocytic mechanisms that are now thought to exist, clathrin-mediated endocytosis remains the most extensively studied. This pathway, which is responsible for the internalization and productive infection of a number of viruses, including alphaviruses, rhabdoviruses, picornaviruses, and orthomyxoviruses, involves the formation of clathrin-coated vesicles at the plasma membrane and leads to delivery of internalized ligands and membrane components to mildly acidic early endosomes (24). Clathrin molecules alone do not have the ability to bind to the cargo proteins that are present in the plasma membrane, and for such activity an association with adaptor proteins (APs) is essential. The APs are also responsible for the correct organization of the clathrin molecules to generate clathrin-coated particles (CCPs). There are several APs, but AP2 is the most abundant in CCPs. AP2 is a large complex composed of four different but related subunits, namely, α, β2, μ2, and σ2. Each subunit has a different and essential function in clathrin-mediated endocytosis (5, 30, 36). The μ2 subunit, in particular, can recognize and bind to specific cytoplasmic motifs of the cargo proteins and is therefore essential for the formation of CCPs.
Here we demonstrate that the nonenveloped virus Bluetongue virus (BTV), a member of the Orbivirus genus of the Reoviridae family, also exploits clathrin-mediated endocytosis for productive infection.
Within the Reoviridae family, reoviruses are also internalized by clathrin-mediated endocytosis (3, 9, 21, 25). For reovirus, the low pH of the late endosomes activates exogenous proteases which are responsible for the cleavage of the outer capsid protein μ1N in order to gain a cell permeabilization-competent structure of the protein (8, 12). On the other hand, the exact mechanism used by rotavirus to enter cells is still debated. In fact, while lysosomotropic agents used to inhibit endosomal acidification initially showed that the virus enters cells by a pH-dependent mechanism (4), additional studies involving the depletion of cholesterol from the cellular membrane also corresponded with the reduction of rotavirus infectivity, indicating an entry mechanism that may also involve lipid rafts (23, 40).
BTV, like the other members of the family, has a complex capsid structure. The virion is comprised of two concentric protein shells enclosing the virus genome, which is made up of 10 double-stranded RNA segments (37). The outer capsid is composed of two proteins, VP2 and VP5, each of which plays a role in virus entry. These two proteins together form a continuous layer that covers the inner capsid or core, which is composed of two major proteins (VP3 and VP7) and three minor proteins (VP1, VP4, and VP6) in addition to the viral genome (37, 38). Cryo-electron microscopy (cryo-EM) studies have shown that the 110-kDa VP2 protein is the most exposed virion protein, with a protruding spike-like structure (29). Indeed, VP2 is responsible for hemagglutination, serotype specificity, and the receptor-binding activity of the virion (13). The second outer capsid protein, VP5 (∼60 kDa), has a globular and almost spherical shape and is less exposed than VP2 (29). We have previously shown that VP5 is involved in cell permeabilization, suggesting an essential role for the protein in the translocation of the transcriptionally active core into the cytoplasm (14). More recently, we demonstrated that VP5 has a pH-dependent fusogenic activity when expressed on the cell surface (10). Together, these findings indicate the ability of the protein to bind to cellular membranes and suggest that BTV entry may require exposure to an acidic pH. Thin-section EM studies previously showed the presence of BTV particles in coated pits, suggesting that clathrin-mediated endocytosis may be used for virus entry (7). It is therefore likely that the low pH of the endosomal compartment triggers VP2 degradation, exposing a functional form of VP5. This structurally altered VP5 then induces the destabilization of the cellular membrane and the release of the core into the cytosol.
In this report, we have undertaken a more detailed investigation of BTV entry to determine how a complex nonenveloped virus proceeds from internalization to uncoating. Using a combination of biochemical and confocal microscopy studies, together with specific inhibitors and RNA interference (RNAi), we show that BTV enters cells by clathrin-mediated endocytosis and pH-dependent penetration.
MATERIALS AND METHODS
Cells and viruses.
Mammalian cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and antibiotics/antimycotics (Sigma). BTV serotype 10 was used in all experiments. Virus stocks were generated by infection of 80 to 90% confluent BSR cells, using a multiplicity of infection (MOI) of 0.1. Infected cells were maintained in medium containing 2% fetal calf serum and incubated at 35°C. At 3 to 4 days postinfection (p.i.) or when total cytopathic effect was visible, cells and supernatant were harvested and centrifuged at low speed (3,000 rpm, 10 min). Virus was released from the cells by three cycles of freezing and thawing. Virus stocks were then stored at 4°C. For time course experiments, synchronized infections were performed. In brief, BTV was added to the cells in cold serum-free medium. Virus attachment was allowed by incubation at 4°C for 1 h. Unbound virus was removed by a series of washes with cold serum-free medium. A zero-time-point sample was then harvested, and the remaining samples were shifted to 37°C by the addition of prewarmed medium and transfer of the samples to a 37°C incubator. Subsequently, samples were harvested at different time points by transfer into cold 4% paraformaldehyde.
Antibodies.
Polyclonal anti-VP5 (gp639), monoclonal anti-VP7 (H1-5), and polyclonal anti-NS2 (R658) were used as previously described (10, 26, 44). Monoclonal anti-BTV 10 VP2 was a gift from J. J. MacLachlan (University of California). Early endosomes were labeled using an anti-early endosomal autoantigen 1 (anti-EEA1) mouse monoclonal antibody, and late endosomes were labeled with an anti-CD63 mouse monoclonal antibody (Abcam). The expression of cellular β-tubulin was monitored by a specific monoclonal antibody (Sigma). Monoclonal anti-μ2 (AP50) was obtained from BD Transduction Laboratories (Lexington, KY). As secondary antibodies for confocal microscopy, fluorescein isothiocyanate (FITC)-conjugated goat anti-guinea pig, tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-mouse, and Alexa 633-conjugated anti-rabbit immunoglobulins G (IgGs) were used (Sigma). Antibodies were used at dilutions of 1:100 for confocal microscopy and 1:5,000 for immunoblotting, with the exception of the anti-μ2 antibody, which was used at a 1:250 dilution for Western blot analysis.
Reagents and inhibition of virus entry.
The mechanism of BTV entry was investigated by using different inhibitors of endocytosis. All reagents were purchased from Sigma. The concentrations of each reagent were standardized before use in order to avoid any toxic effect on the treated cells during the experiment. Nystatin was used at final concentrations of 2.5 and 5 μg/ml to block cholesterol-dependent, raft/caveola-mediated endocytosis (2). Bafilomycin A1 (BAF) was used at final concentrations of 50, 100, and 200 nM to inhibit the vacuolar H+-ATPase (41). Chlorpromazine (CPZ) was used at final concentrations of 5 and 10 μM to disrupt clathrin-mediated endocytosis (31). Nystatin was diluted in sterile water, and BAF and CPZ were diluted in dimethyl sulfoxide (Sigma). Experiments were performed on Vero cells that were grown in 12-well plates to 70 to 80% confluence. After being washed in serum-free medium, cells were pretreated with the inhibitors for 30 min at 37°C. BTV was then added, using an MOI of 1. After 1 h of incubation, the unbound virus was removed by several washes, and fresh medium containing the inhibitor was added to the cells, followed by further incubation. Samples were harvested at 24 h p.i. and normalized for the number of cells present in each. One-third of each sample was used for plaque assay analysis, and the remainder was used for immunoblotting.
Immunoblotting.
For immunoblotting, the samples were clarified by centrifugation and washed twice with phosphate-buffered saline (PBS). Cell pellets were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer and resolved in 10% polyacrylamide gels. Immunoblotting was carried out as previously described (10). To detect proteins that were not highly expressed, a chemiluminescence system was used. Membranes were incubated with the Lumi-Phos WB substrate (Perbio) for 10 min, and the protein bands were visualized by exposing the blots to Hyperfilm (Amersham).
siRNA transfection.
The small interfering RNA (siRNA) designed to target the μ2 subunit of the clathrin-AP2 adaptor complex was described previously (11). In brief, HeLa cells were grown to 30 to 40% confluence in 24-well plates and transfected with 60 pmol of siRNA and 3 μl of Oligofectamine in OptiMEM following the manufacturer's protocol (Invitrogen). As a negative control, a duplex siRNA which targets green fluorescent protein (GFP) was used. Each transfection was performed in duplicate; for immunofluorescence studies, the cells were plated on 13-mm-diameter glass coverslips (VWR). A second transfection was performed 12 h later, using the same protocol as that described above. Thirty-six hours after the second transfection, the cells were infected with BTV, using MOIs of 0.5 for immunoblotting and 10 for morphological studies. Samples were harvested for plaque assay and immunoblotting analysis at 12 and 24 h p.i.
Virus titration.
Supernatants from BTV-infected cells were collected after 24 h, and relative virus titers were determined by plaque assays on BSR cells. Briefly, cells were seeded in 12-well plates and incubated for 45 min with diluted virus in 100 μl serum-free DMEM. After removal of the inoculums, cells were washed once and overlaid with 1 ml of 1% agarose in DMEM containing 2% fetal bovine serum and antibiotics. Plaques were visualized after an incubation period of 2 to 3 days at 35°C by staining with neutral red for several hours.
Tf uptake.
Transferrin conjugated with TRITC (Tf-TRITC; Sigma) was used to monitor the inhibition of clathrin-mediated endocytosis. At 48 h posttransfection, HeLa cells were washed twice with binding buffer (RPMI 1640 without bicarbonate containing 0.2% bovine serum albumin and 10 mM HEPES, pH 7.0) (11) and incubated for 30 min at 37°C to remove endogenous Tf. Cells were then infected with BTV 10, using an MOI of 10, and incubated for 1 h at 4°C to allow virus binding. Unbound virus was removed by washing with ice-cold, serum-free medium. Infections were carried out for 30 min at 37°C. Uptake of Tf-TRITC was allowed by adding the reagent at a final concentration of 100 nM to the infected cells 10 min prior to the end of the incubation. Samples were harvested and processed for immunofluorescence staining and confocal microscopy.
Confocal microscopy.
Mammalian cells were seeded in a 24-well plate on 13-mm-diameter coverslips. After infection, the cells were washed with cold PBS and fixed for 5 to 10 min in 4% paraformaldehyde in PBS. Immunolabeling of each sample was performed as previously described (10). Nuclei were stained using Hoechst 33258 at a dilution of 1:2,000 (Sigma). After being labeled, the samples were mounted using Fluoprep (bioMerieux) and analyzed with a Zeiss LSM 510 confocal microscope, and images were obtained using LSM 510 image browser software and processed using Photoshop Element 2.0 software (Microsoft).
RESULTS
BTV uses a clathrin-mediated pathway to enter cells.
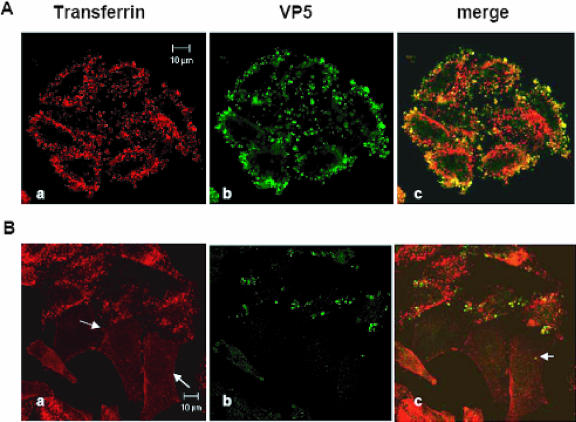
In an earlier EM study, BTV particles were observed in coated vesicles in cells incubated with the virus (7). We therefore investigated whether clathrin-mediated endocytosis is required for BTV infection and replication, using specific inhibitors and RNAi. siRNAs have been used to knock down expression of the μ2 subunit of the clathrin-AP2 adaptor complex and to inhibit, for example, clathrin-mediated endocytosis of Tf (11, 28). For our experiments, we used HeLa cells, which have previously been shown to be susceptible to RNAi and to BTV infection (11, 46). Transfection experiments were carried out as described in Materials and Methods, and an siRNA against GFP was used as a negative control. A functional effect of AP2 depletion on Tf endocytosis was demonstrated by immunofluorescence (Fig. 1B). Cells in which AP2-dependent clathrin-mediated endocytosis was inhibited (arrows) failed to internalize fluorescent Tf into punctate endosomes and to accumulate the receptor on the cell surface. When RNAi-treated cells were incubated with BTV for 30 min and subsequently stained for the viral VP5 protein, only cells that retained the ability to internalize Tf during a 10-min pulse were labeled for virus, which appeared as punctate clusters either at the cell surface or internally. Cells that failed to internalize Tf showed little, if any, labeling of virus (Fig. 1A and B). Note that Tf internalization was seen in some cells in the μ2 RNAi-treated samples, suggesting that these cells had failed to take up the siRNA.
FIG. 1.
BTV entry inhibition by μ2 siRNA. HeLa cells were transfected with the μ2 siRNA for 48 h. Infection was performed as described in Materials and Methods. The effect of the siRNA was investigated by monitoring the cellular uptake of Tf-TRITC by confocal microscopy. BTV particles were stained with a polyclonal anti-VP5 primary antibody and localized with a FITC-conjugated secondary antibody. (A) HeLa cells transfected with the control siRNA, which does not inhibit either Tf uptake (a) or BTV entry (b). (B) Inhibitory effect of μ2 siRNA. In panel a, arrows indicate cells where Tf-TRITC uptake was inhibited, which show no punctate cytoplasmic staining. The arrow in panel c indicates a reduced number of internalized BTV particles in a cell affected by the siRNA.
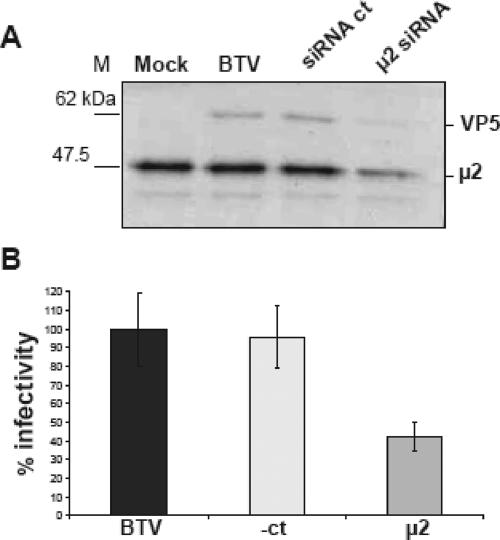
To demonstrate that RNAi reduced μ2 expression, we analyzed cell lysates by Western blotting for the μ2 protein 48 h after the first transfection. After being harvested, the cells were counted, and identical numbers were analyzed by SDS-10% PAGE. Figure 2A shows that μ2 expression was substantially reduced in the cells transfected with the specific siRNA. To analyze the effect of AP2 knockdown on viral entry, we looked for the synthesis of the viral VP5 protein in the BTV-treated cells. Figure 2A also shows that VP5 expression was partially suppressed in the μ2 siRNA-treated cells. No effect on Tf uptake, BTV uptake, or BTV infection was seen in cells treated with the control GFP siRNA (Fig. 2A and B). In addition, expression of the VP5 protein was similar to that seen in BTV-infected cells, indicating that the irrelevant siRNA did not interfere with BTV replication. In addition, the level of μ2 expression was identical to that in uninfected and BTV-infected cells, confirming the specific effect of the siRNA. Additional sets of data were acquired from BTV plaque assays performed on samples collected at the same time point as the samples for protein analyses. The infectivity of each sample was tested in triplicate in BSR cells. The results are expressed as percentages of the plaque numbers seen with samples harvested from untreated BTV-infected cells. The averages of the infectivities in three different experiments indicated that while the control siRNA did not inhibit BTV replication, infectious virus production from the cells transfected with μ2 siRNA was reduced (Fig. 2B). Our data suggest that AP2-dependent clathrin-mediated endocytosis is required for BTV entry and, together with published EM data (7), indicate that endocytosis of BTV in clathrin-coated vesicles is required for productive infection.
FIG. 2.
Effect of siRNA on BTV replication. Transfection of HeLa cells with the μ2 siRNA was carried out for 36 h. Cells were infected with BTV, using an MOI of 0.5, and incubated for 12 h. Infected cells were harvested and counted, and the same number was loaded in each lane for Western blot analysis. (A) SDS-10% PAGE of infected cells stained with anti-VP5 and anti-μ2 antibodies. (B) Plaque assay analysis. Each sample was tested on BSR cells in triplicate as described in Materials and Methods. Results are presented as percentages, where the plaque number seen for untreated cells is taken as 100%.
CPZ is responsible for the inhibition of BTV entry.
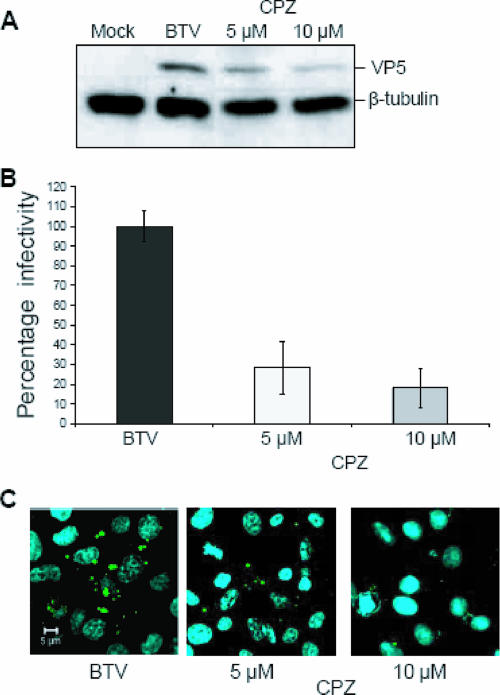
To confirm that BTV entry is indeed dependent on clathrin, we used CPZ, a pharmacological inhibitor of clathrin-mediated endocytosis that has been used extensively by others to demonstrate clathrin-mediated endocytosis of several viruses (1, 19, 20, 35). Experiments were carried out using 5 and 10 μM of CPZ (final concentrations), as described in Materials and Methods. The inhibitor was present during the entire course of the infection in order to obtain a stronger reduction in BTV replication. At the end of the incubation, samples were collected and processed for plaque assay and Western blot analysis. Western blot analysis indicated that cells incubated with 5 and 10 μM CPZ during BTV infection showed a dose-dependent reduction of VP5 expression. To verify that the same amount of protein was loaded in each lane, the blots were also stained with an anti-β-tubulin antibody. Taking into account the variation in loading, the VP5 signal from untreated samples was assumed to be 100% of protein expression. The presence of 5 μM and 10 μM CPZ was therefore responsible for 32% and 52% reductions of the respective signals (Fig. 3A).
FIG. 3.
Chemical inhibition of the clathrin pathway. Vero cells were treated with CPZ as specified in Materials and Methods. Cells were infected with BTV and harvested at 24 h p.i. (A) Immunoblotting of CPZ-treated cells was performed as previously described. To verify that the same amount of protein was loaded in each lane, the blots were also stained with an anti-β-tubulin antibody. The intensity of each band was quantified using ImageQuant software (GE Healthcare) and then normalized. (B) Inhibition of BTV infectivity in the presence of 5 and 10 μM of CPZ. Each sample was tested in triplicate. (C) The effect of CPZ was further confirmed by confocal microscopy. At 24 h p.i., cells were stained with a polyclonal anti-VP5 antibody and a FITC-conjugated secondary antibody (green). Nuclei were stained with Hoechst 33258 (blue). Images were collected as indicated in the legend to Fig. 1. Sample identification and final concentrations of the chemical are indicated.
An additional indication of the inhibitory effect of CPZ on BTV replication was provided by plaque assays of the treated samples in parallel. Each experiment was performed in triplicate, using BSR cells. Since the plaque assay was performed using serial dilutions of the harvested supernatant (released virus), it is unlikely that the inhibitor could influence virus entry at such a level. The results are again expressed as percentages of the number of plaques seen with samples harvested from untreated BTV-infected cells. The results showed an 80% reduction in virus produced from CPZ-treated cells (Fig. 3B). Additional evidence was obtained by confocal microscopy studies. Cells infected with BTV, with or without CPZ, were analyzed by immunofluorescence microscopy 24 h after transfection. In cells infected in the presence of CPZ, very little VP5 staining was seen, in contrast to the case for control cells. The presence of CPZ resulted in a significant reduction in BTV replication, which was likely not due to any loss of cell viability, as the nuclear compartments were intact (Fig. 3C). These findings are consistent with the notion that clathrin-mediated endocytosis is required for BTV entry.
Nystatin does not affect BTV replication.
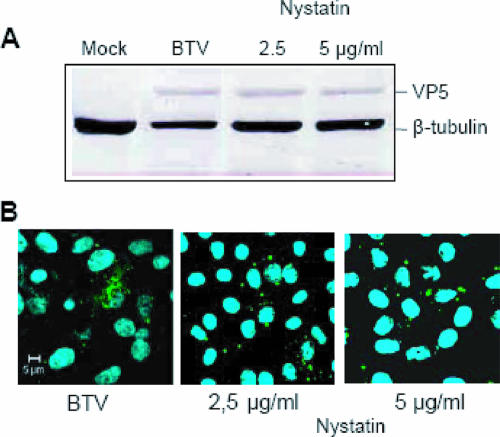
There is now a considerable amount of evidence indicating that many cells exhibit clathrin-independent endocytic pathways, at least some of which can be exploited by different viruses to effect their entry (for a review, see reference 24). To investigate whether cholesterol-dependent (so-called lipid raft-dependent) endocytic mechanisms, including caveola-mediated endocytosis, might be involved in BTV entry, we examined the effect of nystatin, which binds membrane cholesterol, on BTV replication in Vero cells (20, 34). After pretreatment with nystatin, the cells were infected with BTV, using the same procedure as that described for CPZ. Samples were collected after 24 h and analyzed by Western blotting. The results indicated that exposure to 2.5 and 5 μg/ml of nystatin during BTV infection did not have any effect on virus replication. This was further confirmed by plaque assay (data not shown). The data indicate that cholesterol-dependent endocytic mechanisms are not required, thus ruling out the use of this pathway (Fig. 4A). Additional data were provided by confocal microscopy studies, which demonstrated no effect of the drug on BTV replication (Fig. 4B). Indeed, at a concentration of 5 μg/ml, nystatin failed to show any reduction in the number of BTV particles in the infected cells. By staining the cells with Hoechst 33258, we also confirmed that there was no apparent loss of cell viability associated with the inhibitor (Fig. 4B). Since we have not used a positive control, such as simian virus 40 (SV40) (32-34), to confirm the effect of nystatin on raft-dependent virus entry, the results obtained in this set of experiments must be considered preliminary evidence that the BTV entry mechanism does not occur by cholesterol-dependent endocytosis.
FIG. 4.
Abrogation of the caveola pathway does not inhibit BTV replication. Vero cells were incubated with different amounts of nystatin to disrupt the caveola pathway. (A) Western blotting of cells infected with BTV after nystatin treatment. The intensity of each band was quantified using ImageQuant software and then normalized. (B) Confocal microscopy to confirm the above results was performed as described in the legend to Fig. 3. Sample identification and final concentrations of the drug are indicated.
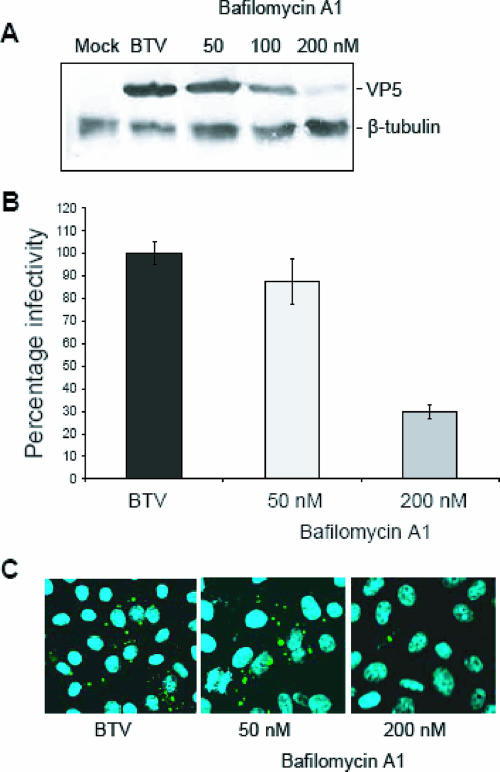
BTV replication is inhibited by BAF.
BTV entry has previously been predicted to be a pH-dependent process (17), and our previous studies also demonstrated that VP5 has a pH-dependent fusogenic activity (10). Together, these results suggest that BTV may undergo low-pH-induced penetration from acidic endosomal vesicles during the infection process. To obtain direct evidence for a role for acidic pH in BTV entry, the effect of BAF on BTV replication was investigated. BAF is a specific inhibitor of the vacuolar H+-ATPase, and its use leads to alkalinization of acidic organelles, including endosomes and lysosomes. For these studies, Vero cells were exposed to three different concentrations of BAF (50, 100, and 200 nM) prior to BTV infection, and the effect of the inhibitor on BTV replication was examined at 24 h p.i. Both plaque assay and Western blotting indicated that at a final concentration of 200 nM, BAF had a strong inhibitory effect on BTV replication (Fig. 5A and B). Additional evidence of the effect of the drug was obtained by confocal microscopy, also demonstrating that the reduction in BTV replication was not due to reduced cell viability (Fig. 5C). The accumulating results provided a clear correlation between increasing BAF concentrations and a reduction of BTV replication, confirming the importance of endosomal acidification in BTV internalization.
FIG. 5.
Inhibition of endosomal acidification. The dependence of BTV entry on low pH was analyzed using increasing concentrations of BAF. Pretreated Vero cells were infected using an MOI of 1 and harvested after 24 h. (A) Cell lysates were analyzed by Western blotting using anti-VP5 and anti-β-tubulin antibodies. (B) Plaque assay of samples collected from BTV infection in the presence of BAF. (C) Confocal microscopy of infected Vero cells was performed as described in the legend to Fig. 3. Sample identification and final concentrations of BAF are indicated.
Upon internalization, VP5 rapidly separates from the core in early endosomes.
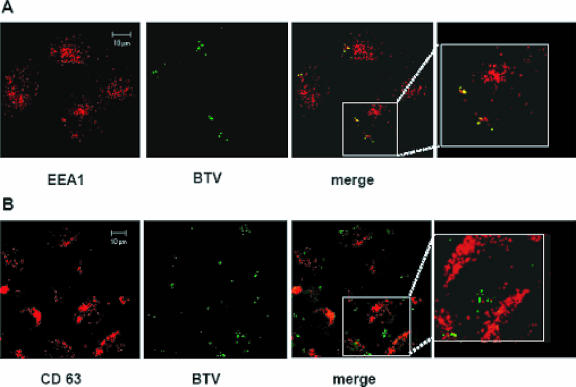
To determine whether internalized BTV particles associate with early or late endosomes, we performed immunofluorescence and confocal microscopy of BTV-infected cells. The presence of BTV particles was monitored with antibodies against the VP5 outer capsid protein, and the early and late endosomes were monitored by labeling for two cellular proteins. Early endosomes were identified with antibodies to EEA1, a FYVE domain protein recruited primarily to early endosomes (39). Late endosomes were identified using antibodies to CD63, a tetraspanin located primarily on the internal membranes of multivesicular late endosomes (11). Synchronized BTV infections were performed as described in Materials and Methods. Confocal microscopy revealed that BTV particles were localized in the early endosomes for up to 30 min p.i. (Fig. 6A). In contrast, samples harvested at the same time points and stained with an anti-CD63 antibody failed to show any localization of BTV in the late endosomes (Fig. 6B). Since BTV particles were monitored using a VP5 antibody, it is possible that the images indicated only the location of the VP5 protein during BTV entry, instead of the whole virus particles. This particular approach could indicate only the specific position of VP5 during entry. It is indeed possible that BTV virions do not migrate to the late endosomes, indicating that the mildly low pH present in the early endosomes could be sufficient to trigger virus uncoating and release of the core into the cytoplasm.
FIG. 6.
BTV localization in endosomal compartments. HeLa cells were infected and prepared for confocal microscopy as described in Materials and Methods. Early and late endosomes were detected using (A) monoclonal anti-EEA1 and (B) monoclonal anti-CD63 antibodies. A TRITC-conjugated antibody was used as the secondary antibody in both cases (red). BTV-infected cells were stained using a polyclonal anti-VP5 antiserum and a FITC-conjugated secondary antibody (green). The displayed pictures represent cells in which the infection was carried out for 30 min. A detailed image of each merged sample is shown in the last panel.
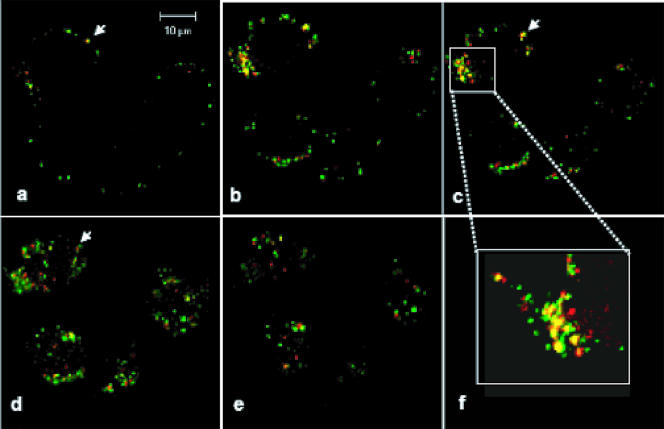
Our data so far suggest that BTV particles enter cells by clathrin-mediated endocytosis and subsequent pH-dependent penetration. In order to obtain a clearer picture about the uncoating of BTV, additional morphological experiments were performed. Cells were harvested at 0, 2, 5, 15, 30, 60, 120, 180, and 240 min p.i., and the exact locations of VP5 and VP7 (the surface protein of BTV core) during BTV entry were monitored by staining the cells with antibodies specific for these two structural proteins. Images of the infected cells taken through the z axis showed that early (by 15 min) after infection, VP7 was already separated from VP5. Progressing from the apical to the basal section of the cells, VP5 appeared to be retained in an area close to the plasma membrane, while VP7 moved distally toward the center of the cytoplasm (Fig. 7 panels a to e). The separation between the two proteins became more evident during the course of the infection. Evidence of such separation was also provided by the increasing number of red dots (representing VP7) visible when proceeding from apical to middle sections. Analysis of the accumulated data suggests that at the early stages of infection, the core particles separate from the outer capsid.
FIG. 7.
Separation between outer capsid and core. HeLa cells were infected using an MOI of 10. Samples were harvested at 15 min p.i. and processed for confocal microscopy. The outer capsid protein VP5 was stained with a polyclonal primary antiserum and a secondary FITC-conjugated antibody (green). The core protein VP7 was labeled with a monoclonal antibody and a TRITC-conjugated secondary antibody (red). Pictures represent a continuous z-stack imaging process, with a stack size of 8 μm and a scale between cellular layers of 1 μm. Images progress from the top of the cell (a) through the middle sections (b to e). Arrows indicate a specific area in which VP5 and VP7 initially colocalized (a) and then separated (d). Panel f shows a detailed view of the localization of the two proteins.
DISCUSSION
Over the past few years, an increasing number of studies have been performed to understand how viruses enter cells and what the underlying mechanisms are. Understanding the strategies used by viruses for their cellular uptake can provide detailed information that might be used to prevent infection. In general, following binding of the virus to a specific host receptor, a complex cascade of intracellular signaling leads to virus internalization (5). This procedure can be achieved by the exploitation of different cellular systems. The best characterized is receptor-mediated, clathrin-dependent endocytosis, by which some viruses enter cells through invaginations of the plasma membrane that are coated with clathrin molecules (24, 33, 36, 43).
BTV enters cells by AP2-dependent clathrin-mediated endocytosis.
Some information regarding BTV entry was reported in previous investigations and was mainly based on ultrastructural EM studies (17). However, to understand the relevance of these observations, it was necessary to undertake a more detailed analysis of BTV entry at a molecular level. The use of the clathrin-mediated pathway was initially investigated by using a siRNA designed to target the μ2 subunit of the clathrin-AP2 complex (11). To monitor whether BTV uses an AP2-dependent clathrin pathway to enter the cells, set of studies were performed. Data obtained from both investigations indicated a direct correlation between reduced clathrin-mediated endocytosis and inhibition of BTV entry. Confocal microscopy revealed that in cells in which Tf uptake was depleted by the μ2 siRNA, there was a corresponding reduction of BTV entry. Interestingly, unlike Tf, which due to the depletion of AP2-specific endocytosis remained associated with the plasma membrane, a few BTV particles were retained on the cellular membrane, as shown by confocal microscopy. Since clathrin vesicles are also used for the recycling of cellular receptors on the plasma membrane, it is possible that the reduction of BTV entry was due to the lack of viral receptor (22). Therefore, it is correct to consider that a percentage of inhibition of virus internalization may be due to the reduction of BTV binding. Further evidence regarding the role of clathrin in BTV entry was provided by measuring the replication of the virus in siRNA-transfected cells. The results obtained from these experiments demonstrated that the reduction of BTV replication was directly associated with the absence of the clathrin pathway. Although these sets of data indicated a relationship between BTV entry and clathrin, it was necessary to undertake additional studies to determine the exact BTV entry pathway.
Clathrin, but not cholesterol, is required for BTV to enter cells.
Although several viruses use clathrin-coated vesicles to enter cells, certain other viruses also use clathrin-independent pathways (18). Endocytosis of SV40 has been found to be mediated by the generation of cholesterol-rich, flask-shaped invaginations or caveolae (32-34). More recent studies revealed the ability of SV40 to use an entry pathway that is non-clathrin-mediated and non-caveola-dependent when the mechanisms that regulate both these processes are disrupted (6). Similarly, influenza virus, which is the prototype for pH-dependent entry, has been recognized to use both clathrin- and non-clathrin-dependent pathways (42). To understand if, like rotavirus, BTV could use a clathrin-independent mechanism to enter cells, we initially explored the ability of CPZ to inhibit the formation of clathrin vesicles. Different sets of experiments revealed that increasing amounts of CPZ during BTV infection resulted in the inhibition of virus replication. The data acquired so far by using μ2 siRNA and CPZ demonstrated that BTV enters cells by using the clathrin-mediated pathway. However, unlike CPZ, nystatin failed to show any reduction in BTV replication, indicating that BTV does not use an alternative cholesterol-dependent mechanism for cell entry. This further suggests that clathrin-mediated endocytosis is likely to be the only route for BTV entry. This particular system of endocytosis usually proceeds with the delivery of the cargo contained in the clathrin vesicles to the early and the late endosomes.
Early endosomal low pH is essential for BTV entry and uncoating.
The relevance of acidic pH in BTV entry was initially suggested by Hyatt et al. (17), who showed that the presence of NH4Cl during BTV replication inhibited virus replication. We have previously shown that when expressed on the cell surface, the outer capsid protein VP5 acquires a low-pH-dependent fusogenic activity (10). This result not only indicated that the outer capsid protein might have an essential role in BTV replication but also suggested that for its activation, BTV particles need to be exposed to low pH. The pH-dependent character of BTV entry was further examined by monitoring the effect of BAF on virus infectivity. The effect of the drug in BTV replication was revealed to be dose dependent. A parallel experiment performed by confocal microscopy also supported such evidence. Since endosomes offer the perfect environment for the activation of pH-dependent viruses (e.g., influenza virus), it is likely that for BTV, such vesicles are the perfect recipients for VP5 activation. Although the effect of BAF indicated the necessity of a low-pH stage of BTV entry, it did not define which vesicles are used by the virus. In fact, virus entry can be triggered by a range of pHs, varying from pH 6.0 to 6.5 in early endosomes and from pH 5.5 to 6.0 in late endosomes. To better understand the internalization route followed by the virus, specific endosomal markers were used. The results obtained demonstrated that in the early stages of the infection, BTV is present in the early endosomes. This event was detectable for at least 30 min without showing further progression of the virion to the late endosomes. Since the presence of virus was monitored by labeling the VP5 protein, we can speculate that due to the membrane-binding activity of the protein, it could remain attached to the membranes of the early endosomes while the core is released into the cytoplasm (10).
The uncoating of BTV by losing the outer capsid proteins was reported to be essential for triggering the transcriptional competence of the core (15, 16, 45). Recent high-resolution cryo-EM analysis and immunoprecipitation studies have produced detailed information about the physical interactions between BTV inner and outer capsid proteins, which could elucidate the importance of BTV uncoating during entry (29). That particular investigation revealed that VP5 trimers are located on top of aqueous channels organized between VP7 trimers in such a way as to occlude them. Considering these channels as the only corridors from which the transcription substrates and cofactors, such as nucleoside triphosphates and Mg2+, can be taken up into the cores for the synthesis of mRNAs and, subsequently, for release into the cytoplasm, the removal of VP2 and VP5 from the virions is an essential step during BTV replication. To confirm the importance of BTV uncoating, we carried out additional investigations. Time course experiments were performed to trace the localization of VP5 and the most exposed core protein, VP7, during BTV infection. The data obtained indicated that from very early stages of infection, VP7, which forms the surface of the core, separates from VP5. The distance between the two proteins gradually increased during the entry of the virus. The accumulated data suggested that at the early stages of the infection, the core separated from VP5, moving toward the cytoplasm. In addition, it appears that early endosomes are the cellular compartments where the VP5 protein and the core separate from each other.
Conclusions.
To date, we do not have enough structural information about VP5; therefore, it is not possible to confirm possible changes in the conformation of the protein during BTV infection. What emerges from these data is that BTV seems to be internalized in a route that progresses from clathrin vesicles to early endosomes, where they are rapidly uncoated. The reason for such a rapid mechanism could be explained by recent studies, in which Mortola et al. demonstrated that the purified BTV VP2 and VP5 proteins can trigger apoptosis in mammalian cells upon attachment to the cell surface (27). This finding may suggest that the VP2 receptor-binding activity and the interaction of VP5 with cellular compartments are necessary to initiate apoptosis. If this is the case, it is possible to assume that the BTV entry mechanism has a dual effect on mammalian cells. For this reason, BTV must replicate as rapidly as possible to avoid the cellular machineries being shut down by apoptosis. The experiments performed here provide sufficient data to establish a model for BTV entry. After VP2 binds to a cellular receptor, BTV is rapidly internalized through clathrin-mediated endocytosis. The BTV virions are then delivered to the early endosomes, where the low pH is responsible for the rearrangement or degradation of VP2, triggering structural modification of VP5, probably by exposing its amphipathic helices. The permeabilization ability of these domains could result in the formation of pores in the endosomal membrane (10, 14). Due to the membrane-binding ability of VP5, the protein is retained in the endosomes, while the transcriptionally active core is released into the cytoplasm to initiate the transcription and, subsequently, the synthesis of viral proteins.
Acknowledgments
This work was supported by a grant from the NIH.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Blanchard, E., S. Belouzard, L. Goueslain, T. Wakita, J. Dubuisson, C. Wychowski, and Y. Rouille. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 80:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brezis, M., S. Rosen, P. Silva, K. Spokes, and F. H. Epstein. 1984. Polyene toxicity in renal medulla: injury mediated by transport activity. Science 224:66-68. [DOI] [PubMed] [Google Scholar]
- 3.Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11:374-382. [DOI] [PubMed] [Google Scholar]
- 4.Chemello, M. E., O. C. Aristimuno, F. Michelangeli, and M. C. Ruiz. 2002. Requirement for vacuolar H+-ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J. Virol. 76:13083-13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 6.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton, B. T., A. D. Hyatt, and S. M. Brookes. 1990. The replication of bluetongue virus. Curr. Top. Microbiol. Immunol. 162:89-118. [DOI] [PubMed] [Google Scholar]
- 8.Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609-24617. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich, M., W. Boll, A. Van Oijen, R. Hariharan, K. Chandran, M. L. Nibert, and T. Kirchhausen. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591-605. [DOI] [PubMed] [Google Scholar]
- 10.Forzan, M., C. Wirblich, and P. Roy. 2004. A capsid protein of nonenveloped bluetongue virus exhibits membrane fusion activity. Proc. Natl. Acad. Sci. USA 101:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraile-Ramos, A., T. A. Kohout, M. Waldhoer, and M. Marsh. 2003. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic 4:243-253. [DOI] [PubMed] [Google Scholar]
- 12.Golden, J. W., J. Linke, S. Schmechel, K. Thoemke, and L. A. Schiff. 2002. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J. Virol. 76:7430-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan, S. H., and P. Roy. 1999. Expression and functional characterization of bluetongue virus VP2 protein: role in cell entry. J. Virol. 73:9832-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan, S. H., C. Wirblich, M. Forzan, and P. Roy. 2001. Expression and functional characterization of bluetongue virus VP5 protein: role in cellular permeabilization. J. Virol. 75:8356-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huismans, H., N. T. Van der Walt, M. Cloete, and B. J. Erasmus. 1983. The biochemical and immunological characterization of bluetongue virus outer capsid polypeptides, p. 165-172. In R. W. Compans and D. H. L. Bishop (ed.), Double-stranded RNA viruses. Elsevier, New York, NY.
- 16.Huismans, H., A. A. Van Dijk, and H. J. Els. 1987. Uncoating of parental bluetongue virus to core and subcore particles in infected L cells. Virology 157:180-188. [DOI] [PubMed] [Google Scholar]
- 17.Hyatt, A. D., B. T. Eaton, and S. M. Brookes. 1989. The release of bluetongue virus from infected cells and their superinfection by progeny virus. Virology 173:21-34. [DOI] [PubMed] [Google Scholar]
- 18.Johannes, L., and C. Lamaze. 2002. Clathrin-dependent or not: is it still the question? Traffic 3:443-451. [DOI] [PubMed] [Google Scholar]
- 19.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kee, S. H., E. J. Cho, J. W. Song, K. S. Park, L. J. Baek, and K. J. Song. 2004. Effects of endocytosis inhibitory drugs on rubella virus entry into VeroE6 cells. Microbiol. Immunol. 48:823-829. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhausen, T., W. Boll, A. van Oijen, and M. Ehrlich. 2005. Single-molecule live-cell imaging of clathrin-based endocytosis. Biochem. Soc. Symp. 2005:71-76. [DOI] [PubMed] [Google Scholar]
- 22.Le Roy, C., and J. L. Wrana. 2005. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell. Biol. 6:112-126. [DOI] [PubMed] [Google Scholar]
- 23.Lopez, S., and C. F. Arias. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271-278. [DOI] [PubMed] [Google Scholar]
- 24.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez, C. G., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modrof, J., K. Lymperopoulos, and P. Roy. 2005. Phosphorylation of bluetongue virus nonstructural protein 2 is essential for formation of viral inclusion bodies. J. Virol. 79:10023-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortola, E., R. Noad, and P. Roy. 2004. Bluetongue virus outer capsid proteins are sufficient to trigger apoptosis in mammalian cells. J. Virol. 78:2875-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motley, A., N. A. Bright, M. N. Seaman, and M. S. Robinson. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nason, E., R. Rothnagel, S. K. Muknerge, A. K. Kar, M. Forzan, B. V. V. Prasad, and P. Roy. 2004. Interactions between the inner and outer capsids of bluetongue virus. J. Virol. 78:8059-8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen, D. J., B. M. Collins, and P. R. Evans. 2004. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20:153-191. [DOI] [PubMed] [Google Scholar]
- 31.Palacios, M., J. Padron, L. Glaria, A. Rojas, R. Delgado, R. Knowles, and S. Moncada. 1993. Chlorpromazine inhibits both the constitutive nitric oxide synthase and the induction of nitric oxide synthase after LPS challenge. Biochem. Biophys. Res. Commun. 196:280-286. [DOI] [PubMed] [Google Scholar]
- 32.Pelkmans, L., and A. Helenius. 2002. Endocytosis via caveolae. Traffic 3:311-320. [DOI] [PubMed] [Google Scholar]
- 33.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 34.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 35.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puertollano, R. 2004. Clathrin-mediated transport: assembly required. EMBO Rep. 5:942-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy, P. 2005. Bluetongue virus proteins and particles and their role in virus entry, assembly and release. Adv. Virus Res. 64:69-123. [DOI] [PubMed] [Google Scholar]
- 38.Roy, P. 2001. Orbiviruses and their replication, p. 1835-1869. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 39.Rubino, M., M. Miaczynska, R. Lippe, and M. Zerial. 2000. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem. 275:3745-3748. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-San Martin, C., T. Lopez, C. F. Arias, and S. Lopez. 2004. Characterization of rotavirus cell entry. J. Virol. 78:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saurin, A. J., J. Hamlett, M. J. Clague, and S. R. Pennington. 1996. Inhibition of mitogen-induced DNA synthesis by bafilomycin A1 in Swiss 3T3 fibroblasts. Biochem. J. 313:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smythe, E. 2003. Clathrin-coated vesicle formation: a paradigm for coated-vesicle formation. Biochem. Soc. Trans. 31:736-739. [DOI] [PubMed] [Google Scholar]
- 44.Tan, B., E. Nason, N. Staeuber, W. Jiang, K. Monastryrskaya, and P. Roy. 2001. RGD tripeptide of bluetongue virus VP7 protein is responsible for core attachment to Culicoides cells. J. Virol. 75:3937-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Dijk, A. A., and H. Huismans. 1988. In vitro transcription and translation of bluetongue virus mRNA. J. Gen. Virol. 69:573-581. [DOI] [PubMed] [Google Scholar]
- 46.Wirblich, C., B. Bhattacharya, and P. Roy. 2006. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J. Virol. 80:460-473. [DOI] [PMC free article] [PubMed] [Google Scholar]