Abstract
Intravenous (i.v.) delivery of recombinant adenovirus serotype 5 (Ad5) vectors for gene therapy is hindered by safety and efficacy problems. We have discovered a new pathway involved in unspecific Ad5 sequestration and degradation. After i.v. administration, Ad5 rapidly binds to circulating platelets, which causes their activation/aggregation and subsequent entrapment in liver sinusoids. Virus-platelet aggregates are taken up by Kupffer cells and degraded. Ad sequestration in organs can be reduced by platelet depletion prior to vector injection. Identification of this new sequestration mechanism and construction of vectors that avoid it could improve levels of target cell transduction at lower vector doses.
For more than a decade, adenovirus serotype 5 (Ad5)-based vectors have been used as intravenous (i.v.) gene transfer vectors. Unfortunately, as a non-blood-borne pathogen, Ad5 has not evolved mechanisms to survive in blood and is rapidly cleared from the circulation, with only a fraction reaching the target tissue (1, 28). Most i.v.-delivered Ad5 is sequestered in the liver, and animal studies indicate that Kupffer cells (KCs) play a major role in this trapping (20, 25, 38, 46, 51). Recent studies have shown that liver sequestration is not mediated by the Ad5 receptor, CAR, but involves either a direct (44) or a blood factor (coagulation factors IX and X and complement protein C4BP)-mediated (34, 35, 40) interaction between the Ad fiber and cellular heparansulfate proteoglycans. While these mechanisms of Ad uptake are less efficient if the Ad5 fiber is replaced with a shorter fiber, such as subspecies B serotype Ad35 (4, 41), the persistence of vectors with the Ad35 fiber in blood does not significantly differ from that of Ad5 (4), indicating that there are other pathways of virus clearance and liver sequestration.
Ad rapidly binds to and activates circulating platelets in vivo.
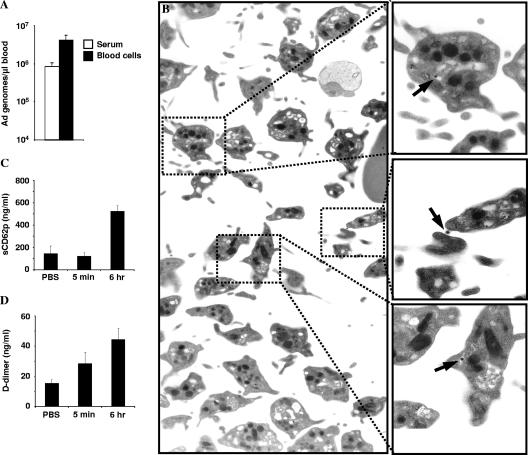
To investigate the early kinetics of Ad clearance from blood, we used a previously described quantitative PCR method (15) to analyze the levels of Ad5 in blood cell and serum fractions (Fig. 1A) after tail vein delivery of 1011 Ad5-cytomegalovirus-green fluorescent protein (43) viral particles (VP) to hCD46Ge transgenic mice (27). All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington, and all viruses were free of replication-competent Ad and endotoxin. Assuming that a 25-g mouse holds 1 ml of blood, the blood cell and serum fractions contained 4.18 and 0.85% of the input dose, respectively, at 5 min postdelivery. To identify the cell type(s) associated with Ad5, we injected [3H]thymidine-labeled Ad5 and after 10 min isolated platelets and fractionated blood samples. Over 95% of 3H-labeled Ad5 was associated with isolated platelet and mixed erythrocyte/platelet fractions (data not shown). Isolated erythrocyte and platelet fractions were fixed in 1/2-strength Karnovsky's fixative for transmission electron microscopy (TEM) analysis. TEM revealed Ad particles associated with external platelet membranes and membranes of the platelet open canalicular system (Fig. 1B) but not erythrocytes (data not shown). Although a previous in vitro study suggested that Ad5 does not potentiate or inhibit platelet aggregation (10), it is well documented that Ad5-based vectors induce thrombocytopenia after i.v. delivery (7, 37, 47, 50), and a recent study suggested that interaction between Ad and platelets in vivo induces thrombocytopenia (33), so it was not surprising to find that platelets were the predominant Ad binding blood cell type.
FIG. 1.
Ad association in blood. hCD46 transgenic mice were injected i.v. with 1011 VP of an Ad5 vector. (A) Blood extracted 5 min after injection was immediately separated into blood cell and serum fractions. Total DNA was extracted from blood cell and serum fractions along with carrier DNA and analyzed for the presence of Ad genomes by quantitative PCR. (B) Platelets purified from blood 10 min after Ad delivery were subjected to TEM analysis. Virus particles associated with the external platelet membrane and membranes of the open canalicular system are shown (arrows). (C) Levels of soluble CD62p in sera were measured by ELISA 5 min and 6 h after Ad delivery. (D) Levels of D-dimer fibrinogen breakdown products in sera were measured by ELISA 5 min and 6 h after Ad delivery. Four mice per group were used.
To explore the downstream effects of this interaction, we looked at platelet activation and initiation of coagulation by measuring CD62p (a platelet and endothelial activation marker) (R&D Systems) and D-dimer (a fibrinogen breakdown product) (Asserachrom D-dimer; Diagnostica Stago) levels in sera by an enzyme-linked immunosorbent assay (ELISA) early and late after Ad injection. For sCD62p, we found significantly elevated levels 6 h (P = 0.0015) but not 5 min after Ad injection (Fig. 1C). For D-dimer, we found significantly elevated levels in sera 5 min (P = 0.047) and 6 h (P = 0.003) after Ad injection (Fig. 1D). Although Ad induces thrombocytopenia in both animals and humans, thrombocytopenia itself is not life threatening, as platelets are readily replenished by megakaryocytes. However, the downstream effects of platelet activation and aberrant initiation of coagulation upon Ad injection are dangerous. Activation of the coagulation cascade can cause disseminated intravascular coagulation, an adverse side effect seen upon clinical Ad injection (37, 39). These observations and those of Othman et al., who demonstrated that upregulation of CD62p expression on circulating platelets (1 and 5 h postinjection [p.i.]) increased levels of circulating von Willebrand factor (1 to 2 h p.i.) and the presence of platelet/leukocyte aggregates (5 h p.i.) after Ad administration (33), suggest a direct interplay between Ad-platelet interaction in blood and subsequent thrombocytopenia, activation of coagulation, endothelial activation, and leukocyte infiltration.
Platelet and Ad sequestration in liver.
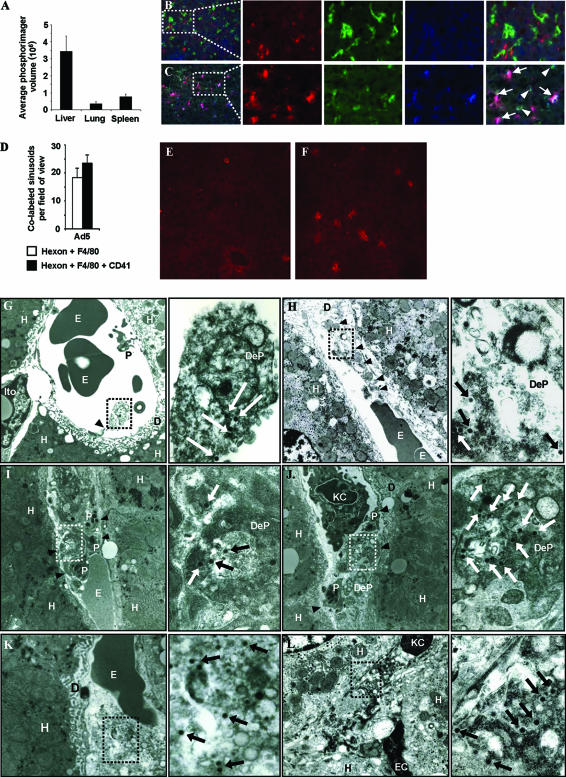
To investigate the fate of Ad-containing platelets in vivo, we first analyzed levels of Ad5 genomes in flushed organs by Southern blot analysis as previously described (45) at 5 min p.i. For comparison, signals were scanned by a phosphorimager (Molecular Dynamics) and quantified using ImageQuant software (Molecular Dynamics) (Fig. 2A). In agreement with earlier findings (48, 51), more Ad genomes were found in the liver than in other organs so we performed immunohistochemistry on livers harvested 5 or 30 min p.i. (without flushing) with antibodies against platelets (MWReg30 [BD Biosciences] and Cy3 conjugated [GE Healthcare]), macrophages/KCs (fluorescein isothiocyanate anti-F4/80 C1:A3-1 [Cedarlane Laboratories]), Ad (anti-hexon 20/11 [Chemicon] developed with AMCA [7-amino-4-methylcoumarin-3-acetic acid]-conjugated secondary 115-155-003 [Jackson Immunoresearch]), and granulocytes/monocytes (PerCP-Cy5.5 anti-Gr-1 RB6-8C5 [BD Pharmingen]). All sections were stained with the corresponding isotype controls. Analysis with antibodies against Ad, platelets, and KCs revealed extensive triple labeling of hexon, CD41, and F4/80 markers throughout the sinusoids of liver sections at a frequency comparable to that for double (hexon plus F4/80) labeling (Fig. 2B to D). This suggested a significant role for platelets in Ad sequestration to and degradation by KCs. While Ad-platelet-KC accumulation in liver was seen as early as 5 min p.i., staining with an anti-Gr-1 antibody showed that infiltrating granulocytes/monocytes appeared only 30 min after Ad injection (Fig. 2D and E).
FIG. 2.
Ad-platelet deposition in liver. hCD46 transgenic mice were injected i.v. with 1011 VP of an Ad5 vector. (A) Total genomic DNA was extracted from organs 5 min after Ad injection. A total of 10 μg was digested with EcoRI and subjected to Southern blot analysis with an Ad5 fiber-specific probe, and signals were quantified by a phosphorimager. (B and C) Livers from phosphate-buffered saline (PBS) (B)- or Ad5 (C)-injected mice were taken 5 min after delivery and stained with antibodies against platelets (mCD41) (red), KCs (F4/80) (green), and Ad capsid (hexon) (blue). Areas labeled with hexon plus F4/80 (arrowheads) or hexon plus F4/80 plus mCD41 (arrows) are shown. (D) Quantification of double- or triple-labeled regions in sections from livers of Ad5-injected mice. Total hepatic sinusoidal regions with hexon-CD41-F4/80 and hexon-F4/80 colabeling were counted for five fields of view. (E and F) Immunohistochemistry for the granulocyte/monocyte marker Gr-1 (red) on liver sections from PBS (E)- or Ad5 (F)-injected animals 30 min p.i. (G to L) Livers from Ad5-injected mice were analyzed by TEM 5 min p.i., and representative images are shown. High-power composite images are shown to the right. VP (arrows) and degranulated platelet/endothelial cell interactions (arrowheads) are shown. D, Disse spaces; DeP, degranulated platelet; E, erythrocyte; EC, endothelial cell; H, hepatocyte; Ito, Ito cell; P, platelet.
To further investigate the Ad-platelet-KC aggregates, livers harvested 5 min p.i. (without flushing) were fixed for TEM analysis. TEM revealed degranulated platelets containing VP within the hepatic sinusoidal spaces (Fig. 2G to L). Virus-loaded platelets could be seen attaching to endothelial cells (Fig. 2G), along with examples of virus-containing platelets flattened against the sinusoidal endothelium (Fig. 2H). In other sinusoids, aggregates containing degranulated, virus-containing platelets were seen (Fig. 2I and J) along with organelle- and virus-containing debris (Fig. 2K). KCs were found in the vicinity of sinusoidal platelet aggregates (Fig. 2J) and with Ad particles in phagosomes (Fig. 2L).
The presence of Ad-containing platelet aggregates in liver indicates that direct interaction between Ad and platelets causes their activation and subsequent aggregation in the hepatic reticuloendothelial system. Earlier reports describe adhesion of activated platelets to reticuloendothelium (16), phagocytosis by macrophages (11, 30), and endothelial activation and cytokine/chemokine release (14). Notably, the release of proinflammatory cytokines/chemokines after i.v. Ad delivery, which plays a major role in pathological changes (vascular leakage, liver damage) and induction of antiviral immune responses (20, 28, 29, 42), may be a consequence of platelet, KC, and endothelial cell interplay.
Effect of platelet depletion on Ad platelet binding and hepatic sequestration.
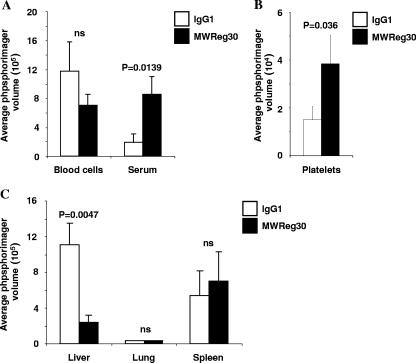
To further confirm a role for platelets in Ad sequestration to tissues, we studied Ad genome biodistribution in platelet-depleted mice. i.v. delivery of 30 μg of MWReg30 (32) antibody (or control rat immunoglobulin G1 [BD Biosciences]) 3 h prior to virus administration caused a 66% ± 6.7% reduction in platelet counts (data not shown) prior to virus injection, while the control antibody had no effect. To assess blood clearance and hepatic sequestration, blood samples and livers were harvested 5 min after Ad delivery and assessed for the presence of Ad genomes by quantitative Southern blot analysis (Fig. 3). At 5 min p.i., the levels of blood cell-associated Ad5 DNA were not significantly different between healthy and platelet-depleted mice; however, more virus DNA was found in the sera of platelet-depleted mice (Fig. 3A). The levels of Ad5 associated with equal numbers of circulating platelets after 5 min were significantly increased in platelet-depleted mice (Fig. 3B). This is not surprising, since the ratio of particles to platelet in circulation increases upon platelet depletion. In liver, hepatic accumulation of Ad5 genomes was greatly reduced upon platelet depletion (Fig. 3C). This corroborates our hypothesis that platelets play a major role in trapping Ad vectors in the liver after i.v. delivery. Notably, no reduction in Ad sequestration to spleen and lung was seen in platelet-depleted mice, indicating that pathways other than platelet-mediated ones are involved in Ad uptake into these organs. Injection of MWReg30 can cause acute lung injury, and we cannot completely exclude that, in addition to platelet depletion, secondary effects of MWReg30 application affect Ad biodistribution.
FIG. 3.
Effect of platelet depletion on sequestration to blood cells and liver. hCD46 transgenic mice were platelet depleted using MWReg30 antibody, and after 3 h, the mice received 1011 VP of Ad5 vector. Blood samples, platelets, and livers were harvested 5 min after virus injection. Equalized amounts of isolated DNA were digested with EcoRI and subjected to Southern blot analysis with an Ad5 fiber-specific probe. Southern blot signals were quantified by a phosphorimager and are shown for blood cells and sera (A), platelets (B), or organs (C). Four mice per group were used. IgG1, immunoglobulin G1.
We also analyzed proinflammatory cytokine/chemokine levels in sera 6 h after Ad delivery to platelet-depleted mice since we hypothesized that platelets may play a role in Ad-mediated innate toxicity. Serum levels of the cytokines/chemokines interleukin-6 and monocyte chemoattractant protein 1, which are critically involved in mediating toxicity in mice upon Ad injection (28) and play roles in disseminated intravascular coagulation and venous thrombosis (19), were assessed. The levels were reduced although not significantly compared to those for immunoglobulin G1-injected control mice (data not shown), so it remains unclear whether interaction with platelets is involved in mediating cytokine/chemokine release.
Consequences of Ad-platelet interactions.
i.v. Ad injection has complex downstream effects, including activation of complement (18), direct Ad uptake by KCs (49), and interaction with and subsequent activation of endothelial cells (21) or blood cells (17). Considering the data obtained in this study, we propose the following scenario. Immediately after i.v. injection, Ad particles bind to circulating platelets. Ad binding causes platelet activation and degranulation, which results in release of cytokines, binding of platelets to endothelial cells, and subsequent endothelial cell activation. Interaction between activated platelets and endothelial cells then promotes platelet aggregation/microthrombus formation in the sinusoids of the liver. Ad-loaded platelets within these microthrombi are then taken up by KCs and possibly by infiltrating monocytes or granulocytes, which results in Ad degradation. Depletion of platelets prior to Ad injection decreases Ad sequestration to liver as well as cytokine/chemokine levels in sera. At this point, it is not clear whether the platelet-mediated pathway of Ad5 uptake into KCs functions independently from the earlier-discovered blood factor-mediated pathway (34, 35, 40) or whether Ad5 interaction with platelets involves soluble blood factors.
Although a role for platelets in the removal of blood-borne infectious agents has not been proposed, it is known that many viral infections (including those caused by hepatitis C virus, human immunodeficiency virus, mumps virus, Dengue virus, human T-cell leukemia virus, human cytomegalovirus, severe acute respiratory syndrome virus, swine fever virus, hantavirus, parvovirus B19, influenza virus, and Epstein-Barr virus) induce thrombocytopenia (36) and that viruses are frequent contaminants of platelet concentrates used for transfusion (8). Platelet activation, aggregation, and subsequent degradation in the reticuloendothelial system have been seen during infections with swine fever virus (3). For Ad, studies with animal models and humans describe decreases in platelet number and circulation time (2, 7, 13, 22, 26, 37, 39, 47, 50), indicating that the platelet-mediated pathway of Ad clearance that we discovered in our mouse model is also relevant for humans. This is supported by observations of thrombotic thrombocytopenic purpura or deep venous thrombosis in patients with disseminated adenoviral infections (5, 12). Furthermore, it is feasible that removal of activated/pathogen-loaded platelets could involve phagocytosis by tissue macrophages, as has been described for idiopathic thrombocytopenic purpura (23, 31).
The discovery of this platelet-mediated delivery pathway to KCs improves our understanding of Ad clearance from blood. Although previous studies have shown interaction between Ad and blood cells in vitro (6, 24), our data suggest a functional interaction between Ad and blood cells in vivo. Our observations also create a basis for the improvement of safety and efficacy for Ad vectors. Furthermore, an improved understanding of the role and mechanism of platelets in virus clearance could be relevant, potentially, for the treatment of infectious diseases. Clearly, our future efforts are focused on the identification of virus capsid protein(s) and residues as well as platelet surface molecules that are involved in this interaction, with the final goal of constructing vectors that are ablated for platelet binding. We speculate that such vectors would be sequestered less by healthy tissue. Notably, unspecific sequestration is a problem that currently limits the efficacy of tumor cell transduction with targeted Ad vectors (9).
Acknowledgments
We thank Pavel Sova, Leonard Meuse, and Lori Cooper for technical assistance. We are grateful to Steve Roffler for helpful discussion.
This study was supported by NIH grants CA080192 and HLA078836.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Alemany, R., K. Suzuki, and D. T. Curiel. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81:2605-2609. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, J. L., P. S. Shirley, W. O. Iverson, A. D. Sherer, J. E. Markovits, L. King, R. M. Lyons, M. Kaleko, and S. Connelly. 2002. Evaluation of the duration of human factor VIII expression in nonhuman primates after systemic delivery of an adenoviral vector. Hum. Gene Ther. 13:1331-1336. [DOI] [PubMed] [Google Scholar]
- 3.Bautista, M. J., E. Ruiz-Villamor, F. J. Salguero, P. J. Sanchez-Cordon, L. Carrasco, and J. C. Gomez-Villamandos. 2002. Early platelet aggregation as a cause of thrombocytopenia in classical swine fever. Vet. Pathol. 39:84-91. [DOI] [PubMed] [Google Scholar]
- 4.Bernt, K. M., S. Ni, A. Gaggar, Z. Y. Li, D. M. Shayakhmetov, and A. Lieber. 2003. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol. Ther. 8:746-755. [DOI] [PubMed] [Google Scholar]
- 5.Castillo Salinas, F., C. Rodriguez Galindo, J. Iglesias Berengue, and I. Calico Bosch. 1991. Disseminated adenovirus infection associated with deep venous thrombosis in a previously healthy female infant. An. Esp. Pediatr. 34:163-164. (In Spanish.) [PubMed] [Google Scholar]
- 6.Cichon, G., S. Boeckh-Herwig, D. Kuemin, C. Hoffmann, H. H. Schmidt, E. Wehnes, W. Haensch, U. Schneider, U. Eckhardt, R. Burger, and P. Pring-Akerblom. 2003. Titer determination of Ad5 in blood: a cautionary note. Gene Ther. 10:1012-1017. [DOI] [PubMed] [Google Scholar]
- 7.Cichon, G., H. H. Schmidt, T. Benhidjeb, P. Loser, S. Ziemer, R. Haas, N. Grewe, F. Schnieders, J. Heeren, M. P. Manns, P. M. Schlag, and M. Strauss. 1999. Intravenous administration of recombinant adenoviruses causes thrombocytopenia, anemia and erythroblastosis in rabbits. J. Gene Med. 1:360-371. [DOI] [PubMed] [Google Scholar]
- 8.Corash, L. 2001. Inactivation of infectious pathogens in labile blood components: meeting the challenge. Transfus. Clin. Biol. 8:138-145. [DOI] [PubMed] [Google Scholar]
- 9.Di Paolo, N., S. Ni, A. Gaggar, R. Strauss, S. Tuve, Z.-Y. Li, D. Stone, D. Shayakhmetov, N. Kiviat, P. Touré, S. Sow, and A. Lieber. 2006. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 13:1072-1081. [DOI] [PubMed] [Google Scholar]
- 10.Eggerman, T. L., T. H. Mondoro, J. N. Lozier, and J. G. Vostal. 2002. Adenoviral vectors do not induce, inhibit, or potentiate human platelet aggregation. Hum. Gene Ther. 13:125-128. [DOI] [PubMed] [Google Scholar]
- 11.Endo, Y., M. Nakamura, Y. Nitta, and K. Kumagai. 1995. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin 1 and tumour necrosis factor. Br. J. Pharmacol. 114:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fassas, A. B., L. N. Buddharaju, A. Rapoport, M. Cottler-Fox, C. Drachenberg, B. Meisenberg, and G. Tricot. 2001. Fatal disseminated adenoviral infection associated with thrombotic thrombocytopenic purpura after allogeneic bone marrow transplantation. Leuk. Lymphoma 42:801-804. [DOI] [PubMed] [Google Scholar]
- 13.Gallo-Penn, A. M., P. S. Shirley, J. L. Andrews, S. Tinlin, S. Webster, C. Cameron, C. Hough, C. Notley, D. Lillicrap, M. Kaleko, and S. Connelly. 2001. Systemic delivery of an adenoviral vector encoding canine factor VIII results in short-term phenotypic correction, inhibitor development, and biphasic liver toxicity in hemophilia A dogs. Blood 97:107-113. [DOI] [PubMed] [Google Scholar]
- 14.Gawaz, M., H. Langer, and A. E. May. 2005. Platelets in inflammation and atherogenesis. J. Clin. Investig. 115:3378-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heim, A., C. Ebnet, G. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228-239. [DOI] [PubMed] [Google Scholar]
- 16.Heyns Adu, P., P. N. Badenhorst, M. G. Lotter, H. Pieters, P. Wessels, and H. F. Kotze. 1986. Platelet turnover and kinetics in immune thrombocytopenic purpura: results with autologous 111In-labeled platelets and homologous 51Cr-labeled platelets differ. Blood 67:86-92. [PubMed] [Google Scholar]
- 17.Higginbotham, J. N., P. Seth, R. M. Blaese, and W. J. Ramsey. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum. Gene Ther. 13:129-141. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, H., Z. Wang, D. Serra, M. M. Frank, and A. Amalfitano. 2004. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 10:1140-1142. [DOI] [PubMed] [Google Scholar]
- 19.Levi, M., T. T. Keller, E. van Gorp, and H. ten Cate. 2003. Infection and inflammation and the coagulation system. Cardiovasc. Res. 60:26-39. [DOI] [PubMed] [Google Scholar]
- 20.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Q., A. K. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. J. Wickham, and D. A. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14:627-643. [DOI] [PubMed] [Google Scholar]
- 22.Lozier, J. N., G. Csako, T. H. Mondoro, D. M. Krizek, M. E. Metzger, R. Costello, J. G. Vostal, M. E. Rick, R. E. Donahue, and R. A. Morgan. 2002. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum. Gene Ther. 13:113-124. [DOI] [PubMed] [Google Scholar]
- 23.Luk, S. C., E. Musclow, and G. T. Simon. 1980. Platelet phagocytosis in the spleen of patients with idiopathic thrombocytopenic purpura (ITP). Histopathology 4:127-136. [DOI] [PubMed] [Google Scholar]
- 24.Lyons, M., D. Onion, N. K. Green, K. Aslan, R. Rajaratnam, M. Bazan-Peregrino, S. Phipps, S. Hale, V. Mautner, L. W. Seymour, and K. D. Fisher. 2006. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 14:118-128. [DOI] [PubMed] [Google Scholar]
- 25.Manickan, E., J. S. Smith, J. Tian, T. L. Eggerman, J. N. Lozier, J. Muller, and A. P. Byrnes. 2006. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 13:108-117. [DOI] [PubMed] [Google Scholar]
- 26.Morral, N., W. K. O'Neal, K. Rice, M. M. Leland, P. A. Piedra, E. Aguilar-Cordova, K. D. Carey, A. L. Beaudet, and C. Langston. 2002. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 13:143-154. [DOI] [PubMed] [Google Scholar]
- 27.Mrkic, B., J. Pavlovic, T. Rulicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157-1166. [DOI] [PubMed] [Google Scholar]
- 29.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 30.Musaji, A., F. Cormont, G. Thirion, C. L. Cambiaso, and J. P. Coutelier. 2004. Exacerbation of autoantibody-mediated thrombocytopenic purpura by infection with mouse viruses. Blood 104:2102-2106. [DOI] [PubMed] [Google Scholar]
- 31.Neiman, J. C., M. J. Mant, and T. K. Shnitka. 1987. Phagocytosis of platelets by Kupffer cells in immune thrombocytopenia. Arch. Pathol. Lab. Med. 111:563-565. [PubMed] [Google Scholar]
- 32.Nieswandt, B., B. Echtenacher, F. P. Wachs, J. Schroder, J. E. Gessner, R. E. Schmidt, G. E. Grau, and D. N. Mannel. 1999. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice. Blood 94:684-693. [PubMed] [Google Scholar]
- 33.Othman, M., A. Labelle, I. Mazzetti, H. S. Elbatarny, and D. Lillicrap. 5 December 2006. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed]
- 34.Parker, A. L., J. H. McVey, J. H. Doctor, O. Lopez-Franco, S. N. Waddington, M. Havenga, S. A. Nicklin, and A. H. Baker. 24 January 2007. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J. Virol. doi: 10.1128/JVI.02786-06. [DOI] [PMC free article] [PubMed]
- 35.Parker, A. L., S. N. Waddington, C. G. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108:2554-2561. [DOI] [PubMed] [Google Scholar]
- 36.Rand, M. L., and J. F. Wright. 1998. Virus-associated idiopathic thrombocytopenic purpura. Transfus. Sci. 19:253-259. [DOI] [PubMed] [Google Scholar]
- 37.Raper, S. E., M. Yudkoff, N. Chirmule, G. P. Gao, F. Nunes, Z. J. Haskal, E. E. Furth, K. J. Propert, M. B. Robinson, S. Magosin, H. Simoes, L. Speicher, J. Hughes, J. Tazelaar, N. A. Wivel, J. M. Wilson, and M. L. Batshaw. 2002. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 13:163-175. [DOI] [PubMed] [Google Scholar]
- 38.Schiedner, G., S. Hertel, M. Johnston, V. Dries, N. van Rooijen, and S. Kochanek. 2003. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol. Ther. 7:35-43. [DOI] [PubMed] [Google Scholar]
- 39.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 40.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2004. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 78:5368-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2005. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J. Immunol. 174:7310-7319. [DOI] [PubMed] [Google Scholar]
- 43.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, T. A., N. Idamakanti, M. L. Rollence, J. Marshall-Neff, J. Kim, K. Mulgrew, G. R. Nemerow, M. Kaleko, and S. C. Stevenson. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14:777-787. [DOI] [PubMed] [Google Scholar]
- 45.Stone, D., S. Ni, Z. Y. Li, A. Gaggar, N. DiPaolo, Q. Feng, V. Sandig, and A. Lieber. 2005. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 79:5090-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao, N., G. P. Gao, M. Parr, J. Johnston, T. Baradet, J. M. Wilson, J. Barsoum, and S. E. Fawell. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 3:28-35. [DOI] [PubMed] [Google Scholar]
- 47.Varnavski, A. N., R. Calcedo, M. Bove, G. Gao, and J. M. Wilson. 2005. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 12:427-436. [DOI] [PubMed] [Google Scholar]
- 48.Vrancken Peeters, M. J., A. L. Perkins, and M. A. Kay. 1996. Method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. BioTechniques 20:278-285. [DOI] [PubMed] [Google Scholar]
- 49.Wolff, G., S. Worgall, N. van Rooijen, W. R. Song, B. G. Harvey, and R. G. Crystal. 1997. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 71:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolins, N., J. Lozier, T. L. Eggerman, E. Jones, E. Aguilar-Cordova, and J. G. Vostal. 2003. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br. J. Haematol. 123:903-905. [DOI] [PubMed] [Google Scholar]
- 51.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]