Abstract
Controversial results have been observed in mouse models regarding the role of lymphoid tissues in prion pathogenesis. To investigate the role of dendritic cells (DC), we used a transgenic mouse model. In this model (CD11c-N17Rac1), a significant reduction of CD8+ CD11chi DC has been described, and the remaining CD8+ DC demonstrate a reduced capacity for the uptake of apoptotic cells. After intraperitoneal prion infection, significantly longer incubation times were observed in CD11c-N17Rac1 mice than in controls, indicating that a defect in CD8+ CD11chi DC significantly impedes neuroinvasion after intraperitoneal infection. In contrast, no distinct differences were observed between CD11c-N17Rac1 mice and controls after oral infection. This provides evidence that oral and intraperitoneal prion infections differ in lymphoreticular requirements.
Immune cells play a role following the peripheral uptake of prions. Using the intraperitoneal route, a role for B lymphocytes and follicular dendritic cells (FDC) (5) has been established in prion pathogenesis. However, experimental data regarding the role of these cells are not conclusive, and other studies have indicated that neither cell type plays a crucial role in prion pathogenesis (12). The link between FDC and prion spread to the central nervous system also remains unclear. Experimental work has shown that prion transfer between FDC and sympathetic nerves takes place with the positioning of FDC and nerves affecting peripheral prion pathogenesis (9). It is unclear how prions reach peripheral nerve endings from FDC located in the germinal center, as mature FDC do not move from the germinal center. It is commonly believed that antigen transport is a task of DC, and it has been shown that the depletion of DC prevents bacterial spread and expansion in the Listeria monocytogenes mouse model (6a). In this context, it has been shown that CD11c+ DC can propagate prions from the periphery to the central nervous system, making DC possible vectors for prions (1). In addition, most of the experimental data created to date have been obtained using the intraperitoneal route of infection. Such results cannot be used to explain the oral route of infection, as studies have indicated that oral and intraperitoneal prion pathogeneses differ in lymphoreticular requirements (10).
The aim of this study was to investigate the role of DC after intraperitoneal and oral prion infections, using a novel mouse model in which a dominant-negative variant of Rac-1, a Rho GTPase, carrying an exchange mutation at amino acid position 17, is expressed under the control of a DC-specific mouse CD11c promoter (4). Rho GTPases play a role in processes such as chemotaxis, the formation of filopodia, receptor-mediated phagocytosis, and endocytosis. In Rho GTPase-related genetic defects, such as Wiscott-Aldrich syndrome, immune functions are seriously disrupted (13, 14). In transgenic mice used in our experiments, the expression of a dominant-negative variant of Rac-1 inhibits the function of the cell-endogenous wild-type Rac-1 selectively in DC. It has been shown that Rac-1 inhibition in transgenic mice (CD11c-N17Rac1) leads to reduced numbers of CD8+ CD11chi DC and that the remaining CD8+ CD11chi DC have a severely reduced capacity for the uptake of apoptotic cells in vivo (4). The reduced capacity of CD8+ CD11chi DC to take up apoptotic cells in vivo has been correlated with decreased efficiency in cross-presentation. It has been shown that the transgene is expressed in all DC subpopulations. The described defects have been observed in splenic DC (4) and lymph node DC (unpublished data), and further work is needed to examine other DC subpopulations. No expression of a dominant variant of Rac1 has been observed in macrophages (4), and subsequently, no defect has been observed in the macrophage population (unpublished data). In these experiments, transgenic CD11c-N17Rac1 mice were inoculated orally and intraperitoneally with the RML prion strain.
To investigate the intraperitoneal route of infection, mice were inoculated intraperitoneally with 100 μl of a 10% brain homogenate of the RML strain. For infection experiments, eight transgenic N17-CD11c-Rac1 mice and eight control mice, nontransgenic littermates, which were backcrossed to C57BL/6 for at least eight generations, were inoculated. For oral infection, mice were deprived of water the day before infection and infected with 200 μl of a 10% brain homogenate of the RML prion strain, prepared from terminally ill mice using a tube. For oral infection experiments, seven transgenic CD11c-N17Rac1 mice and eight control mice, nontransgenic littermates, which were backcrossed to C57BL/6 for at least eight generations, were infected. Western blot analysis of brain homogenates was performed as described elsewhere (8). Briefly, brain tissue was homogenized in 9 volumes of lysis buffer, and after proteinase K (PK) digestion, aliquots were resolved on 12% polyacrylamide gels and immunoblotted. The monoclonal antibody 6h4 (Prionics) was used as the primary antibody. Blots were visualized using an enhanced chemiluminescence system.
To assess histological and immunohistochemical changes induced through prion infection, brain tissue from each mouse was fixed in 4% buffered formaldehyde for a period of 4 days, decontaminated with 98% formic acid for 60 min, postfixed in 4% buffered formaldehyde for 48 h following the protocol of Brown et al. (2), and embedded in paraffin. Sections (2 to 4 μm) were cut on a microtome and then subjected to conventional staining and immunohistochemistry for the detection of glial fibrillary acidic protein. To detect the misfolded disease-associated prion protein (PrPSc), paraffin-embedded tissue blotting was performed as described previously (11).
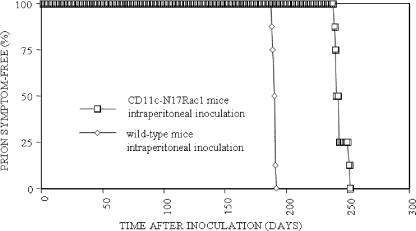
Using the intraperitoneal route of infection, transgenic CD11c-N17Rac1 mice showed significantly longer incubation times than controls (244 ± 5 days versus 190 ± 1 day [mean ± standard deviation]; P = 0.0002) (Fig. 1), with all mice developing prion disease. Brains of terminally diseased mice were analyzed for the presence of PrPSc, and in both transgenic and control mice, PrPSc was detected (Fig. 2). Vacuolation and gliosis were observed in both transgenic mice and controls (Fig. 3). These findings show that a defect in the CD8+ CD11chi DC population leads to a distinct delay in neuroinvasion after intraperitoneal prion infection, indicating that CD8+ CD11chi DC play an important role in peripheral neuroinvasion after intraperitoneal infection.
FIG. 1.
Incubation times in CD11c-N17Rac1 mice and wild-type mice after intraperitoneal inoculation with the RML prion strain. For intraperitoneal infection, mice were infected with 100 μl 10% brain homogenate of the RML prion strain. The vertical axis shows the percentage of mice that were prion symptom free at the indicated points in time. The horizontal axis shows days postinoculation. Groups of eight CD11c-N17Rac1 mice and eight wild-type mice were used for intraperitoneal infection.
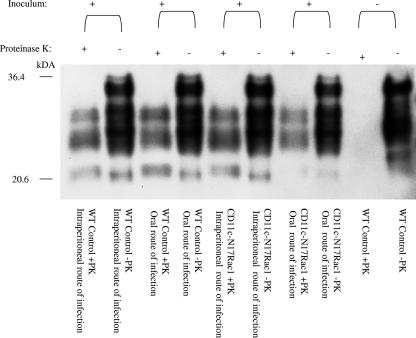
FIG. 2.
Western blot analysis of brains of CD11c-N17Rac1 mice and wild-type mice inoculated intraperitoneally and orally with the RML prion strain. Brain material was electrophoresed without (−) or after digestion with proteinase K (+). Large amounts of PK-resistant prion protein (PrPSc) were detected in all mice that developed prion disease. WT, wild type.
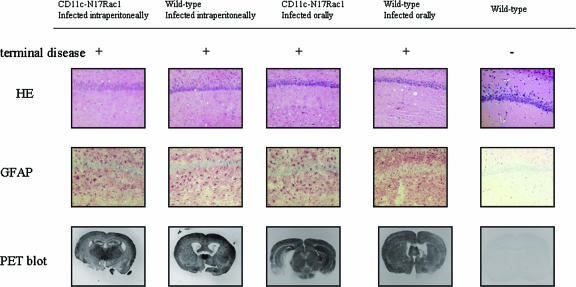
FIG. 3.
Brain histopathology of CD11c-N17Rac1 mice and wild-type mice inoculated intraperitoneally and orally with the RML prion strain. The hippocampus shows the typical morphology of prion disease in terminally ill mice, with vacuolar changes in both CD11c-N17Rac1 mice and wild-type mice (hematoxylin and eosin [HE]) and diffuse gliosis in the hippocampal formation (immunohistochemistry for glial fibrillary acidic protein [GFAP]) after intraperitoneal and oral inoculations with the RML prion strain. Clinically healthy mice showed no vacuolization or gliosis. Paraffin-embedded tissue (PET) blot analysis shows the presence of PK-resistant PrPSc in brains of both CD11c-N17Rac1 mice and wild-type mice after intraperitoneal and oral infections. No PrPSc deposition could be seen in clinically healthy mice.
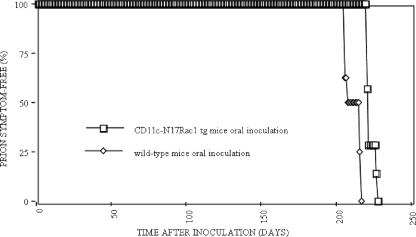
In contrast, after oral infection, no distinct differences in incubation times could be seen between transgenic CD11c-N17Rac1 mice (223 ± 3 days) and controls (212 ± 5 days) (P = 0.0002), with all mice being susceptible to prion disease (Fig. 4). At the time of terminal disease, PrPSc was detected biochemically in both transgenic CD11c-N17Rac1 mice and wild-type controls (Fig. 2) by using Western blot analysis. Brain tissue of terminally ill CD11c-N17Rac1 mice and wild-type controls showed spongiform pathology and reactive gliosis characteristic for prion disease (Fig. 3).
FIG. 4.
Incubation times in CD11c-N17Rac1 mice and wild-type mice after oral inoculation with the RML prion strain. For oral infection, mice were infected with 200 μl 10% brain homogenate of the RML prion strain. The vertical axis shows the percentage of mice that were prion symptom free at the indicated points in time. The horizontal axis shows days postinoculation. Groups of seven CD11c-N17Rac1 mice and eight wild-type mice were used for oral infection. tg, transgenic.
Regarding the intraperitoneal route of infection, one study showed that the use of lymphocytic choriomeningitis virus clone 13, which induces a dysfunction in splenic DEC205+ and CD11c+ DC, did not affect prion transmission (7). However, this defect did not encompass all DC and did not target specific subpopulations. Another study showed that a high infectivity titer is present in a splenocyte population of CD11c/major histocompatibility complex class II+ cells, corresponding to DC from prion-infected mice (1). The infectivity titer in this cell fraction was higher than in a B-cell-enriched fraction and suggests that CD11c+ DC are vectors of prion propagation. Furthermore, the authors of that study showed that a fraction strongly enriched with live infected CD11c+ splenic cells induced disease in Rag-1o/o mice without reconstitution of lymphoid architecture, showing that CD11c+ cells can lead to neuroinvasion without an accumulation step in the germinal center (1). We observed significantly longer incubation times in CD11c-N17Rac1 mice inoculated intraperitoneally than in the respective wild-type controls. These findings show that a defect in the CD8+ CD11chi DC population leads to a distinct delay in neuroinvasion after intraperitoneal prion infection. This indicates that the CD8+ CD11chi DC population plays an important role in peripheral neuroinvasion which can be compensated for by an as-yet-undefined cell type or even by a different route of neuroinvasion. This compensation is achieved at the cost of reduced efficiency of intraperitoneal prion infection, as evidenced by significantly prolonged incubation times in transgenic CD11c-N17Rac1 mice. Regarding the oral route of infection, our studies do not exclude the possibility that DC can acquire prions from the gut lumen and transport them to lymphoid tissue as suggested by Huang et al. (3). It is possible that the intestinal CD8+ CD11chi DC population, even though we know that it expresses the transgene, does not show as pronounced a defect as peripheral DC. However, it is also possible that this postulated step of the infectious process is not rate limiting and can be compensated for through other pathways. The fact that after oral infection no distinct difference could be seen between controls and transgenic CD11c-N17Rac1 mice regarding incubation times, in contrast to after the intraperitoneal route of infection, indicates that the requirement for the CD8+ CD11chi DC population is dependent on the route of infection. Determining which part of the infection process is most susceptible to the defect in the CD8+ CD11chi DC population could be accessed by testing for PrPSc accumulation on FDC, with a comparison of splenic (intraperitoneal) and oral (mesenteric lymph nodes) FDC, to account for infection route-specific differences. These results are in line with previous work indicating differences in lymphoreticular requirements between oral and intraperitoneal prion infections (10). It is conceivable that the oral route uses primarily efferent fibers of the vagus for neuroinvasion, as described previously (6), with no significant role played by the CD8+ CD11chi DC population, in contrast to the intraperitoneal route, which relies on the CD8+ CD11chi DC population for efficient neuroinvasion. We cannot exclude that the difference between the oral and intraperitoneal routes of infection is due partly to different effective infectious doses.
As the mouse model used in this study does not show a complete defect in the CD8+ DC subpopulation, further studies using models in which the CD8+ DC subpopulation is completely depleted and in which other DC subpopulations are impaired have to be established to further elucidate the role of DC in prion infection.
Acknowledgments
We thank Rene Mosquera for maintenance and monitoring of animals and the animal technicians for care of animals. We are indebted to Martin Groschup for supplying us with RML prions.
This work was supported by grants from the state of Bavaria (ForPrion LMU11 to H.K. and T.B.).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Aucouturier, P., F. Geissmann, D. Damotte, G. P. Saborio, H. C. Meeker, R. Kascsak, R. Kascsak, R. I. Carp, and T. Wisniewski. 2001. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Investig. 108:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, P., A. Wolff, and D. C. Gajdusek. 1990. A simple and effective method for inactivating virus infectivity in formalin-fixed tissue samples from patients with Creutzfeldt-Jakob disease. Neurology 40:887-890. [DOI] [PubMed] [Google Scholar]
- 3.Huang, F. P., C. F. Farquhar, N. A. Mabbott, M. E. Bruce, and G. G. MacPherson. 2002. Migrating intestinal dendritic cells transport PrP(Sc) from the gut. J. Gen. Virol. 83:267-271. [DOI] [PubMed] [Google Scholar]
- 4.Kerksiek, K. M., F. Niedergang, P. Chavrier, D. H. Busch, and T. Brocker. 2005. Selective Rac1 inhibition in dendritic cells diminishes apoptotic cell uptake and cross-presentation in vivo. Blood 105:742-749. [DOI] [PubMed] [Google Scholar]
- 5.Mabbott, N. A., F. Mackay, F. Minns, and M. E. Bruce. 2000. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat. Med. 6:719-720. [DOI] [PubMed] [Google Scholar]
- 6.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Neuenhahn, M., K. M. Kerksiek, M. Nauerth, M. H. Suhre, M. Schiemann, F. E. Gebhardt, C. Stemberger, K. Panthel, S. Schroder, T. Chakraborty, S. Jung, H. Hochrein, H. Russmann, T. Brocker, and D. H. Busch. 2006. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25:619-630. [DOI] [PubMed] [Google Scholar]
- 7.Oldstone, M. B. A., R. Race, D. Thomas, H. Lewicki, D. Homann, S. Smelt, A. Holz, P. Koni, D. Lo, B. Chesebro, and R. Flavell. 2002. Lymphotoxin-α- and lymphotoxin-β-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J. Virol. 76:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parchi, P., R. Castellani, P. Cortelli, P. Montagna, S. G. Chen, R. B. Petersen, V. Manetto, C. L. Vnencak-Jones, M. J. McLean, J. R. Sheller, et al. 1995. Regional distribution of protease-resistant prion protein in fatal familial insomnia. Ann. Neurol. 38:21-29. [DOI] [PubMed] [Google Scholar]
- 9.Prinz, M., M. Heikenwalder, T. Junt, P. Schwarz, M. Glatzel, F. L. Heppner, Y. X. Fu, M. Lipp, and A. Aguzzi. 2003. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425:957-962. [DOI] [PubMed] [Google Scholar]
- 10.Prinz, M., G. Huber, A. J. Macpherson, F. L. Heppner, M. Glatzel, H. P. Eugster, N. Wagner, and A. Aguzzi. 2003. Oral prion infection requires normal numbers of Peyer's patches but not of enteric lymphocytes. Am. J. Pathol. 162:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz-Schaeffer, W. J., S. Tschoke, N. Kranefuss, W. Drose, D. Hause-Reitner, A. Giese, M. H. Groschup, and H. A. Kretzschmar. 2000. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am. J. Pathol. 156:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlomchik, M. J., K. Radebold, N. Duclos, and L. Manuelidis. 2001. Neuroinvasion by a Creutzfeldt-Jakob disease agent in the absence of B cells and follicular dendritic cells. Proc. Natl. Acad. Sci. USA 98:9289-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snapper, S. B., and F. S. Rosen. 2003. A family of WASPs. N. Engl. J. Med. 348:350-351. [DOI] [PubMed] [Google Scholar]
- 14.Thrasher, A. J. 2002. WASp in immune-system organization and function. Nat. Rev. Immunol. 2:635-646. [DOI] [PubMed] [Google Scholar]