Abstract
The salivary glands of scrapie-affected sheep and healthy controls were investigated for the presence of the pathological prion protein (PrPSc). PrPSc was detected in major (parotid and mandibular) and minor (buccal, labial, and palatine) salivary glands of naturally and experimentally infected sheep. Using Western blotting, the PrPSc concentration in glands was estimated to be 0.02 to 0.005% of that in brain. Immunohistochemistry revealed intracellular depositions of PrPSc in ductal and acinar epithelia and occasional labeling in the lumina of salivary ducts. The presence of PrPSc in salivary glands highlights the possible role of saliva in the horizontal transmission of scrapie.
Scrapie of sheep and goats is a transmissible spongiform encephalopathy or prion disease. The key feature of this group of fatal neurodegenerative diseases is represented by the posttranslational modification of the normal cellular host-derived, protease-sensitive prion protein, PrPC, into an abnormal protease-resistant isoform named PrPSc (17). Epidemiological and experimental studies suggest that the spread of scrapie among sheep occurs mainly by horizontal transmission, either by direct transmission or by prions being shed into the environment (10). Lateral transmission was observed when scrapie agent-infected sheep were in close contact with healthy sheep which had no previous scrapie exposure (19). For horizontal transmission, the shedding of the scrapie agent from infected body compartments to the external environment has to be postulated. The placentas of infected sheep could represent a source of environmental contamination (18). In sheep, it has also been shown that the cooccurrence of scrapie and lentiviral infection may lead to PrPSc accumulation in ectopic lymphoid follicles of mammary glands (12), pointing to the milk as a possible route by which direct transmission could occur. Until now, salivary glands have not been extensively investigated for the presence of infectivity or PrPSc. Nonetheless, it has previously been shown that the salivary glands of experimentally challenged mice and minks may accumulate prions (5, 7, 20). Traces of infectivity have been detected in the salivary glands of a scrapie-affected goat (6) and of a bovine spongiform encephalopathy-affected kudu (4).
We investigated the salivary glands from 18 sheep with susceptible PrP genotypes (ARQ/ARQ [n = 11], ARQ/AHQ [n = 7]), either naturally infected (n = 9) or experimentally challenged by the oral route (n = 9), and from 15 negative controls (ARQ/ARQ [n = 5], ARR/ARQ [n = 5], ARR/ARR [n = 5]) from a single monitored flock with no history of scrapie. All scrapie-affected sheep were euthanized after showing clinical signs of scrapie. Postmortem examination by Western blotting and immunohistochemistry showed PrPSc deposition in the brains and lymphoid tissues of all affected sheep, while PrPSc was not detected in negative controls. Immunohistochemistry was performed as previously described (23). Sections of salivary glands were immunolabeled with monoclonal antibodies (MAbs) F99/97.6.1 (epitope from amino acids [aa] 220 to 225 [QYQRES] of ovine PrP; diluted 1:250; VMRD), 2G11 (epitope from aa 153 to 158 [YRENMY] of ovine PrP; diluted 1:200; Institute Pourquier), and L42 (epitope from aa 148 to 153 [YEDRYY] of ovine PrP; diluted 1:250; R-Biopharm), applied for 1 h at room temperature. Paraffin-embedded tissue (PET) blotting was performed as previously described (16), using MAb 2G11 (dilution, 1:200). Western blotting was performed as previously described (23) with minor modifications. Briefly, salivary glands were carefully isolated from muscles, lymphoid tissue, and oral epithelium under a dissecting microscope. Isolated glands (0.2 to 1 g) were cut into small pieces and incubated for 2 h in phosphate-buffered saline, pH 7.4, with 2.5 mg/ml collagenase A (Sigma) and 2 mM CaCl2 before being homogenized to 20% (wt/vol) in phosphate-buffered saline. After digestion with proteinase K (50 μg/ml for 1 h at 37°C) in the presence of 2% N-laurylsarcosine (Sigma) and centrifugation at 20,000 × g, pellets were denatured in loading buffer and loaded at 50- to 100-mg tissue equivalents per lane. Collagenase and protease treatments were omitted for the detection of PrPC. Selected samples were deglycosylated with N-glycosidase F (Roche) according to the manufacturer's instructions. The blots were revealed with MAb P4 (0.4 μg/ml; R-Biopharm).
PrPSc was detected in the parotid (nine of nine sheep), mandibular (six of seven), buccal (five of eight), palatine (five of six), and labial (two of two) glands of sheep with natural scrapie as well as in the parotid (seven of nine) and buccal (four or four) glands of experimentally challenged sheep. Overall, there was a good correlation between Western blot and immunohistochemistry results, although PrPSc was detected by immunohistochemistry in only two cases and by Western blotting in only one case. Collectively, 16 out of 18 (89%) scrapie-affected sheep showed PrPSc in at least one salivary gland.
The patterns of immunohistochemistry labeling were similar in naturally and experimentally infected sheep. In both the mucous and serous glands, immunostaining was characterized by multifocal, lobular distribution associated primarily with ductal epithelium and acinar cells (Fig. 1 and 2). Moreover, immunolabeling, associated in some cases with cellular debris, was observed within the lumina of salivary ducts draining saliva into the oral cavity (Fig. 2C). PET blot analysis showed that these positive deposits were proteinase K resistant (Fig. 1 and 2). The intensity and cellular location of immunostaining varied with the antibody used. In parotid glands, granular intracellular deposits in ductal cells were immunolabeled mainly by 2G11, while F99 decorated the luminal surfaces of the same cells (Fig. 1C). Occasionally, F99 and L42 showed immunolabeling in the interstitia of parotid glands. In minor salivary glands, 2G11 showed a stronger intraepithelial labeling than F99 and L42, revealing intracellular granular labeling associated with acinar and ductal epithelia (Fig. 2B and C). Given that the antibodies used recognize different PrP epitopes, these findings suggest that PrPSc accumulating in the various cellular compartments may expose different epitopes in formalin-fixed tissue sections. This could imply, for example, that intracellular PrPSc in ductal cells is conformationally distinct from that present on the luminal surface.
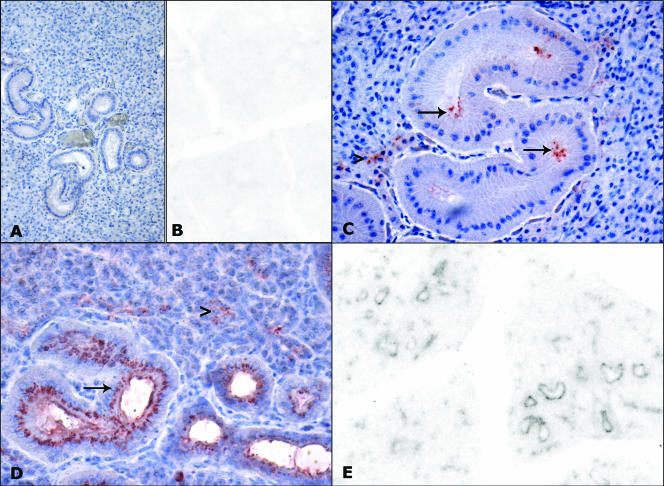
FIG. 1.
Immunohistochemistry and PET blot analysis of PrPSc in parotid glands of scrapie-affected sheep (C, D, E) and controls (A, B). No immunolabeling is evident by immunohistochemistry (A) and PET blot analysis (B) in the parotid glands of control sheep. (C) Immunolabeling of acinar cells (arrowhead) and of the luminal surfaces of ductal cells (arrows), stained by MAb F99 (magnification, ×400). (D) Positive deposits in acinar (arrowhead) and ductal (arrow) cells, stained by MAb 2G11 (magnification, ×200). (E) The same PrP deposits as those shown in panel D are proteinase K resistant by PET blot analysis (magnification, ×40).
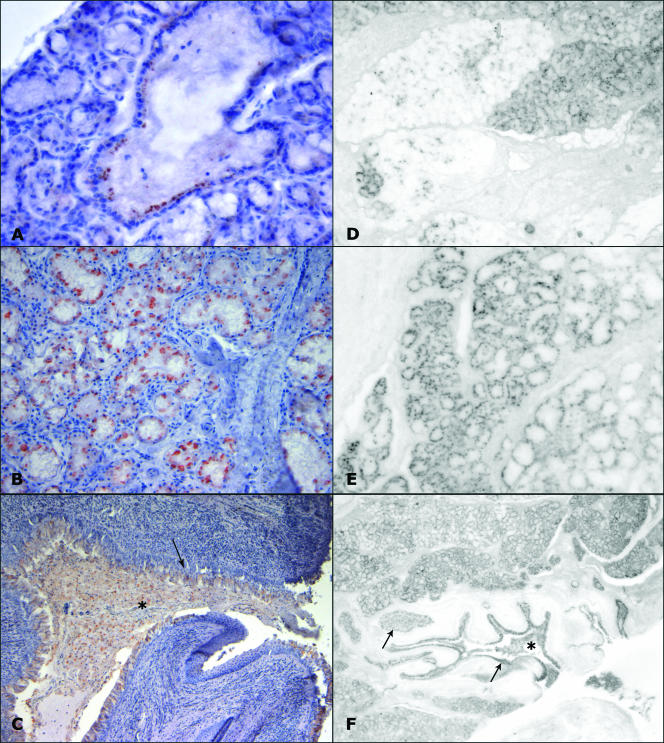
FIG. 2.
PrPSc deposition in the palatine salivary glands of scrapie-affected sheep. (A) Positive immunodeposits in the epithelium of an intralobular duct (MAb L42) (magnification, ×200). (B) Immunolabeling of the acinar epithelium (MAb 2G11) (magnification, ×200). (C) Immunolabeling in the ductal epithelium (arrow) and within the lumen (asterisk) of a major salivary duct draining saliva into the oral cavity (MAb 2G11) (magnification, ×100). (D) Adjacent positive and negative lobuli are evident by PET blot analysis (magnification, ×20). (E) PET blot analysis of a glandular lobule showing proteinase K-resistant immunodeposits in acinar cells (magnification, ×40). (F) PET blot analysis of the same major salivary duct depicted in panel C, showing protease-resistant PrP in the epithelium (arrows) and within the lumen (asterisk) of excretory ducts (magnification, ×10).
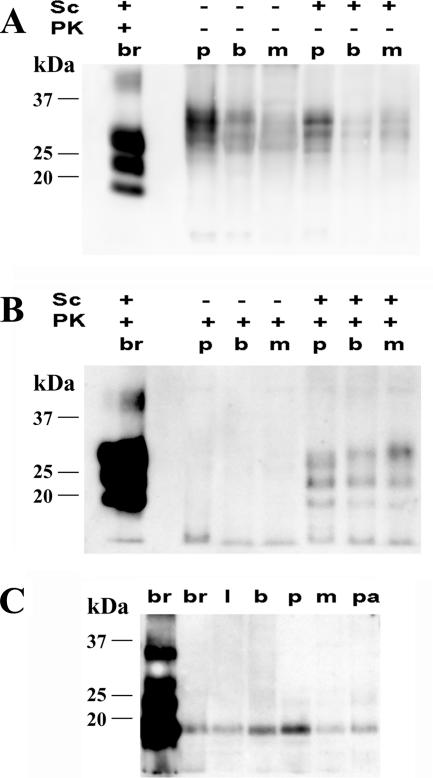
By Western blotting, PrPC was detected in the salivary glands of scrapie-affected and healthy sheep (Fig. 3A). After protease digestion, the typical three-band pattern of PrPSc was detected only in scrapie-affected sheep, in all types of salivary glands investigated (Fig. 3B). Protease-resistant PrPSc from salivary glands and brain displayed similar molecular weights upon deglycosylation (Fig. 3C). By plotting Western blot signals of glands against those of scrapie agent-infected brain, spiked and serially diluted in negative parotid gland homogenate, we determined that the PrPSc concentration in salivary glands ranged between 0.02 and 0.005% of that in brain (Fig. 3C).
FIG. 3.
Western blot analysis of PrPC and PrPSc in the salivary glands of sheep. (A and B) Western blots of PrP in parotid (p), buccal (b), and mandibular (m) salivary gland homogenates from scrapie agent (Sc)-infected (+) and healthy (−) sheep treated (+) or not treated (−) with proteinase K (PK). Samples were loaded at 2-mg equivalents (A) and 80-mg equivalents (B) per lane. In the first lane of each gel is shown a positive brain homogenate (br) diluted 1:500 in negative parotid homogenate. (C) Western blot of PrPSc from labial (l), buccal (b), parotid (p), mandibular (m), and palatine (pa) salivary glands of a scrapie-affected sheep after proteinase K treatment and deglycosylation with N-glycosydase F. In the first two lanes is shown a positive brain homogenate (br) diluted 1:500 and 1:10,000, respectively, in a negative parotid homogenate.
Taken together, these findings clearly show that a significant proportion of sheep with scrapie accumulate low levels of PrPSc in salivary glands.
Data reported herein confirm the presence of PrPC in salivary glands, as previously reported (9, 15). In contrast, PrPSc has not been detected before in the salivary glands of scrapie-affected sheep (9). The discrepancy between our results and those reported by Herrmann et al. (9) could be explained by the different antibodies used, although differences related to the scrapie strain involved cannot be ruled out. In sheep scrapie, PrPSc is detected primarily in nervous and lymphatic cells. Nevertheless, it has also been found recently in muscle spindles (2), trophoblastic cells of placenta (1, 22), papillae of tongue (3), and the interstitium of kidney (21). To our knowledge, this is the first report on the presence of PrPSc within secreting epithelial cells, and this finding may have important implications for understanding the mechanisms by which naturally occurring transmissible spongiform encephalopathies, such as scrapie and chronic wasting disease, spread by horizontal transmission (14, 19). Further studies are needed to investigate the pathway leading to salivary gland involvement. However, the presence of prions in the blood of sheep with scrapie or bovine spongiform encephalopathy (11) suggests a potential route of infection of tissues such as kidney and salivary glands.
To date, prion infectivity has not been described to occur in saliva, nasal secretions, respiratory aerosols, urine, or feces in animals with naturally occurring scrapie infections. However, prion excretion in urine has been demonstrated in prion-infected transgenic mice with chronic lymphocytic inflammation of the kidney (8). Furthermore, it has recently been shown that prions are present in the saliva of deer with chronic wasting disease (13). Our findings indicate that in sheep with scrapie, PrPSc deposits are associated with acinar and ductal epithelial cells of the salivary glands and can be detected in gland secretion. Further investigations by bioassays are in progress to evaluate whether this evidence substantiates the theory that saliva is a potential route for the horizontal transmission of scrapie.
Acknowledgments
This work was supported by grants from the Italian Ministry of Health (Progetti Strategici “Encefalopatie spongiformi trasmissibili dell'uomo e degli animali,” ricerca finalizzata 1%, years 2001 e 2002, and Ricerca Corrente 2005, grant RC-IZSVE 07/05).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Andreoletti, O., C. Lacroux, A. Chabert, L. Monnereau, G. Tabouret, F. Lantier, P. Berthon, F. Eychenne, S. Lafond-Benestad, J. M. Elsen, and F. Schelcher. 2002. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83:2607-2616. [DOI] [PubMed] [Google Scholar]
- 2.Andreoletti, O., S. Simon, C. Lacroux, N. Morel, G. Tabouret, A. Chabert, S. Lugan, F. Corbiere, P. Ferre, G. Foucras, H. Laude, F. Eychenne, J. Grassi, and F. Schelcher. 2004. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat. Med. 10:591-593. [DOI] [PubMed] [Google Scholar]
- 3.Casalone, C., C. Corona, M. I. Crescio, F. Martucci, M. Mazza, G. Ru, E. Bozzetta, P. L. Acutis, and M. Caramelli. 2005. Pathological prion protein in the tongues of sheep infected with naturally occurring scrapie. J. Virol. 79:5847-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham, A. A., J. K. Kirkwood, M. Dawson, Y. I. Spencer, R. B. Green, and G. A. Wells. 2004. Bovine spongiform encephalopathy infectivity in greater kudu (Tragelaphus strepsiceros). Emerg. Infect. Dis. 10:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eklund, C. M., R. C. Kennedy, and W. J. Hadlow. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15-22. [DOI] [PubMed] [Google Scholar]
- 6.Hadlow, W. J., C. M. Eklund, R. C. Kennedy, T. A. Jackson, H. W. Whitford, and C. C. Boyle. 1974. Course of experimental scrapie virus infection in the goat. J. Infect. Dis. 129:559-567. [DOI] [PubMed] [Google Scholar]
- 7.Hadlow, W. J., R. E. Race, and R. C. Kennedy. 1987. Temporal distribution of transmissible mink encephalopathy virus in mink inoculated subcutaneously. J. Virol. 61:3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heikenwalder, M., N. Zeller, H. Seeger, M. Prinz, P. C. Klohn, P. Schwarz, N. H. Ruddle, C. Weissmann, and A. Aguzzi. 2005. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307:1107-1110. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann, L. M., T. V. Baszler, and D. P. Knowles. 2000. PrP(c) mRNA, but not PrP(Sc) is found in the salivary glands of scrapie-infected sheep. Biochim. Biophys. Acta 1479:147-154. [DOI] [PubMed] [Google Scholar]
- 10.Hoinville, L. J. 1996. A review of the epidemiology of scrapie in sheep. Rev. Sci. Tech. Off. Int. Epizoot. 15:827-852. [DOI] [PubMed] [Google Scholar]
- 11.Hunter, N., J. Foster, A. Chong, S. McCutcheon, D. Parnham, S. Eaton, C. MacKenzie, and F. Houston. 2002. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 83:2897-2905. [DOI] [PubMed] [Google Scholar]
- 12.Ligios, C., C. J. Sigurdson, C. Santucciu, G. Carcassola, G. Manco, M. Basagni, C. Maestrale, M. G. Cancedda, L. Madau, and A. Aguzzi. 2005. PrPSc in mammary glands of sheep affected by scrapie and mastitis. Nat. Med. 11:1137-1138. [DOI] [PubMed] [Google Scholar]
- 13.Mathiason, C. K., J. G. Powers, S. J. Dahmes, D. A. Osborn, K. V. Miller, R. J. Warren, G. L. Mason, S. A. Hays, J. Hayes-Klug, D. M. Seelig, M. A. Wild, L. L. Wolfe, T. R. Spraker, M. W. Miller, C. J. Sigurdson, G. C. Telling, and E. A. Hoover. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133-136. [DOI] [PubMed] [Google Scholar]
- 14.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35-36. [DOI] [PubMed] [Google Scholar]
- 15.Moudjou, M., Y. Frobert, J. Grassi, and C. La Bonnardiere. 2001. Cellular prion protein status in sheep: tissue-specific biochemical signatures. J. Gen. Virol. 82:2017-2024. [DOI] [PubMed] [Google Scholar]
- 16.Nonno, R., M. A. Di Bari, F. Cardone, G. Vaccari, P. Fazzi, G. Dell'Omo, C. Cartoni, L. Ingrosso, A. Boyle, R. Galeno, M. Sbriccoli, H. P. Lipp, M. Bruce, M. Pocchiari, and U. Agrimi. 2006. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathogens 2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 18.Race, R., A. Jenny, and D. Sutton. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 178:949-953. [DOI] [PubMed] [Google Scholar]
- 19.Ryder, S., G. Dexter, S. Bellworthy, and S. Tongue. 2004. Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Res. Vet. Sci. 76:211-217. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi, S., S. Katamine, K. Yamanouchi, M. Kishikawa, R. Moriuchi, N. Yasukawa, T. Doi, and T. Miyamoto. 1993. Kinetics of infectivity are dissociated from PrP accumulation in salivary glands of Creutzfeldt-Jakob disease agent-inoculated mice. J. Gen. Virol. 74:2117-2123. [DOI] [PubMed] [Google Scholar]
- 21.Sisó, S., L. Gonzalez, M. Jeffrey, S. Martin, F. Chianini, and P. Steele. 2006. Prion protein in kidneys of scrapie-infected sheep. Vet. Rec. 159:327-328. [DOI] [PubMed] [Google Scholar]
- 22.Tuo, W., K. I. O'Rourke, D. Zhuang, W. P. Cheevers, T. R. Spraker, and D. P. Knowles. 2002. Pregnancy status and fetal prion genetics determine PrPSc accumulation in placentomes of scrapie-infected sheep. Proc. Natl. Acad. Sci. USA 99:6310-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vascellari, M., G. M. Aufiero, R. Nonno, U. Agrimi, G. Vaccari, L. Basilicata, C. Falcaro, M. Mancin, S. Marcon, and F. Mutinelli. 2005. Diagnosis and PrP genotype target of scrapie in clinically healthy sheep of Massese breed in the framework of a scrapie eradication programme. Arch. Virol. 150:1959-1976. [DOI] [PubMed] [Google Scholar]