Abstract
Decoy receptor 3 (DcR3) is a soluble decoy receptor belonging to the tumor necrosis factor receptor superfamily that is overexpressed in various malignant tumor types. DcR3 has been implicated in tumor cell survival by inhibiting apoptosis and by interfering with immune surveillance. A previous study showed that DcR3 expression is associated with Epstein-Barr virus (EBV)-positive lymphomas but rarely with non-EBV-positive B-cell lymphomas, suggesting that the presence of EBV may affect DcR3 expression. Here, we demonstrated enhanced DcR3 expression upon EBV reactivation in P3HR1 cells and in EBV-infected 293 cells. This enhancement, however, could not be detected in 293 cells infected with EBV with BRLF1 deleted. We found that EBV transactivator, Rta, could upregulate DcR3 expression by direct binding to an Rta-responsive element (RRE) located in the DcR3 promoter region and that this RRE is important for Rta-mediated DcR3 expression. Overexpressing CREB-binding protein (CBP) further enhanced Rta-dependent DcR3 expression, suggesting Rta-dependent DcR3 transcription activity is mediated by CBP. Previously, Rta was shown to enhance phosphatidylinositol-3 kinase (PI3-K) activity. However, Rta-transduced PI 3-K activity plays a minor role in DcR3 expression. This is the first report to demonstrate that Rta upregulates a cellular gene by direct binding to an RRE.
Decoy receptor 3 (DcR3)/TR6/M68 is a soluble decoy receptor belonging to the tumor necrosis factor receptor (TNFR) superfamily. Unlike most of the other members of the TNFR family, DcR3 does not contain a transmembrane domain and can be secreted (35). DcR3 is overexpressed in various malignant tumor types arising from the lung, colon, glia, and gastrointestinal tract (4, 17, 35, 44, 52, 54); in normal tissues, however, its expression can be detected only weakly in colon epithelial cells (35) and the placenta (18). DcR3 overexpression in tumor cells can be dependent on (34, 35) or independent of (4, 34) its gene amplification. DcR3 has been postulated to help tumor cells to gain survival advantage by inhibiting apoptosis and by interfering with immune surveillance by neutralizing the cytotoxic and immunomodulatory effects of Fas ligand, LIGHT (homologous to lymphotoxins, shows inducible expression, and competes with HSV glycoprotein D for herpesvirus entry mediator, a receptor expressed by T lymphocytes), and TNF-like molecule 1A (TL1A) (32, 35, 63). By neutralization of TL1A, DcR3 overexpression induces angiogenesis in human umbilical vein endothelial cells, suggesting another important role of DcR3 in tumorigenesis (57, 59). Recently, the DcR3.Fc fusion protein was found to modulate CD14+ monocyte differentiation into macrophages and the functions of dendritic cells (8). Incubation of DcR3.Fc-treated dendritic cells skews naïve T cells toward a T helper cell type 2 phenotype (8, 21), and DcR3 is able to induce actin reorganization and enhance the adhesion of monocytes via cross-linking heparan sulfate proteoglycan to increase ICAM-1 and VCAM-1 expression of endothelial cells (58). All the evidence suggests that DcR3 can not only be a factor responsible for the progression and immune suppression of tumor cells, but can also serve as an effector molecule to modulate pathological and physiological functions. Importantly, the DcR3 expression level is associated with lymph node metastasis and pathological sections in gastric carcinomas (52).
A clue that Epstein-Barr virus (EBV) infection may be linked to DcR3 expression comes from the study by Ohshima et al. (34). In their study, DcR3 expression was found to be associated with EBV-positive B-cell/NK cell lymphomas, while its expression could rarely be found in non-EBV-positive B-cell lymphomas, suggesting EBV-infected cells with DcR3 expression might be selected in the multistep tumorigenesis. EBV, a human gammaherpesvirus, is associated with several human malignancies, including Burkitt's lymphoma, nasopharyngeal carcinoma (NPC), Hodgkin's disease, and lymphoproliferative disorders in immune-compromised patients (24, 41). The monoclonality of resident EBV genomes indicates that EBV infection is an early event in tumorigenesis (3, 37). Latent EBV gene expression in NPC is limited to EBNA1, LMPs (6), and BamHI A transcripts (13, 46). There are, however, uncertainties regarding the direct carcinogenic effect of EBV, and it remains unclear at which stage EBV has a role in the pathogenesis of NPC. A serological survey demonstrated increased EBV-specific antibody titers of immunoglobulin A (IgA) to EBV capsid, and neutralizing antibodies to DNase are associated with a higher incident rate of NPC (11), suggesting that periodic EBV reactivation occurs in EBV-associated NPC.
EBV has two major targets in vivo, B lymphocytes and stratified epithelium, and its infection is predominantly at the latent stage in B cells. Latent EBV is intermittently reactivated to ensure new infection of uninfected cells or new hosts, and the reactivation can be induced by the expression of two immediate-early proteins, Zta and Rta (encoded by BZLF1 and BRLF1) (10, 38, 43, 51), although a full reactivation requires the cooperative functions of both genes (14). Rta is known to have many biological functions. Rta binds to a GC-rich motif known as the Rta-responsive element (RRE) found in viral promoters to activate the expression of BMRF1 (36), BMLF1 (19), and BALF2 (23). It was demonstrated that Rta binds directly to the early lytic EBV gene SM promoter, and the interaction between Rta and CREB-binding protein (CBP) is important for Rta-induced activation of the SM gene in Raji cells (50). Rta forms a complex with Sp1 and MCAF1 on an Sp1-binding site to autoregulate the transcription of BRLF1 and to regulate several host genes in EBV-infected cells (7).
Rta can also activate a class of genes, both cellular (7, 20, 28) and viral (1, 12, 29, 39), that lack any detectable RRE. For example, Rta activates Zta promoter (Zp) via ATF2 activation by increasing the levels of phosphorylated p38 mitogen-activated protein kinase and c-Jun N-terminal kinase (1), and Rta can activate Zp and BMRF1 promoters by phosphatidylinositol 3-kinase (PI3-K) induction (12). In addition, Rta induces expression of fatty acid synthase and c-myc by an indirect mechanism (20, 28). For these genes, it is thought that Rta activates them indirectly by stimulating cell signaling pathways, but it remains unclear how Rta activates these various signal transduction pathways.
There has been significant progress in understanding DcR3 functions in recent years. Normally, DcR3 expression is very low in tissues, but its expression is dramatically increased in various tumors (4, 17, 34, 35). However, little is known of the regulation of DcR3, and exogenous genes, such as those from viruses, may affect DcR3 expression. Since DcR3 expression is higher in EBV-associated lymphomas than in EBV-negative lymphomas, we looked for EBV genes that might regulate DcR3 expression. We demonstrate here that DcR3 expression increases upon viral reactivation. We show that Rta increases DcR3 expression by direct binding to the RRE sequence located in the DcR3 promoter region. Rta-induced PI3-K activity plays a minor role in this activity.
MATERIALS AND METHODS
Cell lines and culture.
Two Burkitt's lymphoma cell lines, P3HR1 (EBV positive) and Akata (EBV negative), were cultured in RPMI 1640 medium (JRH Biosciences) supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) at 37°C with 5% CO2. 293-EBV-wt cells (293 cells infected with wild-type EBV) (7) and 293-EBV-RKO cells (293 cells infected with EBV with BRLF1 deleted) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 100 μg/ml hygromycin at 37°C with 5% CO2 (gifts of S.-T. Liu, Chang-Gung University). SW480, a human colon adenocarcinoma cell line, was grown in Leibovitz 15 culture medium (Invitrogen) supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) at 37°C without CO2.
Plasmids.
The Rta-expressing plasmids pEGFP-Rta-wt, pEGFP-Rta (1-441), pEGFP-Rta-NLSm, and pSG5-Rta were gifts from T. Y. Hsu (National Taiwan University) (22). The reporter plasmid pDcR3-1010 was constructed by cloning the DcR3 promoter +114 to −1010 (+1, initiation site) into pGL3-basic vector (Promega) at the 5′ BglII site and the 3′ HindIII site. This fragment was amplified by PCR with primers 5′-GGGAGATCTACCTTCAGGTTGGTGCCTGG (forward) and 5′-CCCAAGCTTGGTCCTTGCTGGAGCAGGGA (reverse) using the bacterial artificial chromosome CTD3104-L22 (Invitrogen) as the template. pDcR3-249 was constructed by inserting a BamHI/HindIII fragment of pDcR3-1010 into pGL3-basic at the 5′ BglII site and the 3′ HindIII site. pDcR3-113 (+144 to −113) was constructed by self-ligation of KpnI-digested pDcR3-1010-wt. Two ΔRRE mutants contained the mutated RRE sequence ACACAGGCAGCCTGAATG (the mutated nucleotides are underlined). A −249 to +114 fragment with a mutated RRE was amplified from pDcR3-249 by PCR with 5′-TAGGGGATCCACCGACACATTAGGCTGCCTGTGTTGGTCTCTGGG (forward) (the BamHI site is underlined) and 5′-CCCAAGCTTGGTCCTTGCTGGAGCAGGGA (reverse) and cloned into pGL3-Basic at the 5′ BglII site and the 3′ HindIII site to generate pDcR3-249-ΔRRE. A BglII and BamHI fragment containing −1010 to −250 of pDcR3-1010 was inserted into pDcR3-249-ΔRRE at the 5′ BglII site and the 3′ BamHI site to generate pDcR3-1010-ΔRRE.
Transient transfection.
All plasmids for transfection were purified by using the QIAGEN mini kit. Transfection of SW480 cells and 293 cells was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. The amounts of the plasmids used are indicated in the figure legends. Electroporation of Akata cells has been described previously (25). Briefly, 5 × 106 Akata-EBV(−) cells were suspended in serum-free RPMI 1640 medium, washed twice, and resuspended in 400 μl of ice-cold serum-free RPMI 1640 medium containing up to 20 μg of plasmid DNA in a 2-mm-gap electroporation cuvette (BTX). The electroporation was performed with the Electro Cell Manipulator 630 (BTX) set at 180 V, 1 mF, and 25 Ω.
Antibodies and reagents.
Anti-Rta antibody (8C12) recognizing the C-terminus of Rta (Argene) was used to detect full-length Rta or green fluorescent protein (GFP)-Rta. Anti-Rta antibody (no. 467) recognizing the N terminus (a gift from T. Y. Tsu, National Taiwan University) was used to detect Rta (1-441). Anti-DcR3 antibody (3H5) was from BioLegend. Anti-total Akt and phosphorylated Akt (Ser473) were purchased from Cell Signaling Technologies. Anti-GFP antibody was purchased from Clontech. Anti-Flag antibody (M2), anti-β-actin antibody (AC-15), 12-O-tetradecanoylphorbol 13-acetate (TPA), and sodium butyrate were purchased from Sigma. The PI3-K inhibitor LY294002 was purchased from Cayman.
Western blot analysis.
Total cells or nuclear extracts were lysed in sample buffer (3% sodium dodecyl sulfate, 1.6 M urea, 4% β-mercaptoethanol), resolved on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, and electrotransferred onto nitrocellulose membranes. After being blocked in Tris-buffered saline (50 mM Tris-HCl [pH 7.4], 0.2 M NaCl) containing 5% nonfat milk and 0.1% Tween 20 (TBS-T) for 1 h, the membranes were incubated with primary antibodies as indicated overnight at 4°C. The membranes were then washed with TBS-T and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibody (CHEMICON) for 1 h at room temperature. Signals were detected with enhanced-chemiluminescence blotting reagents (Perkin-Elmer). For detecting phosphorylated Akt (Ser 473), membranes were blocked in TBS-T and 5% bovine serum albumin.
Reverse transcription-PCR (RT-PCR).
SW480 cells (4 × 105) were transfected with pEGFP; 0.15, 0.5, and 1.5 μg of pEGFP-Rta-wt; or 1.5 μg of pEGFP-Rta (1-441) plasmid DNA for 48 h; 1 × 106 293-EBV-wt or 293-EBV-RKO cells were transfected with 4 μg of pcDNA-Zta or pSG5-Rta for 48 h. Total RNA was extracted by using REzol reagent (Protech). Five micrograms of total RNA was reverse transcribed into cDNA by using an oligo(dT) primer (Promega) and Super Script III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. PCR for DcR3 was run at 95°C for 30 s, 58°C for 30 s, and 72°C for 40 s with primers 5′-ACGCGGAGTGGCAGAAACAC (forward) and 5′-TCCTCAGCTCCTGGTACCCT (reverse). PCR for β-actin was run at 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s with primers 5′-TGACGGGGTCACCCACACTGTGCCCATCTA (forward) and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG (reverse).
DcR3 ELISA.
The DcR3 concentration in cell-cultured medium was analyzed with a DcR3 enzyme-linked immunosorbent assay (ELISA) kit (BioVender Laboratory Medicine) according to the manufacturer's protocol. All samples were stored at −20°C before detection.
EMSA.
Nuclear extracts were prepared from 2 × 106 SW480 cells transfected with 8 μg of pEGFP or pEGFP-Rta (1-441) plasmid DNA. Nuclei were harvested 48 h posttransfection by using Nuclei EZ lysis buffer (Sigma). Nuclear proteins were extracted by using lysis buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, and 10% glycerol) with vigorous shaking at 4°C for 2 h. The supernatants were harvested, aliquoted, flash frozen, and stored at −20°C. Nuclear protein concentrations were determined by Bradford assay. Single-stranded oligonucleotides were annealed and end labeled with 32P by using T4 polynucleotide kinase (New England Biolabs). The probe for pDcR3-RRE was 5′-CAGAGACCAGCCCAGGCAGCCTGGTGTGTCGGTGGAT. Probes for electrophoretic mobility shift assay (EMSA) positive and negative controls have been described previously (9). Each binding reaction mixture containing 10 μg of nuclear extracts, 10 mM HEPES (pH 7.9), 25 mM KCl, 2.5 mM MgCl2, 0.25 mM EDTA, 1 mM dithiothreitol, 100 ng/μl poly(dI-dC), 10% glycerol and 1 μM of labeled oligonucleotide was incubated at 30°C for 30 min. Anti-Rta antibody (no. 467) and 50 μM of nonradioactive competitor oligonucleotide were used for EMSA supershift and competition reactions, respectively. Reaction mixtures were separated on 4% native polyacrylamide gels in 0.5× Tris-borate-EDTA buffer at 150 V.
Chromatin immunoprecipitation (ChIP) assay.
SW480 cells (5 × 106) were transfected with 24 μg of pEGFP or pEGFP-Rta-wt plasmid DNA for 48 h. P3HR1 cells (2 × 107) were left untreated or treated with 40 ng/ml TPA and 3 mM sodium butyrate for 48 h to induce EBV reactivation. DNA-protein complexes were cross-linked with formaldehyde, sonicated, and then immunoprecipitated with anti-GFP antibody (SW480) or with anti-Rta antibody (P3HR1) overnight at 4°C. The immunoprecipitates were incubated with protein A beads (Amersham) at 4°C for 2 h and then washed once with RIPA A buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) for 5 min, once in RIPA B buffer (50 mM Tris-HCl [pH 9.0], 5 mM EDTA, 300 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) for 10 min, once in LiCl washing buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 250 mM LiCl, 1% NP-40, and 1% sodium deoxycholate) for 15 min, and five times in TE buffer (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). De-cross-linking of DNA-protein complexes was performed by adding 5 M NaCl and heating them at 65°C overnight. DNA fragments were purified by phenol-chloroform extraction. PCR was run at 95°C for 30 s, 57°C for 30 s, and 72°C for 20 s. The primers for pDcR3-RRE were 5′-AGTTGGCAGAGGCCCC (forward) and 5′-ACCCACCTGGTACCATCCC (reverse). The primers for non-RRE fragments were 5′-TCTCAGCCAGCAGCTCCA (forward) and 5′-CTGGCTCACCTGGTACCCT (reverse). Anti-human-IgG antibody (Cappel) was used as the antibody isotype control.
Luciferase assay.
Ten micrograms of DcR3 reporter plasmids and 10 μg of pSG5-Rta or pSG5 were inserted into 5 × 106 Akata cells by electroporation. SW480 cells (2 × 105) were cotransfected with 0.4 μg of DcR3 reporter plasmids and 0.4 μg of different pEGFP-Rta plasmids for 24 h. For Rta and CBP cotransfection, 2 × 105 SW480 cells were transfected with 0.2 μg of pEGFP-Rta plasmid or pEGFP vector plus 0.6 μg of pCMV2-Flag-CBP plasmid or pCMV2 vector. The cells were lysed in cell culture lysis buffer at 4°C for 1 h. A luciferase assay was performed with the Luciferase Assay System (Promega) according to the manufacturer's protocols.
PI3-K inhibition.
SW480 cells (1 × 106) were serum starved for 24 h and then transfected with 4 μg of pEGFP, pEGFP-Rta-wt, or pEGFP-Rta-NLSm plasmid. After being transfected for 24 h, the cells were treated with 20 μM of PI3-K inhibitor LY294002 or dimethyl sulfoxide for 24 h.
RESULTS
DcR3 expression increases upon EBV reactivation.
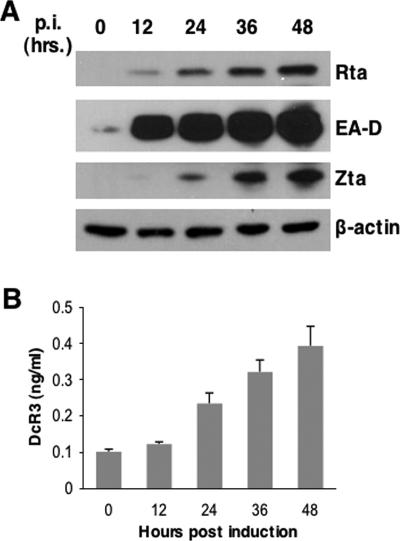
A previous study by Ohshima et al. demonstrated that DcR3 expression is associated with EBV in B-cell/NK cell lymphomas (34), suggesting that EBV genes may increase DcR3 expression. We treated EBV-positive Burkitt's lymphoma P3HR1 cells with TPA and sodium butyrate to induce latent EBV into the lytic cycle. EBV reactivation was monitored in a 48-hour time course by Western blotting of EBV lytic gene products (Fig. 1A), and the amounts of secreted DcR3 in culture media were measured by ELISA (Fig. 1B). We found that DcR3 expression increased in proportion to the degree of EBV reactivation as judged by the expression of EA-D (BMRF1), Rta (BRLF1), and Zta (BZLF1). Amounts of DcR3 increased more than fourfold up to 48 h postinduction. This result indicated that EBV lytic genes, possibly Zta or Rta, could upregulate DcR3 expression.
FIG. 1.
EBV reactivation induces DcR3 expression. (A) Latent EBV in P3HR1 cells was induced into the lytic cycle by TPA (40 ng/ml) and sodium butyrate (3 mM) treatment, and viral reactivation was monitored by the expression of Rta, EA-D, and Zta measured by Western analysis at 12-hour intervals in a 48-hour time course. Western blots were probed with antibodies to Rta, EA-D, Zta, or the control cellular protein β-actin. p.i., postinduction. (B) DcR3 in culture media was measured by ELISA at the indicated time points post-chemical induction. Averages and standard deviations were from measurements taken in triplicate.
Rta alone can enhance DcR3 expression.
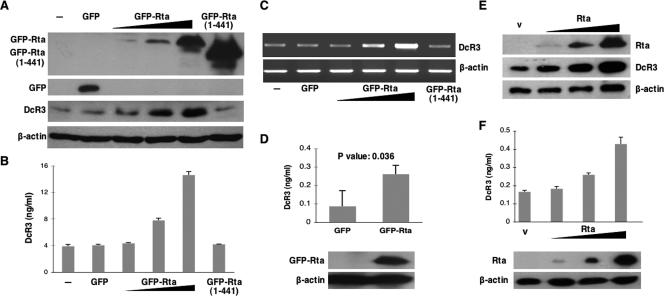
It is well established that the two EBV transactivators Zta and Rta activate each other's promoters (38, 48), and the transcriptional function of either one can trigger downstream gene expression (2, 14, 40, 48). To get a clearer effect of individual EBV genes on DcR3 expression and to avoid the mutual influences of Zta and Rta, we used SW480, an EBV-negative cell line with relatively high DcR3 expression, as our model system. We transfected expression plasmids of GFP-Rta or Zta into SW480 cells and measured DcR3 protein by Western analysis and ELISA. While Zta had no effect on DcR3 expression (data not shown), GFP-Rta enhanced DcR3 protein expression. The increase of DcR3 expression was Rta dosage dependent, as demonstrated either by Western analysis (Fig. 2A) or by ELISA (Fig. 2B). GFP-Rta (1-441) (22), which has an intact DNA binding domain and a truncated transactivation domain, failed to increase DcR3 expression, suggesting the importance of interaction between Rta and the transcription machinery. To confirm that Rta upregulated DcR3 at the transcription level, we measured DcR3 transcripts by RT-PCR. The results demonstrated that DcR3 transcripts increased in the presence of Rta (Fig. 2C). This result, together with the fact that an Rta (1-441) transactivation domain mutant failed to increase DcR3 expression, suggested that Rta may bind to an RRE in the DcR3 promoter region to upregulate DcR3 expression. To demonstrate that Rta can enhance DcR3 expression in EBV-permissive cells, we sent a GFP-Rta expression plasmid (pEGFP-Rta) into Akata cells (EBV negative) by electroporation. ELISA data indicated that Rta could increase DcR3 expression in this cell line compared to a control plasmid (Fig. 2D). A t test was used to test the statistical significance of the DcR3 expression difference in the presence or absence of Rta from three independent experiments. The test revealed a P value of 0.0361. Representative Rta expression in the cells after electroporation was shown by Western analysis (Fig. 2D).
FIG. 2.
Rta enhanced DcR3 expression in SW480 and Akata cells. Up to 4 μg in threefold increments of pEGFP-Rta per 1 × 106 cells was transfected into SW480 cells to demonstrate the dosage effect of Rta on DcR3 expression. An Rta C-terminal deletion mutant, pEGFP-Rta (1-441), was used to demonstrate the importance of the transactivation domain of Rta. pEGFP was used as the vector control. The expression levels of DcR3 were measured by (A) Western analysis for cell-associated DcR3, (B) ELISA for secreted DcR3 in the culture media, and (C) RT-PCR for transcripts of DcR3 and cellular control β-actin derived from SW480 cells. Western blots were probed with antibodies to GFP [for GFP, GFP-Rta, and GFP-Rta (1-441)], DcR3, and the control cellular protein β-actin. All the measurements were taken 48 h posttransfection. Averages and standard deviations were from measurements taken in triplicate. (D) pEGFP-Rta was sent into Akata (EBV-negative) cells by electroporation to demonstrate that Rta can enhance DcR3 expression in EBV-permissive cells. ELISA was used to compare secreted DcR3 levels in the culture media in the presence (GFP-Rta) or absence (GFP) of Rta. Averages and standard deviations were from measurements taken in three independent experiments. The P value of the t test is shown. Representative Rta expression in the cells after electroporation was shown by the Western analysis using anti-GFP antibody. (E and F) Up to 4 μg of pSG5-Rta per 1 × 106 cells was transfected into SW480 cells (E) or sent into Akata cells by electroporation (F) to rule out a GFP effect on DcR3 expression. DcR3 protein expression was measured by Western analysis (E) or by ELISA (F). Rta was detected by anti-Rta antibody in the Western analysis.
To rule out the possible effect of GFP on GFP-Rta-dependent DcR3 expression, we overexpressed an Rta expression plasmid, pSG5-Rta, in SW480 cells (Fig. 2E) and Akata cells (EBV negative) (Fig. 2F). Rta enhanced DcR3 protein expression in a dosage-dependent manner in SW480 cells and Akata cells, as demonstrated by Western analysis (Fig. 2E) and by ELISA (Fig. 2F), respectively. These results, together with the data showing that GFP alone did not enhance DcR3 expression, as seen in Fig. 2A to D, indicated that Rta was responsible for the DcR3 upregulation.
Rta is responsible for DcR3 overexpression upon EBV reactivation.
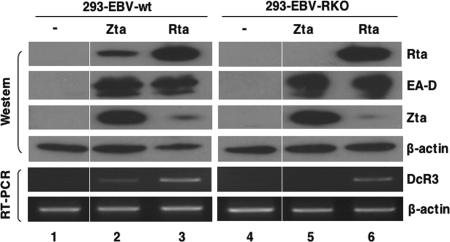
To obtain conclusive evidence that Rta activates DcR3 expression in the context of EBV infection, we investigated the role of Rta in DcR3 expression upon EBV reactivation in 293 cells infected with wild-type EBV (293-EBV-wt) or with EBV with BRLF1 deleted (293-EBV-RKO). A Zta or Rta expression plasmid was transfected into both cell lines, and DcR3 expression was measured using RT-PCR. Either Zta or Rta overexpression induced a lytic program in both cell lines, as judged by the strong expression of EA-D (Fig. 3). Rta induced both Zta expression and DcR3 expression in 293-EBV-wt and 293-EBV-RKO cells (Fig. 3, lanes 3 and 6). As expected, expression of Zta induced Rta expression only in 293-EBV-wt. Importantly, DcR3 was detected only in 293-EBV-wt and not in 293-EBV-RKO cells when transfected with Zta (Fig. 3, lanes 2 and 5). Complementation of 293-EBV-RKO cells with an Rta-expressing plasmid restored DcR3 expression (Fig. 3, lane 6). Comparing DcR3 expression in all settings, we concluded that DcR3 could be detected only in the presence of Rta regardless of the expression level of Zta or EA-D, and the DcR3 expression level was proportional to the amount of Rta expressed, suggesting that Rta was responsible for DcR3 overexpression upon EBV reactivation. Untransfected 293-EBV-wt and 293-EBV-RKO cells provided additional negative controls (Fig. 3, lanes 1 and 4).
FIG. 3.
Rta is responsible for DcR3 overexpression upon EBV reactivation. pcDNA-Zta (Zta) or pSG5-Rta (Rta) was transfected into 293-EBV-wt or 293-EBV-RKO cells for 48 h. DcR3 expression was measured by RT-PCR. Western blots were probed with antibodies to Rta, EA-D, Zta, or the control cellular protein β-actin.
Rta binds to the RRE in the DcR3 promoter region.
To find out if any RRE was present in the DcR3 promoter region, we analyzed the regulatory region of the DcR3 gene. We first mapped the transcription initiation site of DcR3 by rapid amplification of 5′ cDNA ends. After sequence comparison to a genomic clone, we found the initiation site located 14 nucleotides upstream of a GenBank DcR3 clone, NM003823 (data not shown). We analyzed the promoter region of DcR3 and found a sequence that matched the consensus Rta binding element (CRBE) (9) at −222 to −238 of the minus strand in respect to the mapped initiation site (+1) (Fig. 4A). To test if this CRBE was an RRE, we performed EMSA. Since the DNA binding capacity of Rta might be affected by posttranslational modifications or protein-protein interactions, we used extracts of mammalian-cell-expressed Rta in the EMSAs. Full-length Rta was shown to have very weak binding to the RRE of the EBV BMLF1 promoter, and in contrast, a C terminus (55-amino-acid) deletion mutant displayed strong DNA binding activity in vitro (9). Therefore, we used an Rta C terminus deletion clone containing an intact DNA binding domain, pEGFP-Rta (1-441), in the EMSA. Nuclear extracts were prepared from SW480 cells transfected with pEGFP or with pEGFP-Rta (1-441). We found that Rta (1-441) bound to the DcR3 CRBE with an intensity similar to that of the BMLF1 RRE, which was previously demonstrated to have high affinity for Rta (9) (Fig. 4B, lanes 3 and 8). Both of these bindings could be further shifted by the anti-Rta-N-terminus antibody (Fig. 4B, lanes 4 and 9) and could be competed away by an excess of cold probes (Fig. 4B, lanes 5 and 10). None of the Rta-specific binding signals were seen in vector only or when the probe was BaRF1-II, whose CRBE was not bound by Rta (9) (Fig. 4B). We concluded that the CRBE found in the DcR3 promoter is indeed an RRE and hereafter refer to it as DcR3-RRE.
FIG. 4.
Rta can bind to the RRE of the DcR3 promoter in vitro. (A) Sequences of potential RREs found in BMLF1, BaRF1, two viral promoters, and DcR3 were aligned with CRBE (underlined). −238 to −222 marks the location of the DcR3 CRBE found in the DcR3 promoter region in respect to the initiation site (+1). The nucleotide sequences represent the probe sequences used in the EMSA. (B) Nuclear extracts from SW480 cells transfected with pEGFP-Rta (1-441) or control vector were incubated with radiolabeled oligonucleotide probes as indicated. Anti-Rta N-terminal antibody was added to the reaction mixtures in lanes 4 and 9, and bands supershifted by the antibody are shown. A 50-fold excess of cold competitors was added to lanes 5, 10, and 14. BMLF1 was used as a positive Rta binding control, and BaRF1-II was a negative control. The arrows indicate bands of Rta-specific binding or supershifted bands.
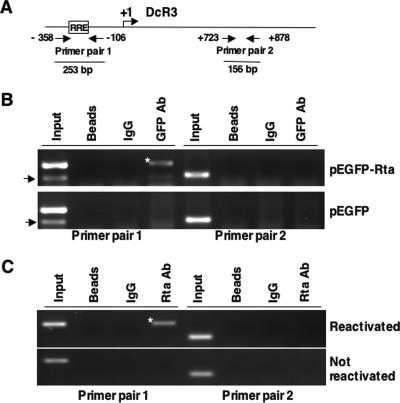
To show that Rta binds to DcR3-RRE in vivo, we performed ChIP assays after the transfection of SW480 cells with pEGFP-Rta-wt expression plasmid or pEGFP control vector. GFP or GFP-Rta from transfected cell lysates was immunoprecipitated by anti-GFP antibody, and cross-linked DNA was PCR amplified using the two primer pairs shown in Fig. 5A. ChIP results revealed that Rta bound to DcR3-RRE specifically as a 253-bp fragment, which covers DcR3-RRE, could be PCR amplified only from anti-GFP antibody precipitates of pEGFP-Rta-transfected cells using primer pair 1 (Fig. 5B). Primer pair 2, which was located approximately 900 bp away from DcR3-RRE, could not amplify this fragment in the PCR. The 253-bp fragment was not amplified from vector control (GFP) lysates, bead controls, or IgG (antibody isotype) immunoprecipitates (Fig. 5B). This result demonstrated the in vivo binding of Rta to the RRE located in the DcR3 promoter region.
FIG. 5.
Rta binds to DcR3 promoter in vivo. (A) Schematic diagram showing the locations of two pairs of primers used in the ChIP assay. Primer pair 1 covers −358 to −106 (253 bp with an RRE), and primer pair 2 covers +723 to +878 (156 bp with no RRE) in respect to the DcR3 initiation site (+1). The size of the expected PCR product of each primer pair is indicated. ChIP assays of Rta were performed (B) after transfection of SW480 cells with GFP-Rta- or GFP-expressing plasmids or (C) after P3HR1 reactivation. P3HR1 cells were induced into the lytic cycle by TPA and sodium butyrate treatment. Anti-GFP antibody was used to immunoprecipitate GFP-Rta from SW480 cells. Rta in reactivated P3HR1 cells was immunoprecipitated by anti-Rta antibody. Primer pair 1 specifically amplified fragments (asterisks) from pEGFP-Rta-transfected SW480 cells and reactivated P3HR1 cells. The arrows indicate bands of nonspecific amplification of primer pair 1; 1/10 of the total lysates was used for input control.
To demonstrate that Rta binds to DcR3-RRE in a more biologically relevant setting, we performed a ChIP assay using an EBV-infected cell line. P3HR1 cells were reactivated with TPA and sodium butyrate for 48 h. Rta of reactivated cell lysates was immunoprecipitated by anti-Rta antibody, and cross-linked DNA was PCR amplified using the primers shown in Fig. 5A. The 253-bp fragment was specifically amplified from immunoprecipitates of reactivated-P3HR1 lysates by anti-Rta antibody using primer pair 1 and was not seen in the negative controls (Fig. 5C). The results indicated that Rta binds to DcR3-RRE upon EBV reactivation.
DcR3-RRE is required for Rta-dependent DcR3 expression.
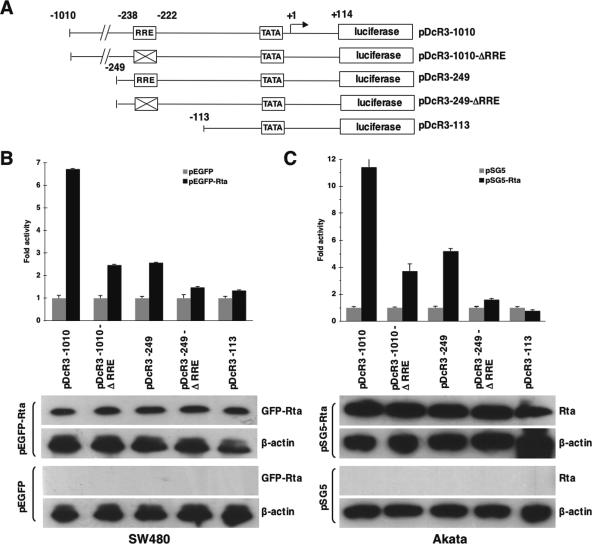
To illustrate the importance of DcR3-RRE for Rta-dependent DcR3 transactivation, luciferase reporter assays were performed in SW480 and Akata cells. Reporters driven by regions of the DcR3 promoter with or without an RRE were constructed in pGL3-basic (Promega) to drive the firefly luciferase gene, as illustrated in Fig. 6A. These constructs were transfected into SW480 cells together with expression plasmids of GFP-Rta or GFP, and luciferase activities were measured. GFP-Rta compared to the GFP control induced about sevenfold more luciferase activity driven by the DcR3 promoter region from −1010 to +114 (pDcR3-1010). An RRE mutant (pDcR3-1010-ΔRRE) that could no longer be bound by Rta (data not shown) dramatically reduced this rise to about twofold. Rta-dependent reporter activity was reduced to less than threefold when the luciferase gene was driven by the DcR3 promoter region from −249 to +114 (pDcR3-249), suggesting that a positive cis element, which is required for Rta-dependent activity, was located between −1010 and −249. Mutation of the RRE and truncation of the promoter region upstream of −113 (pDcR3-113) further reduced the Rta-dependent activity almost to background level (Fig. 6B).
FIG. 6.
Mutation of RRE in the DcR3 promoter reduces Rta-mediated DcR3 transactivation. (A) Schematic illustration showing five luciferase reporter constructs driven by the DcR3 promoter. The DcR3 promoter region in each construct is indicated by numbers in respect to the initiation site (+1). The boxes marked with Xs indicate the mutated RRE sites. TATA, a putative TATA box. (B) SW480 cells were transfected with the indicated reporter construct together with GFP-Rta- or GFP-expressing plasmids (4 μg/106 cells). (C) The indicated reporter constructs together with pSG5-Rta or pSG5 were sent into Akata cells (EBV negative) by electroporation. Luciferase activities were compared among different reporter constructs in the presence (black bars) or absence (gray bars) of Rta. The Rta-induced activity is shown in the bar graphs. Averages and standard deviations were calculated from measurements of luciferase activity taken in triplicate. Western analysis (bottom) showed the expression levels of GFP-Rta, Rta, and the cellular control protein β-actin in the reporter assay. The transfected plasmids are indicated on the left of the Western blots.
To demonstrate that DcR3-RRE is also important for Rta-dependent DcR3 transactivation in EBV-permissive cells, we performed reporter assays similar to those in Fig. 6B in Akata cells (EBV negative). The reporter constructs were sent into Akata cells together with an Rta-expressing plasmid, pSG5-Rta, by electroporation. As seen in SW480 cells, RRE disruption greatly reduced the Rta-mediated reporter activity driven by various regions of the DcR3 promoter (Fig. 6C). These results demonstrated the importance of the RRE found in the DcR3 promoter for DcR3 transactivation by Rta in a cell line-independent manner. These differences were not due to Rta expression variation, since the Rta expression levels indicated by Western blotting were similar in the cell extracts (Fig. 6B and C, bottom).
CBP further enhances Rta-dependent DcR3 expression.
We demonstrated that an Rta transactivation domain mutant (Rta 1-441) failed to enhance DcR3 expression and that the interaction between Rta and the transcription machinery is important for Rta-mediated DcR3 expression (Fig. 2). Rta was shown to interact with a histone acetyltransferase-containing protein, CBP, and this interaction is important for Rta to transactivate of the EBV SM gene (50). To study the significance of the interaction between Rta and CBP for DcR3 expression, a reporter assay was performed. SW480 cells were transfected with the reporter construct pDcR3-1010 (Fig. 6A) in the presence or absence of Rta- and CBP-expressing plasmids. We transfected less of the GFP-Rta expression plasmid used in Fig. 6 to better see the CBP effect on Rta-mediated DcR3 expression. CBP alone did not increase DcR3 expression; however, CBP enhanced DcR3 promoter activity in the presence of Rta in SW480 cells approximately 1.5-fold (Fig. 7A). The degree of Rta enhancement was similar to that of the EBV SM gene in the presence of endogenous and overexpressed CBP (50). Western analysis showed a similar result. CBP alone did not increase DcR3 expression, while Rta plus CBP overexpression increased Rta-dependent DcR3 expression (Fig. 7B). Rta-induced DcR3 expression could be enhanced by treating cells with a histone deacetylase inhibitor, trichostatin A, suggesting that histone acetyltransferase activity positively regulates DcR3 expression (data not shown).
FIG. 7.
CBP enhances Rta-dependent DcR3 expression. (A) pEGFP-Rta (2 μg/106 cells), pCMV-Flag-CBP (6 μg/106 cells), or both were cotransfected with the luciferase reporter pDcR3 −1010 into SW480 cells. Luciferase activity was measured and is presented in relation to that of the mock transfection. Averages and standard deviations were from measurements taken in triplicate. (B) Western blots showing the expression of DcR3 in the presence (+) or absence (−) of Flag-CBP and GFP-Rta. DcR3 expression was quantified, normalized with β-actin expression, and compared to that of the mock infection.
Rta-induced PI3-K activity plays a minor role in DcR3 expression.
We established that Rta could induce DcR3 expression by directly binding to the RRE in its promoter region. Nevertheless, increased reporter activity was detected in the reporter driven by the DcR3 promoter region without the RRE (pDcR3-1010-ΔRRE) in the presence of Rta (Fig. 6). Rta is known to transactivate some EBV genes by a PI3-K signaling pathway in human fibroblasts (12). We therefore investigated the possibility that Rta can upregulate DcR3 expression by PI3-K activation. Serum-starved SW480 cells were transfected with GFP, GFP-Rta, or GFP-Rta-NLSm. GFP-Rta-NLSm is an Rta nuclear localization signal (NLS) mutant (the NLS was converted to AAAA) that was previously shown to be located in the cytoplasm and was thought to be functional for triggering cellular signaling (22). Wild-type Rta enhanced DcR3 expression at a much higher level than Rta-NLSm when examined by Western analysis (Fig. 8A lanes 2 and 3), indicating that Rta entry into nuclei is important. A similar result was observed in the luciferase reporter assay, in which GFP-Rta, GFP-Rta-NLSm, or vector control plasmid was transfected with pDcR3-1010 (Fig. 6A) into SW480 cells. While Rta induced about sixfold more luciferase activity than vector alone, Rta-NLSm induced only less than twofold, confirming the importance of nuclear localization for Rta-mediated DcR3 induction shown in the Western analysis. Figure 8B, bottom, shows the equal expression of GFP-Rta and GFP-Rta-NLSm. Rta-NLSm was still able to induce DcR3 expression, although at lower levels than wild-type Rta (Fig. 8A, lane 3), suggesting that Rta-NLSm could activate DcR3 expression through the signaling transduction pathway. To investigate this possibility, we found that both Rta and Rta-NLSm induced Akt phosphorylation, a downstream target of PI3-K activity. Rta and Rta-NLSm did not increase the expression of the Akt protein in SW480 cells. To test the effect of this Rta-induced Akt phosphorylation on DcR3 expression, we used a PI3-K inhibitor, LY294002. LY294002 reduced phosphorylated Akt induced by wild-type Rta or an Rta NLS mutant as expected, and this led to the reduction of Rta-NLSm-mediated DcR3 expression (Fig. 8A, lanes 3 and 6). However, the levels of Rta-induced DcR3 remained similar with or without inhibitor treatment. These results demonstrated that Rta could induce DcR3 expression via a PI3-K signaling pathway, but the major Rta-mediated DcR3 expression was due to Rta binding to the RRE of the DcR3 promoter. Another PI3-K inhibitor, wortmannin, had effects on Rta-induced DcR3 expression similar to those of LY294002 (data not shown).
FIG. 8.
Rta-dependent-DcR3 expression is mainly from its direct binding to the DcR3 promoter. (A) Serum-starved SW480 cells were transfected with GFP-, GFP-Rta-, or GFP-Rta-NLSm-expressing plasmids. The samples in lanes 1 to 3 were dimethyl sulfoxide solvent controls for the PI 3-K inhibitor (20 μM of LY294002)-treated samples in lanes 4 to 6. The Western blots were probed with antibodies of Rta (for GFP-Rta and GFP-Rta-NLSm), total Akt, phosphorylated Akt (p-Akt), DcR3, or the control cellular protein β-actin. A longer DcR3 exposure was provided to show the basal level of DcR3 expression under starvation conditions. (B) GFP-Rta, GFP-Rta-NLSm, or vector control plasmid (2 μg/106 cells) was transfected with the luciferase reporter driven by the DcR3 promoter region, pDcR3 −1010, into SW480 cells. Luciferase activity was measured and is presented in relation to that of GFP alone. (Bottom) Equal expression of GFP-Rta and GFP-Rta-NLSm by Western analysis. β-Actin was a loading control.
Notably, endogenous DcR3 expression was also inhibited by LY294002 (Fig. 8A, lanes 1 and 4), suggesting that DcR3 could be regulated by the PI3-K signaling pathway. This PI3-K-dependent cis element is possibly located between −1010 and −249, since Rta-dependent activity is much lower in pDcR3-249-ΔRRE than in pDcR3-1010-ΔRRE (Fig. 6). Serum starvation also reduced the basal level of DcR3 expression, as endogenous DcR3 could be detected only by a longer exposure in the Western analysis.
DISCUSSION
DcR3, overexpressed in various cancers, is a soluble TNFR. One of the DcR3 functions is to provide a survival advantage to cancer cells by interfering with the immune response to infected cells (32, 35, 63). A study by Ohshima et al. demonstrated that DcR3 is overexpressed in EBV-associated lymphomas (34), raising the possibility that EBV affects DcR3 expression. In this report, we showed that DcR3 expression was enhanced upon EBV reactivation in P3HR1 cells (Fig. 1). Higher DcR3 levels of EBV-infected peripheral blood mononuclear cells from healthy people were detected in our laboratory (data not shown). These results indicated that EBV infection and reactivation can cause DcR3 elevation. Zta has no effect on DcR3 expression. In contrast, Rta overexpression enhanced DcR3 expression in an EBV-permissive cell line, Akata, as well as in a cell line, SW480, with high endogenous DcR3 expression (Fig. 2). DcR3 could be detected only in the presence of Rta regardless of the expression level of Zta or EA-D in EBV-infected 293 cells (Fig. 3). The results in Fig. 2 and 3 indicated that Rta is responsible for the DcR3 expression upon EBV reactivation. We found a CRBE located in the DcR3 promoter (Fig. 4A). Using EMSA and ChIP assays, we demonstrated binding of Rta to this CRBE both in vitro (Fig. 4) and in vivo (Fig. 5). We concluded that this CRBE is an RRE. Mutating this RRE greatly reduced the activity of an Rta-mediated luciferase reporter driven by the DcR3 regulatory region in the reporter assays (Fig. 6), demonstrating the importance of the RRE to the Rta-mediated DcR3 expression. Overexpression of the transcription coactivator CBP further enhanced Rta-dependent DcR3 expression (Fig. 7). Together, these results showed that Rta can bind to the DcR3 promoter and then recruit CBP to enhance DcR3 transcription. Although a PI3-K signaling pathway regulates endogenous DcR3 and Rta was shown to enhance PI3-K activity, we found that Rta-mediated PI3-K enhancement played a relatively minor role in Rta-mediated DcR3 expression (Fig. 8).
The presence of EBV DNA and the constant expression of EBV-encoded gene products strongly indicate that the virus likely has a role in the pathogenesis of human malignancies. Since DcR3 is overexpressed in various malignant tumor types and EBV is consistently associated with undifferentiated NPC (reviewed in reference 62), while the mRNA of BRLF1 is frequently expressed in NPC tumors (15, 16), it is possible that Rta-mediated DcR3 expression enhances tumor progression by immune suppression. We examined DcR3 expression in three NPC biopsy samples and found strong cytoplasmic DcR3 staining in cancerous epithelial cells in contrast to the weak staining in infiltrated lymphocytes or normal squamous epithelia (data not shown.).
DcR3 gene amplification and its expression level.
Several factors may be involved in determining the amount of DcR3 expression in cells, including the genome copy number, cellular signaling, and exogenous gene regulation, such as by viral genes. DcR3 genomic amplification was originally identified in lung and colon cancers (35). DcR3 was later found to be overexpressed in gastrointestinal tract tumors and lymphomas without gene amplification (4, 34). Our preliminary results found strong DcR3 expression in NPC biopsy specimens, suggesting a correlation of EBV infection and DcR3 expression in EBV-associated tumorigenesis. It will be interesting to find out if DcR3 gene overexpression and amplification are implicated in EBV-associated tumorigenesis, such as NPC.
Notably, P3HR1 cells and Akata cells express much less endogenous DcR3 than SW480 cells, based on the ELISA data (Fig. 1 and 2). Western analysis can readily detect cytoplasmic DcR3 in SW480 but is not sensitive enough to detect smaller amounts of DcR3 in P3HR1, Akata, or 293 cells. One possible reason for this discrepancy is that SW480 contains four or five DcR3 genomic copies (35). We do not know the copy number of the DcR3 gene in the other three cell lines at this point.
Rta enhances DcR3 expression by binding to the RRE.
Rta binds to EBV RREs and transactivates the expression of BMRF1 (36), BMLF1 (19), and BALF2 (23). Rta can also upregulate host and viral genes via Sp1-binding sites by forming complexes with Sp1 and MCAF1 in EBV-infected cells (7). We found putative Sp-1 binding sites in the DcR3 promoter region. However, it is unlikely that Rta-dependent DcR3 expression in our system is through the Sp-1 site, since deletion of Sp-1 sites had no effect on Rta-dependent DcR3 activity in the reporter assays (data not shown). Compare the DcR3 promoter RRE sequence, GCCCAGGCAGCCTGGTG, to that of the proposed optimal RRE, GTCCC/AT/CC/GNA/GNCA/GT/AGGCG; there are 4 nucleotides (underlined) that do not match the optimal sequence. However, our EMSA result (Fig. 4) demonstrated that Rta binds to the DcR3 promoter RRE with an affinity similar to that with which it binds to the BMLF1 RRE. Rta was shown to bind to BMLF1 RRE with high affinity (9). The close correlation between the binding affinity for Rta and the capacity of the RRE to confer a transcriptional response to Rta were shown previously (9). Therefore, it is quite possible that Rta can bind to the DcR3 promoter RRE, which has high affinity for Rta and enhances DcR3 expression in vivo.
Analyzing the DcR3 promoter region, we found putative recombination signal sequence binding protein Jκ (RBP-Jκ) (55, 56) and NF-κB binding sites. EBV EBNA2 activates transcription by both interfering with the function of a corepressor recruited by RBP-Jκ and providing its activation domain (55, 56). Our preliminary data showed that overexpression of EBNA2 alone did not enhance DcR3 expression (data not shown), suggesting that DcR3 may not be regulated by EBNA2 or by RBP-Jκ. LMP-1, another EBV latent gene, is known for its ability to activate NF-κB signaling (27). Whether LMP-1-mediated NF-κB activity regulates DcR3 expression is under investigation.
Tumor viruses and DcR3 expression.
DcR3 was found to be overexpressed in EBV-associated lymphomas and human T-cell lymphotropic virus type 1 (HTLV-1)-associated adult T-cell leukemia lymphoma. One of the possible reasons for increased DcR3 expression is that the DcR3 gene was amplified in these samples (34), yet it is also possible that EBV or HTLV-1 can further enhance DcR3 expression in these cells. Here, we demonstrated that EBV can enhance DcR3 through its transcription activator, Rta. It is not yet known if HTLV-1 plays any role on DcR3 expression in HTLV-1-associated adult T-cell leukemia. It will be important to determine if DcR3 expression levels are increased in other virus-associated malignancies, like hepatitis virus-associated hepatocellular carcinoma, and if any other viral genes regulate DcR3 in their associated tumors (47). The serum DcR3 concentration was found to be elevated in human immunodeficiency virus-infected patients (Y.-M. Chen, personal communication). Whether a human immunodeficiency virus gene regulates DcR3 expression remains unclear. Our preliminary data found that the Rta of Kaposi's sarcoma-associated herpesvirus, a virus implicated in Kaposi's sarcoma found in AIDS patients, did not enhance DcR3 expression in SW480 cells (data not shown).
EBV evades immune responses.
The suppression of systemic and local immune responses has been shown to play a role in tumor pathogenesis. Persistent EBV infection is characterized by stable numbers of latently infected B cells in the blood. The virus is continuously monitored by the immune system, as stable levels of cytotoxic T lymphocytes and serum antibodies to lytic and latent-stage proteins accompany persistent infection (53, 60). On the other hand, EBV develops multiple strategies to evade host immune responses in both latency and the lytic cycle. For example, an EBV latent antigen, EBNA1, blocks antigen presentation and inhibits self-synthesis (26, 30, 61). The EBV lytic gene BCRF1 encodes viral interleukin 10, a human interleukin 10 homologue, to subvert inflammatory responses (5, 45, 49, 64). EBV BHRF1 encodes a bcl-2 homologue that inhibits apoptosis (31). Recently, it was also found that Zta can downregulate TNFR type 1 expression and leads to protection of apoptosis induced by TNF-α (33). However, despite the examples mentioned, the viral strategies that prevent clearance and allow reactivation in the face of persistent immunity are still not well understood. It is possible that, among other strategies used by EBV to avoid the host immune response mentioned earlier, EBV uses DcR3 to escape immunomodulation during tumorigenesis.
It has become clear that humoral immunity alone is not sufficient to confer protection against EBV infection on the host. It has been demonstrated by many studies that HLA class I-restricted CD8+ cytotoxic-T-cell responses play a major role in the control of EBV infection by virtue of their ability to recognize viral antigens associated with B-cell transformation (for a review, see reference 42). People carrying latent EBV in their B cells not only maintain cytotoxic T cells directed against proteins made by the virus during the latent infection but also shed small quantities of infectious virus. How EBV infection avoids an active immune response is an important question. It is possible that by secreting DcR3, EBV-infected cells can reduce cytotoxic-T-lymphocyte cytotoxicity by reducing Fas ligand-Fas interaction.
In conclusion, this is the first report to demonstrate that Rta can upregulate a cellular gene by direct binding to the RRE located in the cellular promoter. Our data also suggest that Rta may contribute to viral survival and tumorigenesis by activating an immunomodulatory factor, DcR3, which prevents infected-cell detection by the host immune system.
Acknowledgments
We thank S.-T. Liu for EBV-infected 293 cell lines and Kenzo Takada for the Akata cell line. We thank T.-Y. Hsu for Rta expression plasmids and C.-H. Tsai for anti-EA-D antibody. We are indebted to Paul Lieberman and C.-H. Tsai for critical readings of the manuscript and helpful suggestions.
This work was supported by the National Science Council (NSC94-2320-B-010-004 and NSC95-2320-B-010-047), the Veterans General Hospitals University System of Taiwan Joint Research Program (VGHUST94-P7-43), and Taipei Veterans General Hospital (V95S5-008).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostopoulos, I., H. Herbst, G. Niedobitek, and H. Stein. 1989. Demonstration of monoclonal EBV genomes in Hodgkin's disease and Ki-1-positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood 74:810-816. [PubMed] [Google Scholar]
- 4.Bai, C., B. Connolly, M. L. Metzker, C. A. Hilliard, X. Liu, V. Sandig, A. Soderman, S. M. Galloway, Q. Liu, C. P. Austin, and C. T. Caskey. 2000. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc. Natl. Acad. Sci. USA 97:1230-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann, M., O. Gires, W. Kolch, H. Mischak, R. Zeidler, D. Pich, and W. Hammerschmidt. 2000. The PKC targeting protein RACK1 interacts with the Epstein-Barr virus activator protein BZLF1. Eur. J. Biochem. 267:3891-3901. [DOI] [PubMed] [Google Scholar]
- 6.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, L. K., J. Y. Chung, Y. R. Hong, T. Ichimura, M. Nakao, and S. T. Liu. 2005. Activation of Sp1-mediated transcription by Rta of Epstein-Barr virus via an interaction with MCAF1. Nucleic Acids Res. 33:6528-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., T. L. Hsu, H. H. Lin, C. C. Chio, A. W. Chiu, N. J. Chen, C. H. Lin, and S. L. Hsieh. 2004. Modulation of macrophage differentiation and activation by decoy receptor 3. J. Leukoc. Biol. 75:486-494. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. W., P. J. Chang, H. J. Delecluse, and G. Miller. 2005. Marked variation in response of consensus binding elements for the Rta protein of Epstein-Barr virus. J. Virol. 79:9635-9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien, Y. C., J. Y. Chen, M. Y. Liu, H. I. Yang, M. M. Hsu, C. J. Chen, and C. S. Yang. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 345:1877-1882. [DOI] [PubMed] [Google Scholar]
- 12.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decaussin, G., F. Sbih-Lammali, M. de Turenne-Tessier, A. Bouguermouh, and T. Ooka. 2000. Expression of BARF1 gene encoded by Epstein-Barr virus in nasopharyngeal carcinoma biopsies. Cancer Res. 60:5584-5588. [PubMed] [Google Scholar]
- 14.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, P., S. H. Chan, M. Y. Soo, D. Liu, M. Guan, E. C. Ren, and H. Hu. 2001. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma: a new serologic parameter for diagnosis. Cancer 92:1872-1880. [DOI] [PubMed] [Google Scholar]
- 16.Feng, P., E. C. Ren, D. Liu, S. H. Chan, and H. Hu. 2000. Expression of Epstein-Barr virus lytic gene BRLF1 in nasopharyngeal carcinoma: potential use in diagnosis. J. Gen. Virol. 81:2417-2423. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, Y., C. Sakakura, K. Shimomura, M. Nakanishi, R. Yasuoka, H. Aragane, A. Hagiwara, T. Abe, J. Inazawa, and H. Yamagishi. 2003. Chromosome arm 20q gains and other genomic alterations in esophageal squamous cell carcinoma, as analyzed by comparative genomic hybridization and fluorescence in situ hybridization. Hepatogastroenterology 50:1857-1863. [PubMed] [Google Scholar]
- 18.Gill, R. M., J. Ni, and J. S. Hunt. 2002. Differential expression of LIGHT and its receptors in human placental villi and amniochorion membranes. Am. J. Pathol. 161:2011-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruffat, H., N. Duran, M. Buisson, F. Wild, R. Buckland, and A. Sergeant. 1992. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J. Virol. 66:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutsch, D. E., K. B. Marcu, and S. C. Kenney. 1994. The Epstein-Barr virus BRLF1 gene product transactivates the murine and human c-myc promoters. Cell Mol. Biol. 40:747-760. [PubMed] [Google Scholar]
- 21.Hsu, T. L., Y. C. Chang, S. J. Chen, Y. J. Liu, A. W. Chiu, C. C. Chio, L. Chen, and S. L. Hsieh. 2002. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J. Immunol. 168:4846-4853. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, T. Y., Y. Chang, P. W. Wang, M. Y. Liu, M. R. Chen, J. Y. Chen, and C. H. Tsai. 2005. Reactivation of Epstein-Barr virus can be triggered by an Rta protein mutated at the nuclear localization signal. J. Gen. Virol. 86:317-322. [DOI] [PubMed] [Google Scholar]
- 23.Hung, C. H., and S. T. Liu. 1999. Characterization of the Epstein-Barr virus BALF2 promoter. J. Gen. Virol. 80:2747-2750. [DOI] [PubMed] [Google Scholar]
- 24.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 25.Komano, J., M. Sugiura, and K. Takada. 1998. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J. Virol. 72:9150-9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitskaya, J., M. Coram, V. Levitsky, S. Imreh, M. P. Steigerwald, G. Klein, M. G. Kurilla, and M. G. Masucci. 1995. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature 375:685-688. [DOI] [PubMed] [Google Scholar]
- 27.Li, H. P., and Y. S. Chang. 2003. Epstein-Barr virus latent membrane protein 1: structure and functions. J. Biomed. Sci. 10:490-504. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., J. Webster-Cyriaque, C. C. Tomlinson, M. Yohe, and S. Kenney. 2004. Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression. J. Virol. 78:4197-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, C., N. D. Sista, and J. S. Pagano. 1996. Activation of the Epstein-Barr virus DNA polymerase promoter by the BRLF1 immediate-early protein is mediated through USF and E2F. J. Virol. 70:2545-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manoury, B., and R. Fahraeus. 2004. Inhibition of synthesis and antigen presentation by protein EBNA1. Med. Sci. 20:5-6. [DOI] [PubMed] [Google Scholar]
- 31.Marshall, W. L., C. Yim, E. Gustafson, T. Graf, D. R. Sage, K. Hanify, L. Williams, J. Fingeroth, and R. W. Finberg. 1999. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J. Virol. 73:5181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migone, T. S., J. Zhang, X. Luo, L. Zhuang, C. Chen, B. Hu, J. S. Hong, J. W. Perry, S. F. Chen, J. X. Zhou, Y. H. Cho, S. Ullrich, P. Kanakaraj, J. Carrell, E. Boyd, H. S. Olsen, G. Hu, L. Pukac, D. Liu, J. Ni, S. Kim, R. Gentz, P. Feng, P. A. Moore, S. M. Ruben, and P. Wei. 2002. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16:479-492. [DOI] [PubMed] [Google Scholar]
- 33.Morrison, T. E., A. Mauser, A. Klingelhutz, and S. C. Kenney. 2004. Epstein-Barr virus immediate-early protein BZLF1 inhibits tumor necrosis factor alpha-induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J. Virol. 78:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohshima, K., S. Haraoka, M. Sugihara, J. Suzumiya, C. Kawasaki, M. Kanda, and M. Kikuchi. 2000. Amplification and expression of a decoy receptor for fas ligand (DcR3) in virus (EBV or HTLV-I) associated lymphomas. Cancer Lett. 160:89-97. [DOI] [PubMed] [Google Scholar]
- 35.Pitti, R. M., S. A. Marsters, D. A. Lawrence, M. Roy, F. C. Kischkel, P. Dowd, A. Huang, C. J. Donahue, S. W. Sherwood, D. T. Baldwin, P. J. Godowski, W. I. Wood, A. L. Gurney, K. J. Hillan, R. L. Cohen, A. D. Goddard, D. Botstein, and A. Ashkenazi. 1998. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396:699-703. [DOI] [PubMed] [Google Scholar]
- 36.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raab, T. N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 38.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragoczy, T., and G. Miller. 2001. Autostimulation of the Epstein-Barr virus BRLF1 promoter is mediated through consensus Sp1 and Sp3 binding sites. J. Virol. 75:5240-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragoczy, T., and G. Miller. 1999. Role of the Epstein-Barr virus RTA protein in activation of distinct classes of viral lytic cycle genes. J. Virol. 73:9858-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr Virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 42.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 43.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth, W., S. Isenmann, M. Nakamura, M. Platten, W. Wick, P. Kleihues, M. Bahr, H. Ohgaki, A. Ashkenazi, and M. Weller. 2001. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 61:2759-2765. [PubMed] [Google Scholar]
- 45.Salek-Ardakani, S., A. D. Stuart, J. E. Arrand, S. Lyons, J. R. Arrand, and M. Mackett. 2002. High level expression and purification of the Epstein-Barr virus encoded cytokine viral interleukin 10: efficient removal of endotoxin. Cytokine 17:1-13. [DOI] [PubMed] [Google Scholar]
- 46.Seto, E., L. Yang, J. Middeldorp, T. S. Sheen, J. Y. Chen, M. Fukayama, Y. Eizuru, T. Ooka, and K. Takada. 2005. Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J. Med. Virol. 76:82-88. [DOI] [PubMed] [Google Scholar]
- 47.Shen, H. W., S. L. Gao, Y. L. Wu, and S. Y. Peng. 2005. Overexpression of decoy receptor 3 in hepatocellular carcinoma and its association with resistance to Fas ligand-mediated apoptosis. World J. Gastroenterol. 11:5926-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart, J. P., and C. M. Rooney. 1992. The interleukin-10 homolog encoded by Epstein-Barr virus enhances the reactivation of virus-specific cytotoxic T cell and HLA-unrestricted killer cell responses. Virology 191:773-782. [DOI] [PubMed] [Google Scholar]
- 50.Swenson, J. J., E. Holley-Guthrie, and S. C. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 interacts with CBP, promoting enhanced BRLF1 transactivation. J. Virol. 75:6228-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahama, Y., Y. Yamada, K. Emoto, H. Fujimoto, T. Takayama, M. Ueno, H. Uchida, S. Hirao, T. Mizuno, and Y. Nakajima. 2002. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer 5:61-68. [DOI] [PubMed] [Google Scholar]
- 53.Tan, L. C., N. Gudgeon, N. E. Annels, P. Hansasuta, C. A. O'Callaghan, S. Rowland-Jones, A. J. McMichael, A. B. Rickinson, and M. F. Callan. 1999. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J. Immunol. 162:1827-1835. [PubMed] [Google Scholar]
- 54.Tsuji, S., R. Hosotani, S. Yonehara, T. Masui, S. S. Tulachan, S. Nakajima, H. Kobayashi, M. Koizumi, E. Toyoda, D. Ito, K. Kami, T. Mori, K. Fujimoto, R. Doi, and M. Imamura. 2003. Endogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinoma. Int. J. Cancer 106:17-25. [DOI] [PubMed] [Google Scholar]
- 55.Waltzer, L., P. Y. Bourillot, A. Sergeant, and E. Manet. 1995. RBP-J kappa repression activity is mediated by a co-repressor and antagonized by the Epstein-Barr virus transcription factor EBNA2. Nucleic Acids Res. 23:4939-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waltzer, L., F. Logeat, C. Brou, A. Israel, A. Sergeant, and E. Manet. 1994. The human J kappa recombination signal sequence binding protein (RBP-J kappa) targets the Epstein-Barr virus EBNA2 protein to its DNA responsive elements. EMBO J. 13:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan, X., G. Shi, M. Semenuk, J. Zhang, and J. Wu. 2003. DcR3/TR6 modulates immune cell interactions. J. Cell Biochem. 89:603-612. [DOI] [PubMed] [Google Scholar]
- 58.Yang, C. R., S. L. Hsieh, F. M. Ho, and W. W. Lin. 2005. Decoy receptor 3 increases monocyte adhesion to endothelial cells via NF-kappa B-dependent up-regulation of intercellular adhesion molecule-1, VCAM-1, and IL-8 expression. J. Immunol. 174:1647-1656. [DOI] [PubMed] [Google Scholar]
- 59.Yang, C. R., S. L. Hsieh, C. M. Teng, F. M. Ho, W. L. Su, and W. W. Lin. 2004. Soluble decoy receptor 3 induces angiogenesis by neutralization of TL1A, a cytokine belonging to tumor necrosis factor superfamily and exhibiting angiostatic action. Cancer Res. 64:1122-1129. [DOI] [PubMed] [Google Scholar]
- 60.Yao, Q. Y., A. B. Rickinson, and M. A. Epstein. 1985. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int. J. Cancer 35:35-42. [DOI] [PubMed] [Google Scholar]
- 61.Yin, Y., B. Manoury, and R. Fahraeus. 2003. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science 301:1371-1374. [DOI] [PubMed] [Google Scholar]
- 62.Young, L. S., and P. G. Murray. 2003. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene 22:5108-5121. [DOI] [PubMed] [Google Scholar]
- 63.Yu, K. Y., B. Kwon, J. Ni, Y. Zhai, R. Ebner, and B. S. Kwon. 1999. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J. Biol. Chem. 274:13733-13736. [DOI] [PubMed] [Google Scholar]
- 64.Zeidler, R., G. Eissner, P. Meissner, S. Uebel, R. Tampe, S. Lazis, and W. Hammerschmidt. 1997. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood 90:2390-2397. [PubMed] [Google Scholar]