Abstract
Cytomegalovirus (CMV), the major viral cause of congenital disease, infects the uterus and developing placenta and spreads to the fetus throughout gestation. Virus replicates in invasive cytotrophoblasts in the decidua, and maternal immunoglobulin G (IgG)-CMV virion complexes, which are transcytosed by the neonatal Fc receptor across syncytiotrophoblasts, infect underlying cytotrophoblasts in chorionic villi. Immunity is central to protection of the placenta-fetal unit: infection can occur when IgG has a low neutralizing titer. Here we used immunohistochemical and function-blocking methods to correlate infection in the placenta with expression of potential CMV receptors in situ and in vitro. In placental villi, syncytiotrophoblasts express the virion receptor epidermal growth factor receptor (EGFR) but lack integrin coreceptors, and virion uptake occurs without replication. Focal infection can occur when transcytosed virions reach EGFR-expressing cytotrophoblasts that selectively initiate expression of αV integrin. In cell columns, proximal cytotrophoblasts lack receptors and distal cells express integrins α1β1 and αVβ3, enabling virion attachment. In the decidua, invasive cytotrophoblasts expressing coreceptors upregulate EGFR, thereby dramatically increasing susceptibility to infection. Our findings indicate that virion interactions with cytotrophoblasts expressing receptors in the placenta (i) change as the cells differentiate and (ii) correlate with spatially distinct sites of CMV replication in maternal and fetal compartments.
Human cytomegalovirus (CMV) is the leading cause of congenital viral infection in children, with an incidence in the United States of about 1 to 3% of live births. Primary CMV infection during gestation poses a 40 to 50% risk of intrauterine transmission (5), whereas reactivated infection in seropositive women rarely causes symptomatic disease, highlighting the role of immunity in fetal protection (16). Symptomatic infants have intrauterine growth restriction, and most survivors (28%) have permanent sequelae, including neurological defects, mental retardation, retinopathy, and sensorineuronal deafness (12). Although virus transmission can occur throughout pregnancy, congenital disease is more severe when primary infection takes place during early gestation (54). Intrauterine growth restriction and loss of the fetus without virus transmission, which are associated with congenital CMV infection, originate in placental pathology (3, 21).
Placentation is a stepwise process whereby specialized cytotrophoblast progenitor cells leave the basement membrane to initiate blood flow, differentiating along two pathways depending on their location (Fig. 1). In floating villi, cells fuse to form a multinucleate syncytial covering attached at one end to the tree-like fetal portion of the placenta. Covered by syncytiotrophoblasts, these villi float in a stream of maternal blood, a source of nutrients and immunoglobulin G (IgG) transported to the fetus. In anchoring villi, cytotrophoblasts switch from an epithelial to an endothelial phenotype controlled through the coordinated actions of numerous interrelated factors (17, 26, 63). The cells express adhesion molecules—integrins, Ig superfamily members, and proteinases that enable invasiveness—and immune-modulating factors for maternal tolerance of the hemiallogeneic fetus (8, 9, 41). Villus cytotrophoblasts express integrin subunits β4, β5, and β6 (63), whereas interstitial invasive cells upregulate expression of integrin α1β1 (11). Endovascular cytotrophoblasts express αVβ3 and vasculogenic factors and receptors, including VE (endothelial)-cadherin and vascular endothelial adhesion molecule 1, that mimic the surface of vascular cells (9, 63). Invasive cytotrophoblasts upregulate matrix metalloproteinase 9, which degrades the extracellular matrix of the uterine stroma (31), and the nonclassical major histocompatibility complex class Ib molecule HLA-G (30, 38) and interleukin-10 for immune tolerance and modulation of metalloproteinases and invasiveness (49, 50).
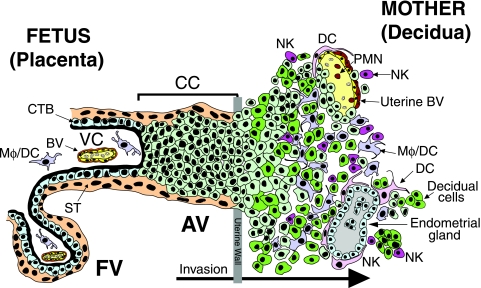
FIG. 1.
Diagram of the placental (fetal)-decidual (maternal) interface near the end of the first trimester of human pregnancy (10 weeks gestational age). A longitudinal section includes floating and anchoring chorionic villi. The floating villus (FV) is bathed by maternal blood. The anchoring villus (AV) functions as a bridge between the fetal and maternal compartments. Cytotrophoblasts in the AV form cell columns that attach to the uterine wall. They invade the decidua and uterine vasculature, anchoring the placenta and gaining access to the maternal circulation. Colors illustrate different cell types: beige, syncytiotrophoblasts (ST); light green, cytotrophoblast (CTB) progenitors and invasive cells; green, decidual cells; yellow, endothelial cells; brown, smooth muscle cells; gray, glandular epithelial cells; light purple/pink, macrophage/dendritic cells (Mφ/DC); red, polymorphonuclear neutrophils (PMN); fuchsia, natural killer cells (NK).
Our studies on intrauterine CMV infection have revealed patterns of replication in the decidua, mirrored in the placenta and dependent in part on maternal immune responses (15, 44). In early gestation, the neonatal Fc receptor transcytoses IgG and some immune complexes of virions across syncytiotrophoblasts that contain CMV glycoprotein B (gB) in caveolae without infection (33). With low neutralizing antibodies, CMV replication proteins are found in decidual and placental compartments. With moderate neutralizing titers, cytotrophoblast infection is reduced in the decidua, and occasional focal infection is found in the placenta. With high neutralizing titers, few cytotrophoblasts stain for viral replication proteins in the decidua, and none stain in the placenta. In syncytiotrophoblasts, the neonatal Fc receptor transcytoses complexes of maternal IgG and CMV virions that infect underlying cytotrophoblasts (33). Consistent patterns of CMV replication in cytotrophoblast progenitor cells in floating villi and invasive cells in the uterine decidua suggest that viral receptors are regulated during development. In vitro studies from other groups reported that viral entry and replication in human fibroblasts require expression of the epidermal growth factor receptor (EGFR) (60) and coreceptors such as integrins αVβ3, α2β1, and α6β1 (14, 59). Inasmuch as CMV glycoproteins gB and gH bind different receptors—EGFR and integrin β1 (gB) and αVβ3 integrin (gH)—virion internalization activates independent signaling pathways (14, 59). Currently, receptors that mediate productive CMV infection in vivo are unknown.
In the present study, we examined the role of potential receptors for CMV virion entry and replication in placentas naturally infected in utero. Receptor expression in cytotrophoblasts was modeled in villus explants ex vivo, and functions were evaluated in differentiating/invading cells infected in vitro. Our results show that cytotrophoblast progenitor cells in chorionic villi and differentiating/invading cells upregulate functional receptors. In contrast, syncytiotrophoblasts and extravillous cytotrophoblasts in cell columns that largely escape infection lack these proteins. Use of different receptors in spatially distant sites suggests that CMV subverts cytotrophoblast development in the maternal and fetal compartments.
MATERIALS AND METHODS
Tissue biopsies and villus explant cultures.
Approval for this project was obtained from the Institutional Review Board of the University of California, San Francisco. First- and second-trimester placentas were obtained from women with normal pregnancies prior to elective termination for nonmedical reasons. Biopsy specimens were analyzed from 45 first-trimester placentas, and villus explant cultures were established from 22 of them. Chorionic villi were dissected from individual placentas as previously described (18). Dissected villi (wet weight, 5 to 10 mg) were cultured on 12-mm (0.4-μm pore) Millicell-CM inserts (Millipore Inc., Bedford, MA) coated with 100 μl of Matrigel (BD Discovery Lab Inc., Bedford, MA) in Dulbecco's modified Eagle's medium-F-12 culture medium (Gibco BRL, Indianapolis, IN) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). Neutralizing titers of IgG purified from conditioned medium of villus explants were quantified as previously reported (37).
Cytotrophoblast isolation.
Cytotrophoblasts were isolated from 10- to 16-week placentas as previously described (31). Purified cells were resuspended in Dulbecco's modified Eagle's medium containing 2% Nutridoma (Roche Diagnostics, Indianapolis, IN) to obtain a density of 106 cells/ml and plated on 6.5-mm Transwell filter inserts with a 5-μm pore size (Corning Inc., Corning, NY) coated with 10 μl of Matrigel. After 12 h, cultures were fixed and stained with rat monoclonal antibody to cytokeratin (an epithelial cell protein) (10), rabbit IgG to von Willebrand factor (an endothelial cell marker), mouse monoclonal antibody (MAb) to vimentin (a fibroblast protein), and mouse MAb CH160 to CMV immediate-early proteins 1 and 2 (IE1 and -2), a marker of viral replication (13). Staining patterns indicated the purification level of isolated cytotrophoblasts, which were used for experiments only when 95 to 99% of cells were positive for cytokeratin. Failure of antibodies to CMV replication proteins to immunostain cytotrophoblasts indicated that cells were uninfected, as reported previously (15, 33, 44, 55). Cytotrophoblasts plated on Matrigel were a replica for differentiating/invading cells that switched their integrin phenotype.
Stock virus and infection.
The genetic content of the clinical viral strain Toledo (6) has been published. Construction and properties of the recombinant virus (Toledorec), which expresses the gene that encodes enhanced green fluorescent protein, have also been published (61). Villus explants were infected with Toledo and Toledorec stock virus (5 × 105 PFU) in serum-free medium. Explants were washed with phosphate-buffered saline (PBS), and fresh culture medium supplemented with 10% fetal bovine serum was added. Cytotrophoblasts from 12-h cultures were infected with CMV strains AD169, Toledo, and Toledorec (5 PFU/cell) in serum-free medium. For virion binding assays, cytotrophoblasts were washed with ice-cold PBS and incubated with filtered (0.45-μm-pore-size) Toledorec-green fluorescent protein at 4°C for 1 h. Cells were fixed, and virion aggregates were visualized by laser confocal microscopy. Binding of Toledorec virions (green) appeared as punctate fluorescence signal comparable to immunostaining of the virion tegument protein pp65 (data not shown). Upon productive replication of Toledorec, infected cells showed intense nuclear and cytoplasmic fluorescence (33, 61).
Serological reagents.
Mouse monoclonal antibodies to CMV-infected-cell proteins were produced in the Pereira laboratory. Pooled antibodies to infected-cell proteins included antibodies to gB (46), gH (CH438, UL75), alkaline nuclease (CH19, UL98/UL99), pp65 (CH65, UL83), IE1 and -2 (CH160, UL122/123), and ICP22 (CH41, US22). Guinea pig antiserum to gB was a gift from Chiron Corp. (Emeryville, CA). Rat anti-human cytokeratin antibody (7D3) was generated in Susan Fisher's laboratory (10). Rabbit IgG to von Willebrand factor was purchased from Novocastra Laboratories Ltd. (Newcastle upon Tyne, United Kingdom), and mouse MAb to vimentin was obtained from Sigma-Aldrich Co. (St. Louis, MO). Function-blocking and immunostaining mouse anti-human antibodies to integrins α1, αVβ3, and α5 were obtained from Chemicon International (Temecula, CA), and antibodies to integrin β1 (AIIB2) were obtained from the Developmental Studies Hybridoma Bank, University of Iowa. Antibodies to EGFR, caveolin-1, and species-specific isotype control IgG were purchased from BD Biosciences (San Diego, CA). Secondary antibodies were goat anti-mouse IgG F(ab′) fragments labeled with fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC), goat anti-rat IgG F(ab′) fragments labeled with TRITC, and goat anti-guinea pig IgG F(ab′) fragments labeled with FITC or TRITC. Nuclei were counterstained with TO-PRO-3 or YO-YO-1 iodides (Molecular Probes, Eugene, OR).
Immunofluorescence assays.
Villus explants, placental biopsy specimens, and isolated cytotrophoblasts were processed for immunohistochemistry as described elsewhere (15). For double labeling, cells were simultaneously incubated with primary antibodies from various species and with secondary antibodies labeled with FITC or TRITC. Tissue sections and cells were analyzed with a Bio-Rad MRC1024 confocal OptiPhot II Nikon microscope using Comos software. Data analysis was performed using NIH Image and Adobe Photoshop software.
Inhibition of CMV infection by antibodies to viral receptors and soluble integrins.
After cytotrophoblasts were isolated, the cells were cultured for 12 h to restore expression of cell surface receptors. Cells were fixed with 3% paraformaldehyde and coimmunostained for cytokeratin, EGFR, and integrins α1, α5, β1, and αVβ3 to verify surface expression of receptors. Function inhibition experiments were performed with CMV strains AD169 and Toledo. First, infection was inhibited by preincubating cytotrophoblasts with antibodies to potential receptors. Cells were washed with ice-cold PBS and preincubated on ice (40 min) with the function-blocking antibodies (Chemicon International Inc.) to integrins α5, α1, β1, αVβ3, and EGFR. Control infected cells were incubated with mouse IgG. Antibodies (20, 40, and 80 μg/ml) were added to serum-free medium. After treatment, the cells were washed with ice-cold PBS and infected (5 PFU/cell) in serum-free medium for 40 min on ice. Cells were washed with ice-cold PBS to remove unbound virions, and warm (37°C) culture medium was added. After 24-h incubation at 37°C, cells were fixed and immunostained for CMV IE1 and -2 proteins and cytokeratin. The second means of inhibiting infection was by preincubating virions with soluble integrins. Virions used as controls were incubated with medium alone. Virus was filtered through a 0.45-μm membrane, incubated at 37°C for 1 h with soluble integrins (10, 20, and 40 μg/ml), and then used to infect cytotrophoblasts. At 24 h after infection, cells were fixed and immunostained for CMV IE1 and -2 proteins and cytokeratin. Nuclei were counterstained with TO-PRO-3. CMV infection was determined by counting the IE1- and -2-positive nuclei to calculate the numbers of them per 200 cytokeratin-positive cytotrophoblasts in 10 to 30 fields. Independent experiments were performed using cytotrophoblasts isolated from three placentas.
Fluorescence in situ hybridization and electron microscopy.
Placental biopsy tissues were processed, fixed, and embedded as described previously (15). Sections (5 to 7 μm) were cut on a Hacker-Slee cryostat and collected on silane-coated glass slides. DNA-DNA hybridization was performed using the DNA Detector FISH kit (KPL, Inc., Gaithersburg, MD), and a CMV DNA probe was labeled with biotin-16-dUTP using a nick-translation kit (Roche Diagnostics Inc.) as described elsewhere (33). For electron microscopy, placental biopsy specimens were fixed in 1.5% Karnovsky medium and postfixed in 1% Palade buffer, dehydrated, and embedded. Sections were stained in uranyl acetate and lead citrate and examined with a JEM-1200EX electron microscope.
RESULTS
Infection patterns in floating villi correlate with CMV receptors expressed in cytotrophoblast progenitor cells.
In the first series of experiments, we correlated the presence of virion proteins and DNA in chorionic villi with expression of potential receptors in placentas naturally infected with CMV in utero. Immunohistochemical analysis of infected-cell proteins in early gestation placentas (15, 44) showed focal replication in underlying cytotrophoblast progenitor cells, as illustrated in Fig. 2a. Placentas were examined for the presence of CMV virions, replication proteins, and cellular proteins that could serve as receptors (Fig. 2b to e). Placental biopsy specimens were fixed and processed immediately after tissue was obtained. Sections were immunostained with a pool of monoclonal antibodies to CMV-infected cell proteins that indicated viral replication (nuclear and cytoplasmic staining) or with guinea pig antiserum to gB, an abundant virion envelope glycoprotein (punctate vesicular staining). In samples from immune donors, we repeatedly detected CMV gB in a punctate staining pattern (red) adjacent to apical microvilli of syncytiotrophoblasts (Fig. 2b). Villous core macrophages internalized virions, as indicated by CMV gB and CD68 staining (data not shown). We did not detect viral replication proteins. Notably, syncytiotrophoblasts expressed EGFR in apical microvilli (Fig. 2b), but neither α1β1 nor αV integrin was detected (data not shown). Occasionally, gB (red) was found in a punctate pattern in cytotrophoblasts intensely reactive with antibodies to EGFR (green) (Fig. 2b). A cluster of underlying cytotrophoblasts also expressed αV integrin (red), and punctate gB (green) was found (Fig. 2c), but α1β1 was not detected (data not shown). Frequently, virion capsids were found clustered near the microvillus surface of syncytiotrophoblasts (Fig. 2d), and CMV DNA was detected by in situ hybridization in a punctate pattern (red) in syncytiotrophoblasts and cytotrophoblasts (Fig. 2e). These results were obtained by analyzing 17 first-trimester placentas. The findings confirmed our earlier observations that CMV capsids, DNA, and gB were often present in syncytiotrophoblasts, without replication, in placentas that contained high-titer neutralizing antibodies (37, 44). The results showed that cytotrophoblasts expressing potential CMV receptors, EGFR and αV integrin, also contain virion gB and DNA in an intense punctate staining pattern.
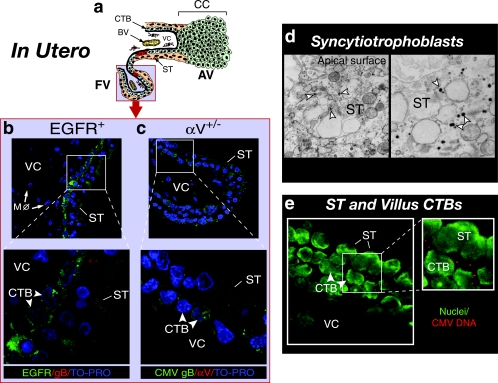
FIG. 2.
Uptake of CMV virions in villus trophoblasts expressing EGFR and αV integrin infected in utero. (a) Diagram of virion proteins and focal infection (red) in trophoblasts of a floating villus. AV, anchoring villi; FV, floating villi; BV, blood vessel; CC, cell column. The light blue field represents the localization of virions in panels b, c, d, and e. The insets in panels b, c, and e correspond to the outlined areas in each panel. (b) CMV gB (red) in syncytiotrophoblasts (ST) and progenitor cytotrophoblasts (CTB) that express EGFR (green) and in villus core (VC) macrophages (Mφ) that sequester gB. (c) CMV gB (green) detected in progenitor cytotrophoblasts reactive with antibodies to integrin αV (red). (d) Virion nucleocapsids (white arrowheads) in syncytiotrophoblasts. Magnification, ×4,000 (left panel) and ×10,000 (right panel). (e) CMV DNA (red) in cytoplasm of syncytiotrophoblasts and select cytotrophoblasts. Nuclei were counterstained with TO-PRO-3 iodide (blue) and YO-YO-1 iodide (green).
We next examined potential receptors expressed in cytotrophoblasts of villus explants infected with Toledorec ex vivo. The villus explant model allowed examination of CMV virion proteins and replication patterns in syncytiotrophoblasts and underlying cytotrophoblast progenitors (floating villi) (Fig. 3a) (33) and extravillous cytotrophoblasts that formed cell columns (anchoring villi) (see Fig. 5a) (18, 31). Virions attached in a punctate (green) pattern to apical microvilli of syncytiotrophoblasts, and some internalized but did not replicate (Fig. 3b). Some adjacent cytotrophoblasts expressed αV integrin (Fig. 3b). When infection was detected in villus cytotrophoblasts, as indicated by the nuclear (green) staining pattern, the cells stained intensely for EGFR (Fig. 3c). However, α1β1 integrin was absent (not shown), which confirmed earlier reports (10, 63). Triple immunostaining showed that Toledorec replicated in cytotrophoblasts that expressed both αV integrin (red) and EGFR (blue) (Fig. 3d). Together these results indicated that CMV virions internalize in syncytiotrophoblasts with EGFR alone. Inasmuch as more than half of our donors were immune to CMV, and neutralizing antibodies are secreted into conditioned medium from villus explants (33), viral replication infrequently occurred (7 of 42 placentas). When placentas contained IgG-CMV virion complexes with low-avidity antibodies, focal replication occurred in susceptible cytotrophoblast progenitor cells that expressed both EGFR and αV integrin, offering an explanation for our earlier observations (33, 44).
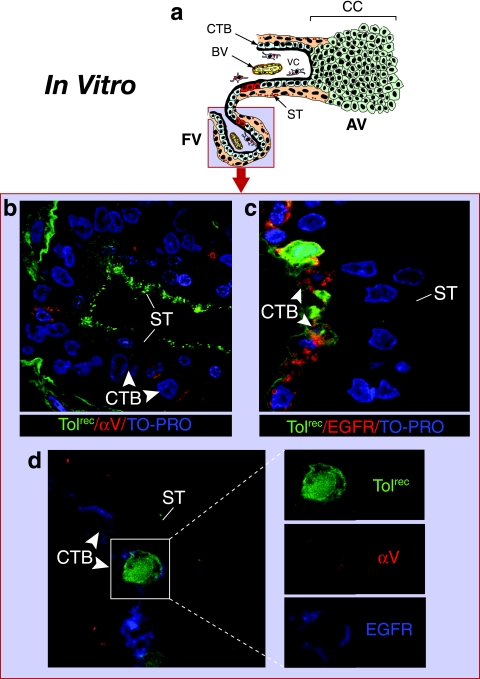
FIG. 3.
CMV replicates in cytotrophoblasts expressing EGFR and αV integrin in villus explants infected in vitro. (a) Diagram showing focal infection in a floating villus. The light blue field represents the localization of CMV structural and replication proteins in panels b and c. Explants were fixed at 24 h postinfection. (b) Toledorec virions bound to apical microvilli of syncytiotrophoblasts (ST). Selected cytotrophoblasts (CTB), but not syncytiotrophoblasts, reacted with antibody to αV integrin (red). (c) Underlying cytotrophoblasts stained intensely with EGFR-specific antibody (red); replicating Toledorec broadly expressed green fluorescent protein in nuclei of infected cells. Nuclei were counterstained with TO-PRO-3 iodide (blue). (d) Villus cytotrophoblasts infected with Toledorec simultaneously expressed αV integrin (red) and EGFR (blue).
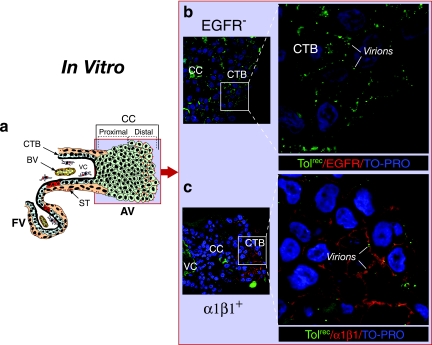
FIG. 5.
CMV virions bind to cytotrophoblast outgrowths in a model of anchoring villus development in vitro. (a) Diagram of an anchoring villus cell column. The light blue field represents the localization of virions in panels b and c. The insets in panels b and c correspond to the outlined areas in each panel. Explants were fixed at 24 h postinfection. (b) Cell column cytotrophoblasts did not react with antibodies to EGFR (red). (c) Toledorec virions (green) bind cytotrophoblasts expressing α1β1 integrin (red) on the edge of a distal cell column. Nuclei were counterstained with TO-PRO-3 iodide (blue).
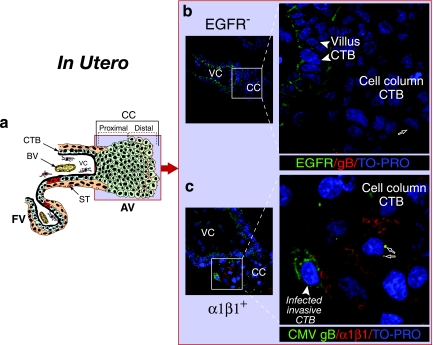
Failure to detect CMV replication in cell column cytotrophoblasts.
In this series of experiments, we examined CMV infection patterns in cell column cytotrophoblasts in placentas naturally infected in utero. As illustrated in Fig. 4a, cytotrophoblasts in cell columns of anchoring villi are most often spared. When early gestation biopsy specimens were stained for potential CMV receptors, proximal cell columns did not express EGFR (green), and punctate staining for virion gB (red) was sometimes detected (Fig. 4b). Interestingly, invasive cytotrophoblasts still adhering to the cell column expressed α1β1 integrin (red) (Fig. 4c). Punctate staining for CMV gB (green) suggested that virions were clustered in membranes (arrows). One cell in this field showed broad cytoplasmic gB staining, which indicated late productive infection. Viral replication in invasive cytotrophoblasts was found in 3 of 10 samples when portions of the decidua were retained. Of 73 placentas analyzed, viral replication proteins were detected in residual pieces of cell columns in 2 highly infected biopsy specimens from donors with primary CMV infection indicated by extremely low or no neutralizing antibodies (44).
FIG. 4.
Cytotrophoblasts in proximal cell columns lack CMV receptors and are uninfected in utero. (a) Diagram of an anchoring villus cell column. The light blue field represents the localization of viral proteins in panels b and c. The insets in panels b and c correspond to the outlined areas in each panel. (b) EGFR (green) staining in villus cytotrophoblast progenitor cells. Cytotrophoblasts within cell columns do not express EGFR. (c) CMV virion gB stained with guinea pig antiserum (green; see black arrows) clusters in membranes of cytotrophoblasts intensely reactive with integrin α1β1-specific antibody (red). One invasive cytotrophoblast shows a broad cytoplasmic gB staining pattern indicating late-stage viral replication. Nuclei were counterstained with TO-PRO-3 iodide (blue).
Next, we examined CMV receptors expressed on cytotrophoblasts in villus explants in model cell columns of anchoring villi infected with Toledorec ex vivo. Like cell columns in intact placentas, extravillous cytotrophoblasts in these outgrowths did not express EGFR (red) (Fig. 5b). Virion attachment was observed as punctate (green) staining in membranes of distal cell columns where potential integrin receptors αV and α1β1 were expressed. Cytotrophoblasts on the edge of distal portions of cell columns upregulated α1β1 (red), and Toledorec virions were bound to the cell membranes (Fig. 5c). αV integrin was also present (data not shown). We failed to detect CMV replication proteins in cytotrophoblast outgrowths of 42 explants infected in vitro for 48 to 96 h. Together the results suggest that cytotrophoblast expression of integrin coreceptors without EGFR coincides with CMV virion binding but is not sufficient for viral entry and replication.
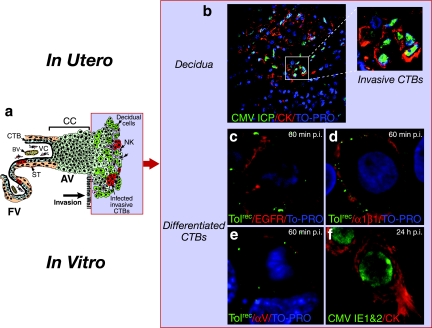
CMV replicates in differentiating/invading cytotrophoblasts that switch their integrin phenotype.
Immunofluorescence analysis of first and second trimester placentas from women with moderate CMV neutralizing titers showed that interstitial cytotrophoblasts that invade the decidua are infected in utero (44). In addition, CMV replicates in cytotrophoblasts from early gestation and term placentas plated on Matrigel in vitro (15, 23, 24, 34, 55). As cytotrophoblasts differentiate, integrins α1β1 and αV are upregulated as the cells progress along the invasive pathway (11, 63). The site of CMV-infected invasive cytotrophoblasts in utero is illustrated in Fig. 6a. Analysis of early gestation decidua from a placenta with low neutralizing titers showed that infected interstitial cytotrophoblasts express viral replication proteins (Fig. 6b). We used cell culture of differentiated cytotrophoblasts to monitor these cells for the expression of potential receptors, binding of Toledorec virions and productive infection in vitro. Virions (punctate green) adsorbed to membranes of cells expressing EGFR (Fig. 6c) and integrins α1β1 (Fig. 6d) and αV (Fig. 6e) at 1 h after infection. In addition, CMV IE1- and -2 proteins were detected in nuclei of Toledo-infected cells at 24 h, suggesting early-stage viral replication (Fig. 6f). These results showed that virion attachment and infection of invasive cytotrophoblasts occur in vitro in cells that express CMV receptors, EGFR and integrins αV and α1β1.
FIG. 6.
CMV replicates in invasive cytotrophoblasts that express virion receptors. (a) Diagram of differentiating/invading cytotrophoblasts at the uterine-placental interface. The light blue field represents the localization of viral proteins in panels b to f. (b) Invasive cells in decidua infected in utero. The inset corresponds to the outlined area in the panel. (c to e) Toledorec virions (green) bind to cytotrophoblast membranes with EGFR (c), integrin α1β1 (d), and integrin αV (e) (red) at 1 h after infection. (f) IE1 and -2 proteins (green) in cytokeratin (CK)-stained cells (red) at 24 h after infection. Nuclei were counterstained with TO-PRO-3 iodide (blue).
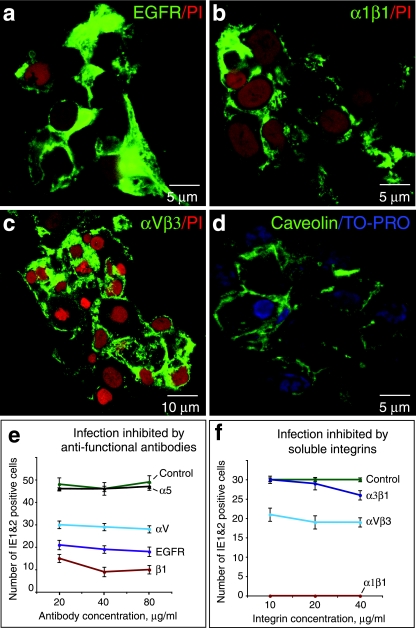
CMV infection of differentiating/invading cytotrophoblasts is reduced by function-perturbing antibodies and soluble integrins.
Immunohistological analysis indicated that expression of developmentally regulated proteins correlates with patterns of CMV infection in utero and in vitro. Using function-perturbing antibodies and soluble integrins, we next evaluated the role of molecules that function as potential receptors in differentiating/invasive cytotrophoblasts in vitro. Intense expression of EGFR and integrins α1β1 and αVβ3 was found as cytotrophoblasts differentiated (Fig. 7a to c). These invasive cells also expressed caveolin-1, as we reported previously for syncytiotrophoblasts and villus cytotrophoblasts where CMV virion gB was localized (33) (Fig. 7d).
FIG. 7.
Function-blocking antibodies and soluble forms of integrins reduce CMV infection of differentiating/invading cytotrophoblasts in vitro. (a to d) Antibodies to EGFR and integrins α1β1, αVβ3, and caveolin (green) immunostain purified cytotrophoblasts after 12 h on Matrigel. Nuclei were counterstained with propidium iodide (PI) (red) or TO-PRO (blue). (e) Preincubation of cytotrophoblasts with function-blocking antibodies to EGFR and integrins. (f) Pretreatment of virions with soluble integrins. The level of CMV replication was quantified in infected cells that express IE1 and -2 proteins.
In the first type of experiment, cytotrophoblasts were incubated with function-blocking antibodies to CMV receptors. Interestingly, we found that infection was reduced to various degrees. Antibody to EGFR blocked 63% of infectivity, whereas antibody to integrin β1 blocked 80% of infectivity and antibody to integrin αV blocked 43% of infectivity (Fig. 7e). Function-blocking antibody to α5 integrin and isotype controls failed to reduce viral replication. Comparable inhibition of infection by increasing concentrations of function-blocking antibodies was found in four experiments using cytotrophoblasts purified from different placentas.
In the second type of experiment, CMV virions were pretreated with soluble forms of specific integrins before infection. When virions were incubated with integrin α1β1 (10 to 40 μg/ml), 100% of virion infectivity was blocked (Fig. 7f). A lower concentration of soluble integrin α1β1 (2.5 μg/ml) partially blocked infectivity (data not shown). Pretreatment with integrin αVβ3 reduced infectivity by approximately 37%, and pretreatment with soluble integrin α3β1 reduced infectivity by 13%. Similar levels of inhibition by soluble integrins were obtained in four experiments. Together the results of function-perturbing experiments confirmed that EGFR and integrins α1β1 and αVβ3—developmentally regulated molecules—function as CMV receptors as cytotrophoblasts progress along the differentiation pathway.
DISCUSSION
Analysis of biopsy specimens from early gestation placentas shows that patterns of CMV replication depend on neutralizing antibody titers and coinfection with other pathogens (33, 37, 44). Whether titers are low, moderate, or high, the topography of virus replication is consistent. CMV infects the uterine vasculature, and virions spread to invasive cytotrophoblasts in the interstitium; in the form of immune complexes, virions spread to cytotrophoblasts underlying syncytiotrophoblasts in floating and anchoring villi (15, 34, 36, 44). Flow cytometric analysis of isolated cytotrophoblasts shows that certain virion receptors—EGFR and integrins α1β1 and αVβ3—are expressed (55). Here we determined that CMV infection of villus explants and purified cytotrophoblasts in vitro depends on functional receptors expressed in distinct populations of progenitor and differentiating cells, similar to virion receptors expressed on placentas naturally infected in utero.
Susceptibility to infection changes as cytotrophoblasts proceed along the differentiation pathway (Fig. 1), upregulate stage-specific proteins, and migrate from the fetal to the maternal compartment (Table 1). On the placental (fetal) side, syncytiotrophoblasts express EGFR but lack coreceptors αVβ3 and α1β1 integrin (2, 11, 51, 63). Nonetheless, immune complexes are internalized and transcytosed by neonatal Fc receptor that binds maternal IgG (33, 52). Selected underlying progenitor cells expressing EGFR become permissive upon initiation of integrin αV expression (Fig. 3). Consequently, focal infection results from coordinated events: few IgG-virion complexes with low neutralizing titers that are internalized by syncytiotrophoblasts are transcytosed to sites where cytotrophoblasts express functional coreceptors (15, 33, 44). Dividing cytotrophoblasts in proximal cell columns without virion receptors were not infected except in profoundly affected cases of first trimester primary congenital infection without maternal neutralizing antibodies. In distal cell columns, cytotrophoblasts adhere and roll over each other, facilitating movement from the placental compartment into the uterine wall (62). Here virions bind to integrin α1β1-expressing cells but do not penetrate (Fig. 5). On the uterine (maternal) side, interstitial cytotrophoblasts express EGFR and integrins α1β1 and αVβ3 as cells invade the interstitium and become highly permissive (Fig. 6; Table 1). Together, the results suggest that spatial and developmental regulation of CMV receptors is a key determinant of infection in distinct cytotrophoblast populations.
TABLE 1.
Sites of human CMV infection correlate with virion receptors expressed in the developing placenta
| Site of infection | Stage-specific protein(s)a | Infection patternb |
|---|---|---|
| Fetal (placental) | ||
| Syncytiotrophoblasts | EGFR, FcRn | IgG-virion uptake and transcytosis |
| Floating villus cytotrophoblasts | EGFR, αVβ6 | Focal infection |
| Anchoring villus | ||
| Proximal cell column | None | None |
| Distal cell column | α1β1, αV | Virion binding |
| Maternal (uterine) interstitial/endovascular invasive cytotrophoblasts | EGFR, α1β1, αVβ3 | Productive replication and cell-cell virus spread |
Our results indicate that EGFR and selected integrin coreceptors mediate CMV infection in cytotrophoblasts, which are unusual cells that switch integrin repertoire from an epithelial to an endothelial type during development (14, 59, 60). Function-perturbing antibodies block virion binding to EGFR (63%) and integrins α1β1 (80%) and αV (43%), indicating receptor activity (Fig. 7). Moreover, antifunction antibody to α1 integrin reduces infection to the level of β1 antibody, emphasizing the importance of these integrins. Cytotrophoblasts do not express coreceptors α2β1 and α6β1 (11, 14), and antifunction antibodies to integrins α5β1 (63) and α9β1 (E. Maidji and L. Pereira, unpublished observation) do not reduce infection. At very high concentration, soluble α3β1 integrin is slightly inhibitory, suggesting steric hindrance (data not shown). With regard to virion ligands, CMV gB binds EGFR (60) and integrin α1β1 (14), and gB neutralizing antibodies strongly inhibit infection (33). Antifunction antibody to integrin αV also perturbs infection, as does soluble αVβ3 integrin, although to a lesser extent than α1β1. Our results suggest that EGFR and integrins α1β1 and αVβ3 function as key receptors in invasive cytotrophoblasts.
Recent reports indicate that gB and gH are major virion ligands for EGFR, α1β1, and αVβ3 (14, 59, 60). Interestingly, villus cytotrophoblasts express αV but not β3, suggesting that other partners could function as coreceptors, particularly integrin subunits β5 and β6 (55, 63). CMV infection in epithelial cell-like progenitor cells could resemble that by foot-and-mouth disease virus, which binds integrins αVβ3 (4) and αVβ6 (25) in epithelia. Temporal and spatial regulation of virion receptors as cytotrophoblasts differentiate limits sites of infection and virus access to the fetal compartment. These findings help explain the discrepancies between frequent detection of viral DNA in the pregnant uterus (80%), reduced levels of virus in the placenta (60%), and a low rate of congenital infection in seropositive women (1 to 3%) (16, 36, 37).
Differential regulation of receptors could explain why the placenta might function as a barrier to viral infection. Syncytiotrophoblasts do not express the coxsackievirus and adenovirus receptor and are resistant to adenovirus entry (29). Cytotrophoblasts in floating villi express this receptor in early gestation but not at later times; receptor-expressing extravillous cytotrophoblasts are easily infected in vitro. Syncytiotrophoblasts lack receptors for herpes simplex virus—HveA, HveB, and HveC—expressed on extravillous cytotrophoblasts, suggesting that intrauterine infection could occur (28). Limited herpes simplex virus type 2 infection was found in interstitial cytotrophoblasts, but not in floating chorionic villi, a result that is in accord with the rarity of congenital infection (1, 36, 44). CMV infection at the maternal-fetal interface presents a different picture. Although syncytiotrophoblasts express EGFR, functional coreceptors are missing; even so, virion-containing immune complexes internalize and colocalize with caveolin-1 vesicles not detected with IgG alone, suggesting that virions drive caveola formation (33). As we reported, CMV gB accumulates in caveosomes, but nucleocapsids and viral DNA remain cytoplasmic without replication. Cytotrophoblasts express caveolin-1 (Fig. 7d) (32), as we previously reported for syncytiotrophoblasts in which caveolae accumulate IgG and CMV virion gB (33). Colocalization of caveolin-1 with gB suggests that binding sites might be exposed upon nucleocapsid release. Notably, U373 glioblastoma, ARPE-19 and HUVEC, specialized cells permissive for CMV replication (34, 56, 57), stain intensely for caveolin-1, which concentrates at the surface membrane of polarized cells plated on permeable filter supports (Maidji and Pereira, unpublished data). In epithelial cells, EGFR triggers caveolin-1 redistribution from the plasma membrane to an early endocytic compartment, inducing tyrosine-14 phosphorylation that stimulates caveola formation (42, 45). CMV gB could engage EGFR in apical microvilli, promote clustering and endocytosis of immune complexes, and trigger assembly of caveolae in syncytiotrophoblasts. Considering that infection occurs via lipid raft microdomains, where molecular complexes coordinate signaling between gB and gH, caveolin-1 could facilitate entry and productive infection when coreceptors are present (35, 59), as reported for coxsackievirus infection through epithelial tight junctions (7).
Developmental regulation of virion receptors in cytotrophoblasts with epithelial and endothelial cell properties reflects different patterns of CMV replication proteins (44). Neutralizing titers and innate immune cells limit the spread of infection in interstitial cytotrophoblasts in decidua, whereas focal infection in villus progenitors depends on sequential expression of virion receptors and the nature of transcytosed immune complexes (33). As predicted, virions could interface differently with surface receptors expressed in these environments. Pathogenic CMV strains encode endothelial cell-tropic properties in the UL131-UL128 locus that merit discussion with regard to congenital infection (19, 20, 22, 48). Open reading frames pUL128 and pUL130 encode small proteins that form complexes with virion envelope glycoproteins gH and gL and, like gH (47), induce neutralizing antibodies (58), albeit less potent than those against gB, the major virion envelope glycoprotein (27, 39, 43). Inasmuch as pUL130 and pUL128 elicit neutralizing antibodies that preclude infection of pathogenic strains, higher-order virion complexes could promote gH- receptor engagement. Synchronous binding of gB and gH to EGFR and integrin αVβ3 enables cross talk between phosphatidylinositol 3-kinase and Src signaling pathways (59). Virion binding downregulates RhoA activity and cofilin phosphorylation and disrupts actin stress fibers, allowing capsid transport to the nucleus followed by infection. Coordination between signaling pathways generates maximal activation, whereas inhibition of signaling from either gB or gH interactions reduces nuclear translocation and infection. One difference in infection of endothelial cells by laboratory strains is the failure of capsids and virion DNA to transport to the nucleus (53). Impaired gH binding could reduce Src signaling and restrict infection by inefficient translocation of capsids to the nucleus. In the context of natural infection, potent gB neutralizing antibodies that preclude virion binding to EGFR could impair one signaling pathway, thereby diminishing or precluding RhoA activation and preventing disruption of actin stress fibers (59). We found that syncytiotrophoblasts and villus cytotrophoblasts that lack virion coreceptors contain capsids but escape infection, suggesting internalization but a failure in nuclear translocation. Our finding that EGFR and integrin α1β1 are essential for viral replication in differentiating/invading cytotrophoblasts suggests that potent neutralizing antibodies to the ligand gB could limit replication in the decidua and help to explain reduced dissemination when pregnant women with primary infection receive early treatment with CMV hyperimmune IgG (40).
Acknowledgments
We are grateful to members of the Pereira and Fisher laboratories and to Dusko Illic and Sharof Tugizov for scientific discussions, to Mirhan Kapidzic for technical assistance, and to Mary McKenney for editing the manuscript.
This project was supported by grants from the National Institutes of Health (AI46657 and AI53782) and the Thrasher Research Fund (02821-7 [L.P.]) and from the Academic Senate of the University of California, San Francisco (E.M.).
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Baldwin, S., and R. J. Whitley. 1989. Intrauterine herpes simplex virus infection. Teratology 39:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Bass, K. E., D. Morrish, I. Roth, D. Bhardwaj, R. Taylor, Y. Zhou, and S. J. Fisher. 1994. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev. Biol. 164:550-561. [DOI] [PubMed] [Google Scholar]
- 3.Benirschke, K., and P. Kaufmann. 2000. Pathology of the human placenta, 4th ed. Springer, New York, NY.
- 4.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrinαVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt, W. J. 1999. Congenital cytomegalovirus infection, p. 269-281. In P. J. Hitchcock, H. T. MacKay, and J. N. Wasserheit (ed.), Sexually transmitted diseases and adverse outcomes of pregnancy. ASM Press, Washington, DC.
- 6.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124:119-131. [DOI] [PubMed] [Google Scholar]
- 8.Cross, J. C., Z. Werb, and S. J. Fisher. 1994. Implantation and the placenta: key pieces of the development puzzle. Science 266:1508-1518. [DOI] [PubMed] [Google Scholar]
- 9.Damsky, C. H., and S. J. Fisher. 1998. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr. Opin. Cell Biol. 10:660-666. [DOI] [PubMed] [Google Scholar]
- 10.Damsky, C. H., M. L. Fitzgerald, and S. J. Fisher. 1992. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Investig. 89:210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damsky, C. H., C. Librach, K. H. Lim, M. L. Fitzgerald, M. T. McMaster, M. Janatpour, Y. Zhou, S. K. Logan, and S. J. Fisher. 1994. Integrin switching regulates normal trophoblast invasion. Development 120:3657-3666. [DOI] [PubMed] [Google Scholar]
- 12.Demmler, G. J. 1996. Congenital cytomegalovirus infection and disease. Adv. Pediatr. Infect. Dis. 11:135-162. [PubMed] [Google Scholar]
- 13.Dondero, D. V., and L. Pereira. 1990. Monoclonal antibody production, p. 101-124. In R. Emmons and N. Schmidt (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association, Washington, DC.
- 14.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler, K. B., S. Stagno, and R. F. Pass. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008-1011. [DOI] [PubMed] [Google Scholar]
- 17.Genbacev, O., A. Krtolica, W. Kaelin, and S. J. Fisher. 2001. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev. Biol. 233:526-536. [DOI] [PubMed] [Google Scholar]
- 18.Genbacev, O., S. A. Schubach, and R. K. Miller. 1992. Villous culture of first trimester human placenta—model to study extravillous trophoblast (EVT) differentiation. Placenta 13:439-461. [DOI] [PubMed] [Google Scholar]
- 19.Gerna, G., E. Percivalle, F. Baldanti, and M. G. Revello. 2002. Lack of transmission to polymorphonuclear leukocytes and human umbilical vein endothelial cells as a marker of attenuation of human cytomegalovirus. J. Med. Virol. 66:335-339. [DOI] [PubMed] [Google Scholar]
- 20.Gerna, G., E. Percivalle, F. Baldanti, S. Sozzani, P. Lanzarini, E. Genini, D. Lilleri, and M. G. Revello. 2000. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J. Virol. 74:5629-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths, P. D., and C. Baboonian. 1984. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br. J. Obstet. Gynaecol. 91:307-315. [DOI] [PubMed] [Google Scholar]
- 22.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 78:10023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halwachs-Baumann, G., M. Wilders-Truschnig, G. Desoye, T. Hahn, L. Kiesel, K. Klingel, P. Rieger, G. Jahn, and C. Sinzger. 1998. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Virol. 72:7598-7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemmings, D. G., R. Kilani, C. Nykiforuk, J. Preiksaitis, and L. J. Guilbert. 1998. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 72:4970-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janatpour, M. J., M. T. McMaster, O. Genbacev, Y. Zhou, J. Dong, J. C. Cross, M. A. Israel, and S. J. Fisher. 2000. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 127:549-558. [DOI] [PubMed] [Google Scholar]
- 27.Kniess, N., M. Mach, J. Fay, and W. J. Britt. 1991. Distribution of linear antigenic sites on glycoprotein gp55 of human cytomegalovirus. J. Virol. 65:138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koi, H., J. Zhang, A. Makrigiannakis, S. Getsios, C. D. MacCalman, J. F. Strauss III, and S. Parry. 2002. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol. Reprod. 67:1572-1579. [DOI] [PubMed] [Google Scholar]
- 29.Koi, H., J. Zhang, and S. Parry. 2001. The mechanisms of placental viral infection. Ann. N. Y. Acad. Sci. 943:148-156. [DOI] [PubMed] [Google Scholar]
- 30.Kovats, S., E. K. Main, C. Librach, M. Stubblebine, S. J. Fisher, and R. DeMars. 1990. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220-223. [DOI] [PubMed] [Google Scholar]
- 31.Librach, C. L., Z. Werb, M. L. Fitzgerald, K. Chiu, N. M. Corwin, R. A. Esteves, D. Grobelny, R. Galardy, C. H. Damsky, and S. J. Fisher. 1991. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 113:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linton, E. A., B. Rodriguez-Linares, F. Rashid-Doubell, D. J. Ferguson, and C. W. Redman. 2003. Caveolae and caveolin-1 in human term villous trophoblast. Placenta 24:745-757. [DOI] [PubMed] [Google Scholar]
- 33.Maidji, E., S. McDonagh, O. Genbacev, T. Tabata, and L. Pereira. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168:1210-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maidji, E., E. Percivalle, G. Gerna, S. Fisher, and L. Pereira. 2002. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 304:53-69. [DOI] [PubMed] [Google Scholar]
- 35.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonagh, S., E. Maidji, H.-T. Chang, and L. Pereira. 2006. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J. Clin. Virol. 35:210-215. [DOI] [PubMed] [Google Scholar]
- 37.McDonagh, S., E. Maidji, W. Ma, H. T. Chang, S. Fisher, and L. Pereira. 2004. Viral and bacterial pathogens at the maternal-fetal interface. J. Infect. Dis. 190:826-834. [DOI] [PubMed] [Google Scholar]
- 38.McMaster, M. T., C. L. Librach, Y. Zhou, K. H. Lim, M. J. Janatpour, R. DeMars, S. Kovats, C. Damsky, and S. J. Fisher. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154:3771-3778. [PubMed] [Google Scholar]
- 39.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. La Vail, and L. Pereira. 1993. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197:143-158. [DOI] [PubMed] [Google Scholar]
- 40.Nigro, G., S. P. Adler, R. La Torre, and A. M. Best. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353:1350-1362. [DOI] [PubMed] [Google Scholar]
- 41.Norwitz, E. R., D. J. Schust, and S. J. Fisher. 2001. Implantation and the survival of early pregnancy. N. Engl. J. Med. 345:1400-1408. [DOI] [PubMed] [Google Scholar]
- 42.Orlichenko, L., B. Huang, E. Krueger, and M. A. McNiven. 2006. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J. Biol. Chem. 281:4570-4579. [DOI] [PubMed] [Google Scholar]
- 43.Pereira, L., M. Hoffman, M. Tatsuno, and D. Dondero. 1984. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology 139:73-86. [DOI] [PubMed] [Google Scholar]
- 44.Pereira, L., E. Maidji, S. McDonagh, O. Genbacev, and S. Fisher. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77:13301-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pol, A., A. Lu, M. Pons, S. Peiro, and C. Enrich. 2000. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J. Biol. Chem. 275:30566-30572. [DOI] [PubMed] [Google Scholar]
- 46.Qadri, I., D. Navarro, P. Paz, and L. Pereira. 1992. Assembly of conformation-dependent neutralizing domains on human cytomegalovirus glycoprotein B. J. Gen. Virol. 73:2913-2921. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen, L. E., R. M. Nelson, D. C. Kelsall, and T. C. Merigan. 1984. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 81:876-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revello, M. G., F. Baldanti, E. Percivalle, A. Sarasini, L. De-Giuli, E. Genini, D. Lilleri, N. Labo, and G. Gerna. 2001. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J. Gen. Virol. 82:1429-1438. [DOI] [PubMed] [Google Scholar]
- 49.Roth, I., D. B. Corry, R. M. Locksley, J. S. Abrams, M. J. Litton, and S. J. Fisher. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth, I., and S. J. Fisher. 1999. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP- 9 production and invasion. Dev. Biol. 205:194-204. [DOI] [PubMed] [Google Scholar]
- 51.Sacks, G. P., L. M. Clover, D. R. Bainbridge, C. W. Redman, and I. L. Sargent. 2001. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta 22:550-559. [DOI] [PubMed] [Google Scholar]
- 52.Simister, N. E., C. M. Story, H. L. Chen, and J. S. Hunt. 1996. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 26:1527-1531. [DOI] [PubMed] [Google Scholar]
- 53.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 54.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 55.Tabata, T., S. McDonagh, H. Kawakatsu, and L. Pereira. 3 July 2006. Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell-matrix and cell-cell adhesion molecules: a quantitative analysis. Placenta doi: 10.1016/j.placenta.2006.05.006. [Epub ahead of print.] [DOI] [PubMed]
- 56.Tugizov, S., E. Maidji, and L. Pereira. 1996. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J. Gen. Virol. 77:61-74. [DOI] [PubMed] [Google Scholar]
- 57.Tugizov, S., D. Navarro, P. Paz, Y. Wang, I. Qadri, and L. Pereira. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263-276. [DOI] [PubMed] [Google Scholar]
- 58.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 102:18153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 11:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Z., C. Mo, G. Kemble, and G. Duke. 2004. Development of an efficient fluorescence-based microneutralization assay using recombinant human cytomegalovirus strains expressing green fluorescent protein. J. Virol. Methods 120:207-215. [DOI] [PubMed] [Google Scholar]
- 62.Zdravkovic, T., O. Genbacev, A. Prakobphol, M. Cvetkovic, A. Schanz, M. McMaster, and S. J. Fisher. 2006. Nicotine downregulates the l-selectin system that mediates cytotrophoblast emigration from cell columns and attachment to the uterine wall. Reprod. Toxicol. 22:69-76. [DOI] [PubMed] [Google Scholar]
- 63.Zhou, Y., S. J. Fisher, M. Janatpour, O. Genbacev, E. Dejana, M. Wheelock, and C. H. Damsky. 1997. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Investig. 99:2139-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]