Abstract
The prion agent has been detected in skeletal muscle of humans and animals with prion diseases. Here we report scrapie infection of murine C2C12 myoblasts and myotubes in vitro following coculture with a scrapie-infected murine neuroblastoma (N2A) cell line but not following incubation with a scrapie-infected nonneuronal cell line or a scrapie brain homogenate. Terminal differentiation of scrapie-infected C2C12 myoblasts into myotubes resulted in an increase in the expression of the disease-specific prion protein, PrPSc. The amount of scrapie infectivity or PrPSc in C2C12 myotubes was comparable to the levels found in scrapie-infected N2A cells, indicating that a high level of infection was established in muscle cells. Subclones of scrapie-infected C2C12 cells produced high levels of PrPSc in myotubes, and the C-terminal C2 polypeptide fragment of PrPSc was found based on deglycosylation and PrPSc-specific immunoprecipitation of cell lysates. This is the first report of a stable prion infection in muscle cells in vitro and of a long-term prion infection in a nondividing, differentiated peripheral cell type in culture. These in vitro studies also suggest that in vivo prion infection of skeletal muscle requires contact with prion-infected neurons or, possibly, nerve terminals.
Prion infection of neuronally derived cell lines has provided insightful information on the cell biology of the disease-specific isoform of the prion protein, PrPSc, in vitro. The standard method to establish prion infection involves incubating a culture of dividing cells with a crude brain homogenate from a prion-infected host or with preparations enriched for PrPSc (7, 12, 45). After several serial passages in vitro, in which the prion agent in the inoculum is removed by dilution, PrPSc is stably produced in these cells lines and prion infectivity can be detected by bioassay in mice (12, 45). Many important contributions toward understanding the biology of prion diseases have been made by studying prion-infected cell lines, including the cellular trafficking of PrPSc (10, 14, 50), mechanism of PrPSc formation and inhibition (14, 44), identification of compounds with antiprion activity (13), and pathways of prion-induced alterations in cellular function or cell death (28, 42, 51).
The majority of cell lines susceptible to prion infection are neuronal in origin or are derived from a prion-infected brain. These include a murine neuroblastoma (N2A) cell line (12, 45), a murine hypothalamic (GT1) cell line (49), a murine cholinergic septal neuronal (SN56) cell line (37), rat PC12 cells (48), and other, less well-defined cell lines isolated from a scrapie-infected mouse brain (26). More complex manipulations have also led to prion infection of cell lines derived from murine Schwann cells (3), rabbit epithelial cells (54), and deer brain cells (46) as well as primary neuronal and astrocyte cultures (21). One feature of prion infection in these cell lines is that infection is stably maintained in dividing cells. However, these cultures have not proven effective for investigating the role of prion infection in differentiated neurons, which is the main target cell in the nervous system. The murine cell line SN56 can be readily infected with the scrapie agent in vitro and induced to differentiate into mature neurons, but one disadvantage is that there is a reduction in PrPSc levels upon induction of cellular maturation (7). The other major target for prion infection in mammalian hosts is the follicular dendritic cell (32, 38), but these long-lived cells have proven difficult to isolate and culture in vitro.
Recently, prion infection in skeletal muscle has been described for a number of natural and experimental prion diseases of animals and humans. PrPSc or prion infectivity is found in skeletal muscle homogenates from rodents experimentally infected with the transmissible mink encephalopathy (TME) or scrapie agent (8, 11, 39, 52), sheep with scrapie or bovine spongiform encephalopathy infections (1, 36), mule deer with chronic wasting disease (2, 29), and humans and macaques with Creutzfeldt-Jakob disease (27, 43). Detection of PrPSc by immunohistochemistry reveals localization to nerve fibers that transverse muscle or, in a few cases, deposition in skeletal muscle cells. In one study, PrPSc was localized to nerve fibers, muscle cells, and the neuromuscular junction in hamsters infected with the HY strain of the TME agent (39). It was postulated that the HY TME agent could spread from the motor nerve endings into the muscle cell via the synapse at the neuromuscular junction. Ultrastructural localization of the cellular isoform of the prion protein, PrPC, determined that it is present on both the presynaptic nerve terminal and postsynaptic muscle cell membrane (25), suggesting that both locations are potential sites of PrPSc formation. Since the prion agent can undergo transynaptic spread between neurons, the distribution of the prion protein in muscle is consistent with the neuromuscular junction acting as a peripheral synapse for cell-to-cell spread of the prion agent.
There is clinical and experimental evidence that PrPC plays a role in muscle physiology and disease. In several human neuromuscular diseases it has been found that there is an upregulation and/or redistribution of PrPC in skeletal muscle cells. These changes include increased PrPC expression in muscle of individuals with inclusion body myositis and neurogenic muscle atrophy (5, 60) as well as increased PrPC immunostaining in regenerating and atrophic muscle fibers in chronic neurogenic lesions, regenerating muscle fibers in muscular dystrophy, the rim of vacuoles in inclusion body myositis, and focal PrPC deposits in myofibrillar myopathy inclusions (34). Furthermore, in transgenic mice, overexpression of wild-type PrPC, or a mutant PrPC transgene that is linked to human prion disease, can induce a necrotizing myopathy (18, 57). It is noteworthy that in sheep scrapie and chronic wasting disease of cervids there is a myopathy and loss of body mass, but the roles of PrPC and PrPSc in these pathologies are unclear. Presently, there is no in vitro model to investigate the effect(s) of prion infection on skeletal muscle cells.
In this study, we report establishment of scrapie infection in a murine myoblast cell line that can undergo differentiation into nondividing, elongated myotubes, which can remain viable for several weeks in vitro. To establish scrapie infection in C2C12 myoblasts, coculture with scrapie-infected N2A cells was necessary, followed by growth in media selective for C2C12 cell survival. Subclones of scrapie-infected C2C12 myoblasts that express high levels of PrPSc upon differentiation into myotubes were identified, and they rapidly produce disease in a mouse scrapie bioassay. The scrapie-infected myotubes produced a C-terminal PrPSc polypeptide fragment characteristic of calpain-dependent cleavage. These are the first studies to demonstrate prion infection of a muscle cell line and long-term maintenance of prion infection in vitro in a terminally differentiated peripheral cell type that also is a target for prion infection in vivo.
MATERIALS AND METHODS
Cell culture.
Murine C2C12 skeletal myoblast cells (American Type Culture Collection, Manassas, VA) were cultured in high-glucose Dulbecco modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 2 mM glutamine (Invitrogen, Carlsbad, CA), 100 IU/ml of penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (CellGro Mediatech, Inc., Herndon, VA). Differentiation to myotubes was achieved by allowing myoblast cultures to grow until confluent, which resulted in cell fusion to produce multinucleated, elongated myotubes. Murine neuroblastoma cells (N2A) cured of scrapie infection were a generous gift from Suzette Priola (Rocky Mountain Laboratories, Hamilton, MT) and were maintained in OptiMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 2 mM glutamine, 100 IU/ml of penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B. An antibiotic-resistant line of C2C12 (C2C12/Zeor) was generated by transfection of muscle cells with the pcDNA3.1/Zeo plasmid (Invitrogen, Carlsbad, CA) and selection in DMEM supplemented with 200 μg/ml Zeocin. Scrapie murine brain (SMB) cells (TSE Resource Center, Institute of Animal Health, Edinburgh, Scotland) were cultured in high-glucose DMEM supplemented with 10% newborn calf serum (Invitrogen, Carlsbad, CA), 5% FBS, 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. All cell lines were grown at 37°C with 5% CO2.
Scrapie infection of cultured cells.
N2A and C2C12 cells were grown in culture medium supplemented with a 0.1% or 1% (wt/vol) brain homogenate from a scrapie strain 22L-infected mouse for 48 h. When cells reached 90% confluence, they were reseeded at a 1:8 dilution, and after the fourth serial passage they were screened for PrPSc by Western blotting. 22L scrapie-infected N2A cells and C2C12 myoblasts were cocultured by plating one in the bottom of a six-well plate while the other cell type was grown on PET membranes mounted above the well bottom (Thin Certs, Bellco Biotechnology, Vineland, NJ). The range in pore size of the PET membrane was 1 to 8 μm. Cells were allowed to grow for 1 week, after which they were detached by trypsinization from either the membrane or plate bottom and were screened for PrPSc by Western blotting at every other passage. To establish scrapie infection by direct coculture, 22L scrapie-infected N2A cells (280,000 cells) were plated with C2C12/Zeor cells (140,000 cells) in 100-mm dishes. Cocultures were grown in C2C12 complete medium that was changed every 2 to 3 days for 8 days, at which time myotubes were visibly present. The cocultures were then trypsinized and reseeded at a 1:2 dilution with C2C12 complete medium containing 200 μg/ml Zeocin. 22L scrapie-infected N2A cells did not survive under these conditions, and neuronal cells were not observed after two additional serial passages. The Zeocin-resistant C2C12 cells were screened for PrPSc, and the purity of the muscle cells was determined with antibodies specific for neuronal and muscle cells by Western blotting. Mock-infected C2C12 cells were established by coculturing C2C12 myoblasts with scrapie-cured N2A cells instead of 22L scrapie-infected N2A cells as described above. 22L scrapie-infected SMB cell and C2C12/Zeor cell cocultures were also performed as described above for 22L N2A and C2C12/Zeor cell cocultures.
Immunofluorescence staining for desmin.
C2C12 myoblasts and myotubes were grown on Nunc Lab-Tek II chamber slides (Fisher Scientific, Hampton, NJ), fixed with cold ethanol, permeabilized with 0.1% Triton X-100, and incubated with rabbit antidesmin polyclonal antibody at a 1:20 dilution (DAKO Corp., Carpenteria, CA) followed by anti-rabbit Alexa Fluor 568 at a 1:200 dilution (Molecular Probes, Eugene, OR). Slides were mounted with ProLong Gold antifade reagent (Molecular Probes, Eugene, OR) and imaged with a Nikon E600 microscope with epi-illumination.
Preparation of lysates for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Cell culture lysates were collected in cell lysis buffer containing 1 mM Tris-HCl (pH 7.4), 140 mM NaCl, 5 mM EDTA, 0.5% sodium deoxycholate, and 0.5% Triton X-100 at 4°C (56). Lysates were subject to centrifugation at 11,000 × g for 10 min at 4°C and the supernatant retained for Western blot analysis. The protein concentration in cell lysates was determined using the Pierce Micro BCA assay kit (Pierce, Rockford, IL). For PrPSc analysis, proteinase K (PK) (Sigma Aldrich, St. Louis, MO) was added to cell lysates to a final concentration of 0.6 units/ml. Samples were incubated at 37°C for 1 h, and phenylmethylsulfonyl fluoride (Sigma Aldrich, St. Louis, MO) was added to a final concentration of 3 mM in order to inhibit PK activity. Following ultracentrifugation at 55,000 rpm for 2 h at 10°C in a TLA-55 rotor (Beckman Inc., Palo Alto, CA), pellets were resuspended in NuPAGE sample buffer and boiled for 10 min prior to polyacrylamide gel electrophoresis.
For removal of carbohydrate residues, cell lysates were precipitated in methanol and the pellet dissolved in 30 μl N-endoglycosidase F (PNGase F) denaturation buffer (New England Biolabs, Beverly, MA.). Samples were boiled for 10 min and subjected to centrifugation in a microcentrifuge for 10 min. To one-third of the supernatant, 75 units of PNGase F was added, and the digestion was performed at 37°C for 16 h. NuPAGE sample buffer was added to samples and analyzed by electrophoresis and Western blotting.
Samples were analyzed on a 12% MOPS (morpholinepropanesulfonic acid) NuPAGE gel (Invitrogen, Carlsbad, CA), and proteins were transferred to a polyvinylidene difluoride membrane and incubated with one of the following antibodies: mouse anti-PrP monoclonal 6H4 antibody (Prionics, Zurich, Switzerland) at a dilution of 1:10,000, mouse anti-PrP monoclonal SAF-32 antibody (Cayman Chemical Company, Ann Arbor, MI) at 1 μg/ml, rabbit anti-desmin polyclonal antibody (DAKO Corp., Carpenteria, CA) at a 1:2,000 dilution, or mouse anti-tubulin III monoclonal antibody (TU-20; Abcam Inc., Cambridge, MA) at a 1:1,000 dilution. The detection system included incubation with either an anti-mouse immunoglobulin (IgG) alkaline phosphatase conjugate (Promega, Madison, WI) at a dilution of 1:20,000 (for PrP antibodies) or 1:5,000 (for TU-20 antibody) or an anti-rabbit IgG alkaline phosphatase conjugate (Cell Signaling, Danvers MA) at a 1:2,000 dilution. Blots were developed using CDP-Star substrate (Applied Biosystems, Foster City, CA) and imaged with a Kodak Image Station 2000MM (Eastman Kodak Company, Rochester, NY).
Subcloning of scrapie strain 22L-infected C2C12 cells.
For subcloning of C2C12 muscle cells, the plating density of C2C12 myoblasts was determined at limiting dilution using standard cell counting and plating techniques. 22L scrapie-infected C2C12 myoblasts at passage 12 (the number of serial passages in Zeocin selection medium following establishment of a coculture of the 22L scrapie-infected N2A cells and uninfected C2C12 cells) were plated at a density of 18 cells per well in a 48-well plate. After cells reached confluence, they were trypsinized and transferred into one well of a six-well plate (a 1:10 split). Upon reaching confluence, the cells were passaged into three 60-mm culture dishes (a 1:6 split). Two of the culture dishes were screened for PK-resistant PrPSc by Western blotting, one as a C2C12 myoblast culture and the other after differentiation into day 5 myotubes. A third culture was passed into a 100-mm dish in order to maintain cultures until the initial screening for scrapie infection was complete.
Immunoprecipitation of PrPSc.
Monoclonal anti-PrP 15B3 IgM antibody was a gift from Alex Räber (Prionics, Zurich, Switzerland) and was used to selectively immunoprecipitate PrPSc as previously described (33, 41). Dynabeads M-450 (rat anti-mouse IgM) (Invitrogen, Carlsbad, CA) were coated with either anti-PrP 15B3 IgM antibody or buffer alone, and standard immunoprecipitation procedures were used as described by the manufacturer (Prionics Inc., Zurich, Switzerland). Briefly, 10 μl of 15B3 IgM-coated Dynabeads was used to immunoprecipitate PrPSc from cell lysates (2.0-cm2 tissue culture equivalents) from N2A cells or C2C12 myotubes. Immunoprecipitates of PrPSc were separated from cell lysates using a Dynal MPC-S magnetic particle concentrator (Invitrogen, Carlsbad, CA) and eluted from the Dynabeads by boiling in NuPAGE sample buffer (Invitrogen, Carlsbad, CA). NuPAGE gels and Western blots were performed with anti-PrP IgG SAF-32 or 6H4 monoclonal antibodies and visualized by CDP-Star chemiluminescence on a Kodak Image Station 2000MM as described above.
Preparation of C2C12 myoblasts and myotubes for scrapie infectivity assay.
Cells from mock- and 22L scrapie-infected N2A cells, C2C12 myoblasts, and C2C12 myotubes were prepared for mouse bioassay. Cells for scrapie infectivity assay were serially passaged greater than 13 times following the scrapie infection step as described above. Based on dilution analysis, the scrapie infectivity from the original scrapie inoculum would have been removed by this passage number. Duplicate 100-mm dishes of each cell line were trypsinized, subject to centrifugation at 200 × g for 5 min, and washed two times in cold phosphate-buffered saline, and each duplicate cell suspension was combined together in 400 μl phosphate-buffered saline. Two hundred microliters was set aside for bioassay, and the remaining cells were screened for PK-resistant PrPSc by Western blotting as described above. Prior to inoculation, samples were submitted to three cycles of sonication at 4°C for 1 minute and several rounds of freezing and thawing in order to lyse the cells.
Animal inoculations and scrapie infectivity bioassay.
Weanling C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were intracerebrally inoculated with 30 μl of a cell lysate (56-cm2 culture plate equivalents per three to six mice) from mock- or scrapie-infected N2A cells, C2C12 myoblasts, or C2C12 myotubes. Following inoculation, mice were observed three times per week for the onset of clinical symptoms of scrapie. The time to the onset of scrapie was reported, and the animals were euthanatized during the early stages of clinical disease. All procedures involving animals were approved by the Montana State University Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals (40).
RESULTS
Endogenous prion protein (PrPC) expression in C2C12 myotubes and myoblasts.
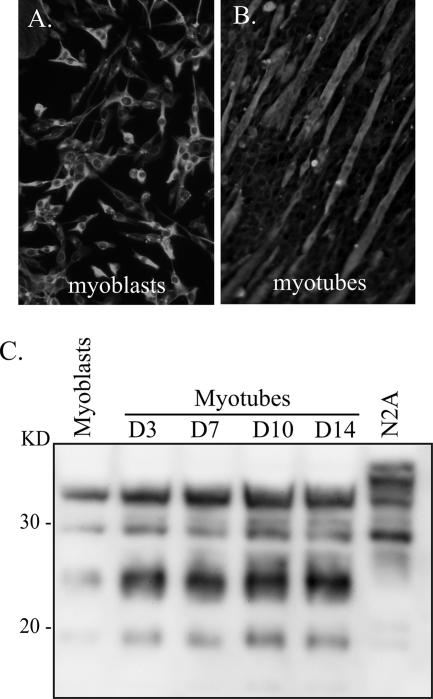
Murine C2C12 muscle cells were previously derived from dystrophic mouse hind limb muscle (59), and the morphologies of the myoblast and myotube cells following immunofluorescent staining for desmin are illustrated in Fig. 1. The C2C12 myoblasts are mononucleated cells with a morphology that is similar to that of fibroblasts, and, under subconfluent conditions, they divide every 14 to 16 h (Fig. 1A). Differentiation to myotubes was achieved by allowing the myoblasts to grow to confluence, at which time cell fusion resulted in multinucleated and elongated muscle cells with a morphology similar to that of myofibrils in vivo (Fig. 1B). Once they undergo differentiation, the C2C12 myotubes do not continue to divide, but they remain viable for approximately 21 days in vitro, after which time spontaneous contraction can occur, causing detachment from the culture surface (6, 20, 35).
FIG. 1.
Morphology and prion protein expression in C2C12 myoblasts and myotubes. (A and B) The morphologies of C2C12 myoblasts (A) and D5 myotubes (B) are illustrated by immunofluorescence staining for the cytoskeletal protein desmin. (C) Lysates from N2A cells (63 μg protein), C2C12 myoblasts (126 μg protein), and C2C12 myotubes (126 μg protein) at days 3, 7, 10, and 14 in vitro were analyzed for total PrP in the absence of PK digestion by Western blotting using anti-PrP 6H4 monoclonal antibody as described in Materials and Methods. Molecular masses are indicated to the left.
In order to determine the relative abundance of the cellular prion protein (PrPC) in the C2C12 cells, Western blotting was performed on cell lysates from myoblasts and myotubes at days 3, 7, 10, and 14 in vitro (Fig. 1C). Four major immunoreactive polypeptides that have molecular masses of 19, 23 to 25, 29, and 34 kDa were found. This PrPC profile was distinct from that found in N2A cells, but the majority of the polypeptides immunoreactive with anti-PrP 6H4 antibody were between 18 and 35 kDa for both cell types. The PrPC expression level in C2C12 muscle cells exhibited an increase upon differentiation of myoblasts into myotubes. Typically there was a two- to fourfold increase in PrPC levels in D7 myotubes, but after this time there was little additional increase of PrPC in myotubes between days 7 and 21 in vitro (Fig. 1C and data not shown).
Scrapie infection of C2C12 myoblasts and myotubes.
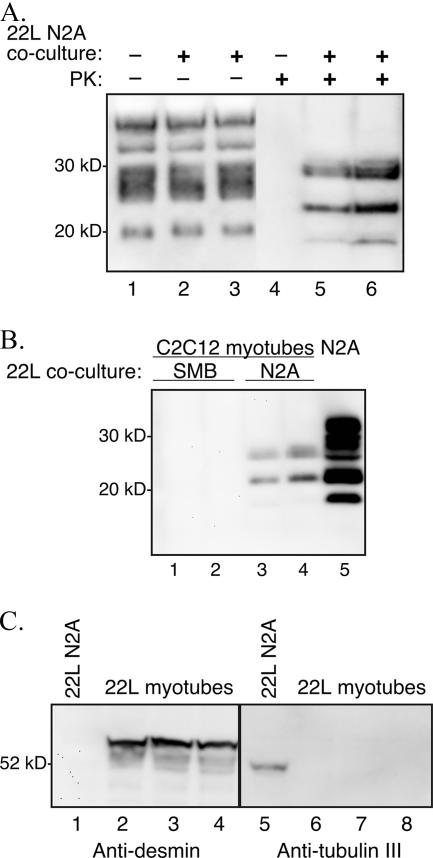
Three approaches were used to establish scrapie infection in C2C12 cells (Table 1). For the first method, murine C2C12 and N2A cells were cultured with brain homogenates (0.1% or 1% [wt/vol] in culture medium) from mice infected with the 22L strain of scrapie, as previously described for establishment of scrapie infection in vitro (12, 45). Despite four attempts, PrPSc was not detected by Western blotting in C2C12 myoblasts and myotubes following 8 to 10 serial passages after the initial plating with infectious scrapie tissue, but PrPSc was consistently found following infection of N2A cells (Table 1 and data not shown). In the second method, 22L scrapie-infected N2A cells were cocultured with C2C12 myoblasts, but the cell types were separated by a membrane with pores that ranged in diameter from 1 to 8 μm. This attempt to enable the 22L scrapie agent to pass through the membrane and infect the C2C12 cells did not result in PrPSc production in the C2C12 myoblasts even though PrPSc was maintained in the 22L scrapie-infected N2A cells (Table 1 and data not shown). In the third method, C2C12 myoblasts were stably transfected with a plasmid expressing Zeocin resistance (C2C12/Zeor) prior to coculture with 22L scrapie-infected N2A cells at a ratio of 1:2 (Table 1). The cocultures were grown for 1 week in medium without Zeocin, after which the cocultures were switched to medium containing 200 μg/ml Zeocin in order to eliminate the antibiotic-susceptible 22L scrapie-infected N2A cells. The C2C12/Zeor myoblasts and myotubes were serially passaged and screened at every other passage for PrPSc by Western blotting. Figure 2A illustrates the presence of PK-resistant PrPSc in C2C12/Zeor day 7 myotubes at passage 10 after addition of Zeocin selection medium. There was no difference in the total PrP polypeptide pattern (i.e., in the absence of experimental PK digestion) from cell lysates of C2C12/Zeor day 7 myotubes cocultured with either mock-infected N2A cells or 22L scrapie-infected N2A cells (Fig. 2A, lanes 1 to 3). However, following PK digestion of the cell lysates, the myotubes cocultured with mock-infected N2A cells showed no PrPSc signal, while the myotubes cocultured with 22L scrapie-infected N2A cells had PK-resistant PrPSc polypeptides of between 19 and 30 kDa (Fig. 2A, lanes 4 to 6). The first appearance of PK-resistant PrPSc polypeptides was observed in cell lysates of both myoblasts and myotubes after four serial passages in Zeocin selection medium (data not shown), indicating that PrPSc production was due to de novo synthesis and not to contamination with the scrapie inoculum. These findings were confirmed in duplicate coculture experiments. The outcome of C2C12 cell infection with either 22L scrapie brain homogenates or 22L scrapie-infected N2A coculture was not due to exposure of C2C12 cells to different doses of scrapie infectivity, since both scrapie sources induced relatively short incubation periods following intracerebral inoculation of mice (Table 2).
TABLE 1.
Approaches to establish 22L scrapie infection in C2C12 myoblasts and myotubes
| Method to establish infection in C2C12 cells | Outcome in:
|
|
|---|---|---|
| N2A cells | C2C12 cells | |
| Add 22L scrapie brain homogenatea | Scrapie infection established | Scrapie infection not established |
| Coculture with 22L scrapie-infected N2A cells separated by a barrierb | Scrapie infection maintained | Scrapie infection not established |
| Coculture with 22L scrapie-infected N2A cells and Zeocin-transfected C2C12 cells | Death due to Zeocin sensitivity | Scrapie infection established in Zeocin-resistant myoblasts and myotubes |
Homogenates at 0.1% to 1% (wt/vol).
One- or 8-μm-pore-diameter membranes.
FIG. 2.
Coculture of C2C12 myoblasts and 22L scrapie-infected N2A cells. C2C12 myoblasts containing a plasmid expressing Zeocin resistance and 22L scrapie-infected N2A cells were cocultured at a ratio of 1:2 for 1 week prior to selection in Zeocin-containing culture medium. The surviving C2C12/Zeor myotubes were assayed for PrPSc by Western blotting after serial passage in vitro. (A) Cell lysates from C2C12 D5 myotubes at passage 10 in Zeocin-containing selection medium following coculture with either uninfected N2A cells (lanes 1 and 4) or 22L scrapie-infected N2A cells (lanes 2, 3, 5, and 6). The cellular lysates were either undigested (−) or digested (+) with 0.6 U/ml of PK prior to analysis. (B) Western blot of PK-digested lysates from C2C12 D5 myotubes at passage 6 in Zeocin-containing selection medium following coculture with either 22L scrapie-infected SMB cells (lanes 1 and 2) or 22L scrapie-infected N2A cells (lanes 3 and 4). Control 22L N2A cells (lane 5) were in the absence of C2C12 cell coculture and Zeocin selection. (C) Western blotting for desmin and tubulin III was performed on cell lysates from C2C12 D6 myotubes following coculture of 22L scrapie-infected N2A cells and C2C12 myoblasts at passage 4 in Zeocin selection medium (lanes 2 to 4 and 6 to 8). Eighty-six micrograms of 22L scrapie-infected N2A cells (lanes 1 and 5) and 22L scrapie-infected C2C12 D6 myotubes was analyzed using anti-PrP 6H4 monoclonal antibody. Molecular mass markers are shown on the left.
TABLE 2.
Scrapie incubation period in murine neuroblastoma (N2A) and C2C12 myoblast and myotube cell lysates
| Samplea | Trial 1b
|
Trial 2
|
||||
|---|---|---|---|---|---|---|
| Incubation period, days (mean ± SD)c | No. affected/no. inoculated | No. of times cultures were passed after scrapie infection | Incubation period, days (mean ± SD) | No. affected/no. inoculated | No. of times cultures were passed after scrapie infection | |
| 22L scrapie-infected brain | 137 ± 7d | 5/5 | NAe | NDf | NA | NA |
| 22L scrapie-infected N2A cells | 125 ± 7.6* | 5/5 | 19 | 138 ± 2.2 | 5/5 | 15 |
| 22L scrapie-infected C2C12 myoblasts | 138 ± 6.2* | 6/6 | 17 | 145 ± 4.3 | 6/6 | 14 |
| 22L scrapie-infected C2C12 myotubes | 132 ± 2.2 | 5/5 | 17 | 137 ± 1.7 | 4/4 | 14 |
| 22L A3 scrapie-infected C2C12 myoblasts | ND | NA | NA | 139 ± 6.4 | 4/4 | 18 |
| 22L A3 scrapie-infected C2C12 myotubes | ND | NA | NA | 133 ± 0.6 | 3/3 | 18 |
| C2C12 myoblasts | >250 | 0/5 | 17 | >205 | 0/3 | 14 |
| C2C12 myotubes | ND | NA | NA | >205 | 0/3 | 14 |
For the brain sample, a 1% (wt/vol) homogenate was used. For all other samples, a 56-cm2 tissue culture at ∼90% to 100% confluence was trypsinized and resuspended in phosphate-buffered saline, and the total cell lysate was inoculated into each group of mice.
Trials represent C2C12 muscle cells and N2A cells from independent scrapie infection experiments.
Brain homogenates or tissue lysates were intracerebrally inoculated into C57Bl/6 mice and the time to onset of clinical disease was measured. *, Tukey's Studentized range test, P < 0.05.
The presence of PrPSc in the brains of all clinically ill mice was confirmed by Western blotting.
NA, not applicable.
ND, not done.
To investigate whether transfer of scrapie infection to C2C12 cells from other scrapie-infected cell lines could be achieved, cocultures between C2C12/Zeor cells and a 22L SMB cell line was performed as described above. The SMB cell line is a nonneuronal cell line, and following coculture and growth in Zeocin selection medium, C2C12/Zeor myoblasts and myotubes did not show evidence of PrPSc production by the eighth serial passage (Fig. 2B, lanes 1 and 2). In the control cocultures using 22L N2A cells, PrPSc was detected in C2C12/Zeor cells by the sixth serial passage (Fig. 2B, lanes 3 and 4). These findings indicate that a scrapie-infected neuronal cell line was able to transfer infection to C2C12 cells, while a scrapie-infected nonneuronal cell line did not establish infection in C2C12 cells upon coculture.
To determine whether cocultures of 22L scrapie-infected N2A cells and C2C12/Zeor cells had any remaining N2A cells after the fourth serial passage in Zeocin-containing growth medium, we tested for the presence of a muscle marker (desmin) and neuronal marker (tubulin III) in cell lysates by Western blotting. The Zeocin-resistant 22L scrapie-infected day 5 myotubes were immunopositive for desmin at 54 kDa (Fig. 2B, lanes 2 to 4) but not for tubulin III (Fig. 2B, lanes 6 to 8), indicating an absence of the neuronal marker in these PrPSc-positive cells. Control cultures of 22L scrapie-infected N2A cells that were not grown in Zeocin-containing medium did not immunoreact for desmin (Fig. 2B, lane 1) but did contain tubulin III at 52 kDa (Fig. 2B, lane 5). Similar results were obtained in a duplicate coculture experiment. No viable cells were found in control cultures of 22L scrapie-infected N2A cells after growth in culture medium containing Zeocin at 200 μg/ml (data not shown).
Subcloning of 22L scrapie-infected C2C12 myoblasts.
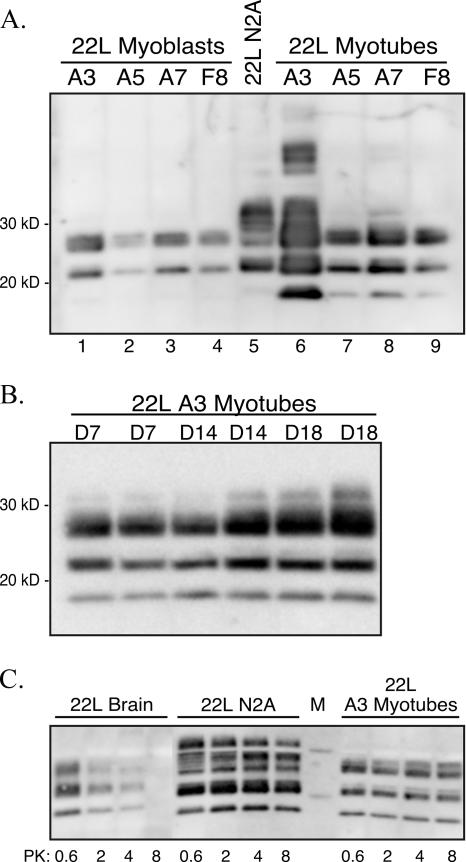
To identify a population of 22L scrapie-infected myoblasts that expressed high levels of PrPSc, 22L scrapie-infected myoblasts were plated at limiting dilution in a 48-well plate, and when ∼70% confluent they were serially passaged three times into larger culture plates. Five PrPSc-expressing subclones were identified by screening of cell lysates from myoblasts and day 5 myotubes by Western blotting (Fig. 3A). While two prominent PrPSc polypeptides were found in subclones of 22L scrapie-infected myoblasts at 22 kDa and 26 to 28 kDa, in the myotubes a third, lower-molecular-mass PrPSc polypeptide at 18 kDa was also apparent (Fig. 3A, lanes 6 to 9). Subclone 22L A3 had the highest level of PK-resistant PrPSc polypeptides in day 5 myotubes (Fig. 3A, lane 6), even though 22L A3 scrapie-infected myoblasts had PrPSc levels comparable to those of the other myoblast subclones (Fig. 3A, lanes 1 to 4). Additional immunoreactive PrPSc polypeptides were found in the 22L A3 myotube subclone at higher molecular masses (Fig. 3A, lane 6), which are likely due to the larger quantity of PrPSc analyzed in this sample. A second round of subcloning starting with the 22L A3 myoblasts resulted in identification of seven additional PrPSc-positive clones out of 12 limiting-dilution groups. These subclones expressed comparable or lower levels of PrPSc than the original 22L A3 C2C12 subclone (data not shown).
FIG. 3.
Subcloning of 22L scrapie-infected C2C12 cells. (A) 22L scrapie-infected C2C12 myoblasts were subcloned by limiting dilution, and cell lysates (∼48-cm2 tissue culture plate equivalents at >90% confluence) from myoblasts (lanes 1 to 4) and D5 myotubes (lanes 6 to 9) were digested with proteinase K prior to screening for PrPSc by Western blotting with monoclonal anti-PrP 6H4 antibody. PK-digested lysates from 22L scrapie-infected N2A cells are in lane 5 (∼24-cm2 tissue culture plate equivalents at >90% confluence). (B) Western blot for PrPSc in PK-digested lysates (∼7.5-cm2 tissue culture plate equivalents at >90% confluence) from 22L C2C12 myotubes at days 7, 14, and 18 in vitro. (C) PrPSc Western blot of 22L N2A cells (∼12-cm2 tissue culture plate equivalents at >90% confluence), 22L C2C12 day 5 myotubes (∼12-cm2 tissue culture plate equivalents at >90% confluence), and 22L scrapie-infected mouse brain (300 μg protein prior to PK digestion) following digestion with 0.6, 2, 4, or 8 U/ml of PK.
The amount of PrPSc increases in scrapie-infected C2C12 cells upon differentiation of myoblasts into myotubes.
Based on the higher expression level of PrPC in C2C12 myotubes versus myoblasts (Fig. 1C), the amount of PrPSc in 22L A3 scrapie-infected C2C12 cells was measured. Lysates of scrapie-infected 22L A3 myotubes at days 7, 14, and 18 in vitro were analyzed for PrPSc by Western blotting (Fig. 3B). Approximately 700 μg of protein from each cell lysate was digested with 0.6 units/ml of PK for 1 hour at 37°C. There was a progressive increase in PrPSc levels of approximately two- to fourfold in myotubes between days 7 and 18 (Fig. 3B). Despite the increase in PrPSc accumulation in 22L A3 scrapie-infected C2C12 cells upon differentiation, no overt cytopathology was observed in these cultures (data not shown).
22L scrapie-infected C2C12 myotubes are highly resistant to PK digestion.
To compare the relative resistance of PrPSc to PK degradation from the neuronal and muscle cell types, lysates (containing 580 μg of protein) from 22L scrapie-infected N2A cells and 22L A3 scrapie-infected C2C12 day 5 myotubes were digested with either 0.6, 2, 4, or 8 units/ml of PK at 37°C for 1 hour and analyzed for PrPSc by Western blotting (Fig. 3C). For comparison, 300 μg of protein from a 22L scrapie-infected mouse brain was also digested with PK under similar conditions (Fig. 3C, left panel). In the 22L scrapie-infected N2A cells and the 22L scrapie-infected A3 myotubes, the PrPSc signal did not diminish considerably as the concentration of PK was increased from 0.6 to 8 U/ml (Fig. 3C, middle and right panels), but there was a loss of PrPSc signal in the 22L scrapie-infected murine brain following digestion with 8 U/ml of PK. These findings indicate that PrPSc derived from these cell lines is highly resistant to PK digestion and suggests that PrPSc in 22L scrapie-infected myotubes and PrPSc in scrapie-infected N2A cells have similar biochemical properties.
22L scrapie-infected C2C12 myoblasts and myotubes are infectious in mouse bioassay.
In order to measure the amount of scrapie infectivity in the 22L scrapie-infected C2C12 myoblasts and myotubes, cell lysates were intracerebrally inoculated into C57BL/6 mice and the incubation period was determined using a mouse bioassay. In trial 1, lysates from both uncloned 22L scrapie-infected C2C12 myoblasts and myotubes induced clinical disease in recipient mice between 130 and 140 days postinoculation (Table 2). This incubation period was similar to that caused by inoculation of 30 μl of a 1% (wt/vol) brain homogenate from a 22L scrapie-infected mouse at clinical disease. The 22L scrapie-infected N2A cell lysate produced a statistically different incubation period compared to the 22L scrapie-infected C2C12 myoblasts (125 ± 7.6 days versus 138 ± 6.2 days) but not compared to 22L scrapie-infected C2C12 myotubes (125 ± 7.6 days versus 132 ± 2.2 days) in the mouse bioassay (Table 2). By the 12th serial passage of the C2C12 myoblasts after establishment of cocultures, the original 22L scrapie-infected N2A cell inoculum was diluted by a factor of greater than 109. Since C2C12 cell lysates from serial passage 14 or later were tested in the mouse bioassay, the ability of 22L scrapie-infected C2C12 myoblast and myotube lysates to cause prion disease in mice was not due to residual scrapie inoculum (Table 2). C2C12 myoblasts that were not infected with the 22L scrapie agent did not cause disease in recipient mice after 250 days postinoculation. In trial 2, which represents a mouse bioassay using C2C12 cell lysates from an independent scrapie coculture experiment, there was a 1-week difference in incubation period between the uncloned 22L scrapie-infected C2C12 myoblasts and myotubes (Table 2). The 22L A3 C2C12 subclone derived from the uncloned 22L scrapie-infected myoblasts had a slightly shorter incubation period than the uncloned cell lysates; however, there was no statistical difference in incubation period between these two sources of inoculum or between the 22L A3 C2C12 myoblasts and myotubes. These findings indicate that 22L scrapie-infected C2C12 cells can maintain a high level of scrapie infection.
N-terminal truncation of PrPC and PrPSc in C2C12 myotubes.
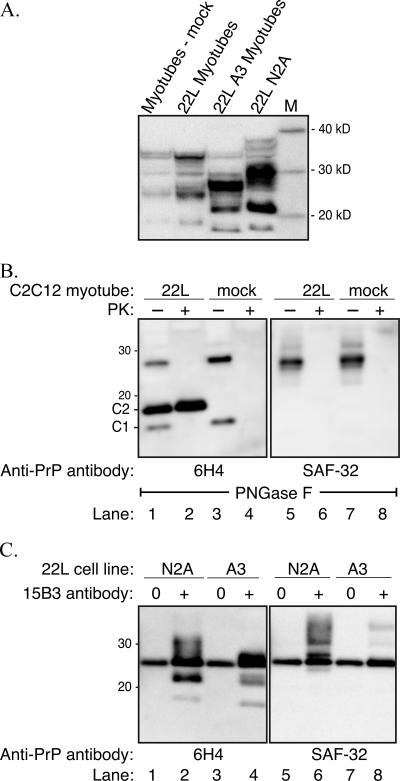
An immunoblot for total PrP in mock-infected C2C12 myotubes, uncloned 22L scrapie-infected C2C12 myotubes, and the A3 subclone of the 22L scrapie-infected C2C12 myotubes revealed a distinct polypeptide pattern in the A3 subclone (Fig. 4A). The PrPSc polypeptide patterns of the mock-infected and uncloned 22L scrapie-infected myotubes were similar to that shown in Fig. 2A and were characterized by four major immunoreactive polypeptides at approximately 19, 23 to 25, 29, and 34 kDa (Fig. 2A and 4A). For the 22L A3 scrapie-infected C2C12 myotubes, the major PrP polypeptides were found at approximately 18, 22, and 26 to 28 kDa. Hence, there appeared to be truncation of the PrP polypeptides in the 22L A3 scrapie-infected myotubes compared to the mock and uncloned 22L scrapie-infected myotubes. The total PrP profile of the 22L scrapie-infected N2A cells also had three major polypeptides that were slightly larger than those in the 22L A3 scrapie-infected myotubes (Fig. 4A). Since it has previously been demonstrated that PrPSc can be truncated in lysosomes or by calpain-dependent endoproteolytic cleavage in scrapie-infected cell lines in vitro or in vivo (15, 16, 58) and since disintegrins can truncate PrPC (19, 55), additional studies were designed to investigate cellular processing of PrPC and PrPSc in the mock-infected and 22L A3 scrapie-infected C2C12 myotubes.
FIG. 4.
Deglycosylation and immunoprecipitation of the prion protein reveal N-terminal truncation in C2C12 myotubes. (A) Total PrP polypeptides in lysates (200 μg protein) of mock-infected myotubes, uncloned 22L scrapie-infected C2C12 myotubes, 22L A3 scrapie-infected C2C12 myotubes, and 22L scrapie-infected N2A cells were immunoblotted with anti-PrP 6H4 monoclonal antibody. (B) PNGase F deglycosylation of mock-infected myotubes (lanes 3, 4, 7 and 8) and 22L A3 scrapie-infected C2C12 myotubes (lanes 1, 2, 5 and 6) in the absence (−) or presence (+) of PK digestion. Cell lysates were immunoblotted with anti-PrP 6H4 (lanes 1 to 4) or anti-PrP SAF-32 (lanes 5 to 8) monoclonal antibodies. (C) 22L scrapie-infected cell lysates immunoprecipitated with anti-PrP 15B3 IgM monoclonal antibody (+) or uncoated Dynabeads (0) were immunoblotted with anti-PrP 6H4 (lanes 1 to 4) or anti-PrP SAF-32 (lanes 5 to 8) monoclonal antibodies. Immunoprecipitation of PrPSc was performed on 22L scrapie-infected N2A cells (lanes 1, 2, 5, and 6) and 22L A3 scrapie-infected C2C12 myotubes (lanes 3, 4, 7, and 8). A 25-kDa nonspecific polypeptide was immunoprecipitated by Dynabeads that were not coated with anti-PrP 15B3 IgM monoclonal antibody and from uninfected cell lysates (data not shown). Lane M, molecular mass markers.
To further assess the molecular masses of PrPC and PrPSc in the C2C12 myotubes, and specifically the cleavage into C1 or C2 polypeptides (16), the N-linked carbohydrates were removed following enzymatic digestion with PNGase F. In mock-infected C2C12 myotubes two PrPC polypeptides were observed, at approximately 16 and 27 kDa, following PNGase F digestion and Western blotting with anti-PrP 6H4 monoclonal antibody (Fig. 4B, lane 3). While the larger PrPC polypeptide is consistent with the full-length unglycosylated PrPC molecule, the 16-kDa polypeptide is characteristic of the C1 PrPC fragment (55). Following PK digestion, the PrPC polypeptides were degraded and immunoreactivity to anti-PrP 6H4 antibody was lost (Fig. 4, lane 4). Analysis of the A3 subclone of the 22L scrapie-infected C2C12 myotubes revealed the 16- and 27-kDa polypeptides that were present in the mock-infected cells and an additional polypeptide band at 18 kDa (Fig. 4, lane 1). While the PrPC polypeptides were no longer immunoreactive after PK digestion in the 22L A3 scrapie-infected myotubes, the 18-kDa polypeptide band was not further reduced in molecular mass after PK digestion (Fig. 4, lane 2) indicating that it was truncated in the muscle cells. This 18-kDa PrPSc polypeptide is consistent with the previously described C2 PrPSc fragment, but this C-terminal polypeptide was reported to have a molecular mass of 21 kDa (16).
Western blotting of a set of deglycosylated cell lysates similar to that illustrated in Fig. 4B, lanes 1 to 4, was performed using anti-PrP SAF-32 monoclonal antibody, which recognizes the octapeptide repeat region of murine prion protein located between amino acids 52 and 92 of the N-terminal region of the prion protein. This octapeptide region is partially degraded following limited PK digestion of PrPSc and results in a reduction or loss of SAF-32 antibody immunoreactivity. For both the PNGase F-treated mock-infected and 22L A3 scrapie-infected myotubes, only the 27-kDa PrPC polypeptide was immunoreactive in the non-PK-digested samples, indicating that the 16-kDa C1 PrPC polypeptide is truncated at the N-terminal end (Fig. 4B, lanes 5 and 7). Following PK digestion, the 27-kDa PrPC polypeptide is degraded (Fig. 4B, lanes 6 and 8). Interestingly, the 18-kDa C2 PrPSc polypeptide in the 22L scrapie-infected myotubes also does not immunoreact with anti-PrP SAF-32 antibody (Fig. 4B, lanes 5 and 6), indicating that it too is truncated at its N terminus. Therefore, in C2C12 myotubes, in addition to full-length PrPC, both PrPC and PrPSc undergo N-terminal truncation.
A second approach was used to examine the molecular mass of PrPSc polypeptides in 22L A3 scrapie-infected C2C12 myotubes. PrPSc was selectively immunoprecipitated from 22L scrapie-infected myotubes and 22L scrapie-infected N2A cell lysates with anti-PrP 15B3 antibody, which does not recognize PrPC (33, 41). These PrPSc polypeptides were further analyzed by immunoblotting with either anti-PrP 6H4 antibody (Fig. 4C, lanes 1 to 4) or anti-PrP SAF-32 antibody (Fig. 4C, lanes 5 and 8). In the anti-PrP 6H4 immunoblot, PrPSc polypeptides were found at approximately 18, 22, and 26 kDa (Fig. 4C, lane 4), excluding the nonspecific immunoprecipitation of a 25-kDa polypeptide band (Fig. 4C, lane 3). An immunoblot of the 15B3-immunoprecipitated polypeptides from 22L A3 scrapie-infected C2C12 myotubes with anti-PrP SAF-32 antibody revealed no immunoreactivity with these polypeptide bands and weak detection of two PrPSc polypeptides at 31 and 35 kDa (Fig. 4C, lane 8). This reduction in immunoreactivity with SAF-32 antibody indicates that the majority of PrPSc in the 22L scrapie-infected myotubes was truncated at the N-terminal end. A comparison with 22L scrapie-infected N2A cells reveals a similar pattern of immunoreactivity with anti-PrP 6H4 and SAF-32 monoclonal antibodies following immunoprecipitation with anti-PrPSc 15B3 monoclonal antibody (Fig. 4, lanes 2 and 6).
DISCUSSION
In an attempt to develop a cell culture model for scrapie infection in muscle, we investigated the murine C2C12 myoblast cell line, since it can undergo myogenic differentiation into nondividing myotubes, which are similar to myofibrils in vivo (59). In this study we demonstrate that scrapie infection and PrPSc production can be established in murine C2C12 myoblasts and myotubes in vitro. The properties of C2C12 cells infected with 22L scrapie were similar to those found in scrapie-infected N2A cells, including a high level of PrPSc resistance to PK degradation, the production of C1 and C2 PrP polypeptide fragments, and the ability to induce scrapie in mice upon intracerebral inoculation. Based on the similarities of scrapie infection in these cell lines, we propose that 22L scrapie-infected C2C12 myoblasts and myotubes will provide a useful tissue culture model for studying prion infection of skeletal muscle in vivo. The recent report of prion infection in muscle of deer with chronic wasting disease indicates a food safety risk to humans who consume venison (2).
In order to establish scrapie infection in C2C12 cells, it was necessary to coculture myoblasts with 22L scrapie-infected N2A cells and then selectively grow the myoblasts in Zeocin-containing growth medium, which does not support N2A cell survival. Attempts to establish scrapie infection in C2C12 myoblasts and myotubes by direct incubation with a scrapie brain homogenate were unsuccessful in four independent experiments even though homogenates contained high scrapie titers that were able to establish scrapie infection of N2A cells. These findings suggested that the 22L scrapie agent, or induction of PrPSc formation, was directly transferred between the infected N2A cells and uninfected myoblasts or myotubes. It is likely that scrapie infection requires direct contact between these cell types, since separation of these cell types by a porous membrane did not result in scrapie infection of the C2C12 myoblasts. The type of cell that can transfer scrapie infection to C2C12 myoblasts and myotubes also appears to be an important factor, since coculture with 22L scrapie-infected SMB cells did not result in infection of C2C12/Zeor cells. However, previous coculture studies demonstrated that SMB cells infected with the 79A strain of the scrapie agent can transfer infection to uninfected SMB cells (31); cell-to-cell contact is not needed to infect SMB cells, since infection can be established using the standard method of incubating the dividing cells with a scrapie brain homogenate (31). These findings suggest that the ability of a scrapie-infected neuronal cell line, but not a nonneuronal cell line, to infect C2C12 myoblasts or myotubes may be due to the formation of a neuromuscular junction in vitro. Both N2a cells and C2C12 myotubes have been demonstrated to form neuromuscular junctions in nerve-muscle cell line cocultures (17, 61). Transynaptic spread of the prion agent has been demonstrated along motor pathways (9). Localization of PrPSc to the neuromuscular junction in prion-infected muscle cells in vivo (39) and of PrPC to both the presynaptic and postsynaptic membranes in the neuromuscular junction provides support for a role of the neuromuscular junction in prion agent spread. The transfer of scrapie infection via exosomes (47) and release of scrapie infectivity into the supernatant (7, 49) represents another possible mechanism for prion infection of C2C12 cells, but this is less likely to have been the case in the current study since we observed an absence of measurable PrPSc in C2C12 cells when they were cocultured with 22L scrapie-infected N2A cells that were separated by a porous membrane. However, we cannot exclude the possibility that these mechanisms of cell-to-cell spread are involved in scrapie infection of C2C12 muscle cells.
In C2C12 muscle cells there was evidence of N-terminal truncation of the full-length prion protein into either a 16-kDa PrPC polypeptide in both mock- and 22L scrapie-infected C2C12 cells or an 18-kDa PrPSc polypeptide in 22L scrapie-infected C2C12 myotubes. The size and cleavage properties of these truncated PrP polypeptides are consistent, but not precisely matched, with the 17-kDa C1 and 21-kDa C2 PrP polypeptide fragments, respectively, that have been described in mock- and prion-infected brain and cell lines (16, 30, 55, 58). The apparent discrepancy in the size of the C2 PrPSc polypeptide between 22L C2C12 myotubes and other scrapie-infected cells or brain tissue could be due to the use of different polyacrylamide gel separation systems or, perhaps, to differences in cleavage specificity among tissue types. Prior studies demonstrated that N-terminal truncation of PrPSc into the C2 polypeptide is mediated by calpain-dependent endoproteolytic cleavage to generate the C2 PrPSc polypeptide (58). Calpains play an essential role in muscle cell differentiation and, in myoblasts, they have been associated with cell adhesion and spreading (23). In C2C12 cells, inhibition of calpains prevented myocyte migration and fusion into multinucleated myotubes (22). High calpain activity during myogenic differentiation is consistent with cleavage into the C2 PrPSc polypeptide in 22L scrapie-infected C2C12 myotubes.
Our findings that scrapie infection can be established in both dividing myoblasts and nondividing myotubes in vitro directly illustrates that the scrapie agent can replicate in muscle cells. This study supports previous reports of direct detection of PrPSc in myocytes of sheep with scrapie, in skeletal muscle cells of the tongue in rodents experimentally infected with the prion agent (24, 39, 53), and in cardiomuscle fibers of humans with Creutzfeldt-Jakob disease (4) or deer with chronic wasting disease (29). The C2C12 cell model for scrapie infection could be used to investigate prion infection of muscle, including the mechanism of scrapie infection in muscle cells, the pathway of PrPSc formation and trafficking in a terminally differentiated cell type, and the relationship of prion infection in muscle cells to prion-induced myopathy that is found in scrapie of sheep and chronic wasting disease of deer and elk.
Acknowledgments
This work was supported by Public Health Service grant R01 AI-055043 from the National Institute of Allergy and Infectious Diseases; by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (grant 2006-35201-16626); by U.S Fish and Wildlife Service grant 1448-60181-03-G739; and by NIH grant P20 RR020185-01 from the National Center for Research Resources.
Special thanks go to Crista DeJoia, Kimberly O'Connell, and Becca Sorg for technical assistance with scrapie bioassay experiments; to Renee Arens for animal care; and to Glenn Telling and members of our laboratory for helpful discussions. We also thank Prionics for their gift of the 15B3 antibody kit.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Andreoletti, O., S. Simon, C. Lacroux, N. Morel, G. Tabouret, A. Chabert, S. Lugan, F. Corbiere, P. Ferre, G. Foucras, H. Laude, F. Eychenne, J. Grassi, and F. Schelcher. 2004. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat. Med. 10:591-593. [DOI] [PubMed] [Google Scholar]
- 2.Angers, R. C., S. R. Browning, T. S. Seward, C. J. Sigurdson, M. W. Miller, E. A. Hoover, and G. C. Telling. 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117. [DOI] [PubMed] [Google Scholar]
- 3.Archer, F., C. Bachelin, O. Andreoletti, N. Besnard, G. Perrot, C. Langevin, A. Le Dur, D. Vilette, A. Baron-Van Evercooren, J. L. Vilotte, and H. Laude. 2004. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J. Virol. 78:482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashwath, M. L., S. J. Dearmond, and T. Culclasure. 2005. Prion-associated dilated cardiomyopathy. Arch. Intern. Med. 165:338-340. [DOI] [PubMed] [Google Scholar]
- 5.Askanas, V., M. Bilak, W. K. Engel, R. B. Alvarez, F. Tome, and A. Leclerc. 1993. Prion protein is abnormally accumulated in inclusion-body myositis. Neuroreport 5:25-28. [DOI] [PubMed] [Google Scholar]
- 6.Bardouille, C., J. Lehmann, P. Heimann, and H. Jockusch. 2001. Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Appl. Microbiol. Biotechnol. 55:556-562. [DOI] [PubMed] [Google Scholar]
- 7.Baron, G. S., A. C. Magalhaes, M. A. Prado, and B. Caughey. 2006. Mouse-adapted scrapie infection of SN56 cells: greater efficiency with microsome-associated versus purified PrP-res. J. Virol. 80:2106-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2002. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J. Virol. 76:5759-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchelt, D. R., M. Scott, A. Taraboulos, N. Stahl, and S. B. Prusiner. 1990. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J. Cell Biol. 110:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosque, P. J., C. Ryou, G. Telling, D. Peretz, G. Legname, S. J. DeArmond, and S. B. Prusiner. 2002. Prions in skeletal muscle. Proc. Natl. Acad. Sci. USA 99:3812-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler, D. A., M. R. Scott, J. M. Bockman, D. R. Borchelt, A. Taraboulos, K. K. Hsiao, D. T. Kingsbury, and S. B. Prusiner. 1988. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol. 62:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caughey, B., and R. E. Race. 1992. Potent inhibition of scrapie-associated PrP accumulation by congo red. J. Neurochem. 59:768-771. [DOI] [PubMed] [Google Scholar]
- 14.Caughey, B., and G. J. Raymond. 1991. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 266:18217-18223. [PubMed] [Google Scholar]
- 15.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, S. G., D. B. Teplow, P. Parchi, J. K. Teller, P. Gambetti, and L. Autilio-Gambetti. 1995. Truncated forms of the human prion protein in normal brain and in prion diseases. J. Biol. Chem. 270:19173-19180. [DOI] [PubMed] [Google Scholar]
- 17.Chen, T. J., S. S. Chen, R. E. Wu, D. C. Wang, and C. H. Lin. 2005. Implication of nNOS in the enlargement of AChR aggregates but not the initial aggregate formation in a novel coculture model. Chin. J. Physiol. 48:129-138. [PubMed] [Google Scholar]
- 18.Chiesa, R., A. Pestronk, R. E. Schmidt, W. G. Tourtellotte, B. Ghetti, P. Piccardo, and D. A. Harris. 2001. Primary myopathy and accumulation of PrPSc-like molecules in peripheral tissues of transgenic mice expressing a prion protein insertional mutation. Neurobiol. Dis. 8:279-288. [DOI] [PubMed] [Google Scholar]
- 19.Cisse, M. A., C. Sunyach, S. Lefranc-Jullien, R. Postina, B. Vincent, and F. Checler. 2005. The disintegrin ADAM9 indirectly contributes to the physiological processing of cellular prion by modulating ADAM10 activity. J. Biol. Chem. 280:40624-40631. [DOI] [PubMed] [Google Scholar]
- 20.Cooper, S. T., A. L. Maxwell, E. Kizana, M. Ghoddusi, E. C. Hardeman, I. E. Alexander, D. G. Allen, and K. N. North. 2004. C2C12 co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil. Cytoskel. 58:200-211. [DOI] [PubMed] [Google Scholar]
- 21.Cronier, S., H. Laude, and J. M. Peyrin. 2004. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl. Acad. Sci. USA 101:12271-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dedieu, S., G. Mazeres, S. Poussard, J. J. Brustis, and P. Cottin. 2003. Myoblast migration is prevented by a calpain-dependent accumulation of MARCKS. Biol. Cell 95:615-623. [DOI] [PubMed] [Google Scholar]
- 23.Dedieu, S., S. Poussard, G. Mazeres, F. Grise, E. Dargelos, P. Cottin, and J. J. Brustis. 2004. Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp. Cell Res. 292:187-200. [DOI] [PubMed] [Google Scholar]
- 24.DeJoia, C., B. Moreaux, K. O'Connell, and R. A. Bessen. 2006. Prion infection of oral and nasal mucosa. J. Virol. 80:4546-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gohel, C., V. Grigoriev, F. Escaig-Haye, C. I. Lasmezas, J. P. Deslys, J. Langeveld, M. Akaaboune, D. Hantai, and J. G. Fournier. 1999. Ultrastructural localization of cellular prion protein (PrPc) at the neuromuscular junction. J. Neurosci. Res. 55:261-267. [DOI] [PubMed] [Google Scholar]
- 26.Haig, D. A., and M. C. Clarke. 1971. Multiplication of the scrapie agent. Nature 234:106-107. [DOI] [PubMed] [Google Scholar]
- 27.Herzog, C., J. Riviere, N. Lescoutra-Etchegaray, A. Charbonnier, V. Leblanc, N. Sales, J. P. Deslys, and C. I. Lasmezas. 2005. PrPTSE distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt-Jakob disease. J. Virol. 79:14339-14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetz, C., M. Russelakis-Carneiro, K. Maundrell, J. Castilla, and C. Soto. 2003. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22:5435-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jewell, J. E., J. Brown, T. Kreeger, and E. S. Williams. 2006. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J. Gen. Virol. 87:3443-3450. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Huete, A., P. M. Lievens, R. Vidal, P. Piccardo, B. Ghetti, F. Tagliavini, B. Frangione, and F. Prelli. 1998. Endogenous proteolytic cleavage of normal and disease-associated isoforms of the human prion protein in neural and non-neural tissues. Am. J. Pathol. 153:1561-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanu, N., Y. Imokawa, D. N. Drechsel, R. A. Williamson, C. R. Birkett, C. J. Bostock, and J. P. Brockes. 2002. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 12:523-530. [DOI] [PubMed] [Google Scholar]
- 32.Kitamoto, T., T. Muramoto, S. Mohri, K. Doh-Ura, and J. Tateishi. 1991. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J. Virol. 65:6292-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korth, C., B. Stierli, P. Streit, M. Moser, O. Schaller, R. Fischer, W. Schulz-Schaeffer, H. Kretzschmar, A. Raeber, U. Braun, F. Ehrensperger, S. Hornemann, R. Glockshuber, R. Riek, M. Billeter, K. Wuthrich, and B. Oesch. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74-77. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs, G. G., O. Kalev, E. Gelpi, C. Haberler, J. Wanschitz, M. Strohschneider, M. J. Molnar, L. Laszlo, and H. Budka. 2004. The prion protein in human neuromuscular diseases. J. Pathol. 204:241-247. [DOI] [PubMed] [Google Scholar]
- 35.Langen, R. C., A. M. Schols, M. C. Kelders, E. F. Wouters, and Y. M. Janssen-Heininger. 2003. Enhanced myogenic differentiation by extracellular matrix is regulated at the early stages of myogenesis. In Vitro Cell Dev. Biol. Anim. 39:163-169. [DOI] [PubMed] [Google Scholar]
- 36.Lezmi, S., F. Ronzon, A. Bencsik, A. Bedin, D. Calavas, Y. Richard, S. Simon, J. Grassi, and T. Baron. 2006. PrP(d) accumulation in organs of ARQ/ARQ sheep experimentally infected with BSE by peripheral routes. Acta Biochim. Pol. 53:399-405. [PubMed] [Google Scholar]
- 37.Magalhaes, A. C., G. S. Baron, K. S. Lee, O. Steele-Mortimer, D. Dorward, M. A. Prado, and B. Caughey. 2005. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 25:5207-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBride, P. A., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168:413-418. [DOI] [PubMed] [Google Scholar]
- 39.Mulcahy, E. R., J. C. Bartz, A. E. Kincaid, and R. A. Bessen. 2004. Prion infection of skeletal muscle cells and papillae in the tongue. J. Virol. 78:6792-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 41.Nazor, K. E., F. Kuhn, T. Seward, M. Green, D. Zwald, M. Purro, J. Schmid, K. Biffiger, A. M. Power, B. Oesch, A. J. Raeber, and G. C. Telling. 2005. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J. 24:2472-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostlund, P., H. Lindegren, C. Pettersson, and K. Bedecs. 2001. Altered insulin receptor processing and function in scrapie-infected neuroblastoma cell lines. Brain Res. Mol. Brain Res. 97:161-170. [DOI] [PubMed] [Google Scholar]
- 43.Peden, A. H., D. L. Ritchie, M. W. Head, and J. W. Ironside. 2006. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am. J. Pathol. 168:927-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priola, S. A., B. Caughey, R. E. Race, and B. Chesebro. 1994. Heterologous PrP molecules interfere with accumulation of protease-resistant PrP in scrapie-infected murine neuroblastoma cells. J. Virol. 68:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Race, R. E., L. H. Fadness, and B. Chesebro. 1987. Characterization of scrapie infection in mouse neuroblastoma cells. J. Gen. Virol. 68:1391-1399. [DOI] [PubMed] [Google Scholar]
- 46.Raymond, G. J., E. A. Olsen, K. S. Lee, L. D. Raymond, P. K. Bryant, 3rd, G. S. Baron, W. S. Caughey, D. A. Kocisko, L. E. McHolland, C. Favara, J. P. Langeveld, F. G. van Zijderveld, R. T. Mayer, M. W. Miller, E. S. Williams, and B. Caughey. 2006. Inhibition of protease-resistant prion protein formation in a transformed deer cell line infected with chronic wasting disease. J. Virol. 80:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson, C., S. A. Booth, D. R. Beniac, M. B. Coulthart, T. F. Booth, and A. McNicol. 2006. Cellular prion protein is released on exosomes from activated platelets. Blood 107:3907-3911. [DOI] [PubMed] [Google Scholar]
- 48.Rubenstein, R., R. I. Carp, and S. M. Callahan. 1984. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J. Gen. Virol. 65:2191-2198. [DOI] [PubMed] [Google Scholar]
- 49.Schatzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71:8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taraboulos, A., A. J. Raeber, D. R. Borchelt, D. Serban, and S. B. Prusiner. 1992. Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell 3:851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatzelt, J., J. Zuo, R. Voellmy, M. Scott, U. Hartl, S. B. Prusiner, and W. J. Welch. 1995. Scrapie prions selectively modify the stress response in neuroblastoma cells. Proc. Natl. Acad. Sci. USA 92:2944-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomzig, A., C. Kratzel, G. Lenz, D. Kruger, and M. Beekes. 2003. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 4:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomzig, A., W. Schulz-Schaeffer, C. Kratzel, J. Mai, and M. Beekes. 2004. Preclinical deposition of pathological prion protein PrPSc in muscles of hamsters orally exposed to scrapie. J. Clin. Investig. 113:1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent, B., E. Paitel, P. Saftig, Y. Frobert, D. Hartmann, B. De Strooper, J. Grassi, E. Lopez-Perez, and F. Checler. 2001. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J. Biol. Chem. 276:37743-37746. [DOI] [PubMed] [Google Scholar]
- 56.Vorberg, I., A. Raines, and S. A. Priola. 2004. Acute formation of protease-resistant prion protein does not always lead to persistent scrapie infection in vitro. J. Biol. Chem. 279:29218-29225. [DOI] [PubMed] [Google Scholar]
- 57.Westaway, D., S. J. DeArmond, J. Cayetano-Canlas, D. Groth, D. Foster, S. L. Yang, M. Torchia, G. A. Carlson, and S. B. Prusiner. 1994. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 76:117-129. [DOI] [PubMed] [Google Scholar]
- 58.Yadavalli, R., R. P. Guttmann, T. Seward, A. P. Centers, R. A. Williamson, and G. C. Telling. 2004. Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J. Biol. Chem. 279:21948-21956. [DOI] [PubMed] [Google Scholar]
- 59.Yaffe, D., and O. Saxel. 1977. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725-727. [DOI] [PubMed] [Google Scholar]
- 60.Zanusso, G., G. Vattemi, S. Ferrari, M. Tabaton, E. Pecini, T. Cavallaro, G. Tomelleri, M. Filosto, P. Tonin, E. Nardelli, N. Rizzuto, and S. Monaco. 2001. Increased expression of the normal cellular isoform of prion protein in inclusion-body myositis, inflammatory myopathies and denervation atrophy. Brain Pathol. 11:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong, Z. G., M. Noda, H. Takahashi, and H. Higashida. 1999. Overexpression of rat synapsins in NG108-15 neuronal cells enhances functional synapse formation with myotubes. Neurosci. Lett. 260:93-96. [DOI] [PubMed] [Google Scholar]