Abstract
The mechanisms of cellular entry of dengue and West Nile viruses are not well characterized. We show that both these viruses enter HeLa cells by clathrin-dependent endocytosis and require vacuolar acidic pH. Inhibition of the GTPase Rab 5 or 7, which regulates transport to early or late endosomes, respectively, demonstrated that Rab 5 was essential for survival of both dengue and West Nile virus. These data broaden our understanding of the pathways required for productive dengue and West Nile virus infection and may facilitate new strategies for combating disease.
Dengue virus (DNV) and West Nile virus (WNV) are medically important flaviviruses that contain class II viral envelope proteins. Several aspects of the intracellular survival of DNV and WNV are known; however, the molecular mechanisms governing their entry into host cells have not been fully delineated (2, 4, 7). Viruses with class II envelope proteins require acidic pH in different intracellular compartments to trigger viral fusion with the host membrane (13, 22). An earlier study, however, reported that DNV entry occurred by direct fusion with the plasma membrane in both mammalian and mosquito cells (12, 20). Because of this discrepancy, further investigations are required to characterize the exact mechanisms involved in the cellular entry of DNV. WNV is reported to enter cells through clathrin-dependent endocytosis and colocalize with both early and late endosomes (3). Microtubule disruption impairs WNV infectivity, and because early to late endosomal transport requires microtubules, it was interpreted that WNV requires transport from early to late endosomes for infection (3). However, microtubule disruption may affect multiple cellular functions, and hence, the specificity of the requirement of early and late endosomal compartments for the completion of the life cycle of WNV is not known. In this report, we characterize the precise cellular uptake pathways and compartments involved in the entry of DNV and WNV into HeLa cells, using chemical inhibitors, dominant-negative mutant gene overexpression, and RNA interference (RNAi).
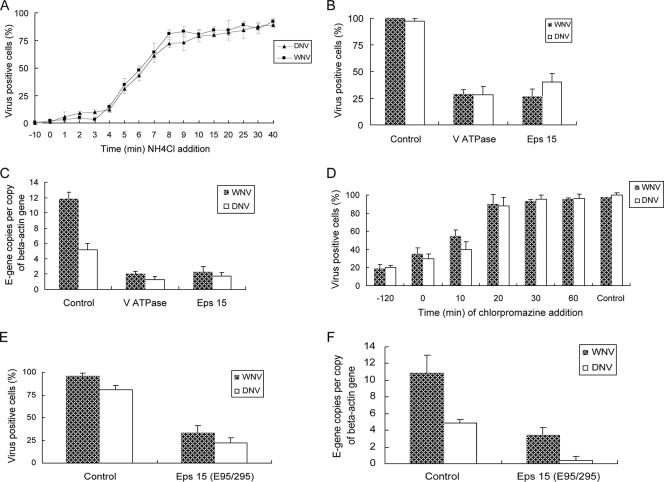
We first tested the requirement of low endosomal pH for the entry of these viruses using the endosomotropic weak base NH4Cl, because it instantaneously raises the pH of cellular vacuolar compartments (23, 24). HeLa cells preadsorbed with the viruses for 1 h at 4°C (multiplicity of infection [MOI] of 10) were transferred to 37°C, and then culture medium containing 20 mM NH4Cl was added and incubated for 16 h or 10 h for DNV and WNV, respectively. The 2741 strain of WNV (John F. Anderson, Connecticut Agricultural Experiment Station) (1) and DNV serotype 2 (New Guinea C strain) (Aravinda de Silva, University of North Carolina) were used throughout. NH4Cl strongly inhibited the infection of both DNV and WNV (∼90%; P < 0.05) when added prior to, or up to 5 min after, incubation at 37°C, suggesting that their infections have an early pH-dependent step (Fig. 1A). We further tested the involvement of acidic pH in the entry of DNV and WNV by silencing the gene encoding vacuolar ATPase (VATPase), a proton pump key to establishing the low pH of endosomal compartments (15). HeLa cells transfected with 50 nM of small interfering RNAs (siRNAs) targeting VATPase and a nonspecific sequence (Dharmacon) for 4 days were exposed to either DNV or WNV (MOI of 10) for 16 or 10 h (at 37 °C), respectively, and analyzed by both immunofluorescence and quantitative reverse transcription-PCR (Q-RT-PCR). siRNA-treated cells were completely viable throughout the experimental period, and knockdown was verified at the transcript and protein levels (not shown). Both immunofluorescence- and Q-RT-PCR-based quantifications demonstrated up to 80% (P < 0.05) inhibition of DNV or WNV in VATPase-silenced cells, confirming the requirement of low endosomal pH for infection (Fig. 1B and C). The requirement of low vacuolar pH suggests that DNV may be entering HeLa cells by endocytosis. Treatment of HeLa cells with the receptor-mediated endocytosis inhibitor chlorpromazine (10 μg/ml) reduced infection of DNV and WNV by up to 80% (P < 0.01) (Fig. 1D). Both NH4Cl and chlorpromazine were not cytotoxic within the experimental time frame. RNAi-based silencing (Fig. 1B and C) and dominant-negative mutant overexpression (Fig. 1E and F and 2D to F or P to R) of Eps15, a gene required for clathrin-dependent endocytosis (18), also reduced infection of DNV and WNV by up to 80% (P < 0.05) and 70% (P < 0.05), respectively. The inhibitory effect of chlorpromazine and the dominant-negative Eps15 mutant on WNV infection observed in this study are similar to those in a previous report (3).
FIG. 1.
Effect of NH4Cl (A) and chlorpromazine (D) and depletion of VATPase and Eps15 (B, C, E, and F) on DNV or WNV infectivity of HeLa cells. (A and D) HeLa cells preadsorbed with the viruses for 1 h at 4°C (MOI of 10) were transferred to 37°C and incubated for the desired time periods, culture medium containing 20 mM NH4Cl or 10 μM chlorpromazine was added, and the cells were grown for 16 h or 10 h for DNV or WNV, respectively. After the incubation, cells were fixed and immunofluorescence was performed. (B and C) HeLa cells transfected with siRNA against VATPase and Eps15 for 4 days were infected with DNV and WNV (MOI of 10) for 16 h or 10 h, respectively, and analyzed by immunofluorescence (B) or Q-RT-PCR to quantify viral E-gene copies (C). (E and F) HeLa cells transfected with pEGFP-C vector expressing a dominant-negative mutant of Eps15 (E95/295) for 30 h were infected with DNV or WNV (MOI of 10) for 16 h or 10 h, respectively, and analyzed by immunofluorescence (E) or Q-RT-PCR (F) to quantify viral E-gene copies. E-gene copies were normalized using beta actin gene copies. Images were captured using a magnification ×40 objective by fluorescence microscopy. Quantification was done by counting the total number of infected cells in each captured image (corresponds to an average of 100 cells per image) and expressed as percent infected cells of the total cells. Results are expressed as means ± standard deviations from triplicates of a representative experiment.
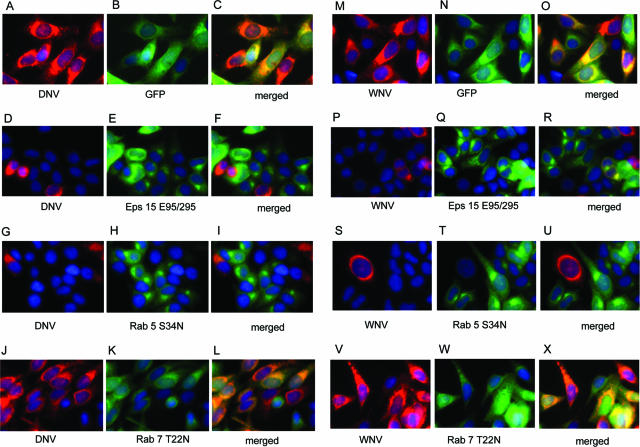
FIG. 2.
Effect of overexpression of green fluorescent protein (GFP)-tagged dominant-negative mutant of Eps15 (D, E, and F or P, Q, and R), Rab 5 (G, H, and I or S, T, and U), or Rab 7 (J, K, and L or V, W, and X) on DNV or WNV infection, respectively. HeLa cells transfected with EGFP-C vector expressing a dominant-negative mutant gene of Eps15 (E95/295) or pQCXIP vector having a dominant-negative Rab 5 or 7 mutant (Rab 5 S34N or Rab 7 T22N) for 30 h was infected with DNV or WNV (MOI of 10) for 16 or 10 h, respectively, and analyzed by immunofluorescence. Control cells were transfected with GFP vector (A, B, and C or M, N, and O). Images were captured by fluorescence microscopy using an ×40 objective lens. Green, either the mutant gene or GFP alone (control); red, virus; blue, nucleus.
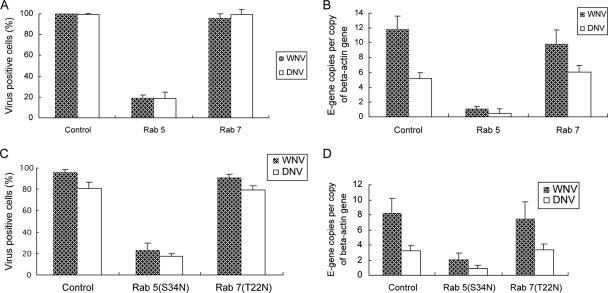
Rab 5 and 7 GTPases are the key regulators of transport to early and late endosomes (8, 19, 25), and ablation of Rab 5 and 7 has been used to study viral entry (14, 21). To identify the exact endosomal compartment(s) traversed by endocytosed DNV and WNV, we silenced Rab 5 and 7 using RNAi. Rab 5 and 7 were strongly repressed at the RNA level (74% for Rab 5 [P < 0.05] and 81% for Rab 7 [P < 0.05]) and protein level (72% and 77%, respectively) by RNAi. Lysosomal degradation of internalized horseradish peroxidase by HeLa cells was used to validate that knockdown of Rab 5 and 7 indeed blocks transport through the respective compartments (not shown). Immunofluorescence showed that only up to 20% (P < 0.05) of the total cells treated with Rab 5 siRNA were permissive to either DNV or WNV (Fig. 4A). Using Q-RT-PCR, we also detected a 10-fold (P < 0.001) or 11-fold (P < 0.05) reduction in the multiplication of DNV and WNV, respectively, in Rab 5-repressed cells (Fig. 4B). Depletion of Rab 7 expression did not have any impact on the infectivity of either DNV or WNV (Fig. 4A and B). We also utilized dominant-negative mutants of Rab 5 (Rab 5 S34N) and 7 (Rab 7 T22N) to assess the requirement of transport to early and late endosomes in the entry of DNV and WNV. Similar to the results of knockdown experiments, Rab 5 dominant-negative overexpression reduced the infection by DNV and WNV up to 70% (P < 0.05), as determined by both immunofluorescence (Fig. 4C and 2G to I and S to U) and Q-RT-PCR (Fig. 4D). Rab 7 mutant overexpression did not impair DNV and WNV infection (Fig. 4C and D and 2J to L and V to X). Thus, these two lines of evidence suggest that DNV and WNV require expression of Rab 5 but not Rab 7 for infection of HeLa cells.
FIG. 4.
Effect of functional repression of Rab 5 and 7 on DNV or WNV infection, using RNAi (A and B) or dominant-negative mutant overexpressions (C and D). HeLa cells treated with the corresponding siRNA for 4 days were infected with DNV for 16 h or WNV for 10 h (MOI of 10) and processed for immunofluorescence (A) or Q-RT-PCR to quantify viral E-gene copies (B). Images were captured by fluorescence microscopy (×40 objective). Panels C and D shows the results of the effect of Rab 5 and Rab 7 dominant-negative mutant overexpression on DNV and WNV infections. HeLa cells transfected with mutant genes for 30 h were infected with DNV or WNV (MOI of 10) for 16 h or 10 h, respectively, and analyzed by immunofluorescence (C) or Q-RT-PCR to quantify viral E-gene copies (D). Control cells were transfected with GFP vector. E-gene copies were normalized with beta actin gene copies. Results in panels A and C were prepared by visually counting three random fields of images from representative experiments and expressed as percent infected cells of the total cells. Results are expressed as mean ± standard deviations from a representative experiment.
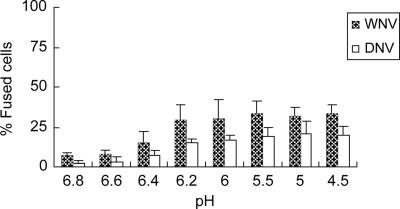
We then used a virus-induced cell-cell fusion assay to examine the fusion-initiating pH threshold of these two highly related viruses as an indirect measure of viral fusion location. Virus-infected and virus-preadsorbed HeLa cells labeled with CellTracker Green CMFDA (Molecular Probes) fluorescent dye were mixed together and exposed to buffers of various pHs (9, 16). As shown in Fig. 3, the number of cells engaged in fusion induced by both DNV and WNV increased notably at pH 6.4 or below (up to 29% [p = 0.05] for WNV and 21% [p < 0.05] for DNV).
FIG. 3.
Virus-mediated, pH-induced cell-cell fusion by DNV and WNV. Cells (labeled with the fluorescent probe CellTracker Green CMFDA; Molecular Probes) infected with virus for 20 h (MOI of 10) or preadsorbed with virus on surface by incubation with virus at an MOI of 200 for 1 h at 4°C (both labeled with the fluorescent probe CellTracker Green CMFDA) were mixed at a 1:1 ratio at 1 × 106 cells/ml in buffers of various pHs for up to 5 min at 37°C. Soon after the incubation, the low-pH buffer was replaced with serum-free medium. The cell mixture was then plated onto 384-well plates at a density of 9,000 cells per well, spun down at 220 × g for 2 min, and imaged under a fluorescence microscope using a ×10 objective lens. Results were prepared by visually counting three random fields of images from representative experiments and expressed as percentages of the total cells involved in cell-cell fusion. Results are expressed as means ± standard deviations from a representative experiment.
In this report, we demonstrated that both DNV and WNV enters HeLa cells through clathrin-dependent endocytosis and that after endocytosis, both DNV and WNV require transport to early—but not late—endosomes before an acidic pH-dependent step presumably results in the release of the viral genome into the cytoplasm, leading to successful infection. In addition, we determined that the pH thresholds for membrane fusion of both DNV and WNV are in the same range, thus further indicating that they fuse presumably in the same pH compartment within the cells. Both viruses induce notable cell-cell fusion below pH 6.4, reaching a maximum at 6.2 or lower, a pH similar to that of early endosomes (17). Earlier studies also demonstrated that WNV fuses with cellular membranes with various efficiencies below pH 6.7 (5, 6, 10, 11). The findings of this study are notable because previous studies reported that DNV enters its host cells by direct fusion with the plasma membrane, though none of those studies involved HeLa cells (12, 20). In summary, this report broadens our understanding of the host cellular entry mechanisms adopted by DNV and WNV, two important human pathogens. It is reasonable to presume that related flaviviruses may use similar mechanisms, which can potentially be exploited for the development of novel therapeutics.
Acknowledgments
We thank Alice Dautry-Varsat for the Eps 15 mutant and Tatjana Dragic for Rab 5 and 7 mutants.
This work was funded by grants from the NIH.
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Wakem, R. A. French, A. E. Garmendia, and H. J. Van Kruiningen. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331-2333. [DOI] [PubMed] [Google Scholar]
- 2.Brinton, M. A. 2002. The molecular biology of West Nile virus: a new invader of the western hemisphere. Annu. Rev. Microbiol. 56:371-402. [DOI] [PubMed] [Google Scholar]
- 3.Chu, J. J., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clyde, K., J. L. Kyle, and E. Harris. 2006. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J. Virol. 80:11418-11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gollins, S. W., and J. S. Porterfield. 1985. Flavivirus infection enhancement in macrophages: an electron microscopic study of viral cellular entry. J. Gen. Virol. 66:1969-1982. [DOI] [PubMed] [Google Scholar]
- 6.Gollins, S. W., and J. S. Porterfield. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J. Gen. Virol. 67:157-166. [DOI] [PubMed] [Google Scholar]
- 7.Halstead, S. B., F. X. Heinz, A. D. Barrett, and J. T. Roehrig. 2005. Dengue virus: molecular basis of cell entry and pathogenesis, 25-27 June 2003, Vienna, Austria. Vaccine 23:849-856. [DOI] [PubMed] [Google Scholar]
- 8.Jordens, I., M. Marsman, C. Kuijl, and J. Neefjes. 2005. Rab proteins, connecting transport and vesicle fusion. Traffic 6:1070-1077. [DOI] [PubMed] [Google Scholar]
- 9.Kielian, M. C., S. Keranen, L. Kaariainen, and A. Helenius. 1984. Membrane fusion mutants of Semliki Forest virus. J. Cell Biol. 98:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura, T., S. W. Gollins, and J. S. Porterfield. 1986. The effect of pH on the early interaction of West Nile virus with P388D1 cells. J. Gen. Virol. 67:2423-2433. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, T., and A. Ohyama. 1988. Association between the pH-dependent conformational change of West Nile flavivirus E protein and virus-mediated membrane fusion. J. Gen. Virol. 69:1247-1254. [DOI] [PubMed] [Google Scholar]
- 12.Lim, H. Y., and M. L. Ng. 1999. A different mode of entry by dengue-2 neutralisation escape mutant virus. Arch. Virol. 144:989-995. [DOI] [PubMed] [Google Scholar]
- 13.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meertens, L., C. Bertaux, and T. Dragic. 2006. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 80:11571-11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson, N. 2003. A journey from mammals to yeast with vacuolar H+-ATPase (V-ATPase). J. Bioenerg. Biomembr. 35:281-289. [DOI] [PubMed] [Google Scholar]
- 16.Ogino, M., K. Yoshimatsu, H. Ebihara, K. Araki, B. H. Lee, M. Okumura, and J. Arikawa. 2004. Cell fusion activities of Hantaan virus envelope glycoproteins. J. Virol. 78:10776-10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachse, M., G. Ramm, G. Strous, and J. Klumperman. 2002. Endosomes: multipurpose designs for integrating housekeeping and specialized tasks. Histochem. Cell Biol. 117:91-104. [DOI] [PubMed] [Google Scholar]
- 18.Salcini, A. E., H. Chen, G. Iannolo, P. De Camilli, and P. P. Di Fiore. 1999. Epidermal growth factor pathway substrate 15, Eps15. Int. J. Biochem. Cell. Biol. 31:805-809. [DOI] [PubMed] [Google Scholar]
- 19.Schimmoller, F., I. Simon, and S. R. Pfeffer. 1998. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273:22161-22164. [DOI] [PubMed] [Google Scholar]
- 20.Se-Thoe, S. Y., A. E. Ling, and M. M. Ng. 2000. Alteration of virus entry mode: a neutralisation mechanism for Dengue-2 virus. J. Med. Virol. 62:364-376. [DOI] [PubMed] [Google Scholar]
- 21.Sieczkarski, S. B., and G. R. Whittaker. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4:333-343. [DOI] [PubMed] [Google Scholar]
- 22.Sieczkarski, S. B., and G. R. Whittaker. 2005. Viral entry. Curr. Top. Microbiol. Immunol. 285:1-23. [DOI] [PubMed] [Google Scholar]
- 23.Sturzenbecker, L. J., M. Nibert, D. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vonderheit, A., and A. Helenius. 2005. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 3:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]